Abstract
Patients with social anxiety disorder (SAD) experience anxiety and avoidance in face-to-face interactions. We performed a meta-analysis of functional magnetic resonance imaging (fMRI) studies in SAD to provide a comprehensive understanding of the neural underpinnings of face perception in this disorder. To this purpose, we adopted an innovative approach, asking authors for unpublished data. This is a common procedure for behavioral meta-analyses, which, however has never been used in neuroimaging studies. We searched Pubmed with the key words “Social Anxiety AND faces” and “Social Phobia AND faces.” Then, we selected those fMRI studies for which we were able to obtain data for the comparison between SAD and healthy controls (HC) in a face perception task, either from the published papers or from the authors themselves. In this way, we obtained 23 studies (totaling 449 SAD and 424 HC individuals). We identified significant clusters in which faces evoked a higher response in SAD in bilateral amygdala, globus pallidus, superior temporal sulcus, visual cortex, and prefrontal cortex. We also found a higher activity for HC in the lingual gyrus and in the posterior cingulate. Our findings show that altered neural response to face in SAD is not limited to emotional structures but involves a complex network. These results may have implications for the understanding of SAD pathophysiology, as they suggest that a dysfunctional face perception process may bias patient person-to-person interactions.
Keywords: Face perception, social phobia, functional magnetic resonance imaging, meta-analysis, amygdala, anxiety
Introduction
Social anxiety disorder (SAD)—also known as social phobia—is a relatively common anxiety disorder in which patients experience high levels of anxiety during social situations, which are consequently avoided.1 Typically, patients experience an excessive wariness of others’ judgment and are afraid to appear socially inadequate or awkward. Among the most frequent clinical features observed in patients with SAD are increased anxiety and avoidance behaviors in social face-to-face interactions. SAD patients are mostly afraid of finding negative expressions on the faces of their interlocutors. Moreover, behavioral results highlight an increased bias toward negative expressions in SAD patients,2–4 as well as biased memory encoding and recall of negative expressions.5
Face perception is a highly complex and sophisticated process underpinned by a distributed neural system that comprises several brain areas, including the fusiform gyrus, superior temporal sulcus (STS), insula, amygdala, and temporal poles.6,7 Behavioral and neurobiological studies consistently suggest that such a system may be affected in SAD.8–10
While a hyperreactivity in the amygdala has been a consistent finding across most studies on face perception in SAD (e.g. in the literature8,11–14), results diverged regarding the potential role of other structures that are involved in face perception (e.g. in the literature8,12,15). As a matter of fact, a possible explanation for these partially discrepant results can be ascribed to the fact that most brain imaging studies, with only a few exceptions (e.g. in the literature8,12), used faces as a major and powerful tool to elicit an emotional response in SAD patients, in the same way one would use spiders to trigger a response in arachnophobias. In this sense, the goal was not to understand brain correlates of face (or spider) processing but simply of their emotional effects. Thus, experimental paradigms in SAD were designed to detect the dysfunctional response within the emotional brain rather than to map neural processing in the face perception network.
This very same “emotional brain dysfunction” line of research was the focus of a recent meta-analysis by Hattingh et al. on differential brain activity in SAD patients as compared to healthy controls (HCs) in response to emotionally relevant stimuli (either faces or statements).16 In their analysis, the authors did not just look at faces, but at all socially relevant stimuli that may trigger an altered response in SAD. The strength of this analysis resides in the fact that a consistent pattern of brain response emerged irrespectively of the kind of paradigms utilized, either faces or statements. The meta-analysis highlighted activations, in particular, in the bilateral amygdala, parahippocampus, and ventral anterior cingulate cortex, strengthening the idea of the pivotal role of the amygdala in fear conditioning and, more generally, of the role of the limbic system in anxiety disorders.
Another recent meta-analytic review of neurobiological studies in SAD tried to cover all the studies on SAD including functional connectivity, activation, response to treatment, and structural ones.17 The purpose was to provide further evidence for the neurobiological model of phobias and anxiety disorders developed by Etkin and Wager.18 The results confirmed the hyperrecruitment of fear circuits, as well as the involvement of medial parietal and occipital regions and the disconnection among parietal, limbic, and executive network.17
Aim of the present study
Although these meta-analyses investigated the topic of emotional reactivity in SAD, they did not provide any information about the functional neuroanatomy of face perception processes in this disorder, in spite of the potential role that this phenomenon likely plays in the psychopathology of SAD. To fill this gap, we aimed to find a pattern of neural alterations that may be specifically related to disrupted face processing in SAD. We investigated the hypothesis that an abnormal neural response in SAD patients may not be limited to the amygdala but rather may affect the extended cortical system for face perception as well. Specifically, alterations within social cognition and theory of mind areas, as well as in areas related to empathy, may represent the neurobiological correlates of the abnormal features typically observed in clinical and experimental studies in SAD patients. To this purpose, we conducted a meta-analysis taking into account all the functional magnetic resonance imaging (fMRI) studies on face perception in which a direct comparison between SAD patients and HCs had been carried out, regardless of the emotional characteristics of faces used in the experimental tasks and the contrasts chosen for the analysis and also including unpublished data, a strategy commonly used in behavioral meta-analysis.
Recently, another meta-analysis, published by Binelli et al. in 2014, has assessed this topic comparing face perception in SAD patients versus controls and in patients with William’s syndrome versus controls.19 Although the methodology for the inclusion criteria of this meta-analysis is similar to the present work, there are also fundamental differences. In particular, to our knowledge, ours is the first study in which authors of individual papers were asked to provide unpublished data for the meta-analysis. Specifically, we asked authors of studies that used faces as stimuli, but in which a direct comparison between SAD versus HCs had not been reported in the original paper, to provide us with the coordinates for this contrast. This strategy has potentially important consequences for the results. Asking for unpublished coordinates allowed us to increase the number of studies entered into the meta-analysis and to reduce publication bias. Publication bias is the tendency to avoid publishing negative results, which indeed may play quite a relevant role in neuroimaging studies. Furthermore, the meta-analysis by Binelli et al. also used data from ROI-based studies. This choice could also introduce a bias in the results since the meta-analysis approach in neuroimaging calculates some parameters (including smoothing and suggested cluster size), assuming that whole brain is considered. For this reason we excluded ROI studies, unless authors could provide also results for the whole brain analysis.
Methods
The selection process took place in three stages. In the first stage, two independent investigators searched Pubmed (www.pubmed.gov) with the key words “social anxiety” AND “faces” and “social phobia” AND “faces” for the time frame up to January 2015. In the second step, we refined the search assessing from the title and abstract whether the studies: (1) were fMRI studies; (2) used faces for the experimental paradigm; (3) included both a group of patients with social phobia and a control group. In this phase, we also excluded narrative reviews on the topic. Following this selection, we obtained 43 studies, for which we recovered the full texts. Out of these, in a third step, we further excluded those papers that used particular type of patients (e.g. autism patients with social anxiety;20 subclinical social anxiety21) or particular type of faces (e.g. own-face perception22) and we included only those papers in which the results of the comparison between SAD and HC in a face perception task were reported. For those studies in which the basic contrast was not reported in the paper, we asked the authors to provide it, if possible with the same statistical level used in each original paper. For the studies that only considered the SAD > HC contrast, we also requested the contrast HC > SAD if available. For studies based on ROI analysis, we also requested the author to provide whole brain results if available. In case of absence of response or impossibility to provide data for a whole brain analysis, we excluded the paper from the analysis (Table 2). Finally, we excluded any study that explicitly reported having used, even partially, data from another published study already included in our meta-analysis.
Table 2.
