Abstract
Background
Cancer screening recommendations for patients with Lynch-like syndrome (LLS) are not well defined. We evaluated adherence to Lynch syndrome (LS) screening recommendations, cancer risk perceptions, and communication within the families among colorectal cancer (CRC) survivors with LLS.
Methods
Thirty-four participants with LLS completed a questionnaire about risk perception, adherence to LS screening recommendations, and communication with relatives. Clinical data were obtained from medical records.
Results
Most participants (76%) believed they should undergo colonoscopy every 1-2 years. Only 41% correctly interpreted their genetic tests as uninformative negative or as variant of unknown significance for LS. Less than half had had an upper GI endoscopy for screening purpose. Among female participants, 86% had been screened for endometrial cancer and 71% for ovarian cancer. Most participants had informed relatives about the CRC diagnosis and advised them to undergo CRC screening, but only 50% advised female relatives to be screened for endometrial cancer and only one-third advised relatives to have genetic counseling.
Conclusions
Most CRC survivors with LLS follow the same cancer screening recommended for LS patients but do not understand the meaning of LLS. Greater care must be devoted to communicating the implications of non-diagnostic germline mutation testing among patients with LLS.
Keywords: Lynch syndrome, HNPCC, Lynch-like syndrome, screening, colorectal cancer
Introduction
Lynch syndrome (LS) is an autosomal dominant syndrome resulting from a mutation in one of the following DNA mismatch repair (MMR) genes: MLH1, MSH2, MSH6, or PMS2, or in the EPCAM gene [1]. LS predisposes individuals to several cancers, mainly early-onset colorectal cancer (CRC). Other LS-associated cancers include but are not limited to endometrial (EC), gastric (GC), and ovarian (OC) [1-13].
LS is diagnosed based on the presence of a pathogenic germline mutation in the DNA MMR genes or the EPCAM gene. Genetic counseling and testing should be offered to those meeting the clinical criteria, including tumors that show microsatellite instability (MSI) or loss of expression of DNA MMR protein [14].
Because LS mutation carriers are at increased risk for CRC, EC, and other cancers at an early age, screening and preventive measures are recommended [14-16]. According to the United States Multi--Society Task Force on CRC (USMSTF) [14], a colonoscopy is recommended every 1-2 years, beginning at 20-25 or 2-5 years before the youngest CRC diagnosis in the family (whichever is younger). For women, an annual pelvic examination with endometrial sampling and a transvaginal ultrasound (TVU) are recommended beginning at 30-35 or 10 years younger than the earliest known EC or OC diagnosis in the family (whichever is younger). Additionally, an esophagogastroduodenoscopy (EGD) every 2-3 years beginning at 30-35 can be considered on the basis of patient risk factors, and an annual urinalysis beginning at 30-35 is recommended. Hysterectomy and bilateral salpingo-oophorectomy are recommended as a preventive measure for women with LS who have completed childbearing or at age 40. Most of these recommendations are in agreement with the National Comprehensive Cancer Network guidelines [15] and the Mallorca Group guidelines [16].
Most studies examining adherence to screening recommendations in the LS population have focused on CRC. Results have generally shown high rates of colonoscopy adherence in LS mutation carriers [17-23], with some exceptions [24-26]. A few studies have examined adherence to gynecologic screening. Among women with LS, adherence rates for CRC screening are reported to be about 25% higher than those for gynecologic screening (76.4% for CRC, 52.9% for endometrial biopsy, and 51.5% for TVU) [27].
Lynch-like syndrome (LLS), also known as tumor-Lynch, refers to a subgroup of patients with CRC or other LS-related tumors that manifest MSI without MLH1 promoter hypermethylation and show absence of immunohistochemistry (IHC) staining of the DNA MMR proteins but do not have germline mutations or deletions in the DNA MMR genes or in EPCAM. This group is estimated to account for as much as 70% of suspected LS patients [28,29]. The average age of onset of CRC in LLS patients is similar to that of LS patients (53.7 years compared with 48.5 years for LS) and lower than that of sporadic CRC patients (68.8 years, p = .004) [28]. However, the standardized incidence ratio for CRC is lower in LLS patients than in LS patients (2.12 compared with 6.04, p < .001), although still higher than in the general population (1.20, p < .001) [28,29]. Little is known about the perceived risk and current screening behaviors among LLS patients.
