Abstract
The expression of hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channel isoforms varies among species, cardiac tissues, developmental stages, and disease generation. However, alterations in the HCN channels during aging remain unclear. We investigated the protein expressions of HCN channel isoforms, HCN1-HCN4, in the sinoatrial nodes (SANs) from young (1-month-old), adult (4-month-old), and aged (30-month-old) rats. We found that HCN2 and HCN4 proteins were present in rat SAN using immunohistochemistry; therefore, we quantitatively analyzed their expression by Western blot. Aim to correlate protein expression and pacemaking function, specific blockade of HCN channels with 3 µmol/L ivabradine prolonged the cycle length in the intact rat heart. During the senescent process, the HCN2 and HCN4 protein levels declined, which was accompanied with a decreased effect of ivabradine on rat SAN automaticity. These results indicated the age-associated expression and relative function of HCN channel isoforms.
Keywords: Aging, sick sinus syndrome, ion channels, sinoatrial node
Introduction
Sick sinus syndrome (SSS) is commonly seen in the aged population.1 The aged sinoatrial node (SAN) has a reduced velocity of diastolic and phase 0 depolarization, with a lengthened action potential duration,2,3 manifesting in a decreased SAN function with a reduced intrinsic heart rate (IHR), prolonged sinoatrial conduction time (SACT), and sinoatrial node recovery time (SNRT).4 However, the underlying age-associated electrical remodeling of SAN remains uncertain.
Diverse data support a specific role of pacemaker current (If) carried by the hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channel in pacemaking and heart rate control. The specific HCN channel blocker, ivabradine, acting as a “pure heart rate reducing” agent, is used today as a therapeutic tool in chronic stable angina and heart failure.5 Also, the pacemaking capability of HCN channels has been the main rationale behind the development of “biological” pacemakers, whose aim is to eventually replace the electronic pacemakers implanted today.6 In human, HCN dysfunction can result in the genesis of symptomatic sinus bradycardia, tachycardia–bradycardia syndrome, atrioventricular node block, atrial fibrillation, etc. However, the precise expression and function of each HCN channel isoform related to physiology and pathology remain debated.
The mammalian genome encodes four HCN channels, that is HCN1–HCN4, which share ∼80% of homology and differ mainly in activation kinetics and sensitivity to cAMP.7–10 The expression of these HCN channel isoforms assumes species, developmental, and tissue heterogeneity.7,11 In adult SAN, HCN4 is the dominant HCN isoform of all species investigated including rabbit, murine, and canine, accounting for ∼80% of the total HCN message.12,13 HCN1 takes up 18% of the total HCN mRNA in rabbit SAN13 but only at very low level in mouse SAN.12 HCN2 transcripts are also expressed in mouse SAN. However, they are equally distributed among atria and SAN at a low to moderate level, unlike the dominant distribution of HCN4 transcripts in the nodal region.12 In addition, the dynamic transcriptions of HCN channel isoforms during normal cardiac development and disease generation have been revealed. During mouse cardiac development, embryonic atrium and ventricle revealed abundant HCN4 transcription but other HCN transcripts were almost absent. Toward birth, HCN4 was downregulated in the atrium and almost vanished from the ventricle. At postnatal day 10, HCN4 was highest in the SAN, being twofold higher than HCN1 and fivefold higher than HCN2. This developmental and tissue-specific transcription pattern of HCN isoforms may be required to establish and ensure a stable heart rhythm and to prevent atrial and ventricular arrhythmogenesis in adult.14 In hypertrophied or failing hearts, transcripts of HCN2 and HCN4 were upregulated in working myocardium, which may be related to a higher incidence of arrhythmias.15,16 All above findings lead to the hypothesis that an age-associated expression of HCN channel isoforms may trigger, at least in part, the declined function of aging SAN.
This set of experiments is aimed to identify the age-associated expression of the HCN channel isoforms at the protein level in rat SAN. Our results indicate that the HCN2 and HCN4 isoforms are expressed in SAN cells, but they are virtually absent in the surrounding atrial myocytes. During the senescent process, the HCN2 and HCN4 protein levels declined, which was accompanied with a decreased effect of ivabradine, a blocker of HCN channel, on rat SAN automaticity.
Materials and methods
Animal preparation
Animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals issued by the Institute of Laboratory Animal Resources (NIH publication 85-23, revised 1996). The study was also approved by the Xi’an Jiaotong University Animal Care and Use Committee.
Male Sprague-Dawley rats, which were one (young) and four months old (adult), were obtained from the Xi’an Jiaotong University Laboratory Animal Center, and 30-month-old rats (aged) were purchased from Shanghai Laboratory Animal Center, Chinese Academy Sciences (Shanghai, China). All rats were housed in a temperature-controlled room under a 12 h/12 h light/dark and were allowed access to standard rat chow and tap water ad libitum.
