Abstract
The transcription factor MYC, which is dysregulated in the majority of gliomas, is difficult to target directly. Deubiquitinase ubiquitin-specific protease 28 (USP28) stabilizes oncogenic factors, including MYC. However, the contribution of USP28 in tumorigenesis, particularly in glioma, is unknown. Here, we determined the expression of USP28 and assessed its clinical significance in human glioma. We found that USP28 is overexpressed in human glioma but not in normal brain tissue. The level of USP28 protein expression in human glioma tissues was directly correlated with glioma grade. Meanwhile, the level of USP28 protein expression in human glioblastoma tissues was inversely correlated with patient survival. Enforced USP28 expression promotes SW1783 glioma cell proliferation. Moreover, gliomas that arose from USP28-transfected SW1783 cells displayed tumorigenicity in nude mouse model systems. Inhibition of USP28 expression in glioblastoma U373 cells suppressed anchorage-independent growth in vitro and tumorigenicity in vivo. Furthermore, USP28 regulates the expression of MYC protein, which is essential in USP28-induced cell growth in glioma cells. These results showed that USP28 is overexpressed in human glioblastomas and it contributes to glioma tumorigenicity. Therefore, USP28 could be a new target of therapy for human malignant glioma.
Keywords: Ubiquitin-specific protease 28, glioma, MYC
Introduction
Gliomas encompass all primary central nervous system tumors of glial cell origin.1 Despite recent advances in both diagnostic modalities and therapeutic strategies, glioma remains one of the deadliest human cancers. The five-year survival rate in patients with glioma is among the lowest for all cancers. In patients with glioblastoma multiforme (GBM), the median survival duration ranges from 9 to 12 months.2,3 Advances in the treatment of malignant glioma will require an improvement in the understanding of the biological mechanisms of glioma development and progression.
Knowledge of the molecular pathways associated with glioma carcinogenesis and development of cancer is crucial for identification of new diagnostic and prognostic biomarkers.4–6 Ubiquitin-specific protease 28 (USP28), an ubiquitin-specific protease, is an important regulator of oncogene and tumor suppressor.7,8 Researchers have reported that USP28 controls MYC stability through antagonistic effects on the activity of the SCF-Fbxw7 ubiquitin ligase complex. Moreover, stabilization of MYC by USP28 is required for proliferation of several tumor cell types and inhibition of cell differentiation in colon carcinoma.9,10 MYC is a central regulator of cell growth, proliferation, and apoptosis. Enhanced levels of MYC are thought to contribute to genesis of many human tumors, including glioma.11–13
These results suggested that USP28 plays a role in the oncogenesis of some malignancies. However, whether USP28 expression contributes to glioma development and progression is unknown. In the present study the mechanism by which USP28 regulates the growth of glioma is determined. Aberrant USP28 expression was found to directly affect the tumorigenicity of human gliomas.
Materials and methods
Samples, cells, and antibodies
Human normal brain tissues and glioma tissue samples were obtained from patients who underwent surgery therapeutic procedures at Department of Neurosurgery, Weifang People’s Hospital. The adult normal brain tissues as normal controls were obtained from surgical resections of three trauma patients. Clinicopathological features and treatment strategies of all the glioma patients are shown in supplemental Table 1. All experiments were approved by the ethics committee of Weifang People’s Hospital and informed consent was obtained from all patients prior to specimen collection. Human glioma cell lines, SW1783, U87 MG, U373, LN-229, SW1088, and Hs 683, were obtained from American Type Culture Collection (ATCC, Manassas, VA). The glioblastoma cell line HFU251 MG and immortalized normal human astrocyte line NHA-E6/E7/hTERT, which were described previously,14 were also used. These cells were maintained in Minimum Essential Medium (MEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen). Mouse monoclonal USP28, MYC, and β-actin antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA).
Plasmid construction and transfection
For overexpression, the cDNA representing the complete open reading frame of USP28 was cloned into the pBabe vector to generate the USP28 expression plasmid. The expression plasmid was verified by sequencing both strands and was used to transfect the SW1783 cells to establish the USP28 overexpression cell line. For USP28 RNA interference, the control (pSuper) and pSuper-sh USP28 plasmids were purchased from OligoEngine Biotechnology (Seattle, USA) and was used to transfect the U373 cells to establish the USP28 knockdown cell line. The transfection efficiency of USP28 was confirmed by Western blotting and quantitative reverse transcription PCR (qRT-PCR) analyses.
MTT assay
A 3 -(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium (MTT) assay was used to assess cell proliferation. The cells were seeded and 20 mL of the MTT solution (5 mg/mL) was then added to each well at the indicated time. The absorbance at 490 nm was measured using a microplate reader (Bio-Rad, Hercules, CA).
