Abstract
In this study, we looked at the possible effects of low-level laser therapy (LLLT) on blood flow velocity, and serotonin (5-HT) and cholinesterase levels in patients with chronic headache associated with temporomandibular disorders (TMD). LLLT has been clinically applied over the past years with positive results in analgesia and without the report of any side effects. The understanding of biological mechanisms of action may improve clinical results and facilitate its indication. Ten patients presenting headache associated with TMD completed the study. An 830-nm infrared diode laser with power of 100 mW, exposure time of 34 s, and energy of 3.4 J was applied on the tender points of masseter and temporal muscle. Blood flow velocity was determined via ultrasound Doppler velocimetry before and after laser irradiation. The whole blood 5-HT and cholinesterase levels were evaluated three days before, immediately, and three days after laser irradiation. Pain score after treatment decreased to a score of 5.8 corresponding to 64% of pain reduction (P < 0.05). LLLT promoted a decrease in the blood flow velocity (P < 0.05). In addition, the 5-HT levels were significantly increased three days after LLLT (P < 0.05). The cholinesterase levels remained unchanged at the analyzed time points (P > 0.05). Our findings indicated that LLLT regulates blood flow in the temporal artery after irradiation and might control 5-HT levels in patients suffering with tension-type headache associated to TMD contributing to pain relief.
Keywords: Cholinesterase, myogenous TMD, near-infrared laser, temporomandibular disorders, tension-type headache
Introduction
Low-level laser therapy (LLLT) is currently being used as a therapeutic procedure in a variety of medical situations such as control of edema and inflammation, chronic joint disorders, pain, wound healing, among others.1–5
One of the clinical conditions that could benefit from the effects of LLLT would be chronic headache, which is a condition that, depending on the statistics, may affect up to 2.5% of the world population.6 This disorder is also an obvious burden for patient’s quality of life, besides putting them in risk of pain killers’ addiction.6,7 Chronic headaches are often associated with temporomandibular disorders (TMD),8 and according to recent studies, the prevalence of TMD is increasing despite of improvement in general oral health.9,10 Therefore, the benefits of having a non-pharmacological therapy for such conditions is evident, and several studies have pointed out the positive effects of LLLT on TMD.11–13
Many attempts have been made to understand the mechanisms of LLLT at the molecular and cellular level,14–16 and this therapy has been tested with success in clinical trials.4 At the cellular level, LLLT presents a wide range of different effects, and its fundamental mechanism of action is difficult to establish.15 Furthermore, in different clinical situations (e.g. pain relief or wound healing), the effect on the target area may also be different.15 Within cells, there is substantial evidence that LLLT would act primarily on the mitochondria, increasing its metabolism, modulating reactive oxygen species formation and inducing transcription factors by changing the cell redox state.15,16
On the other hand, studies investigating LLLT mechanism in patients are not often encountered. Recently, Mitchell and Mack17 demonstrated an immediate increase in venous nitric oxide levels after near-infrared laser irradiation in healthy subjects, underlining one of the possible pathways that would explain LLLT clinical effects.
Among the biological effects of LLLT reported in the literature, an increase in local microcirculation,18 release of endogenous opioids,19 and alteration in cholinesterase levels20 have been found, including tension-type headache.21,22 These mechanisms may be involved in pain relief and can be linked with the clinical results, but most of the studies are performed in an animal model,19 in a cell culture model,20 or in healthy subjects.17,18
In this study, our purpose was to investigate possible analgesic mechanisms in patients referred to treatment for chronic pain due to tension-type headache. We hypothesized that LLLT would affect blood flow, serotonin (5-HT) and/or cholinesterase levels in patients, seeking mitigation of chronic headache associated to TMD with masticatory muscles involvement.
Material and methods
Patients
Ten Caucasian females patients suffering from recurrent headache associated with TMD volunteered to join in this trial. The study was conducted in a private dental clinic, and inclusion criteria were age between 20 and 50 years, headache complaint for at least six months before the inclusion, and completion of a diagnostic headache diary for at least one month before inclusion in accordance with International Classification of Headache Disorders-2nd edition (ICHD-II).23 Patients were excluded if they reported orofacial trauma history, drug or alcohol abuse, arterial hypertension, use of drugs with cerebrovascular action, and history of current treatment for headache.
The present study was approved by the IPEN Institutional review board no. 0.514. All participants received both verbal and written information about the procedures and objectives of the research and provided written informed consent.