SAN function
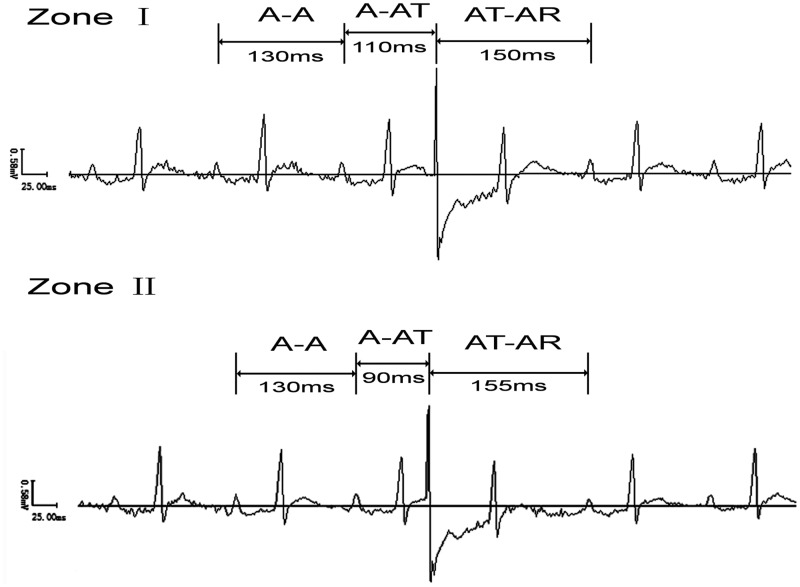
ECGs were recorded under anesthesia with sodium pentobarbitone (40 mg/kg, i.p., Sigma, USA). The stimuli were applied to the right atrial appendage by a bipolar stimulus through the esophagus. SACT was tested by premature atrial stimulation reported by Strauss et al.17 The stimuli were introduced late in atrial diastole and moved progressively earlier in 3–5 ms increments, until the entire atrial diastolic period was scanned. When the spontaneous sinus cycle was interrupted by an atrial premature depolarization (APD), the following atrial intervals were measured: (1) spontaneous sinus cycle length (CL) (A-A), i.e. the interval between the last two spontaneous sinus P waves that preceded the APD; (2) the test cycle (A-AT), i.e. the interval between the last spontaneous sinus P wave and the APD; (3) the return cycle (AT-AR), i.e. the interval between the APD and the following spontaneous sinus P wave. Earlier in atrial diastole, not only did progressive shortening of the test cycle (A-AT) fail to result in reciprocal lengthening of the return cycle (AT-AR) but the return cycle duration remained nearly constant. The return cycle was no longer fully compensatory but was greater than one spontaneous sinus cycle (A-A). This change in return cycle response marked the transition from zone I to zone II. Then the difference between AT-AR and A-A reflects conduction time into and out of the SAN. The SACT was calculated to be approximately the product of one half the distance. SNRT was obtained by 30 s of overdrive pacing, beginning at a frequency of 6.5 Hz, with a 0.5 Hz increment, until the SNRT was no longer lengthened.18 The longest time interval from the last paced atrial depolarization to the first spontaneous sinus cycle was designated as the SNRTmax. The rate-corrected maximal sinoatrial node recovery time (CSNRTmax) was determined by subtracting the spontaneous sinus cycle from the SNRTmax.
The IHR in vivo
The IHR was tested under complete blockade of the cardiac autonomic nervous system with propranolol (3 mg/kg/mL i.v., Sigma, USA) and atropine (2 mg/kg/mL i.v., Sigma, USA) as previously reported.19 Additionally, the IHR was recorded and averaged for 1 min.
The CL in Langendorff hearts
Excised hearts were quickly mounted on a Langendorff apparatus at a constant pressure of 80 mmHg with warm (36 ± 0.5℃), oxygenated, modified Krebs–Henseleit bicarbonate (KHB) buffer as described previously.20 The composition of the modified KHB was as follows: (in mmol/L) NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, glucose 11.0, CaCl2 2.0, pyruvate 2.0, NaHCO3 25, and insulin 5 U/L (PH 7.4 ± 0.5). Two recording electrodes were attached to the atrial appendages, and data were sampled with a BL-420 Electrophysiolograph (Chengdu Taimeng Technology Co., Ltd, Chengdu, Sichuan, China). Electrocardiographies were recorded at baseline and at the end of 25 min perfusion of 3 µmol/L ivabradine (Sigma, USA) which demonstrated to reduce about 60% If without obvious effect on T-type and L-type calcium current and delayed rectifier potassium current21; subsequently, the ivabradine was washed out with KHB buffer for 25 min. After perfusion, heart samples were removed, blotted dry, and weighed. The CL was averaged over 3 min.