Immunohistochemistry
Paraffin-embedded sections were deparaffinized, blocked, and incubated with antibody at 4℃ overnight. Horse-radish peroxidase-conjugated secondary antibody (1:500) was then added and further incubated for 1 h at room temperature. The sections were developed using a 3,3’-diaminobenzidine (DAB) tetrahydrochloride substrate kit at room temperature for 1–5 min and then counterstained with hematoxylin. The extent of immunostaining was quantified by counting the percentage of positive cells and classified into four groups: 0, less than 25% positive cells; 1, 25–50% positive cells; 2, 51–75% positive cells; and 3, more than 75% positive cells.15
Colony formation assay
The cells were seeded in 6-cm dishes at a density of 300 cells per dish. After incubation for 14 days, the colonies were fixed with methanol for 10 min and stained with crystal violet for 15 min, after which point the number of colonies containing more than 50 cells was scored.5
Western blot assay
Equal amounts of protein were separated using sodium dodecyl sulfate (SDS)–polyacrylamide gels and were electrotransferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). The membranes were immunoblotted overnight at 4℃ with primary antibodies, followed by their respective secondary antibodies. β-actin was used as the loading control.
Quantitative reverse-transcription PCR
RNA was extracted using TRIzol reagent, according to the manufacturer’s recommended protocol (Invitrogen). qRT-PCR was performed using Applied Biosystems (Foster City, CA) StepOne and StepOne Plus Real-Time PCR Systems. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. The experiments were repeated a minimum of three times to confirm the results.
Immunofluorescence staining
The cells were grown on the sterile coverslips, and the cells were fixed with 4% paraformaldehyde and permeabilized using 0.1% Triton-X100. Cells were blocked with primary antibody followed by hodamine-conjugated anti-rabbit secondary antibody. Finally, the cells were further stained with 4,6-diami-dino-2-phenylindole (DAPI).
In vivo tumorigenesis assays
The in vivo tumorigenesis and metastasis assays were performed, as previously described.16 Briefly, 1 x 106 cells were injected subcutaneously into the right flanks of severe combined immunodeficient (SCID) mice. Tumor length (L) and width (W) were measured every three days, and tumor volume was calculated using the equation: volume = (W2 × L)/2. After five weeks, the mice were killed and the tumor volume and weight were measured. All of the animal experiments were performed with the approval of the Weifang People’s Hospital Animal Care and Use Committee.
Statistical analysis
Experimental data are shown as mean ± standard deviation (SD). The results from different treatment groups were compared using a two-tailed Student’s t-test. The Kaplan–Meier method was used to estimate the probability of patient survival, and differences in the survival of subgroups of patients were compared using Mantel’s log-rank test. Differences were considered to be statistically significant at a value of P less than 0.05. Statistical analysis was done with SPSS/Win11.0 software (SPSS, Inc., Chicago, IL).
Results
Expression of USP28 directly correlates with glioma grade and decreased patient survival
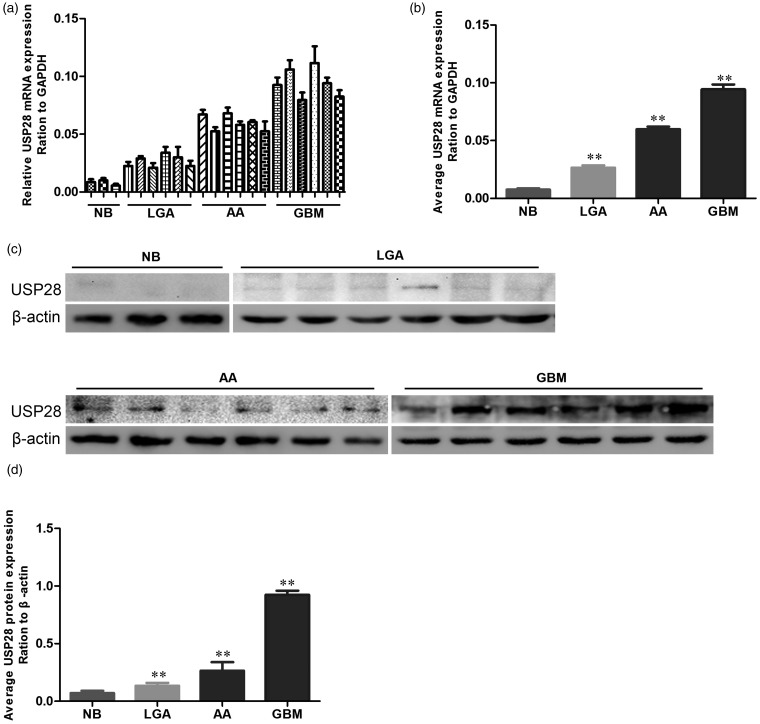
To determine the relative expression of USP28 mRNA in human normal brain and glioma tissues, qRT-PCR analysis with specific primers was employed. As shown in Figure 1a and b, USP28 mRNA expression in normal brain tissue specimens is lower than that in glioma tissues. Predominant expression of USP28 mRNA was found in glioma specimens. Moreover, the levels of USP28 mRNA in GBM specimens were 1.7 times higher than that in the anaplastic astrocytoma specimens (P < 0.001) and 4.3 times higher than that in the low-grade astrocytoma specimens (P < 0.001). To establish whether USP28 protein is expressed in human GBM samples, Western blot for USP28 expression was performed on three normal human brains, six low-grade astrocytoma, six anaplastic astrocytoma, and six GBM specimens (Figure 1c). The protein levels of USP28 were upregulated in glioma samples compared with the normal brain samples. Densitometric evaluation of the relative expression showed that the expression level of USP28 was significantly higher in anaplastic astrocytoma than in low-grade astrocytoma. GBM expressed the highest USP28 (Figure 1d).
