Abstract
Renal fibrosis is a progressive pathological change characterized by tubular cell apoptosis, tubulointerstitial fibroblast proliferation, and excessive deposition of extracellular matrix (ECM). miR-21 has been implicated in transforming growth factor-β (TGF-β)-stimulated tissue fibrosis. Recent studies showed that sphingosine kinase/sphingosine-1-phosphate (SphK/S1P) are also critical for TGF-β-stimulated tissue fibrosis; however, it is not clear whether SphK/S1P interacts with miR-21 or not. In this study, we hypothesized that SphK/S1P signaling is linked to upregulation of miR-21 by TGF-β. To verify this hypothesis, we first determined that miR-21 was highly expressed in renal tubular epithelial cells (TECs) stimulated with TGF-β by using qRT-PCR and Northern blotting. Simultaneously, inhibition of miR-21, mediated by the corresponding antimir, markedly decreased the expression and deposition of type I collagen, fibronectin (Fn), cysteine-rich protein 61 (CCN1), α-smooth muscle actin, and fibroblast-specific protein1 in TGF-β-treated TECs. ELISA and qRT-PCR were used to measure the S1P and SphK1 levels in TECs. S1P production was induced by TGF-β through activation of SphK1. Furthermore, it was observed that TGF-β-stimulated upregulation of miR-21 was abolished by SphK1 siRNA and was restored by the addition of exogenous S1P. Blocking S1PR2 also inhibited upregulation of miR-21. Additionally, miR-21 overexpression attenuated the repression of TGF-β-stimulated ECM deposition and epithelial–mesenchymal transition by SphK1 and S1PR2 siRNA. In summary, our study demonstrates a link between SphK1/S1P and TGF-β-induced miR-21 in renal TECs and may represent a novel therapeutic target in renal fibrosis.
Keywords: miRNA-21, sphingosine kinase, sphingosine-1-phosphate, tubular epithelial cells, renal fibrosis
Introduction
Renal fibrosis is a progressive and complicated pathological change that is characterized by tubular cell apoptosis, tubulointerstitial fibroblast proliferation, leukocytic cell infiltration, and excessive production and deposition of extracellular matrix (ECM). As the common final result of chronic kidney disease, renal fibrosis can be caused by a range of diseases, including diabetes, atherosclerosis, and nephritis.1 Renal tubular epithelial cells (TECs) play a key role in the maintenance of kidney function. Following injury, TECs are able to undergo a phenotypic transformation, which is similar to epithelial–mesenchymal transition (EMT), to become matrix-producing fibroblasts in pathologic states and to produce more ECM, which contributes to deleterious renal fibrosis and dysfunction.2,3
Transforming growth factor-β (TGF-β), one of profibrotic growth factors produced by epithelial cells and macrophages, plays a crucial role in the pathogenesis of tissue fibrosis. Accumulating evidence indicates that TGF-β can regulate a set of signaling molecules leading to activation of various downstream signaling pathways and can also cause matrix deposition by promoting expression of ECM genes and can reduce matrix degradation by suppressing expression of matrix metalloproteinases.4,5 As a potent inducer of renal fibrosis, expression of TGF-β was identified as being increased in nephric tubules in both experimental and human kidney disease. Increased TGF-β levels in plasma enhance renal fibrosis of mice, and blocking TGF-β protects mice with diabetes from renal dysfunction, especially glomerular hypertrophy and fibrosis.6
Sphingosine-1-phosphate (S1P) is a bioactive lysophospholipid and can be produced by active sphingosine kinase (SphK). By acting as an intracellular second messenger or a ligand for the family of G-protein-coupled receptors, S1P has been proven to mediate numerous signaling pathways related to a wide range of biological responses, such as cell proliferation, differentiation, migration, and survival.7 Recent studies showed that SphK and S1P are critical for TGF-β-stimulated tissue fibrosis. TGF-β increases SphK1 and S1P synthesis in human lung fibroblasts, and inhibition of SphK1 reduces S1P production and TGF-β-mediated signal transduction.8,9 Additionally, TGF-β increases collagen production in cardiac fibroblasts by activating SphK and S1P signaling.10
MicroRNAs (miRNAs) are short (21–23 nucleotides), endogenous, non-coding, single-stranded RNAs that constitute a large class of important endogenous regulators of gene expression and cellular activity. Most miRNAs negatively regulate gene expression in the key processes of cell growth, differentiation, and apoptosis at the post-transcriptional level by blocking protein translation or by inducing targeted mRNA to be cleaved by binding to targets with different degrees of complementarity.11,12 Various studies have suggested that miR-21 is involved in TGF-β-stimulated renal fibrosis. Upregulation of miR-21 by TGF-β could promote ECM expansion and kidney fibrosis by silencing metabolic pathways, regulating MMP-9 and TIMP1 expression, and by activating PTEN/AKT/TORC1 signaling and other pathways.13–15 TGF-β may elevate the miR-21 level by initiating the transcription of genes that encode the essential regulatory elements for miRNA maturation. Smad3 is an upstream molecule of miR-21 that executes TGF-β-induced tissue fibrosis. To date, highlighted papers focus on Smad3; however, Smad3 is not the unique transcription factor of miR-21 and other signaling pathways are likely involved in the TGF-β-mediated regulation of miR-21.16
In this report, we hypothesized that the SphK/S1P pathway is directly or indirectly linked to upregulation of miR-21 by TGF-β, and we investigated the interaction between TGF-β/SphK1/S1P and miR-21. Furthermore, we also measured the combined effect of TGF-β, SphK1/S1P, and miR-21 on the pathological changes related to renal fibrosis of renal TECs.
