Abstract
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS), characterized by infiltrating myelin-reactive T lymphocytes and demyelinating lesions. Experimental autoimmune encephalomyelitis (EAE) is the animal model widely utilized to study MS. EAE is mediated by CD4+ T cells and can be induced in EAE-susceptible mice through immunization with a myelin antigen, such as proteolipid protein 139–151 (PLP139-151) in SJL mice. In this PLP-induced EAE model, autoreactive CD4+ T cells migrate from peripheral tissues into the CNS where they are reactivated resulting in CNS damage. Th1 and Th17 cells produce the pro-inflammatory cytokines IFNγ and IL-17, respectively, that have been shown to have pathogenic roles in EAE and MS. Anti-inflammatory Th2, IL-4 secreting cells, have been indicated to inhibit EAE exacerbation. However, given the inflammatory environment of EAE, Th2 effector cells are outnumbered by Th1/Th17 cells. Regulatory CD4+ T cells suppress immune reactions and have been demonstrated to be dysfunctional in MS patients. Opioid growth factor (OGF), chemically termed [Met5]-enkephalin, is a negative growth factor that interacts with the OGF receptor. The OGF-OGFr axis can be activated through exogenous administration of OGF or a low dosage of naltrexone (LDN), an opioid antagonist. We have previously demonstrated that modulation of the OGF-OGFr axis results in alleviation from relapse-remitting EAE, and that CNS-infiltrating CD3+ T cells are diminished with exogenous OGF or intermittent blockade with LDN administration. In this paper, we aimed to determine whether OGF or LDN alter the Th effector responses of CD4+ T lymphocytes within the CNS in established EAE. We report in these studies that the numbers of CD4+ T lymphocytes in the CNS of EAE mice are decreased following treatment with OGF for five days but not LDN. However, modulation of the OGF-OGFr axis did not result in changes to CD4+ Th effector cell responses in the CNS of EAE mice.
Keywords: OGF, low-dose naltrexone, spinal cord, flow cytometry, IL-17, IL-2, IL-4, cytokines, multiple sclerosis
Introduction
Multiple sclerosis (MS) is an autoimmune disorder1 affecting approximately 2.3 million individuals worldwide.2,3 MS is characterized by inflammatory cells, mainly autoreactive lymphocytes and macrophages, infiltrating into the central nervous system (CNS) and giving rise to an inflammatory cascade that results in demyelinated plaques.4 The etiology of MS is unknown. Experimental autoimmune encephalomyelitis (EAE), the animal model of MS, is a CD4+ T-cell mediated disorder that effects the immune system and has pathophysiological similarities to MS, including infiltrating lymphocytes, demyelination, and neurodegeneration.5 EAE can be actively induced in the SJL mouse, a highly EAE-susceptible strain, through immunization with proteolipid peptide 139-151 (PLP139–151), resulting in activation of PLP-specific CD4+ T cells.6 The myelin-specific CD4+ T cells migrate to the CNS by crossing the blood–brain barrier (BBB) and are reactivated resulting in a series of events leading to demyelination.7
The effector cells and their associated cytokines that play a pathogenic and ameliorative role in EAE are Th1 (IFNγ), Th17 (IL-17) and Th2 (IL-4), and regulatory T cells (Tregs), respectively. Pro-inflammatory cells, Th1 and Th17, are found in high frequency in lymph nodes, spleens and spinal cords of EAE mice during acute disease.8–10 Prior to the discovery of IL-17-secreting Th17 cells, IFNγ-secreting Th1 cells were considered the primary cell responsible for the development of EAE.11
In 2005, Langrish et al. examined the role of IL-23 in EAE by inducing disease in IL-23p19 deficient mice.12 The IL-23p19−/− mice were resistant to EAE, suggesting an important role for IL-23 in CNS autoimmune diseases.12 Furthermore, CD4+ T cells isolated from IL-23 deficient mice displayed reduced IL-6, TNF, and undetectable IL-17 production, despite the presence of IFNγ within the CNS.12 In vitro studies confirmed these findings, demonstrating that in vivo antigen-primed lymphocytes cultured in the presence of IL-23 produce IL-17 by CD4+ cells.12 These findings suggested that Th1 is not a requirement for EAE development, and that Th17 is critical for EAE development. However, more recent studies indicate that both Th1 and Th17 are involved in disease manifestation.13
A primary focus of MS therapeutic research has been to shift from the T1/Th17 to a Th2/regulatory T-cell (Treg) paradigm.14 Th2 and Treg T cells are anti-inflammatory cells that help suppress immune responses, a response that is impaired in autoimmune disease such as EAE/MS.15,16 Animal studies have confirmed that IL-4, the cytokine secreted by Th2 cells, alleviates EAE development.17 Constantinescu et al. compared myelin basic protein (MBP)-stimulated lymphocytes isolated from EAE-susceptible SJL/J mice with those in BALB/c mice resistant to EAE, looking specifically at Th1 and Th2 cytokines, IFNγ and IL-4 production.18 These authors found that MBP-stimulated lymphocytes from SJL/J mice produced predominantly IFNγ and insignificant amounts of IL-4, while lymphocytes from BALB/c mice exhibited the inverse.18 Proteins encoded by the MBP gene are associated with myelin formation in both the central and peripheral nervous system. Immunization using MBP as the antigen may increase blood–brain barrier permeability and facilitate T-cell activation. Thus, the data suggest that the IL-4 cytokine is important for immune protection because BALB/c mice that secrete this cytokine are EAE resistant.