List of the papers excluded at the third step of selection and principal reason for exclusion
| Title | First Author | Date | Reason for exclusion |
|---|---|---|---|
| fMRI reveals amygdala activation to human faces in social phobics | Birbaumer N | 1998 | No direct comparison between phobics and controls in a face perception study |
| Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans | Veit R. | 2002 | No direct comparison between phobics and controls in a face perception study |
| Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study | Straube T. | 2004 | ROI study—not full brain cover |
| Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? | Cooney RE | 2006 | ROI study |
| Time-varying amygdala response to emotional faces in generalized social phobia | Campbell DW | 2007 | ROI study—analysis on temporal dynamics and not on magnitude of response |
| Activity in medial prefrontal cortex during cognitive evaluation of threatening stimuli as a function of personality style | Rubino V. | 2007 | Target population is phobic prone subjects which cannot be considered as patients with social phobia |
| Common and distinct amygdala-function perturbations in depressed vs anxious adolescents | Beesdo K | 2009 | Anxiety adolescents group is heterogeneous |
| Beyond amygdala: Default mode network activity differs between patients with social phobia and healthy controls | Gentili C. | 2009 | Same data-set used for another paper already in the meta-analysis |
| Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder | Labuschagne I. | 2010 | No direct comparison between phobics and healthy controls but just drug*group interactions Same data-set used for another paper already in the meta-analysis |
| Is social phobia a “mis-communication” disorder? Brain functional connectivity during face perception differs between patients with social phobia and healthy control subjects | Danti S. | 2010 | Same data-set already entered the meta-analysis |
| Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders | Kleinhans NM | 2010 | Target population is autistic patients with or without social anxiety |
| Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety. A multicenter fMRI study | Demenescu LR | 2011 | Anxiety patients group is heterogeneous |
| Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament | Blackford JU | 2013 | Target population is inhibited temperament subjects which cannot be considered as patients with social phobia |
| Neural response to the observable self in social anxiety disorder | Pujol J. | 2013 | Task involved own-face perception |
| Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI | Sladky R. | 2013 | Contrast for SAD versus HC not available neither in the manuscript nor from the authors |
| Self-referential and anxiety-relevant information processing in subclinical social anxiety: an fMRI study | Abraham A. | 2013 | Subjects with subclinical social anxiety |
| Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder | Demenescu L.R. | 2013 | No main effect for the diagnosis of SAD (only for the diagnosis of panic disorder) |
| Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder | Klumpp H. | 2013 | Same data-set used for another paper already in the meta-analysis |
| Serotonin transporter gene alters insula activity to threat in social anxiety disorder | Klumpp H. | 2014 | Same data-set used for another paper already in the meta-analysis |
| Classifying social anxiety disorder using multivoxel pattern analyses of brain function and structure | Frick A. | 2014 | Same data-set used for another paper already in the meta-analysis |
Thus, ultimately we included 23 fMRI studies with a total of 873 subjects 449 with SAD and 424 HCs in our meta-analysis (Table 1). For 16 studies, we used published data, while for seven studies we used, partially or totally, the unpublished analysis provided by the authors.13,23,24,27,31,32,40 Table 1 reports the characteristics of each study of the present meta-analysis including type of faces, type of contrasts, and type of task used.8,11,13–15,23–27,29–41 Table 2 reports the 20 studies excluded in the second step of the selection and the main reason why they were not included in the final analysis.
Table 1.
Characteristics of the papers entered the meta-analysis
| SAD |
HC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. | M/F | Age (mean ± SD) | SAD severity* (mean ± SD) | N. | M/F | Age (mean ± SD) | SAD severity* (mean ± SD) | Type of faces | Type of contrast† | Table number | Notes | |
| Fonzo et al.23 | 14 | 4/10 | 25.4 ± 8.5 | Not evaluated | 15 | 6/9 | 30.0 ± 10.2 | Not evaluated | H, F, A | F > HF > A | Data provided by authors | |
| Wheaton et al.24 | 23 | 16/7 | 26.1 ± 6.7 | 70.7 ± 15.1 | 24 | 11/13 | 25.0 ± 5.6 | 6.8 ± 5.6 | N, F, A | F > NA > N | Data provided by authors | Each of the contrast was considered for the two conditions of the original experiment. High and low cognitive load. |
| Ziv et al.25 | 67 | 35/32 | 33.0 ± 8.8 | 84.1 ± 17.5 | 28 | 15/13 | 32.6 ± 9.5 | 15.3 ± 9.1 | A, C | Faces > fixation asterisks | 3 | The whole group analysis was conducted on 27 SAD patients (with highest scores at LSAS) and 27 HC |
| Klumpp et al.26 | 29 | 11/18 | 24.9 ± 6.3 | 77.3 ± 15.4 | 27 | 12/15 | 24.9 ± 5.9 | 24.9 ± 5.9 | H, F, A | H > shF > shA > sh | Data provided by authors | |
| Phan et al.27 | 21 | 8/13 | 25.9 ± 5.5 | 82.29 ± 13.02 | 19 | 10/9 | 26.9 ± 8.1 | 9.17 ± 7.40 | H, F, A | F > H A > H | Data provided by authors | |
| Prater et al.27 | 20 | 9/11 | 25.9 ± 5.3 | 79.35 ± 15.41 | 17 | 7/10 | 25.7 ± 7.1 | 7.94 ± 7.05 | H, F, A | F > H A > H | Data provided by authors | |
| Pantazatos et al.28,‡ | 16 | 2/14 | 33.6 ± 7.1 | Not provided | 19 | 11/8 | 31.7 ± 8 | Not provided | F, N, MF, MN | All faces versus baseline | S4 | |
| Pantazatos et al.28 | 14 | 4/10 | 27.3 ± 7.5 | 86.7 ± 18.1 | 17 | 7/10 | 31 ± 10.7 | 7.8 ± 5.3 | A, H, N | All faces versus baseline | S4 | |
| Frick et al.29 | 14 | Not provided | 32.4 ± 8.8 | 72.1 ± 25.7 | 12 | Not provided | 28.0 ± 8.2 | Not provided | F,N | F > N | 2 | |
| Labuschagne et al.30 | 18 | 18/0 | 29.4 ± 9.0 | >70 | 18 | 18/0 | 29.9 ± 10.2 | Not provided | S, H, N | S > N H > N | 2 | Contrast obtained for the placebo condition. No significant regions for H > N were found |
| Klumpp et al.31 | 29 | 12/17 | 24.7 ± 5.9 | 81.0 ± 15.2 | 26 | 10/16 | 26.2 ± 6.3 | 8.2 ± 7.7 | F,H | F > H | 2 | |
| Hahn et al.32 | 10 | 9/1 | 28.6 ± 4.3 | Not evaluated | 27 | 11/16 | 27.7 ± 7.2 | Not evaluated | A, F, D, S, Su, H, N | Emotional faces > baseline | Data in the text and provided by authors | Contrast obtained for the nine men with SAD compared with 11 HC |
