Abstract
Hypoxia-induced cardiomyocyte apoptosis contributes significantly to the development of numerous cardiac diseases, such as ischemic heart disease, heart failure, etc. Promoting cell survival by inhibiting apoptosis is one of the available strategies to attenuate cardiac dysfunction caused by cardiomyocyte loss. Previous studies have been demonstrated that miR-138 and lipocalin-2 (Lcn2) play important roles in cardiomyocyte apoptosis and survival. We presently determined whether Lcn2 is a target gene of miR-138 involved in hypoxia-induced cardiomyocyte apoptosis. Firstly, mimics of miR-138 were transfected into HL-1 cells to investigate its effect on cell apoptosis. Using 3-(4,5-dimethyl-thiazol-2-y1) 2,5-diphenyl tetrazolium bromide (MTT) and Annexin V-FITC/PI flow cytometer assays, over-expression of miR-138 significantly enhanced the cell growth and significantly attenuated the cell apoptosis in hypoxic conditions. Dual-luciferase reporter gene and western blot results confirmed Lcn2 was a direct target of miR-138. Then, the recombinant plasmid, pcDNA3.1/Lcn2 was transfected into the HL-1 cells that over-expressed miR-138. We further observed that the over-expression of Lcn2 diminished the protection of miR-138 over-expression from hypoxia-induced cell survival and apoptosis. In conclusion, our study demonstrated that up-regulation of miR-138 inhibits the hypoxia-induced cardiomyocyte apoptosis via down-regulating the pro-apoptotic gene expression of Lcn2.
Keywords: miR-138, lipocalin-2, hypoxia, apoptosis, cardiomyocyte
Introduction
Ischemic heart disease, with a high incidence and prevalence, is one of the leading causes of death worldwide.1 Oxidative phosphorylation is severely impaired during ischemia.2 Mitochondria are vulnerably damaged in cardiomyocytes under hypoxia, leading to the excessive production of reactive oxygen species (ROS), which decrease cell antioxidant defenses.3–5 And also, many other heart diseases are associated with ROS, including myocardial infarction, cardiac hypertrophy, and heart failure. The dysfunction of mitochondria could release apoptotic proteins into the cytosol, and then evoke the apoptotic pathway, leading to cardiac apoptosis.6 A variety of animal and human studies have demonstrated that apoptosis contributes significantly to cardiomyocyte loss during the development and progression of heart diseases.7,8 Currently, overcoming hypoxia-induced cardiac apoptosis remains challenging for the treatment of various heart diseases.
MicroRNAs (miRNAs) are naturally occurring, short, non-coding RNAs that negatively regulate gene expression.9 In mammals, mature miRNAs consist of 21–24 nucleotides, integrate into RNA-inducing silencing complexes, and pair with the 3′untranslated regions (3′UTRs) of target mRNAs to suppress translation or induce degradation of the target mRNAs.10 miR-138 is one of the miRNAs that functions in various biological processes, such as heart development.11 Dysregulation of miR-138 may lead to disrupted heart morphogenesis and cardiac function.11 Interestingly, over-expression of miR-138 was able to rescue the heart defects generated by citrinin.12 Recent studies have shown the miR-138 expression changed in hypoxic pulmonary artery smooth muscle cells13 or cardiomyocytes,14 and then regulated the hypoxia-induced cell apoptosis. However, the mechanics underlying the miR-138 that protects cardiomyocytes from hypoxia-induced apoptosis was not completely elucidated.
Lipocalin-2 (Lcn2) is a small, secreted adipokine and belongs to a diverse family of lipocalins that has the characteristics of binding and transporting small molecules such as retinoid, steroid, fatty acid, and iron.15 It was suggested that Lcn2 may mediate the innate immune responses in the pathogenesis of heart failure.16,17 Lcn2 expression is significantly enhanced in patients with coronary heart disease and myocardial infarction.18,19 After carotid artery injury in rats and in a heterotopic mouse with transplanted heart after ischemia/reperfusion, plasma Lcn2 was augmented.20,21 Most recently, it was demonstrated that Lcn2 directly induced cardiomyocyte apoptosis.22 However, little is presently known about regulation of the Lcn2 gene. The aim of the current study was to determine whether miR-138 protects cardiomyocytes from hypoxia-induced cell apoptosis via targeting Lcn2 gene. HL-1 cells represent a cardiac myocyte cell line that can be repeatedly passaged and yet maintain a cardiac-specific phenotype,23 and the HL-1 cells were used as cell model in this study; our results revealed miR-138 can directly target the Lcn2 gene, and its over-expression inhibits the hypoxia-induced cell apoptosis via down-regulating the expression of Lcn2.
