Abstract
Endoplasmic reticulum (ER) stress-induced unfolded protein response plays a critical role in inflammatory diseases, including allergic airway disease. However, the benefits of inhibiting ER stress in the treatment of allergic airway disease are not well known. Herein, we tested the therapeutic potential of a chemical chaperone, tauroursodeoxycholic acid (TUDCA), in combating allergic asthma, using a mouse model of house dust mite (HDM)-induced allergic airway disease. TUDCA was administered during the HDM-challenge phase (preventive regimen), after the HDM-challenge phase (therapeutic regimen), or therapeutically during a subsequent HDM rechallenge (rechallenge regimen). In the preventive regimen, TUDCA significantly decreased HDM-induced inflammation, markers of ER stress, airway hyperresponsiveness (AHR), and fibrosis. Similarly, in the therapeutic regimen, TUDCA administration efficiently decreased HDM-induced airway inflammation, mucus metaplasia, ER stress markers, and AHR, but not airway remodeling. Interestingly, TUDCA administered therapeutically in the HDM rechallenge regimen markedly attenuated HDM-induced airway inflammation, mucus metaplasia, ER stress markers, methacholine-induced AHR, and airway fibrotic remodeling. These results indicate that the inhibition of ER stress in the lungs through the administration of chemical chaperones could be a valuable strategy in the treatment of allergic airway diseases.
Keywords: ER stress, asthma, inflammation, airway hyperresponsiveness, house dust mite
asthma affects millions of people across the world, leading to significant morbidity, mortality, and health care costs (2, 4, 13, 43). Allergic asthma, the most common type of asthma, is a chronic inflammatory disease characterized by airway inflammation, mucus metaplasia, fibrosis, and airway hyperresponsiveness (AHR), all of which contribute to altered structure of the lungs and decreased lung function (5, 21, 35, 43, 56).
Endoplasmic reticulum (ER) plays a critical role in the synthesis, folding, modification, and transportation of proteins (29, 63). Disruption in ER homeostasis affects protein folding and transportation, leading to the accumulation of unfolded and/or misfolded proteins, a condition termed as ER stress (10, 11, 63). In turn, ER stress activates the unfolded protein response, resulting in the activation of downstream signaling pathways to restore ER homeostasis (10, 11). Furthermore, ER stress plays a critical role in several inflammatory disease conditions, including airway inflammation, chronic airway disorders, and remodeling (9, 15, 25, 33, 34, 51).
Our group has previously shown that house dust mite (HDM) induces components of ER stress, including activating transcription factor 6 (ATF6) α and endoplasmic reticulum resident protein 57 (ERp57), in airway epithelial cells, both in vitro and in vivo (24). Moreover, small-interfering RNA-mediated knockdown of ATF6α and ERp57 in mice during the development of allergic airway pathophysiology resulted in a decrease in ER stress, inflammation, airway fibrosis, and AHR, the fundamental features of allergic airway disease in mice, indicating the significant role of these ER stress mediators in the development of allergic airway disease (23, 24). Furthermore, increases in ERp57 level in the airway epithelium from asthmatic patients compared with healthy normal individuals, and the decrease in HDM-induced airway inflammation, fibrosis, and AHR, in ERp57-deficient mice compared with wild-type mice, supports the significance of ER stress in the development of allergic airway diseases (23).
Chemical chaperones that inhibit ER stress have been shown to exert anti-inflammatory activities in animal models (20, 42, 54). Among the ER stress inhibitor chaperones, tauroursodeoxycholic acid (TUDCA) is clinically used in the treatment of cholelithiasis and cholestatic liver diseases (28). In addition, TUDCA is being used in clinical trials evaluating its efficacy in patients with amyotrophic lateral sclerosis, transthyretin amyloid cardiomyopathy, and hepatobiliary disease in cystic fibrosis (NCT no. 01855360, NCT no. 01855360, and NCT no. 00004441; https://clinicaltrials.gov). To date, several studies in animals have shown that TUDCA, an endogenous ambiphilic bile acid, can inhibit unfolded protein response dysfunction and ameliorate ER stress (46, 58, 61). TUDCA has inhibitory effects on inflammatory diseases, including atherosclerosis, diabetes, obesity, adipocyte inflammation and insulin resistance, stroke, gastrointestinal disorders, cardiovascular diseases, renal injury, and pancreatitis (3, 8, 17, 26, 30, 37, 46, 52, 58). Furthermore, TUDCA exhibited antiapoptotic and cytoprotective activities in animal models of neurodegenerative disorders with associated ER stress (16, 31, 32, 59). Despite these benefits, the therapeutic potential of TUDCA in alleviating allergic airway diseases has not been carefully evaluated.
Our previous findings indicating the importance of ER stress in the development of allergic airway disease, and the beneficial effect of TUDCA on other inflammatory diseases led us to hypothesize that TUDCA would attenuate pathophysiology associated with allergic asthma. Therefore, this study was designed to investigate the therapeutic efficacy of TUDCA in attenuating HDM-induced airway inflammation and remodeling in mice. Our data show that TUDCA administration attenuates HDM-induced ER stress, airway inflammation, mucus metaplasia, airway remodeling, and methacholine-induced AHR.
MATERIALS AND METHODS
Animals.
Eight-week-old female wild-type C57BL/6 NJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in pathogen-free conditions, and the experiments were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
HDM-induced allergic airway disease mouse model.
Mice were anesthetized using isoflurane and exposed to 25 μg of HDM (item no. XPB70D3A2.5, lot no. 269026, containing 35 endotoxin units/mg, normalized to protein content; GREER, Lenoir, NC) extract suspended in PBS through intranasal administration on day 1 and boosted again on day 8. Mice were then administered 25 μg of HDM consecutively on days 15-19 (Figs. 1A, 5A, and 9A). Another set of mice received two additional doses of HDM on days 25 and 27 (see Fig. 9A). Control groups received sterile PBS (vehicle) at all time points.
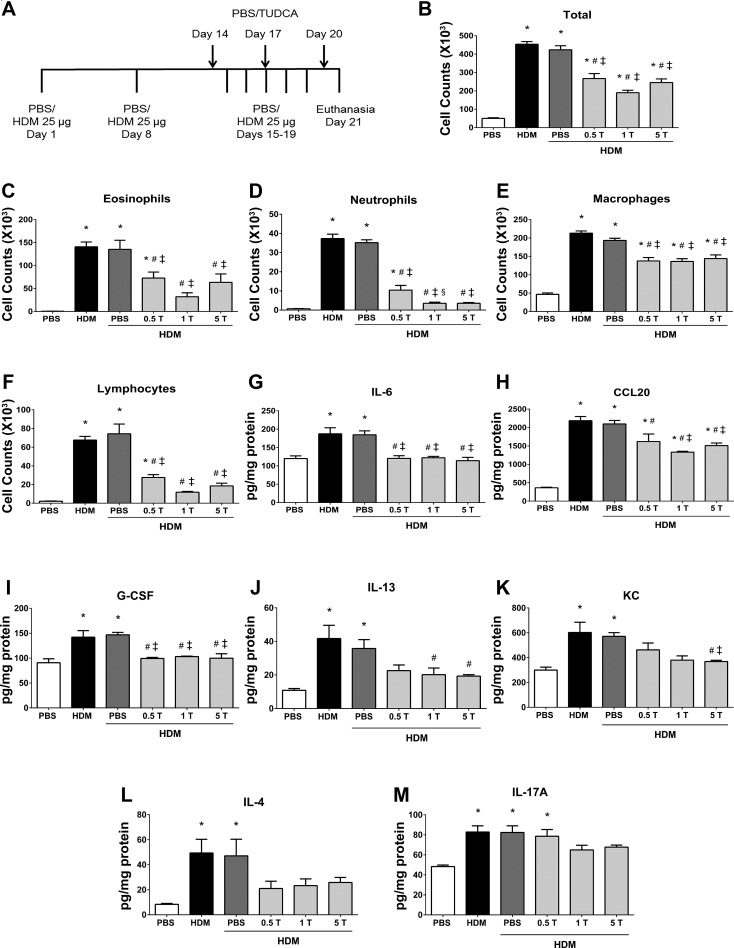
Fig. 1.
Preventive regimen of tauroursodeoxycholic acid (TUDCA) attenuates house dust mite (HDM)-induced airway inflammation. A: schematic representing the time points of HDM or PBS instillation and TUDCA treatment. HDM (25 μg/mouse) was instilled intranasally while TUDCA (0.5, 1, and 5 mg/kg body wt) was administered via nasopharynx during the HDM challenge phase. B–F: analysis of inflammatory and immune cells in the bronchoalveolar lavage fluid (BALF). Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-untreated HDM-challenged mice. ‡P < 0.05 compared with vehicle-treated HDM-challenged mice. §P < 0.05 compared with mice treated with 0.5 mg/kg TUDCA. G–M: analysis of inflammatory cytokines and chemokines in lung lysates. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-untreated HDM-challenged mice. ‡P < 0.05 compared with vehicle-treated HDM-challenged mice.
