Abstract
Since Chungkookjang, a short-term fermented soybean, is known to improve glucose metabolism and antioxidant activity, it may prevent the neurological symptoms and glucose disturbance induced by artery occlusion. We investigated the protective effects and mechanisms of traditional (TFC) and standardized Chungkookjang fermented with Bacillus licheniformis (BLFC) against ischemia/reperfusion damage in the hippocampal CA1 region and against hyperglycemia after transient cerebral ischemia in gerbils. Gerbils were subjected to either an occlusion of the bilateral common carotid arteries for 8 min to render them ischemic or a sham operation. Ischemic gerbils were fed either a 40% fat diet containing 10% of either cooked soybean (CSB), TFC, or BLFC for 28 days. Neuronal cell death and cytokine expression in the hippocampus, neurological deficit, serum cytokine levels, and glucose metabolism were measured. TFC and BLFC contained more isoflavonoid aglycones than CSB. Artery occlusion increased the expressions of IL-1β and TNF-α as well as cell death in the hippocampal CA1 region and induced severe neurological symptoms. CSB, TFC, and BLFC prevented the neuronal cell death and the symptoms such as dropped eyelid, bristling hair, reduced muscle tone and flexor reflex, and abnormal posture and walking patterns, and suppressed cytokine expressions. CSB was less effective than TFC and BLFC. Artery occlusion induced glucose intolerance due to decreased insulin secretion and β-cell mass. TFC and BLFC prevented the impairment of glucose metabolism by artery occlusion. Especially TFC and BLFC increased β-cell proliferation and suppressed the β-cell apoptosis by suppressing TNF-α and IL-1β which in turn decreased cleaved caspase-3 that caused apoptosis. In conclusion, TFC and BLFC may prevent and alleviate neuronal cell death in the hippocampal CA1 region and neurological symptoms and poststroke hyperglycemia in gerbils with artery occlusion. This might be associated with increased isoflavonoid aglycones.
Keywords: Chungkookjang, cytokine, artery occlusion, hyperglycemia, neurological symptoms
Introduction
Stroke is a leading cause of mortality worldwide and is one of the foremost contributors to functional disability.1 Disability from stroke is major public health problem that is growing in importance with the increasingly aging population. Previous studies have estimated that 45% of stroke patients experience post-stroke hyperglycemia, as defined as a fasting serum glucose level of 7 mmol/L or more.2 Only 10–20% patients with poststroke hyperglycemia have been previously diagnosed with diabetes and most patients do not have a previous impairment of glucose metabolism.3 Hyperglycemia after stroke was independently associated with infarct volume in magnetic resonance and spectroscopy studies, and it induces poor functional outcome.4–6 These results suggest that stroke itself leads to the impairment of glucose metabolism that exacerbates poststroke disability.
The incidence of stroke can be modified with modifiable managing risk factors such as hypertension, thrombosis, and fibrosis.7 The management of risk factors can decrease the incidence of stroke but it may not prevent poststroke hyperglycemia. It is important to determine underlying pathophysiologic mechanisms in order to limit the risk of poststroke hyperglycemia. Ischemic stroke accounts for 87% of all stroke events and it results from an interruption of blood flow to the brain due to a clot in the cerebral circulation.8 During the event, both the occlusion itself and the restoration of blood flow (reperfusion) generate the production of reactive oxygen species (ROS).9 ROS induce oxidative stress and inflammation. Productions of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 are also increased which activate the inflammatory cascades and they contribute to the progression of ischemic damage.10 These cytokines increase adhesion molecules and leukocytes infiltration to exacerbate the brain infarction to exacerbate the outcomes. Thus, all of these many factors are potentially involved in poststroke hyperglycemia.
Increased oxidative stress and inflammation may result in hyperglycemia in addition to increasing stress hormones during cerebral ischemic event, which in turn cause hyperglycemia due to both increased insulin resistance and impaired β-cell function and mass.11 However, the mechanism has not been determined. The possible mechanisms include increased circulating cytokines, nerve degeneration, and/or damage to brain regions that regulate glucose metabolism.11 Previous studies have demonstrated that pancreatic islets and neuronal cells closely interact to reciprocally maintain the function and mass of neuronal cells and islets, but ischemic stroke induces β-cell apoptosis.12,13 Therefore, poststroke hyperglycemia is related to the reduction of β-cell function and impairment of β-cell mass.
Soybeans (Glycine max MERILL) have long been consumed as a major protein source in Asia. They also contain isoflavonoids that are beneficial against metabolic diseases.14,15 Since soybeans are fermented for long-term storage in Asian countries, they contain a lot of salt as a preservative. However, traditional Chungkookjang is a soy food that is fermented with rice straw for short periods (2–3 days) without salt and is predominantly fermented with Bacillus species. During fermentation, isoflavonoids are converted from glycosides into the corresponding aglycones and most proteins are degraded into small peptides and amino acids.16,17 It is difficult to maintain consistent quality control when making traditional Chungkookjang. Inoculated Chungkookjang has been developed with fermentation started by inoculation with different major Bacillus species that are found in traditional Chungkookjang such as Bacillus amyloliquefaciens, Bacillus subtilus, and Bacillus licheniformis (BLFC). Previous studies have demonstrated that soybean fermented with BLFC have the greatest antidiabetic efficacy and the oral consumption of traditional Chungkookjang and Chungkookjang fermented with BLFC potentiates β-cell function and increases β-cell mass via decreased β-cell apoptosis and elevated β-cell proliferation in partially pancreatectomized rats, a type 2 diabetic animal model.18 Therefore, we hypothesized that traditional and BLFC-inoculated Chungkookjang may have neuroprotective effects against ischemia/reperfusion (I/R) damage in the hippocampal CA1 region and prevent hyperglycemia after transient ischemia. We tested the hypothesis in sand gerbils with transient ischemia and explored the mechanism of neuroprotection and glucose homeostasis by determining insulin secretion and insulin resistance.
Material and methods
Preparation of traditionally fermented Chungkookjang (TFC) and BLFC-inoculated Chungkookjang
BLFC SCD 111067P was obtained from Institute of Sunchang Fermented Soybean Products (Sunchang, Korea). It was cultivated in Luria-Bertani broth at 37 ℃ with shaking (128 r/min, Jeio Tech, Daejeon, Korea) to expand the number of BLFC and was used for Chungkookjang fermentation immediately after the culture.
TFC was prepared using a traditional processing method in the Sunchang region known to produce Chungkookjang with potent antidiabetic efficacy at Institute of Sunchang Fermented Soybean Products (Sunchang, Korea) as described in the previous studies.17,18 Boiled soybeans were cooled to 40 ℃ and fermented with rice straw at 42 ℃ for 48 h to make traditional Chungkookjang.17 For making standardized Chungkookjang, soaked soybeans were sterilized at 121 ℃ for 1 h, cooled to 40 ℃, and inoculated with 1% (v/w) BLFC SCD 111067P at concentrations of 107–108 CFU/mL, and the inoculated soybeans were incubated at 42 ℃ for 48 h.18 The fermented soybeans were lyophilized using a freezing dryer (Il Shin, Korea).
Isoflavonoid and peptide contents of soybeans and Chungkookjangs
CSB, TFC, and BLFC were extracted in 70% methanol containing 0.1% acetic acid, and isoflavonoids in the supernatant of the extracts were detected using HPLC (PU 980, JASCO, Japan) equipped with an ODS A303 (4.6 mm × 250 mm, 5 µm, YMC, USA) reversed phase column and monitored at a wavelength of 254 nm with a UV detector. Elution was carried out at a flow rate of 1.0 mL/min with water and acetonitrile containing 0.1% acetic acid. Peaks were identified by comparing to 12 reference isoflavonoids (Sigma Co., St Louis, MO) and Fujico (Tokyo, Japan).17
Animals and diets
Progeny of male Mongolian gerbils (Meriones unguiculatus) was obtained from the Experimental Animal Center, Hallym University (Chuncheon, Korea). The animals were housed under a 12 h light/dark cycle at 23 ℃ with 60% humidity and provided free access to food and water. The procedures for handling animals and their care conformed to NIH Guidelines and were approved by the Hoseo University Animal Care and Use Committee.