Material and methods
Cell culture
Mouse HL-1 cells (ATCC, USA) were cultured. In brief, cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA). After serum-starved overnight, cells were placed in an Invivo200 cultivator (Ruskin Technology Ltd, UK) containing a gaseous mixture of 94% N2, 5% CO2, and 1% O2 at 37℃ for durations of 0 h, 12 h, 24 h, 48 h, and 96 h, respectively. Cells in normoxia group were incubated under the same conditions except for 21% O2 concentrations.
Micro RNA mimics transfection
The miR-138 mimics and miRNA negative control (miR-NC) were purchased from a commercial manufacturer (GenePharm, China). HL-1 cells were seeded in a six-well plate (2 × 105/well) 24 h before transfection, and cells were transfected with miRNA mimics (50 nM) or miR-NC (50 nM) using FuGENE® HD transfection reagent (Promega, USA) in accordance with the manufacturer’s instruction.
MTT assay
Cell survival was evaluated using the 3-(4,5-dimethyl-thiazol-2-y1) 2,5-diphenyl tetrazolium bromide (MTT) (Sigma, USA) colorimetric assay. Cells were seeded in 96-well tissue culture plates at 2 × 104 cells per well and incubated in hypoxic conditions for various hours. Cells were first washed with phosphate-buffered saline and then incubated in 100 µL of 5 mg/mL MTT solution (Invitrogen Inc., USA) for 3 h. MTT is converted into purple-colored formazan in living cells which was then solubilized with dimethylsulfoxide (DMSO) (Invitrogen Inc., USA), and absorbance of solution was taken at 450 nm using the microplate reader Thermo Plate (Rayto Life and Analytical Science C. Ltd, Germany).
Flow cytometry
Cell apoptosis was determined by Annexin V assay. HL-1 cells incubated in hypoxic conditions for 48 h were collected, washed, and suspended in Annexin V binding buffer. Fluorescein isothiocyanate (FITC) conjugated Annexin V and propidium iodide (PI; Beyotime, China) were added to the cells successively. After incubation, Annexin V binding buffer was added, and cells were analyzed by flow cytometry.
Dual-luciferase reporter gene assay
The 3′UTR of Lcn2 harboring either the miR-138 binding site (Lcn2-3′UTR-WT) or a mutant (Lcn2-3′UTR-MUT) was cloned into the psiCHECK-2 vectors (Promega, USA) immediately downstream of the stop codon of the luciferase gene to generate the psiCHECK-lcn2-3′UTR luciferase reporter plasmid. Mutagenesis was performed when the seed region was mutated to remove all complementarity to nucleotides of miR-138. Plasmid DNA and mimics/miR-NC were co-transfected into HL-1 cells by using Lipofectamine 2000 (Invitrogen Inc., USA).
Western blot assay
Polyclonal anti-Lcn2 was purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (Bioss, China) was used as an internal control. Proteins of cells were extracted with sodium dodecyl sulfate (SDS) lysis buffer (Beyotime, China) and separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) gel. Subsequently, protein samples were transferred to polyvinylidene difluoride (PVDF; Roche). Membranes were probed with primary antibodies at 4℃ overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Beyotime, China) and detected by enhanced chemiluminescence (ECL) (Beyotime, China).
Cell transfection with pcDNA3.1/Lcn2
The full-length cDNA encoding human Lcn2 was amplified, and the recombinant plasmid, pcDNA3.1/Lcn2 was constructed (Invitrogen Inc., USA). HL-1 cells (miR-138 mimics or miR-NC) at 75% confluency were transfected with pcDNA3.1/Lcn2 (miR-138 + Lcn2 cells) using Lipofectamine 2000 in a six-well plate for 48 h, and transfection with pcDNA3.1(-) alone was performed for the control group (miR-138 + vector cells). Cells were then trypsinized and seeded onto a 10-cm dish. Stable transfection cells were subjected to G418 selection (400 mg/ml), and after two weeks, single-cell clones were selected and expanded. After G418 selection, identification of Lcn2 was performed in six selected cell clones in each group. And one clone in each group was selected to be used in the subsequent study.