Experimental protocol
The patients had a clinical dental examination to ensure they fulfilled the inclusion criteria and none of the exclusion criteria. Patient examination followed the guiding principle express by the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD).24 The clinical assessment of the temporomandibular joint (TMJ) and the masticatory muscles included evaluation of mandibular range of motion, pain during movements, presence of joint sounds or tinnitus, pain on palpation of related muscles and/or joints, and intraoral examination. Patients were submitted to one treatment session and were evaluated before and after irradiation. The pain intensity was measured using the Visual Analog Scale (VAS), which is set for “no pain” (score 0) and “worst possible pain” (score 10). After the end of the study, the patients were reevaluated and treated with occlusal splint whenever it was indicated.
Laser irradiation
To ensure muscle penetration of light, irradiation was performed with a near-infrared diode laser (Thera Lase, DMC, São Carlos) in continuous wave mode and wavelength of 830 nm (P = 100 mW). Laser irradiation was applied onto the tender points of the masseter and temporal muscle chosen ad hoc according to palpation, with the probe gently positioned on the skin (Φ = 0.2 cm). Patients were irradiated during 34 s, and energy of 3.4 J was delivered corresponding to an energy density of about 110 J/cm2. The energy and energy density given to each muscle point is within the range described for painful musculoskeletal conditions.25,26
Ultrasound Doppler velocimetry
Ultrasound Doppler velocimetry (DV) measurements were performed by an experienced physician who was not aware about the research protocol, using an Acuson Antares ultrasound (Siemens, Germany) with a linear multifrequency transducer (VF13-5) from 4.4–13 MHz and presets for flow endowed with pulsed Doppler. Before DV evaluation, patients were installed in lying supine position at room temperature. The transducer was placed in the anterior portion of auricular tragus given the best signal/noise ratio for the superficial temporal artery blood flow. Following artery localization, the probe was kept in position during 1 min before laser irradiation to obtain the baseline flow values for each patient. This procedure was repeated for three times, and the peak systolic velocity was recorded in both right and left sides.
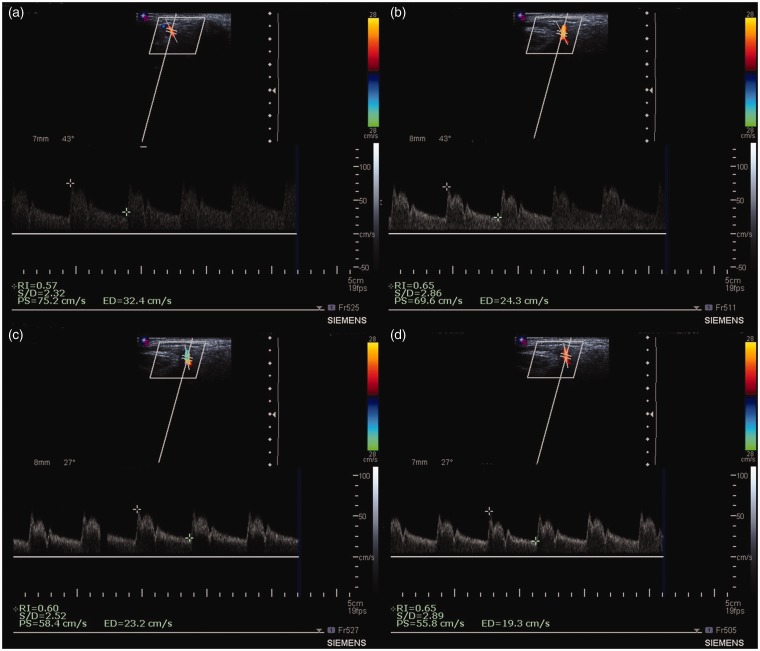
Laser irradiation was performed over the trigger points with the abovementioned parameters. After irradiation, the DV was performed during 2 min looking for arterial flow alterations that could happen after irradiation (Figure 1).
Figure 1.
Representative Doppler images of blood flow of a female patient with recurrent headache. (a) Before LLLT; (b) immediately after LLLT; (c) 1 min after LLLT; and (d) 2 min after LLLT. PS: peak systolic velocity; ED: end diastolic velocity; S/D: PS/ED; RI: resistive index (PS-ED/PS). (A color version of this figure is available in the online journal.)