Tregs, which express Foxp3, have been studied thoroughly in EAE models, and have been reported to prevent disease development and lessen disease severity.19 Tregs also have been identified in MS patients’ peripheral blood and are thought to play a role in immune homeostasis by negatively regulating inflammatory responses through secretion of TGFβ or IL-10.15
Opioid growth factor (OGF), chemically termed [Met5]-enkephalin, is a tonically active neuropeptide, that is produced in an autocrine fashion and secreted.20 OGF has a potent inhibitory growth effect mediated through interaction with the opioid growth factor receptor (OGFr).20 OGFr is a non-classical opioid receptor that is ubiquitously expressed.20 The OGF-OGFr axis is present in T and B lymphocytes in vitro.21,22 Stimulated T and B cells were treated with OGF and displayed a significant decrease in proliferation, indicating that the OGF-OGFr axis is present in activated T and B cells and can be modulated to impair proliferation.21,22 We have previously demonstrated that regulation of the OGF-OGFr axis with both OGF and low doses of naltrexone (LDN) decreases clinical disease severity, as well as diminishes the pathological damage found within the spinal cord of both chronic and relapse-remitting (RR) EAE mice.23–27 Since it is known that CD4+ Th cells play a dominant role in the development and/or alleviation of EAE pathogenesis, and that the OGF-OGFr axis is present in T cells, we sought to determine whether modulation of this axis alters CD4+ T-lymphocyte effector responses. This study determined whether OGF or LDN administration beginning at the time of immunization alters the immune response in the CNS. We treated mice with established PLP-induced EAE with OGF or LDN for five days to determine whether modulation of the OGF-OGFr axis alters Th effector responses in the CNS. Brain and spinal cord tissue were harvested at peak disease and CD4+ T lymphocytes were stained intracellularly for Th cytokine production (IFNγ, IL-4, IL-17) and Foxp3 transcription factor, followed by flow cytometry analysis.
Materials and methods
Animals
Female, 6–8-week-old SJL/JOrlCRL mice (Charles River Labs, Wilmington, MA) were housed five per cage under standard conditions in a room separated from other rodents, and acclimated for one week prior to disease induction; food and sterile water were available ad libitum. As the course of EAE progressed, soft food and HydroGel (ClearH2O, Portland, ME) were placed on the floor of the cages. All experiments were conducted following NIH guidelines on animal care, and were approved by the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee.
Induction of EAE
EAE was induced in 6–8-week-old female SJL/JOrlCRL (SJL) mice by subcutaneous immunization with 100 µg of myelin proteolipid protein 139–151 (PLP139-151) (Peptides International, Louisville, KY), as previously published.26,27 Mice were injected with an emulsion containing PLP139–151 in 0.15 ml sterile phosphate buffered saline (PBS) and 0.15 ml incomplete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO); this solution (1:1) was supplemented with 250 µg Mycobacterium tuberculosis (H37RA, Difco Laboratories, Detroit, MI). Mice were anesthetized with 3% isoflurane (Vedco, Inc., St. Joseph, MO) and injected subcutaneously (s.c.) at multiple regions of the back with 100 μl (total volume of 300 μl) of PLP139–151. Intraperitoneal (i.p.) injections of 200 ng pertussis toxin (List Biological Laboratories, INC., Campbell, CA) were administered on days 0 and 2 post immunization.
Drug treatments
Mice were randomly assigned to treatment groups beginning at the time of established EAE (behavioral score of 0.5 or greater for 2 consecutive days). Animals received i.p. injections (0.1 ml) of 10 mg/kg OGF (Polypeptide Laboratories, Torrance, CA) (EAE + OGF), 0.1 mg/kg naltrexone (EAE + LDN) (Sigma-Aldrich, Indianapolis, IN), or sterile saline (EAE + Saline).
