Abstract
Energy intake can affect the metabolism. But it is not very clear that how and to what degree the metabolism can be changed by energy intake quantity and change. Here we applied four feeding patterns in male Sprague–Dawley rats—normal ad libitum diet (NFal), high-fat diet (HFal), caloric restriction (CR) after HFal (HFal-NFcr), and refeeding from CR to ad libitum (HFal-NFcr-NFal). Food intake and body weight, along with fat mass, insulin sensitivity, fasting plasma insulin, and glucose level were used to calculate the energy efficiency and compared the quantitative effects of energy intake. Energy intake changed little in NFal or HFal group; while it changed greatly and suddenly in HFal-NFcr or HFal-NFcr-NFal group. All the parameters we detected were different between these four feeding patterns. Excess of energy intake from high-fat diet induced adverse outcomes with low energy efficiency. CR reversed the impairment of high-fat diet with very high energy efficiency in a short period. However, dramatic response with high energy efficiency induced by recovery to feeding ad libitum after CR, which was possible harmful to health. In conclusion, energy intake quantity and change are key determinants of metabolism. Different energy intake quantity and change affect body weight, white adipose tissue weight, insulin sensitivity, etc. at different degrees and speeds because of different energy efficiency.
Keywords: Energy efficiency, caloric restriction, refeeding, high-fat diet, insulin sensitivity, fat mass
Introduction
Overweight and obesity have been recognized as a major public health concern throughout the world,1 with serious co-morbidities such as the metabolic syndrome, a cluster of risk factors associated with insulin resistance, and heightened cardiovascular and diabetes risk2 and thereby place a substantial burden on the health care system. High-energy, dense diets over the medium or long term are one of the most important factors associated with increased risk of overweight and obesity over the past half century.3–5 Therefore, obesity begins as a consequence of a positive energy balance,3 which leads to an increase in fat mass, in particular abdominal obesity, characterized by deposition of abdominal fat, that has been a prominent health hazard associated with the metabolic syndrome.4,5
In contrast to the detrimental effects of overeating energy-dense foods, a reduction in energy intake without malnutrition defined as caloric restriction (CR) has a wide range of benefits. Weight loss is a cornerstone to the prevention and treatment of metabolic syndrome.2,6,7 CR is the most effective nutritional intervention for preventing or delaying the occurrence of a wide range of chronic diseases in rodents.8–11 In non-human and human primates, CR with adequate nutrition protects against abdominal obesity, diabetes, hypertension, and cardiovascular diseases.12–16 Interestingly, even monkeys, which had glucose metabolism impairment prior to initiation of CR, showed no impairment of glucose homeostasis years later.12
However, restricting food intake for a greater part of one’s life will be difficult if not impossible to sustain. The questions arise of whether and for how long the beneficial effects of CR persist, in other words, what happens in caloric-restricted animals after being refed. This is an important medical problem, since, for instance, most obese patients in weight-reduction programs (food restriction) start to regain weight shortly after the end of active therapy (simply after refeeding).17 In addition to the real problems of overweight patients, the growing lean esthetic ideal has induced many people to make repeated attempts at dieting. In reality, many of these attempts end in “yo-yo dieting” that consists of cycles of weight loss or nutrition deprivation followed by weight gain or nutrition promotion. Essentially, shifting from an environment of food scarcity to one where food is abundant motivates catch-up growth and increases the risks for insulin resistance-related diseases later in life.18,19
Therefore, the energy intake quantity and change—change little or change greatly and suddenly—both can affect body weight and other metabolism-related parameters. But it is not very clear that how and to what degree the metabolism can be changed by energy intake quantity and change.
To address this question, we applied four feeding patterns with different energy intake quantity and changes in rat model to mimic lifestyles in human population—normal diet and fed ad libitum (NFal), high-fat diet and fed ad libitum (HFal), 45% CR with normal diet as NFal group after two-month ad libitum high-fat diet (HFal-NFcr), and then refed ad libitum to normal diet after two-month HFal-NFcr (HFal-NFcr-NFal). In NFal and HFal groups, energy intake had no significant changes; while in HFal-NFcr and HFal-NFcr-NFal groups, energy intake changed greatly and suddenly, either decreased in HFal-NFcr or increased in HFal-NFcr-NFal group. We recorded the food intake and body weight every week and determined the fat mass, insulin sensitivity, and fasting plasma insulin (FPIns) and serum glucose level, then calculated the energy efficiency and compared the quantitative effects of energy intake on each parameter in these different situations.