| Blair et al.33,§ | 25 | 10/15 | 32.2 ± 9.1 | 73.2 ± 20.4 | 25 | 13/10 | 29.7 ± 8.3 | Not provided | A, F, H | A, F > H | 2 | Main effect of diagnosis and diagnosis X emotion interaction were considered |
| Blair et al.33,** | 14 | 7/7 | 13.3 ± 3.4 | 21.7 ± 4.8 | 14 | 9/7 | 14.9 ± 2 | Not provided | A, F, H | A, F > H | 2 | Main effect of diagnosis and diagnosis X emotion interaction were considered |
| Schneier et al.34 | 16 | 6/10 | 29.8 ± 9.0 | 81.4 ± 15.6 | 16 | 6/10 | 30.3 ± 9.7 | 8.2 ± 5.4 | N | Direct > averted gaze | 3 | |
| Klumpp et al.35 | 12 | Not provided | 28.2 ± 8.6 | Not provided | 12 | Not provided | 33.6 ± 9.6 | Not provided | A,F,D | A,F,D > baseline | 1 | Data from amygdala obtained by ROI analysis |
| Goldin et al.36 | 15 | 6/9 | 31.6 ± 9.7 | 67.6 ± 21.1 | 17 | 8/9 | 32.1 ± 9.3 | 29.3 ± 20.9 | HA, NS | HA > NS | 2 | |
| Evans et al.37 | 11 | 4/7 | 29 ± 7.5 | 82.4 ± 21.5 | 11 | 4/7 | 27.9 ± 10.6 | Not provided | A,H,N | A > N | 2,3 | Schematic faces |
| Gentili et al.8 | 8 | 4/4 | 39 ± 7 | 69.6 ± 1.01 | 7 | 4/3 | 30 ± 7 | 24.7 ± 1.25 | N,H,D,F,A | All faces > scrambled | 1 | |
| Blair et al.13 | 17 | 9/8 | 29 ± 8.7 | 68.3 ± 20.7 | 17 | 9/8 | 31.2 ± 9.1 | 57.2 ± 26.7 | A,F,H | A, F > H | Data provided by the authors | Main effect of diagnosis and diagnosis X emotion interaction were considered |
| Yoon et al.38 | 11 | 5/6 | 27 ± 6 | 70.91 ± 19.98 | 11 | 5/6 | 26.9 ± 6.1 | 9.64 ± 8.42 | A,F,D,H** | High > low†† | Data in the text | Data from amygdala obtained by ROI analysis |
| Phan et al.14 | 10 | 5/5 | 26.7 ± 6.8 | 72.1 ± 20.6 | 10 | 5/5 | 26.6 ± 6.8 | 9.8 ± 8.9 | A, F, D, S, H, N | A,F,D, S > H | Data in the text | |
| Amir et al.15 | 11 | 3/8 | 24.1 ± 5.2 | Not provided | 11 | 3/8 | 23.9 ± 5.7 | Not provided | D, N | D > N | 2 | |
| Straube et al.39 | 9 | 4/5 | 25.7 8 3.4 | Not provided | 9 | 4/5 | 22.7 8 2.6 | Not provided | A, F, H, N | A, F, H, N > fixation cross | 1 | Data from ROIAuthors reported no other clusters outside the ROI |
| Stein et al.11 | 15 | 10/5 | 39.1 ± 14.3 | 87.7 ± 25.7 | 15 | 10/5 | 39.3 ± 12.3 | 15.5 ± 13.4 | A,F,H,C | A,F,C > H | 2 | |
A: angry faces; C: contemptuous faces; D: disgusted faces; F: fearful faces; H: happy faces; HC: healthy controls; MF: masked fearful faces; MN: masked neutral faces; N: neutral faces; S: sad faces; SAD: patients with social anxiety disorders; Sh: shapes; Su: surprised faces; LSAS: Liebowitz Social Anxiety Scale.
SAD severity was evaluated with the Liebowitz Social Anxiety Scale in all the study but in the adolescent group of the study by Blair et al.33 where the Pediatric Anxiety Rating Scale was used: all the values are mean ± SD with the exclusion of Labuschagne et al.30 who reported only that all SAD patients score over 70 at the LSAS.
Type of contrast used for the comparison between SAD and HC.
Two independent groups with two different tasks were used in the study. The analysis assessed the main effect of diagnosis (SAD versus HC) on all faces perception.
Demographic data for the adult groups.
Demographic data for the adolescent groups.
Faces were presented with a low and a high degree of emotional intensity.
Activation likelihood estimation (ALE) meta-analysis was conducted using the GingerALE software (version 2.3 http://www.brainmap.org/ale/).
ALE meta-analysis is a coordinate-based meta-analysis approach, which is widely adopted in neuroimaging. ALE has been typically used to identify concordance across studies or to compare results across distinct tasks or groups of subjects. ALE models the probability of localizing active foci with Gaussian probability density distributions. Distributions map are derived by each data-set entering in the meta-analysis. The ALE value, generated by the union of each distribution at a voxel level, is an estimate of the likelihood that at least one of the foci in a data-set was truly located at a given voxel of the final ALE map (for a more detailed description of the method refer to Turkeltaub et al.42 and Eickhoff et al.43).
We considered the foci from the contrasts in SAD versus HC during face perception. We were able to obtain the contrast SAD > HC for all the 23 studies. As far as the HC > SAD, 12 out of the 23 included studies explicitly stated that they had performed the comparison HC > SAD. Among these studies the comparison yielded significant differences in only six studies8,28,33,35–37 and non-significant results in the other six. For the studies that did not explicitly report the contrast, we asked authors for the results of the contrast. We obtained a response for each of the remaining 11 papers: three had obtained non-significant results for the comparison HC > SAD13,23,39; for additional three papers, it was not possible to recover the original data to run the new analysis15,34,38; for the remaining five papers, we recovered original unpublished data for the comparison HC > SAD.
Finally, 11 out of the 23 papers used in this meta-analysis are from previous publications of the authors of the present paper. More in particular, regarding the seven studies for which we used unpublished data provided under request, four of them are studies from the authors of the present paper, while three were provided by independent researchers (see “Acknowledgment” section). The meta-analysis was run by one author who was not involved in the analysis for the original papers (see “Acknowledgment” section).
We used Talairach coordinates for the meta-analysis and accordingly converted coordinates from papers using MNI with the GingerALE foci converter tool. We calculated the Random Effects Model according to Turkeltaub et al.42 and the p values according to Eickhoff et al.43 We used an False Discovery Rate (FDR)-corrected p value of 0.01 to compute ALE maps and we also considered a minimum cluster size of 32 mm3 in order to minimize type I and II errors. The cluster size was chosen according to the simulations provided by the GingerALE software. Specifically, the cluster volume was determined through a Monte Carlo simulation performed by the software on simulated data created from the results data-set. The obtained cluster size was calculated to allow only a 5% of false positive at the given statistical threshold of FDR 0.01.
AFNI toolbox44 was used to display the results.
Results
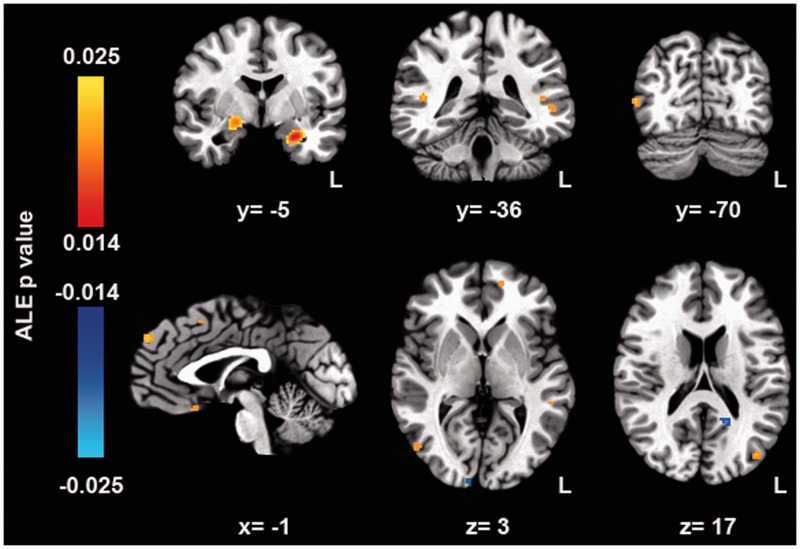
The meta-analysis highlighted clusters in which SAD patients showed a higher activation for face perception as compared to HC. Namely, significant clusters were located in the two amygadalae, in the STS, in the prefrontal cortex (inferior frontal gyrus, medial frontal gyrus, superior frontal gyrus, and in the subgenual cingulate), and in the visual cortex (lingual gyrus, middle occipital, and middle temporal gyrus) (Figure 1 and Table 3). Of note, the right amygdala cluster extended to the region of the globus pallidus.