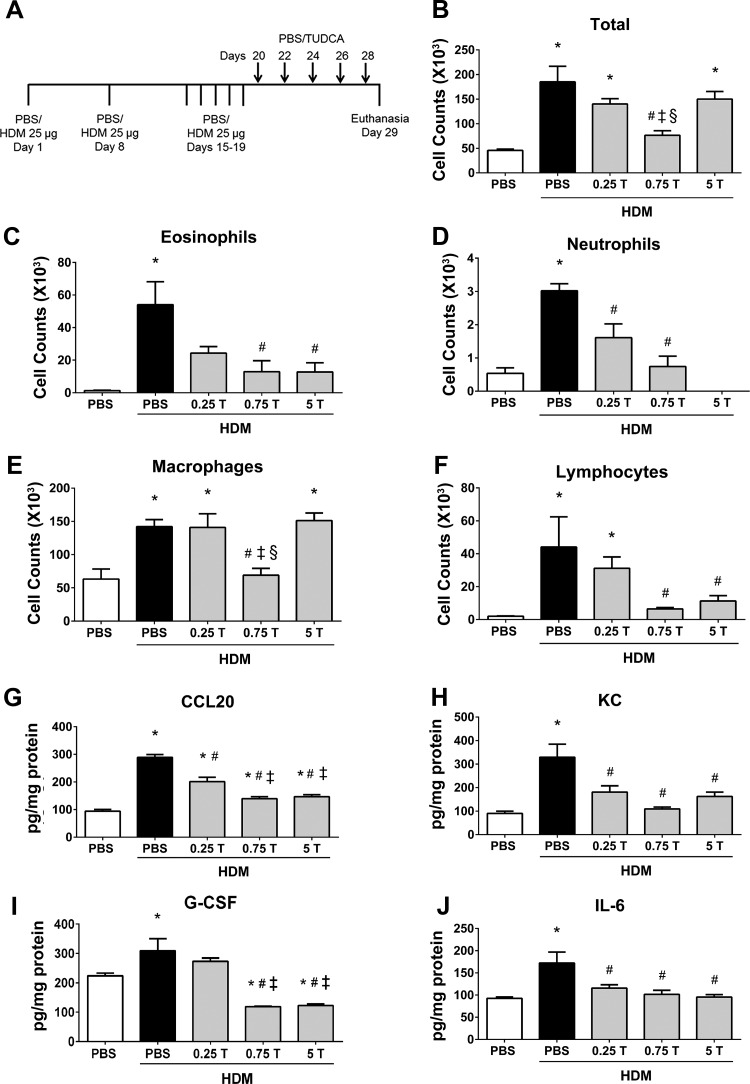
Fig. 5.
Therapeutic regimen of TUDCA decreases HDM-induced airway infiltration of inflammatory cells and decreases inflammatory cytokines and chemokines. A: schematic representing the time points of HDM or PBS instillation and TUDCA treatment. HDM (25 μg/mouse) was instilled intranasally while TUDCA (0.25, 0.75, and 5 mg/kg body wt) was administered via nasopharynx after the HDM challenge phase. B–F: analysis of inflammatory and immune cells in the BALF. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with mice treated with 0.25 mg/kg TUDCA. §P < 0.05 compared with mice treated with 5 mg/kg TUDCA. G–J: ELISA for cytokines and chemokines. Data are means ± SE of 4–6 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with mice treated with 0.25 mg/kg TUDCA.
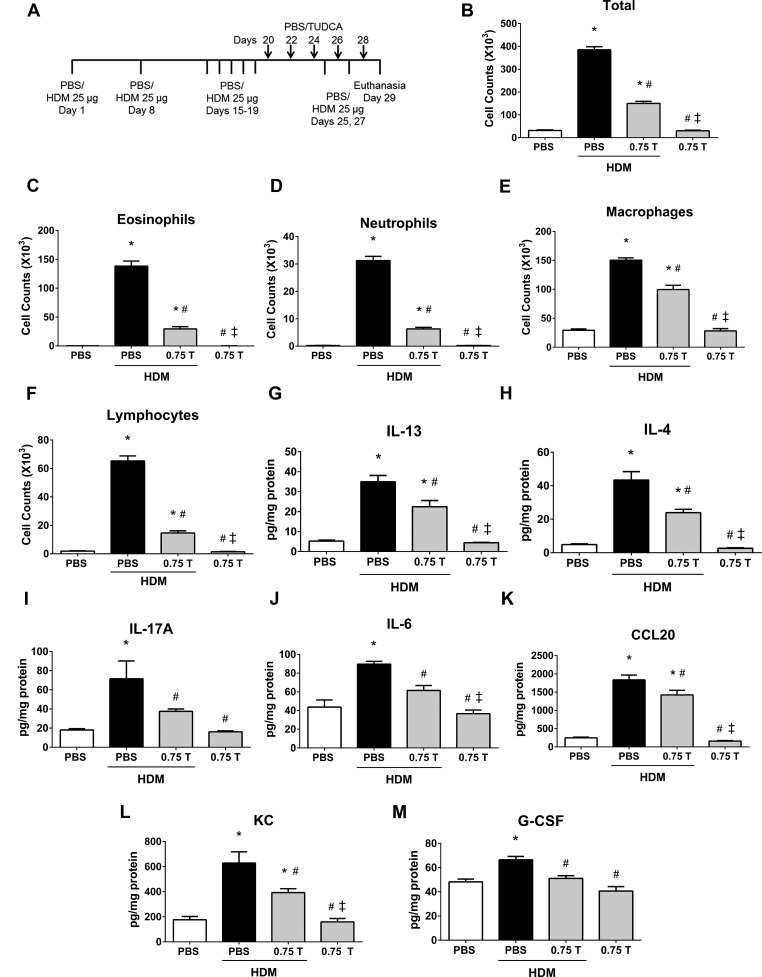
Fig. 9.
Therapeutic regimen of TUDCA decreases HDM-induced airway infiltration of inflammatory cells and decreases inflammatory cytokines and chemokines, regardless of HDM rechallenge. A: schematic representing the time points of HDM or PBS instillation and TUDCA treatment. HDM (25 μg/mouse) was instilled intranasally while TUDCA (0.75 mg/kg body wt) was administered via nasopharynx after the HDM challenge phase. B–F: analysis of inflammatory and immune cells in the BALF. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with HDM-challenged mice treated with 0.75 mg/kg TUDCA. G–M: ELISA for cytokines and chemokines. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with HDM-challenged mice treated with 0.75 mg/kg TUDCA.
TUDCA treatment.
In the experiments testing the preventive regimen, treatment groups were administered TUDCA (0.5, 1, and 5 mg/kg body wt; dissolved in PBS, pH 7.2) via the nasopharynx on days 14, 17, and 20, during the HDM-challenge phase (Fig. 1A) and killed 24 h following the final TUDCA treatment. In the therapeutic regimen experiments, treatment groups received different doses of TUDCA (0.25, 0.75, and 5 mg/kg body wt) on days 20, 22, 24, 26, and 28 (Fig. 5A), and the mice were killed 24 h following the final TUDCA treatment. In the experiments testing the therapeutic regimen along with HDM rechallenge, the treatment group and drug alone control group received 0.75 mg/kg TUDCA on days 20, 22, 24, 26, and 28 (Fig. 9A), and the mice were killed 24 h following the final TUDCA treatment. Administration of TUDCA did not show any overt distress and mortality even at the highest dose (5 mg/kg) tested in this study.
Assessment of AHR.
Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (90 mg/kg), tracheotomized using an 18-gauge cannula, and then mechanically ventilated at 200 breaths/min using a FlexiVent computer-controlled small animal ventilator (SCIREQ). While on the ventilator, mice also received the paralytic pancuronium bromide. The parameters Newtonian resistance, tissue resistance/damping, and tissue stiffness/elastance were calculated as previously described (6, 40). Airway responsiveness is represented as the average of the three peak measurements for each animal, obtained at incremental methacholine doses.
Bronchoalveolar lavage processing.
Bronchoalveolar lavage fluid (BALF) was collected by lavaging lungs with 1.0 ml of sterile PBS. Total and differential cell counts were performed as previously described (23). Briefly, cells were isolated by centrifugation, and total cell counts were enumerated using the Advia 120 Automated Hematology Analyzer System. Differential cell counts were obtained via cytospins using Hema3 stain reagents (Fisher Scientific). Differentials were performed on a minimum of 300 cells/animal.
ELISA.
Lung protein samples were assayed for interleukin (IL)-13, IL-17A, IL-4 (eBioscience, San Diego, CA), IL-6, granulocyte colony-stimulating factor (G-CSF), CCL20 (macrophage inflammatory protein-3), and keratinocyte-derived chemokine [(KC)-CXCL1; chemokine (C-X-C motif) ligand 1] (R & D Systems, Minneapolis, MN) by ELISA according to the manufacturer's instructions. The data are normalized to their total protein levels.
Analysis of mRNA expression from lung tissue samples.
Right lung lobes were flash-frozen and pulverized, and total RNA was isolated using Tri-reagent (Sigma) and purified using the RNeasy kit (Qiagen, Hilden, Germany). One microgram of RNA was reverse transcribed to cDNA for quantitative assessment of gene expression using SYBR green (Bio-Rad, Hercules, CA). Expression values were normalized to the housekeeping gene Gapdh. The primers used in this study are listed in Table 1.
Table 1.
Sequences of primers used for the RT-qPCR analysis
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Atf6 | 5′-ggcactgaggaagcaagaac-3′ | 5′-ggtggccagtctcttgtgat-3′ |
| Chop | 5′-tcttgagcctaacacgtcgat-3′ | 5′-tccggctgttattctggctc-3′ |
| Erp57 | 5′-tatgatgggcctaggactgc-3′ | 5′-tgctggctgcttttaggaat-3′ |
| Gapdh | 5′-aggtcggtgtgaacggatttg-3′ | 5′-tgtagaccatgtagttgaggtca-3′ |
| Gob5 | 5′-actaaggtggcctacctccaa-3′ | 5′-ggaggtgacagtcaaggtgaga-3′ |
| Grp78 | 5′-ccttgtgtttgacctgggtg-3′ | 5′-ccatgacccgctgatcaaag-3′ |
| Muc5ac | 5′-cagtgaattctggaggccaacaaggtagag-3′ | 5′-ctaagcttagatctggttgggacagcagc-3′ |
| Xbp1 s | 5′-ctgagtccgcagcaggtg-3′ | 5′-gacagggtccaacttgtcca-3′ |
Atf6, activating transcription factor 6; Chop, C/EBP homologous protein; Erp57, endoplasmic reticulum protein 57; Grp7, glucose-regulated protein 78; Grp94, glucose-regulated protein 94; Xbp1, X-box-binding protein-1.
Western blot analysis.