Materials and methods
Cell culture
The human renal TEC line HK-2 was purchased from American Type Culture Collection and cultured in HK2 medium supplemented with 10% fetal bovine serum (Gibco, Rockville, MD), 100 µg/mL streptomycin, 100 IU/mL penicillin, 2 mg/L fibroblast growth factor (Invitrogen, Carlsbad, CA), and 0.5 mg/L epidermal growth factor (Sigma, St. Louis, MO) at 37℃ in a humidified 5% CO2 atmosphere. Cells were then serum starved for 16 h, followed by incubation with 10 ng/mL TGF-β (R&D Shanghai, china) for 24 h or were not treated. S1P was obtained from Avanti Polar Lipids (Alabaster, AL, USA).
Cell transfection
miR-21 antimir, miR-21 mimic, the control miRNA, and siRNA of SphK1 and specific S1P receptor type 2 (S1PR2) were synthesized by Takara (Dalian, China). TECs grown to approximately 80% confluence were transfected with the miR-21 mimic or antimir, as well as the control miRNA, using Xfect MicroRNA Transfection Reagent (Clontech, Mountain View, CA). Transfection complexes were prepared according to the manufacturer’s instructions. Transfection of SphK1 and S1PR2 siRNA was performed in the presence or absence of the miR-21 mimics using Lipofectamine 2000 (Invitrogen). Transfection complexes were prepared according to the manufacturer’s instructions. After 48 h, cells were treated with 10 ng/mL TGF-β for 24 h. Vehicle was applied as the control.
Cell viability assay
The 3 -(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method was used to measure cell viability transfected with RNA oligonucleotides as described in Musser and Oseroff.17 Cells were washed with PBS, and then incubated with MTT (0.5 mg/mL) in PBS containing glucose (5.5 mM) and CaCl2 (11 mM) for 1 h at 37℃. Absorbance was measured at 570 nm.
Northern blotting
Northern blotting analysis of miR-21 was performed as described in Wang et al.18 with minor modifications. Trizol-extracted total RNA (10 µg) from TECs was separated on a precast 15% denaturing polyacrylamide gel (BioRad, Hercules, CA), and then electrophoretically transferred to Hybond-N+ blotting membranes (Roche). Probes modified with 5′- and 3′-digoxigenin (DIG) labeled LNA were carried out in Ultrahyb buffer overnight at 37℃ to be prehybridized and hybridized. After washing and blocking, the membrane was incubated with anti-DIG HRP antibody (1:2000, Roche) for 1 h at room temperature. The bands were detected with enhanced chemiluminescence (ECL) Advance Kit, and U6 was used to normalize the expression levels.
Quantitative RT-PCR
Total RNA was isolated from cells using Trizol reagent (Sigma) and was enriched for the miR-21 fraction using the High Pure miRNA Isolation kit (Roche). The preparation of cDNA and qRT-PCR was performed using the MicroRNA First-Strand Synthesis and miRNA Quantitation kits (Takara, Dalian, China) according to the instructions. Expression of SphK1, S1PR2, type I collagen, fibronectin (Fn), cysteine-rich protein 61 (CCN1), α-smooth muscle actin (α-SMA), and E-cadherin was detected using the CellAmp Direct RNA Prep kit for qPCR and the Protein Analysis (Takara). Ct values of U6 were used to normalize the relative expression of miR-21, and GAPDH was used as the internal control to normalize the relative expression of target genes. Relative expression levels are presented using the 2−ΔΔCT method. All PCRs were performed in triplicate.
