Abstract
Carbon monoxide (CO) attenuates lung ischemia reperfusion injury (IRI) via inhalation, and as an additive dissolved in flush/preservation solution. This study observed the effects of lung inflation with CO on lung graft function in the setting of cold ischemia. Donor lungs were inflated with 40% oxygen + 60% nitrogen (control group) or with 500 ppm CO + 40% oxygen + nitrogen (CO group) during the cold ischemia phase and were kept at 4℃ for 180 min. Recipients were sacrificed by exsanguinations at 180 min after reperfusion. Rats in the sham group had no transplantation and were performed as the recipients. Compared with the sham group, the oxygenation determined by blood gas analysis and the pressure–volume curves of the lung grafts decreased significantly, while the wet weight/dry weight (W/D) ratio, inflammatory reaction, oxidative stress, and cell apoptosis increased markedly (P < 0.05). However, compared to the control group, CO treatment improved the oxygenation (381 ± 58 vs. 308 ± 78 mm Hg) and the pressure–volume curves (15.8 ± 2.4 vs. 11.6 ± 1.7 mL/kg) (P < 0.05). The W/D ratio (4.6 ± 0.6) and the serum levels of interleukin-8 (279 ± 46 pg/mL) and tumor necrosis factor-α (377 ± 59 pg/mL) in the CO group decreased significantly compared to the control group (5.8 ± 0.8, 456 ± 63 pg/mL, and 520 ± 91 pg/mL) (P < 0.05). In addition, CO inflation also significantly decreased malondialdehyde activity and apoptotic cells in grafts, and increased the superoxide dismutase content. Briefly, CO inflation in donor lungs in the setting of cold ischemia attenuated lung IRI and improved the graft function compared with oxygen.
Keywords: Carbon monoxide, lung inflation, cold ischemia phase, ischemia reperfusion injury, lung transplantation
Introduction
Donor quality is an important factor for ischemia reperfusion injury (IRI) and lung graft function in lung transplantation (LTx).1 Hypothermia preservation is an effective therapy that improves lung graft function and decreases the incidence of primary graft dysfunction (PGD) after LTx, but many events, including oxidative stress and intracellular calcium overload,2,3 occur in the setting of cold ischemia that result in cell death and the release of inflammatory cytokines, which aggravate IRI.
Methods utilized to protect donor lungs during cold ischemia include lung preservation solutions, such as low-potassium dextran (LPD) solution,1 and different flushing methods, such as antegrade flushing and retrograde flushing.4 Substances may be added to LPD solution to improve donor quality and decrease the incidence of PGD.5,6 Additionally, lung inflation during the cold ischemia phase improved the pulmonary function after reperfusion compared with lung collapse, and lung inflation with oxygen showed protective effects on lung IRI compared with nitrogen.7,8 Studies showed that carbon monoxide (CO) inhalation at 250–500 parts per million (ppm) ameliorated lung injury induced by ischemia reperfusion and mechanical ventilation.9,10 Therefore, the present study was to explore the effects of lung inflation with CO in the setting of cold ischemia on lung graft IRI.
Methods and materials
Animals
All protocols were approved by the Institutional Animal Care and Use Committee of Harbin Medical University. Adult male pathogen-free Sprague-Dawley rats weighing 260–300 g were purchased from Vital River Laboratories (Beijing, China) and were immediately allowed to acclimate to 12 h light/dark cycles in a temperature-controlled room before being used for experiments. The rats were fed a standard diet and were allowed free access to water.
Experimental protocol
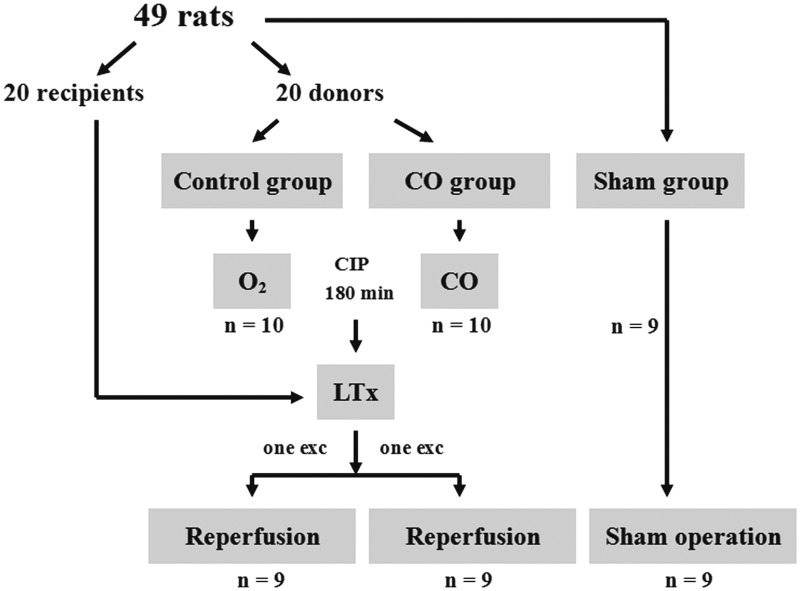
In total, 49 rats including donors and recipients were randomly assigned to one of three groups. During cold ischemia, the donor lungs in the control group were inflated using 40% oxygen + 60% nitrogen, and the donor lungs in the CO group were inflated using 500 ppm CO + 40% oxygen + nitrogen (Liming Gas Corporation, Harbin, China). Donor lungs were kept in 4℃ LPD solution for 180 min. The inflation gas was replaced at doses of 5 mL/kg every 30 min.8 The rats in the sham group were ventilated under the same conditions as the control group and underwent left thoracotomies, but they were not transplant recipients (Figure 1).