Serum levels of 5-HT and cholinesterase
5-HT and cholinesterase levels were evaluated three days before, immediately after, and three days post laser irradiation. All patients were instructed to follow a nutritional program to avoid high-5-HT foods as, for instance, avocado, banana, eggplant, nuts, pickles, and tomato, prior to blood sampling. Venous blood was collected and kept in tubes containing disodium ethylenediamine tetraacetic acid (EDTA) and ascorbic acid. The blood tests were conducted in an accredited clinical analyses laboratory (Sabin Laboratory, Brasília, DF, Brazil) with international certifications (ISO 9001 and ISO 14001). All samples were handled within 2 h maximum after puncture. The samples were analyzed according to the laboratory guidelines for measurements of these substances on blood, for 5-HT high-performance liquid chromatography and kinetic method for cholinesterase evaluation.
Statistical analysis
Data are represented as means, and bars represent the standard deviations (SD). Data normality and variance homogeneity were verified with the Shapiro-Wilk W and Brown-Forsythe tests, respectively. Paired t-test was used to verify the effect of treatment on pain control and peak systolic velocity. 5-HT and cholinesterase levels were compared through one-way repeated measures analysis of variance (ANOVA), and to identify the significant differences, the Fisher’s least significant difference (LSD) test was employed. The significance level was set to P < 0.05.
Results
Ten Caucasian females with a mean age of 33.9 ± 9 years and tension-type headache from myogenic bilateral TMD completed the study. Headache episodes on these patients were in average 20 (±2) a month for at least six months. Clinical examination of the TMJ revealed that among all the 10 patients, some of them suffered from bruxism (n = 3 patients), irregular jaw opening (n = 6), crepitus (n = 4), pain in the TMJ (n = 2), dental malocclusion (n = 1), pain in the masticatory muscles (n = 2), earache (n = 1), tinnitus (n = 2), and teeth clenching (n = 4).
All the patients reported pain relief after experiment. In fact, the pain intensity before treatment was 9.1 ± 0.9 (mean ± SD), and after experiment, the mean value of the pain intensity was 3.3 ± 1.2. The result of the paired t-test pointed out to a significant analgesia with a 5.8 score, which is equivalent to a 64% of pain reduction (P < 0.001).
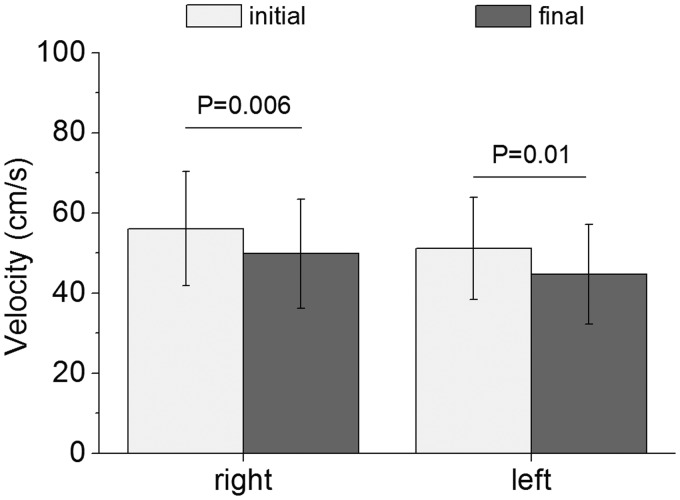
Figure 2 shows the initial peak systolic velocity values from right (57.58 ± 14.56) and left temporal arteries (50.54 ± 12.63). Two minutes after laser irradiation, the velocity showed statistically significant reduction (P = 0.0006 and P = 0.01, correspondingly, calculated via paired t-test) in both sides. In fact, on the right side, final velocity was 49.82 ± 13.5 and on left side, 44.78 ± 9.85. Blood flow velocity significantly decreased approximately 13.5% and 11.4% in right and left sides of the superficial temporal artery, respectively.
Figure 2.