Western blot
Cell lysates were treated with 200 µL lysis buffer containing 25 mM MgCl2, 5 mM KCl, 20 mM HEPES, 0.5% (v/v) complete protease inhibitor, and Triton X-100. The debris was then removed by centrifugation at 12,000 × g at 4℃ for 10 min. Protein concentrations were determined by the bicinchoninic acid method using bovine serum albumin as the standard. Equal amounts of cell protein (typically 80 µg) were separated using 8% precast SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) gels (Invitrogen) and electrophoretically transferred to PVDF (polyvinylidene fluoride) membrane. Blots were blocked for 1 h in blocking buffer at room temperature. Antibodies (rabbit anti-SphK1, α-SMA, E-cadherin, 1:500 dilution, Abcam; mouse anti-CCN1, 1:500, R&D; mouse anti-type I collagen, FN, 1:500, Sigma; mouse anti-GAPDH, 1:3000 dilution, Acris) were incubated with blots overnight at 4℃. Secondary antibodies conjugated with horseradish peroxidase were reacted with the blots at 1:2000 for 1 h at room temperature followed by ECL assay (Amersham Pharmacia, NJ). The intensity of bands was measured using Image Quant software.
ELISA
The endogenous S1P level was measured using an S1P ELISA kit (Eastbiopharm, Hangzhou, China) and was performed according to the manufacturer’s recommendations. An ELISA kit (X-Y Biotechnology, Shanghai, China) was applied to determine the level of fibroblast-specific protein (FSP1) in cultured TECs.19
Statistical analysis
SPSS 11.0 statistical software was used to analyze the significance of differences between groups by Student’s t-test. Statistical comparisons of the results were performed using ANOVA. Data are expressed as mean ± SEM. Differences were considered statistically significant at P < 0.05. All experiments were performed independently at least three times.
Results
miR-21 is upregulated in TECs stimulated with TGF-β
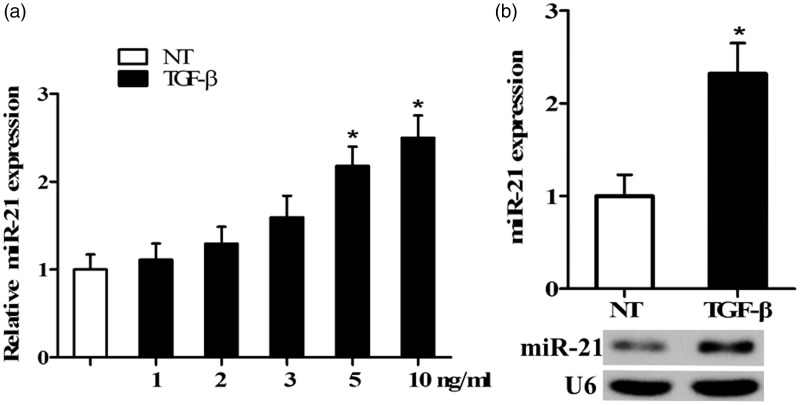
miR-21 expression was examined by qRT-PCR in TECs cultures treated with different concentrations of TGF-β for 24 h. miR-21 expression was significantly upregulated in TECs stimulated by TGF-β in a dose-dependent manner, peaking (2–3 folds) with an optimal dose of 10 ng/mL compared to untreated cells (NT; P < 0.05; Figure 1(a)). This result was also verified by Northern blotting as shown in Figure 1(b).
Figure 1.
Expression of miR-21 in TECs treated with TGF-β. (a) qRT-PCR was used to analyze the expression of miR-21 in cultures treated with 1, 2, 3, 5, and 10 ng/mL TGF-β for 24 h or not treated (NT). (b) The level of miR-21 in cells treated with 10 ng/mL TGF-β was measured by Northern blotting, as shown in representative northern blots. (n = 5/group, *P < 0.05 versus NT)
Inhibition of miR-21 decreases TGF-β-induced ECM protein expression and EMT in TECs
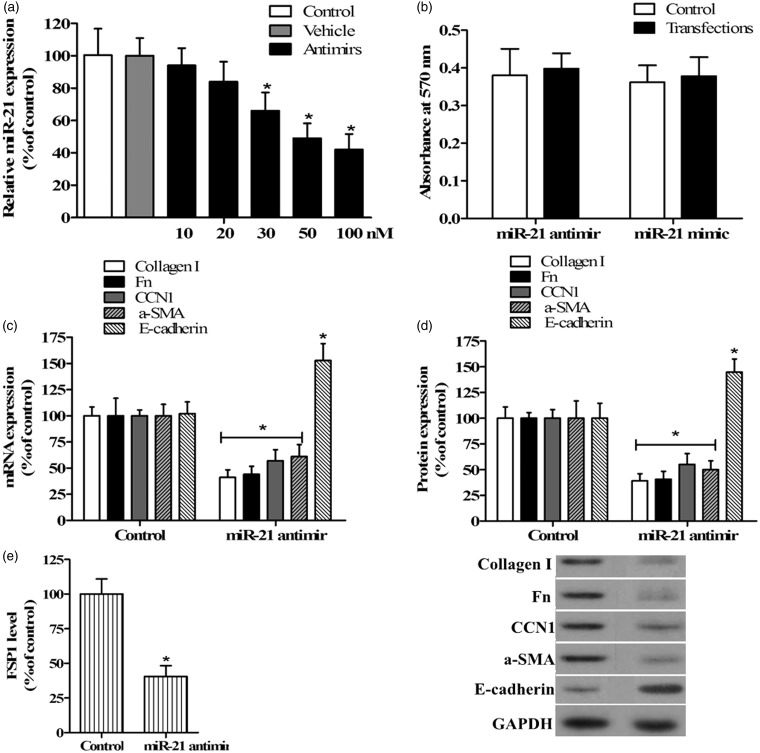