Methods and materials
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Biomedical Ethics Committee of Beijing Hospital and Beijing Institute of Geriatrics, Ministry of Health, Beijing, China. All surgeries were performed under 10% chloral hydrate anesthesia, and all efforts were made to minimize suffering.
Animals
Male Sprague–Dawley rats (n = 84) were purchased from the Institute of Laboratory Animal Sciences CAMS and PUMC (Beijing, China) at two months of age. After a one-week acclimation, all rats were randomly assigned to two groups: ad libitum, normal-fat diet (NFal group, n = 28) and ad libitum, high-fat diet (HFal group, n = 56). After two months, half of the rats from HFal group were randomly changed to 45% CR as in NFal group with normal-fat diet (HFal-NFcr group, n = 18). After another two months, 10 rats from HFal-NFcr group were randomly assigned to refeeding as in NFal (HFal-NFcr-NFal group) for about two months (Figure 1(a)). Two to three rats were housed in each cage, except in HFal-NFcr group that the rats were housed individually.
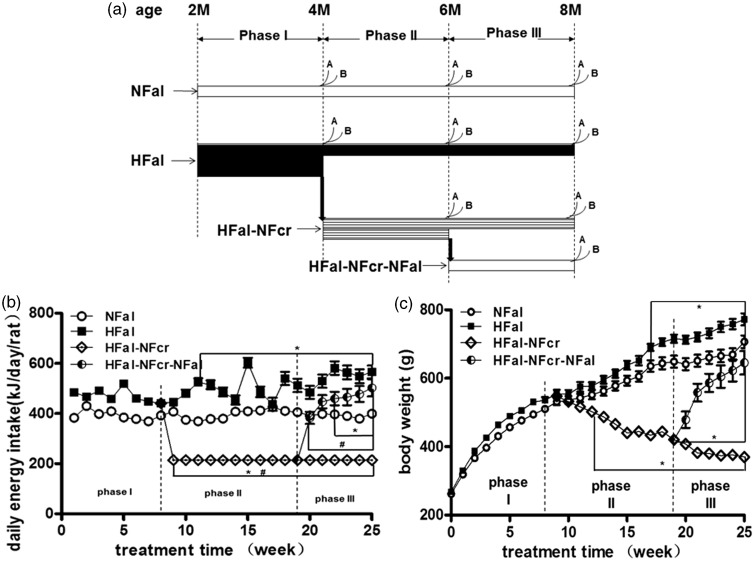
Figure 1.
The groups, the average daily energy intake per rat (DEI), and body weight of the animal model. (a) The diagram of the animal model. Male Sprague–Dawley (SD) rats were assigned into four groups: NFal—feed ad libitum with normal chow diet (n = 28, 19, and 10 in phase I, II, and III, respectively), HFal—feed ad libitum with high-fat diet (n = 56, 19, and 10 in phase I, II, and III, respectively), HFal-NFcr—caloric restriction with normal chow diet after HFal for about two months (n = 18 and 9 in phase II and III, respectively) and HFal-NFcr-NFal—refeeding ad libitum with normal chow diet after CR for about two months (n = 10). A: for blood and fat pad, n = 4–5; B: for euglycemic–hyperinsulinemic clamp technique, n = 5. (b) The average daily energy intake per rat of the four groups in each week. The food intake per rat was measured once a week and the daily energy intake per rat was calculated as described in “Methods and Materials” section. *: P < 0.05 versus NFal at the same time point; #: P < 0.05 versus the start point of the same group. (c) The body weight of the four groups in each week. n = 9–56 as shown in Figure 1(a). *: P < 0.05 versus NFal at the same time point
All the animals were housed at 21℃ in a 12-h light/12-h dark cycle. We recorded the body weight and food intake once a week during the study. The diet intake in each cage was calculated as Wd = W0 (initial food weight)–W1 (leftover food weight)–W2 (spilled food weight). The spilled food was weighed after feces had been picked out and spilled food had been dried. The daily energy intake (DEI) per rat (kJ/day/rat) was calculated as: Wd / 7 / n * Et (Et is total energy of the chow diet which is 15.3 kJ/g in normal diet and 21.5 kJ/g in high-fat diet) (Table 1), n is the number of rats in the cage).
Table 1.