Figure 1.
Study design. O2: lung inflation with 40% oxygen; CO: lung inflation with carbon monoxide; CIP: cold ischemia phase; LTx: lung transplantation; exc: exclude
Donor preparation and orthotopic left LTx
Donor rats were anesthetized using sodium pentobarbital (60 mg/kg) and were intubated via tracheotomy. After the injection of sodium heparin (200 U/kg), the donor lungs were flushed with 20 mL 4℃ LPD preservation solution (prepared by Harbin Medical University) with a pressure of 20 cm H2O. The left lungs were subsequently isolated and inflated with the gas mixtures according to the experimental protocol. Orthotopic left LTx was performed using the cuff technique as described previously.9 Recipients were anesthetized, and femoral artery was cannulated for blood pressure monitoring and sample collection. Recipients were ventilated with 40% oxygen + 60% nitrogen (Liming Gas Corporation, Harbin, China) at a tidal volume of 10 mL/kg, but their rates were adjusted to maintain an arterial carbon dioxide tension (PaCO2) of 35–45 mm Hg. Mean arterial blood pressure (MAP) and body temperature were continuously recorded (AS/3, Datex, Helsinki, Finland). Anesthesia and complete muscle relaxation were maintained with additional doses of sodium pentobarbital and pipecuronium bromide (0.4 mg·kg−1·h−1). The recipients were sacrificed 180 min after reperfusion by exsanguination.
CO concentration detection
CO metabolism during cold ischemia in the donor lung was measured in a preliminary experiment. Donor lungs were inflated with 500 ppm CO + 40% oxygen + nitrogen (5 mL/kg), using a 5 mL airtight injector (Agilent Corporation, CA, USA) via a t-junction connected to the endotracheal tube at either 0 min or 150 min following recovery. Gas was withdrawn from the donor lungs using a 1 mL airtight injector after being preserved at 4℃ for 30 min for concentration detection via gas chromatography (4890D, Agilent Corporation). A total of 16 rats were included in the preliminary experiments.
Blood gas analysis
Arterial blood gas analyses were completed before transplantation and following reperfusion. Time points were recorded as T0–T7 in recipients, points that corresponded to baseline (5 min before transplantation) and 3 min, 30 min, 60 min, 90 min, 120 min, 150 min, and 180 min following reperfusion. At the end of the experiment, blood was collected from the left pulmonary vein and used for blood gas analysis (Rapid Lab 348, Bayer, MA).
Measurement of pulmonary P–V curves
Median sternotomies were performed immediately after the recipients euthanized via exsanguination, and the lungs were connected to an apparatus to measure static pressure–volume (P–V) curves. Airway pressure was increased to 30 cm H2O before being decreased to 0 cm H2O in stepwise intervals of 5 cm. Lung volumes were recorded following 1 min of stabilization. Volume measurements were corrected for gas compression in the apparatus.11
Measurement of W/D ratios and inflammatory cytokines
The upper lobes of the lung grafts were desiccated at 80℃ for 72 h to measure wet weight/dry weight (W/D) ratios. The inferior lobes were frozen at −80℃ and homogenized to measure myeloperoxidase (MPO) activity using a special reagent box (Jiancheng Bio-Technology, Nanjing, China) and expressed as units per gram of lung tissue (U/g). Serum levels of interleukin (IL)-8 and tumor necrosis factor (TNF)-α were measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN).
Measurement of oxidation–reduction indicators
Superoxide dismutase (SOD) activity, expressed as units per milligram of protein (U/mg protein), and malonaldehyde (MDA) activity, expressed as nanomoles per milligram of protein (nmol/mg protein) were determined for the supernatants of the graft homogenates using the appropriate kits (Jiancheng Bio-Technology).
Histological examination and scoring
Paraformaldehyde-fixed, paraffin-embedded left lung grafts were cut into 6 µm thick sections and stained with hematoxylin and eosin. All sections were evaluated via light microscopy by a pathologist who was blinded to this study. The evaluation was based on the following criteria: (1) neutrophil infiltrate, (2) airway epithelial cell damage, (3) interstitial edema, (4) hyaline membrane formation, and (5) hemorrhage. Each section had five scores corresponding to the following criteria based on the degree of deterioration: normal = 0, minimal change = 1, mild change = 2, moderate change = 3, and severe change = 4. A lung injury score (LIS) was recorded for each criterion.12
Cellular apoptosis detection
Alveolar epithelial cellular apoptosis was examined using a terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay (Zhongshan Golden Bridge Biotechnology, Beijing, China). The numbers of positive cells per section were counted and evaluated using the apoptotic index (AI). AI is a measure of the number of positive cells for every 100 cells counted in five random high-power (×40) fields in the same section.13 The protein expression levels of caspase-3 in the alveolar epithelial cells were measured using immunohistochemical staining (Zhongshan Golden Bridge Biotechnology, Beijing, China). The numbers of positive cells per section were counted in five random high-power (×40) fields in every specimen to calculate immunohistochemical scores (IHS), which were determined by multiplying the quantity score (an estimation of the percentage of immunoreactive cells) by the staining intensity score (an estimation of the staining intensity). Quantity scores were as follows: no staining was scored a 0; 1–10% of cells were scored as a 1; 11–50% were scored as a 2; 51–80%, a 3; and 81–100%, a 4. Staining intensity was rated on a scale of 0–3: 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. All sections were examined by a pathologist using a single-blind method.14
Statistical analysis
Data were expressed as the mean values ± standard deviations (SDs) or medians (interquartile ranges) based on their distributions. Differences in the measured variables between groups were assessed by one-way analysis of variance (ANOVA) or by the Kruskal–Wallis test. Repeated data measurements were analyzed via repeated-measures ANOVA. P < 0.05 was considered statistically significant.