Figure 1.
USP28 is overexpressed in human gliomas. (a) qRT-PCR assay of USP28 mRNA in NB, LGAs, AA, and GBMs. (b) Average USP28 mRNA expression in NB, LGAs, AA, and GBMs for ‘a’. (c) Western blot assay of USP28 protein in NB, LGAs, AA, and GBMs. (d) Average USP28 protein expression ratio to β-actin in NB, LGAs, AA, and GBMs for ‘c’. **P < 0.01 is based on Student's t-test. All results are from three or four independent experiments. Error bars indicate standard deviation. NB, normal brain tissue; LGA, low-grade astrocytoma; AA, anaplastic astrocytoma; GBM, glioblastoma multiforme
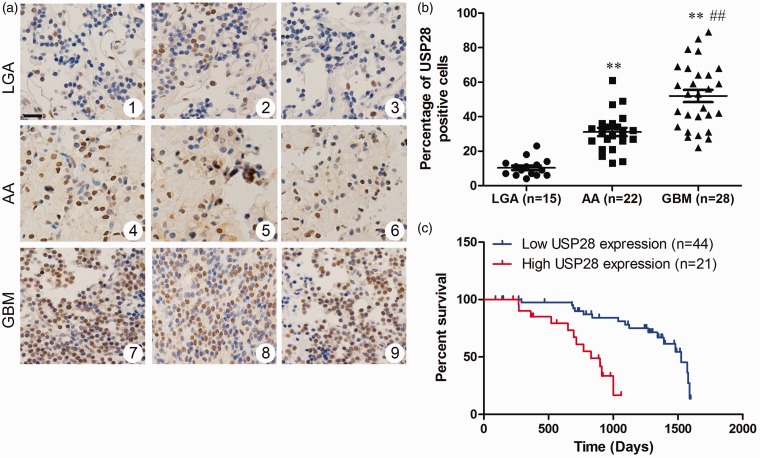
USP28 expression was analyzed by immunohistochemistry. No stain was detected in the normal human brain samples, and USP28 protein was expressed in all human glioma samples. The protein was localized in the nuclei of the tumor cells (Figure 2a). The ratio of USP28-positive cells in high-grade glioma samples was higher than that in low-grade glioma samples (Figure 2b). These results indicate that USP28 expression in the glioma cells was grade-dependent. A high level of USP28 expression was significantly more common in glioma tissues with high pathologic grade than that with low pathologic grade (P = 0.003, supplemental Table 1). No significant association was found between USP28 expression and gender, diameter, tumor locus, and age at diagnosis. Kaplan–Meier survival analysis showed significant survival extension in USP28 low-expression gliomas compared with USP28 high-expression gliomas (Figure 2c). The cut-off point was established using the X-tile software program (version 3.6.3; Yale University School of Medicine, CT). The results indicated that increased expression of USP28 was significantly associated with poor overall survival of GBM patients.
Figure 2.
Overexpressed USP28 is associated with decreased overall survival in human gliomas. (a) Immunohistochemical staining of USP28 expression in human glioma (1–3: LGA; 4–6: AA; 7–9: GBM). (f) Ratio of USP28 positive cells in LGAs, AA, and GBMs tissues. (b) Kaplan–Meier analyses of overall survival periods among 65 resected glioma patients are shown to be stratified according to USP28 expression. LGA, low-grade astrocytoma; AA, anaplastic astrocytoma; GBM, glioblastoma multiforme. (A color version of this figure is available in the online journal.)