It is well known that TECs play an important role in renal fibrosis via EMT and by excessively synthesizing ECM proteins that result in structural damage and dysfunction of the kidney.3 We designed an antimir of miR-21 to evaluate the effects of miR-21 on TGF-β-induced EMT marker and ECM protein expression in TECs. Antimir and negative control miRNA were, respectively, transfected into TECs for 48 h before treatment with TGF-β. The levels of miR-21 were confirmed by qRT-PCR. The results indicated that the antimir was specific and that it markedly downregulated the level of miR-21 in a dose-dependent manner, with a significant decrease observed at 30 nM and the maximum effect at 100 nM, while the control miRNA had no effect on miR-21 levels (Figure 2(a)). The miR-21 antimir had no effect on cellular viability, implying that the exogenous oligonucleotide resulted in no cytotoxic effects on TECs (Figure 2(b)).
Figure 2.
Inhibition of miR-21 affected ECM protein and EMT marker molecule expression in TGF-β-stimulated TECs. (a) qRT-PCR was used to analyze the expression of miR-21 in TECs transfected with 10, 20, 30, 50, and 100 nM miR-21 antimir and 100 nM control miRNA (vehicle). (b) The MTT method was used to determine the viability of cells transfected with miR-21 antimir or mimic, and absorbance was measured at 570 nm. Untransfected cells were used as the control in a and b. qRT-PCR (c) and Western blotting (d) were used to analyze the expression of type I collagen, Fn, CCN1, α-SMA, and E-cadherin in TECs stimulated with TGF-β after transfection with miR-21 antimir or control miRNA, as shown in representative Western blots. FSP1 level was detected by ELISA (e). Cells transfected with control miRNA were used as the control in c, d, and e (n = 5/group, *P < 0.05 versus control)
qRT-PCR analysis was then used to evaluate the expression of ECM proteins, including type I collagen, Fn, and CCN1, and EMT markers α-SMA and E-cadherin in TECs transfected with 100 nM miR-21 antimir or control miRNA and treated with 10 ng/mL TGF-β, while the FSP1 level was detected by ELISA. Expression of ECM proteins type I collagen, Fn, and CCN1 was induced by TGF-β in TECs whereas it was dramatically decreased after inhibition of miR-21 (P < 0.05; Figure 2(c)). This result was verified by Western blotting analysis (Figure 2(d)). Furthermore, as seen in Figure 2(c) to (e), the levels of α-SMA and FSP1, a marker specific for EMT, were also significantly decreased by repression of miR-21 in TECs treated with TGF-β. However, the expression of E-cadherin, an epithelial cell marker, was markedly upregulated compared to the control when miR-21 expression was abolished by the addition of antimir. These findings indicate that miR-21 may contribute to myofibroblast activity, and the process of EMT may be involved.