Following dissection, right lung lobes were flash-frozen for protein analysis. Lungs were pulverized and lysed in buffer containing 137 mM Tris·HCl (pH 8.0), 130 mM NaCl, and 1% Nonidet P-40. Proteins from cell lysates were prepared in the same buffer. Insoluble proteins were pelleted via centrifugation. Following protein quantitation of the supernatant, samples were resuspended in loading buffer with dithiothrietol and resolved by SDS-PAGE. Proteins were transferred to PVDF, and membranes were probed using a standard immunoblotting protocol. The primary antibodies glucose-regulated protein 78 (GRP78) and CCAAT/enhancer-binding protein homologous protein (CHOP) were purchased from Abcam, ERp57 and glucose-regulated protein 94 (GRP94) were from Stressgen, and β-actin was from Sigma. The quantification of protein expression was performed by densitometry using ImageJ software (imagej.nih.gov/ij/).
Assessment of mucus metaplasia.
Paraffin blocks of lung tissues were cut into 5-μm sections and mounted to slides, and mucus metaplasia was assessed by periodic acid-Schiff staining (PAS). The images were captured using an Olympus BX50 light microscope, and mucus metaplasia was measured by quantifying positively stained area of airway for PAS staining, using MetaMorph software, as described in the previous section. Muc5ac, Gob5, and Xbp1s mRNA expression was assessed via real-time PCR analysis.
Measurement of collagen by hydroxyproline assay.
Collagen content of the lung was measured by determining hydroxyproline content using a method described previously (60). Briefly, the lung lobes were pulverized and hydrolyzed overnight in 2 ml of 6 N HCl at 110°C in sealed glass tubes. The samples were neutralized and incubated with chloramine T solution at room temperature for 20 min. Next, 1.0 ml of perchloric acid solution was added, vortexed, and incubated at room temperature for 5 min. To this, Ehrlich's reagent solution was added and incubated at 60°C for 20 min, and the absorbance was measured at 561 nm. All reagents were obtained from Sigma.
α-Smooth muscle actin immunohistochemistry.
The left lung was inflated by injecting 10% neutral buffered formalin via the trachea and fixed in the same fixative followed by paraffin imbedding. Paraffin blocks were cut into 5-μm sections and mounted to slides. Immunohistochemistry was performed on the paraffin-embedded lung sections to determine the α-smooth muscle actin (α-SMA) content, as described previously (23). Images were captured at a magnification of ×20 using an Olympus BX50 light microscope and analyzed for quantifying α-SMA-positive area of airway using MetaMorph software.
For each sample, three airway were randomly imaged from the top, middle, and bottom regions of lung sections. Using the software, the pixel distances were first calibrated (1 pixel = 0.368 μm) with the loaded lens of an Olympus BX50 Q imaging objective (×1 magnification) for all of the images. For all of the measurements, the color intensity range corresponding to the α-SMA staining was chosen using the settings on three image scales: hue (180/255), saturation (0/240), and intensity (0/104). To measure the color intensity within an airway, the outer and inner layers of airway (labeled area 1 and 2, respectively) were traced using a tracing tool. The inner area was traced to eliminate the lumen of the airway from the calculation. The measurements were exported into a spreadsheet with which total area and color intensities were calculated. The total area was calculated by subtracting the inner outline (area 2) from the outer outline (area 1) of the airway. The color intensity was calculated in a similar manner. The percentage of positive staining was calculated by dividing the threshold color intensity by the total area of airway and multiplying by 100. Averages of each sample were clustered into specific groups, and graphs were plotted.
Statistics.
Data were analyzed by analysis of variance using the Tukey's test to adjust for multiple comparisons. Data from multiple experiments were averaged and expressed as mean values ± SE.
RESULTS
Preventive regimen of TUDCA attenuates HDM-induced airway inflammation.
To evaluate the potential of inhibiting ER stress through the administration of the chemical chaperone TUDCA on pathophysiology associated with allergic asthma, we instilled mice with various doses of TUDCA via the nasopharynx during the HDM challenge phase (Fig. 1A). Analysis of the inflammatory profiles in BALF revealed a significant decrease in total cell counts, eosinophils, neutrophils, macrophages, and lymphocytes in TUDCA-treated HDM-challenged mice compared with vehicle-treated or untreated HDM-challenged mice (Fig. 1, B–F). Our analysis also showed that, although the effects of TUDCA were not dose dependent, there was a marked decrease in total cell and inflammatory cell counts in mice that received the 1 mg/kg TUDCA (Fig. 1, B–F).
Next, we tested whether the levels of inflammatory cytokines and chemokines corresponded to the inflammatory cell counts in these mice. Interestingly, all three doses of TUDCA significantly decreased the levels of IL-6, CCL20, and G-CSF in the lungs from TUDCA-treated HDM-challenged mice compared with vehicle-treated or untreated HDM-challenged mice (Fig. 1, G–I). In addition, 1 and 5 mg/kg TUDCA significantly attenuated IL-13, a Th2-type cytokine (Fig. 1J), whereas only the 5 mg/kg TUDCA significantly decreased the levels of neutrophil chemokine KC (Fig. 1K). Furthermore, all three doses showed trends toward decreasing the Th2-type cytokine IL-4 (Fig. 1L), whereas there was a slight decrease in the Th17-type cytok ine IL-17A with 1 and 5 mg/kg TUDCA (Fig. 1M). These results indicate that the anti-inflammatory effects of TUDCA on airway inflammation are mediated via the attenuation of proinflammatory cytokines and chemokines in the lung. Because we observed similar extent of robust and significant increases in inflammatory cells and cytokines in both vehicle-untreated and vehicle-treated HDM-challenged mice, from here on we have selected vehicle-treated HDM-challenged mice for comparison.
Preventive regimen of TUDCA attenuates HDM-induced ER stress.
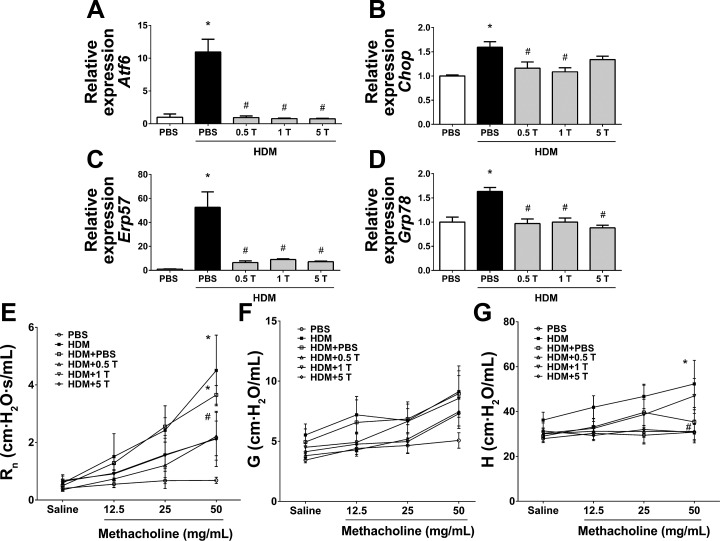
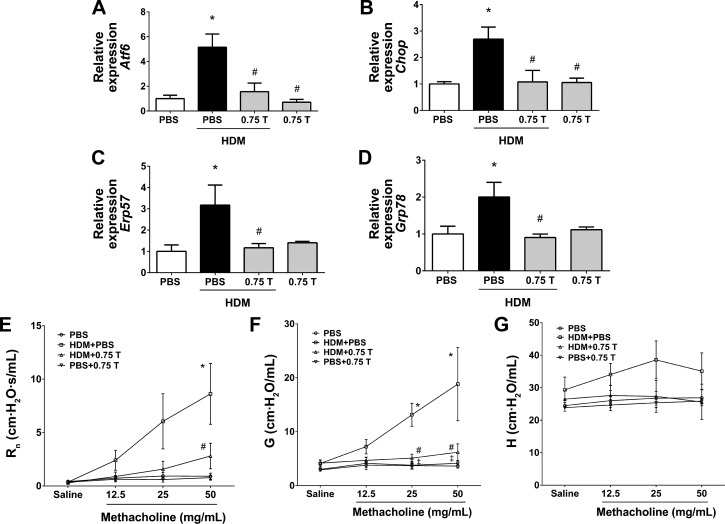
Analysis of the mRNA expression of ER stress markers in the lung lysates indicated significant decrease in HDM-induced expression levels of Atf6, Chop, Erp57, and Grp78 mRNAs in all three groups of TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 2, A–D), indicating that TUDCA effectively decreases HDM-induced ER stress.
Fig. 2.
Preventive regimen of TUDCA inhibits HDM-induced endoplasmic reticulum (ER) stress and protects from methacholine-induced airway hyperresponsiveness (AHR). A–D: mRNA expression analysis of ER stress marker genes in whole lung lysates of vehicle-treated and TUDCA-treated HDM-challenged mice. GAPDH served as housekeeping gene, and data were normalized to GAPDH. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. E–G: analysis of methacholine-induced AHR in mice. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls and #P < 0.05 compared with vehicle-treated or untreated HDM-challenged mice.
Preventive regimen of TUDCA protects from methacholine-induced AHR.
We further assessed the effect of TUDCA on AHR to increasing doses of inhaled methacholine (12.5, 25, and 50 mg/ml). There was a significant decrease in central airway resistance in all three groups of TUDCA-treated mice compared with vehicle-treated or untreated HDM-challenged mice (Fig. 2E). Furthermore, tissue stiffness/elastance was significantly decreased in HDM-challenged mice that received 0.5 and 5 mg/kg TUDCA compared with vehicle-treated or untreated HDM-challenged mice (Fig. 2F). However, we did not observe statistically significant differences in tissue resistance/damping (Fig. 2G). Taken together, these results suggest that TUDCA improves methacholine-induced hyperresponsiveness.