The semi-purified diets were prepared by modifying a base AIN-93 formulation for experimental diets.19 Since a high fat diet is reported to exacerbate ischemic damage,20,21 gerbils in each group were fed a high fat diet at one week prior to the ischemia and four weeks after ischemia. The diets in the CSB, TFC, and BLFC groups contained 10% lyophilized CSBs, traditionally fermented Chungkookjang or standardized Chungkookjang, respectively, as a main protein source. CSBs and two different kind of Chungkookjang were analyzed for carbohydrates, protein, and lipids. According to the results of analysis, each diet was tailored to contain identical macronutrient compositions by adding soybean oil and cellulose. All diets approximately consisted of 40 energy percent (En%) carbohydrates, 20 En% protein, and 40 En% fats (Table 1). Isoflavonoid contents in CSB, TFC, and BLFC were also given in Table 1.
Table 1.
Composition of experimental diets
| Casein diet | Cooked soybeans (CSB) diet | Chungkookjang (TFC/BLFC) diet | |
|---|---|---|---|
| Corn starch | 310 | 280 | 286 |
| Sucrose | 200 | 200 | 200 |
| Casein | 200 | 165 | 167 |
| Methionine | 3 | 3 | 3 |
| Corn oil | 50 | 40 | 38 |
| Shortening | 150 | 150 | 150 |
| Cellulose | 34 | 13 | 12 |
| Mineral | 35 | 31 | 30 |
| Vitamin | 10 | 10 | 10 |
| Choline | 2 | 2 | 2 |
| Soybean powder* | 0 | 100 | 100 |
| Total isoflavonoids (%) | – | 0.035 | 0.026 |
| Isoflavonoid aglycones (%) | – | 0.001 | 0.015 |
Assigned dried powder of cooked soybeans or traditionally made Chungkookjang (TFC) and Chungkookjang fermented with Bacillus licheniformis (BLFC) for each group.
Induction of transient forebrain ischemia
Mongolian gerbils are an experimental model of cerebral I/R injury, and the severity and duration of brain damage following I/R injury have been established in our laboratory as described.13 Neuronal cell death is affected by several factors such as the type of anesthesia and low body temperature during transient artery occlusion.22,23 We have used proper surgical methods to minimize the protecting factors. Gerbils at 15 weeks of age with a body weight of 50–60 g had anesthetization with an intraperitoneal injection of 400 mg/kg chloral hydrate (Sigma, St Louis, MO, USA) that has minimal preventive impact on forebrain ischemia.22 Common carotid arteries were isolated bilaterally and occluded using non-traumatic aneurysm clips and the complete interruption of blood flow was confirmed by observing the central artery in the retina using an ophthalmoscope. The aneurysm clips were removed after the 8 min occlusion to induce neuronal cell death in the CA1 region of the hippocampus.13 Since the decline of body temperature by 2–3 ℃ during transient artery occlusion surgery is another factor that could prevent neuronal cell death, body temperature was maintained during the surgery. Rectal body temperature under free-regulating or normothermic (37 ± 0.5 ℃) conditions was monitored with a rectal temperature probe (TR-100; Fine Science Tools, Foster City, CA, USA) and maintained using a thermometric blanket before, during, and after the surgery until the animals completely recovered from anesthesia. Thereafter, the animals were kept in a thermal incubator (Mirae Medical Industry, Seoul, Korea) to maintain their body temperature until they were euthanized. Sham gerbils underwent artery occlusion surgery using identical surgical procedures, except that the common carotid arteries were not occluded.
Experimental design and metabolic analysis
Gerbils with artery occlusion were randomly divided into four groups as follows: (1) artery occlusion + casein diet (control), (2) artery occlusion + CSB diet, (3) artery occlusion + TFC diet, and (4) artery occlusion + Chungkookjang fermented with BLFC diet. Gerbils with sham operations had casein diet as the normal-control group. On day 2 after artery occlusion, blood was collected from the tail to measure serum IL-1β and TNF-α levels by enzyme-linked immunosorbent assay using a commercial kit (Quantikine, R&D Systems, Minneapolis, MN, USA).
Overnight-fasted serum glucose levels, food and water intakes, and body weights were measured weekly at 10 AM during the experimental period. Three days before euthanasia (on day 27 after ischemia), overnight-fasted animals were underwent an oral glucose tolerance test (OGTT). OGTT was performed by orally administering 2 g/kg body weight of glucose after overnight fasting, and tail blood serum glucose levels were determined at 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 120 min after glucose loading. During OGTT, serum insulin levels were determined at 0, 10, 30, 60, 90, and 120 min. The average areas under the curve (AUC) of glucose and insulin were calculated.
After finishing the OGTT, animals were provided food for 6 h and then food was withheld. The next day after intraperitoneal insulin tolerance test, blood was collected from overnight-fasted animals and serum separated by centrifugation at 3,000 × g** for 15 min. After blood collection, the six gerbils were anesthetized with the mixture of ketamine and xylazine and human regular insulin (5 U/kg body weight) was injected through their inferior vena cava. After the insulin injection, tissues were rapidly collected and immediately frozen in liquid nitrogen, and stored at −70 ℃ for further experiments. Serum glucose levels were determined with a Glucose Analyzer II (Beckman, Palo Alto, CA, USA) and serum insulin levels were measured using a commercial RIA kit (Linco Research, St Charles, MO, USA).
Neurological severity score
The neurological evaluation scores suggested by Lawner et al.24 were determined with different clinical signs of ischemia, including ptosis: eyelid drooped (no symptom = 0, one eyelid partially drooped = 1, one eyelid totally drooped = 2, both eyelids partially drooped = 3, both eyelids totally drooped = 4); hair bristling (no symptom = 0, hair bristled = 1); decreased muscular tone (no symptom = 0, diminished muscular tone or strength in the limbs = 1); flexor reflex (no symptom = 0, hind limbs slightly withdrawn when pinch = 1, no withdrawal of hind limbs when pinched = 2), posture (normal = 0, hunched = 1); and walking pattern (normal = 0, slow = 1, no walking = 2).
Cresyl violet staining
The neuronal cell death due to ischemic injuries was evaluated in the hippocampal sections from gerbils with and without artery occlusion by staining them with cresyl violet at the end of the 28-day experimental period. The sections were mounted on gelatin-coated microscopy slides. Cresyl violet acetate (1.0% w/v, Sigma) was dissolved in distilled water and glacial acetic acid (Sigma). After staining the sections with cresyl violet solution for 2 min at room temperature, they were washed twice in distilled water. The fixed brain tissue samples were dehydrated by immersion in a graded series of ethanol baths at room temperature and mounted with Permount (Fisher Scientific Inc., Pittsburgh, PA, USA).
Determination of lipid peroxidation
The hippocampal levels of malondialdehyde (MDA) were determined as an indicator of lipid peroxidation. The MDA production and hence lipid peroxidation were assessed in the tissues. MDA forms a colored complex in the presence of TBA, which is detectable by measurement of absorbance at 532 nm. Absorbance was measured with spectrophotometer (Perkin Elmer). 1,1′,3,3′-Tetraethoxypropane was used as a standard and the results were expressed in tissue as µmol/g protein.25
Islet isolation
At the end of the experimental period, pancreatic islets of six gerbils from each group were isolated by collagenase digestion. Three milliliters of 1.0 mg/mL collagenase P (Roche Molecular Biochemicals, Indianapolis, Indiana, USA) in DMEM-high glucose was injected through the pancreatic duct into the pancreas of gerbils anesthetized with sodium pentobarbital. The pancreas was immediately removed and incubated at 37 ℃ for 15 min. The digested pancreas was washed with DMEM-high glucose four times on ice, and islets were isolated with a separation medium consisting of gradient Ficoll reagents (Sigma)26 and pooled from two or three gerbils from each group.