Immunohistochemistry
As previously reported,22 the leading pacemaker site recognized by optical mapping was isolated, fixed, dehydrated, and embedded in optimal cutting temperature compound (Tissue-Tek, NL). Frozen 10 µm sections were serially cut across the leading pacemaker site perpendicular to the crista terminalis. Serial sections were labeled with HE and Masson stains, rabbit polyclonal antibodies against connexin 43 (Cx43) (1:2000; Sigma, USA), and the hyperpolarization-activated cyclic nucleotide-gated potassium channel 1-4 (HCN1-4) (1:200; Alomone Labs, Israel), respectively. The FITC-conjugated goat anti-rabbit IgG (Zhongshan Golden Bridge Biotechnology Corporation, Beijing, China) was used as the secondary antibody with a dilution factor of 1:100. For positive control experiments, rat brain tissue was used. For the negative control experiments, the primary antibody was substituted by horse serum (4%) alone. The sections were analyzed under a Leica TCS SP 2 laser scanning confocal microscope (Leica, Germany) in a blinded manner by two senior pathologists from Pathology Department of First Affiliated Hospital of Xi’an Jiaotong University.
Western blot assay
Membrane proteins were extracted from the leading pacemaker site of SAN, by centrifugation on a sucrose buffer (including protease inhibitor cocktail) according to the protocol published by Alomone Labs. Western blot was performed as described previously.23 The rabbit polyclonal antibodies to rat HCN2 and HCN4 (1:200 dilution, respectively) or mouse monoclonal antibody to rat β-actin (1:2000 dilution, Santa Cruz Biotechnology, Inc., Texas, USA) were used as the primary antibodies. The goat anti-rabbit IRDye® 800CW or goat anti-mouse IRDye® 680 LT secondary antibody (1:2000 dilution) (Li-COR Biosciences, NE, USA) was used as the secondary antibodies. Bands were then detected by using Odyssey for Infrared Fluorescent Western Blots from Li-COR Bioscience (Lincoln, NE). Quantitative analysis of the Western blot results was performed with the Odyssey software 3.0 (Li-COR Bioscience, Lincoln, Nebraska). For positive control experiments, membrane proteins of rat brain tissue were used. For the negative control experiments, the primary antibody was substituted by phosphate buffered saline alone.
Statistical analysis
Data are expressed as the means ± SEM. The significance of difference was determined by analysis of variance and a post hoc multiple comparison test using the SPSS 11. 0 software. Statistical significance was established at p < 0.05.
Results
Age-associated changes of the physical characteristics of SAN
The intrinsic SAN function was examined in three ages of rats, young, adult, and aged, with recording of SACT and CSNRT under autonomic blockage (n = 16 for each age group). Figure 1 shows a representative measurement trace of SACT by atrial premature stimulation through esophagus in vivo from the young group. The stimuli were introduced earlier in atrial diastole when progressive shortening of the test cycle (A-AT) failed to result in reciprocal lengthening of the return cycle (AT-AR) but the return cycle duration remained nearly constant. The return cycle was no longer fully compensatory but was greater than one spontaneous sinus cycle (A-A). This change in return cycle response marked the transition from zone I to zone II. Then the difference between AT-AR and A-A reflects conduction time into and out of the SAN. SACT = [(AT-AR)-(A-A)]/2. The SACT and CSNRTmax were significantly lengthened from youth to senescence. The resting heart rate (RHR) and IHR, which reflect the intrinsic SAN activity, were also decreased with increasing age (Table 1).
Figure 1.
Measurement of sinoatrial conduction time (SACT) by premature atrial stimulation through esophagus in vivo (from the young group). The stimuli were introduced earlier in atrial diastole when progressive shortening of the test cycle (A-AT) failed to result in reciprocal lengthening of the return cycle (AT-AR) but the return cycle duration remained nearly constant. The return cycle was no longer fully compensatory but was greater than one spontaneous sinus cycle (A-A). This change in return cycle response marked the transition from zone I to zone II. Then the difference between AT-AR and A-A reflects conduction time into and out of the SAN
Table 1.
Age-associated changes of the physical characteristics and the SAN function
| Characteristics | Young | Adult | Senescent |
|---|---|---|---|
| BW (g) | 50 ± 1.2 | 350 ± 1.5* | 500 ± 4.7# |
| HW (mg) | 292 ± 14 | 1236 ± 11* | 1797 ± 15# |
| HBWR (mg/g) | 5.91 ± 0.04 | 3.36 ± 0.03* | 3.52 ± 0.03 |
| RHR (bpm) | 530 ± 7 | 382 ± 5* | 331 ± 4# |
| IHR (bpm) | 430 ± 4 | 310 ± 2* | 255 ± 5# |
| SACT (ms) | 12.5 ± 0.6 | 17.7 ± 0.2* | 22. 5 ± 0.4# |
| CSNRTmax (ms) | 33.9 ± 1.0 | 50.7 ± 3.0* | 78.1 ± 4.9# |
BW: body weight; CSNRTmax: rate-corrected maximal sinoatrial node recovery time; HBWR: heart-to-body weight ratio; HW: heart weight; IHR: intrinsic heart rate; RHR: resting heart rate; SACT: sinoatrial conduction time.
Mean ± SEM, n = 16, *P < 0.05 versus young group; #P < 0.05 versus adult group.