We evaluated adherence to LS surveillance and screening recommendations among a sample of CRC survivors with LLS. Secondary aims included assessing communication between participants and their relatives about their cancer, genetic test results, and screening recommendations.
Methods
Participants
The study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Participants were recruited through MD Anderson’s cancer registry and included CRC patients who had undergone tumor studies and genetic testing for a LS mutation and were classified as having LLS.
Inclusion criteria were: (a) CRC diagnosis; (b) 18 years or older; (c) able to read English; (d) able to be contacted by mail; (e) having a tumor that showed MSI-high (MSI-H) and/or abnormal IHC staining for 1 or more of the DNA MMR proteins; (f) having undergone genetic tests for LS and had no mutation or a variant of unknown significance identified; and (g) having undergone genetic counseling at MD Anderson from 1996-2012, which included an explanation of LLS and recommendations for cancer screening as if the patients had LS.
Exclusion criteria were: (a) negative or uninformative tumor studies for MSI or DNA MMR proteins; (b) absence of available genetic test results; (c) pathogenic mutation, deletion, or insertion found in genetic tests for DNA MMR genes or the EPCAM gene (indicating LS); or (d) either BRAF mutation or MLH1 promoter hypermethylation detected in tumor samples taken from patients with abnormal staining for MLH1 (indicating sporadic CRC).
Data Collection Methods
Data were collected using a mailed questionnaire. Non-respondents were called 3 weeks after mailing and given the option to receive a new questionnaire or complete the survey over the phone. For individuals whom we were unable to contact by phone after 5 attempts, a follow-up mailing was sent. An additional call was made at 6 weeks with the option to complete the questionnaire over the phone.
Study Measures
Demographic data were obtained through self-report. MD Anderson medical records and registry data were used to obtain participant and family medical data.
Surveillance and screening adherence
Participants self-reported the use of colonoscopy, EGD, endometrial biopsy, and TVU within the past 6 months, 1 year, 2 years, and 3 years or more before the survey (or never). Participants also were asked why they did or did not have these tests.
Importance and need for screening
Participants were asked about the importance of CRC, EC, OC, and GC screening. Responses ranged from not at all to very important. Participants also were asked whether they believed they needed to be screened on the basis of personal or family history or their genetic test results. Participants were asked whether they had been told to be screened and by whom.
Understanding the meaning of tumor studies and genetic test results
Participants were asked to interpret their genetic test results. Possible responses were positive (a gene mutation compatible with LS was discovered); uninformative negative (a gene mutation was not discovered); variant of unknown significance (a gene mutation was discovered but the mutation was not known to be associated with LS), or not sure. Participants also were asked whether they believed they were at increased risk for LS.
Perceived risk of CRC relapse
Participants self-reported their perceived risk for CRC relapse compared with other CRC survivors or with people their age who had never had CRC. Responses ranged from much lower to much higher. Participants were asked how often they should undergo a colonoscopy; possible responses were every 5 years, 3 years, 1-2 years, or 6 months.
Worry about CRC relapse
Three items from the Lerman cancer worry scale [30] were used to measure participants’ relapse worries. Responses ranged from not at all worried to worried almost all the time. Responses were dichotomized as “worried” or “not worried”.
Communicating with relatives about cancer, screening, and genetic tests
Participants were asked to fill out a table about their communication with their relatives regarding their CRC diagnosis and genetic tests. Participants also indicated whether they had recommended that the relative undergo CRC and EC screening, genetic counseling, or genetic testing.
Statistical Analysis
Primary outcomes were adherence to LS cancer screening recommendations for CRC, EC, OC, and GC. Secondary outcomes were need and importance of being screened for the above cancers, interpretation of genetic test results, perceived risk of and worry about CRC relapse. Summary statistics were calculated for continuous variables; frequencies were calculated for categorical variables. Exploratory association tests were conducted using the Fisher exact test to compare 2 categorical variables, the Kruskal-Wallis test to compare categorical and continuous variables, and Spearman correlation to compare 2 continuous variables (p < .05 was considered statistically significant). Stata v13.1 software (StataCorp LP, College Station, TX) was used for all analyses.