Immunohistochemistry and islet morphometry
Four gerbils from each group were treated with 5-bromo-2-deoxyuridine (BrdU; Roche Molecular Biochemicals; 100 µg/kg body weight) at the end of the 28-day experimental period. Six hours postinjection, pancreas samples were prepared and evaluated as previously described.27 The pancreas was dissected, fixed in a 4% paraformaldehyde solution (pH 7.2) overnight at room temperature, and embedded in paraffin blocks. Serial 5 µm paraffin-embedded tissue sections were mounted on slides. To prevent selection of sections with the same islet from occurring twice, after rehydration every sixth or seventh section was selected to determine β-cell area, BrdU incorporation, and apoptosis. Three sections per each rat were randomly chosen for immunostaining as described previously.28
Endocrine β-cells were identified by incubating paraffin-embedded pancreatic sections with a guinea pig anti-insulin antibody. β-cell proliferation was examined by the incorporation of BrdU into β-cells from gerbils injected with BrdU. Total area of the section of the pancreas and the area of anti-insulin antibody staining were determined and the percentage of the stained area was calculated. This incorporation was determined by immunohistochemistry with anti-insulin (Zymed Laboratories, South San Francisco, CA, USA) and anti-BrdU antibodies (Roche, Mannheim, Germany). Apoptosis of the β-cells was measured by TUNEL assay (Roche) using hematoxylin and eosin to visualize islets.23,24 Quantification of the β-cell area, BrdU+ cells, and apoptotic bodies in islets was performed as described previously.28
Real-time quantitative reverse transcriptase- polymerase chain reaction (RT-PCR) in islets
Total RNA was isolated from the islets using a monophasic solution of phenol and guanidine isothiocyanate (Trizol reagent, Gibco-BRL, Rockville, MD), followed by extraction and precipitation with isopropyl alcohol. The cDNA was synthesized from equal amounts of total RNA with superscript III reverse transcriptase, and PCR was performed with high fidelity Taq DNA polymerase. Equal amounts of cDNA were mixed with Sybergreen mix and were analyzed using a RT-PCR machine (BioRad Laboratories, Hercules, CA). The gene expression level in unknown samples was quantitated using the comparative CT method.27 The following primers were used for PCR reactions (5′–3′), mouse IL-1β: sense 5′-TGCAGAGTTCCCCAACTGGTACATC-3′, antisense 5′-GTGCTGCCTAATGTCCCCTTGAATC-3′, mouse TNF-α: sense 5′-CCTGTAGCCCACGTCGTAGC-3′, antisense: 5′-TTGACCTCAGCGCTGAGTTG-3′, mouse β-actin: sense 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and antisense 5′-ATGGAGCCACCGATCCACA-3′. The primers were designed to sandwich at least one intron in order to distinguish between the products derived from mRNA and genomic DNA.
Immunoblot analysis
The pooled islets of three mice were lysed in 20 mM Tris buffer (pH 7.4) containing 2 mM EDTA, 137 mM NaCl, 1% NP40, 10% glycerol, 12 mM α-glycerol phosphate, and protease inhibitors. After 30 min on ice, the lysates were centrifuged for 10 min at 12,000 r/min at 4 ℃. After measuring the protein content of lysates using a Bio-Rad protein assay kit, lysates containing equal amounts of protein (30–50 µg) were resolved by SDS-PAGE, and immunoblotting was performed using specific antibodies against caspase-3, cleaved caspase-3, and β-actin (Cell Signaling Technology) as previously described.28 Band intensities were quantified using Imagequant TL (Amersham Biosciences, Piscataway, NJ, USA) and the relative intensity of interested protein was calculated based on β-actin.
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical analysis was performed using SAS software. The significance of supplementation effects on neurological impairment and glucose homeostasis after artery occlusion was determined by one-way ANOVA followed by Tukey’s test. In this analysis normal-control group was included. Differences among groups with a P < 0.05 were considered statistically significant.
Results
Isoflavonoid contents in CSB, TFC, and BLFC
The contents of diadzin, genistin, and glycitin, isoflavonoid glycosides, were higher in the ascending order of CSB, BLFC, and TFC and on contrarily, the contents of malonyl daidzin, malonyl glycitin, and malonyl genistin were opposite of isoflavonoid glycosides. The contents of isoflavonoid aglycones, daidzein, glycitein, and genistein were much higher in TFC and BLFC than CSB. As a result, total isoflavonoids were decreased during fermentation, but the ratio of isoflavonoid aglycones was greatly increased in TFC and BLFC (Table 2).
Table 2.
Isoflavone contents in soybeans and Chungkookjang
| CSB | TFC | BLFC | |
|---|---|---|---|
| Daidzin | 492 ± 18a | 152 ± 21b | 62.6 ± 5.3c |
| Glycitin | 151 ± 17a | 76.6 ± 10.7b | 33.5 ± 1.2c |
| Genistin | 841 ± 23a | 257 ± 38b | 107 ± 13c |
| Malonyl daidzin | 124 ± 10c | 168 ± 21b | 215 ± 17a |
| Malonyl glycitin | 24.5 ± 5.2c | 34.3 ± 3.2b | 62.4 ± 4.2a |
| Malonyl genistin | 141 ± 13a | 33.5 ± 10.4c | 95.3 ± 15.4b |
| Acetyl daidzin | 105 ± 10a | 19.3 ± 2.2c | 45.4 ± 3.5b |
| Acetyl glycitin | 61.5 ± 3.4 | – | – |
| Acetyl genistin | 146 ± 4.2a | 13.1 ± 1.8c | 18.4 ± 2.2b |
| Total glycosides | 2,086 ± 268a | 754 ± 85b | 640 ± 71a |
| Daidzein | 24.2 ± 4.3b | 177 ± 24a | 166 ± 16a |
| Glycitein | 13.2 ± 2.7c | 67.4 ± 8.5b | 78.7 ± 6.7a |
| Genistein | 22.5 ± 3.5c | 125 ± 18a | 98.2 ± 5.3b |
| Total aglycones | 59.9 ± 7.8b | 369 ± 48a | 343 ± 41a |
| Total isoflavones | 2,146 ± 225a | 1,123 ± 128b | 983 ± 92c |
BLFC: Chungkookjang fermented with Bacillus licheniformis; CSB: cooked soybeans; TFC: traditionally fermented Chungkookjang.
Values are mean ± SD (n = 3).
Means in the same row with different superscripts were significantly different by Tukey’s test at P < 0.05.
Consumption of soybean and isoflavonoids
Gerbils in the CSB and TFC/BLFC groups daily consumed about 35–40 and 25–30 mg total isoflavonoids/kg body weight, respectively, whereas the intake of isoflavonoid aglycones was approximately 1 mg/kg body weight for CSB and 15–20 mg/kg body weight, as calculating from food intake and contents of isoflavonoids in the diets (Table 1). This was equivalent of about 20–25 g dried soybeans or Chungkookjang on a daily basis in humans using a conversion coefficient for human dosage from experimental animal studies suggested by the US FDA.29
Cresyl violet staining and neurological severity score
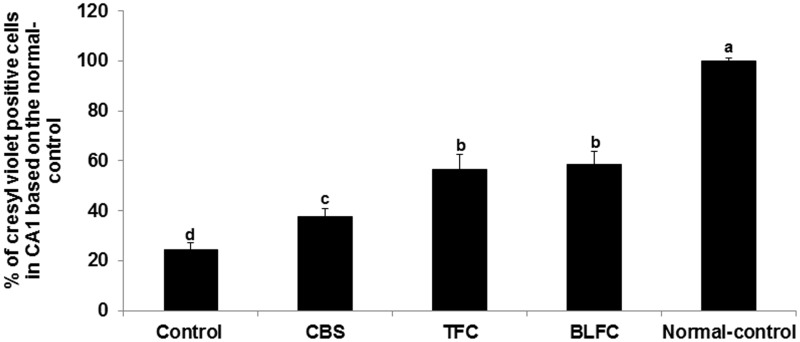
There were 58% fewer cresyl violet-positive neurons, in the CA1 regions of the hippocampus in the control group compared to the normal-control group (Figure 1). The number of cresyl violet-positive neurons in the CA1 regions was increased in the ascending order of the control, CSB, TFC, and BLFC but the number of live neurons was not as high as in the normal-control group (Figure 1).