Peak systolic velocity measured during the study (n = 10). The ultrasound transducer was placed in the anterior portion of auricular tragus given the best signal/noise ratio for the superficial temporal artery blood flow. Data are means, and bars are SD values. Initial and final represent the measures before and 2 min after laser irradiation, respectively
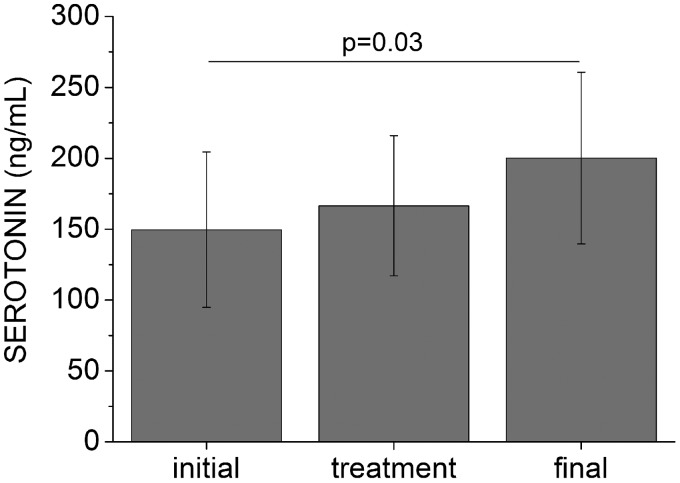
We also observed a progressive increase of about 34% (50.5 ng/mL) in the 5-HT levels after laser irradiation in comparison to the initial level (Figure 3). The mean value measured three days before, immediately after, and three days after laser irradiation were (149.60 ± 54.9) ng/mL, (166.50 ± 49.5) ng/mL, and (200.10 ± 60.6) ng/mL, respectively. However, significant difference was only observed three days after laser irradiation (P = 0.043) compared to baseline, which was verified by Fisher’s LSD test following one-way ANOVA with repeated measures.
Figure 3.
Serotonin levels measured during the study (n = 10). Data are means, and bars are SD values. Initial, treatment, and final represent the measures at three days before, immediately after, and three days after laser irradiation, respectively
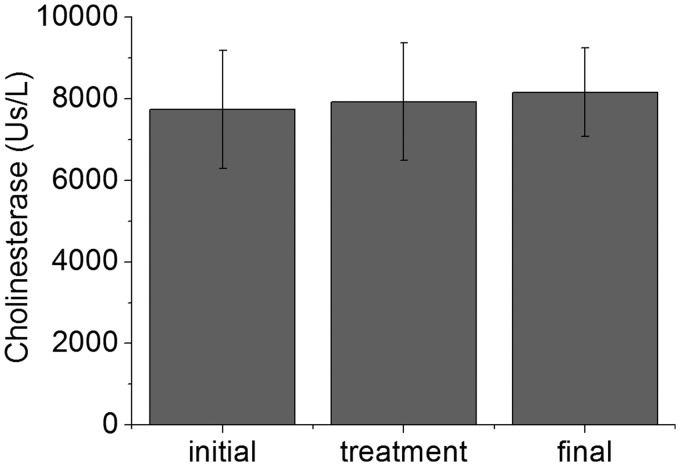
Figure 4 exhibits cholinesterase levels measured at different evaluation times. The mean values (7738.5 ± 1445.3 Us/L, 7925.5 ± 1442.2 Us/L, and 8160.0 ± 1085.8 Us/L) at three days before, immediately after, and three days after laser irradiation, respectively, showed a slight increase (about 5%) in cholinesterase levels during the study. No statistically significant differences were detected among the time points (P = 0.62 initial x treatment, P = 0.24 initial x final, and P = 0.54 treatment x final) using the same test above stated.
Figure 4.
Cholinesterase levels measured during the study (n = 10). Data are means, and bars are SD values. Initial, treatment, and final represent the measures at three days before, immediately after, and three days after laser irradiation, respectively
Discussion
LLLT biological mechanisms are an open field for investigation, given that clinical and preclinical trials have reported positive results with the use of this therapy.1–3 Although this therapy has been studied since late 1960s, so far there is not a consensus about its exact mechanisms of action.15
Our results demonstrated a significant diminution of the pain score after irradiation. In addition, there was a decrease in the blood flow velocity probably due to an increase on the temporal artery diameter,27 which led to the lower pressure inside the vessel. Also, the levels of 5-HT sustainably increased three days after irradiation. We also verified the levels of cholinesterase at the three proposed moments of evaluation: three days before treatment, immediately after, and three days after treatment, and we did not find significant differences among the values.