Results
Patient characteristics
78 questionnaires were mailed, 37 (47%) were returned, and the final sample included 34 participants (see Figure 1). Demographic and clinical characteristics are reported in Table 1.
Figure 1.
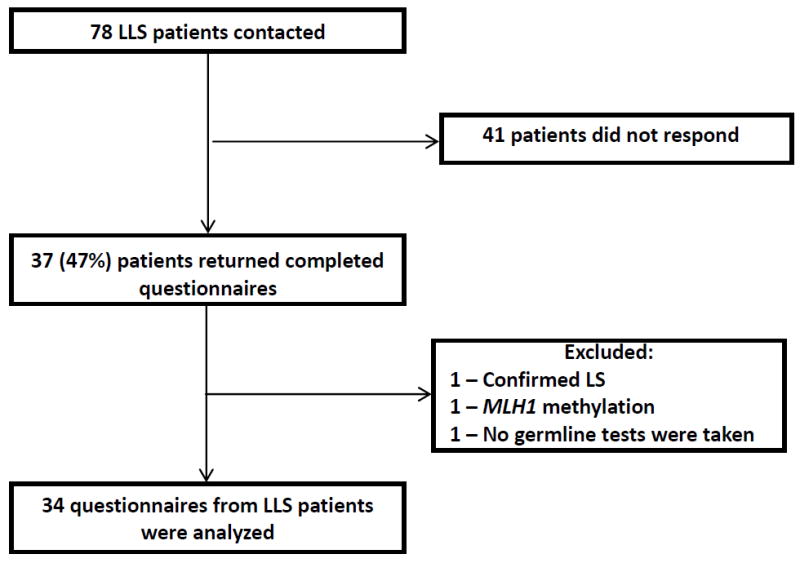
Flowchart showing response rate and exclusions for the survey data collected.
Table 1.
Participant demographic and clinical characteristics (n = 34)
| Characteristic | No. (%) |
|---|---|
| Mean age (standard deviation, range) | 53.9 years (11.5 years, 35.1-77.7 years) |
| Mean age at colorectal cancer diagnosis (standard deviation, range) | 47.6 years (10.9 years, 31-75 years) |
| Female | 17 (50) |
| White | 29 (85) |
| Married | 29 (85) |
| Education >high school | 30 (88) |
| Income >$50,000 | 26 (76) |
| Personal history of another cancer | 12 (35) |
| Family history of cancer | 32 (94) |
| Cancer involved the right colon | 25 (74) |
| Synchronous tumor | 0 (0) |
| Synchronous polyps | 6 (18) |
| Stage III or IV | 16 (47) |
| MSI-H (n=31) | 31 (100) |
| Immunohistochemistry staining | |
| Normal | 3 (9) |
| MLH1 | 20 (59) |
| MSH2 | 8 (24) |
| MSH6 | 1 (3) |
| MLH1 and MSH6 | 1 (3) |
| MSH6 and PMS2 | 1 (3) |
| Genetic testing | |
| Uninformative negative | 22 (65) |
| Variant of unknown significance | 12 (35) |
Table 2 describes the CRC relapse risk perceptions and worry by our participants. While most participants estimated their risk of CRC relapse to be higher than people their age who had never had CRC, less than 1/3 believed that their CRC relapse risk was higher than that of other CRC survivors. Compared with participants who only had CRC, those with CRC and another type of cancer were more likely to believe that they had an increased risk for CRC relapse on the basis of their tumor test results (p = .038). Cancer worry was not associated with any clinical parameter, including time from CRC diagnosis. Interestingly, approximately 1/3 of participants were not sure whether their tumor studies or genetic test results affected their relapse risk.
Table 2.
Colorectal cancer (CRC) relapse risk perceptions and worry among participants (n = 34)
| Item | Response | No. (%) |
|---|---|---|
| Risk for CRC relapse compared with risk of developing CRC in age-matched peers who have never had it | Higher or much higher | 26 (76) |
| Risk for CRC relapse compared with other CRC survivors | Higher or much higher | 10 (29) |
| Increased risk for CRC relapse on the basis of tumor studies | Yes | 18 (53) |
| Increased risk for CRC relapse on the basis of genetic test results | Yes | 16 (47) |
| Risk of CRC relapse on the basis of family history of cancer compared with other people | Higher or much higher | 18 (53) |
| Worries about CRC relaps | Sometimes, often, or all the time | 25 (74) |
| Influence of worry on mood | Somewhat or a lot | 6 (18) |
| Influence of worry on daily activities | Somewhat or a lot | 3 (9) |
Screening behavior
26 participants (76%) believed they should undergo colonoscopy every 1-2 years; 3 participants (9%) thought they should do it even more frequently (every 6 months), and the remaining participants (15%) believed they should repeat it every 3 years or more. CRC relapse worry was associated with the belief that shorter intervals of screening are needed (p = .006), but not with adherence. Almost all participants reported undergoing a colonoscopy within the last 2 years.