Behavioral observations
Mice were observed daily for clinical disease presentation by two individuals (one observer masked to treatment). Behavior was assessed according to previously published protocols and utilized a 10-point scale consisting of analysis of gait, limb strength, tail tonicity and righting reflex.24,26,27 Disease onset was defined as the second consecutive day that a mouse had a behavioral score of 0.5 or greater.
Isolation of mononuclear cells from CNS
Following anesthesia with ketamine (30 mg/kg), xylazine (5 mg/kg) and acepromazine (2 mg/kg) diluted in sterile water, mice were perfused with cold PBS. Brains and spinal cords were collected and mononuclear cells were isolated following published protocols.28,29 CNS tissue was digested in DMEM supplemented with 2.5 mg/ml collagenase D (Roche Diagnostics, Indianapolis, IN) and 10 µg DNAse I (Sigma-Aldrich, St. Louis, MO) at 37℃ for 30 min. The digestion was deactivated with 0.5 mM EDTA (Affymetrix USB, Cleveland, OH). A single cell suspension was prepared utilizing a 70 µm cell strainer and centrifugation for 5 min at 1500 r/min at room temperature (RT). Fresh Percoll gradients were prepared for each assay. Cells were resuspended in 4 ml of 70% Percoll and gently overlayed with 4 ml of 37% Percoll (plus 0.5 ml DMEM for color contrast) and then 4 ml of 30% Percoll and centrifuged at 1800 r/min (500 g), for 30 min at RT (slow accelerator and break off). The interface between 70% and 37% layers including the distinct white ring in the middle was transferred to a 15 ml conical tube and washed thoroughly with 0.5 mM PBS/EDTA; red blood cells remained in the tube. Cells were resuspended in trypan blue (1:5 dilution) and counted using a hemocytometer.
Intracellular cytokine staining
Cells (1–2 × 106) were stimulated for 4 h at 37℃ in a 6-well plate with 3 ml IMDM, 5 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich), and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of 2 µl/well brefeldin A (eBioscience, San Diego, CA). Harvested cells were spun down and resuspended in 2.4g2 Fc block and placed on ice for 15 min. Cells were surface stained with anti-CD4 FITC (1:100, RM4-5, eBioscience) for 15 min, fixed and permeabilized (Fix & Perm kit, GAS003, Invitrogen, Camarillo, CA). Final staining was completed using the following antibodies: 1:100, Foxp3 APC (FJK-16 s), IL-4 PE (11B11), IFNγ APC (XMG1.2), and IL-17 A PerCP Cy 5.5 (eBio17B7), or appropriate isotype controls (all antibodies were from eBioscience).28 Cells were sorted by the FacsCalibur (BD Biosciences, San Jose, CA); data were analyzed utilizing BD CellQuest Pro software (BD Bioscience).
Immunohistochemistry
Mice with EAE were humanely sacrificed after 40 days of treatment and perfused with 4% paraformaldehyde (PFA). Lumbar spinal cords were dissected out of the column and embedded in OCT compound and stored at −80℃ according to previously published protocols.26,27 Spinal cords were sectioned at 10 µm on a cryostat and collected on glycerol-subbed slides. Tissues were acclimated to room temperature, washed twice in sodium phosphate buffer (SPB), and blocked for 2 h with a solution of 1% bovine serum albumin (BSA), 5% normal goat serum (NGS) and SPB at room temperature. Spinal cords were double labeled with primary antibodies to rabbit anti-CD3 and rat anti-CD4 (1:200, Abcam, Cambridge, MA) at 4℃ overnight (at least 20 h). Slides were washed in 1% NGS/SPB twice for 15 min each, and blocked with 5% NGS/1% BSA in SPB for 1 h at room temperature. Tissues were incubated for 1 h at 4℃ with secondary goat-anti-rabbit TRITC (1:1000) and goat-anti-rat FITC (1:1000, Molecular Probes, Grand Island, NY) antibodies and counterstained with DAPI (1:5000). Slide Book Pro was used to acquire images at 20× magnification of the central ventral white matter. ImageJ (NIH) software was utilized for analysis. All CD3 + CD4+ double-labeled cells within the central ventral white matter of each section were counted. Positive cells were identified as DAPI-labeled nuclei surrounded by both TRITC and FITC. Two to three sections per mouse, 3–5 mice per treatment group were analyzed.
Statistical analysis
Data were analyzed by one-way analysis of variance (1-way ANOVA) or Student’s t-test where appropriate in GraphPad Prism 6 software (La Jolla, CA). Significance was established with p values less than 0.05.