Arterial blood flow was investigated since a possible circulatory effect of the irradiation could lead to a relaxation on the irradiated muscles. The subjects in this study suffered from tension-type headache, and during tensional periods, the blood flow tends to increase.28 Local muscle ischemia, disturbances in the local metabolism, mitochondrial dysfunction, and microcirculation alterations on the tender points in individuals presenting tensional headache and other forms of myofascial pain have been suggested as possible mechanisms for trigger points formation and pain.29 Our results demonstrated an immediate effect on blood flow over the irradiated site. Since alterations on blood flow, due to altered sympathetic depletion to blood vessels in striated muscles, is identified as one symptom observed in patients with tensional headache,29,30 we may suggest that LLLT might regulate blood flow at the tender sites resulting in diminished pain at the area.
The regulatory effect of LLLT on blood flow in the tender areas may be one of the mechanisms that results in pain relief reported in literature by patients after irradiation.11–13 The non-pharmacological approach to treat patients with any type of chronic pain per se is worth to try since the risk for drug addiction in this group of people is high.6,7 Therefore, it is very relevant to investigate and understand the exact scope of LLLT in these cases, although our study has dealt only with pain related to muscle tension.
The results of our investigation also point out to an increase in serum 5-HT levels three days after irradiation. In 1983, Walker31 published her results evaluating the catabolite of 5-HT in the urine of patients with chronic pain after laser irradiation, and her findings presented an augmentation on the catabolite levels during the course of LLLT that were linked with the pain relief reported by the patients.
Among the neurotransmitters in the brain, 5-HT has been involved to tension-type headache. In fact, patients with tension-type headache show low 5-HT serum levels that can conduce to high indexes of reactive and personal anxiety and high depression level.32 In spite of the fact that no anxiety and depression have been measured in this study, our results demonstrate an increase in 5-HT levels three days after irradiation. This finding added to the results reported by Walker31 during the course of several LLLT sessions may suggest a possible balance on 5-HT metabolism that could be responsible for the results of the therapy in chronic pain.
We also investigate the levels of cholinesterase looking for alterations promoted by LLLT that could be responsible for muscle relaxation, since we were dealing with patients who presented tension-type headache. Our results did not point out to any interference on cholinesterase values in the analyzed periods that could be linked with the therapy. Conversely, Kujawa et al.20 reported changes on erythrocyte membrane acetylcholinesterase activity after irradiation suggesting a biphasic dose-dependent change. In addition, Onac et al.33 showed an increase in serum cholinesterase in an animal model that reached a peak ten days after irradiation. In both studies, the authors presented a dose-dependent response for cholinesterase alterations.
Interestingly, Chung et al.15 reported that the effects of LLLT obey the Arndt-Schulz law postulating that small doses and higher doses can present a particular effect, but the doses in between cannot produce the same results forming a biphasic dose response. Therefore, we may assume that, for the cholinesterase values, we might have been caught in the biphasic response for this parameter, while for serum 5-HT levels and for blood circulation, the dose was appropriate.
A limitation of this study is the lack of a control group. It is well known that pain relief can occur due to a placebo effect.34 As this study is a preliminary report, the sample size was not big enough to add a placebo group, and in the literature, it is possible to find placebo-controlled, randomized studies with positive effect of LLLT on pain related to TMD and chronic pain.13,35,36 Although the placebo effect may have some immediate effect on pain relief, our results showed blood flow decrease in real time and a sustainable effect was observed three days after irradiation on 5-HT levels.
According to our results, we suggest that, as the LLLT has multiple and simultaneous mechanisms of action, the dose that is appropriate for one mechanism may not be appropriate for another. This could explain some negative results of the therapy since the research usually focus on one or other mechanism and not the full range that can be occurring. Further studies are warranted to confirm our results in a large series of patients exploiting the exact mechanism of action of LLLT on patients with chronic pain.
Within the scope of our study, we conclude that LLLT has an immediate biological effect on local circulation when applied over the injured area and that 5-HT levels are augmented until three days following LLLT in patients with tension-type headache. These findings can provide basis for clinical trials aiming to understand the exact mechanisms of phototherapy working as a non-pharmacological analgesic agent.
Acknowledgements
The authors thank Dr Manoel Aparecido Gomes da Silva, Dr Sandra Santana Soares Costa, Dr Lídia Freire Abdala, and Dr Janete Ana Ribeiro Vaz for technical assistance in this work. Authors also thank Marily SR Pascoaloto for professional reading of the manuscript. The authors received no financial support for the research, authorship, and/or publication of this article.