SphK1 and S1P are involved in the upregulation of miR-21 induced by TGF-β in TECs
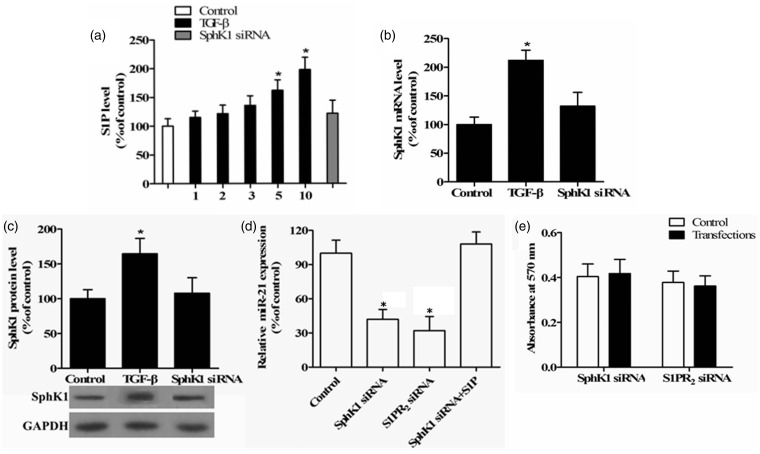
TGF-β, a profibrotic growth factor, has been proven to increase SphK expression and subsequent S1P production in the liver, and during pulmonary and cardiac fibrosis.8–10 To investigate whether SphK and S1P are regulated by TGF-β during renal fibrosis in TECs, we first evaluated the S1P level using an ELISA assay with TECs treated with 1, 2, 3, 5, and 10 ng/mL TGF-β. Figure 3(a) shows that TGF-β induced S1P production in a dose-dependent manner, with an approximate twofold increase at 10 ng/mL TGF-β. Similarly, SphK1 mRNA and protein expression were also markedly upregulated in TECs treated with TGF-β compared to untreated cells (P < 0.05; Figure 3(b) and (c)). In addition, the TGF-β-induced SphK1 and S1P levels were abolished by the addition of the SphK1 siRNA (Figure 3(a) to (c)), suggesting that TGF-β stimulates S1P production by increasing SphK1 expression in renal TECs.
Figure 3.
Effects of S1P and SphK1 on miR-21 expression. (a) An ELISA was used to investigate S1P levels in TECs treated with 1, 2, 3, 5, or 10 ng/mL TGF-β for 24 h or transfected with 100 nM SphK1 siRNA when 10 ng/mL TGF-β was added. TECs without treatment were used as the control. The expression of SphK1 was measured by qRT-PCR (b) and Western blotting (c) in TECs treated with 10 ng/mL TGF-β for 24 h and transfected with 100 nM SphK1 siRNA or not, as shown in a representative Western blot. (d) The levels of miR-21 were measured by qRT-PCR in TECs treated with 10 ng/mL TGF-β for 24 h after transfection with 100 nM SphK1 and S1PR2 siRNA, plus untreated control, or treated with 1 µM S1P. (e) The MTT method was used to determine the viability of cells transfected with SphK1 and S1PR2 siRNA, and absorbance was measured at 570 nm (n = 5/group, *P < 0.05 versus control)
Considering the ability of TGF-β to regulate SphK/S1P and miR-21 expression, we hypothesized that the SphK/S1P pathway is directly or indirectly linked to TGF-β-stimulated upregulation of miR-21, and we investigated the interaction between TGF-β/SphK/S1P and miR-21 in renal TECs. We measured the miR-21 expression by qRT-PCR. The TGF-β-induced miR-21 expression was significantly reduced by SphK1 siRNA in TECs compared to the control (P < 0.05; Figure 3(d)). A similar change in miR-21 expression was observed in TECs transfected with S1PR2 siRNA (P < 0.05). However, this inhibition of miR-21 induced by SphK1 siRNA was attenuated by exogenous S1P. These findings suggest that SphK1 and S1P are involved in the upregulation of miR-21 induced by TGF-β in TECs. Additionally, no reduction of TEC viability was observed in response to these siRNAs (Figure 3(e)).
TGF-β induces ECM protein expression and EMT in TECs via the SphK1/S1P and miR-21 pathway
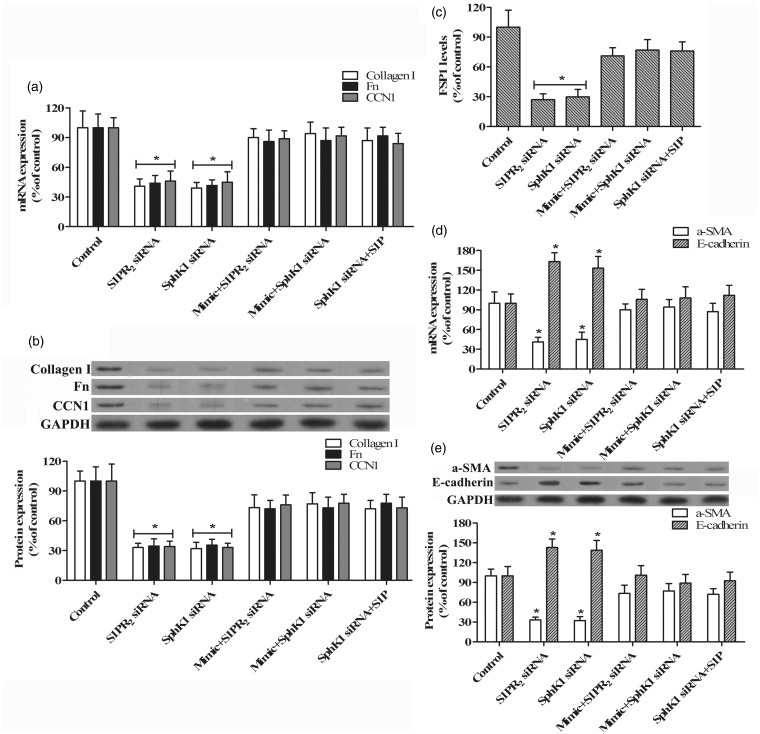
Excessive production and deposition of ECM protein is the primary cause of tissue fibrosis. To further investigate the role of SphK1/S1P and miR-21 in the response of TECs to TGF-β stimulation and renal fibrosis, we evaluated the expression of type I collagen, Fn, and CCN1, which are major proteins of the ECM, in TECs treated with TGF-β after transfection with SphK1 and S1PR2 siRNA in the presence or absence of the miR-21 antimir and mimic. qRT-PCR analysis indicated that inhibition of either SphK1 or S1PR2 with siRNA significantly decreased TGF-β-stimulated type I collagen, Fn, and CCN1 expression in TECs compared with the control (P < 0.05; Figure 4(a)). However, these expression changes induced by SphK1 and S1PR2 siRNA were attenuated by the overexpression of miR-21 induced by transfection with the miR-21 mimic. A similar pattern induced by SphK1 siRNA was also observed when exogenous S1P was added. These results were verified by Western blotting analysis (Figure 4(b)).