Transfection efficiency of USP28 in glioma cell lines
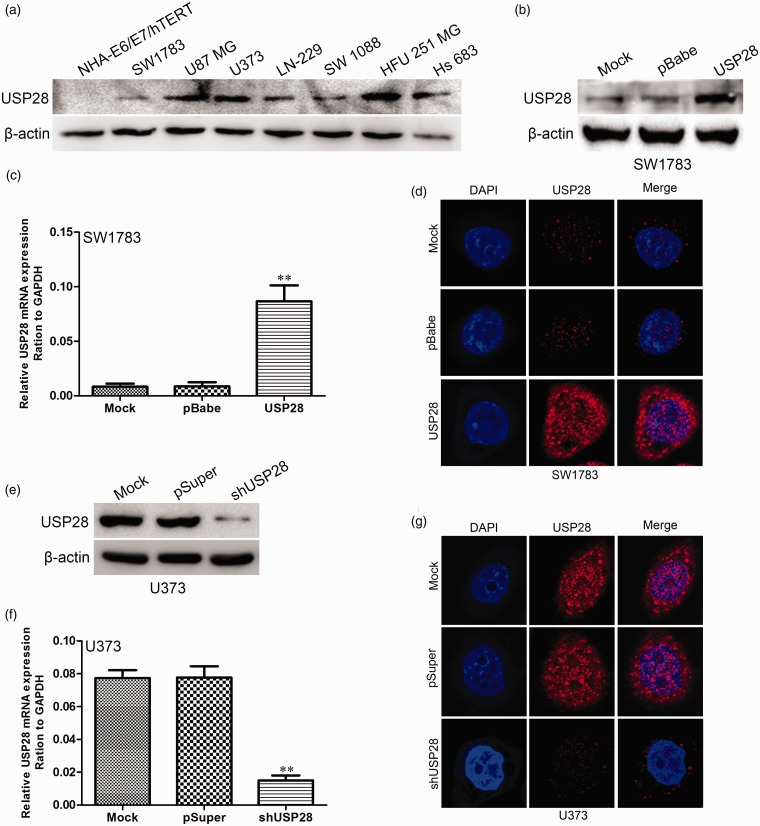
The expression levels of USP28 protein in the immortalized normal human astrocyte cell line NHA-E6/E7/hTERT and glioma cell lines SW1783, U87MG, U373, LN-229, SW1088, HFU-251MG, and Hs683 were investigated. Significantly higher expression of USP28 protein was evident in U87MG, U373, Hs683, and HFU-251MG glioblastoma cells than in SW1783, LN-229, and SW1088 cells. The immortalized normal human astrocytes had a negligible level of USP28 protein expression (Figure 3a). USP28 expression plasmid pBabe-USP28 was transfected into SW1783 cells. After selection with puromycin, USP28 expression was assayed by Western blot (Figure 3b), qRT-PCR (Figure 3c), and immunofluorescence microscopy (Figure 3d). U373 is a highly tumorigenic human glioma cell line commonly used in glioma research. This cell line was infected with pSuper-shUSP28 and pSuper (as control) to investigate the effect of USP28 knockdown on proliferation and colony formation of glioma cells. Western blot (Figure 3e), qRT-PCR (Figure 3f), and immunofluorescence microscopy (Figure 3g) demonstrated that the protein expression level of USP28 was significantly suppressed in cells infected with pSuper-shUSP28.
Figure 3.
Transfection efficiency of USP28 in glioma cell lines. (a) USP28 protein levels in human immortalized normal astrocytes (NHA-E6/E7/hTERT) and glioma cell lines (SW1783, U87 MG, U373, LN-229, SW 1088, HFU 251 MG, and Hs 683) were revealed by Western blot. The transfection efficiency of USP28 in SW1783 cells were analyzed by Western blot (b), qRT-PCR (c), and immunofluorescence staining (d). The transfection efficiency of USP28 shRNA or control vector in U373 cells were analyzed by Western blot (e), qRT-PCR (f), and immunofluorescence staining (g). **P < 0.01 is based on Student's t-test. All results are from three or four independent experiments. Error bars indicate standard deviation. (A color version of this figure is available in the online journal.)
USP28 promotes glioma cell proliferation
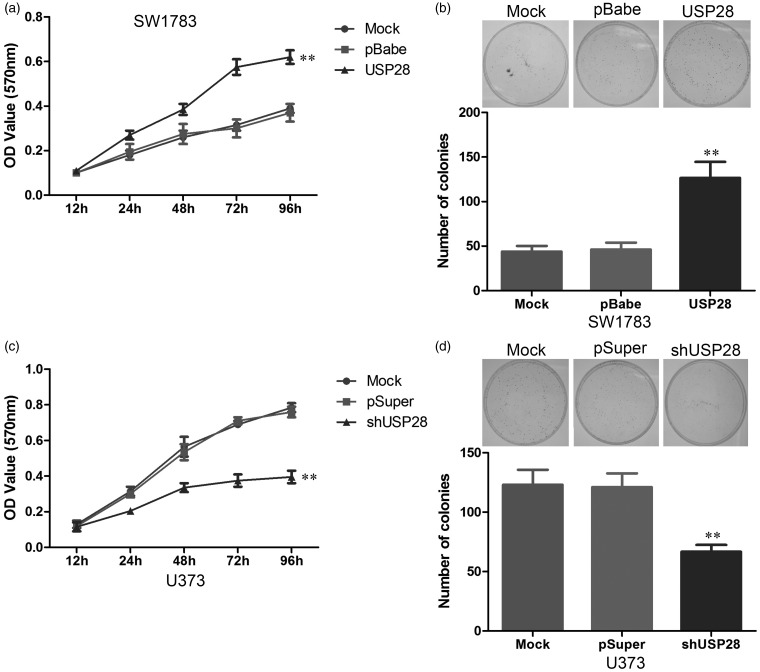
The effect of USP28 overexpression was determined to characterize the functions of USP28 in glioma cells. Results of MTT assay showed that SW1783-pBabe-USP28 cells displayed higher proliferation rates than control cells (i.e. parent SW1783 cells and SW1783-pBabe cells) (Figure 4a). The ability of USP28-expressing SW1783 cells to form spheres was analyzed to determine the function of USP28 in cellular transformation. Results of the colony formation assay revealed that USP28 overexpression significantly increased the number of colonies in SW1783-pBabe-USP28 cells (Figure 4b). The results of the MTT assay also showed that USP28 suppression was associated with decreased cell proliferation (Figure 4c), and the results of the colony formation assay revealed that USP28 suppression significantly decreased the number of formed colonies (Figure 4d). These results further confirm that USP28 was involved in the proliferation of glioma cells.