Results
Behavior and general observations
OGF and LDN were capable of diminishing clinical disease scores, and decreased the number of CD3+ T lymphocytes within the lumbar spinal cord in an established EAE model.26,27 In previous studies, observations were limited to immunohistochemical analysis of the central ventral white matter of the lumbar spinal cord, which gave insight into how OGF and LDN altered EAE. However, in order to determine whether these findings were consistent throughout the CNS of EAE mice, or limited to the lumbar spinal cord, and whether OGF or LDN was capable of altering the CD4+ effector T lymphocytes during the effector phase of disease, mice were treated with OGF or LDN after they developed clinical disease in the current study. The CNS was evaluated at peak disease (day 5 of treatment when behavioral scores ranged >3), in order to detect the highest numbers of CNS infiltrates.
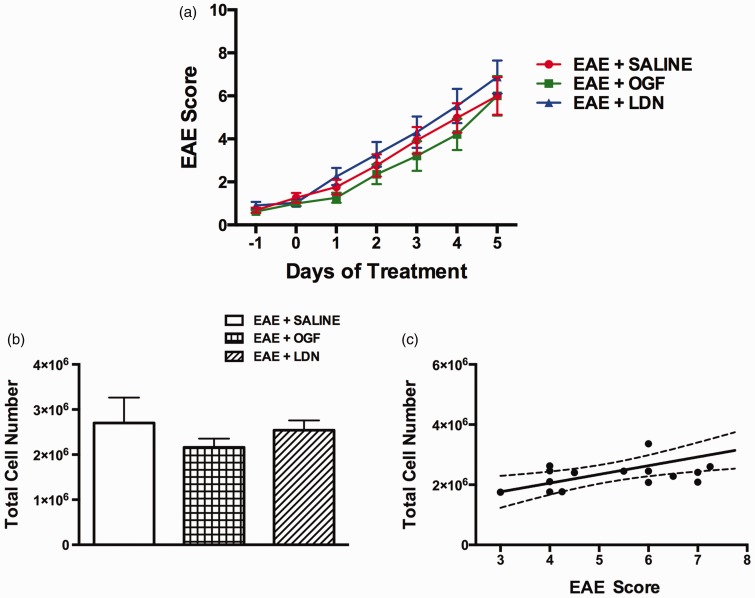
No animals died from immunizations; one mouse developed a small lesion at an injection site. OGF or LDN treatment for only five days beginning at the time of established disease had no effect on behavioral development (Figure 1(a)), a finding consistent with previous studies.26,27 However, longer exposure to OGF and LDN did alter the course of clinical disease presentation.27,27
Figure 1.
Modulation of the OGF-OGFr axis in animals with established EAE. SJL mice were immunized with PLP139–151 and allowed to develop disease. Mice were treated on the second consecutive day of clinical disease with 10 mg/kg OGF (EAE + OGF), 0.1 mg/kg naltrexone (EAE + LDN) or saline (EAE + SALINE). (a) Mean clinical disease scores over the course of the 14-day experiment. (b) Total number of mononuclear cells isolated from CNS (brain + spinal cord) is shown. (c) Correlation between clinical disease score and total mononuclear cells of all EAE mice is shown, Spearman r = 0.4898, p = 0.04.
EAE: experimental autoimmune encephalomyelitis; OGF: opioid growth factor; LDN: low dosage of naltrexone. (A color version of this figure is available in the online journal.)
Lack of effect on the number of CNS-infiltrating mononuclear cells by OGF or LDN in established EAE
Five days of exposure to OGF (10 mg/kg) or LDN (0.1 mg/kg naltrexone) did not alter the number of mononuclear cells in the CNS of mice with established EAE. Total cell numbers were calculated per tube, and each tube contained the brain and spinal cord from two mice. Inasmuch as possible, the brain and spinal cords were comparable for each tube of tissue; tissues were dissected carefully to include the entire brain and the same lumbar region of spinal cord. There were 2.7 million (±5.6 × 105) (SEM) mononuclear cells per tube of CNS tissue from EAE + saline mice, 2.1 million (±1.7 × 105) mononuclear cells per tube of CNS tissue from EAE mouse treated with OGF and 2.6 million (±2.2 × 105) for each EAE + LDN mouse (Figure 1(b)).
The number of infiltrated mononuclear cells into the CNS of EAE mice correlated to clinical disease scores (Figure 1(c)) as determined by Spearman correlation analysis (coefficient = 0.5925 and a p value of 0.0096). All EAE mice were combined for behavioral correlation analysis.