Age-associated effect of ivabradine on the CL in Langendorff hearts
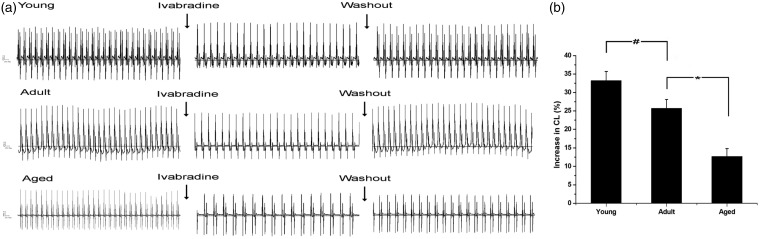
A pseudo-ECG was recorded from the surface of the left and right atrial appendages (Figure 2(a)). The physiological significance of HCN channels in automaticity was analyzed with the application of ivabradine, which is known to inhibit If specifically. Three µmol/L ivabradine significantly prolonged CL by 33.2 ± 2.5, 25.7 ± 2.4, and 12.7 ± 2.1% in the young, adult, and aged rats, respectively (p < 0.001 versus age-matched control group, n = 5) (Figure 2(b)).
Figure 2.
Effect of ivabradine on the cycle length (CL) in Langendorff hearts from the young, adult, and aged rats. (a) Representative electrocardiogram recorded before, during, and after (washout with KHB buffer) 3 µmol/L ivabradine perfusion. (b) Declined effect of ivabradine on CL with age. #p < 0.05 versus the young group; *p < 0.001 versus the adult group; n = 5
Expression of HCN channel isoforms in rat SAN
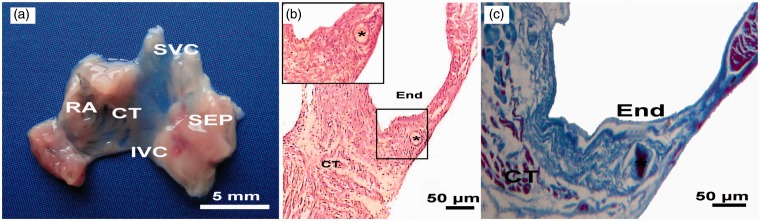
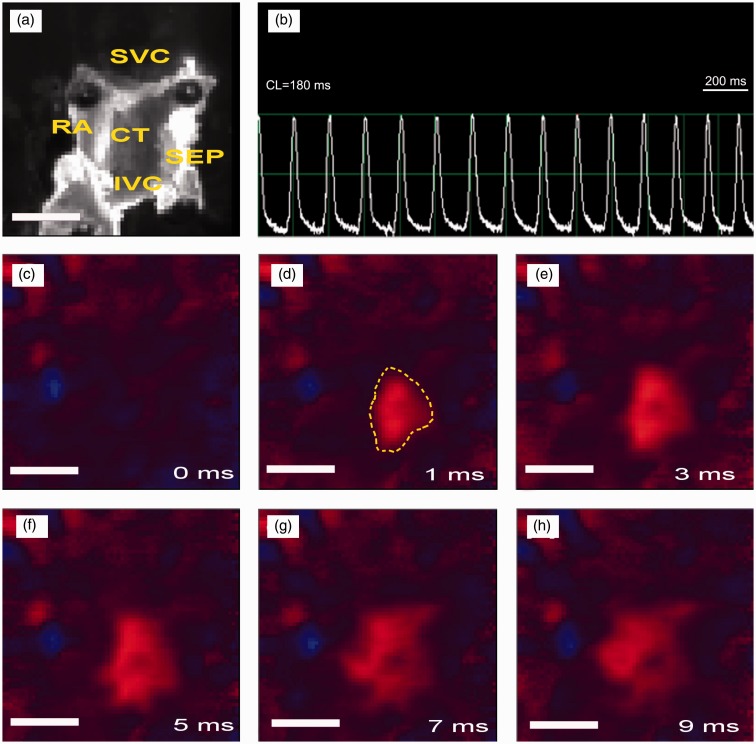
The rat SAN is located in the posterior wall of the right atrium; a coarse anatomical definition identifies this structure as the intercaval region adjacent to the atrial muscle of the crista terminalis, extending from the superior to near the inferior vena cava (Figure 3(a)). With HE staining, the SAN cells are small and empty compared to the surrounding atrial cells (Figure 3(b)). Masson staining reveals a heterogeneous architecture with myocytes that are sparsely distributed in a dense connective matrix. A characteristic feature of the SAN is substantial connective tissue, mainly collagen and fibroblasts (Figure 3(c)).
Figure 3.
Histology of rat SAN tissue (from the young group). (a) The image of the raw SAN preparation. (b) HE staining of the SAN tissue. (c) Masson staining of the SAN tissue. * indicates SAN artery. CT: crista terminalis; End: endocardium; IVC: inferior vena cava; RA: right atrium; SEP: septum; SVC: superior vena cava. (A color version of this figure is available in the online journal.)