Authors’ contributions
All authors participated in the study design, data interpretation, and analysis and review of the manuscript; MTM and MSR conducted the experiments; ITK, SCN, and MSR wrote the manuscript, and all authors discussed the results.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Meneguzzo DT, Lopes LA, Pallota R, Soares-Ferreira L, Lopes-Martins RA, Ribeiro MS. Prevention and treatment of mice paw edema by near-infrared low-level laser therapy on lymph nodes. Lasers Med Sci 2013; 28: 973–80. [DOI] [PubMed] [Google Scholar]
- 2.Barretto SR, de Melo GC, dos Santos JC, de Oliveira MG, Pereira-Filho RN, Alves AV, Ribeiro MA, Lima-Verde IB, Quintans Júnior LJ, de Albuquerque-Júnior RL, Bonjardim LR. Evaluation of anti-nociceptive and anti-inflammatory activity of low-level laser therapy on temporomandibular joint inflammation in rodents. J Photochem Photobiol B 2013; 129: 135–42. [DOI] [PubMed] [Google Scholar]
- 3.Núñez SC, França CM, Silva DF, Nogueira GE, Prates RA, Ribeiro MS. The influence of red laser irradiation timeline on burn healing in rats. Lasers Med Sci 2013; 28: 633–41. [DOI] [PubMed] [Google Scholar]
- 4.Alghadir A, Omar MT, Al-Askar AB, Al-Muteri NK. Effect of low-level laser therapy in patients with chronic knee osteoarthritis: a single-blinded randomized clinical study. Lasers Med Sci 2014; 29: 749–55. [DOI] [PubMed] [Google Scholar]
- 5.França CM, França CM, Nuñez SC, Prates RA, Noborikawa E, Faria MR, Ribeiro MS. Low-intensity red laser on the prevention and treatment of induced-oral mucositis in hamsters. J Photochem Photobiol B 2009; 94: 25–31. [DOI] [PubMed] [Google Scholar]
- 6.Pozo-Rosich P. Chronic migraine: its epidemiology and impact. Rev Neurol 2012; 54(Suppl 2): S3–11. [PubMed] [Google Scholar]
- 7.Latimer KM. Chronic headache: stop the pain before it starts. J Fam Pract 2013; 62: 126–33. [PubMed] [Google Scholar]
- 8.da Silva Junior AA, Krymchantowski AV, Gomes JB, Leite FM, Alves BM, Lara RP, Gómez RS, Teixeira AL. Temporomandibular disorders and chronic daily headaches in the community and in specialty care. Headache 2013; 53: 1350–5. [DOI] [PubMed] [Google Scholar]
- 9.Köhler AA, Hugoson A, Magnusson T. Clinical signs indicative of temporomandibular disorders in adults: time trends and associated factors. Swed Dent J 2013; 37: 1–11. [PubMed] [Google Scholar]
- 10.Köhler AA, Helkimo AN, Magnusson T, Hugoson A. Prevalence of symptoms and signs indicative of temporomandibular disorders in children and adolescents. A cross-sectional epidemiological investigation covering two decades. Eur Arch Paediatr Dent 2009; 10(Suppl 1): 16–25. [DOI] [PubMed] [Google Scholar]
- 11.Maia ML, Bonjardim LR, Quintans Jde S, Ribeiro MA, Maia LG, Conti PC. Effect of low-level laser therapy on pain levels in patients with temporomandibular disorders: a systematic review. J Appl Oral Sci 2012; 20: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melis M, Di Giosia M, Zawawi KH. Low level laser therapy for the treatment of temporomandibular disorders: a systematic review of the literature. Cranio 2012; 30: 304–12. [DOI] [PubMed] [Google Scholar]
- 13.Ahrari F, Madani AS, Ghafouri ZS, Tunér J. The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Med Sci 2014; 29: 551–7. [DOI] [PubMed] [Google Scholar]
- 14.Prindeze NJ, Moffatt LT, Shupp JW. Mechanisms of action for light therapy: a review of molecular interactions. Exp Biol Med 2012; 237: 1241–8. [DOI] [PubMed] [Google Scholar]
- 15.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 2012; 40: 516–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B 2014; 140: 344–58. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell UH, Mack GL. Low-level laser treatment with near-infrared light increases venous nitric oxide levels acutely: a single-blind, randomized clinical trial of efficacy. Am J Phys Med Rehabil 2013; 92: 151–6. [DOI] [PubMed] [Google Scholar]