19 participants (56%) reported undergoing EGD within the last 3 years. Only 10 participants (53%) who had an UGI endoscopy did so as a part of routine screening or because of family history. Cancer worry was associated with a stronger belief that upper gastrointestinal tract screening is needed (p = .001), but not with adherence to EGD recommendations.
17 women participated in our study. Ten (59%) had undergone hysterectomy, oophorectomy, or both; however, only one-third of them had done it to prevent cancer. Most other female participants (6 patients, 86%) believed they needed to be screened for EC and OC. In fact, almost all (6 participants, 86%) had undergone an endometrial biopsy within the last year, and 5 (71%) had undergone a TVUS within the same period.
Table 3 summarizes participants’ beliefs about cancer screening and screening behavior. We found statistically significant differences in beliefs about the importance of screening by cancer type (p = .001). Unsurprisingly, screening behaviors also differed by cancer type (p = .004).
Table 3.
Screening behavior and beliefs of participants
| Item | Cancer type, no. (%)*
|
p | |||
|---|---|---|---|---|---|
| CRC | UGI | EC† | OC† | ||
| Believe screening is somewhat or very important | 32/33 (97) | 18/33 (55) | 6/7 (86) | 6/7 (86) | 0.001 |
| Told by someone to be screened | NA | 7/34 (21) | 7/7 (100) | 4/7 (57) | <0.001 |
| Last screening test occurred recently‡ | 31/33 (94) | 19/34 (56) | 6/7 (86) | 5/7 (71) | 0.004 |
| Test was specifically for routine screening†† | 28/34 (82) | 10/21 (48) | 6/6 (100) | 4/6 (75) | 0.053 |
CRC, colorectal cancer; UGI, upper gastrointestinal cancer; EC, endometrial cancer; OC, ovarian cancer.
n = 7 (responses analyzed only from patients eligible for the screening).
For colonoscopy, within the 2 years prior to taking the survey; for UGI endoscopy, within the last 3 years; for endometrial biopsy, within the last year; for transvaginal ultrasound, within the last year.
Denominator is the number of participants who underwent the test This group also included participants who underwent testing owing to “increased risk of cancer in my family”.
Understanding the Meaning of Tumor Studies and Genetic Test Results
Most participants believed they had an increased risk for LS based on personal or family history (23 participants, 68%) or tumor studies and genetic test results (25 participants, 74%); however, 59% did not understand the meaning of their tumor studies and genetic test results. Twelve participants (37%) interpreted these results as positive for LS and 7 (22%) were not sure what they meant. Only 13 (41%) recognized that the meaning of the test results was either uninformative negative or variant of unknown significance.
Patient’s Risk perception and screening recommendations for family members
Table 4 describes our participant perceptions of relatives’ cancer risks and recommended screening. Most participants believed their first-degree relatives are at higher risk for CRC as well as LS (61% and 58%, respectively) compared to other people their age, and therefore should be screened every 1-2 years. The rates were smaller for second- and third-degree relatives.
Table 4.
Participant perceptions of relatives’ cancer risks and recommended screening, by type of relative
| Belief | No. of participants with the belief (%)
|
|
|---|---|---|
| For first-degree relatives | For second- and third- degree relatives | |
| Relatives have a higher risk than other people their age to develop colorectal cancer | 20 (61) † | 12 (40) ‡ |
| Relatives should undergo colonoscopy every 1-2 years or less | 20 (61) † | 11 (38) †† |
| Relatives may be at increased risk for Lynch syndrome* | 19 (58) † | 11 (37) ‡ |
Correctly understood test results as uninformative negative or variant of unknown significance.
n = 33 responses.
n = 30 responses.
n = 29 responses.