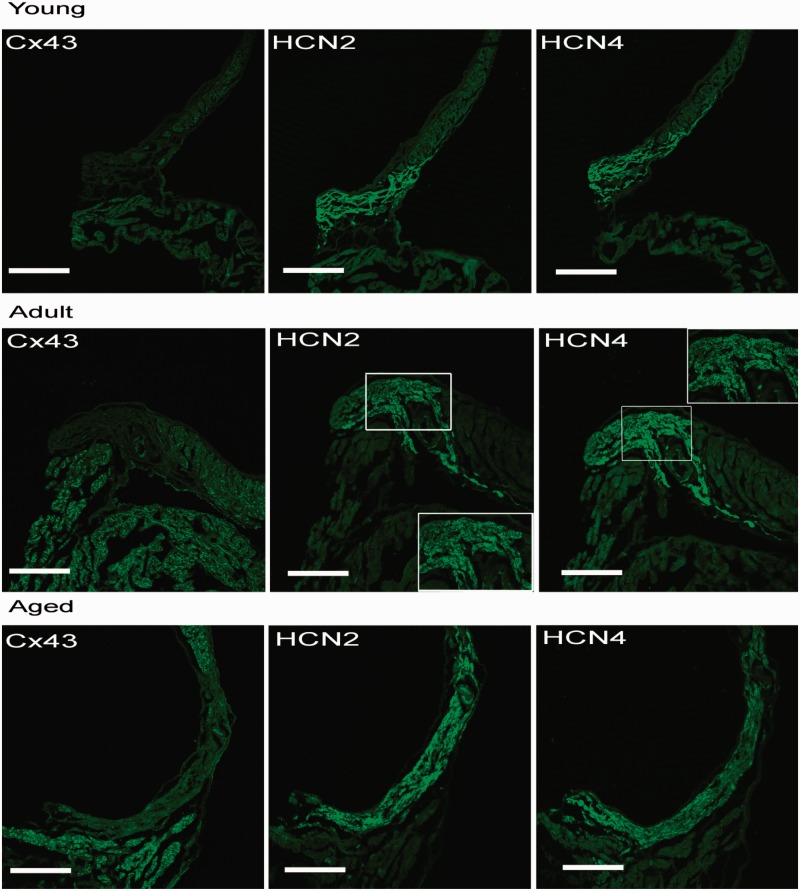
SAN cells lack Cx43. Instead, adjacent atrial working myocytes take on positive staining of Cx43. As illustrated in Figure 4, the SAN tissues have robust HCN2 and HCN4 staining with negative HCN1 and HCN3 staining (data not shown).
Figure 4.
Confocal images of serial sections of SAN tissues labeled with Cx43, HCN2, and HCN4 from the young, adult, and aged rats. SAN tissues have robust HCN2 and HCN4 staining. Scale bar = 300 µm. (A color version of this figure is available in the online journal.)
Age-associated expression of HCN channel isoforms in rat SAN
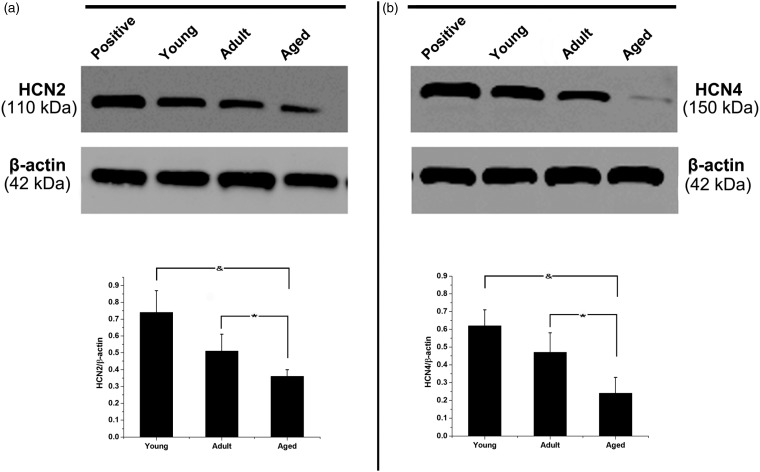
The pacemaker activity and activation conduction were recorded by voltage-sensitive fluorescent signals from 64 × 64 sites simultaneously at a rate of 910 frames/s over the SAN and adjacent atrium (Figure 5). The mapping field was 20 mm × 20 mm. Only the leading pacemaker site as surrounded by yellow line in Figure 5(d) was harvested for further Western blot assay. As shown in Figure 6, HCN2 and HCN4 in the SAN were further evaluated by Western blot analysis. The protein expression of HCN2 was reduced from 0.74 ± 0.13 in the young rats to 0.51 ± 0.10 in the adult rats and then further decreased to 0.36 ± 0.04 in the aged rats (aged versus young, p < 0.01; aged versus adult, p < 0.05; n = 20 for each group). Similarly, the HCN4 expression in the SAN was also decreased during aging (from [0.62 ± 0.09] in the young rats to [0.47 ± 0.11] in the adult rats and then to [0.24 ± 0.09] in the aged rats) (aged versus young, p < 0.01; aged versus adult, p < 0.05; n = 20 for each group).