Figure 1.
Neuronal cell death of the hippocampal CA1 region measured by cresyl violet staining. Gerbils that underwent the ischemic by carotid artery occlusion for 8 min were randomly divided into four groups, as follows: artery occlusion-induced I/R (I/R) + a high-fat diet (HFD), I/R + cooked soybean in HFD (CSB), I/R + traditionally made Chungkookjang (TFC) in HFD, I/R + Chungkookjang fermented with Bacillus licheniformis in HFD (BLFC). Sham-operated gerbils were had a HFD as normal control. At end of the experiments live neuronal cells were measured by cresyl violet staining and the intensity was measured by image analyzers. The percentage of cresyl violet positive neuronal cells (live cells) in the I/R group was calculated based on those of the normal-control group. Bars represent mean ± SD (n = 10). a,b,c,dMeans on the bars with different superscripts were significantly different by Tukey’s test at P < 0.05.
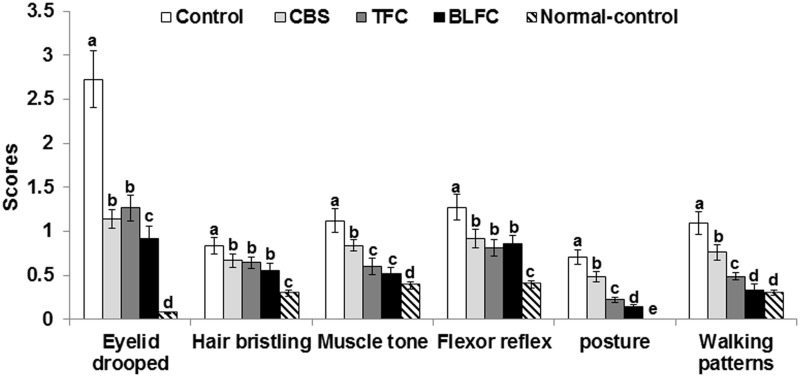
Artery occlusion significantly increased neurological severity scores including dropped eyelid, bristling hair, less muscle tone and flexor reflex, and abnormal posture and walking patterns (Figure 2). CBS, TFC, and BLFC had lower scores than the controls but not as low as the normal-control group. CSB, TFC, and BLFC had different impacts on the scores: all three diets improved hair bristling and flexor reflex. BLFC exhibited the lowest scores for eyelid drooped, muscle tone, and posture and walking pattern.
Figure 2.
Neurological severity scores. Gerbils that underwent the ischemic by carotid artery occlusion for 8 min were randomly divided into four groups, as follows: artery occlusion-induced I/R (I/R) + a high-fat diet (HFD), I/R + cooked soybean in HFD (CSB), I/R + traditionally made Chungkookjang (TFC) in HFD, I/R + Chungkookjang fermented with Bacillus licheniformis in HFD (BLFC). Sham-operated gerbils were had a HFD as normal control. After 28 days after artery occlusion, the neurological severity scores were determined. Bars represent mean ± SD (n = 10). a,b,c,d,eMeans on the bars with different superscripts were significantly different by Tukey’s test at P < 0.05.
The circulating levels of cytokines
To determine the modification of cytokines induced by artery occlusion at two days after ischemia, IL-1β and TNF-α levels in the circulation were markedly increased in overnight-fasted I/R gerbils in comparison to Sham gerbils whereas TFC and BLFC treatment suppressed their increase (Table 1). CSB, unfermented soybean, inhibited the increase of TNF-α levels but not IL-1β levels. Thus, the increases in serum levels of IL-1β and TNF-α in I/R gerbils were associated with I/R-induced inflammation and TFC and BLFC substantially suppressed inflammation in gerbils with artery occlusion. The levels of cytokines in the circulation were consistent with the cell apoptosis in the CA1 of the hippocampus in I/R gerbils.
Lipid peroxide contents and cytokine expression in the hippocampus
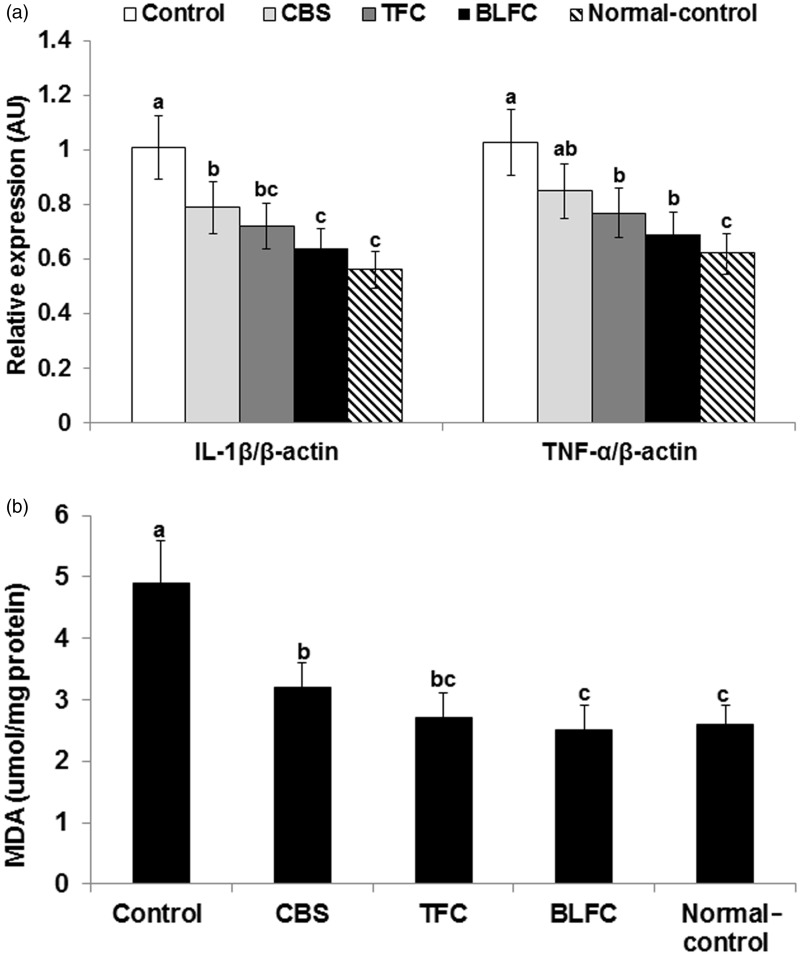
Since the increase of neuronal cell death by I/R may be related to increased lipid peroxide contents and cytokines, MDA contents and relative mRNA expression of IL-1β and TNF-α, the major pro-cytokines, were measured in the hippocampus. Similar to serum concentration, mRNA levels of IL-1β and TNF-α in the hippocampus were much greater in I/R than sham gerbils and CSB, TFC, and BLFC inhibited the increase of their expression (Figure 3(a)). BLFC-treated I/R gerbils had similar levels of IL-1β and TNF-α as Sham gerbils. In addition to cytokine expression, the MDA concentrations, an index of lipid peroxide, were much greater in control gerbils than sham gerbils and CBS, TFC, and BLFC prevented their increase in the hippocampus (Figure 3(b)).
Figure 3.
Hippocampal mRNA expression of IL-1β and TNF-α and lipid peroxide contents. Gerbils that underwent the ischemic by carotid artery occlusion for 8 min were randomly divided into four groups, as follows: artery occlusion-induced I/R (I/R) + a high-fat diet (HFD), I/R + cooked soybean in HFD (CSB), I/R + traditionally made Chungkookjang (TFC) in HFD, I/R + Chungkookjang fermented with Bacillus licheniformis in HFD (BLFC). Sham-operated gerbils were had a HFD as normal control. At end of experiment, the mRNA expression of IL-1β and TNF-α (a; n = 5) and the contents of malondialdehyde (MDA) as an indicator of lipid peroxides (b; n = 10) were measured in the hippocampus by real-time PCR. Bars represent means ± SD. a,b,cMeans on the bars with different superscripts were significantly different by Tukey’s test at P < 0.05.