Statistical analysis
For quantitative data, all results are expressed as the mean ± SEM. Statistical significance between groups was determined using one-way analysis of variance (ANOVA) or an unpaired Student’s t-test using SPSS 18.0 (SPSS, USA). Each experiment was repeated at least three times. And P value of <0.05 was considered statistically significant.
Results
Expression of miR-138 in hypoxic cardiomyocytes
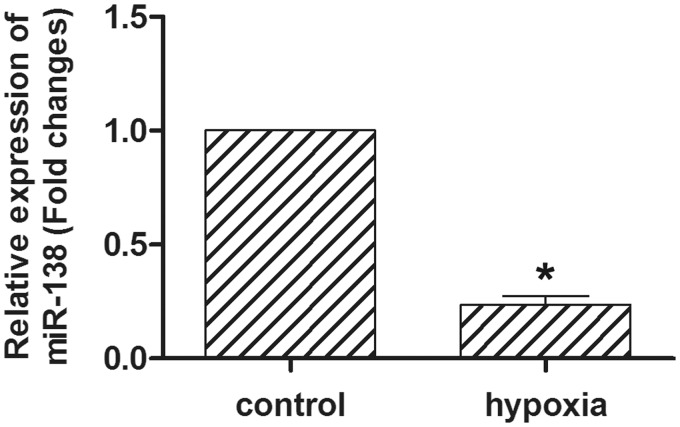
To determine the miR-138 level in hypoxic HL-1 cells, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed (Figure 1). HL-1 cells were exposed to hypoxia for 24 h at 1% oxygen concentration. The expression of miR-138 was significantly decreased to 23% of that in normoxia controls (P < 0.05). The results suggested that miR-138 was down-regulated by hypoxia in cardiomyocytes.
Figure 1.
Expression of miR-138 in hypoxic cardiomyocytes. qRT-PCR revealed that the expression of miR-138 was significantly decreased in the hypoxic HL-1 cells. HL-1 cells were cultured in 1% O2 and 5% CO2. *P < 0.05.
Effect of miR-138 on cell growth and apoptosis in hypoxic cardiomyocytes
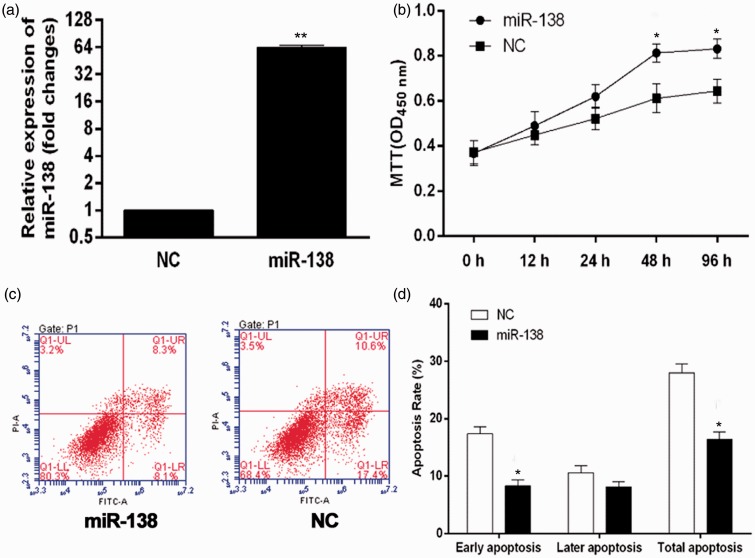
The miR-138 was over-expressed in HL-1 cells using miR-138 mimics (Figure 2(a)). After miR-138 upon mimetic transfection, the cell survival in hypoxic conditions was tested by MTT assay (Figure 2(b)). Over-expression of miR-138 promoted HL-1 cells proliferation and reached 132% of that in miR-NC group at 24 h, with a statistical significance (P < 0.05). Cardiomyocytes are highly susceptible to hypoxia-induced cell apoptosis. To determine whether over-expression of miR-138 was protective against hypoxia-induced apoptosis, the effect of miR-138 upon mimetic transfection on cell apoptosis was examined by the Annexin V-FITC/PI assay (Figure 2(c)). The results showed that over-expression of miR-138 significantly decreased hypoxia-induced cell apoptosis compared with miR-NC groups, especially in the level of early apoptosis (Figure 2(d)).Thus, miR-138 over-expression significantly enhanced cell survival and inhibited cell apoptosis in the hypoxic conditions.