Figure 4.
USP28 promoted glioma cell proliferation. (a) Cell proliferation after USP28 overexpression in SW1783 cells was measured using MTT assays. (b) Cell proliferation after USP28 overexpression in SW1783 cells was measured using colony formation assays. (c) Cell proliferation after USP28 knockdown in U373 cells was measured using MTT assays. (d) Cell proliferation after USP28 knockdown in U373 cells was measured using colony formation assays. **P < 0.01 is based on Student's t-test. All results are from three or four independent experiments. Error bars indicate standard deviation
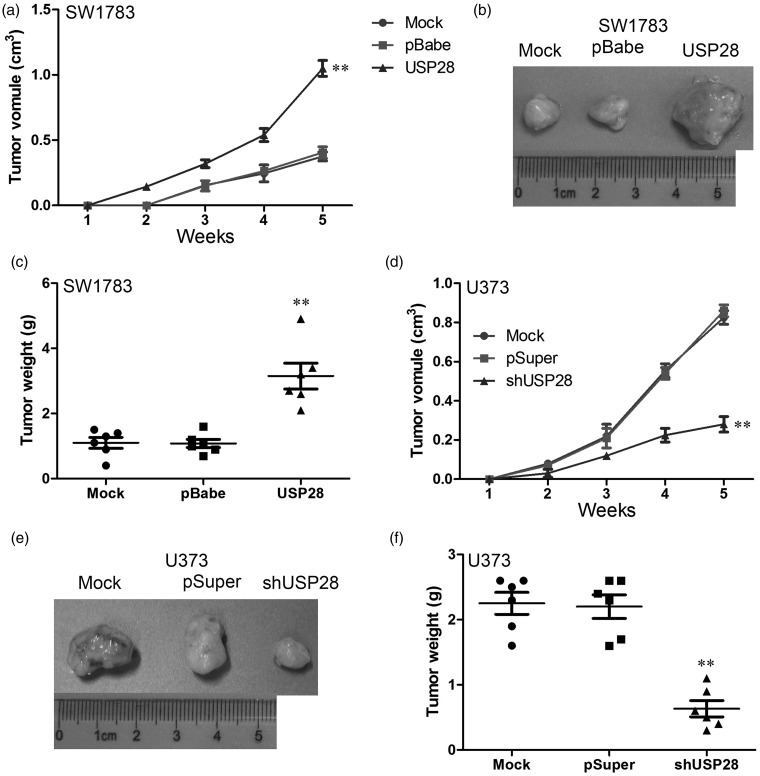
USP28 promotes tumorigenesis in vivo
To determine the effects of USP28 on tumorigenesis in vivo, different cell lines were injected subcutaneously into the flanks of nude mice. The diameters of the tumors were measured every seven days. The mice that had been injected with USP28-overexpressing SW1783 cells formed tumors on the second week, whereas the mice that had been injected with control cells did not form tumors until the third week (Figure 5a and b). Similar results were observed in U373 cells. The control cells formed tumors earlier, and tumor volumes were much larger in those that were formed from control cells than in those that were formed from USP28 knockdown cells (Figure 5d and e). As shown in Figure 5c, the tumor volume of tumors formed from SW1783 cells was much lower than that formed from USP28-overexpressing cells. Similar with these, the control group was dramatically larger than the shUSP28 group in U373 cells (Figure 5f). These results suggested that USP28 promotes formation and growth of glioma cell xenograft in vivo.
Figure 5.