Glucose metabolism
Overnight fasted serum glucose levels were higher in I/R control gerbils than sham gerbils, whereas pretreatment of TFC and BLFC prevented their increase in I/R gerbils, but the levels were not as low as Sham gerbils (Table 3). Serum insulin levels at the overnight fasting state were lower in I/R control gerbils than Sham gerbils whereas TFC and BLFC suppressed their decrease in I/R gerbils and the levels in gerbils having TFC and BLFC reached the levels of Sham gerbils (Table 3). These results indicated that the increase of serum glucose levels in I/R gerbils was partly associated with decreased serum insulin levels and TFC and BLFC pretreatment prevented the attenuation of insulin secretion.
Table 3.
The cytokine and glucose and insulin levels in the circulation
| Control (n = 10) | CSB (n = 10) | TFC (n = 10) | BLFC (n = 10) | Normal control (n = 10) | |
|---|---|---|---|---|---|
| Serum glucose levels (mM) | 6.9 ± 0.8a | 6.7 ± 0.7a | 6.0 ± 0.6b | 6.0 ± 0.5b | 5.4 ± 0.6c |
| Serum insulin levels (ng/mL) | 0.92 ± 0.12b | 0.93 ± 0.11b | 1.11 ± 0.13a | 1.08 ± 0.12a | 1.17 ± 0.13a |
| HOMA-IR | 6.4 ± 0.8 | 6.2 ± 0.7 | 6.6 ± 0.5 | 6.5 ± 0.6 | 6.3 ± 0.7 |
| Serum interlukin-1β levels (pg/mL) | 9.2 ± 1.1a | 8.5 ± 0.9ab | 7.7 ± 0.9b | 7.5 ± 0.9b | 7.6 ± 0.8b |
| Serum tumor necrosis factor-α levels (pg/mL) | 20.6 ± 2.5b | 17.2 ± 1.9c | 16.5 ± 1.8a | 16.3 ± 1.9a | 16.6 ± 1.9a |
Gerbils that underwent the ischemic by carotid artery occlusion for 8 min were randomly divided into four groups, as follows: artery occlusion-induced I/R (I/R) + a high-fat diet (HFD), I/R + cooked soybean in HFD (CSB), I/R + traditionally made Chungkookjang (TFC) in HFD, I/R + Chungkookjang fermented with Bacillus licheniformis in HFD (BLFC). Sham-operated gerbils were had a HFD as normal control. Values are means ± SD (n = 10).
Values in the same row with different superscripts were significantly different by Tukey’s test at P < 0.05.
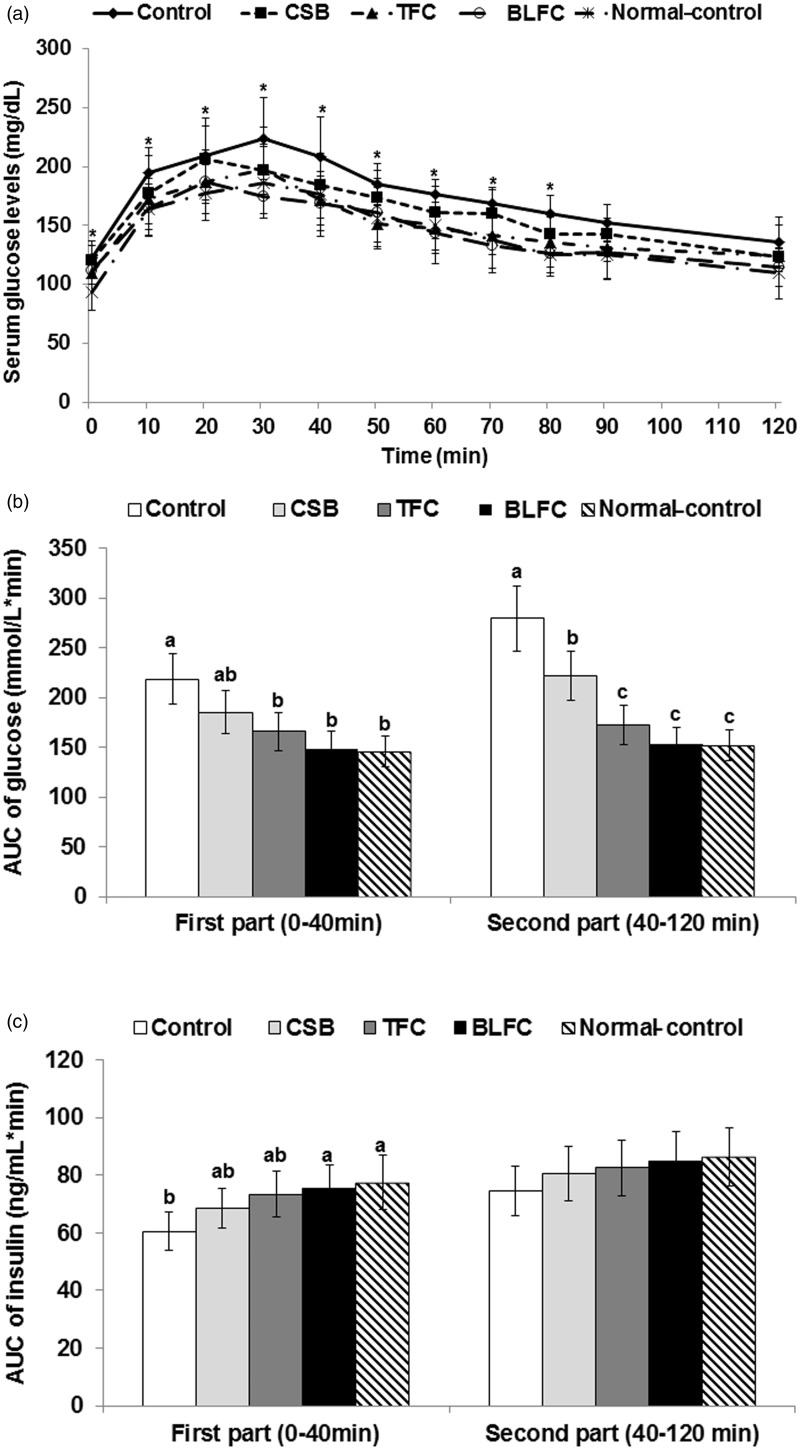
During OGTT, peak serum glucose levels were higher in I/R control gerbils than in sham gerbils, and CSB, TFC, and BLFC treatments lowered the peak levels in I/R gerbils (Figure 4(a)). The changes in serum glucose levels after glucose loading revealed glucose intolerance in I/R gerbils and the glucose intolerance was prevented by soy foods in the ascending order of CSB, TFC, and BLFC. Serum glucose levels after peak levels were decreased in all groups but the decrease was slightly lower in the I/R control gerbils than Sham gerbils. CSB, TFC, and BLFC treatment in the I/R group decreased serum glucose levels similar to those of Sham gerbils.
Figure 4.
Oral glucose tolerance testing (OGTT) in ischemic gerbils. Gerbils that underwent the ischemic by carotid artery occlusion for 8 min were randomly divided into four groups, as follows: artery occlusion-induced I/R (I/R) + a high-fat diet (HFD), I/R + cooked soybean in HFD (CSB), I/R + traditionally made Chungkookjang (TFC) in HFD, I/R + Chungkookjang fermented with Bacillus licheniformis in HFD (BLFC). Sham-operated gerbils were had a HFD as normal control. On day 25 after ischemia, gerbils underwent an OGTT with 2 g glucose/kg body weight after overnight fasting. The changes of serum glucose levels during 120 min (a) and area under the curve (AUC) of serum glucose (b) and insulin (c) in the first part (0–40 min) and second part (40–120 min) during the oral glucose tolerance test. Bars and dots represent means ± SD (n = 10). *Significantly different among the groups by one-way ANOVA at P < 0.05. a,b,cMeans on the bars with different superscripts were significantly different by Tukey’s test at P < 0.05.