Results
CO concentrations in donor lungs
The CO concentration in the donor lung was 300 ± 7 ppm at 30 min following inflation with 500 ppm CO at 0 min and 264 ± 7 ppm at 180 min after inflation with 500 ppm CO at 150 min in the setting of cold ischemia. Therefore, the CO concentration in the donor lung was maintained within the 250–500 ppm range for 30 min following inflation.
Experiment-related data
Ten pairs of rats in the control group and CO group underwent surgery, but eventually nine pairs were included in both groups due to failure to separate lung hila; nine rats were included in the sham group. Total ischemia time (including cold ischemia and transplantation time) was 206 ± 10 min in the control group and 207 ± 8 min in the CO group, a difference that was not statistically significant. MAP and heart rates (HRs) were stable among the three groups, and there were no significant differences among the groups.
Blood gas analysis
Blood gas analysis indices, including the oxygenation index, which was equal to the partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2), base excess (BE), and pH value, were stable in the sham group. These indices were lower in the control group compared with the sham group (P < 0.05). However, compared with the control group, the PaO2/FiO2, BE, and pH values of the CO group were significantly higher (P < 0.05, Table 1). Pulmonary venous oxygen tension (PvO2)/FiO2, BE, and pH values from the left pulmonary venous circulation exhibited the same trends as the values listed above (Table 2).
Table 1.
The indices of blood gas analysis in recipients (mean ± SD, n = 9)
| Group | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
|---|---|---|---|---|---|---|---|---|---|
| PaO2/FiO2 (mm Hg) | Sham group | 445 ± 12 | 438 ± 12 | 442 ± 8 | 447 ± 12 | 448 ± 10 | 455 ± 10 | 448 ± 10 | 442 ± 8 |
| Control group | 443 ± 13 | 420 ± 51 | 345 ± 44* | 334 ± 48* | 315 ± 75* | 311 ± 84* | 313 ± 77* | 308 ± 78* | |
| CO group | 440 ± 17 | 426 ± 52 | 358 ± 77* | 362 ± 54* | 388 ± 55*† | 384 ± 56*† | 386 ± 63*† | 381 ± 58*† | |
| pH value | Sham group | 7.40 ± 0.02 | 7.39 ± 0.02 | 7.40 ± 0.01 | 7.39 ± 0.02 | 7.40 ± 0.02 | 7.40 ± 0.02 | 7.38 ± 0.03 | 7.40 ± 0.02 |
| Control group | 7.37 ± 0.05 | 7.38 ± 0.03 | 7.33 ± 0.03 | 7.32 ± 0.04* | 7.27 ± 0.04* | 7.21 ± 0.07* | 7.11 ± 0.05* | 7.12 ± 0.04* | |
| CO group | 7.38 ± 0.04 | 7.39 ± 0.02 | 7.35 ± 0.04 | 7.33 ± 0.04* | 7.33 ± 0.04*† | 7.31 ± 0.04*† | 7.33 ± 0.04*† | 7.34 ± 0.04*† | |
| BE value (mmol/L) | Sham group | 0.10 ± 0.06 | 0.11 ± 0.03 | 0.09 ± 0.03 | 0.11 ± 0.06 | 0.08 ± 0.04 | 0.11 ± 0.03 | 0.10 ± 0.03 | 0.12 ± 0.03 |
| Control group | 0.13 ± 0.03 | 0.13 ± 0.04 | −0.89 ± 0.09 | −2.11 ± 0.29* | −2.57 ± 0.75* | −3.08 ± 0.73* | −3.58 ± 0.60* | −3.92 ± 0.52* | |
| CO group | 0.10 ± 0.05 | 0.12 ± 0.04 | −0.86 ± 0.08 | −2.01 ± 0.27* | −2.17 ± 0.51*† | −2.39 ± 0.71*† | −2.30 ± 0.58*† | −2.15 ± 0.58*† | |
| PaCO2 (mm Hg) | Sham group | 39.7 ± 2.3 | 39.9 ± 2.8 | 40.8 ± 3.0 | 40.0 ± 2.7 | 38.6 ± 2.2 | 40.4 ± 3.6 | 39.5 ± 3.2 | 41.1 ± 2.7 |
| Control group | 41.7 ± 3.2 | 39.5 ± 3.4 | 38.9 ± 2.2 | 39.7 ± 3.2 | 39.4 ± 3.0 | 38.9 ± 2.8 | 40.0 ± 3.4 | 39.1 ± 2.3 | |
| CO group | 39.3 ± 2.6 | 38.8 ± 2.4 | 38.7 ± 2.7 | 38.8 ± 2.7 | 37.7 ± 1.8 | 39.0 ± 1.8 | 39.4 ± 4.0 | 41.1 ± 2.7 |
T0–T7 represented the following time points: the baseline, 3 min, 30 min, 60 min, 90 min, 120 min, 150 min, and 180 min after reperfusion. PaO2/FiO2: partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2); BE: base excess; PaCO2: arterial carbon dioxide tension.