Figure 5.
Optical mapping over rat SAN preparation (from the young group). (a) Image of the raw SAN preparation. CT: crista terminalis; IVC: inferior vena cava; RA: right atrium; SEP: septum; SVC: superior vena cava. (b) Representative optical signals recorded at the leading pacemaking site. CL = cycle length. (c to h) Activation sequence over SAN from 0 to 9 ms. The area surrounded by yellow line in d is the leading pacemaker site which was harvested for further Western blot assay. Scale bar = 5 mm. (A color version of this figure is available in the online journal.)
Figure 6.
Western blot assays of HCN2 and HCN4 for each group. The HCN2 (a) and HCN4 (b) protein expression levels were reduced with aging. Membrane proteins of rat brain tissue were used as positive control. &indicates p < 0.01 versus the young group; * indicates p < 0.05 versus the adult group; n = 20 for each group
Discussion
Our results revealed that (1) the presence of HCN2 and HCN4 proteins in the rat SAN and (2) there is an age-associated decline in the expression of HCN2 and HCN4 proteins accompanied with a reduction in their relative contribution to SAN pacemaking.
The normal heart rate is triggered by rhythmic spontaneous depolarization of SAN during the diastole. The hyperpolarization-activated cyclic nucleotide-gated cation current, termed If, carried by HCN channel, plays a key role in the pacemaker activity.24,25 To date, four HCN channel isoforms have been identified in the heart. Furthermore, the relevance of each HCN channel isoform to maintaining a stable heart rate and regular beat-to-beat variation has been confirmed in genetic mouse models in vivo.26–29 Mice lacking HCN1 display sinus node dysrhythmia and recurrent sinus pauses pronounced at low heart rates and nearly absent at the high heart rates observed during high physical activity or after pharmacological stimulation by isoproterenol.27 HCN2-deficient mice have similar mean heart rate to wild-type mice at rest and during spontaneous activity.28 However, the intervals between successive heart beats of HCN2-deficient mice varied widely at rest and completely abolished during enhanced spontaneous activity and stimulation of cardiac β-adrenergic receptors by isoproterenol or block of muscarinic receptors by atropine increased the heart rate. The HCN4-null mice as well as mice with a selective deletion of HCN4 in cardiomyocytes died between embryonic days 9.5 and 11.5. HCN4-deficient embryos have a slower heart rate than wild-type embryos and could not be stimulated by cAMP.29 By contrast, HCN3-deficient mice display normal cardiac pacemaker activity with an abnormality in the ventricular repolarization.30 In human, the physiological significance of HCN channel in the normal function of SAN has also been confirmed. Mutations in the HCN4 gene were found in patients with congenital SSS, which suggests that a genetic defect in HCN channel can lead to SSS.31,32 Currently, increasing evidence is highlighting that the HCN channel functions not only in the impulse formation but in the impulse propagation of SAN.21,33 HCN1-deficient mouse have prolonged SACT. The prolonged SACT in HCN1−/− mice could result from a more negative maximal diastolic potential, which increases the distance to the threshold at which an action potential is generated. In this situation, more current and more time are required for a cell to charge the cell membrane of an adjacent cell to reach the threshold potential for an action potential and therefore slows the action potential conduction. This effect outweighs the competing effect of a more negative maximal diastolic potential to increase the availability of L-type Ca2+ channels and voltage-gated Na+ channels. In the present study, we confirmed the contribution of HCN channels to the automaticity of the heart. Blockade of HCN channels with ivabradine prolonged CL of all age groups in Langendorff hearts. Furthermore, we revealed the age-associated effect of ivabradine on CL, reflecting a compromised function of HCN channels in pacemaking during aging.
The heterogeneity of four HCN channel isoforms within the heart has been revealed. The transcripts of the HCN channel isoforms in the SAN were detected by different methods, including the RNAse protection assay, Northern blot analysis, in situ hybridization, quantitative polymerase chain reaction, and non-quantitative PCR. The HCN4 transcript is by far the most highly expressed in the SAN in adult rabbits, mice, and canines with lower levels of HCN2 and HCN1 transcripts.12,13,34,35 This expression profile correlates well with findings in HCN knockout models. HCN4-knockout mice display a ∼70% reduction in SAN If,20 whereas deletion of HCN1 results in ∼40% reduction and deletion of HCN2 results in ∼30% reduction.27,28 In human, HCN4 was also most abundant in SAN.36 However, transcription is far away from functional ion channel protein. Due to the complex structure of SAN, little information is available as to the protein expression of the HCN channel isoforms in the SAN tissue, especially during aging. Functionally, the SAN can be divided into central and peripheral regions (central is adapted for pacemaking only, whereas peripheral is adapted to protect the center and drive the atrial muscle as well as pacemaking).37 Tellez et al. found that HCN1 and HCN4 mRNA were significantly more abundant in the rabbit SAN center than in both the SAN periphery and atrial muscle.34 Our previous study revealed the presence of HCN2 and HCN4 transcripts in laser-captured rat SAN pacemaker cells from the central of SAN and their downregulated transcriptions with increased age. In the present study, we found HCN2 and HCN4 proteins in the rat SAN using immunofluorescence but failed to detect HCN1 and HCN3. The leading pacemaker site of the SAN tissue was identified by optical mapping for further Western blot analysis, which mainly consists of pacemaker cells within the central region of the SAN. In agreement with the functional results, Western blot analysis showed reduced expression of HCN2 and HCN4 proteins in the rat SAN during aging. Several studies also demonstrated a temporal and spatial expression of HCN channel isoforms during cardiac development. Yasui et al. showed that an extensive expression of HCN4 and an If current, similar to the If of adult SAN, is present in early embryonic mouse ventricular myocytes, which declined later on during the second half of embryonic development.38 As reported by Schweizer et al., transcriptions of HCN channel isoforms also varied in atrium, ventricle, and SAN during mouse cardiac development.14 An investigation based on the overexpression of HCN2 channels in embryonic rat ventricular myocytes has shown a large increase of the rate of spontaneous activity correlated to the expression of pacemaker channels.39 We speculate that reduction in If caused by declined HCN channel expression may at least in part involved in the lower IHR during aging. Further If patch clamp recording of single isolated SAN cells is needed.