During the early phase of OGTT (0–40 min), the AUC of serum glucose levels was higher in I/R gerbils than Sham gerbils whereas TFC and BLFC treatments lowered the AUC in I/R gerbils (Figure 4(b)). The AUC of glucose also remained higher in I/R gerbils during the late phase of OGTT (40–120 min), but its increase was prevented by CBS < TFC and BLFC in I/R gerbils and TFC and BLFC lowered the AUC as much as Sham gerbils (Figure 4(b)). Serum insulin levels in the early phase were lower in I/R gerbils and BLFC prevented the decrease in insulin levels. However, the AUC of serum insulin levels during the late phase tended to be lower in I/R gerbils, but it was not significantly different among the groups (Figure 4(c)) and the soybean-based treatments did not affect the levels.
β-cell mass
Although serum glucose levels were higher in the I/R group than Sham group, serum insulin levels were lower in the I/R gerbil, indicating that insulin secretion may be insufficient and it may be related to β-cell mass. The islet morphometry was assessed revealing that β-cell area was markedly lower in the I/R gerbils than in the Sham gerbils (Table 4). TFC and BLFC treatment in I/R gerbils completely prevented the loss of β-cell mass which was the same as Sham gerbils, whereas CBS partly suppressed its decrease (Table 4). The increase of β-cell area is related to the sum of β-cell number and size. Despite of the decrease in β-cell area, the individual size of β-cells was greater in the I/R group than the Sham group and the treatment of CSB, TFC, and BLFC inhibited the increase (Table 4). This indicated that I/R induced hypertrophy and suppressed hyperplasia. The β-cell number is regulated by the balance of proliferation and apoptosis. The β-cell proliferation measured by BrdU+ incorporation was not different between the I/R and Sham groups but it was increased by TFC and BLFC treatment in I/R gerbils (Table 4). Apoptosis of β-cells was much greater in I/R gerbils than Sham gerbils, whereas it was suppressed in the ascending order of CSB < TFC and BLFC (Table 4). Thus, I/R-induced β-cell apoptosis and TFC and BLFC prevented it at the levels of Sham gerbils.
Table 4.
Expression of cytokines and islet morphometry at the end of experimental periods
| Control (n = 4) | CSB (n = 4) | TFC (n = 4) | BLFC (n = 4) | Normal control (n = 4) | |
|---|---|---|---|---|---|
| β-cell area (%) | 19.5 ± 3.6b | 23.3 ± 3.7b | 26.7 ± 3.5a | 27.2 ± 4.1a | 27.4 ± 3.4a |
| Absolute β-cell mass (mg) | 0.94 ± 0.12c | 1.12 ± 0.14b | 1.26 ± 0.14a | 1.28 ± 0.15a | 1.24 ± 0.14a |
| Individual β-cell size (µm2) | 7.5 ± 0.9a | 6.3 ± 0.7b | 6.5 ± 0.8b | 6.4 ± 0.8b | 6.1 ± 0.7b |
| BrdU +cells (% BrdU+ cells of islets) | 5.3 ± 0.7b | 5.9 ± 0.7ab | 6.2 ± 0.7a | 6.3 ± 0.7a | 5.7 ± 0.6 |
| Apoptosis (% apoptotic bodies of islets) | 20.9 ± 2.5a | 16.5 ± 2.1b | 15.4 ± 2.0b | 15.0 ± 1.8b | 14.8 ± 1.9b |
Gerbils that underwent the ischemic by carotid artery occlusion for 8 min were randomly divided into four groups, as follows: artery occlusion-induced I/R (I/R) + a high-fat diet (HFD), I/R + cooked soybean in HFD (CSB), I/R + traditionally made Chungkookjang (TFC) in HFD, I/R + Chungkookjang fermented with Bacillus licheniformis in HFD (BLFC). Sham-operated gerbils were had a HFD as normal control. Values are means ± SD.
Values in the same row with different superscripts were significantly different among I/R gerbil groups by Tukey’s test at P < 0.05.
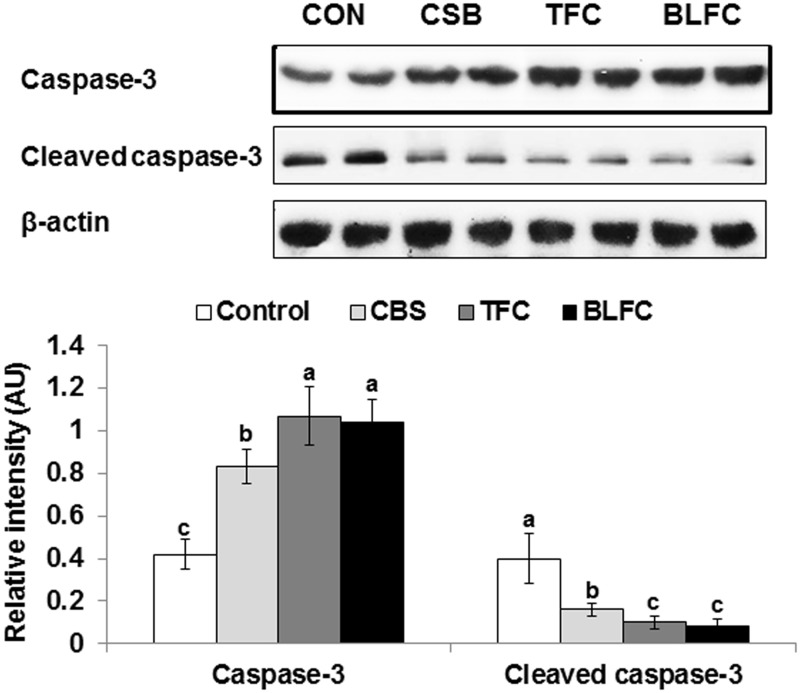
Since the increase in β-cell apoptosis by I/R may be related to increased cleaved caspase-3 production, the protein expression of caspase-3 and cleaved caspase-3 was measured. The caspase-3 expression in the islets was increased in I/R gerbils but the levels of cleaved caspase-3 were opposite of the caspase-3 expression (Figure 5). These changes in the I/R gerbils were reversed by TFC and BLFC and the CSB did not prevent the increase of cleaved caspase-3 in I/R gerbils (Figure 5).
Figure 5.
Caspase-3 and cleaved caspase-3 protein levels in the isolated islets. Gerbils that underwent the ischemic by carotid artery occlusion for 8 min were randomly divided into four groups, as follows: artery occlusion-induced I/R (I/R) + a high-fat diet (HFD), I/R + cooked soybean in HFD (CSB), I/R + traditionally made Chungkookjang (TFC) in HFD, I/R + Chungkookjang fermented with Bacillus licheniformis in HFD (BLFC). Sham-operated gerbils were had a HFD as normal control. At the end of experiment islets were isolated and the protein levels of caspase-3 and cleaved caspase-3 were measured from their lysate by immunoblotting assay and the relative intensity of interested protein was calculated based on β-actin. Values are means ± SD (n = 4). a,b,cMeans on the bars with different superscripts were significantly different by Tukey’s test at P < 0.05
Discussion
Artery occlusion caused extensive cell death in the hippocampal CA1 region and induced severe neurological symptoms in gerbils in comparison to Sham gerbils. The cell death was associated with higher IL-1β and TNF-α levels. Fermentation of soybeans remarkably increased isoflavonoid aglycones in comparison to unfermented soybeans. CSB, TFC, and BLFC prevented the neuronal cell death and the symptoms such as dropped eyelid, bristling hair, reduced muscle tone and flexor reflex, and abnormal posture and walking patterns. All animals fed soy foods lowered levels of pro-inflammatory cytokines, but CSB was less effective than TFC and BLFC. Artery occlusion induced glucose intolerance primarily due to decreased insulin secretion and β-cell mass. TFC and BLFC prevented the impairment of glucose metabolism by artery occlusion. Especially TFC and BLFC increased β-cell proliferation and suppressed the β-cell apoptosis, probably due to lower IL-1β and TNF-α resulting in decreased cleaved caspase-3 which increases apoptosis. Therefore, TFC and BLFC may prevent the neuronal cell death in the hippocampal CA1 region and neurological symptoms and poststroke hyperglycemia in gerbils with artery occlusion. The improvement of cerebral ischemic symptoms might be related to compositional changes of fermented soybeans, TFC and BLFC.