Energy resources (kJ/100 g) and ratio (%) of the diets
| Protein | Carbohydrate | Fat | Total | Increment | |
|---|---|---|---|---|---|
| Normal chow diet | 331.5 (21.6%) | 1004.4 (65.6%) | 195.9 (12.8%) | 1531.8 (100%) | – |
| High-fat diet | 323.1 (15.0%) | 582.6 (27.1%) | 1256.8 (57.9%) | 2152.4 (100%) | 620.6 (40.5%) |
The chow diet was purchased from the Institute of Laboratory Animal Sciences CAMS and PUMC. Energy resources and ratio of the diets were shown in Table 1. The diets were in the form of pellets and the components were similar to D12450B (normal diet) and D12492 (high-fat diet) (Research Diets Inc., NJ, USA). Protein is from casein and l-cystine, fat is from lard and soybean oil. Carbohydrate is from sucrose, corn starch and maltodextrin in the normal diet and from maltodextrin and sucrose in the high-fat diet.
Blood plasma extraction and analysis
At the end of the feeding schedule, four to five rats in each group were anesthetized with 10% chloral hydrate intraperoneal injection after fasted for 12 h. The whole blood was collected from abdominal aorta. After storage at 4℃ for less than 2 h with or without EDTA for anticoagulation, the blood was centrifuged at 3000 r/min, 4℃ for 10 min, then the supernatant was collected and stored at –80℃. The serum glucose was determined by Hitachi 7180 chemistry analyze and hexokinase method (11876899, Roche, Basel, Switzerland). The blood plasma insulin was determined by chemiluminescence method (IMMULITE/IMMULITE 1000 Insulin, SIEMENS, Munich, Germany). All of these measurements were undertaken in National Center for Clinical Laboratory, Beijing Hospital, Beijing, PR China.
Epididymal fat pad weight
After blood extraction, left side epididymal fat pad was dissected and water was absorbed with cotton gauze and weighted.
Euglycemic–hyperinsulinemic clamp technique
We detected insulin sensitivity via in vivo insulin-stimulated glucose uptake measured by euglycemic–hyperinsulinemic clamp technique as described previously.20–22 We got glucose infusion rate (GIR, mg/kg/min) of each group after treatment for two, four, and six months. At the end of the feeding schedule, five rats in each group were performed euglycemic–hyperinsulinemic clamp technique. One day prior to the clamp procedure, rats were anesthetized with 10% chloral hydrate intraperitoneal injection and catheters were inserted into the right jugular vein and left carotid artery for blood infusions and sampling, respectively. Rats were fasted overnight before the clamp procedure. Before the onset of the infusion protocol, blood samples were obtained to determine preinfusion glucose concentration. Insulin (4 mU/kg body weight/min) and glucose (12.5%) were infused intravenously for 147 min. During the clamp study, blood glucose concentrations were measured every 7 min and maintained at the basal level with a variable infusion of glucose. The means of GIR of the last five times were regarded as indices of the insulin action in peripheral tissues, because a plateau in the GIR was achieved during these periods, as reported previously.20–22
Energy efficiency calculation
Energy efficiency of body weight (EE-BW) was calculated as weight gained in NFal, HFal, and HFal-NFcr-NFal groups or weight lost in HFal-NFcr group (energy expenditure efficiency) in mg divided by energy intake in kJ.19,23–26 It means the gained body weight from taking 1 kJ from food in NFal, HFal, and HFal-NFcr-NFal groups; while in HFal-NFcr group, the EE-BW means the lost body weight from taking 1 kJ less energy compared with NFal group. Similar to EE-BW, energy efficiency of left epididymal fat pad weight (EE-LEFPW) was calculated as weight gained (in NFal, HFal, and HFal-NFcr-NFal) or weight lost in HFal-NFcr (energy expenditure efficiency) in mg divided by energy intake in kJ; energy efficiency of fasting plasma insulin (EE-FPIns) was calculated as concentration elevated (in NFal, HFal, and HFal-NFcr-NFal) or reduced (in HFal-NFcr) in uIU/mL divided by energy intake in kJ.
Energy efficiency of glucose infusion rate (EE-GIR) was calculated as rate decreased in NFal, HFal, and HFal-NFcr-NFal or increased in HFal-NFcr in µg/kg/min divided by energy intake in kJ. The direction of the change of GIR by energy intake is contrary to the other parameters—the more energy intake, the lower GIR is. So EE-GIR in NFal, HFal, or HFal-NFcr-NFal group is the decrement of GIR from taking 1 kJ from food; while the EE-GIR in HFal-NFcr group is the increment of GIR from taking 1 kJ less energy compared with NFal group.
The energy efficiency of each parameter was calculated in the period between the first and the last time point the parameter was measured.
Statistical analysis
SPSS software (version 17.0) was used for statistical analysis. Two-way ANOVA was used to estimate difference among groups, followed by Tukey’s post hoc test. Also, the non-parametric Spearman correlation coefficient was calculated between parameters. Data were presented as group mean ± S.E. A two-tailed P < 0.05 was considered statistically significant.