Figure 4.
Effects of SphK1/S1P and miR-21 on ECM proteins and EMT marker molecules expression. qRT-PCR (a) and Western blotting (b) were used to determine the expression of type I collagen, Fn, and CCN1 in TECs treated with 10 ng/mL TGF-β for 24 h after transfection with 100 nM SphK1 siRNA, S1PR2 siRNA, miR-21 mimic plus SphK1 siRNA, miR-21 mimic plus S1PR2 siRNA and vehicle (control), or addition with 1 µM S1P, as shown in representative Western blots. (c) ELISA was used to detect the FSP1 levels. The expression of α-SMA and E-cadherin was measured by qRT-PCR (d) and Western blotting (e) in TECs treated with the same conditions as in a and b. (n = 5/group, *P < 0.05 versus control)
Next, we determined the levels of α-SMA, E-cadherin, and FSP1, which are markers of EMT, in TECs treated as above. Figure 4(c) to (e) demonstrates that the levels of α-SMA and FSP1 induced by TGF-β were significantly decreased by inhibition of SphK1 or S1PR2 with siRNA in TECs compared to the control (P < 0.05), whereas this reduction induced by SphK1 and S1PR2 siRNA was attenuated by overexpression of miR-21. Consistently, the addition of exogenous S1P attenuated the decreasing of α-SMA and FSP1 expression in response to SphK1 siRNA. In contrast, there was a significant increase in E-cadherin expression when SphK1 or S1PR2 was abolished by siRNA in TGF-β-stimulated TECs. Attenuation of increased E-cadherin expression induced by siRNA was observed when the miR-21 mimic or exogenous S1P was administered. These findings indicate that TGF-β may upregulate miR-21 by activating the SphK1/S1P pathway, and subsequently stimulating the excessive expression of ECM proteins and EMT in renal TECs.
Discussion
As regulators of gene expression, miRNAs play central roles in the diverse physiological processes of development and disease. miR-21 is a prominent miRNA that may be involved in the genesis and progression of tissue fibrosis. The role of miR-21 in fibrosis was first reported in myocardial disease.20 Thum et al. showed that miR-21 stimulates MAP kinase signaling in fibroblasts, thus contributing to myocardial disease. The upregulation of miR-21 is also observed in patients with idiopathic pulmonary fibrosis and in human fibrotic livers.21,22 In the kidney, various studies have suggested that miR-21 is crucial in TGF-β-stimulated renal fibrosis. The levels of miR-21 could be increased by TGF-β and could subsequently stimulate the extra deposition of ECM proteins, thus resulting in the fibrotic dysfunction of kidney by different downstream signaling pathways.23,24 Consistent with these findings, the data presented in this report also demonstrate that miR-21 is significantly upregulated in TECs stimulated with TGF-β in vitro. We then employed an antimir of miR-21 to further investigate the effect of miR-21 on the response of TGF-β-treated TECs. As seen in Figure 2(b) and (c), repression of miR-21 markedly decreased the expression of ECM proteins type I collagen, Fn, and CCN1, as well as EMT markers α-SMA and FSP1, in TECs treated with TGF-β.