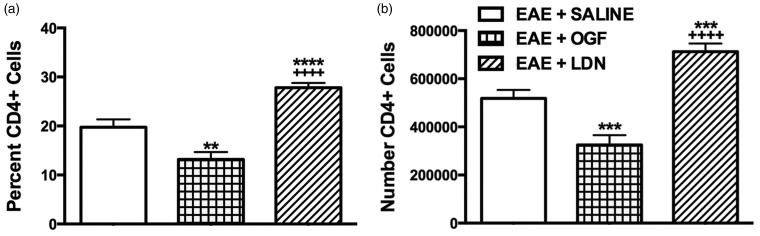
OGF reduces the number of CD4+ T lymphocytes in the CNS
Treatment of mice for five days with LDN beginning two days after the first appearance of clinical disease resulted in animals with increased numbers of CD4+ T lymphocytes in the brain and spinal cord, whereas the numbers of CD4+ T lymphocytes were decreased significantly in EAE mice that received OGF in comparison to saline-treated controls. Mice with EAE and treated with saline or LDN had an average of 5.2 × 105 or 7.1 × 105 CD4+ T lymphocytes, respectively, whereas EAE + OGF mice had an average of 3.3 × 105 CD4+ T lymphocyte infiltrates (Figure 2(b)). The average percentage of cells from EAE + saline mice that were CD4 positive was 19.8% and 13.2% for mice in the EAE + OGF group; EAE mice receiving LDN had 27.8% CD4+ cells (Figure 2(a)).
Figure 2.
Modulation of the OGF-OGFr axis alters the numbers of CD4+T lymphocytes present in the CNS of mice with established EAE. (a) Percent of total cells that are CD4+ isolated from CNS tissue of EAE mice treated with OGF, LDN or saline for five days from disease onset. (b) Number of CD4+ T lymphocytes isolated from the CNS of EAE mice. Significantly different from EAE + SALINE at p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****). Significantly different from EAE + OGF at p < 0.0001 (++++).
EAE: experimental autoimmune encephalomyelitis; OGF: opioid growth factor; LDN: low dosage of naltrexone
Modulation of the OGF-OGFr axis does not play a role in CD4+ T lymphocyte effector responses
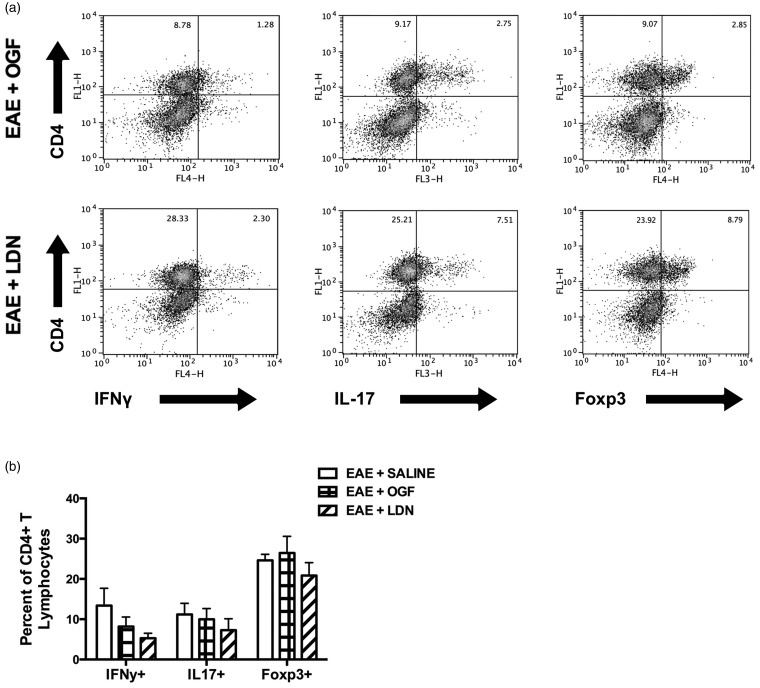
Intermittent blockade of the OGF-OGFr axis with LDN or exogenous OGF administration for five days to mice with established EAE did not alter the presence of CD4+ Th1, Th2 (data not shown), Th17 or Treg infiltrates within the CNS (Figure 3(b)).
Figure 3.
Effector CD4+ T lymphocytes isolated from the brain and spinal cord of EAE mice after five days of treatment (10 mg/kg OGF, 0.1 mg/kg naltrexone (LDN), or saline), corresponding to 15 days post immunization. (a) Intracellular cytokine staining of IFNγ, IL-17, and Foxp3 in mononuclear cells isolated from CNS tissue gated on live cells after ex vivo stimulation with PMA/ionomycin and brefeldin A for 4 h. (b) Percentage of CD4+ T lymphocytes that are positive for IFNγ, IL-17, and Foxp3 are shown.