Results
The characteristics of energy intake under four different feeding patterns
Because of the different feeding patterns, the rats had different characteristic of energy intake status as shown in Figure 1(b). In NFal and HFal groups, the average DEI per rat of the whole time changed little (P > 0.05) and was 395.3 ± 3.3 (369.1–430.4) kJ/day/rat and 501.1 ± 9.4 (436.9–602.7) kJ/day/rat, respectively. In HFal group, it was 1.27-fold as in NFal group. We restricted the DEI of HFal-NFcr group to 214.4 kJ/day/rat, which was ∼54.2% (P < 0.01) as in NFal group and 45.5% (P < 0.01) as before CR. In HFal-NFcr-NFal group, the DEI increased dramatically in the first two weeks: it surpassed NFal group and twice as before refeeding (P < 0.01). The DEI of the whole time in HFal-NFcr-NFal was 455.9 ± 16.5 kJ/day/rat (382.9–502.4), which was 2.13-fold (P < 0.01) as in HFal-NFcr group and 1.15-fold (P < 0.05) as in NFal group, but 8.3% lower than in HFal (P < 0.01). Thus, the energy intake had no significant change in NFal or HFal group but changed greatly in a short period in both HFal-NFcr and HFal-NFcr-NFal group.
The effects of energy intake pattern on body weight
At the start point, the body weight was 261.9 ± 1.0 and 267.6 ± 1.1 g in NFal and HFal groups, respectively (P > 0.05) (Figure 1(c)). In average, the rat gained 444.7 g body weight in NFal group and 504.6 g in HFal group at the end of six-month treatment. The rats in HFal group were 5.1% (P > 0.05), 10.4% (P < 0.05), and 9.3% (P < 0.05) heavier than those in NFal group at four, six, and eight months old, respectively. In HFal-NFcr group, the body weight decreased ∼10% (9.2–11.9%, P < 0.05) in each month in the first three months, while only 3.1% (P > 0.05) in the last month. After CR for two and four months, the rats in HFal-NFcr group were only 65.8% (P < 0.01) and 52.3% (P < 0.01) as those in NFal group at the same age. After recovery, the body weight increased dramatically, especially in the first two weeks: gained >10% (13.9 and 16.5%, P < 0.05) in each week; while gained no more than 5% (2.9–5.0%, P > 0.05) in each week in the following four weeks. Before refeeding, the body weight was only 64.8% (P < 0.05) as that of NFal group at the same age; while at the end of the treatment, there was only 8.7% (P < 0.05) lighter than that of NFal at the same age. Thus, comparing the changes of body weight, the speed of decrease after ∼45% CR was lower than the speed of increase after refeeding with energy intake doubled.
The effects of energy intake pattern on fat mass weight
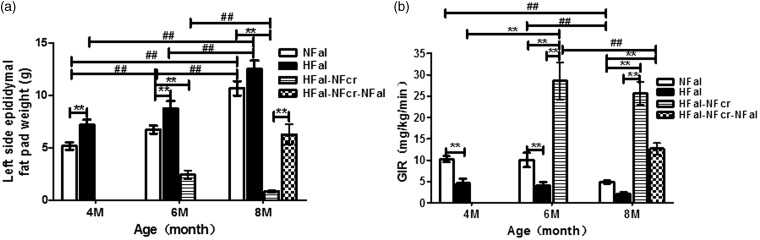
The LEFP gained weight with energy accumulation in both NFal and HFal groups and the increments were similar at each time point between these two groups: 1.56, 1.57 and 3.93, 3.77 g from four to six months and from six to eight months old, respectively (P < 0.01) (Figure 2(a)). The LEFP in HFal was ∼ 2.0 g heavier than that in NFal at all the three time points (P < 0.01 at four and six months). After CR for two and four months, the LEFP weight reduced 65.8 and 65.1%, respectively (P < 0.01); it was only 36.5 and 8.0% as that of NFal at the same age, respectively (P < 0.01). On the contrary, the LEFP weight increased dramatically after refeeding: it increased 1.53-fold (P < 0.01) within two months and there was no significant difference with NFal group (P > 0.05). Therefore, to some degree, the changing tendency of LEFP weight was similar to that of body weight.
Figure 2.