Communicating with Relatives about Cancer, Screening, and Genetic Testing
All participants reported their CRC diagnosis to at least 1 family member (mean ± standard deviation number of relatives informed: 8.7 ± 6.1; median: 7; range: 1-30), and all patients except 1 reported genetic test results to at least 1 relative. Genetic test results were disclosed to 242 relatives (68%). The three most common reasons for nondisclosure were: 1) not in contact with the relative, 2) assumption that the relative’s parents had told the relative, or 3) belief that the relative was too old to need the information. The number of relatives participants told about the CRC diagnosis was not associated with personal or family history of another cancer or disease stage.
30 participants recommended CRC screening to 223 of 300 of their second- and third-degree relatives (74%), and 22 participants recommended EC screening to 45 of 90 of their second- and third-degree female relatives (50%). 63% Participants advised 67 of 202 relatives (33%) to undergo genetic counseling. Although the genetic test results were all inconclusive, 21 participants reported recommending genetic testing to 85 of 208 relatives (41%).
Discussion
Among CRC survivors with LLS, most worried about and believe that they were at increased relapse risk. Therefore, they adhered to CRC screening recommendations as if they had been diagnosed with LS. Adherence to gynecologic cancer screening recommendations also was high; however, most CRC survivors with LLS in our study had not undergone EGD to screen for GC. Participants adhered to the screening recommendations in parallel with a lack of understanding of the meaning of the genetic test results and the impact of those results on screening recommendations. Participants informed most of their relatives about the CRC diagnosis and advised them to undergo CRC screening; however, only half of the female relatives were advised to be screened for EC, and one-third of relatives were recommended to undergo genetic counseling.
There are three potential explanations for how LLS-associated cancers show MSI and lack DNA MMR proteins but do not show DNA MMR germline mutations [29,31]. First, individuals with LLS may have other, non DNA MMR germline mutations; second, they may have DNA MMR mutations that are not identifiable by current detection methods; third, a different pathologic process within the tumors, not involving germline mutations, such as biallelic somatic mutation [32-34], biallelic MLH1 promoter methylation, or mutation coupled with loss of heterozygosity [33,34] may cause the tumors to manifest MSI. Other possible causes of LLS-associated cancers could include false-positive results showing the MSI [34] or biallelic mutation of the MUTYH gene [35].
Thus, LLS is a heterogeneous group, including patients with both hereditary and sporadic cancers. The only way to distinguish between these two groups is by sequencing the tumor’s DNA MMR genes, which is not routinely done. Consequently, clinicians are limited in their knowledge of which LLS patients should and should not receive LS screening. Until this issue is resolved, the USMSTF guidelines recommend that LLS patients and their relatives continue LS surveillance [14]; the NCCN guidelines state that no consensus has been reached, and genetic testing of the corresponding gene be performed on tumor DNA to assess somatic mutations, although the efficacy of this method has not been proven [15]. Therefore, it is not surprising that physicians lack confidence in the genetic diagnosis of these patients and are unsure which screening recommendations should be offered, if any. LLS is particularly challenging for physicians who, even for patients with a clear LS diagnosis, do not always make the correct recommendations [27,36,37]. Our results indicate that LLS patients believe they should be screened, and most did undergo screening for LS-related cancers, even though they did not understand the meaning or screening implications of their genetic test results.
Participants had a high rate of adherence to LS-associated cancer screening recommendations. These high rates of adherence among LLS patients are consistent with the high rates of colonoscopy adherence among LS patients [17-23], as well as with studies showing that female LS patients have higher rates of adherence to CRC screening than gynecologic screening [27].
Stoffel et al reported that among 174 LS probands, 98% reported disclosing their LS status to at least 1 first-degree relative; however, only 62% reported having disclosed the information to a second- or third-degree relative. The main predictor of information sharing was a mutation-positive proband [38]. In our study, all LLS participants (none having a positive mutation status) reported their CRC diagnosis to at least 1 relative, and only 1 did not report genetic test results to relatives. Only about 2% of participants’ first-degree relatives were not informed of the CRC diagnosis; in contrast, about 30% of second-degree and 40% of third-degree relatives were not informed. Our results show that LLS patients behave similarly to LS patients when it comes to disclosing information to relatives, even in the absence of a definitive (genetic-based) LS diagnosis.