Figure 1.
ALE map of the significant cluster for face perception in SAD patients versus HC. All the clusters were significant at a p value of 0.01 (FDR corrected) and of > 35 mm3 volume. Warm colors indicate higher activity in SAD while cold ones indicate higher activity in HC. Higher ALE probability values are related to more significant clusters, while lower ALE probability values are related to less significant clusters. (A color version of this figure is available in the online journal.)
Table 3.
Significant ALE clusters for face perception in SAD patients versus HC. All the clusters were significant at a p value of 0.01 (FDR corrected) and of > 35 mm3 volume
| Hemi-sphere | Region | BA | Center of mass |
Peak |
Peak ALE p-value | Volume (mm3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||||
| SAD > HC | |||||||||||
| R | IFG | 47 | 29.21 | 14.43 | −14 | 30 | 16 | −14 | 0.014 | 40 | |
| L | MedFG | 9 | .45 | 53.79 | 36.26 | 0 | 54 | 36 | 0.02 | 240 | |
| L | SFG | 8 | −2.53 | 15.47 | 48.12 | −2 | 16 | 48 | 0.017 | 120 | |
| R | MedFG | 10 | 6.99 | 43.03 | −7.98 | 6 | 44 | −8 | 0.016 | 96 | |
| L | MedFG | 10 | −13 | 51.03 | 3 | −14 | 52 | 2 | 0.015 | 64 | |
| L | SubACC | 25 | −2.19 | 17.76 | −14.25 | −2 | 18 | −14 | 0.017 | 200 | |
| SubACC | 25 | −2 | 14 | −12 | 0.015 | ||||||
| R | GP/amygdala | 20.72 | −4.76 | −8.05 | 18 | −2 | −8 | 0.021 | 952 | ||
| L | amygdala | −23.65 | −3.84 | −18.04 | −24 | −4 | −18 | 0.025 | 760 | ||
| L | MTG | 19 | −38.13 | −74.38 | 18 | −38 | −74 | 18 | 0.017 | 128 | |
| R | MTG/MOG | 19 | 49.41 | −68.67 | 6.02 | 50 | −68 | 6 | 0.017 | 192 | |
| R | STS | 41 | 43.57 | −36.87 | 9.76 | 44 | −36 | 10 | 0.016 | 144 | |
| L | STS | 22 | −50.36 | −35.5 | 1.66 | −50 | −36 | 2 | 0.016 | 96 | |
| L | STS | 41 | −42.86 | −35.15 | 8.85 | −44 | −36 | 8 | 0.014 | 56 | |
| R | Lingual gyrus | 18 | 22.78 | −86.82 | −6.38 | 22 | −86 | −6 | 0.014 | 40 | |
| HC > SAD | |||||||||||
| L | Precuneus | 30 | −15.21 | −49.04 | 16.33 | −16 | −50 | 16 | 0.013 | 216 | |
| R | Lingual gyrus | 17 | 11.56 | −92.7 | 1.64 | 10 | −94 | 2 | 0.012 | 184 | |
BA: Broadmann areas; GP: globus pallidus; IFG: inferior frontal gyrus; MedFG: medial frontal gyrus; MOG: middle occipital gyrus; MTG: middle temporal gyrus; SFG: superior frontal gyrus; SubACC: subgenual anterior cingulate cortex.
We also identified two significant clusters for the contrast HC > SAD in the precuneus and in the lingual gyrus.
Discussion
The aim of the present meta-analysis was to evaluate the presumed altered recruitment of the extended face perception system in SAD patients. Specifically, we intended to verify whether neural abnormalities would occur beyond those attributed to “emotional” and/or “threat” processing in prior published narrative and meta-analytic reviews17,19 and would extend to the perceptive, attentive, and cognitive systems involved in face perception.6 To this purpose, we considered all the fMRI studies that conducted a direct comparison between SAD and HC using a face perception task, regardless of the face emotional expressions and task used. Our study highlighted a higher activation of SAD patients in regions related to emotional recognition and processing (STS), related to attention (superior frontal gyrus) and emotional regulation (subgenual anterior cingulate and medial frontal gyrus). We also found an abnormally higher response to faces in the visual cortex. Of note, our meta-analysis highlighted for the first time also areas that are more active in HC as compared to SAD during face perception, namely in a cluster of the occipital visual cortex (lingual gyrus) and in the posterior cingulate, Finally, consistently with the other available meta-analyses and with the vast majority of functional brain imaging studies, we identified an abnormal activation in the amygdalae of SAD patients as compared to HCs in response to face stimuli (e.g. in the literature 8,11,12,14,35,45).
Specifically, as expected, we found that bilateral amygdala was more active in SAD patients as compared to HCs. An altered amygdala response has been consistently reported by brain imaging studies in SAD patients, as well as in subjects with other anxiety disorders, both by ROI12,39 and whole brain studies.16 Although amygdala alterations are almost always present in SAD studies involving faces, it is possible to underline different patterns of altered brain activity. For instance, some studies highlighted bilateral amygdala hyperactivity in SAD (e.g. in the literature11,13,14,31,33,35,37,38), while others found a lateralized greater amygdala response. For instance, several works identified an altered response in the right amygdala only,13,14,31,37 while others reported an opposite pattern (e.g. Gentili et al.8).
As a matter of fact, it is well established that the two amygdalae have different functions. For instance, Zalla et al.46 showed a differential amygdala response in healthy individuals while they were performing a competitive computer game: neural activity in the left amygdala became progressively greater in a parametrically increasing winning condition while the opposite behavior was shown by the right one. Other studies reported that the two amygdalae reacted differently to diverse types of mood induction, suggesting a different role in emotional processing.47 Finally, a differential effect of gender on amygdala-lateralized discharge during face perception has been shown.48 Thus, it is possible that differences in experimental designs may account for differences in amygdala activations. It is also possible that the two amygdalae may play different roles in face perception in SAD. However, the meta-analytic approach cannot directly support this hypothesis, because it evaluates common patterns among studies and does not single out particularities from individual studies.
It is worth noting that the right amygdala cluster also included the globus pallidus. Interestingly, the globus pallidus was found to be altered also in a previous meta-analysis on the neurobiological dysfunctions of emotional processing in SAD,16 as well as in a meta-analysis of specific phobias.49 Although globus pallidus is primarily related to motor control, it has been linked also to emotional regulation.50,51 Recently, neural activity in the right globus pallidus was found to correlate with a better short-term memory for faces portraying negative emotions (namely anger).52 The recruitment of this area may be related to the involvement of the salience circuit, which is more prominent for faces conveying information about a potential threat.52,53 Thus, the increased discharge in the globus pallidus and amygdala in SAD patients may be related to an increased salience attribution to faces and to the worry of a potential threat.