The fat pad weight and insulin sensitivity at different time points in each group. (a) The left side epididymal fat pad weight (n = 4–5). (b) The insulin sensitivity was detected by euglycemic–hyperinsulinemic clamp technique and presented with glucose infusion rate (GIR). (n = 5) NFal—feed ad libitum with normal chow diet, HFal—feed ad libitum with high-fat diet, HFal-NFcr—caloric restriction with normal chow diet after HFal for two months and HFal-NFcr-NFal—refeeding ad libitum with normal chow diet after CR for two months. 4 M, 6 M, and 8 M: the age of four, six, and eight months old. **: P < 0.01 versus other groups at the same age; ##: P < 0.01 versus the same groups at different ages. All values are means ± S.E
The effects of energy intake pattern on insulin sensitivity
In both NFal and HFal groups, there was no significant change on GIR from four to six months old (P > 0.05), but the GIR decreased ∼50% (51.4 and 49.2%, respectively) in the last two months (P < 0.01) (Figure 2(b)). However, the GIR in HFal group was <50% (40.2–45.0%, P < 0.01) as in NFal group at the same age. After CR for only two months, the GIR was elevated 5.2-fold (P < 0.05), while there was no significant difference between CR for two and four months (P > 0.05). On the other hand, after refeeding for two months, the GIR declined 55.4% (P < 0.01). These data indicated that insulin sensitivity was highly responsive to the change of energy intake.
The effects of energy intake pattern on blood insulin and glucose
We also detected two other metabolic parameters related with insulin sensitivity. As to the FPIns (Table 2), it was stable from four to six months (P > 0.05) and increased only a little from six to eight months old in both NFal and HFal groups: 17.1 and 10.1%, respectively (P > 0.05). The FPIns in HFal group was 18.2, 13.5, and 6.8% higher than in NFal group at four, six, and eight months old, respectively (P > 0.05). However, CR reduced FPIns to 32.9% (P < 0.05) as in NFal group after CR for four months. The FPIns was elevated to 1.98-fold (P < 0.05) after refeeding for only two months.
Table 2.
Fasting plasma insulin concentration (uIU/mL, upper) and fasting serum glucose concentration (mM, lower) at different time points in each group
| Groups | Age | 4 M | 6 M | 8 M |
|---|---|---|---|---|
| NFal | Insulin (uIU/mL) | 5.54 ± 0.39 | 5.74 ± 1.39 | 6.72 ± 0.55 |
| Glucose (mM) | 5.45 ± 0.09 | 5.21 ± 0.16 | 5.22 ± 0.12 | |
| HFal | Insulin(uIU/mL) | 6.55 ± 0.70 | 6.52 ± 2.50 | 7.18 ± 0.76 |
| Glucose (mM) | 5.51 ± 0.09 | 5.30 ± 0.06 | 5.68 ± 0.10 | |
| HFal-NFcr | Insulin(uIU/mL) | – | 2.68 ± 0.31 | 2.21 ± 0.66* |
| Glucose (mM) | – | 4.71 ± 0.14 | 4.58 ± 0.18*‡ | |
| HFal-NFcr-NFal | Insulin(uIU/mL) | – | – | 5.31 ± 0.16† |
| Glucose (mM) | – | – | 5.00 ± 0.15 |
All values are mean ± S.E. uIU/mL. 4 M, 6 M, and 8 M: the age of four, six, and eight months old.
: versus AL-8 M; †: versus CR-6 M; ‡: versus HF-8 M. P < 0.01, calculated by a further post hoc multiple comparisons between groups according to the equal variances assumed or not. n = 4–5.
For fasting serum glucose (FG) (Table 2), it was stable under both NFal and HFal conditions (P > 0.05) and there were no significant differences between these two groups (P > 0.05). The FG level was reduced 14.5% (P > 0.05) and 16.9% (P < 0.05) after CR for two and four months, respectively. In HFal-NFcr group, it was 9.6% (P > 0.05) and 12.2% (P < 0.05) lower than in NFal group at six and eight months old, respectively. However, refeeding elevated FG level to close to NFal group (P > 0.05 versus NFal) within two months. There was no significant difference between HFal-NFcr-NFal and NFal group at the end of this study (P > 0.05).
The energy efficiency on different parameters
Compared to NFal group, the EE-BW was 10.0% lower in HFal group, 82.5 and 26.8% higher in HFal-NFcr-NFal and HFal-NFcr group, respectively; the EE-LEFPW was 25.0% lower in HFal group, 70.7% and 1.52-fold higher in HFal-NFcr-NFal and HFal-NFcr groups; the EE-GIR in HFal group was 63.1% lower than in NFal group. The EE-GIR in HFal-NFcr-NFal and HFal-NFcr was 6.25 - and 7.51-fold higher than in NFal group. The EE-FPIns was 60.0% lower in HFal group, while 4.48 - and 6.99-fold higher in HFal-NFcr-NFal and HFal-NFcr group compared to NFal group (Table 3). Thus, among all these four groups, HFal-NFcr group had the highest energy efficiency on almost all these parameters, except that it was the second high on body weight; HFal-NFcr-NFal group also had high energy efficiency on LEFPW, GIR, and FPIns just following HFal-NFcr and had highest EE-BW; while HFal group had the lowest energy efficiency on all these parameters.