USP28 promotes tumorigenesis in vivo. (a) Tumor growth curves in mice (n = 5/group) inoculated with USP28 overexpressed SW1783 cells and it control cells at indicated days. (b) At the experimental endpoint, USP28 overexpressed SW1783 cells and its control cell tumors were dissected and photographed as indicated. (c) Each tumor formed by USP28 overexpressed SW1783 cells and it control cells was weighed. (d) Tumor growth curves in mice (n = 5/group) inoculated with USP28 knockdown and it control U373 cells at indicated days. (e) At the experimental endpoint, USP28 knockdown and it control U373 cells tumors were dissected and photographed as indicated. (f) Each tumor formed by USP28 knockdown and its control U373 cells was weighed. **P < 0.01 is based on Student's t-test. All results are from three or four independent experiments. Error bars indicate standard deviation
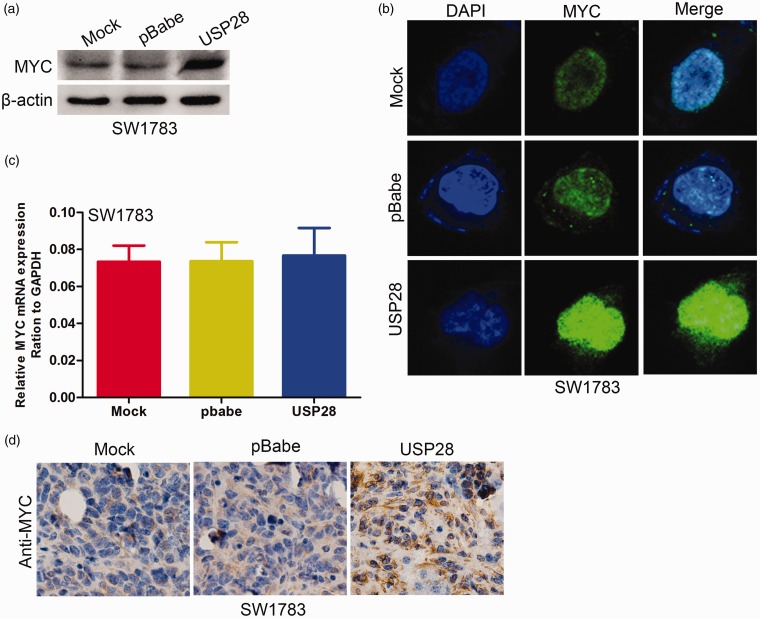
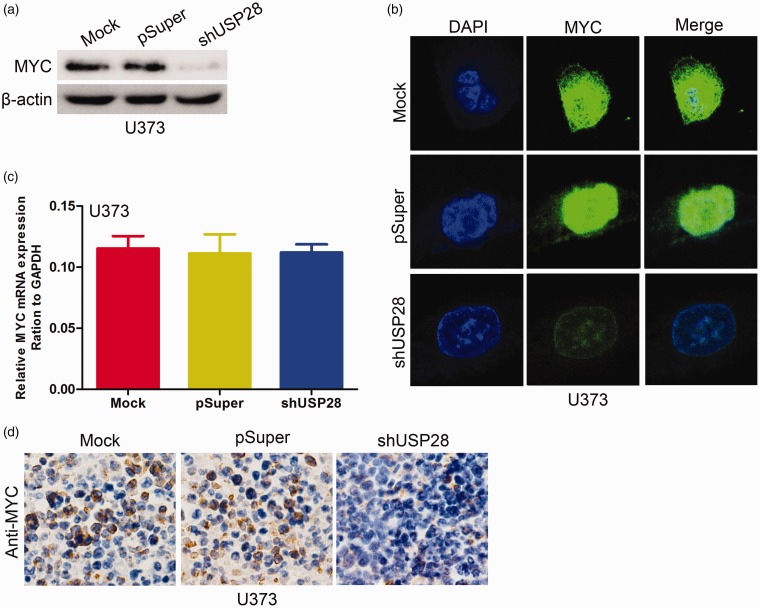
Activation of MYC contributes to USP28-induced oncogenic activities
Previous studies showed that MYC is induced in USP28-expressing breast cancer cells.9 Thus, the present study investigated whether MYC is induced by USP28 in glioma cells. USP28 overexpression in SW1783 cells significantly increased MYC protein levels (Figure 6a and b) but not mRNA expression (Figure 6c). By contrast, USP28 knockdown in U373 cells significantly decreased the expression levels of MYC protein (Figure 7a and b) but did not alter the mRNA (Figure 7c). The MYC expression was examined by immunohistochemistry staining in xenograft tumor tissues. As shown in Figure 6d, the overexpression of USP28 in SW1783 cells significantly upregulated MYC staining. In addition, knockdown of USP28 in U373 cells dramatically downregulated the staining of MYC (Figure 7d). These results suggested that USP28 promotes formation and growth of glioma cell xenograft in vivo partly by MYC upregulation.
Figure 6.
Overexpressed USP28 increased MYC expression. The expression of MYC protein in USP28 overexpression SW1783 cells was examined using Western blotting (a) and immunofluorescence staining (b). (c) The expression of MYC mRNA in USP28 overexpression SW1783 cells was examined using qRT-PCR. (d) Expression of MYC was measured by immunohistochemistry staining in USP28 overexpressed SW1783 and its control cells in xenograft tumor tissues. All results are from three or four independent experiments. Error bars indicate standard deviation. (A color version of this figure is available in the online journal.)
Figure 7.
Knockdown USP28 decreased MYC expression. The expression of MYC protein in USP28 knockdown U373 cells was examined using Western blotting (a) and immunofluorescence staining (b). (c) The expression of MYC mRNA in USP28 knockdown U373 cells was examined using qRT-PCR. (d) Expression of MYC was measured by immunohistochemistry staining in USP28 knockdown U373 and its control cells xenograft tumor tissues. All results are from three or four independent experiments. Error bars indicate standard deviation. (A color version of this figure is available in the online journal.)