Contrary to the findings from the other meta-analysis on the topic,19 we identified several clusters in the prefrontal cortex that are more active in patients as compared to HC. The inferior frontal gyrus (IFG) is a region that has been linked to regulation and modulation of emotions both in social54 and non-social contexts55 and which mediates aspects of empathy.56 A similar role has been postulated for the subgenual anterior cingulate.57 It has been suggested that this region gathers information about pain, threat, punishment and, more in general, negative feedback, triggering fear and anxiety, influencing goal-directed behaviors, and biasing the balance between self-focused and other-focused attention in favor of the first.55 For this reason, it is not surprising that the cingulate cortex activity has been found altered in several psychiatric disorders, including not only SAD,58,59 but also other anxiety and mood disorders.57,60 Moreover, medial prefrontal cortex clusters, including medial frontal gyrus and superior frontal gyrus, were hyperrecruited in SAD as compared to HC. These regions are typically involved not only in emotional modulation but also in cognitive and attentive processes including decision-making and autobiographical memory61,62 and seem to contribute to neuro-vegetative control and to the sympathovagal balance.63 Moreover, several studies highlighted their recruitment (together with the subgenual cingulate) while subjects practiced cognitive strategies of emotional modulation.64–66 Finally, their abnormal activity seems to be specifically involved in SAD given its role in fear conditioning and extinction in phobias.67 These frontal areas also seem to modulate self-focused attention—the attention to inner sensations and thoughts, which typically is abnormally increased in anxiety disorders.68,69 The higher response in these areas in SAD may be interpreted as the neurobiological counterpart of altered emotional regulation processing while patients are exposed to socially anxious stimuli. It is interesting to note that a recent meta-analysis in healthy individuals also found bilateral amygdala, globus pallidus, and areas belonging to the IFG and the ventral prefrontal cortex to be more activated during negative social evaluation of faces.70 We suggest that such an overlap may be related to the increased proneness to rate faces as negative, which is typical behavioral feature in SAD patients.71,72 Moreover, these results are also in line with the cortico-limbic dysfunction hypothesis of SAD.73
Concerning the extended network for face perception, we also identified significant clusters in the bilateral STS in which response for faces was higher in SAD as compared to HC. STS plays a fundamental role in several components of the face perception process,6 including gaze perception,10,74 emotion recognition,75,76 and mental state attribution.75 The existing evidence consistently points to the role of the STS in linking variant face characteristics (expression, gaze, etc.) to their social and communicative meanings.77 Thus, it is not surprising that STS was also found to be hyperactive in studies on social phobia. Straube et al., for instance, showed a significantly greater activity in STS in SAD patients as compared to HCs in a wide range of conditions and independently from the type of face image (schematic draw or picture) and task (explicit or implicit face perception task) used.39,78 Gentili et al.8 found an hyperactivation of bilateral STS for face perception as compared to scrambled images in SAD versus HC. A significantly stronger activation in STS in SAD patients than in healthy matched controls also was found by Amir et al.15 in a study focused on the role of cingulate cortex during the perception of disgusted/contemptuous faces. Overall, hyperactivation in STS is thought to reflect an increased wariness to socially relevant stimuli. This increased wariness may be related to the perceptual bias toward negative emotions, a seemingly core characteristic of SAD.8,79
Our results also showed significant differences in brain activity within the visual cortex. Namely, clusters in the lingual gyrus and in the temporal cortex were more active in SAD while another cluster, again in the lingual gyrus, was more active in HC. We believe that this complex pattern of altered response to faces in the visual cortex is related to the dysfunctional perceptive process in SAD. In this sense, it is interesting to note that Giménez et al. found an increased activation of visual areas in social phobics while they were under scrutiny by others.80 Pujol et al. reported an increased activation in the primary visual cortex during a self-recognition task in SAD patients,22 which was interpreted as an increased arousal due to negative self-judgment, typically present in these patients. Given the importance of early visual areas in face processing (particularly in the case of aversive emotions),81 it is possible that this cluster of activation may also be related to the increased arousal and wariness toward faces. An alternative interpretation for this finding implies that altered face processing in SAD could also involve early visual processing areas. Some studies suggested that activity in the fusiform face area is disrupted in SAD.8,20 Consequently, this alteration may represent the neurobiological counterpart of the altered scan path usually detected in SAD patients while exploring faces.9 We can hypothesize that because of the altered functionality in the fusiform gyrus, face recognition processes would rely on a partially different path within the distributed ventro-temporal cortex.6
Apart from the above-mentioned cluster in the lingual gyrus, we also found another region, the precuneus, in which HC showed a higher response to faces as compared to SAD. Precuneus activity seems to be related to the sense of self82 and the self-attribution of emotions, as compared to when emotions are attributed to others.83 Moreover, abnormal activity and connectivity in precuneus has been reported in anxiety disorders, including SAD.84–88 Studying the switch between rest and a face perception task, Gentili et al. reported a weakened deactivation in SAD as compared to HC.89 This lack of deactivation was considered a possible neurobiological correlate of self-focused attention. Finally, resting state activity in this region as measured by means of fALFF and Hurst exponent seems to be modulated by social anxiety severity in a group of healthy volunteers.90
Methodological considerations and comparison with other published meta-analyses
Emotional processing in SAD is a very relevant topic in social neuroscience. In the last few years, a narrative review91 and three meta-analyses16,17,19 have focused on this topic. Hattingh and coworkers investigated brain responses to socially relevant stimuli and compared emotional faces and statements to neutral ones.16 In this way, the emotional dysregulation in SAD was assessed in a consistent and coherent way, independently from the characteristics of the specific stimuli. Brühl et al., in the same framework, tried to be as inclusive as possible and create an inclusive network-based model of brain abnormal responses in SAD.17 On the other hand, we decided to focus on the functional neuroanatomy of face perception and not to include studies with other stimuli, as we predicted that face perception in SAD would be a unique phenomenon with distinctive abnormalities.
As expected, some of the regions we identified in our meta-analysis (for instance the bilateral amygdala and the globus pallidus) are consistent with Hattinigh et al.’s and Brühl et al.’s results.16,17 These areas may be related to the altered emotional regulation process, which is a relevant core aspect in SAD and is evoked whenever patients cope with any socially relevant stimuli. Interestingly, as hypothesized, we found altered neural activity in additional regions, including STS, and prefrontal cortical areas, which are more active in SAD, as well as a reduced activation in the precuneus and lingual gyrus. We argued these regions could be more strictly related to the specific alteration in the face perception process rather than to emotional dysregulation, because while we found significant differences here, these were absent in the two above-mentioned meta-analyses. However, we acknowledge that the attempt to disentangle the dysfunctions in face perception processes from those associated with emotional regulation is rather speculative, as the two are intrinsically intertwined.55 It may be that face perception in SAD induces specific increases in arousal and alterations in emotional regulation regions, while, in turn, alterations in these regions may interfere with the normal exploration of faces.
The third recent meta-analysis tackles a very similar research question to our present work.19 Binelli et al. were interested in evaluating face processing in SAD and William’s syndrome patients, in order to explore the complete spectrum of emotional reactivity to faces. As expected, results partially overlapped with our own (specifically, in the bilateral amygdalae), but they also differed from the present study for several aspects. First of all, they found a greater recruitment in the insula for the contrast SAD versus HC, possibly driven by ROI-based studies. On the other hand, we found higher activation in bilateral STS, in the superior frontal gyrus and in the IFG which were not found by Binelli et al. Moreover, we identified clusters in medial frontal gyrus, anterior cingulate, and visual cortex that, although similar to those found by Binelli et al., were not overlapping. Finally and, in our opinion, most relevantly, we also found areas in which SAD patients had a significantly lower response as compared to HC, namely the precuneus and lingual gyrus.