Figure 2.
Effect of miR-138 over-expression on cell growth and apoptosis in hypoxic cardiomyocytes. (a) The over-expression of miR-138 in miR-138 upon mimetic transfection was validated using qRT-PCR. HL-1 cells transfected with empty plasmid were used as a negative control (NC). *P < 0.05. (b) After transfected with miR-138 mimics, HL-1 cells were cultured in 1% O2 and 5% CO2 (hypoxia) for different duration, and cell survival curve was measured by MTT. *P < 0.05. (c) Exposed to hypoxia for 48 h, cell apoptosis was tested by Annexin V-FITC/PI flow cytometry, and the proportion of apoptosis cells was measured. *P < 0.05. (d) Cells treated with miR-138 mimics versus cells treated with miR-NC.
MTT: 3-(4,5-dimethyl-thiazol-2-y1) 2,5-diphenyl tetrazolium bromide. (A color version of this figure is available in the online journal)
Lcn2 is a target gene of miR-138
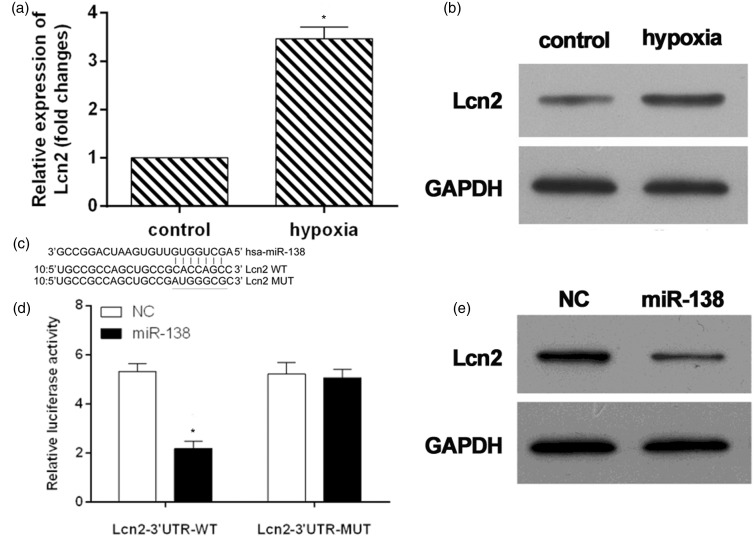
In the hypoxic conditions for 48 h, the mRNA level of Lcn2 increased threefolds (Figure 3(a)). The protein expression of Lcn2 was also enhanced (Figure 3(b)). To verify whether Lcn2 is a direct target of miR-138, TargetScan algorithm was used to predict target genes of miR-138, and a dual-luciferase reporter system was employed. The 3′UTR of Lcn2 was inserted downstream of the luciferase gene and transfected into HL-1 cells together with miRNAs mimics or miR-NC (Figure 3(c)). The results showed that miR-138 could down-regulate the luciferase activity of the reporter (Figure 3(d)). In order to further proved its reliability, mutants of Lcn2 3′UTR was constructed by deleting the miR-138 targets site (Figure 3(c)) and co-transfected into HL-1 cells together with miR-138 mimics/miR-NC. The luciferase expression of mutant 3′UTR of Lcn2 was no longer subject to be regulated by miR-138 (Figure 3(d)). These results suggested that this site in the 3′UTR of Lcn2 was exact regulation site for miR-138. The decrease of Lcn2 expression after miR-138 upon mimetic transfection for 48 h further confirmed that Lcn2 was a target gene of miR-138 (Figure 3(e)).
Figure 3.
Lcn2 was target gene of miR-138. The mRNA (a) and protein (b) expressions of Lcn2 in hypoxic cardiomyocytes. (c) Sequence alignment of miR-138 and 3'UTR of Lcn2 using TargetScan algorithm. (d) HL-1 cells were co-transfected with miR-138 mimics and a luciferase reporter containing a fragment of the Lcn2 3'UTR harboring either the miR-138 binding site (Lcn2-3′UTR-WT) or a mutant (Lcn2-3′UTR-MUT). The assays showed that luciferase activity in the Lcn2-3′UTR-WT group was significantly decreased compared to the luciferase activity of the mutant groups. (e) Western blot detection of Lcn2 expression in HL-1 cells treated with miR-138-overexpression or NC constructs. *P < 0.05.
GADPH: glyceraldehyde 3-phosphate dehydrogenase; NC: negative control.