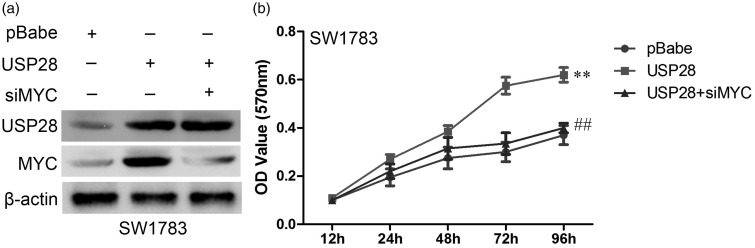
To test whether MYC is involved in UPS28-induced proliferation, we measured the role of MYC in USP28-induced proliferation by knocking down MYC expression with the use of siRNA in SW1738 cells. The MYC knockdown efficiency was detected using Western blot at 48 h after transfection (Figure 8a). As shown in Figure 8b, the proliferation induced by USP28 was obviously reversed following MYC knockdown using siRNA. These results confirmed that MYC is involved in USP28-mediated proliferation ability in glioma cells.
Figure 8.
Activation of MYC contributes to USP28-induced cell proliferation. (a) The MYC knockdown efficiency was detected using Western blot at 48 h after transfection. (b) Cell proliferation after USP28 overexpression and MYC knockdown in SW1783 cells was measured using MTT assays. **P < 0.01 vs. pBabe group; ##P < 0.01 vs. USP28 overexpression group is based on Student's t-test. All results are from three or four independent experiments. Error bars indicate standard deviation
Discussion
Glioma cells are a mixture of heterogeneous cell populations and numerous factors are likely to be involved in dictating their recurrence, progression, and patient survival.17 However, the molecular mechanisms of the initiation and progression of glioma are unclear. This study characterized the functions of USP28 in glioma.
Previous results showed that USP28 is expressed differentially in normal and cancer tissues.12,18 USP28 overexpression has been associated with progression of bladder and breast cancer.13,19 However, the effect of altered USP28 expression on the progression of glioma cells remains unclear. This study examined the expression of USP28 in glioma cells and investigated the relationship between USP28 expression and the clinicopathological characteristics of patients with glioma.
This study found a significant difference in USP28 expression at both protein and mRNA levels between glioma and normal tissue. Furthermore, USP28 mRNA is upregulated remarkably in cancerous tissues compared with the USP28 protein in non-cancerous tissues. This observation suggests that dysregulation at the transcriptional level could be the primary source of USP28 expression in glioma cells. Immunohistochemistry demonstrated that glioma cells showed weak to strong nuclear staining. These results were similar to those of previous studies on other human cancers. Furthermore, intense expression of USP28 in glioma cells correlated with its clinicopathologic features, including tumor recurrence rate, histopathological classification, and clinical stage. Thus, overexpression of USP28 protein is correlated with poor prognosis for patients with glioma. Disease-free survival rate was significantly different between the two groups, and the results showed that higher expression of USP28 protein entails lower the disease-free survival rate.
USP28 overexpression was also found to increase glioma cell proliferation and promote tumorigenesis in vitro and in vivo, whereas USP28 knockdown by shRNA inhibited tumorigenesis. These data are consistent with a recent study that investigated the function of USP28 in bladder and breast carcinogenesis. All of these findings are supported by a recent study, which reported that overexpression of USP28 protein in breast cancer alters tumor cell fate and promotes tumor progression and metastasis.
The initiation and progression of glioma involve a series of genetic events including activation of oncogenes and inactivation of tumor suppressors.20,21 Genetic studies have shown that c-MYC proto-oncogene encodes a transcription factor, MYC, which is a central regulator of cell growth, proliferation, and apoptosis.13,12 Enhanced levels of MYC are thought to contribute to the formation of many human tumors. Correspondingly, expression of MYC is regulated at multiple levels. The action of FBW7 is antagonized by USP28, which forms a complex with MYC and FBW7. USP28 overexpression could deubiquitinate FBW7 through deubiquitination of their substrates such as MYC, because USP28 is a member of the ubiquitin-specific protease family.9,22
In this study, USP28 overexpression significantly increased MYC expression, whereas USP28 knockdown produced an opposite effect in glioma cells. The inhibition of MYC by siRNA reversed the induction of USP28 on glioma cell proliferation. Thus, aberrant USP28 in gliomas may enhance the oncogenic effects of activated MYC. However, the mechanisms involved in the expression profile of the gene need further investigation. Additional studies are required to explore the relationships of the USP28 gene with other genes, such as p53 and HIF-1, and its relationship to other molecules that may be associated with glioma.
Conclusions
In conclusion, this study demonstrated for the first time that USP28 was overexpressed in glioma tissues. USP28 overexpression increased the growth of glioma cells in vitro and promoted glioma tumorigenesis in vivo. Therefore, USP28 is potentially an important molecular target for the design of novel antiglioma therapy.