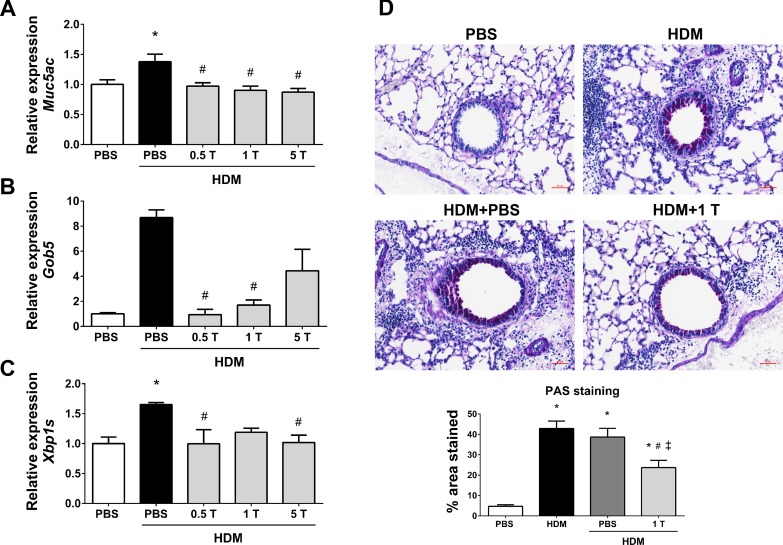
TUDCA in preventive regimen mitigates HDM-induced mucus metaplasia.
ER stress-activated transcription factor X-box-binding protein-1 (XBP-1) is known to regulate mucus production in airway and intestinal epithelial cells (44). Therefore, the mRNA expression of Muc5ac and Gob5, genes associated with mucus production and secretion, were analyzed along with the spliced form of XBP-1 (Xbp1s) in lung lysates. Remarkably, Muc5ac, Gob5, and Xbp1s mRNA expressions were downregulated in TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 3, A–C). All three doses of TUDCA exhibited similar extents of effect on the mRNA expression of Muc5ac and Xbp1s (Fig. 3, A and C). However, we did not observe significant decreases in Gob5 mRNA expression in 5 mg/kg TUDCA-treated HDM-challenged mice compared with other mice (Fig. 3B). Based on these results, we selected 1 mg/kg TUDCA for the PAS staining to explore the extent of mucus metaplasia. Interestingly, TUDCA at 1 mg/kg significantly decreased mucus metaplasia as revealed by the significant reduction in the intensity of the PAS-stained epithelial cells in the airway of the lung sections of TUDCA-treated HDM-challenged mice compared with vehicle-treated or untreated HDM-challenged mice (Fig. 3D). These results suggest that the treatment with TUDCA might be useful in alleviating mucus metaplasia in allergic asthma.
Fig. 3.
Preventive regimen of TUDCA downregulates HDM-induced increases in mucus genes and mitigates mucus metaplasia. A–C: mRNA expression analysis of the genes associated with mucus production and secretion, in whole lung lysates of vehicle-treated and TUDCA-treated HDM-challenged mice. Data were normalized to the housekeeping gene Gapdh. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls and #P < 0.05 compared with vehicle-treated HDM-challenged mice. D: representative images of periodic acid-Schiff (PAS)-stained lung tissue sections of vehicle-treated or untreated and TUDCA (1 mg/kg body wt dose)-treated HDM-challenged (×20 magnification; scale bars = 50 μm) mice and the quantification of percentage of area that positively stained for PAS staining. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-untreated HDM-challenged mice. ‡P < 0.05 compared with vehicle-treated HDM-challenged mice.
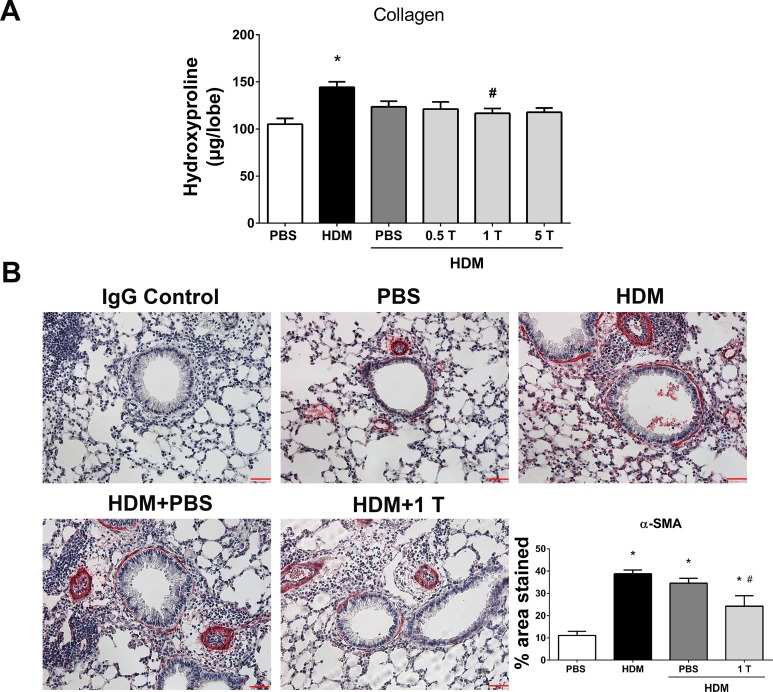
Preventive regimen of TUDCA reduces HDM-induced airway fibrotic remodeling.
The measurement of collagen content by the hydroxyproline assay indicated a decrease in collagen in the lungs of TUDCA-treated HDM-challenged mice compared with vehicle-treated or untreated HDM-challenged mice (Fig. 4A). Our analysis revealed that 1 mg/kg TUDCA alone significantly decreased the collagen content. Therefore, we analyzed the α-SMA content in these mice compared with vehicle-treated or untreated HDM-challenged mice. Analysis of α-SMA immunostaining exhibited significant decreases in α-SMA levels in the peribronchiolar region of lung sections from TUDCA-treated HDM-challenged mice compared with vehicle-treated or untreated HDM-challenged mice (Fig. 4B). Collectively, these experiments strongly support the impact of HDM-induced ER stress in airway inflammation, mucus metaplasia, and subepithelial airway remodeling and demonstrate the potential of TUDCA to alleviate ER stress, methacholine-induced AHR, and remodeling.
Fig. 4.
Preventive regimen of TUDCA decreases HDM-induced airway fibrosis. A: measurement of collagen by hydroxyproline assay. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls and #P < 0.05 compared with vehicle-untreated HDM-challenged mice. B: representative images of α-smooth muscle actin (α-SMA)-stained lung tissue sections of vehicle-treated or untreated and TUDCA (1 mg/kg body wt dose)-treated HDM-challenged mice (×20 magnification; scale bars = 50 μm) and the quantification of percentage of area that positively stained for α-SMA immunostaining. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls and #P < 0.05 compared with vehicle-untreated HDM-challenged mice.
TUDCA in therapeutic regimen decreases HDM-induced airway inflammation.
Based on the preventative effect of TUDCA on the development of allergic airway disease, next we sought to investigate the therapeutic potential of TUDCA administration after the establishment of allergic airway disease. To address this, we administered different doses of TUDCA via the nasopharynx after the HDM challenge phase (Fig. 5A). Analysis of inflammatory cells in BALF indicated that all three doses of TUDCA significantly decreased eosinophils and neutrophils (Fig. 5, C and D). Interestingly, TUDCA at 0.75 mg/kg body wt markedly decreased total cell counts, macrophages, and lymphocytes in HDM-challenged mice (Fig. 5, B, E, and F). Next, we investigated whether the levels of inflammatory cytokines and chemokines. Interestingly, CCL20, KC, G-CSF, and IL-6 were significantly decreased in the lungs from TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 5, G–J). However, we could not detect Th2- and Th17-type cytokines in this regimen. These results indicate that the therapeutic treatment of TUDCA decreased HDM-induced airway inflammation and cytokines.
Therapeutic regimen of TUDCA inhibits HDM-induced ER stress.
ER stress was measured by analyzing the expression of ER stress marker proteins in lung lysates. The expression levels of GRP94 and GRP78 were significantly decreased in TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 6, A–C). Furthermore, there was a trend toward decreased expression of CHOP and ERp57 (Fig. 6, A, D and E). In addition, TUDCA markedly downregulated HDM-induced ER stress, as measured by mRNA expression of Xbp1s, a spliced form of Xbp1 (Fig. 7C).
Fig. 6.
Therapeutic regimen of TUDCA attenuates HDM-induced ER stress and AHR. A: Western blot analysis for ER stress markers in whole lung lysates of vehicle-treated or untreated and TUDCA-treated HDM-challenged mice. β-Actin was used as a loading control. B–E: protein bands were subjected to densitometry, and fold change is represented after normalizing to β-actin. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with HDM-challenged mice. ‡P < 0.05 compared with mice treated with 0.25 mg/kg TUDCA. F–H: analysis of methacholine-induced AHR in mice. Data are means ± SE of 8–10 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice.
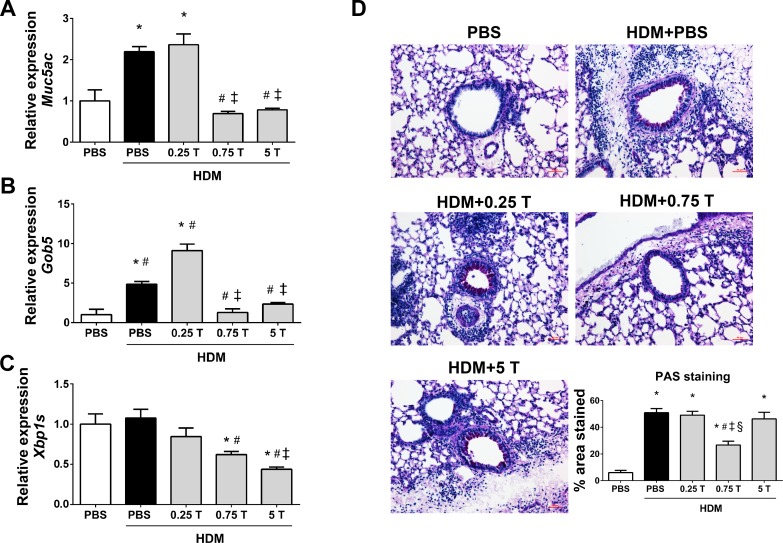
Fig. 7.