An interesting observation is that CCN1 is induced in TGF-β-stimulated TECs (data not shown) and could be downregulated by inhibition of miR-21. The matricellular protein CCN1 is a connective-tissue growth factor that can inhibit type I procollagen synthesis and can induce collagen degradation via upregulation of collagenase matrix metalloproteinase-1 (MMP-1).25 CCN1 is highly accumulated in livers of human patients with cirrhosis and may limit fibrogenesis and promote regression of liver fibrosis via the accumulation of reactive oxygen species.26 The upregulation of CNN1 induced by TGF-β in TECs suggests that CNN1 may contribute to the regression of renal fibrosis in the early stage of kidney injury.
In terms of the interaction between TGF-β and miR-21, studies have focused on Smad3, an upstream molecule of miR-21 that executes TGF-β-induced tissue fibrosis. Zhong and colleagues showed that Smad3 interacts with the promoter region of the miR-21 gene in the presence of TGF-β and mediates the upregulation of miR-21.16,27 However, Smad3 is not the unique transcription factor of miR-21, and other signaling pathways are likely to be involved in the regulation of TGF-β to miR-21.
As one of the bioactive lysophospholipids, S1P is produced by active SphK and mediates various downstream signaling pathways related to a wide range of biological processes. Recent studies suggested that TGF-β increases SphK1 and S1P production in many tissues, and subsequently stimulates tissue fibrosis in tissues including the human lung, liver, and heart.8–10
In light of these findings, and the TGF-β-stimulated SphK1/S1P production in renal TECs seen in Figure 3, we hypothesized that the SphK1/S1P pathway is linked to upregulation of miR-21 by TGF-β, and we sought to investigate the interaction between TGF-β/SphK1/S1P and miR-21 in renal TECs. As shown in Figure 3, when the TGF-β-stimulated increase in SphK1 expression was inhibited with siRNA, TGF-β-stimulated upregulation of miR-21 was abolished. However, this inhibition of miR-21 expression was attenuated by exogenous S1P, suggesting that S1P production is necessary for TGF-β-stimulated upregulation of miR-21 in TECs. To further clarify this idea, we employed siRNAs to block the specific S1P receptor type 2 (S1PR2), which is linked to S1P-stimulated collagen expression. The blocking of S1PR2 inhibited TGF-β-stimulated upregulation of miR-21. In contrast, neither repression nor overexpression of miR-21 had an effect on TGF-β-stimulated SphK1 and S1PR2 expression (data not shown). This implicates the role of SphK1/S1P pathway in the upregulation of miR-21 induced by TGF-β in TECs.
To further elucidate this finding, we determined the effects of SphK1/S1P and miR-21 on the expression of ECM proteins and EMT markers in the TEC response to TGF-β stimulation. As seen in Figure 4, when the induction of SphK1 expression stimulated by TGF-β was inhibited with siRNA, the TGF-β-stimulated increase in ECM protein expression was abrogated. The addition of S1PR2 siRNA abrogated the TGF-β-stimulated ECM protein and EMT marker expression in TECs, whereas the expression changes induced by SphK1 siRNA were attenuated by miR-21 overexpression and exogenous S1P. These findings suggest that TGF-β may stimulate excessive expression of ECM proteins and EMT via the upregulation of miR-21 by activating the SphK1/S1P signaling pathway in renal TECs.
Our study is not without its limitation. Although it was initially hypothesized that the SphK/S1P pathway is directly or indirectly linked to TGF-β-stimulated miR-21, we did not determine the mechanism of interaction between SphK/S1P and miR-21 in TECs. No binding site of SphK/S1P/S1PR was predicted in the promoter region of miR-21 using a bioinformatics approach. In some reports, however, S1P was proven to activate Smad signaling and to promote TGF-β-induced cell responses in mesangial cells.28,29 This implies that Smad signaling might be involved in the upregulation of miR-21 induced by the SphK/S1P pathway. Additionally, previous reports indicate that miR-21 stimulates the fibrotic processes in different organs to generate the fibrosis by activating different downstream signaling pathways. miR-21 has been proven to mediate mesangial cell hypertrophy and high glucose-induced renal cell pathology by regulating PTEN and AKT/mTORC1 signaling.13 The miR-21/programmed cell death protein 4/activation protein-1 (miR-21/PDCD4/AP-1) autoregulatory loop is one of the main driving forces for hepatic fibrosis and myocardial fibrosis.21 Besides, MMP9/TIMP1, silencing metabolic pathways, and Smad 7 signaling might be also implicated in the miR-21-mediated fibrosis.14,15 However, the specific mechanism and regulatory network that involved in the modulation of miR-21 to fibrotic dysfunction of kidney is not well defined and is the next focus point in our subsequent study.