Chungkookjang is traditionally made by natural fermentation with rice straw as the source of ambient bacteria in the environment, and during the traditional fermentation the predominant bacteria is Bacillus subtilis. Previous study has shown that the improvement of efficacy of TFC and BLFC for antidiabetes is associated with compositional changes of soybeans by fermentation. TFC and BLFC contain more isoflavonoid aglycones, soyasaponins, lysoposphatidylcholines, dipeptides, and polyglutamate than CSB.18 Previous studies have shown that TFC improves insulin sensitivity and potentiates insulinotropic action better than CSB.17,30 However, it is difficult to standardize the quality of TFC since the fermentation bacteria cannot be modulated, but rather depends on the bacteria present in the local environment. The antidiabetic properties of Chungkookjang have been studied using several Bacillus species as fermentation starters. BLFC retains the antidiabetic properties of the most efficacious traditional Chungkookjang, and it is even more effective for potentiating glucose-stimulated insulin secretion and preventing decreases in β-cell mass than TFC.18 Therefore, Chungkookjang has the potential to prevent poststroke hyperglycemia that is related to attenuated β-cell function and mass, as shown in previous studies.13,28 The present study demonstrated that TFC and BLFC prevented the decrease in β-cell mass by decreasing β-cell apoptosis and increasing β-cell proliferation in I/R gerbils fed a high-fat diet better than CSB. Thus, the enhancement of prevention was related to changes of compositional changes of soybeans during fermentation.
The Mongolian gerbil is a useful animal model for studying artificially induced transient cerebral ischemia.31 The identified mechanism of cerebral ischemia is oxidative stress and inflammation in transient I/R gerbils.9,10 Artery occlusion in gerbils induces global cell death, and it can be easily detected in the cell death in the hippocampal CA1 region by cresyl violet staining. It has been reported that artery occlusion increases ROS production due to hypoxia.9 Increased ROS induces neuronal cell death in the brain by increasing oxidation of unsaturated fatty acids in the membrane and DNA, as indicated by increased lipid peroxidation by-products.9 Chungkookjang has exhibited antioxidant properties in previous animal studies. Kim et al.32 reported that oral administration of CKJ extract (800 mg/kg/day) for two weeks potently inhibited the formation of MDA, the damage of DNA, and the formation of micronucleated reticulocytes in KBrO3-treated mice. In the present study, the contents of MDA, an index of lipid peroxidation, were higher in the hippocampus of the control group than that of the Sham group. TFC and BLFC prevented the increase in I/R gerbils, although CSB was not as effective as BLFC. Thus, TFC and BLFC may prevent neuronal cell death by suppressing ROS production in I/R gerbils.
In addition to increased oxidative stress, hypoxia is closely associated with inflammation and the suppression of inflammation is the molecular target of cerebral ischemia.33 Pro-inflammatory cytokines, particularly IL-1β and TNF-α are upregulated in the blood, cerebrospinal fluids, and brain tissues during both focal and global cerebral ischemic events.34,35 IL-1β-deficient mice exhibit much less cerebral infarct than wild-type mice and IL-1β administration results in greater ischemic damage in rats.35 Inhibition of TNF-α also appears to reduce ischemic brain damage and administration of TNF-α after stroke worsens brain damage.36 Secretion patterns of IL-1β and TNF-α are similar during and after cerebral ischemia; both are markedly increased over 72 h after ischemia and then slowly decrease.33,34 Consistent with these studies, the current results demonstrate that serum levels of IL-1β and TNF-α were much higher after two days of transient artery occlusion in gerbils. Chungkookjang is reported to attenuate inflammation by inhibiting NF-κB-dependent iNOS, TNF-α, and IL-6 production induced by toll-like receptor ligands.36 The current study demonstrated that TFC and BLFC decreased the levels, indicating that soybeans fermented in the traditional manner and that BLFC fermented soybeans might be useful for preventing neuroinflammation during cerebral ischemia.
Although poststroke hyperglycemia has not been proven to be a causal factor for poor outcome after stroke, several mechanisms have been identified through which hyperglycemia could aggravate cerebral damage in ischemic stroke.37,38 During acute brain ischemia, hyperglycemia may increase blood coagulation by stimulating thrombin production and it may decrease fibrolytic activity of tissue plasminogen activator by increasing the production of plasminogen activator inhibitor-1.39 Furthermore, elevated insulin resistance may lead to prothrombotic and pro-inflammatory states that impair fibrinolysis and increases platelet activation as well as exacerbating the disturbance of glucose metabolism.40,41 The deleterious effect of insulin resistance may contribute to creating a vicious cycle of incident and/or recurrent strokes. Insulin resistance can lead to hyperglycemia and hyperinsulinemia that are associated with increased risk of stroke.39 In the present study, a high diet was provided to increase insulin resistance to exacerbate the outcome of the transient artery occlusion in gerbils. Thus, the prevention of insulin resistance may ameliorate the outcomes of artery occlusion including global neuronal cell death and neurological symptoms as well as glucose metabolism.
Some fermented soybean foods such as natto, douchi, tempeh, and Chungkookjang may contain fibrinolytic enzymes such as nattokinase which act on thrombotic and fibrinolytic factors.42–45 Most studies have been in vitro studies.42,43 Therefore, the fibrinolytic activity may not be available to animals fed the fermented soybeans since the fibrinolytic enzymes are about 20–30 kDA proteins that cannot be absorbed intact. However, Yamashita et al.44 demonstrated that 14-week consumption of Bacillus natto productive protein decreases thrombus volume possibly by enhancing endogenous thrombolysis in a dose-dependent manner in a He–Ne laser-induced thrombosis animal model. In addition, Hsia et al.45 demonstrated that oral administration of two capsules of nattokinase (2000 fibrinolysis units per capsule) daily for two months decreases fibrinogen, factor VII, and factor VIII by about 7–19% in healthy humans and patients with cardiovascular risk factors. Chungkookjang has also shown thrombolysis in vitro studies46,47 and it may have thrombolysis in vivo activity in animals. In the present study, Chungkookjang may have contributed to the attenuated neuronal cell death by preventing thrombosis, although thrombosis may not be a major casual factor for cerebral ischemia in the transient artery occlusion animal model.
In conclusion, TFC and BLFC improved the glucose metabolism by potentiating β-cell survival much better than neuronal cell death and neurological symptoms. This suggested that TFC and BLFC directly enhanced the glucose metabolism to overcome the changes by the cerebral ischemia. Therefore, the consumption of traditionally fermented Chungkookjang and especially standardized Chungkookjang fermented with BLFC may attenuate the adverse outcomes associated with cerebral I/R better than non-fermented CSBs.
Authors’ contribution
Conceived and designed the experiments: SP. Performed the experiments: DSK, SK, BRM. Analyzed the data: DSK, SK, SP. Wrote the paper: SP. All authors participated in review of the manuscript.
Acknowledgements
We acknowledge funding from the Korean Research Foundation in Korea (NRF-2015R1D1A3A01019577).