Therapeutic regimen of TUDCA downregulates the expression of HDM-induced mucin genes and mucus production. A–C: mRNA analysis of the genes associated with mucus production and secretion in whole lung lysates of vehicle-treated and TUDCA-treated HDM-challenged mice. Data are means ± SE of 5–6 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with mice treated with 0.25 mg/kg TUDCA. D: representative images of PAS-stained lung tissue sections of vehicle-treated or untreated and TUDCA-treated HDM-challenged mice (×20 magnification; scale bars = 50 μm) and the quantification of percentage of area that positively stained for PAS staining. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with mice treated with 0.25 mg/kg TUDCA. §P < 0.05 compared with mice treated with 5 mg/kg TUDCA.
Therapeutic regimen of TUDCA improves methacholine-induced hyperresponsiveness.
Based on the significant therapeutic effects observed, we chose the 0.75 mg/kg TUDCA for AHR studies. Analysis of AHR data revealed that TUDCA markedly decreased methacholine-induced AHR, as indicated by significantly decreased central airway resistance, tissue resistance, and tissue stiffness in HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 6, F–H). These results indicate the beneficial effects of TUDCA-mediated ER stress inhibition in airway inflammation, and AHR.
Therapeutic regimen of TUDCA mitigates HDM-induced mucus metaplasia.
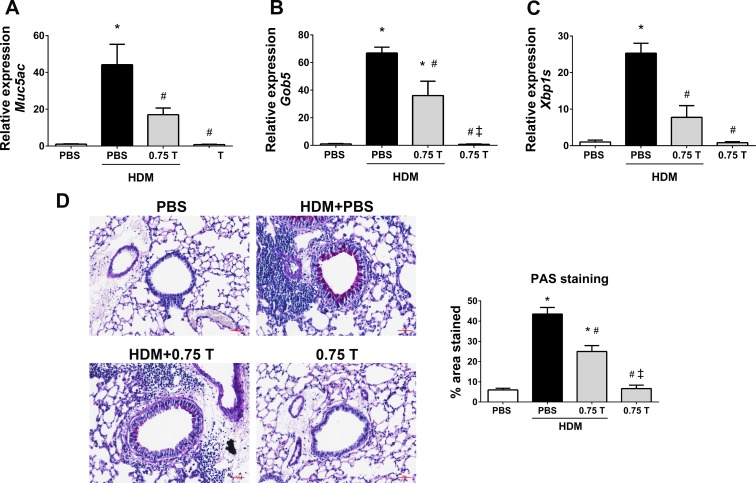
The mRNA expression analysis revealed that the expression of genes associated with mucus, such as Muc5ac, Gob5, and Xbp1s, was significantly downregulated in TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 7, A–C). Furthermore, 0.75 mg/kg TUDCA significantly decreased the intensity of PAS-stained airway epithelial cells in lung sections from TUDCA-treated HDM-challenged mice compared with the vehicle-treated HDM-challenged mice (Fig. 7D). These data indicate that TUDCA could be useful in relieving mucus metaplasia.
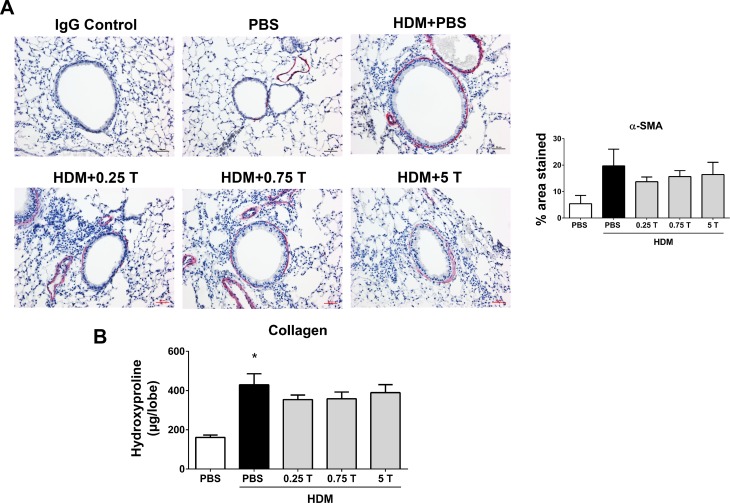
Therapeutic regimen of TUDCA does not protect from HDM-induced airway fibrotic remodeling.
To examine the therapeutic efficacy of TUDCA in reversing structural airway remodeling elicited by allergen exposure, we assessed the airway smooth muscle and collagen content in TUDCA-treated and vehicle-treated HDM-challenged mice. The therapeutic regimen of TUDCA did not alleviate airway fibrosis, as indicated by the absence of changes in peribronchial α-SMA levels in TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 8A). Similarly, analysis of hydroxyproline content did not show a significant change in collagen levels in TUDCA-treated HDM-challenged mice than vehicle-treated HDM-challenged mice (Fig. 8B). Collectively, these results indicate that the therapeutic treatment of TUDCA does not improve HDM-induced airway fibrotic remodeling.
Fig. 8.
Therapeutic regimen of TUDCA does not decrease HDM-induced airway fibrosis. A: representative images of α-SMA-stained lung tissue sections of vehicle-treated and TUDCA-treated HDM-challenged mice (×20 magnification; scale bars = 50 μm) and the quantification of percentage of area that positively stained for α-SMA immunostaining. Statistical differences were not significant between groups. B: measurement of collagen by hydroxyproline assay. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. Statistical differences were not significant between vehicle-treated and TUDCA-treated HDM-challenged mice.
TUDCA decreases airway inflammation regardless of HDM rechallenge.
Although therapeutic regimen of TUDCA effectively attenuated major features of allergic asthma, the inflammatory and immune cell counts and methacholine-induced AHR (central airway resistance) were markedly decreased (∼48 and 70%, respectively) in HDM-challenged mice in the therapeutic regimen experiments compared with HDM-challenged mice in the preventive regimen experiments (Fig. 13, A and B). These comparisons suggested that the disease phenotype may be self-resolving in mice in the therapeutic regimen experiments. Therefore, another set of experiments was carried out similar to that of the therapeutic regimen, in which HDM-challenged mice received two additional HDM rechallenges during the TUDCA treatment phase (Fig. 9A). Here, we chose the most effective (0.75 mg/kg) TUDCA dose for evaluating its efficacy in attenuation of allergic airway disease features. Furthermore, we also used a group of mice receiving 0.75 mg/kg TUDCA without HDM challenge as a control group.
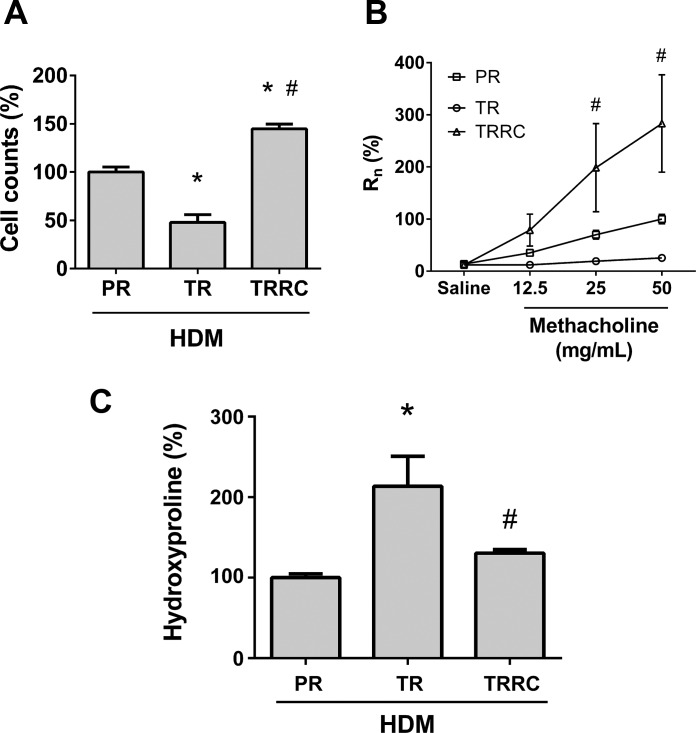
Fig. 13.
Comparison of total cell counts and AHR between HDM-challenged mice. A: percentage of difference in total cell counts in HDM-challenged mouse BALF from preventive (PR) and therapeutic (TR) regimen and therapeutic regimen with subsequent HDM rechallenge (TRRC) experiments. *P < 0.05 compared with PR and #P < 0.05 compared with TR. B: percent difference in methacholine-induced central airway resistance (Rn) of HDM-challenged mice from preventive and therapeutic regimen and therapeutic regimen with subsequent HDM rechallenge experiments. #P < 0.05 compared with TR. C: percent difference in hydroxyproline content of HDM-challenged mice from preventive and therapeutic regimen and therapeutic regimen with subsequent HDM rechallenge experiments. *P < 0.05 compared with PR and #P < 0.05 compared with TR.
Analysis of cells in BALF demonstrated that TUDCA significantly attenuated airway infiltration of inflammatory cells in the rechallenge regimen (Fig. 9B). Interestingly, eosinophils, neutrophils, macrophages, and lymphocytes were markedly decreased in TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 9, C–F). Further investigations indicated that TUDCA significantly attenuated HDM-induced increases in IL-13 and IL-4, the Th2-type cytokines, IL-17A, the Th17-type cytokine, as well as IL-6, CCL20, KC, and G-CSF in the lungs from TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 9, G–M). Furthermore, 0.75 mg/kg TUDCA alone did not alter either inflammatory cell counts or cytokines compared with the PBS alone group (Fig. 9). These results indicate that 0.75 mg/kg TUDCA is effective in the attenuation of airway inflammation in the lung, irrespective of the stages of disease.
TUDCA attenuates allergen-induced ER stress.