In summary, this report provides new evidence that miR-21 is an important mediator of TGF-β-stimulated renal fibrosis through its effects on the TEC response and deposition of ECM proteins by regulating downstream signaling pathways. These data suggest that SphK1 and S1P play a role in the stimulation of fibrosis in kidneys by TGF-β and support the hypothesis that TGF-β stimulates upregulation of miR-21 via increased SphK1/S1P production and activation, which then promotes the excessive expression of ECM proteins and the pathological changes to fibrosis of renal TECs. This is the first report demonstrating a link between SphK1/S1P and TGF-β-induced miR-21 in renal TECs and may represent a novel therapeutic target in renal fibrosis.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (No. 81260114), Natural Science Fund Projects of Jiangxi Province (No. 20142BAB205007) and Military Medical Scientific Research Projects (NO. 14ZD28).
Authors’ contributions
All of the authors participated in the design, interpretation of the studies, and review of the manuscript; XL has overall responsibility for this manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Cho MH. Renal fibrosis. Kor J Pediatr 2010; 53: 735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell MT, Hile KL, Zhang H, Asanuma H, Vanderbrink BA, Rink RR, Meldrum KK. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res 2011; 168: e61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rastaldi MP. Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis. J Nephrol 2006; 19: 407–12. [PubMed] [Google Scholar]
- 4.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004; 18: 816–27. [DOI] [PubMed] [Google Scholar]
- 5.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-β in hepatic fibrosis. Front Biosci 2002; 7: d793–807. [DOI] [PubMed] [Google Scholar]
- 6.Dey N, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. TGFbeta-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS One 2012; 7: e42316–e42316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Yue S, Yang L, Liu X, Han Z, Zhang Y, Li L. Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol 2013; 59: 114–23. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, Li L, Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol 2009; 50: 1174–83. [DOI] [PubMed] [Google Scholar]
- 9.Huang LS, Natarajan V. Sphingolipids in pulmonary fibrosis. Adv Biol Regulat 2014;57:55–63. [DOI] [PMC free article] [PubMed]
- 10.Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts. Cardiovasc Res 2009; 82: 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 2007; 100: 1579–88. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey N, Das F, Mariappan MM, Mandal CC, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. J Biol Chem 2011; 286: 25586–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 2012; 4: 121ra18–121ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem Biophys 2013; 67: 537–46. [DOI] [PubMed] [Google Scholar]
- 16.Eddy AA. The TGF-β route to renal fibrosis is not linear: the miR-21 viaduct. J Am Soc Nephrol 2011; 22: 1573–5. [DOI] [PubMed] [Google Scholar]
- 17.Musser DA, Oseroff AR. The use of tetrazolium salts to determine sites of damage to the mitochondrial electron transport chain in intact cells following in vitro photodynamic therapy with photofrin II. Photochem Photobiol 1994; 59: 621–26. [DOI] [PubMed] [Google Scholar]
- 18.Wang YS, Li SH, Guo J, Mihic A, Wu J, Sun L, Davis K, Weisel RD, Li RK. Role of miR-145 in cardiac myofibroblast differentiation. J Mol Cell Cardiol 2014; 66: 94–105. [DOI] [PubMed] [Google Scholar]
- 19.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol-Renal Physiol 1997; 273: 563–74. [DOI] [PubMed] [Google Scholar]
- 20.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008; 456: 980–4. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Zha Y, Hu W, Huang Z, Gao Z, Zang Y, Chen J, Dong L, Zhang J. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem 2013; 288: 37082–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 2010; 207: 1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glowacki F, Savary G, Gnemmi V, Buob D, der Hauwaert CV, Lo-Guidice JM, Bouye S, Hazzan M, Pottier N, Perrais M, Aubert S, Cauffiez C. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One 2013;8:e58014. DOI: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed]
- 24.Zarjou A, Yang SZ, Abraham E, Agarwal A, Liu G. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol-Renal Physiol 2011;301:F793–801. DOI: 10.1152/ajprenal.00273.2011. [DOI] [PMC free article] [PubMed]
- 25.Quan T, Qin Z, Shao Y, Xu Y, Voorhees JJ, Fisher GJ. Retinoids suppress cysteine-rich protein 61 (CCN1), a negative regulator of collagen homeostasis, in skin equivalent cultures and aged human skin in vivo. Exp Dermatol 2011; 20: 572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol 2013; 33: 2078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 2011; 22: 1668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, Schafer-Korting M, Pfeilschifter J, Huwiler A. Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem 2004; 279: 35255–62. [DOI] [PubMed] [Google Scholar]
- 29.Sauer B, Vogler R, von Wenckstern H, Fujii M, Anzano MB, Glick AB, Schafer-Korting M, Roberts AB, Kleuser B. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J Biol Chem 2004; 279: 38471–9. [DOI] [PubMed] [Google Scholar]