Several agents were developed for their ability to selectively reduce heart rate act by a specific inhibition of HCN channel; these substances have a potential for the treatment of diseases such as angina and heart failure. In the near future, new agents to focus on to facilitate the expression and function of HCN channel or devices based on the delivery of HCN channels in situ, or of a cellular source of HCN channels (biological pacemakers), will likely be developed for use in therapies for diseases of heart rhythm with the aim of replacing electronic pacemakers.
Conclusions
In summary, the expression of HCN channel isoforms may serve to maintain the physiological SAN pacemaker in rats. The aging-induced remodeling of HCN channel isoforms expression may be an important contributor to the declined SAN function in aging. Therapies that target HCN expression and/or HCN-based currents may be an interesting approach for treating age-related arrhythmias.
Acknowledgements
This work was supported by grants from The National Science Foundation of China (NSFC) (No.81100132), Shaanxi Province Science and Technology Research and Development Program (No. 2011k14-01-05) and the First Affiliated Hospital of XJTU science program (No. 2010yk10). The authors would like thanking Dr Jie Liu and Pengbo Yu for providing technical assistance and Prof. Zhiquan Liu for helpful suggestions during the preparation of the manuscript.
Authors’ contributions
AM designed the study. XH and AM wrote the manuscript. PY, HZ and ZY researched and and analyzed the data.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Gregoratos G. Permanent pacemakers in older persons. J Am Geriatr Soc 1999; 47: 1125–35. [DOI] [PubMed] [Google Scholar]
- 2.Schmidlin O, Bharati S, Lev M, Schwartz JB. Effects of physiological aging on cardiac electrophysiology in perfused Fischer 344 rat hearts. Am J Physiol 1992; 262: H97–105. [DOI] [PubMed] [Google Scholar]
- 3.Alings AM, Bouman LN. Electrophysiology of the ageing rabbit and cat sinoatrial node—a comparative study. Eur Heart J 1993; 14: 1278–88. [DOI] [PubMed] [Google Scholar]
- 4.ME J. Clinical cardiac electrophysiology: techniques and interpretations, 2nd ed Philadelphia: Lea and Febeger, 1993. [Google Scholar]
- 5.Scicchitano P, Cortese F, Ricci G, Carbonara S, Moncelli M, Iacoviello M, Cecere A, Gesualdo M, Zito A, Caldarola P, Scrutinio D, Lagioia R, Riccioni G, Ciccone MM. Ivabradine, coronary artery disease, and heart failure: beyond rhythm control. Drug Des Dev Ther 2014; 8: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiFrancesco D. Funny channel gene mutations associated with arrhythmias. J Physiol 2013; 591: 4117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biel M, Schneider A, Wahl C. Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc Med 2002; 12: 206–12. [DOI] [PubMed] [Google Scholar]
- 8.Clapham DE. Not so funny anymore: pacing channels are cloned. Neuron 1998; 21: 5–7. [DOI] [PubMed] [Google Scholar]
- 9.Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Annu Rev Physiol 2001; 63: 235–57. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature 1998; 393: 587–91. [DOI] [PubMed] [Google Scholar]
- 11.Stieber J, Hofmann F, Ludwig A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc Med 2004; 14: 23–8. [DOI] [PubMed] [Google Scholar]
- 12.Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem/FEBS 2001; 268: 1646–52. [DOI] [PubMed] [Google Scholar]
- 13.Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res 1999; 85: e1–6. [DOI] [PubMed] [Google Scholar]
- 14.Schweizer PA, Yampolsky P, Malik R, Thomas D, Zehelein J, Katus HA, Koenen M. Transcription profiling of HCN-channel isotypes throughout mouse cardiac development. Basic Res Cardiol 2009; 104: 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlak J, Thum T. Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J 2003; 17: 1592–608. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Velasco M, Goren N, Benito G, Blanco-Rivero J, Bosca L, Delgado C. Regional distribution of hyperpolarization-activated current (If) and hyperpolarization-activated cyclic nucleotide-gated channel mRNA expression in ventricular cells from control and hypertrophied rat hearts. J Physiol 2003; 553: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss HC, Saroff AL, Bigger JT, Jr, Giardina EG. Premature atrial stimulation as a key to the understanding of sinoatrial conduction in man. Presentation of data and critical review of the literature. Circulation 1973; 47: 86–93. [DOI] [PubMed] [Google Scholar]