Table 3.
Energy efficiency of different parameters in each group
| Parameter | EE-BW | EE-LEFPW | EE-GIR | EE-FPIns |
|---|---|---|---|---|
| (mg/kJ) | (mg/kJ) | (µg/kg/min/kJ) | (nIU/mL/kJ) | |
| NFal | 6.4 | 0.116 | 0.114 | 0.025 |
| HFal | 5.6 | 0.087 | 0.042 | 0.010 |
| HFal-NFcr-NFal | 11.7 | 0.198 | 0.827 | 0.137 |
| HFal-NFcr | 7.7 | 0.293 | 0.970 | 0.200 |
EE-BW: energy efficiency on body weight; EE-FPIns: energy efficiency on fasting plasma insulin concentration; EE-GIR: energy efficiency on glucose infusion rate; EE-LEFPW: energy efficiency on left epididymal fat pad weight.
Discussion
To investigate the effects of different energy intake quantity and change, we applied four feeding patterns on rat model. We omitted some other groups, such as group of rats that would be calorie restricted on a HF diet, and refed on an ad libitum HF diet, etc. However, the current four groups can mimic lifestyles in human population—normal fat diet and high fat diet fed ad libitum, overweight and obese people on diet to reduce body weight, and those back to normal diet after CR. Besides, they can also stand for different changes of energy intake: changed little or changed greatly and suddenly. Among these four groups, all the outcome parameters were quantitatively different, including body weight, fat mass, insulin sensitivity, FPIns, and serum glucose level. Here we provided evidence on the questions that how and to what degree energy intake affected metabolism-associated parameters in rats.
To assess the effects of energy intake on different metabolic parameters, we applied the concept of “energy efficiency” for quantification. Energy efficiency was usually evaluated only on body weight in previous studies.19,23–26 The actual energy efficiency is influenced by many factors, such as age, physical activity, body size, type of food, duration under calculation, etc.19,23–26 Here, we extended this index to other metabolism-associated parameters, including epididymal fat pad weight, insulin sensitivity, etc. “Energy efficiency of body weight” has been used previously19,23–26; however, it is the first time that energy efficiency of the other parameters is used. Correlation analysis indicates that these parameters have correlation with body weight (Table S1). We calculated the energy efficiency with the changes of each parameter and energy intake. It provided a convenient and practical way to evaluate and compare the effects between different energy intake patterns, especially between the patterns that energy intake changes drastically as in HFal-NFcr or HFal-NFcr-NFal group and those that energy intake changes little as in NFal and HFal groups.
Our data showed that insulin sensitivity changed dramatically with quick change of energy intake (Figure 2). Moreover, the fluctuation of insulin sensitivity was bigger than that of plasma insulin and glucose level. It suggested that to keep the plasma glucose homeostasis after the energy intake pattern changed, the response of insulin effect was more sensitive and more important than that of insulin amount. Sirt1 and adiponectin can increase insulin sensitivity27–29 and response to nutrient availability as well, including CR and high-fat diet.30–33 So the relationship between energy intake and insulin sensitivity may be regulated by change of adiponectin or sirt1, as reported in our other works.34,35 The high response of insulin sensitivity to energy status also demonstrates that CR effects on insulin sensitivity cannot sustain for a long time after the termination of CR.
From the analysis of the change of average DEI (Figure 1(b)), we found that, when fed ad libitum, no matter normal diet or high-fat diet, the energy intake changed little during the whole process. However, the energy intake changed greatly and acutely in the beginning of CR from HFal or refeeding from CR, which induced dramatic changes of the other parameters in a short term. These findings suggest that compared to food composition, the way of food intake—quantity and change—has more potent effect on metabolic parameters. Indeed, the food intake pattern of HFal-NFcr group included two facets of changes, reduction of energy and a change of diet from the HF diet to normal diet. Since we did not set a group of HFal-HFcr—from HFal group to high-fat diet and 45% CR, we cannot rule out the effects of the change of diet composition. However, in fact, decades of studies found that application of only food restriction in quantity but not alteration in the food composition could bring many beneficial effects, including insulin sensitivity increase, oxidative stress reduction, mitochondrial biogenesis motivation, etc. (reviewed in Speakman and Mitchell36).