Our study has several limitations. It is based on a limited number of patients and does not compare screening behavior between LLS and LS patients. Furthermore, the variation in time since testing and the range time of when testing was done could have an impact on what information was provided during counseling. However, it is the first study, to the best of our knowledge, to address LLS from the patients’ perspective. Our findings enable health care providers who counsel LLS patients to better understand their knowledge, beliefs, and behaviors to prevent recurrence and additional LS-associated cancers for both themselves and their relatives. Further studies are needed to better understand how beliefs and behaviors differ between LLS and LS patients, as well as how information about cancer screening is communicated within LLS families.
Our results indicate that most LLS-associated CRC survivors believe they are at increased risk for relapse, follow most of the LS screening recommendations, and communicate with their relatives about their CRC and genetic test results; however, most do not attribute their risk to the results of their tumor studies or genetic testing and do not recommend that their relatives undergo genetic counseling. Greater care must be devoted to communicating the implications of MSI-H status and nondiagnostic germline mutation test results to help patients understand the complex and uncertain implications of the testing. This can be done by defining the clinical terms to make them be more understandable by patients. Better understanding of the genetic basis and natural history of LLS is needed to tailor genetic counseling and screening recommendations for LLS-affected CRC survivors.
Acknowledgments
Support provided, in part, by the Patient-Reported Outcomes, Survey, and Population Research (PROSPR) Shared Resource and through a Cancer Center Support Grant, CA 16672, from the National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest: All the authors declare they don’t have any conflict of interest.
References
- 1.Kohlmann W, Gruber SB. Lynch syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews. Seattle, WA: University of Washington, Seattle; 2004. Feb 05, pp. 1993–2014. updated 2014 May 22 [online] [PubMed] [Google Scholar]
- 2.Kastrinos F, Stoffel EM, Balmana J, et al. Phenotype comparison of MLH1 and MSH2 mutation carriers in a cohort of 1,914 individuals undergoing clinical genetic testing in the United States. Cancer Epidemiol. Biomarkers Prev. 2008;17(8):2044–2051. doi: 10.1158/1055-9965.EPI-08-0301. [DOI] [PubMed] [Google Scholar]
- 3.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009;75:141–149. doi: 10.1111/j.1399-0004.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129:415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation negative family members. J Clin Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 7.Park YJ, Shin KH, Park JG. Risk of gastric cancer in hereditary nonpolyposis colorectal cancer in Korea. Clin Cancer Res. 2000;6:2994–2998. [PubMed] [Google Scholar]
- 8.Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–1627. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444–449. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol. 2012;30:958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch HT, Lynch JF, Attard TA. Diagnosis and management of hereditary colorectal cancer syndromes: Lynch syndrome as a model. CMAJ. 2009;181:273–280. doi: 10.1503/cmaj.071574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindor NM, Peterson GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch Syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 13.Lynch H, Lynch JF, Lynch PM, Attard T. Hereditary colorectal cancer syndromes: molecular genetics, genetic counseling, diagnosis, and management. Fam Cancer. 2008;7:27–39. doi: 10.1007/s10689-007-9165-5. [DOI] [PubMed] [Google Scholar]
- 14.Giardiello FM, Allen JI, Axilbund JE, et al. US Multi-Society Task Force on Colorectal Cancer. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014;147:502–526. doi: 10.1053/j.gastro.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colorectal Cancer Screening. Version 2. 2014 doi: 10.6004/jnccn.2010.0003. Available at http://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. [DOI] [PubMed]
- 16.Vasen HF, Blanco I, Aktan-Collan K, et al. Mallorca group. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62:812–823. doi: 10.1136/gutjnl-2012-304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes Halbert C, Lynch H, Lynch J, et al. Colon cancer screening practices following genetic testing for Hereditary Nonpolyposis Colon Cancer (HNPCC) mutations. Arch Intern Med. 2004;164:1881–1887. doi: 10.1001/archinte.164.17.1881. [DOI] [PubMed] [Google Scholar]