EAE: experimental autoimmune encephalomyelitis; OGF: opioid growth factor; LDN: low dosage of naltrexone
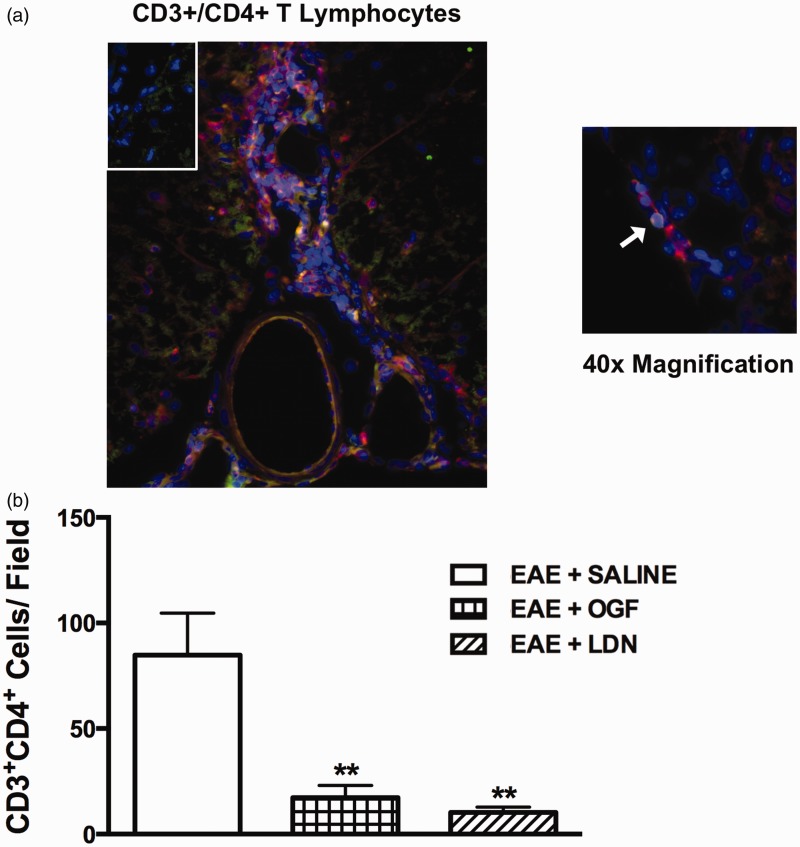
The numbers of CD3+/CD4+ T lymphocyte infiltrates in the lumbar spinal cord are limited by both OGF and LDN
Sections of lumbar spinal cord were stained with antibodies against CD3 and CD4 after 40 days of treatment with OGF, LDN, or saline to determine the presence of CD4+ T lymphocytes. The number of CD4+ T cells within the central ventral white matter of the lumbar spinal cord was decreased in mice with EAE after 40 days of treatment with OGF (17.3 ± 5.7 CD3+CD4+ cells, mean ± SEM) or LDN (10.3 ± 2.5 CD3+CD4+ cells) in comparison to the number of cells recorded for mice receiving saline (84.9 ± 19.8 CD3+CD4+ cells) (Figure 4(b)).
Figure 4.
CD3+/CD4+ T lymphocytes in the lumbar spinal cord of established EAE mice treated for 40 days with OGF, LDN or saline. (a) Lumbar spinal cord sections were stained with antibodies for CD3 (TRITC, red) and CD4 (FITC, green), as well as DAPI (blue) for nuclear identification and imaged at 20 × (left) and 40 × (right) magnification; a cell positive for both CD3+ and CD4+ is indicated by the arrow. Inset is stained with secondary antibody only as a control. (b) Number of CD3+/CD4+ cells in the central ventral white matter of lumbar spinal cord after 40 days of treatment. Significantly different from EAE + SALINE at p < 0.01 (**). (A color version of this figure is available in the online journal.)