- 18.Breithardt G, Seipel L, Loogen F. Sinus node recovery time and calculated sinoatrial conduction time in normal subjects and patients with sinus node dysfunction. Circulation 1977; 56: 43–50. [DOI] [PubMed] [Google Scholar]
- 19.Safa-Tisseront V, Ponchon P, Laude D, Elghozi JL. Contribution of the autonomic nervous system to blood pressure and heart rate variability changes in early experimental hyperthyroidism. Eur J Pharmacol 1998; 352: 247–55. [DOI] [PubMed] [Google Scholar]
- 20.Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, Catterall WA, Scheuer T. An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci USA 2003; 100: 3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol 1996; 118: 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Yang P, Du Y, Zhang J, Ma A. Age-related down-regulation of HCN channels in rat sinoatrial node. Basic Res Cardiol 2007; 102: 429–35. [DOI] [PubMed] [Google Scholar]
- 23.Jia B, Choy E, Cote G, Harmon D, Ye S, Kan Q, Mankin H, Hornicek F, Duan Z. Cyclin-dependent kinase 11 (CDK11) is crucial in the growth of liposarcoma cells. Cancer Lett 2014; 342: 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 1993; 55: 455–72. [DOI] [PubMed] [Google Scholar]
- 25.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 2003; 65: 453–80. [DOI] [PubMed] [Google Scholar]
- 26.Baruscotti M, Bucchi A, Viscomi C, Mandelli G, Consalez G, Gnecchi-Rusconi T, Montano N, Casali KR, Micheloni S, Barbuti A, DiFrancesco D. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc Natl Acad Sci USA 2011; 108: 1705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenske S, Krause SC, Hassan SI, Becirovic E, Auer F, Bernard R, Kupatt C, Lange P, Ziegler T, Wotjak CT, Zhang H, Hammelmann V, Paparizos C, Biel M, Wahl-Schott CA. Sick sinus syndrome in HCN1-deficient mice. Circulation 2013; 128: 2585–94. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 2003; 22: 216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F, Ludwig A. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA 2003; 100: 15235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fenske S, Mader R, Scharr A, Paparizos C, Cao-Ehlker X, Michalakis S, Shaltiel L, Weidinger M, Stieber J, Feil S, Feil R, Hofmann F, Wahl-Schott C, Biel M. HCN3 contributes to the ventricular action potential waveform in the murine heart. Circ Res 2011; 109: 1015–23. [DOI] [PubMed] [Google Scholar]
- 31.Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. New Engl J Med 2006; 354: 151–7. [DOI] [PubMed] [Google Scholar]
- 32.Schulze-Bahr E, Neu A, Friederich P, Kaupp UB, Breithardt G, Pongs O, Isbrandt D. Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest 2003; 111: 1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahl-Schott C, Fenske S, Biel M. HCN channels: new roles in sinoatrial node function. Curr Opin Pharmacol 2014; 15: 83–90. [DOI] [PubMed] [Google Scholar]
- 34.Tellez JO, Dobrzynski H, Greener ID, Graham GM, Laing E, Honjo H, Hubbard SJ, Boyett MR, Billeter R. Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circ Res 2006; 99: 1384–93. [DOI] [PubMed] [Google Scholar]
- 35.Zicha S, Fernandez-Velasco M, Lonardo G, L’Heureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 2005; 66: 472–81. [DOI] [PubMed] [Google Scholar]
- 36.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation 2007; 115: 1921–32. [DOI] [PubMed] [Google Scholar]
- 37.Boyett MR, Dobrzynski H, Lancaster MK, Jones SA, Honjo H, Kodama I. Sophisticated architecture is required for the sinoatrial node to perform its normal pacemaker function. J Cardiovasc Electrophysiol 2003; 14: 104–6. [DOI] [PubMed] [Google Scholar]
- 38.Yasui K, Liu W, Opthof T, Kada K, Lee JK, Kamiya K, Kodama I. I(f) current and spontaneous activity in mouse embryonic ventricular myocytes. Circ Res 2001; 88: 536–42. [DOI] [PubMed] [Google Scholar]
- 39.Qu J, Barbuti A, Protas L, Santoro B, Cohen IS, Robinson RB. HCN2 overexpression in newborn and adult ventricular myocytes: distinct effects on gating and excitability. Circ Res 2001; 89: E8–14. [DOI] [PubMed] [Google Scholar]