Effect of Lcn2 expression on miR-138 over-expression-enhanced cell survival and miR-138 over-expression-inhibited cell apoptosis in hypoxic conditions
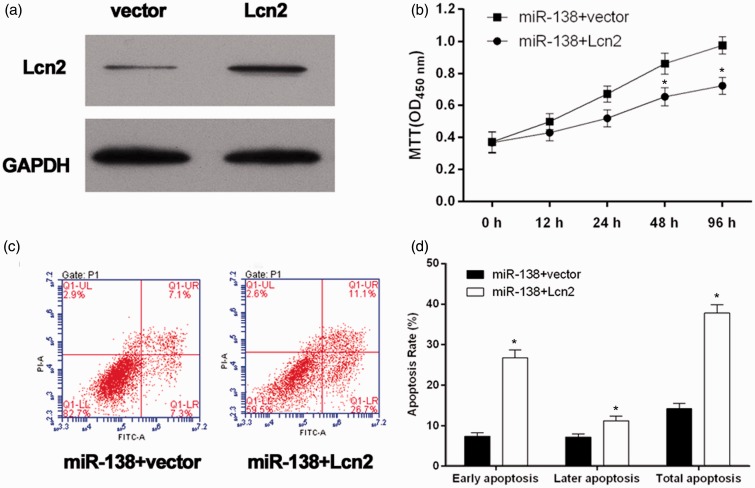
To identify the increased expression of Lcn2 in HL-1 cells, Lcn2 was examined using western blot analysis (Figure 4(a)). The results showed an over-expression of Lcn2 in miR-138 + Lcn2 cells. Using MTT assay, we found that miR-138 over-expression-promoted HL-1 cells proliferation in the hypoxia conditions was significantly decreased by Lcn2 over-expression (Figure 4(b)). To determine whether over-expression of Lcn2 could attenuate the protection of miR-138 over-expression from hypoxia-induced apoptosis, the Annexin V-FITC/PI assay was performed (Figure 4(c)). The results showed that over-expression of Lcn2 significantly increased the apoptosis of miR-138 over-expression cells (Figure 4(d)). Taken together, miR-138 over-expression significantly enhanced cell survival and inhibited cell apoptosis in the hypoxic conditions via targeting Lcn2.
Figure 4.
Validation of the role of Lcn2 in the over-expression of miR-138-influenced cell growth and apoptosis of hypoxic cardiomyocytes. (a) The Lcn2 levels were analyzed by western blot. Lcn2 was high expressed in miR-138 + Lcn2 cells. (b) The miR138 + Lcn2 cells/miR138 + vector cells were cultured in 1% O2 and 5% CO2 (hypoxia) for different duration, and cell survival curve was measured by MTT. *P < 0.05. (c) Exposed to hypoxia for 48 h, cell apoptosis was tested by Annexin V-FITC/PI flow cytometry, and proportion of apoptosis cells was measured. *P < 0.05. (d) miR138 + Lcn2 cells versus miR-138 + vector cells.
GADPH: glyceraldehyde 3-phosphate dehydrogenase; MTT: 3-(4,5-dimethyl-thiazol-2-y1) 2,5-diphenyl tetrazolium bromide. (A color version of this figure is available in the online journal)
Discussion
Literatures have demonstrated that Lcn2 was elevated during ischemia-reperfusion processes and in coronary heart disease and myocardial infarction16,18,19,21 and might be a useful biomarker of the severity of heart diseases. In the current study, we demonstrated that Lnc2 expression in the cardiomyocytes regulated by miR-138 and over-expression of miR-138 protects cardiomyocytes from hypoxia-induced apoptosis via targeting Lcn2.
Using qRT-PCR, we observed a decrease of the expression of miR-138 in hypoxic HL-1 cells (Figure 1). When miR-138 over-expressed in HL-1 cells, the hypoxia-induced cell apoptosis was dramatically attenuated (Figure 2). Also, the over-expression of miR-138 significantly enhanced the cell survival. It was suggested the over-expression of miR-138 to be beneficial for protection of cardiomyocyte from hypoxia.