EAE: experimental autoimmune encephalomyelitis; OGF: opioid growth factor; LDN: low dosage of naltrexone
Discussion
In an established relapse-remitting model of EAE, we have previously shown that OGF or LDN decreased the presence of CD3 positive infiltrates in sections of lumbar spinal cord after treatment for 40 days but neither treatment had an effect after five days when compared to saline-treated controls.27 In this study, we examined whether OGF or LDN had any differential effects on CD4+ T-lymphocyte cytokine responses, given that these cells predominate in CNS infiltrates in both the acute and relapse phases of EAE in SJL mice.30
We have found that mice with EAE and treated with OGF for five days beginning from the day of disease onset exhibit diminished numbers of mononuclear cells in their brain and spinal cord. This correlated with flow analysis showing that the absolute number of CD4+ T cells was significantly reduced in EAE mice exposed to OGF in comparison to mice with EAE and treated with saline or LDN. Immunohistochemical evaluation of lumbar spinal cord sections from OGF and LDN-treated mice with established EAE revealed decreased numbers of CD3+CD4+ T lymphocytes in the central ventral white matter after 40 days of treatment compared to saline controls. Even though OGF treatment resulted in decreased number of CD4+ T-cell infiltrates, it did not alter effector CD4+ T-cell responses. The frequency of IL-4+CD4+ T cells was undetectable, which is expected due to the inflamed environment.
Our laboratory has previously reported that OGF administration in vitro to PHA-stimulated splenic T lymphocytes decreased proliferative activity.21 However, OGF did not have an effect on non-stimulated cell cultures.21 We also demonstrated that naltrexone had no effect on either PHA-stimulated or non-stimulated T lymphocytes, while in other cell types continuous opioid-receptor blockade using naltrexone increases cell replication.21 These data suggest that the OGF-OGFr axis is only active in the immune system after T cells have been activated. In vivo studies using mice with myelin oligodendrocytic glycoprotein (MOG)-induced EAE examined the role of OGF or LDN on proliferation of T and B cell subpopulations derived from the spleen or inguinal lymph nodes.30 At 5 and 12 days of treatment, both OGF and LDN suppressed the number of total splenic lymphocytes, as well as the number of peripheral CD4+ and CD8+ T cells relative to saline-treated MOG-immunized EAE mice suggesting that this therapy inhibits T-cell proliferation in the periphery, thus reducing the population of cells migrating to the CNS.30 In the present study, we examined the actions of the OGF-OGFr axis on CD4+ T lymphocytes during the effector phase of EAE by delaying treatment until disease onset. With this approach, OGF treatment decreased CD4+ T lymphocytes and LDN increased CD4+ T lymphocytes in EAE mice. However, only one time point following drug treatment was monitored; thus, the LDN exposure may have completely blocked OGFr and thus increased cell proliferation. Future studies are required to refine these responses. Neither OGF nor LDN treatment altered the Th effector cell responses in EAE, but treatment was only five days in length. If the OGF-OGFr axis is solely functioning as a mediator of proliferation, then modulation of the axis should not alter T-cell subsets individually. Our data support this hypothesis in the SJL-PLP139–151 model of EAE.
To further examine interactions of the OGF-OGFr axis in PLP-reactive CD4+ T cells, studies should utilize other strategies for EAE induction including adoptive transfer of activated CFSE-labeled PLP-specific CD4+ T cells or CD4+ T cells from PLP TCR transgenic mice into recipient mice.31 This would allow examination of trafficking of myelin-specific lymphocytes to the CNS. Finally, to eliminate contributions from OGF interactions with other classical opioid receptors as immunomodulatory agents, a triple opioid receptor (MOR, DOR and KOR) knock-out mouse model that could be immunized to generate EAE should be established.
ACKNOWLEDGEMENTS
This work was supported by funding from The Paul K. and Anna M. Shockey Family Foundation (ISZ, PJM), the LDN Surgery Fund (ISZ, PJM), and the H.G. Barsumian M.D. Memorial Fund (HPW).
Authors’ contributions
LAH performed the research, analyzed data, and drafted the manuscript as part of her doctoral research. PJM designed the overall study, interpreted data and composed the final paper. HW and ISZ contributed to the design of the overall research, provided important discussion on data analyses and interpretation and critically revised the manuscript.