TUDCA in the rechallenge regimen significantly decreased HDM-induced expression of Atf6, Chop, Erp57, Grp78, and Xbp1s mRNAs in lung lysates from TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Figs. 10, A-D and 11C), indicating that TUDCA effectively attenuates HDM-induced ER stress.
Fig. 10.
Therapeutic regimen of TUDCA attenuates HDM-induced ER stress and AHR, regardless of HDM rechallenge. A–D: mRNA expression analysis of ER stress marker genes in lung lysates of vehicle-treated and TUDCA-treated HDM-challenged mice. Data were normalized to the housekeeping gene GAPDH. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. E–G: analysis of methacholine-induced AHR in mice. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls and #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with HDM-challenged mice treated with 0.75 mg/kg TUDCA.
TUDCA protects from methacholine-induced AHR.
Analysis of methacholine-induced responsiveness data revealed that TUDCA in the rechallenge regimen markedly decreased methacholine-induced AHR, as indicated by decreased central airway resistance and tissue resistance in HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 10, E and F). Although tissue stiffness showed a trend toward decrease with TUDCA treatment, the effects were not statistically significant (Fig. 10G). Moreover, TUDCA alone in the control group did not affect the parameters of AHR (Fig. 10, E–G). These results suggest that TUDCA plays a protective role in methacholine-induced AHR.
TUDCA alleviates HDM-induced mucus metaplasia.
Therapeutic regimen of TUDCA despite HDM rechallenge significantly downregulated the expression of genes that are associated with mucus production and secretion, including Muc5ac, Gob5, and Xbp1s, in TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 11, A–C). Furthermore, TUDCA markedly decreased the intensity of PAS-stained epithelial cells in the airways of TUDCA-treated HDM-challenged mice than the vehicle-treated HDM-challenged mice (Fig. 11D). Of note, TUDCA alone in the control group did not promote mucus metaplasia (Fig. 11).
Fig. 11.
Therapeutic regimen of TUDCA downregulates the expression of HDM-induced mucin genes and mucus production, regardless of HDM rechallenge. A–C: mRNA analysis of the genes associated with mucus production and secretion in whole lung lysates of vehicle-treated and TUDCA-treated HDM-challenged mice. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with HDM-challenged mice treated with 0.75 mg/kg TUDCA. D: representative images of PAS-stained lung tissue sections of vehicle-treated and TUDCA-treated HDM-challenged mice (×20 magnification; scale bars = 50 μm) and the quantification of percentage of area that positively stained for PAS staining. *P < 0.05 compared with their respective PBS controls. #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with HDM-challenged mice treated with 0.75 mg/kg TUDCA.
TUDCA protects from HDM-induced airway fibrotic remodeling.
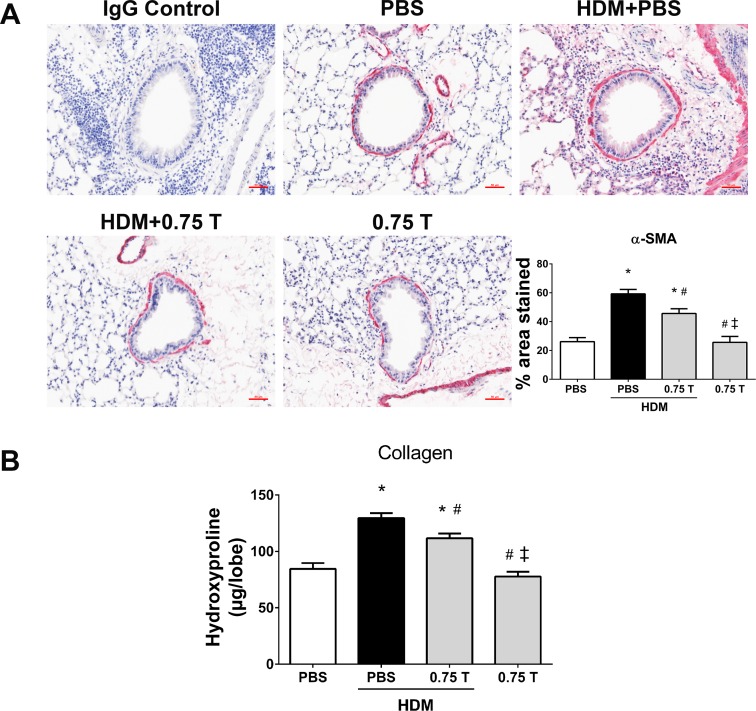
The therapeutic regimen of TUDCA with subsequent HDM rechallenge significantly attenuated airway fibrosis, as indicated by the decrease in peribronchial α-SMA levels in TUDCA-treated HDM-challenged mice compared with vehicle-treated HDM-challenged mice (Fig. 12A). Furthermore, hydroxyproline content of the lungs from TUDCA-treated HDM-challenged mice exhibited a significant decrease in collagen levels compared with vehicle-treated HDM-challenged mice (Fig. 12B). Moreover, TUDCA alone in the control group did not affect any of these parameters (Fig. 12). Taken together, these results strongly indicate that TUDCA at the 0.75 mg/kg body wt dose is safe and effective in attenuating HDM-induced allergic airway disease pathologies, including airway inflammation, mucus metaplasia, airway fibrotic remodeling, and methacholine-induced AHR in mice.
Fig. 12.
Therapeutic regimen of TUDCA decreases HDM-induced airway fibrotic remodeling, regardless of HDM rechallenge. A: representative images of α-SMA-stained lung tissue sections of vehicle-treated and TUDCA (0.75 mg/kg body wt dose)-treated HDM-challenged mice (×20 magnification; scale bars = 50 μm) and the quantification of percentage of area that positively stained for α-SMA immunostaining. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls and #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with HDM-challenged mice treated with 0.75 mg/kg TUDCA. B: measurement of collagen by hydroxyproline assay. Data are means ± SE of 6–8 mice/group. *P < 0.05 compared with their respective PBS controls and #P < 0.05 compared with vehicle-treated HDM-challenged mice. ‡P < 0.05 compared with HDM-challenged mice treated with 0.75 mg/kg TUDCA.
DISCUSSION
Herein, we investigated the potential protective role of TUDCA in alleviating allergic asthma in mice, when administered during development of the disease (preventive regimen), following manifestation of disease features (therapeutic regimen), and the therapeutic regimen with subsequent HDM rechallenge. Our results show that TUDCA in all three regimens efficiently attenuates HDM-induced ER stress, airway inflammation, mucus metaplasia, and methacholine-induced AHR. Interestingly, TUDCA in the preventive regimen, and therapeutic regimen with subsequent HDM rechallenge, but not the therapeutic regimen, significantly reduced airway fibrosis in HDM-challenged mice.
Proper ER function is critical for cell homeostasis due to its indispensable function in protein folding and transport. ER stress is a condition triggered subsequent to disruption of ER homeostasis (10, 11). ER stress has been shown to participate in inflammation and inflammatory diseases, including atherosclerosis, diabetes, obesity, autoimmune, infectious, neurodegenerative, and cardiovascular diseases (11, 58). In addition, our laboratory has demonstrated that HDM-induced allergic airway disease pathologies are linked to ER stress (24). Furthermore, in our previous study, airway epithelial-specific deletion of ERp57 significantly decreased HDM-induced airway inflammation, ER stress, and airway fibrosis (23). Because ER stress contributes to the pathogenesis of inflammatory diseases, the inhibition of ER stress might be advantageous as a therapeutic strategy in the management of allergic airway disease.
This is consistent with several other reports suggesting beneficial effects of reducing ER stress in other diseases. Fu et al. demonstrated that a small-molecule compound, azoramide, which possesses antidiabetic activity, improves protein folding capacity of ER and activates ER chaperone capacity to protect cells against ER stress (20). The chemical chaperones 4-phenylbutyric acid (4-PBA), trimethylamine-N-oxide (TMAO), trehalose, and glycerol are shown to attenuate airway inflammation, mucus metaplasia, and airway remodeling by attenuating ER stress in a mouse model of ovalbumin-induced allergic airway inflammation (42). In another study, 4-PBA significantly decreased ER stress, inflammatory cytokines, dendritic cell infiltration, airway inflammation, and AHR in the mouse model of ovalbumin-induced eosinophil-dominant asthma (33). However, 4-PBA and TMAO have not been used in clinical trials due to their toxicity and/or off-target effects (12, 19, 38, 41, 45, 50, 55, 57, 62). On the other hand, as we stated earlier, TUDCA is currently being used in clinical trials (NCT no. 01855360, NCT no. 01855360, and NCT no. 00004441; https://clinicaltrials.gov). Furthermore, TUDCA has been shown to inhibit acute lung injury-induced inflammation and fibrosis by downregulating the expression of CHOP in the mouse model of bleomycin-induced pulmonary fibrosis (54). Therefore, we have systematically evaluated the efficacy of TUDCA in alleviating hallmarks of allergic asthma. To the best of our knowledge, this is the first report on prophylactic and therapeutic efficacy of TUDCA in attenuating HDM-induced ER stress, inflammation, mucus metaplasia, airway fibrotic remodeling, and methacholine-induced AHR in allergic airway disease.