- 18.Samoilova KA, Zhevago NA, Petrishchev NN, Zimin AA. Role of nitric oxide in the visible light-induced rapid increase of human skin microcirculation at the local and systemic levels: II. healthy volunteers. Photomed Laser Surg 2008; 26: 443–9. [DOI] [PubMed] [Google Scholar]
- 19.Hagiwara S, Iwasaka H, Hasegawa A, Noguchi T. Pre-irradiation of blood by gallium aluminum arsenide (830 nm) low-level laser enhances peripheral endogenous opioid analgesia in rats. Anesth Analg 2008; 107: 1058–63. [DOI] [PubMed] [Google Scholar]
- 20.Kujawa J, Zavodnik L, Zavodnik I, Bryszewska M. Low-intensity near-infrared laser radiation-induced changes of acetylcholinesterase activity of human erythrocytes. J Clin Laser Med Surg 2003; 21: 351–5. [DOI] [PubMed] [Google Scholar]
- 21.Bendtsen L, Fernandez-de-la-Penas C. The role of muscles in tension-type headache. Curr Pain Headache Rep 2011; 15: 451–8. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Araguz A, Bustamante-Martinez C, de Pedro-Pijoan JM. Treatment of chronic tension type headache with mirtazapine and amitriptyline. Rev Neurol 2003; 37: 101–5. [PubMed] [Google Scholar]
- 23.Olesen J, Steiner TJ. The international classification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry 2004; 75: 808–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992; 6: 301–55. [PubMed] [Google Scholar]
- 25.Simunovic Z. Low level laser therapy with trigger points technique: a clinical study on 243 patients. J Clin Laser Med Surg 1996; 14: 163–7. [DOI] [PubMed] [Google Scholar]
- 26.Núñez SC, Garcez AS, Suzuki SS, Ribeiro MS. Management of mouth opening in patients with temporomandibular disorders through low-level laser therapy and transcutaneous electrical neural stimulation. Photomed Laser Surg 2006; 24: 45–9. [DOI] [PubMed] [Google Scholar]
- 27.Bai CH, Chen JR, Chiu HC, Pan WH. Lower blood flow velocity, higher resistance index, and larger diameter of extracranial carotid arteries are associated with ischemic stroke independently of carotid atherosclerosis and cardiovascular risk factors. J Clin Ultrasound 2007; 35: 322–30. [DOI] [PubMed] [Google Scholar]
- 28.Schraml C, Schwenzer NF, Martirosian P, Claussen CD, Schick F. Temporal course of perfusion in human masseter muscle during isometric contraction assessed by arterial spin labeling at 3T. MAGMA 2011; 24: 201–9. [DOI] [PubMed] [Google Scholar]
- 29.Ashina S, Bendtsen L, Ashina M. Pathophysiology of tension-type headache. Curr Pain Headache Rep 2005; 9: 415–22. [DOI] [PubMed] [Google Scholar]
- 30.Ashina M, Stallknecht B, Bendtsen L, Pedersen JF, Galbo H, Dalgaard P, Olesen J. In vivo evidence of altered skeletal muscle blood flow in chronic tension-type headache. Brain 2002; 125(Pt 2): 320–6. [DOI] [PubMed] [Google Scholar]
- 31.Walker J. Relief from chronic pain by low power laser irradiation. Neurosci Lett 1983; 43: 339–44. [DOI] [PubMed] [Google Scholar]
- 32.Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia 2007; 27: 1293–300. [DOI] [PubMed] [Google Scholar]
- 33.Onac I, Pop L, Ungur R, Giurgiu I. Implications of low-power He-Ne laser and monochromatic red light biostimulation in the metabolism of proteins and glucosides. Proc SPIE 2001; 4430: 684–690. [Google Scholar]
- 34.Murray D, Stoessl AJ. Mechanisms and therapeutic implications of the placebo effect in neurological and psychiatric conditions. Pharmacol Ther 2013; 140: 306–18. [DOI] [PubMed] [Google Scholar]
- 35.Sattayut S, Bradley P. A study of the influence of low intensity laser therapy on painful temporomandibular disorder patients. Laser Ther 2012; 21: 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armagan O, Tascioglu F, Ekim A, Oner C. Long-term efficacy of low level laser therapy in women with fibromyalgia: a placebo-controlled study. J Back Musculoskelet Rehabil 2006; 19: 135–40. [Google Scholar]