P < 0.05 vs. sham group.
P < 0.05 vs. control group.
Table 2.
The indices of blood gas analysis from pulmonary vein in recipients (mean ± SD, n = 9)
| PvO2/FiO2 (mm Hg) | pH value | BE value (mmol/L) | PCO2 (mm Hg) | |
|---|---|---|---|---|
| Sham group | 433 ± 29 | 7.41 ± 0.03 | 0.09 ± 0.02 | 39.6 ± 2.5 |
| Control group | 322 ± 77* | 7.21 ± 0.04* | −3.07 ± 0.18* | 40.2 ± 2.6 |
| CO group | 387 ± 51† | 7.36 ± 0.05† | −1.50 ± 0.11† | 39.8 ± 3.2 |
PvO2/FiO2: pulmonary venous oxygen tension (PvO2)/fraction of inspired oxygen (FiO2).
P < 0.05 vs. sham group.
P < 0.05 vs. control group.
Inflammatory response and oxidative stress index in recipients
The W/D ratio of the control group was higher than the sham group, and compared with the control group, the W/D ratio of the CO group was lower (P < 0.05, Table 3). Serum levels of IL-8 and TNF-α in the control group were higher than the sham group and these indices were lower in the CO group than the control group (P < 0.05, Table 3). The MPO levels of the control group were higher than the sham group, and the values of the CO group were lower than the control group (P < 0.05, Table 3). In the control group, the MDA levels were significantly higher and SOD activities were significantly lower than those in the sham group (P < 0.05), and compared with the control group, the MDA levels were decreased and SOD activities were increased in the CO group (P < 0.05, Table 3).
Table 3.
The indices of inflammatory response and oxidative stress in each group (mean ± SD, n = 9)
| W/D ratio | MPO (U/g) | IL-8 (pg/mL) | TNF-α (pg/mL) | MDA (nmol/mg prot) | SOD (U/mg prot) | |
|---|---|---|---|---|---|---|
| Sham group | 4.2 ± 0.3 | 0.6 ± 0.2 | 236 ± 32 | 320 ± 42 | 3.4 ± 0.6 | 76 ± 13 |
| Control group | 5.8 ± 0.8* | 1.4 ± 0.6* | 456 ± 63* | 520 ± 91* | 8.1 ± 3.4* | 40 ± 9* |
| CO group | 4.6 ± 0.6† | 0.9 ± 0.3† | 279 ± 46† | 377 ± 59† | 4.9 ± 1.1† | 64 ± 17† |
W/D ratio: wet weight/dry weight ratio; MPO: myeloperoxidase; IL-8: interleukin-8; TNF-α: tumor necrosis factor-α; MDA: malondialdehyde; SOD: superoxide dismutase.
P < 0.05 vs. sham group.
P < 0.05 vs. control group.
Lung injury scores
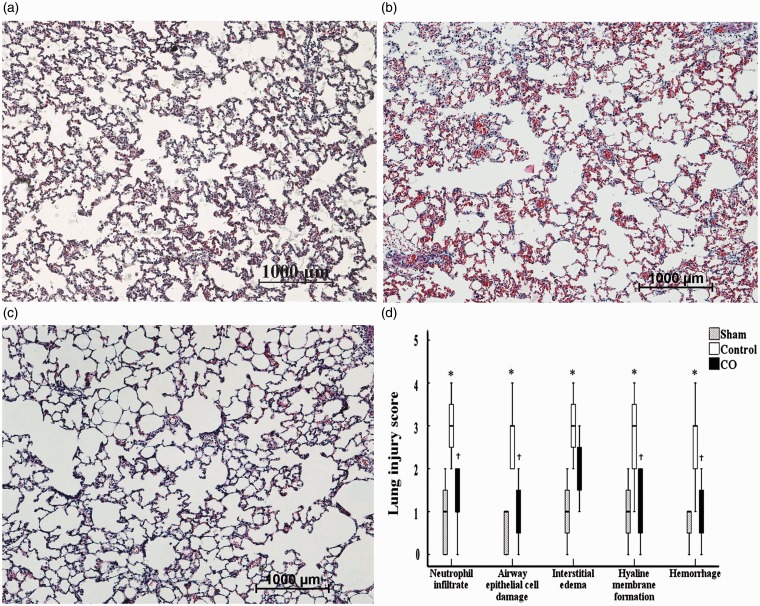
The lung tissues in the sham group were almost completely normal and did not exhibit any interstitial edema or intra-alveolar hemorrhages (Figure 2a). However, numerous abnormalities were noted in the control group, including severe interstitial edema, hyaline membrane formation, and intra-alveolar hemorrhages (Figure 2b); smaller changes were observed in the grafts of the CO group (Figure 2c). Similarly, the LIS pertaining to neutrophil infiltration in the control group (3 [2 to 4]) was higher than that noted in the sham group (1 [0 to 2]) (P < 0.05), and the LIS pertaining to neutrophil infiltration in the CO group (1 [0 to 2]) was lower than the control group (P < 0.05). Similar tendencies were noted for the LIS of the other criteria (Figure 2d).