We believe that discrepancy in results is mostly due to the different research questions investigated by the two papers. The work by Binelli et al.19 was interested in assessing face perception process in two distinct disorders, which are at the opposite extremes of the continuum of social fear, SAD, and William’s syndrome. As in other studies in the literature, the Binelli’s review seems specifically interested in assessing emotional response to faces. On the other hand, we were more interested in assessing the whole face perception process in SAD. These differences are not trivial as for instance, for our purposes we were also very interested in the contrast HC > SAD, as differences in this direction may also indicate a face perception dysfunction in SAD. However, several studies did not report such a contrast, with the consequence that the Binelli’s meta-analysis found no significant clusters. We believe that these two different approaches did play an effect on inclusion/exclusions criteria for individual studies and search methodology, producing two very relevant differences between the two meta-analyses:
The meta-analysis by Binelli et al.19 considered also ROI studies in which the whole brain was not covered, as well as a priori regions (with a priori significant thresholds) were considered. For instance, the study by Straube et al.,12 which considered ROIs in the insula, amygdala, and fusiform gyrus, was conducted without a complete brain coverage.
We had the opportunity to use unpublished contrasts for some papers available in the literature leading to an increase of the number of studies and to a better control of publication bias, while also allowing us to focus on the comparison HC > SAD which is typically less evaluated and often considered less important.
As a result, our present work includes 23 studies, of which only 15 are in common with those reported by Binelli et al.19
Study limitations
Out of the 23 papers included in this meta-analysis, 11 are co-authored by at least one of the authors of the present paper. Thus, a potential critical issue is that we could be biased toward the confirmation of our own findings. This phenomenon, known as “allegiance” is well known in clinical research, especially when psychotherapies are compared, and may constitute an important source of bias.92–94 In neuroimaging meta-analyses this problem has not yet been raised. However, it is less likely for neuroimaging data to be affected by this type of bias, given that the type of measures (discrete, as spatial coordinates are) is different as compared to those used in behavioral meta-analysis (continuous, as psychological scales are). In our specific case, moreover, the inclusion also of unpublished data from studies that were not conducted with the aim of comparing faces in SAD versus HC minimizes this potential bias even further. Finally, as far as the unpublished contrasts are concerned, it is relevant to underline that the authors who performed these new analyses are not those who performed the GingerALE meta-analytic one, as indicated in the “Acknowledgment” section.
A limitation of our study, as well as of the other recent meta-analyses, is that we did not calculate ALE maps for face perception separately for HC and SAD patients prior to performing a contrast meta-analysis. Although this is a more robust way to conduct this type of meta-analysis, neither any of the previous meta-analyses,16,17,19 nor the present one were able to perform this type of analysis, as all the published papers just presented results for the comparison between the two groups. Further meta-analyses requesting this type of results from the authors may help to confirm the findings obtained in the present work. Moreover, in our meta-analysis we did not consider single typology of contrasts between faces (for instance negative versus neutral faces). On the one hand, this provides a pattern of activation that may be considered specifically related to general face processing in SAD; on the other hand, this makes it impossible to evaluate subtle differences.11,16 However, our statement should be weighted also considering that dissecting the emotional component of face perception from the strictly sensory-perceptive one is obviously difficult (if not impossible), given the intertwined relation between these two aspects. One can speculate that an experimental approach using only neutral faces could allow to identify more specifically the sensory-perceptive component. However, that neutral faces are truly neutral, meaning without any emotional content, especially when SAD patients are concerned, is debated.95
Another possible methodological limitation is that the heterogeneity of the contrasts is a hard to control condition. Particularly, some contrasts are more frequently used than others across the studies we considered (e.g. the fearful versus neutral contrast is more present and therefore would weight more in the results as compared to the happy versus neutral faces). This may have biased the meta-analysis. Nevertheless, these limits do not reduce the general meaning of our results, indicating an alteration within the face perception neural pathway that can be highlighted despite differences across specific experimental designs.
Finally, our meta-analysis suffers from the limitations intrinsic to the ALE meta-analysis methods. For instance, data were collected and provided with different threshold and different corrections for multiple comparisons (and sometimes included no correction at all). Indeed, a unique approach to meta-analysis for fMRI data is still missing and methodological studies should look into the effects of using ROI studies and results from different thresholds, in order to produce a consensus methodological agreement. However, we believe that our innovative approach, derived from meta-analyses of behavioral data, could be extended to control for these issues, by asking authors for original data at a given pre-set threshold, and not limited to ROIs.
Conclusions
Our work expanded the available neurobiological models of SAD.17,73,91 Recently, it has been underlined how the neural abnormalities in SAD may involve a wider network that still remains to be characterized.73 To address this need, here we have examined the neurobiological alterations in SAD related to face perception processing.
In this meta-analysis, we used comparisons between SAD patients and HCs for all types of face perception contrasts reported in the fMRI studies published to date. Thus, we believe that the brain areas that emerged are consistently related to face perception and provide a comprehensive knowledge of face perception alterations in SAD patients. As a matter of fact, meta-analytic approach in neuroimaging allows to “average” brain activations which are consistent among studies.43 For this reason, we think that gathering together different contrasts would dilute the activations due to specific contrasts and strengths into those related to the common face perception neural pathway.
In this meta-analysis, as compared to other similar ones, we adopted an innovative approach, which is widely used in behavioral meta-analyses. Considering the relevant differences that emerged in this work, using unpublished data, as compared to the commonly used approach with only published data19 we believe that requesting supplementary results from authors should become a routine in fMRI meta-analyses. Specifically, we had the opportunity to increase the number of studies included into the meta-analysis and to minimize potential publication biases.
In line with recent positions on meta-analyses, we believe that the results of this meta-analysis would be a valid tool to define ROIs for functional connectivity studies.96,97 In particular, ROI selection is a relevant issue in SAD since this approach is widely used in the study of this psychopathological condition (e.g. Straube et al.12 and Straube et al.39). Our results found a significant cluster at a meta-analytic level in the bilateral amygdala and STS, which are often used in ROI studies. However, we failed to detect significant clusters in other ROIs, such as the fusiform gyrus, which are often used as well.
To conclude, the foci identified in our meta-analysis support the idea that a complex network belonging to the extended neural system for face perception is altered in SAD.6 Thus, while our results also provide additional support to the hypothesis that amygdala hyperactivity is a consistent marker of SAD patient response to faces, they clearly indicate that alterations of face perception in this disorder are more than just a dysfunction in amygdala discharge, in line with previous original reports, including those from our own lab.8,89 These findings may have potential implications at a clinical level, both for a wider understanding of the pathogenesis of SAD and for the development of novel psychotherapeutic approaches, as they suggest that person-to-person contact in SAD patients may be biased by a dysfunctional perception of faces and reflect a broader alteration in social cognition.
Acknowledgements
The Authors wish to thank the following researchers for providing unpublished contrasts for the present meta-analysis: Karina Blair (National Institute of Mental Health, Bethesda, US), Gregory Fonzo (Stanford University, US), Andreas Hahn and Rupert Lanzenberger (Medical University of Vienna, Austria), Thomas Straube (Muenster University, Germany).
Authors’ contribution
Paper conception: CG, IAC, PP
Article search: CG, LT
Data analysis for meta-analysis: CG
Original data-set re-analysis: LT, MA, HK, KLP
Manuscript writing: CG, IAC, PP
Manuscript reviewing: All
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
Part of the present research was supported by Fondazione IRIS, Castagneto Carducci, Livorno, Italy. Ioana A. Cristea was supported by a Young Researcher Grant (GTC_34064/2013), awarded by the Babes-Bolyai University, Romania.
References
- 1.APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Pub, 2000.