Previous studies have demonstrated the association between inflammatory and ER stress signaling. Thapsigargin-mediated ER stress upregulated IL-6 and KC expression, and 4-PBA attenuated thapsigargin-induced expression of these proinflammatory cytokines in myeloid-derived suppressor cells (36). Moreover, the activation of XBP-1 has been shown to increase the levels of IL-6 in alveolar macrophages from patients with cystic fibrosis (39). In addition, our laboratory has demonstrated that the HDM-induced ER stress is linked to increased CCL20 and IL-6 in wild-type mice (23). Furthermore, the proinflammatory cytokines (IL-6), Th2 cytokines (IL-13 and IL-4), Th17 cytokines (IL-17A), and chemokines, including CCL20, KC, and G-CSF, have been shown to play a critical role in the pathogenesis of allergic airway disease (1, 7, 18, 47, 48, 64). In this study, TUDCA significantly decreased HDM-induced inflammatory cells in BALF and also significantly decreased IL-13, IL-4, IL-17A, IL-6, CCL20, KC, and G-CSF levels in HDM-challenged mice, either during the preventive regimen or rechallenge regimen. In addition, TUDCA (0.75 mg/kg) administered during the therapeutic regimen phase without HDM rechallenge efficiently decreased HDM-induced inflammatory cells and IL-6, CCL20, KC, and G-CSF levels in mice. The comparison of total cell counts from HDM-challenged mice that did not receive TUDCA indicated a significant decrease in HDM-induced inflammatory cell counts (∼48%) in the therapeutic regimen experiments compared with the preventive regimen experiments (Fig. 13A). However, HDM rechallenges in the therapeutic regimen (rechallenge regimen) experiments markedly increased the inflammatory cell counts compared with both the preventive regimen and therapeutic regimen without HDM rechallenge (∼45 and 300%, respectively; Fig. 13A). Interestingly, TUDCA administered during all three treatment regimens significantly decreased the HDM-induced inflammatory and immune cell counts, inflammatory cytokines and chemokines. These results indicate that TUDCA may play a critical role in attenuation of recruitment and/or activation of inflammatory cells, and the production of inflammatory cytokines and chemokines that are crucial in immune responses.
TUDCA is a well-known ER stress inhibitor chaperone that has been shown to exhibit anti-inflammatory effects (8, 17, 46, 54, 61). Hence, we hypothesized that TUDCA-mediated regulation of immune responses might be mediated through the attenuation of ER stress in the lungs. Interestingly, TUDCA markedly downregulated the expression of HDM-induced ER stress marker genes, including Atf6, Erp57, Xbp1s, Chop, and Grp78 in the lung lysates, both during the preventive and therapeutic regimen with subsequent HDM rechallenge phases. Furthermore, TUDCA (0.75 mg/kg) administered during the therapeutic regimen phase without HDM rechallenge significantly decreased the protein levels of GRP78 and GRP94, and, also, there was a trend toward decrease in CHOP and ERp57 in HDM-challenged mice. Taken together, the effect of TUDCA on ER stress markers corresponds with the decrease in inflammatory cells, cytokines and chemokines, indicating that TUDCA-mediated attenuation of ER stress might play a critical role in attenuating airway inflammation.
Mucus metaplasia is a well-known feature of airway remodeling associated with allergic airway disease (5, 22, 43, 51, 56). ER stress transducer IRE1β-dependent mucin production is mediated by activation of XBP-1, and XBP-1-dependent transcription of anterior gradient homolog 2 (AGR2), a gene associated with mucin production (44). Furthermore, AGR2 has been shown to interact with immature mucin in the ER, and the lack of AGR2 impairs allergen-induced MUC5AC and MUC5B overproduction (51). Here, we found that TUDCA administered during all three treatment regimens markedly decreased the HDM-induced mucus production in airway epithelial cells. In addition, TUDCA significantly downregulated Muc5ac, Gob5, and Xbp1s mRNA expression in HDM-challenged mice. These results indicate that TUDCA attenuates mucus metaplasia by downregulating mucin genes in HDM-induced allergic airway disease. Interestingly, 5 mg/kg TUDCA significantly did not decrease HDM-induced mucus metaplasia, although it decreased mucin genes in therapeutic regimen without HDM rechallenge experiments (Fig. 7). These observations suggest that the higher concentration of TUDCA may have unintended effects, such as increasing mucin gene expression other than Muc5ac, and also the disruption of epithelial barrier functions in the lung, resulting in reverse effect of 0.75 mg/kg TUDCA. However, these parameters have to be substantiated with more experimental evidence in the future.
Airway remodeling is characterized by abnormal subepithelial collagen deposition along airway walls, smooth muscle hypertrophy, epithelial cell hyperplasia, mucus metaplasia, and basement membrane thickening (22). Increased levels of the profibrotic growth factor TGF-β, collagen, and α-SMA in the lung drive airway fibrosis (23, 56). Our results showed that TUDCA in both the preventive regimen and therapeutic regimen with subsequent HDM rechallenge significantly decreased collagen/hydroxyproline content and peribronchiolar fibrosis in the lungs of HDM-challenged mice. Conversely, TUDCA administered during the therapeutic regimen phase without HDM rechallenge did not improve airway fibrotic remodeling, measured by α-SMA or collagen/hydroxyproline content. However, there was a trend toward decrease in α-SMA and hydroxyproline content in these mice.
Interestingly, hydroxyproline quantitation data from HDM-challenged mice without TUDCA treatment indicated a significant increase in HDM-induced airway fibrosis (∼200%) in therapeutic regimen experiments compared with preventive regimen experiments (Fig. 13C). However, airway fibrosis was significantly decreased in the therapeutic regimen with subsequent HDM rechallenge experiments compared with therapeutic regimen without HDM rechallenge (∼60%; Fig. 13C). These observations suggest that the lesser magnitude of airway fibrosis in the presence of active inflammation during the HDM challenge phase could be mitigated by the administration of TUDCA. In contrast, the exacerbated collagen deposition in the absence of active inflammation during the resolution phase might not be efficiently attenuated by TUDCA. In this context, Johnson et al. have demonstrated the increased levels of collagen deposition during the resolution phase compared with the 3 wk HDM-challenge phase, suggesting the role for inflammation in airway remodeling (27). Based on these results, we speculate that TUDCA alone may not be sufficient for the resolution of airway fibrotic remodeling. Nevertheless, TUDCA in combination with antifibrotic agents (53) could be helpful in attenuating all of the pathologies of allergic airway disease.
Furthermore, our findings suggest that TUDCA protects against methacholine-induced hyperresponsiveness, both during and after the establishment of allergic airway disease. TUDCA in all three regimens efficiently protected mice from methacholine-induced AHR, as indicated by decreases in central airway resistance, tissue resistance, and tissue elastance in these mice. The comparison of central airway resistance data from HDM-challenged mice without TUDCA treatment revealed decreases in methacholine-induced AHR in the therapeutic regimen experiments compared with preventive regimen experiments (∼70%; Fig. 13B). On the other hand, central airway resistance was significantly increased in the therapeutic regimen with subsequent HDM rechallenge experiments compared with therapeutic regimen without HDM rechallenge (∼175%; Fig. 13B). These comparisons indicate that the disease phenotype in therapeutic regimen experiments might be self-resolving, and HDM rechallenge in therapeutic regimen settings results in increased methacholine-induced AHR.
Narrowing of the central airway is more likely to be effected by features of airway remodeling such as airway smooth muscle hypertrophy/hyperplasia. Of note, TUDCA significantly attenuated airway fibrosis in both the preventive regimen and the therapeutic regimen with HDM rechallenge by decreasing total collagen content and α-SMA content. Furthermore, α-SMA and hydroxyproline content showed a trend toward decrease in TUDCA-treated HDM-challenged mice compared with HDM-challenged mice. Furthermore, it is well known that the airway epithelium acts as a barrier to inhaled spasmogens, limiting their interaction with airway smooth muscle (14). Therefore, any effect of TUDCA on epithelial integrity is likely to protect against the extent of airway smooth muscle activation and thus central airway resistance. Additionally, TUDCA attenuated the HDM-induced increases in the levels of IL-13, IL-4, IL-17A, IL-6, and chemokines that play crucial roles in asthma pathophysiology (1, 7, 18, 47–49, 64). Henceforth, we speculate that the protective effect of TUDCA on central airway resistance might be mediated through its effect on inflammatory cytokines/chemokines. Furthermore, increased airway responsiveness in mice is primarily due to increased airway closure (40). Therefore, the protective effect of TUDCA on peripheral airway function is likely due to reduced airway closure related to the reported reduction in mucus metaplasia. However, the exact mechanisms underlying the protective role of TUDCA on AHR remain to be determined. Collectively, our findings suggest that treatment with the chemical chaperone TUDCA has an important beneficial effect on both central and peripheral AHR.
In conclusion, the present investigation demonstrates the therapeutic efficacy of TUDCA in alleviating allergen-induced airway inflammation, ER stress, mucus metaplasia, airway fibrotic remodeling, and methacholine-induced AHR, suggesting that the inhibition of ER stress by chemical chaperones could be an important strategy in the management of allergic airway diseases.
GRANTS
This project is supported by grants from the National Heart, Lung, and Blood Institute (R01-HL-122383), the Parker B. Francis Foundation, and the Department of Pathology and Laboratory Medicine-University of Vermont to V. Anathy. G. K. Rattu received a Summer Research Award from the University of Vermont and received payment for research from that award. D. G. Chapman is a recipient of a CJ Martin Fellowship from the National Health and Medical Research Council of Australia (1053790).
DISCLOSURES
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
J.M.S., E.M.N., M.E.P., and V.A. conception and design of research; J.M.S., B.R.M., S.M.H., G.K.R., N.C., J.M.C., K.G.L., N.D., M.A., and V.A. performed experiments; J.M.S. and V.A. analyzed data; J.M.S., E.M.N., D.G.C., D.H.D., and V.A. interpreted results of experiments; J.M.S. and V.A. prepared figures; J.M.S. and V.A. drafted manuscript; J.M.S., E.M.N., B.R.M., S.M.H., G.K.R., J.M.C., K.G.L., D.G.C., D.H.D., M.E.P., and V.A. edited and revised manuscript; J.M.S., E.M.N., B.R.M., S.M.H., G.K.R., N.C., J.M.C., K.G.L., N.D., M.A., D.G.C., D.H.D., M.E.P., and V.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Vermont Lung Center phenotyping core (P30-GM-103532), and UVM microscopy imaging facility.