Figure 2.
Histological analyses of the lung tissues. Paraformaldehyde-fixed sections of the lung grafts were stained with hematoxylin and eosin. a: sham group; b: control group; c: CO group; d: lung injury score (LIS) of every criterion applied to the lung tissues. The figure represents × 10 the original magnification. Lung tissues in the sham group had nearly normal tissue, and the LIS for every criterion were ameliorated in the CO group compared with the control group. *P < 0.05 vs. sham group; †P < 0.05 vs. control group. (A color version of this figure is available in the online journal.)
Lung graft apoptosis
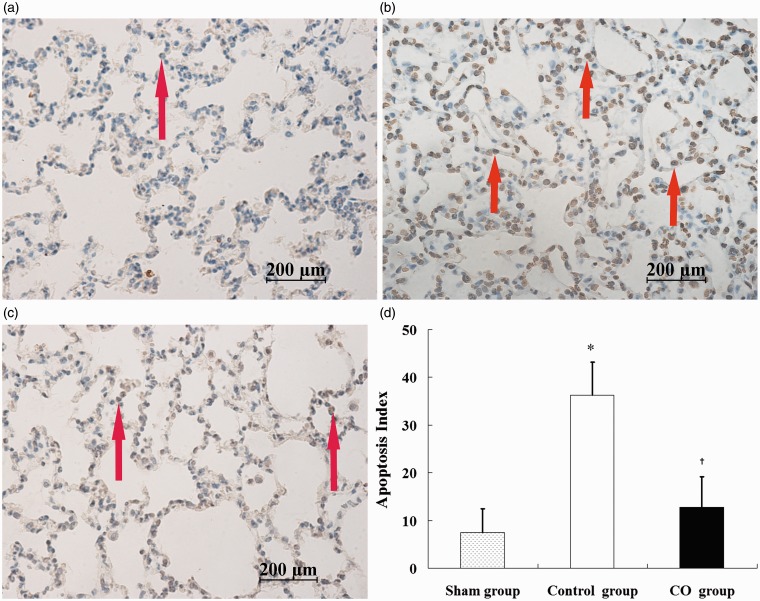
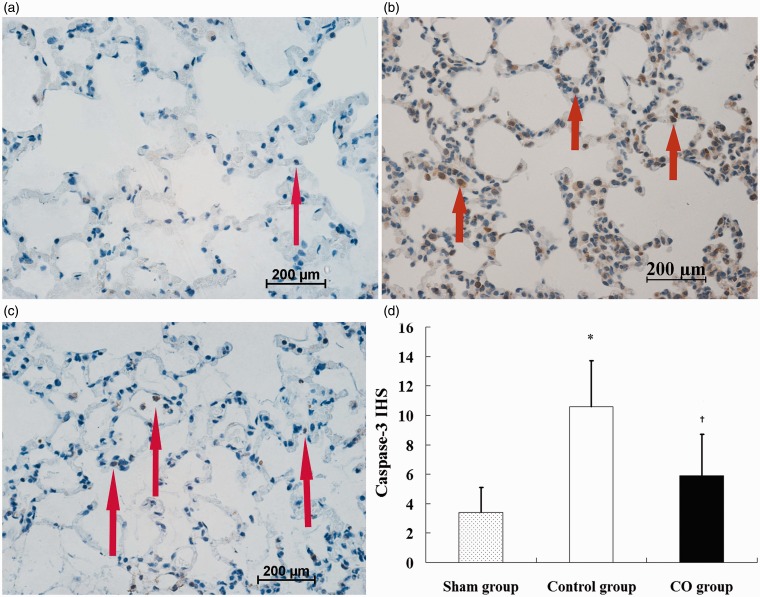
The lung sections in the sham group demonstrated almost no TUNEL positive cells. The numbers of TUNEL positive cells in the control group (Figure 3b) and the CO group (Figure 3c) were higher than the sham group (Figure 3a), and fewer TUNEL positive cells were noted in the CO group compared with the control group. The AI in the control group (36.3 ± 6.9) was significantly higher compared with the sham group (7.5 ± 4.9), and the AI in the CO group (12.8 ± 6.4) was lower compared with the control group (P < 0.05, Figure 3d). Similarly, the IHS of caspase-3 of the control group (10.6 ± 3.1) was higher than the sham group (3.4 ± 1.7), and the IHS of the CO group (5.9 ± 2.8) was significantly lower than the control group (P < 0.05, Figure 4d).
Figure 3.