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Dreyer R, Murugiah K, Nuti SV, Dharmarajan K, Chen SI, Chen R, Wayda B, Ranasinghe I. Most important outcomes research papers on stroke and transient ischemic attack. Circ Cardiovasc Qual Outcomes 2014; 7: 191–204. [DOI] [PubMed] [Google Scholar]
- 2.Allport L, Baird T, Butcher K, Macgregor L, Prosser J, Colman P, Davis S. Frequency and temporal profile of poststroke hyperglycemia using continuous glucose monitoring. Diabetes Care 2006; 29: 1839–44. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheimer SM, Hoffbrand BI, Oswald GA, Yudkin JS. Diabetes mellitus and early mortality from stroke. BMJ (Clin Res Ed) 1985; 291: 1014–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van KF, Hoogerbrugge N, Naarding P, Koudstaal PJ. Hyperglycemia in the acute phase of stroke is not caused by stress. Stroke 1993; 24: 1129–32. [DOI] [PubMed] [Google Scholar]
- 5.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol 2002; 52: 20–8. [DOI] [PubMed] [Google Scholar]
- 6.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426–32. [DOI] [PubMed] [Google Scholar]
- 7.Allen CL, Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke 2008; 3: 105–16. [DOI] [PubMed] [Google Scholar]
- 8.Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin 2008; 26: 871–95. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 201; 47: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doll DN, Barr TL, Simpkins JW. Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis 2014; 5: 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dali-Youcef N, Mecili M, Ricci R, Andrès E. Metabolic inflammation: connecting obesity and insulin resistance. Ann Med 2013; 45: 242–53. [DOI] [PubMed] [Google Scholar]
- 12.Olerud J, Kanaykina N, Vasylovska S, King D, Sandberg M, Jansson L, Kozlova EN. Neural crest stem cells increase beta cell proliferation and improve islet function in co-transplanted murine pancreatic islets. Diabetologia 2009; 52: 2594–601. [DOI] [PubMed] [Google Scholar]
- 13.Park S, Kim DS, Kang S, Kwon DY. Ischemic hippocampal cell death induces glucose dysregulation by attenuating glucose-stimulated insulin secretion which is exacerbated by a high fat diet. Life Sci 2011; 88: 766–73. [DOI] [PubMed] [Google Scholar]
- 14.Nikander E, Tiitinen A, Laitinen K, Tikkanen M, Ylikorkala O. Effects of isolated isoflavonoids on lipids, lipoproteins, insulin sensitivity, and ghrelin in postmenopausal women. J Clin Endocrinol Metab 2004; 89: 3567–72. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YB, Li LN, Zhao XY, Chen WH, Guo JJ, Fu ZH, Yang Y, Na XL. Effect of soy isoflavone crude extract supplementation on high fat diet-induced insulin resistance in ovariectomized rats. Biomed Environ Sci 2014; 27: 49–51. [DOI] [PubMed] [Google Scholar]
- 16.Kwon DY, Daily JW, 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res 2010; 30: 1–13. [DOI] [PubMed] [Google Scholar]
- 17.Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH, Park S. The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-gamma activity in vitro. Biofactors 2006; 26: 245–58. [DOI] [PubMed] [Google Scholar]
- 18.Yang HJ, Kim HJ, Kim MJ, Kang S, Kim DS, Daily JW, Jeong do Y, Kwon DY, Park S. Standardized chungkookjang, short-term fermented soybeans with Bacillus licheniformis, improves glucose homeostasis as much as traditionally made chungkookjang in diabetic rats. J Clin Biochem Nutr 2013; 52: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr 1993; 123: 1939–51. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Prakash R, Chawla D, Du W, Didion SP, Filosa JA, Zhang Q, Brann DW, Lima VV, Tostes RC, Ergul A. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol 2013; 304: R1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhungana H, Rolova T, Savchenko E, Wojciechowski S, Savolainen K, Ruotsalainen AK, Sullivan PM, Koistinaho J, Malm T. Western-type diet modulates inflammatory responses and impairs functional outcome following permanent middle cerebral artery occlusion in aged mice expressing the human apolipoprotein E4 allele. J Neuroinflammation 2013; 10: 102–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudetz JA, Pagel PS. Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth 2010; 24: 131–42. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab 2007; 27: 1879–94. [DOI] [PubMed] [Google Scholar]
- 24.Lawner P, Laurent J, Simeone F, Fink E, Rubin E. Attenuation of ischemic brain edema by pentobarbital after carotid ligation in the gerbil. Stroke 1979; 10: 644–7. [DOI] [PubMed] [Google Scholar]
- 25.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–8. [DOI] [PubMed] [Google Scholar]
- 26.Park S, Dong X, Fisher TL, Dunn S, Omer AK, Weir G, White MF. Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem 2006; 281: 1159–68. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Kim DS, Kang S. Exercise training attenuates cerebral ischemic hyperglycemia by improving hepatic insulin signaling and β-cell survival. Life Sci 2013; 93: 153–60. [DOI] [PubMed] [Google Scholar]
- 29.Center for Drug Evaluation and Research. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers, Washington, DC: US Department of Health and Human Services Food and Drug Administration, 2005. [Google Scholar]
- 30.Kwon DY, Jang JS, Hong SM, Lee JE, Sung SR, Park HR, Park S. Long-term consumption of fermented soybean-derived Chungkookjang enhances insulinotropic action unlike soybeans in 90% pancreatectomized diabetic rats. Eur J Nutr 2007; 46: 44–52. [DOI] [PubMed] [Google Scholar]
- 31.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 1982; 23: 57–69. [DOI] [PubMed] [Google Scholar]
- 32.Kim NY, Song EJ, Kwon DY, Kim HP, Heo MY. Antioxidant and antigenotoxic activities of Korean fermented soybean. Food Chem Toxicol 2008; 46: 1184–9. [DOI] [PubMed] [Google Scholar]
- 33.Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 1999; 19: 87–98. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 1994; 25: 1481–8. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 1995; 26: 676–80. [DOI] [PubMed] [Google Scholar]
- 36.Lee WH, Wu HM, Lee CG, Sung DI, Song HJ, Matsui T, Kim HB, Kim SG. Specific oligopeptides in fermented soybean extract inhibit NF-κB-dependent iNOS and cytokine induction by Toll-like receptor ligands. J Med Food 2014; 17: 1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calleja AI, Garcia-Bermejo P, Cortijo E, Bustamante R, Rojo Martinez E, Gonzalez Sarmiento E, Fernández-Herranz R, Arenillas JF. Insulin resistance is associated with a poor response to intravenous thrombolysis in acute ischemic stroke. Diabetes Care 2011; 34: 2413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosentino F, Battista R, Scuteri A, De Sensi F, De Siati L, Di Russo C, Camici GG, Volpe M. Impact of fasting glycemia and regional cerebral perfusion in diabetic subjects: a study with technetium-99m-ethyl cysteinate dimer single photon emission computed tomography. Stroke 2009; 40: 306–8. [DOI] [PubMed] [Google Scholar]
- 39.Pandolfi A, Giaccari A, Cilli C, Alberta MM, Morviducci L, De Filippis EA, Buongiorno A, Pellegrini G, Capani F, Consoli A. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol 2001; 38: 71–6. [DOI] [PubMed] [Google Scholar]
- 40.Yoo DS, Chang J, Kim JT, Choi MJ, Choi J, Choi KH, Park MS, Cho KH. Various blood glucose parameters that indicate hyperglycemia after intravenous thrombolysis in acute ischemic stroke could predict worse outcome. PLoS One 2014; 9: e94364–e94364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res 2014; 5: 442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CT, Ji BP, Li B, Nout R, Li PL, Ji H, Chen LF. Purification and characterization of a fibrinolytic enzyme of Bacillus subtilis DC33, isolated from Chinese traditional Douchi. J Ind Microbiol Biotechnol 2006; 33: 750–8. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto S, Fujii T, Morimiya T, Johdo O, Nakamura T. The fibrinolytic activity of a novel protease derived from a tempeh producing fungus, Fusarium sp. BLB. Biosci Biotechnol Biochem 2007; 71: 2184–9. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita T, Oda E, Giddings JC, Yamamoto J. The effect of dietary bacillus natto productive protein on in vivo endogenous thrombolysis. Pathophysiol Haemost Thromb 2003; 33: 138–43. [DOI] [PubMed] [Google Scholar]
- 45.Hsia CH, Shen MC, Lin JS, Wen YK, Hwang KL, Cham TM, Yang NC. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr Res 2009; 29: 190–6. [DOI] [PubMed] [Google Scholar]
- 46.Chang JH, Shim YY, Kim SH, Chee KM, Cha SK. Fibrinolytic and immunostimulating activities of Bacillus spp. strains isolated from Chungkuk-jang. Kor J Food Sci Technol 2005; 37: 255–60. [Google Scholar]
- 47.Shin SH, Hong SW, Chung KS. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens HC188. Kor J Microbiol Biotechnol 2013; 41: 33–43. [Google Scholar]