- 18.Claes E, Denayer L, Evers-Kiebooms G, et al. Predictive testing for hereditary nonpolyposis colorectal cancer: subjective perception regarding colorectal and endometrial cancer, distress, and health-related behavior at one year post-test. Genetic Testing. 2005 Spring;9:54–65. doi: 10.1089/gte.2005.9.54. [DOI] [PubMed] [Google Scholar]
- 19.Collins VR, Meiser B, Ukoumunne OC, Gaff C, St John DJ, Halliday JL. The impact of predictive genetic testing for hereditary nonpolyposis colorectal cancer: three years after testing. Genet Med. 2007 May;9:290–297. doi: 10.1097/gim.0b013e31804b45db. [DOI] [PubMed] [Google Scholar]
- 20.Pylvanainen K, Kairaluoma M, Mecklin JP. Compliance and satisfaction with long-term surveillance in Finnish HNPCC families. Fam Cancer. 2006;5:175–178. doi: 10.1007/s10689-005-5442-3. [DOI] [PubMed] [Google Scholar]
- 21.Wagner A, van Kessel I, Kriege MG, et al. Long term follow-up of HNPCC gene mutation carriers: compliance with screening and satisfaction with counseling and screening procedures. Fam Cancer. 2005;4:295–300. doi: 10.1007/s10689-005-0658-9. [DOI] [PubMed] [Google Scholar]
- 22.Burton-Chase AM, Hovick SR, Peterson SK, et al. Changes in screening behaviors and attitudes toward screening from pre-test genetic counseling to post-disclosure in Lynch syndrome families. Clinical Genetics. 2013;83:215–220. doi: 10.1111/cge.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 24.Hadley DW, Jenkins JF, Dimond E, de Carvalho M, Kirsch I, Palmer CG. Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2004;22:39–44. doi: 10.1200/JCO.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 25.Ersig AL, Williams JK, Hadley DW, Koehly LM. Communication, encouragement, and cancer screening in families with and without mutations for Hereditary Non-Polyposis Colorectal Cancer: a pilot study. Genetics in Medicine. 2009;11:728–734. doi: 10.1097/GIM.0b013e3181b3f42d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoffel EM, Mercado RC, Kohlmann W, et al. Prevalence and predictors of appropriate colorectal cancer screening in Lynch syndrome. The American Journal of Gastroenterology. 2010;105:1851–1860. doi: 10.1038/ajg.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton-Chase AM, Hovick SR, Sun CC, et al. Gynecologic cancer screening and communication with health care providers in women with Lynch syndrome. Clinical Genetics. 2014;86:185–189. doi: 10.1111/cge.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Soler M, Pérez-Carbonell L, Guarinos C, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144:926–932. doi: 10.1053/j.gastro.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Carethers JM. Differentiating Lynch-like from Lynch syndrome. Gastroenterology. 2014;146:602–604. doi: 10.1053/j.gastro.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med. 1991;114:657–661. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- 31.Boland CR. The mystery of mismatch repair deficiency: lynch or lynch-like? Gastroenterology. 2013;144:868–870. doi: 10.1053/j.gastro.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sourrouille I, Coulet F, Lefevre JH, et al. Somatic mosaicism and double somatic hits can lead to MSI colorectal tumors. Fam Cancer. 2013;12:27–33. doi: 10.1007/s10689-012-9568-9. [DOI] [PubMed] [Google Scholar]
- 33.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146:643–646. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147:1308–1316. doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillejo A, Vargas G, Castillejo MI, et al. Prevalence of germline MUTYH mutations among Lynch-like syndrome patients. Eur J Cancer. 2014;50:2241–2250. doi: 10.1016/j.ejca.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Schroy PC, III, Barrison AF, Ling BS, Wilson S, Geller AC. Family history and colorectal cancer screening: A survey of physician knowledge and practice patterns. American Journal of Gastroenterology. 2002;97:1031–1036. doi: 10.1111/j.1572-0241.2002.05624.x. [DOI] [PubMed] [Google Scholar]
- 37.Batra S, Valdimarsdottir H, McGovern M, Itzkowitz S, Brown K. Awareness of genetic testing for colorectal cancer predisposition among specialists in gastroenterology. The American Journal of Gastroenterology. 2002;97:729–733. doi: 10.1111/j.1572-0241.2002.05556.x. [DOI] [PubMed] [Google Scholar]
- 38.Stoffel EM, Ford B, Mercado RC, et al. Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol. 2008;6:333–338. doi: 10.1016/j.cgh.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