Alveolar epithelial cellular apoptosis in lung tissues as measured via TUNEL staining. a: sham group; b: control group; c: CO group; d: apoptotic index (AI) of the lung tissues. Figures represent × 40 the original magnification. Data represent five rats. Apoptosis is represented by brown-yellow nuclear staining (arrows). *P < 0.05 vs. sham group; †P < 0.05 vs. control group. (A color version of this figure is available in the online journal.)
Figure 4.
Expression of caspase-3 protein in lung tissues. a: sham group; b: control group; c: CO group; d: immunohistochemical scores (IHS) of caspase-3 protein in lung tissues. Figures represent × 40 the original magnification. Data represent five rats. Positive cells are represented by brown granules in the cytoplasm (arrows). *P < 0.05 vs. sham group; †P < 0.05 vs. control group. (A color version of this figure is available in the online journal.)
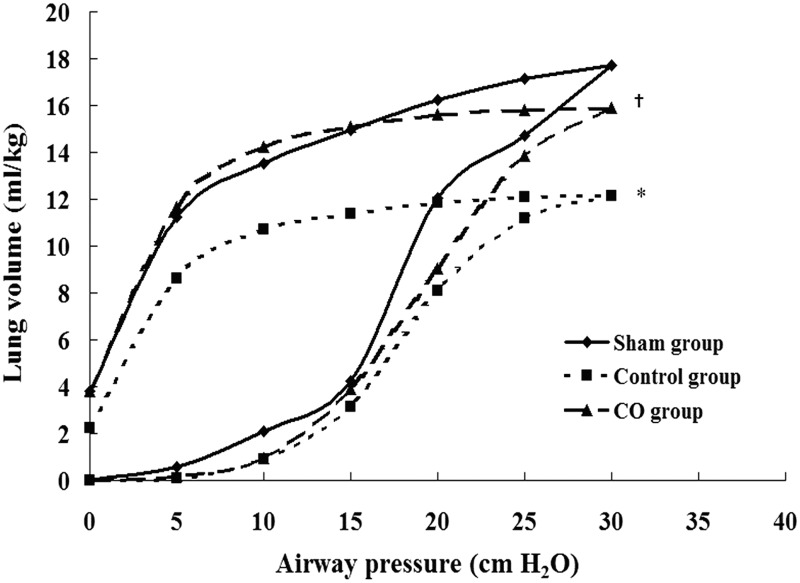
The static P–V curves of the lung grafts
The values of the P–V curves of the control group were significantly lower than the sham group, and the values of the CO group were higher than the control group (P < 0.05). At a pressure of 30 cm H2O, the volumes in the sham group, the control group, and the CO group were 17.4 ± 1.1 mL/kg, 11.6 ± 1.7 mL/kg, and 15.8 ± 2.4 mL/kg, respectively (Figure 5).
Figure 5.
Static pressure–volume (P–V) curves of lung tissues. Values are means: standard deviation bars were omitted for clarity. Data represent four rats. The values of the P–V curves of the control group were lower than those of the sham group, and the values of the P–V curves of the CO group were significantly improved compared with those of the control group. *P < 0.05 vs. sham group; †P < 0.05 vs. control group
Discussion
In this study, donor lungs were inflated with 500 ppm CO in the setting of cold ischemia, which resulted in reduced W/D ratios, LIS, inflammatory responses, oxidant stress, and cell apoptosis in the lung grafts. The inflation with CO also ameliorated lung graft injury as evaluated by histopathology and improved lung graft function as evaluated by oxygenation and P–V curves. Previous studies found that CO inhalation in the donors for a short time had a long protective effect on the recipients.15,16 Therefore, we inferred that CO inflation during the cold ischemia phase might have a long duration of protection. In this study, the oxygenation index in the control group was larger than 300 mm Hg and the rats in the control group had no PGD according to previous study,9 the cause might be the donor lung had no injury before harvested. Therefore, CO inflation in donor lung from no heart beating donors or brain dead donors might be more meaningful.
The lungs are a unique organ system and enable oxygen to be derived from the alveoli, in addition to the circulation. CO is capable of acting as a second messenger and moves freely through the alveolar membrane.17 This study obtained an initial data about CO metabolism in the donor lung and provided an innovative strategy for donor lung protection in the setting of cold ischemia, that is, CO inflation in donor lung. Compared with CO inhalation, CO inflation may represent a simple and feasible method of donor protection, especially in the process of long-distance transport. The CO concentration was maintained in therapeutic concentration (250–500 ppm9,10) when replaced every 30 min during this study. Additionally, CO has already been used in clinical treatments such as endotoxemia and chronic obstructive pulmonary diseases,18,19 but the dosage was lower and the applied time was shorter. Therefore, the method used in this study should be explored further before being applied in clinical practice.
The release of pro-inflammatory cytokines (e.g. IL-8 and TNF-α) played a critical role in IRI.20 Kohmoto et al.9 reported that CO (250 ppm) inhalation decreased the upregulation of TNF-α and IL-6 mRNA in lung grafts. Song et al.21 found that CO inhalation decreased MPO activity in lung grafts, as well as the expression of IL-6 mRNA induced by ischemia reperfusion in serum. In this study, the inflammatory mediators (IL-8 and TNF-α) in the serum decreased during CO inflation, and MPO activity, an index of neutrophil infiltration,21 decreased in the CO group. Therefore, CO inflation in the donor lungs in the setting of cold ischemia exerted protective effects against IRI in grafts via local and systemic anti-inflammatory effects.