- 2.Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. J Abnormal Psychol 2004; 113: 160–5. [DOI] [PubMed] [Google Scholar]
- 3.Schofield CA, Inhoff AW, Coles ME. Time-course of attention biases in social phobia. J Anxiety Disord 2013; 27: 661–9. [DOI] [PubMed] [Google Scholar]
- 4.Schofield CA, Johnson AL, Inhoff AW, Coles ME. Social anxiety and difficulty disengaging threat: evidence from eye-tracking. Cogn Emot 2012; 26: 300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foa EB, Gilboa-Schechtman E, Amir N, Freshman M. Memory bias in generalized social phobia: remembering negative emotional expressions. J Anxiety Disord 2000; 14: 501–19. [DOI] [PubMed] [Google Scholar]
- 6.Haxby JV, Hoffman E, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci 2000; 4: 223–33. [DOI] [PubMed] [Google Scholar]
- 7.Natu V, O’Toole AJ. The neural processing of familiar and unfamiliar faces: a review and synopsis. Brit J Psychol 2011; 102: 726–47. [DOI] [PubMed] [Google Scholar]
- 8.Gentili C, Gobbini MI, Ricciardi E, Vanello N, Pietrini P, Haxby JV, Guazzelli M. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull 2008; 77: 286–92. [DOI] [PubMed] [Google Scholar]
- 9.Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Res 2004; 127: 43–53. [DOI] [PubMed] [Google Scholar]
- 10.Weeks JW, Howell AN, Goldin PR. Gaze avoidance in social anxiety disorder. Depress Anxiety 2013; 30: 749–56. [DOI] [PubMed] [Google Scholar]
- 11.Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59: 1027–34. [DOI] [PubMed] [Google Scholar]
- 12.Straube T, Kolassa I, -T, Glauer M, Mentzel H-J, Miltner WHR. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry 2004; 56: 921–30. [DOI] [PubMed] [Google Scholar]
- 13.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJR, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry 2008; 165: 1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 2006; 59: 424–9. [DOI] [PubMed] [Google Scholar]
- 15.Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 2005; 57: 975–81. [DOI] [PubMed] [Google Scholar]
- 16.Hattingh CJ, Ipser J, Tromp SA, Syal S, Lochner C, Brooks SJ, Stein DJ. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front Hum Neurosci 2012; 6: 347–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev 2014; 47: 260–80. [DOI] [PubMed] [Google Scholar]
- 18.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binelli C, Subirà S, Batalla A, Muñiz A, Sugranyés G, Crippa JA, Farré M, Pérez-Jurado L, Martín-Santos R. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: a systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia 2014; 64C: 205–17. [DOI] [PubMed] [Google Scholar]
- 20.Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, Aylward E. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia 2010; 48: 3665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham A, Kaufmann C, Redlich R, Hermann A, Stark R, Stevens S, Hermann C. Self-referential and anxiety-relevant information processing in subclinical social anxiety: an fMRI study. Brain Imaging Behav 2013; 7: 35–48. [DOI] [PubMed] [Google Scholar]
- 22.Pujol J, Giménez M, Ortiz H, Soriano-Mas C, López-Solà M, Farré M, Deus J, Merlo-Pich E, Harrison BJ, Cardoner N, Navinés R, Martín-Santos R. Neural response to the observable self in social anxiety disorder. Psychol Med 2013; 43: 721–31. [DOI] [PubMed] [Google Scholar]
- 23.Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Letamendi A, Simmons AN, Paulus MP, Stein MB. Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br J Psychiatry 2015; 206: 206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheaton MG, Fitzgerald DA, Phan KL, Klumpp H. Perceptual load modulates anterior cingulate cortex response to threat distractors in generalized social anxiety disorder. Biol Psychol 2014; 101: 13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord 2013; 3: 20–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol Mood Anxiety Disord 2013; 3: 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phan KL, Coccaro EF, Angstadt M, Kreger KJ, Mayberg HS, Liberzon I, Stein MB. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biol Psychiatry 2013; 73: 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantazatos SP, Talati A, Schneier FR, Hirsch J. Reduced anterior temporal and hippocampal functional connectivity during face processing discriminates individuals with social anxiety disorder from healthy controls and panic disorder, and increases following treatment. Neuropsychopharmacology 2014; 39: 425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frick A, Howner K, Fischer H, Kristiansson M, Furmark T. Altered fusiform connectivity during processing of fearful faces in social anxiety disorder. Transl Psychiatry 2013; 3: e312–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int J Neuropsychopharmacol 2012; 15: 883–96. [DOI] [PubMed] [Google Scholar]
- 31.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol 2012; 89: 273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 2011; 56: 881–9. [DOI] [PubMed] [Google Scholar]
- 33.Blair KS, Geraci M, Korelitz K, Otero M, Towbin K, Ernst M, Leibenluft E, Blair RJR, Pine DS. The pathology of social phobia is independent of developmental changes in face processing. Am J Psychiatry 2011; 168: 1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneier FR, Pomplun M, Sy M, Hirsch J. Neural response to eye contact and paroxetine treatment in generalized social anxiety disorder. Psychiatry Res 2011; 194: 271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Res 2010; 183: 167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry 2009; 66: 170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety 2008; 25: 496–505. [DOI] [PubMed] [Google Scholar]
- 38.Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res 2007; 154: 93–8. [DOI] [PubMed] [Google Scholar]
- 39.Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology 2005; 52: 163–8. [DOI] [PubMed] [Google Scholar]
- 40.Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety 2013; 30: 234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantazatos SP, Talati A, Pavlidis P, Hirsch J. Decoding unattended fearful faces with whole-brain correlations: an approach to identify condition-dependent large-scale functional connectivity. PLoS Comput Biol 2012; 8: e1002441–e1002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapp 2012; 33: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapp 2009; 30: 2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–73. [DOI] [PubMed] [Google Scholar]
- 45.Campbell DW, Sareen J, Paulus MP, Goldin PR, Stein MB, Reiss JP. Time-varying amygdala response to emotional faces in generalized social phobia. Biol Psychiatry 2007; 62: 455–63. [DOI] [PubMed] [Google Scholar]
- 46.Zalla T, Koechlin E, Pietrini P, Basso G, Aquino P, Sirigu A, Grafman J. Differential amygdala responses to winning and losing: a functional magnetic resonance imaging study in humans. Eur J Neurosci 2000; 12: 1764–70. [DOI] [PubMed] [Google Scholar]
- 47.Dyck M, Loughead J, Kellermann T, Boers F, Gur RC, Mathiak K. Cognitive versus automatic mechanisms of mood induction differentially activate left and right amygdala. Neuroimage 2011; 54: 2503–13. [DOI] [PubMed] [Google Scholar]
- 48.Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, Loth E, Banaschewski T, Barbot A, Barker G, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Mallik C, Mann K, Artiges E, Paus T, Poline JB, Rietschel M, Reed L, Smolka MN, Spanagel R, Speiser C, Strohle A, Struve M, Schumann G, Buchel C. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage 2011; 56: 1847–53. [DOI] [PubMed] [Google Scholar]
- 49.Ipser JC, Singh L, Stein DJ. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci 2013; 67: 311–22. [DOI] [PubMed] [Google Scholar]
- 50.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci 2003; 3: 207–33. [DOI] [PubMed] [Google Scholar]