The previous studies have also indicated that the hypoxia could induce the cardiomyocyte apoptosis, which is a main reason for the cardiac disease.4,8,12 Therefore, in this study, we established the cardiomyocyte apoptosis model by using the hypoxia. Our data also demonstrated that hypoxia induced the expression of Lcn2 in HL-1 cells in both mRNA and protein levels (Figure 3(a) and (b)). Lcn2 is a novel protein involved in iron transport.24 Lcn2 has the capability to bind iron, and the complex can internalize through binding to 24p3R and/or megalin.25,26 Lcn2-induced cardiomyocyte apoptosis is in part mediated via an increase in intracellular iron levels and induction of the intrinsic mitochondrial pathway via translocation and activation of Bax.22 However, little is presently known about regulation of the Lcn2 gene. To verify whether Lcn2 is a direct target of miR-138, we used a dual-luciferase reporter system. The 3′UTR of Lcn2 was inserted downstream of the luciferase gene and transfected into HL-1 cells together with miRNAs mimics or miR-NC (Figure 3(c)). The results showed that miR-138 could down-regulate the luciferase activity of the reporter (Figure 3(d)). In order to further prove its reliability, mutants of Lcn2 3′UTR were constructed by deleting the miR-138 targets site (Figure 3(c)) and co-transfected into HL-1 cells together with miR-138 mimics/miR-NC. The luciferase expression of mutant 3′UTR of Lcn2 was no longer subject to be regulated by miR-138 (Figure 3(d)). These results suggested that this site in the 3′UTR of Lcn2 was exact regulation site for miR-138. The decrease of Lcn2 expression after miR-138 upon mimetic transfection for 48 h further confirmed that Lcn2 was a target gene of miR-138 (Figure 3(e)).
In addition, our data using fluorescence activated cell sorting (FACS) analysis of Annexin V/PI binding to MTT assay clearly demonstrate that Lcn2 over-expression attenuated the cell proliferation and increased the cell apoptosis in HL-1 cells that over-expressed miR-138 (Figure 4(b) and (d)). The role of Lcn2 in the protection of miR-138 over-expression from hypoxia-induced cardiomyocyte apoptosis was confirmed.
It is very interesting to note that the role of Lcn2 and miR-138 in regulating apoptosis is stage-dependent. Over-expression of miR-138 predominantly inhibited the hypoxia-induced cell apoptosis at early stage (Figure 2(d)). However, the Lcn2 over-expression primarily attenuated the protection of miR-138 over-expression at later stage (Figure 4(d)). Also, there were some limitations for the present study, such as this study is only performed in the level in vitro and has no profound investigation for the mechanism. Therefore, the further experiments may attempt to understand fully the mechanism of miR-138 and Lcn2 on apoptosis on the level in vivo model.
In conclusion, over-expression of miR-138 inhibits the hypoxia-induced cardiomyocyte apoptosis via down-regulating pro-apoptotic gene expression of Lcn2. Manipulating the expression level of miR-138 and/or its downstream factor Lcn2 in injured/failing heart may lead to intervention of novel therapeutic strategies.
Author contribution
HX and ZG conceived and designed the study; HX, TL, and WH performed laboratory experiments; HX, DX, HL, and ML analyzed data and interpreted the results; HX and JL created the figures and drafted the manuscript; HX, TL, DX, and ZG edited and revised manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of China (81370380), the Natural Science Foundation of Guangdong province of China (S2013010014739) and the Science and Technology Foundation of Guangdong province of China (2012B091100155).
Conflict of interest
None declared.