Oxidative stress induced by IRI causes tissue lipid peroxidation and uncontrollable reactive oxygen species (ROS) generation, leading to a ROS imbalance and cell dysfunction, which eventually result in cellular necrosis and organ failure.22 MDA is a common indicator of oxidative damage, and SOD is an important antioxidant. In this study, both SOD activity and the inhibition of MDA activity were noted in the lung grafts of the CO group, indicating that lung graft lipid peroxidation was alleviated. These results were consistent with those of our previous study.11 We determined that CO inhalation ameliorated oxidant stress in the setting of lung graft injury involving tissues obtained from brain-dead donors and also improved lung graft function. Additionally, Liu et al.23 reported that CO inhalation (250 ppm) increased SOD activity and decreased MDA levels in lipopolysaccharide-induced lung injury in rats. Wang et al.24 found that CO treatment ameliorated hyperoxia-induced lung injury and inhibited ROS formation. Therefore, the protective effects of CO inflation in the setting of cold ischemia are related to its antioxidant effects.
Apoptosis plays a critical role in lung dysfunction, and the levels of alveolar epithelial apoptotic cells were increased in the lung grafts in the setting of cold ischemia.25 Additionally, inflammatory responses and subsequent tissue injury may be aggravated by apoptotic cells. Zhang et al.26 found that cell apoptosis was decreased by CO treatment in the setting of lung IRI. Wang et al.24 also found that CO protected against hyperoxia-induced cellular apoptosis. In this study, the numbers of apoptotic cells in alveolar epithelial cells detected by caspase-3 protein expression and TUNEL decreased during CO inflation in the setting of cold ischemia. Therefore, the inhibition of apoptosis may be an additional mechanism by which CO protects against IRI.
The exact signaling pathways in which CO is involved, as well as its molecular targets, have not been fully elucidated. Several signaling pathways have been studied in an effort to uncover evidence of CO’s protective effects against lung injury. CO-mediated decreases in lung IRI have been linked to p38 mitogen-activated protein kinase (MAPK),26 as well as the activated signal transducers and activators of transcription (STAT) 3 pathway in epithelial cells in hyperoxia-induced lung injury models.27 Additionally, heme oxygenase-1 (HO-1), a molecule with potent antioxidant properties, has been linked to CO’s protective effects in the setting of lung IRI.27 In our previous study, CO inhalation exerted protective effects via p38-MAPK in lung grafts obtained from brain-dead donors.28 However, the underlying mechanisms by which CO inflation acts were not explored in this study.
This study had several limitations. First, only 3 h elapsed during cold ischemia and 3 more hours elapsed following reperfusion; a longer period of time may be required to observe the long-term effects of lung inflation with CO on lung graft function. Second, only one CO concentration was used in this study. Whether different CO concentrations exert different effects should be explored, and the optimal CO concentration should be determined. It is also needed to be confirmed the role of CO in the air in future. Third, the levels of CO in the grafts or carboxyhemoglobin (COHb) in the blood were not evaluated, but a previous study showed that CO inhalation at low concentration had no systemic sequelae.9 At last, the specific mechanism by which CO confers lung protection was not clarified, and further experiment, such as isolated cell culture, are required.
In conclusion, donor lungs inflated with CO in the setting of cold ischemia released lung IRI and improved the graft function compared with oxygen, and the protective effects exerted by CO were associated with anti-inflammatory mediators, anti-oxidative, and anti-apoptotic effects.
Acknowledgment
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the China Postdoctoral Science Foundation (Grant 201104453) and the National Nature Science Foundation of China (Grant 30901391).
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data, review of the manuscript, and approval the version to be published. CM, RL, JX, and HZ conducted the experiments and collected the data, LM, XC, and HZ supplied critical reagents and analysis of the data, CM, JL, and HZ wrote the manuscript.