Visceral adipose tissue is more pathogenic than subcutaneous abdominal adipose tissue.37 Dysregulated adipose metabolism has been suggested to play an important role in insulin resistance. Besides, it is found from a systematic review that acute CR produces early preferential loss of visceral adipose tissue with weight loss relative to subcutaneous abdominal fat.38 We weighted part of visceral abdominal fat–epididymal fat pad which is easily to be separated completely. We also observed that the change of insulin sensitivity is closely related to that of LEFP weight (Figure 2(a) and (b)). In this study we presented more details on their relationship. First, when energy intake quantity changes, no matter slowly and slightly as in NFal or HFal group, or suddenly and drastically as in HFal-NFcr or HFal-NFcr-NFal group, white adipose tissues sense and response to the energy intake change faster than the whole body weight (Table 3). Second, when the energy intake quantity changed greatly in a short period as in HFal-NFcr-NFal group, the big amount and fast speed of body fat accumulation depended on high energy efficiency rather than more energy intake. In today’s environment of food abundance, the high energy efficiency on fat is a factor that contributes to the relapse of obesity after therapeutic slimming, hence to the poor efficacy of dietary restriction in the management of obesity and to excessive fat accumulation during catch-up growth.39 On the other hand, the high EE-LEFPW and EE-BW in HFal-NFcr group indicated that the mobilization of the stored energy was quick and efficient. Compared with HFal-NFcr-NFal group, the EE-BW in HFal-NFcr group was lower, while the EE-LEFPW was higher. It suggests that to accomplish the energy demands after CR, the mobilization of the energy reserved in white adipose tissue is efficient and important. Calorie restriction promotes a reduction of the adipocyte size. The adipocyte size was shown to be associated with basal and stimulated lipolysis rate and insulin-stimulated lipogenesis and, also, with secretion and expression of several adipokines (reviewed in Rossmeislova et al.40). Furthermore, under conditions of CR and refeeding after CR, the white adipose tissue mass was efficiently regulated by the change of energy intake.
In conclusion, the way of energy intake, including quantity and changes, is a key determinant of metabolism. Under the above four different feeding patterns in rats, energy intake has different quantitative effects on body weight, fat mass, insulin sensitivity, and fasting plasma glucose and insulin concentration. Excess of energy intake from high-fat diet induces adverse outcomes with low energy efficiency. It is promising that CR can reverse the impairment of HFal and even surpass NFal. CR is high energy efficient. However, to sustain the beneficial effects of CR, people should keep CR as routine regimen, which may be a big challenge for most people. Since recovery to ad libitum feeding after CR induced acute response in a short time, which was possible to do harm to the health and should be avoided.
ACKNOWLEDGEMENTS
The authors thank Dr Ping Zeng for advice in statistical analysis. This work was supported by the Research Special Fund for Public Welfare Industry of Health to T.M.Z. (No. 201302008) and National Natural Science Foundation of China to H.G. (No. 81300693).
Authors’ contributions
All authors participated in the design, interpretation of the studies, and analysis of the data and review of the manuscript; HG, YZ, EYZ, and YL conducted the experiments; HG and TMZ wrote the manuscript; and HG, LS, and YWH participated in analytical work.
References
- 1.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i–xii,1–253. [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006; 23: 469–80. [DOI] [PubMed] [Google Scholar]
- 3.Duarte FO, Sene-Fiorese M, Cheik NC, Maria AS, de Aquino AE, Jr, Oishi JS, Rossi EA, Garcia de Oliveira Duarte AC, Damaso AR. Food restriction and refeeding induces changes in lipid pathways and fat deposition in the adipose and hepatic tissues in rats with diet-induced obesity. Exp Physiol 2012; 97: 882–94. [DOI] [PubMed] [Google Scholar]
- 4.Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr 2000; 84: 417–27. [DOI] [PubMed] [Google Scholar]
- 5.Westerterp KR. Perception, passive overfeeding and energy metabolism. Physiol Behav 2006; 89: 62–5. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol 2004; 24: e19–24. [DOI] [PubMed] [Google Scholar]
- 7.Stone NJ, Saxon D. Approach to treatment of the patient with metabolic syndrome: lifestyle therapy. Am J Cardiol 2005; 96: 15E–21E. [DOI] [PubMed] [Google Scholar]
- 8.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev 2005; 126: 913–22. [DOI] [PubMed] [Google Scholar]