REFERENCES
- 1.Acciani TH, Suzuki T, Trapnell BC, Le Cras TD. Epidermal growth factor receptor signalling regulates granulocyte-macrophage colony-stimulating factor production by airway epithelial cells and established allergic airway disease. Clin Exp Allergy 46: 317–328, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet 372: 1073–1087, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Amin A, Choi SK, Galan M, Kassan M, Partyka M, Kadowitz P, Henrion D, Trebak M, Belmadani S, Matrougui K. Chronic inhibition of endoplasmic reticulum stress and inflammation prevents ischaemia-induced vascular pathology in type II diabetic mice. J Pathol 227: 165–174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldacci S, Maio S, Cerrai S, Sarno G, Baiz N, Simoni M, Annesi-Maesano I, Viegi G. Allergy and asthma: Effects of the exposure to particulate matter and biological allergens. Respir Med 109: 1089–1104, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. New therapies for asthma: is there any progress? Trends Pharmacol Sci 31: 335–343, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Bates JH, Cojocaru A, Haverkamp HC, Rinaldi LM, Irvin CG. The synergistic interactions of allergic lung inflammation and intratracheal cationic protein. Am J Respir Crit Care Med 177: 261–268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilal S, Haworth O, Wu L, Weylandt KH, Levy BD, Kang JX. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochim Biophys Acta 1812: 1164–1169, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Z, Li F, Gong W, Liu W, Duan Q, Chen C, Ni L, Xia Y, Cianflone K, Dong N, Wang DW. Endoplasmic reticulum stress participates in aortic valve calcification in hypercholesterolemic animals. Arterioscleros Thromb Vasc Biol 33: 2345–2354, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet 19: 111–121, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Cao SS, Kaufman RJ. Unfolded protein response. Curr Biol 22: R622–R626, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Cao SS, Luo KL, Shi L. Endoplasmic reticulum stress interacts with inflammation in human diseases. J Cell Physiol 231: 288–294, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carducci MA, Gilbert J, Bowling MK, Noe D, Eisenberger MA, Sinibaldi V, Zabelina Y, Chen TL, Grochow LB, Donehower RC. A Phase I clinical and pharmacological evaluation of sodium phenylbutyrate on an 120-h infusion schedule. Clin Cancer Res 7: 3047–3055, 2001. [PubMed] [Google Scholar]
- 13.Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet 386: 1086–1096, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Coyle AJ, Uchida D, Ackerman SJ, Mitzner W, Irvin CG. Role of cationic proteins in the airway. Hyperresponsiveness due to airway inflammation. Am J Respir Crit Care Med 150: S63–71, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Delmotte P, Sieck GC. Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca(2+) regulation in airway smooth muscle (ASM). Can J Physiol Pharmacol 93: 97–110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan WM, Rodrigues CM, Zhao LR, Steer CJ, Low WC. Tauroursodeoxycholic acid improves the survival and function of nigral transplants in a rat model of Parkinson's disease. Cell Transplan 11: 195–205, 2002. [PubMed] [Google Scholar]
- 17.Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, Mathis D, Hotamisligil GS. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci Trans Med 5: 156–211, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol 184: 1663–1674, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fogelman AM. TMAO is both a biomarker and a renal toxin. Circ Res 116: 396–397, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu S, Yalcin A, Lee GY, Li P, Fan J, Arruda AP, Pers BM, Yilmaz M, Eguchi K, Hotamisligil GS. Phenotypic assays identify azoramide as a small-molecule modulator of the unfolded protein response with antidiabetic activity. Sci Trans Med 7: 292–298, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol 32: 402–411, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 144: 1026–1032, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman SM, Chapman DG, Lahue KG, Cahoon JM, Rattu GK, Daphtary N, Aliyeva M, Fortner KA, Erzurum SC, Comhair SA, Woodruff PG, Bhakta N, Dixon AE, Irvin CG, Janssen-Heininger YM, Poynter ME, Anathy V. Protein disulfide isomerase-endoplasmic reticulum resident protein 57 regulates allergen-induced airway inflammation, fibrosis, and hyperresponsiveness. J Allergy Clin Immunol 137: 822–832, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME, Anathy V. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir Res 14: 141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu KJ, Turvey SE. Functional analysis of the impact of ORMDL3 expression on inflammation and activation of the unfolded protein response in human airway epithelial cells. Allergy Asthma Clin Immunol 9: 4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao P, Ma J, Feng B, Zhang H, Diehl JA, Chin YE, Yan W, Xu H. FFA-induced adipocyte inflammation and insulin resistance: involvement of ER stress and IKKbeta pathways. Obesity 19: 483–491, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 169: 378–385, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59: 1899–1905, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13: 1211–1233, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep 2: 799, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington's disease. Proc Natl Acad Sci USA 99: 10671–10676, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keene CD, Rodrigues CM, Eich T, Linehan-Stieers C, Abt A, Kren BT, Steer CJ, Low WC. A bile acid protects against motor and cognitive deficits and reduces striatal degeneration in the 3-nitropropionic acid model of Huntington's disease. Exp Neurol 171: 351–360, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kim SR, Kim DI, Kang MR, Lee KS, Park SY, Jeong JS, Lee YC. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kappaB activation. J Allergy Clin Immunol 132: 1397–1408, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Kim SR, Lee YC. Endoplasmic reticulum stress and the related signaling networks in severe asthma. Allergy Asthma Immunol Res 7: 106–117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 18: 684–692, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Lee BR, Chang SY, Hong EH, Kwon BE, Kim HM, Kim YJ, Lee J, Cho HJ, Cheon JH, Ko HJ. Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells. Oncotarget 5: 12331–12345, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhang H, Jiang C, Xu M, Pang Y, Feng J, Xiang X, Kong W, Xu G, Li Y, Wang X. Hyperhomocysteinemia promotes insulin resistance by inducing endoplasmic reticulum stress in adipose tissue. J Biol Chem 288: 9583–9592, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T, Kuljaca S, Tee A, Marshall GM. Histone deacetylase inhibitors: multifunctional anticancer agents. Cancer Treat Rev 32: 157–165, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Lubamba BA, Jones LC, O'Neal WK, Boucher RC, Ribeiro CM. X-box binding protein 1 modulates innate immune responses of cystic fibrosis alveolar macrophages. Am J Respir Crit Care Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med 175: 768–774, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maguire JA, Mulugeta S, Beers MF. Endoplasmic reticulum stress induced by surfactant protein C BRICHOS mutants promotes proinflammatory signaling by epithelial cells. Am J Respir Cell Mol Biol 44: 404–414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makhija L, Krishnan V, Rehman R, Chakraborty S, Maity S, Mabalirajan U, Chakraborty K, Ghosh B, Agrawal A. Chemical chaperones mitigate experimental asthma by attenuating endoplasmic reticulum stress. Am J Respir Cell Mol Biol 50: 923–931, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Martinez FD, Vercelli D. Asthma. Lancet 382: 1360–1372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O'Neal WK, Ribeiro CM. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol 6: 639–654, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizukami T, Orihashi K, Herlambang B, Takahashi S, Hamaishi M, Okada K, Sueda T. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J Vasc Surg 52: 1580–1586, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piyadasa H, Altieri A, Basu S, Schwartz J, Halayko AJ, Mookherjee N. Biosignature for airway inflammation in a house dust mite-challenged murine model of allergic asthma. Biology Open 5: 112–121, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Post S, Rozeveld D, Jonker MR, Bischoff R, van Oosterhout AJ, Heijink IH. ADAM10 mediates the house dust mite-induced release of chemokine ligand CCL20 by airway epithelium. Allergy 70: 1545–1552, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci 8: 1281–1290, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roque T, Boncoeur E, Saint-Criq V, Bonvin E, Clement A, Tabary O, Jacquot J. Proinflammatory effect of sodium 4-phenylbutyrate in deltaF508-cystic fibrosis transmembrane conductance regulator lung epithelial cells: involvement of extracellular signal-regulated protein kinase 1/2 and c-Jun-NH2-terminal kinase signaling. J Pharmacol Exp Ther 326: 949–956, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell Mol Biol 47: 178–185, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seyhun E, Malo A, Schafer C, Moskaluk CA, Hoffmann RT, Goke B, Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 301: G773–G782, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Szabo H, Fiorino G, Spinelli A, Rovida S, Repici A, Malesci AC, Danese S. Anti-fibrotic agents for the treatment of Crohn's disease: lessons learnt from other diseases. Alimen Pharmacol Ther 31: 189–201, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka Y, Ishitsuka Y, Hayasaka M, Yamada Y, Miyata K, Endo M, Kondo Y, Moriuchi H, Irikura M, Tanaka K, Mizushima T, Oike Y, Irie T. The exacerbating roles of CCAAT/enhancer-binding protein homologous protein (CHOP) in the development of bleomycin-induced pulmonary fibrosis and the preventive effects of tauroursodeoxycholic acid (TUDCA) against pulmonary fibrosis in mice. Pharmacol Res 99: 52–62, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116: 448–455, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tully JE, Hoffman SM, Lahue KG, Nolin JD, Anathy V, Lundblad LK, Daphtary N, Aliyeva M, Black KE, Dixon AE, Poynter ME, Irvin CG, Janssen-Heininger YM. Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. J Immunol 191: 5811–5821, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ufnal M, Zadlo A, Ostaszewski R. TMAO: A small molecule of great expectations. Nutrition 31: 1317–1323, 2015. [DOI] [PubMed] [Google Scholar]
- 58.Vang S, Longley K, Steer CJ, Low WC. The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of nonliver diseases. Global Advan Health Med 3: 58–69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viana RJ, Nunes AF, Castro RE, Ramalho RM, Meyerson J, Fossati S, Ghiso J, Rostagno A, Rodrigues CM. Tauroursodeoxycholic acid prevents E22Q Alzheimer's Abeta toxicity in human cerebral endothelial cells. Cell Mol Life Sci 66: 1094–1104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93: 440–447, 1961. [DOI] [PubMed] [Google Scholar]
- 61.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36: 592–601, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Xu R, Wang Q, Li L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genomics 16, Suppl 7: S4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol 111: 227–243, 2003. [DOI] [PubMed] [Google Scholar]