References
- 1.Simões EA, Cardoso PF, Pêgo-Fernandes PM, Canzian M, Pazetti R, Braga KA, Nepomuceno NA, Jatene FB. An experimental rat model of ex vivo lung perfusion for the assessment of lungs regarding histopathological findings and apoptosis: low-potassium dextran vs. histidine-trytophan-ketoglutarate. J Bras Pneumol 2012; 38: 461–9. [DOI] [PubMed] [Google Scholar]
- 2.Kayano K, Toda K, Naka Y, Pinsky DJ. Identification of optimal conditions for lung graft storage with Euro-Collins solution by use of a rat orthotopic lung transplant model. Circulation 1999; 100: II257–61. [DOI] [PubMed] [Google Scholar]
- 3.Pickford MA, Gower JD, Doré C, Fryer PR, Green CJ. Lipid peroxidation and ultrastructural changes in rat lung isografts after single-passage organ flush and 48-hour cold storage with and without one-hour reperfusion in vivo. Transplantation 1990; 50: 210–8. [DOI] [PubMed] [Google Scholar]
- 4.Bitu-Moreno J, Francischetti I, Siemer R, Matheis G, Baretti R, Maffei FH, Kreitmayr B, Beyersdorf F. Influence of different routes of flush perfusion on the distribution of lung preservation solutions in parenchyma and airways. Eur J Cardiothorac Surg 1999; 15: 481–9. [DOI] [PubMed] [Google Scholar]
- 5.Sommer SP, Gohrbandt B, Fischer S, Hohlfeld JM, Warnecke G, Avsar M, Strüber M. Glutathione improves the function of porcine pulmonary grafts stored for twenty-four hours in low-potassium dextran solution. J Thorac Cardiovasc Surg 2005; 130: 864–9. [DOI] [PubMed] [Google Scholar]
- 6.de Perrot M, Fischer S, Liu M, Jin R, Bai XH, Waddell TK, Keshavjee S. Prostaglandin E1 protects lung transplants from ischemia-reperfusion injury: a shift from pro- to anti-inflammatory cytokines. Transplantation 2001; 72: 1505–12. [DOI] [PubMed] [Google Scholar]
- 7.Sakuma T, Tsukano C, Ishigaki M, Nambu Y, Osanai K, Toga H, Takahashi K, Ohya N, Kurihara T, Nishio M, Matthay MA. Lung deflation impairs alveolar epithelial fluid transport in ischemic rabbit and rat lungs. Transplantation 2000; 69: 1785–93. [DOI] [PubMed] [Google Scholar]
- 8.van der Kaaij NP, Kluin J, Lachmann RA, den Bakker MA, Lambrecht BN, Lachmann B, de Bruin RW, Bogers AJ. Alveolar preservation with high inflation pressure and intermediate oxygen concentration reduces ischemia-reperfusion injury of the lung. J Heart Lung Transplant 2012; 31: 531–7. [DOI] [PubMed] [Google Scholar]
- 9.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, Billiar TR, Choi AM, Murase N, McCurry KR. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery 2006; 140: 179–85. [DOI] [PubMed] [Google Scholar]
- 10.Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflamatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med 2004; 170: 613–20. [DOI] [PubMed] [Google Scholar]
- 11.Zhou H, Qian H, Liu J, Zhu D, Ding W, Pan P, Jin D, Wang J, Li W. Protection against lung graft injury from brain-dead donors with carbon monoxide, biliverdin, or both. J Heart Lung Transplant 2011; 30: 460–6. [DOI] [PubMed] [Google Scholar]
- 12.Pirat A, Zeyneloglu P, Aldemir D, Yücel M, Ozen O, Candan S, Arslan G. Pretreatment with simvastatin reduces lung injury related to intestinal ischemia-reperfusion in rats. Anesth Analg 2006; 102: 225–32. [DOI] [PubMed] [Google Scholar]
- 13.Lu MP, Du LZ, Gu WZ, Chen XX. Nitric oxide inhalation inhibits inducible nitric oxide synthase but not nitrotyrosine formation and cell apoptosis in rat lungs with meconium-induced injury. Acta Pharmacol Sin 2005; 26: 1123–9. [DOI] [PubMed] [Google Scholar]
- 14.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000; 89: 2637–45. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein pinase pathway and involves caspase 3. J Biol Chem 2003; 278: 1248–58. [DOI] [PubMed] [Google Scholar]
- 16.Sahara H, Shimizu A, Setoyama K, Okumi M, Oku M, Samelson-Jones E, Yamada K. Carbon monoxide reduces pulmonary ischemia–reperfusion injury in miniature swine. J Thorac Cardiovasc Surg 2010; 139: 1594–601. [DOI] [PubMed] [Google Scholar]
- 17.Siriussawakul A, Chen LI, Lang JD. Medical gases: a novel strategy for attenuating ischemia-reperfusion injury organ transplantation? J Transplant 2012; 2012: 819382–819382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, Wagner O, Jilma B. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med 2005; 171: 354–60. [DOI] [PubMed] [Google Scholar]
- 19.Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J 2007; 30: 1131–7. [DOI] [PubMed] [Google Scholar]
- 20.Lemay S, Rabb H, Postler G, Singh AK. Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation 2000; 69: 959–63. [DOI] [PubMed] [Google Scholar]
- 21.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, Choi AM. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol 2003; 163: 231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta 2006; 1762: 256–65. [DOI] [PubMed] [Google Scholar]
- 23.Liu SH, Ma K, Xu B, Xu XR. Carbon monoxide inhalation protects lung from lipopolysaccharide induced injury in rat. Sheng Li Xue Bao 2006; 58: 483–9. [PubMed] [Google Scholar]
- 24.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 2007; 282: 1718–26. [DOI] [PubMed] [Google Scholar]
- 25.Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JF, Keshavjee S. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med 2000; 162: 1932–9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and bcl-2 family proteins via the p38 mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem 2003; 278: 22061–70. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Shan P, Jiang G, Zhang SS, Otterbein LE, Fu XY, Lee PJ. Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J 2006; 20: 2156–8. [DOI] [PubMed] [Google Scholar]
- 28.Zhou H, Liu J, Pan P, Jin D, Ding W, Li W. Carbon monoxide inhalation decreased lung injury via anti-inflammatory and anti-apoptotic effects in brain death rats. Exp Biol Med 2010; 235: 1236–43. [DOI] [PubMed] [Google Scholar]