- 9.Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol 2009; 44: 41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science 2010; 328: 321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett 2011; 585: 1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Alison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009; 325: 201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann NY Acad Sci 2007; 1114: 434–55. [DOI] [PubMed] [Google Scholar]
- 14.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 2006; 47: 398–402. [DOI] [PubMed] [Google Scholar]
- 15.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 2004; 101: 6659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol 2007; 42: 709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodrick GK, Foreyt JP. Why treatments for obesity don't last. J Am Diet Assoc 1991; 91: 1243–7. [PubMed] [Google Scholar]
- 18.Duarte FO, Sene-Fiorese M, Manzoni MS, de Feitas LF, Cheik NC, Garcia de Oliveira Duarte AC, Nonak KO, Damaso AR. Caloric restriction and refeeding promoted different metabolic effects in fat depots and impaired dyslipidemic profile in rats. Nutrition 2008; 24: 177–86. [DOI] [PubMed] [Google Scholar]
- 19.Chen LL, Hu X, Zheng J, Kong W, Zhang HH, Yang WH, Zhu SP, Zeng TS, Zhang JY, Deng XL, Hu D. Lipid overaccumulation and drastic insulin resistance in adult catch-up growth rats induced by nutrition promotion after undernutrition. Metabolism 2011; 60: 569–78. [DOI] [PubMed] [Google Scholar]
- 20.Jiang HY, Koike T, Li P, Wang ZH, Kawata Y, Oshida Y. Combined effects of short-term calorie restriction and exercise on insulin action in normal rats. Horm Metab Res 2010; 42: 950–4. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Koike T, Qin B, Kubota M, Kawata Y, Jia YJ, Oshida Y. A high-fructose diet impairs Akt and PKCzeta phosphorylation and GLUT4 translocation in rat skeletal muscle. Horm Metab Res 2008; 40: 528–32. [DOI] [PubMed] [Google Scholar]
- 22.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 2006; 55: 390–7. [DOI] [PubMed] [Google Scholar]
- 23.Handayani D, Chen J, Meyer BJ, Huang XF. Dietary shiitake mushroom (Lentinus edodes) prevents fat deposition and lowers triglyceride in rats fed a high-fat diet. J Obes 2011; 2011: 258051–258051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So M, Gaidhu MP, Maghdoori B, Ceddia RB. Analysis of time-dependent adaptations in whole-body energy balance in obesity induced by high-fat diet in rats. Lipids Health Dis 2011; 10: 99–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin D. The effect of seamustard on blood lipid profiles and glucose level of rats fed diet with different energy composition. Nutr Res Pract 2009; 3: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjorntorp P, Yang MU. Refeeding after fasting in the rat: effects on body composition and food efficiency. Am J Clin Nutr 1982; 36: 444–9. [DOI] [PubMed] [Google Scholar]
- 27.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol 2009; 5: 367–73. [DOI] [PubMed] [Google Scholar]
- 28.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008; 118: 2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941–6. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol 2004; 39: 1049–59. [DOI] [PubMed] [Google Scholar]
- 31.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One 2008; 3: e1759.–e1759.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang X, Sweeney G. Mechanisms regulating energy metabolism by adiponectin in obesity and diabetes. Biochem Soc Trans 2006; 34: 798–801. [DOI] [PubMed] [Google Scholar]
- 33.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab 2012; 16: 180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang J, Gong H, Xi C, Fan W, Dai Y, Zhang TM. Poly(ADP-ribose) polymerase 1 is involved in glucose toxicity through SIRT1 modulation in HepG2 hepatocytes. J Cell Biochem 2011; 112: 299–306. [DOI] [PubMed] [Google Scholar]
- 35.Dai Y, Pang J, Gong H, Fan W, Zhang TM. Roles and tissue source of adiponectin involved in lifestyle modifications. J Gerontol A Biol Sci Med Sci 2013; 68: 117–28. [DOI] [PubMed] [Google Scholar]
- 36.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med 2011; 32: 159–221. [DOI] [PubMed] [Google Scholar]
- 37.Tchkonia T, Thomou T, Zhu Y, Zhu Y, Karagiannids I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013; 17: 644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 2008; 32: 619–28. [DOI] [PubMed] [Google Scholar]
- 39.Crescenzo R, Bianco F, Falcone I, Tsalouhidou S, Yepuri G, Mougios V, Dulloo AG, Lieverini G, Iossa S. Hepatic mitochondrial energetics during catch-up fat with high-fat diets rich in lard or safflower oil. Obesity (Silver Spring) 2012; 20: 1763–72. [DOI] [PubMed] [Google Scholar]
- 40.Rossmeislova L, Malisova L, Kracmerova J, Stich V. Adaptation of human adipose tissue to hypocaloric diet. Int J Obes 2013; 37: 640–50. [DOI] [PubMed] [Google Scholar]