- 51.Sztainberg Y, Kuperman Y, Justice N, Chen A. An anxiolytic role for CRF receptor type 1 in the globus pallidus. J Neurosci 2011; 31: 17416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson MC, Wolf C, Johnston SJ, Raymond JE, Linden DEJ. Neural correlates of enhanced visual short-term memory for angry faces: an FMRI study. PLoS One 2008; 3: e3536–e3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA 2011; 108: E255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grecucci A, Giorgetta C, Bonini N, Sanfey AG. Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Front Hum Neurosci 2013; 7: 523–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci 2015; 9: 58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liakakis G, Nickel J, Seitz RJ. Diversity of the inferior frontal gyrus–a meta-analysis of neuroimaging studies. Behav Brain Res 2011; 225: 341–7. [DOI] [PubMed] [Google Scholar]
- 57.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev 2013; 37: 2529–53. [DOI] [PubMed] [Google Scholar]
- 58.Arnold Anteraper S, Triantafyllou C, Sawyer AT, Hofmann SG, Gabrieli JD, Whitfield-Gabrieli S. Hyper-connectivity of Subcortical Resting State Networks in Social Anxiety Disorder. Brain Connect 2013; 4(2): 81–90. [DOI] [PubMed] [Google Scholar]
- 59.Ball TM, Sullivan S, Flagan T, Hitchcock CA, Simmons A, Paulus MP, Stein MB. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. Neuroimage 2012; 59: 1879–87. [DOI] [PubMed] [Google Scholar]
- 60.Fredrikson M, Faria V. Neuroimaging in anxiety disorders. Mod Trends Pharmacopsychiatry 2013; 29: 47–66. [DOI] [PubMed] [Google Scholar]
- 61.Araujo HF, Kaplan J, Damasio A. Cortical midline structures and autobiographical-self processes: an activation-likelihood estimation meta-analysis. Front Hum Neurosci 2013; 7: 548–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bissonette GB, Powell EM, Roesch MR. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res 2013; 250: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 2012; 36: 747–56. [DOI] [PubMed] [Google Scholar]
- 64.Farb NAS, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Can J Psychiatry 2012; 57: 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linden DEJ. Brain imaging and psychotherapy: methodological considerations and practical implications. Eur Arch Psychiatry Clin Neurosci 2008; 258: 71–5. [DOI] [PubMed] [Google Scholar]
- 66.Hofmann SG, Ellard KK, Siegle GJ. Neurobiological correlates of cognitions in fear and anxiety: a cognitive-neurobiological information-processing model. Cogn Emot 2012; 26: 282–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marek R, Strobel C, Bredy TW, Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 2013; 591: 2381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boehme S, Miltner WHR, Straube T. Neural correlates of self-focused attention in social anxiety. Soc Cogn Affect Neurosci 2014; 10(6): 856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spurr JM, Stopa L. Self-focused attention in social phobia and social anxiety. Clin Psychol Rev 2002; 22: 947–75. [DOI] [PubMed] [Google Scholar]
- 70.Mende-Siedlecki P, Said CP, Todorov A. The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Soc Cogn Affect Neurosci 2013; 8: 285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange W-G, Allart E, Keijsers GPJ, Rinck M, Becker ES. A neutral face is not neutral even if you have not seen it: social anxiety disorder and affective priming with facial expressions. Cogn Behav Ther 2012; 41: 108–18. [DOI] [PubMed] [Google Scholar]
- 72.Lange W-G, Rinck M, Becker ES. To be or not to be threatening, but what was the question? Biased face evaluation in social anxiety and depression depends on how you frame the query. Front Psychol 2013; 4: 205–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freitas-Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MHN, Nardi AE, Crippa JAS. Neuroimaging in social anxiety disorder: a systematic review of the literature. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 565–80. [DOI] [PubMed] [Google Scholar]
- 74.Carlin JD, Calder AJ. The neural basis of eye gaze processing. Curr Opin Neurobiol 2013; 23: 450–5. [DOI] [PubMed] [Google Scholar]
- 75.Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci 2007; 19: 1803–14. [DOI] [PubMed] [Google Scholar]
- 76.Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the human brain. J Neurosci 2010; 30: 10127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Said CP, Haxby JV, Todorov A. Brain systems for assessing the affective value of faces. Philos Trans R Soc Lond B Biol Sci 2011; 366: 1660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Straube T, Mentzel H-J, Miltner WHR. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry 2006; 59: 162–70. [DOI] [PubMed] [Google Scholar]
- 79.Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex 2007; 17: 1595–603. [DOI] [PubMed] [Google Scholar]
- 80.Giménez M, Pujol J, Ortiz H, Soriano-Mas C, López-Solà M, Farré M, Deus J, Merlo-Pich E, Martín-Santos R. Altered brain functional connectivity in relation to perception of scrutiny in social anxiety disorder. Psychiatry Res 2012; 202: 214–23. [DOI] [PubMed] [Google Scholar]
- 81.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia 2007; 45: 174–94. [DOI] [PubMed] [Google Scholar]
- 82.Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Front Hum Neurosci 2013; 7: 647–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneider F, Bermpohl F, Heinzel A, Rotte M, Walter M, Tempelmann C, Wiebking C, Dobrowolny H, Heinze HJ, Northoff G. The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience 2008; 157: 120–31. [DOI] [PubMed] [Google Scholar]
- 84.Danti S, Ricciardi E, Gentili C, Gobbini MI, Pietrini P, Guazzelli M. Is social phobia a “mis-communication” disorder? Brain functional connectivity during face perception differs between patients with social phobia and healthy control subjects. Front Syst Neurosci 2010; 4: 152–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao X-H, Wang P-J, Li C-B, Hu Z-H, Xi Q, Wu W-Y, Tang X-W. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol 2007; 63: 373–8. [DOI] [PubMed] [Google Scholar]
- 86.Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S, Gong Q, Zhang W. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 2010; 52: 1549–58. [DOI] [PubMed] [Google Scholar]
- 87.Pannekoek JN, Veer IM, van Tol M-J, van der Werff SJA, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SARB, van der Wee NJA. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol 2013; 23: 186–95. [DOI] [PubMed] [Google Scholar]
- 88.Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, Kalin NH. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatry 2013; 18: 700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M. Beyond amygdala: Default mode network activity differs between patients with social phobia and healthy controls. Brain Res Bull 2009; 79: 409–13. [DOI] [PubMed] [Google Scholar]
- 90.Gentili C, Vanello N, Cristea I, David D, Ricciardi E, Pietrini P. Proneness to social anxiety modulates neural complexity in the absence of exposure: A resting state fMRI study using Hurst exponent. Psychiatry Res 2015; 232: 135–44. [DOI] [PubMed] [Google Scholar]
- 91.Machado-de-Sousa JP, Arrais KC, Alves NT, Chagas MH, de Meneses-Gaya C, Crippa JA, Hallak JE. Facial affect processing in social anxiety: tasks and stimuli. J Neurosci Methods 2010; 193: 1–6. [DOI] [PubMed] [Google Scholar]
- 92.Munder T, Brütsch O, Leonhart R, Gerger H, Barth J. Researcher allegiance in psychotherapy outcome research: an overview of reviews. Clin Psychol Rev 2013; 33: 501–11. [DOI] [PubMed] [Google Scholar]
- 93.Munder T, Flückiger C, Gerger H, Wampold BE, Barth J. Is the allegiance effect an epiphenomenon of true efficacy differences between treatments? A meta-analysis. J Couns Psychol 2012; 59: 631–7. [DOI] [PubMed] [Google Scholar]
- 94.Munder T, Gerger H, Trelle S, Barth J. Testing the allegiance bias hypothesis: a meta-analysis. Psychother Res 2011; 21: 670–84. [DOI] [PubMed] [Google Scholar]
- 95.Cooney RE, Atlas LY, Joormann J, Eugène F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res 2006; 148: 55–9. [DOI] [PubMed] [Google Scholar]
- 96.Di X, Biswal BB. Identifying the default mode network structure using dynamic causal modeling on resting-state functional magnetic resonance imaging. Neuroimage 2013; 86: 53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB. Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci 2013; 7: 232–232. [DOI] [PMC free article] [PubMed] [Google Scholar]