References
- 1.Song T, Lv LY, Xu J, Tian ZY, Cui WY, Wang QS, Qu S, Shi XM. Diet-induced obesity suppresses sevoflurane preconditioning against myocardial ischemia-reperfusion injury: role of AMP-activated protein kinase pathway. Exp Biol Med 2011; 236: 1427–36. [DOI] [PubMed] [Google Scholar]
- 2.Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta 1998; 1366: 79–94. [DOI] [PubMed] [Google Scholar]
- 3.Li YG, Zhu W, Tao JP, Xin P, Liu MY, Li JB, Wei M. Resveratrol protects cardiomyocytes from oxidative stress through SIRT1 and mitochondrial biogenesis signaling pathways. Biochem Biophys Res Commun 2013; 438: 270–6. [DOI] [PubMed] [Google Scholar]
- 4.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radical Bio Med 2011; 51: 1271–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res 2004; 61: 461–70. [DOI] [PubMed] [Google Scholar]
- 6.Mancuso DJ, Abendschein DR, Jenkins CM, Han X, Saffitz JE, Schuessler RB, Gross RW. Cardiac ischemia activates calcium-independent phospholipase a2beta, precipitating ventricular tachyarrhythmias in transgenic mice: rescue of the lethal electrophysiologic phenotype by mechanism-based inhibition. J Biol Chem 2003; 278: 22231–6. [DOI] [PubMed] [Google Scholar]
- 7.Kitsis RN, Peng CF, Cuervo AM. Eat your heart out. Nat Med 2007; 13: 539–41. [DOI] [PubMed] [Google Scholar]
- 8.Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 2000; 87: 118–25. [DOI] [PubMed] [Google Scholar]
- 9.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120: 15–20. [DOI] [PubMed] [Google Scholar]
- 10.Lai EC. Micro rnas are complementary to 3′ utr sequence motifs that mediate negative post-transcriptional regulation. Nat Genet 2002; 30: 363–4. [DOI] [PubMed] [Google Scholar]
- 11.Morton SU, Scherz PJ, Cordes KR, Ivey KN, Stainier DY, Srivastava D. Microrna-138 modulates cardiac patterning during embryonic development. PNAS 2008; 105: 17830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu TS, Yang JJ, Yu FY, Liu BH. Cardiotoxicity of mycotoxin citrinin and involvement of microrna-138 in zebrafish embryos. Toxicol Sci 2013; 136: 402–12. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Ran Y, Zhang D, Chen J, Li S, Zhu D. Microrna-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem J 2013; 452: 281–91. [DOI] [PubMed] [Google Scholar]
- 14.He S, Liu P, Jian Z, Li J, Zhu Y, Feng Z, Xiao Y. Mir-138 protects cardiomyocytes from hypoxia-induced apoptosis via mlk3/jnk/c-jun pathway. Biochem Biophys Res Commun 2013; 441: 763–9. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz N, Michaelson JS, Putterman C. Lipocalin-2, tweak, and other cytokines as urinary biomarkers for lupus nephritis. Ann NY Acad Sci 2007; 1109: 265–74. [DOI] [PubMed] [Google Scholar]
- 16.Yndestad A, Landro L, Ueland T, Dahl CP, Flo TH, Vinge LE, Espevik T, Froland SS, Husberg C, Christensen G, Dickstein K, Kjekshus J, Oie E, Gullestad L, Aukrust P. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J 2009; 30: 1229–36. [DOI] [PubMed] [Google Scholar]
- 17.Poniatowski B, Malyszko J, Bachorzewska-Gajewska H, Malyszko JS, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in patients with chronic heart failure and coronary artery disease. Kidney Blood Press Res 2009; 32: 77–80. [DOI] [PubMed] [Google Scholar]
- 18.Choi KM, Lee JS, Kim EJ, Baik SH, Seo HS, Choi DS, Oh DJ, Park CG. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol 2008; 158: 203–7. [DOI] [PubMed] [Google Scholar]
- 19.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol 2006; 26: 136–42. [DOI] [PubMed] [Google Scholar]
- 20.Bu DX, Hemdahl AL, Gabrielsen A, Fuxe J, Zhu C, Eriksson P, Yan ZQ. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappab. Am J Pathol 2006; 169: 2245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aigner F, Maier HT, Schwelberger HG, Wallnofer EA, Amberger A, Obrist P, Berger T, Mak TW, Maglione M, Margreiter R, Schneeberger S, Troppmair J. Lipocalin-2 regulates the inflammatory response during ischemia and reperfusion of the transplanted heart. Am J Transplant 2007; 7: 779–88. [DOI] [PubMed] [Google Scholar]
- 22.Xu G, Ahn J, Chang S, Eguchi M, Ogier A, Han S, Park Y, Shim C, Jang Y, Yang B, Xu A, Wang Y, Sweeney G. Lipocalin-2 induces cardiomyocyte apoptosis by increasing intracellular iron accumulation. J Biol Chem 2012; 287: 4808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo HJ. Hl-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 1998; 95: 2979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson DR. 24p3 and its receptor: dawn of a new iron age? Cell 2005; 123: 1175–7. [DOI] [PubMed] [Google Scholar]
- 25.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 2005; 123: 1293–305. [DOI] [PubMed] [Google Scholar]
- 26.Elangovan N, Lee YC, Tzeng WF, Chu ST. Delivery of ferric ion to mouse spermatozoa is mediated by lipocalin internalization. Biochem Biphys Res Commun 2004; 319: 1096–104. [DOI] [PubMed] [Google Scholar]