References
- 1.Ramagopalan S, Sadovnick A. Epidemiology of multiple sclerosis. Neurol Clin 2011; 29: 207–17. [DOI] [PubMed] [Google Scholar]
- 2.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014; 83: 1022–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Jr, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Ingelese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AER, montalban X, O’Connor PW, Petkau J, Possilli C, Rudick RA, Sormani MP, Stuve O, Waubant E, Polman CH. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurol 2014; 83: 278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafler DA. Multiple sclerosis. J Clin Invest 2004; 113: 788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.’t Hart BA, Gran B, Weissert R. EAE: imperfect but useful models of multiple sclerosis. Trends Mol Med 2011; 17: 119–25. [DOI] [PubMed] [Google Scholar]
- 6.Tuohy VK, Zhijian L, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol 1989; 142: 1523–7. [PubMed] [Google Scholar]
- 7.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm 2006; 113: 477–85. [DOI] [PubMed] [Google Scholar]
- 8.Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immunol 1998; 161: 4437–46. [PubMed] [Google Scholar]
- 9.Hofstetter HH, Karulin AY, Forsthuber TG, Ott PA, Tary-Lehmann M, Lehmann PV. The cytokine signature of MOG-specific CD4 cells in the EAE of C57BL/6 mice. J Neuroimmunol 2005; 170: 105–14. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhao X, Hao H, Shaw MK, Tse HY. T cells that trigger acute experimental autoimmune encephalomyelitis also mediate subsequent disease relapses and predominantly produce IL-17. J Neuroimmunol 2011; 230: 26–32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Codarri L, Greter M, Becher B. Communication between pathogenic T cells and myeloid cells in neuroinflammatory disease. Trends Immunol 2013; 34: 114–9. [DOI] [PubMed] [Google Scholar]
- 12.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domingues HS, Mues M, Lassmann H, Wekerle H, Gurumoorthy K. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One 2010; 5: e15531–e15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Nun A, Kaushansky N, KawakamiN, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H. From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J Autoimmun 2014; 54: 33–50. [DOI] [PubMed] [Google Scholar]
- 15.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 2004; 199: 971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rostami A, Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J Neurol Sci 2013; 333: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 2006; 129: 1953–71. [DOI] [PubMed] [Google Scholar]
- 18.Constantinescu CS, Hilliard B, Ventura E, Wysocka M, Showe L, Lavi E, Fujioka T, Scott P, Trinchieri G, Rostami A. Modulation of susceptibility and resistance to an autoimmune model of multiple sclerosis in prototypically susceptible and resistant strains by neutralization of IL-12 and IL-4, respectively. Clin Immunol 2001; 98: 23–30. [DOI] [PubMed] [Google Scholar]
- 19.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Röcken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med 1994; 180: 1961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr). Brain Res Rev 2002; 38: 351–76. [DOI] [PubMed] [Google Scholar]
- 21.Zagon IS, Donahue RN, Bonneau RH, McLaughlin PJ. T lymphocyte proliferation is suppressed by the opioid growth factor ([Met(5)]-enkephalin)-opioid growth factor receptor axis: implication for the treatment of autoimmune diseases. Immunobiology 2011; 216: 579–90. [DOI] [PubMed] [Google Scholar]
- 22.Zagon IS, Donahue RN, Bonneau RH, McLaughlin PJ. B lymphocyte proliferation is suppressed by the opioid growth factor-opioid growth factor receptor axis: implication for the treatment of autoimmune diseases. Immunobiology 2011; 216: 173–83. [DOI] [PubMed] [Google Scholar]
- 23.Rahn KA, McLaughlin PJ, Zagon IS. Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis. Brain Res 2011; 1381: 243–53. [DOI] [PubMed] [Google Scholar]
- 24.Campbell AM, Zagon IS, McLaughlin PJ. Opioid growth factor arrests the progression of clinical disease and spinal cord pathology in established experimental autoimmune encephalomyelitis. Brain Res 2012; 1472: 138–48. [DOI] [PubMed] [Google Scholar]
- 25.Zagon IS, Rahn KA, Bonneau RH, Turel AP, McLaughlin PJ. Opioid growth factor suppresses expression of experimental autoimmune encephalomyelitis. Brain Res 2010; 1310: 154–61. [DOI] [PubMed] [Google Scholar]
- 26.Hammer LA, Zagon IS, McLaughlin PJ. Treatment of a relapse-remitting model of multiple sclerosis with opioid growth factor. Brain Res Bull 2013; 98: 122–31. [DOI] [PubMed] [Google Scholar]
- 27.Hammer LA, Zagon IS, McLaughlin PJ. Improved clinical behavior of established relapsing-remitting experimental autoimmune encephalomyelitis following treatment with endogenous opioids: implications for the treatment of multiple sclerosis. Brain Res Bull 2015; 112: 42–51. [DOI] [PubMed] [Google Scholar]
- 28.Rothhammer V, Heink S, Petermann F, Srivastava R, Claussen MC, Hemmer B, Korn T. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J Exp Med 2011; 208: 2465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 2005; 11: 355–9. [DOI] [PubMed] [Google Scholar]
- 30.Soellner IA, Rabe J, Mauri V, Kaufmann J, Addicks K, Kuerten S. Differential aspects of immune cell infiltration and neurodegeneration in acute and relapse experimental autoimmune encephalomyelitis. Clin Immunol 2013; 149: 519–29. [DOI] [PubMed] [Google Scholar]
- 31.Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc Natl Acad Sci 2000; 97: 3412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]