Acknowledgements
This project was supported by National Natural Science Foundation of China (81171488).
Authors’ contributions
ZW and SQ designed the experiments. ZW and QS performed the experiments. JX and YZ performed the statistical analysis. ZW and QS wrote the manuscript. All authors approved the final draft of this manuscript.
Declaration of conflicting interest
No conflicts of interest exist.
References
- 1.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Ann Rev Pathol 2014; 9: 1–25. [DOI] [PubMed] [Google Scholar]
- 2.Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci 2014; 15: 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed R, Oborski MJ, Hwang M, Lieberman FS, Mountz JM. Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res 2014; 6: 149–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondo Y, Katsushima K, Ohka F, Natsume A, Shinjo K. Epigenetic dysregulation in glioma. Cancer Sci 2014; 105: 363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G, Wei J. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Mol Cancer 2014; 13: 137–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang P, Liu Z, Wang Q, Wen M, Wang Y, Yuan H, Mao JH, Wei G. CUL4A overexpression enhances lung tumor growth and sensitizes lung cancer cells to Erlotinib via transcriptional regulation of EGFR. Mol Cancer 2014; 13: 252–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valero R, Bayes M, Francisca Sanchez-Font M, Gonzalez-Angulo O, Gonzalez-Duarte R, Marfany G. Characterization of alternatively spliced products and tissue-specific isoforms of USP28 and USP25. Genome Biol 2001;2:RESEARCH0043. [DOI] [PMC free article] [PubMed]
- 8.Zhen Y, Knobel PA, Stracker TH, Reverter D. Regulation of USP28 de-ubiquitinating activity by SUMO conjugation. J Biol Chem 2014;289(50):34838–50. [DOI] [PMC free article] [PubMed]
- 9.Popov N, Herold S, Llamazares M, Schulein C, Eilers M. Fbw7 and Usp28 regulate myc protein stability in response to DNA damage. Cell Cycle 2007; 6: 2327–31. [DOI] [PubMed] [Google Scholar]
- 10.Diefenbacher ME, Popov N, Blake SM, Schulein-Volk C, Nye E, Spencer-Dene B, Jaenicke LA, Eilers M, Behrens A. The deubiquitinase USP28 controls intestinal homeostasis and promotes colorectal cancer. J Clin Invest 2014; 124: 3407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popov N, Wanzel M, Madiredjo M, Zhang D, Beijersbergen R, Bernards R, Moll R, Elledge SJ, Eilers M. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol 2007; 9: 765–74. [DOI] [PubMed] [Google Scholar]
- 12.Bidwell GL, 3rd, Perkins E, Hughes J, Khan M, James JR, Raucher D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PloS one 2013; 8: e55104–e55104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, Hjelmeland AB, Rich JN. c-Myc is required for maintenance of glioma cancer stem cells. PloS one 2008; 3: e3769–e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonoda Y, Ozawa T, Hirose Y, Aldape KD, McMahon M, Berger MS, Pieper RO. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res 2001; 61: 4956–60. [PubMed] [Google Scholar]
- 15.Wang Q, Wang Y, Zhang Y, Zhang Y, Xiao W. The role of uPAR in epithelial-mesenchymal transition in small airway epithelium of patients with chronic obstructive pulmonary disease. Respir Res 2013; 14: 67–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Wen M, Kwon Y, Xu Y, Liu Y, Zhang P, He X, Wang Q, Huang Y, Jen KY, LaBarge MA, You L, Kogan SC, Gray JW, Mao JH, Wei G. CUL4A induces epithelial-mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expression. Cancer Res 2014; 74: 520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64: 252–71. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Zaugg K, Mak TW, Elledge SJ. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell 2006; 126: 529–42. [DOI] [PubMed] [Google Scholar]
- 19.Guo G, Xu Y, Gong M, Cao Y, An R. USP28 is a potential prognostic marker for bladder cancer. Tumour Biol 2014; 35: 4017–22. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Wang Y, Ma G, Wang Q, Wei G. CUL4A is overexpressed in human pituitary adenomas and regulates pituitary tumor cell proliferation. J Neuro-oncol 2014; 116: 625–32. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Ma G, Wang Q, Wen M, Xu Y, He X, Zhang P, Wang Y, Yang T, Zhan P, Wei G. Involvement of CUL4A in regulation of multidrug resistance to P-gp substrate drugs in breast cancer cells. Molecules 2013; 19: 159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annibali D, Whitfield JR, Favuzzi E, Jauset T, Serrano E, Cuartas I, Redondo-Campos S, Folch G, Gonzalez-Junca A, Sodir NM, Masso-Valles D, Beaulieu ME, Swigart LB, Mc Gee MM, Somma MP, Nasi S, Seoane J, Evan GI, Soucek L. Myc inhibition is effective against glioma and reveals a role for Myc in proficient mitosis. Nat Commun 2014; 5: 4632–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]