Abstract
Libby amphibole (LA) causes a unique progressive lamellar pleural fibrosis (LPF) that is associated with pulmonary function decline. Pleural fibrosis among the LA-exposed population of Libby, MT, has been associated with the production of anti-mesothelial cell autoantibodies (MCAA), which induce collagen production from cultured human mesothelial cells. We hypothesized that the progressive nature of LPF could be at least partially attributed to an autoimmune process and sought to demonstrate that LA-induced MCAA trigger collagen deposition in vivo. C57BL/6 mice were exposed to LA for 7 mo, and serum was tested for MCAA by cell-based ELISA on primary mouse mesothelial cells. When treated in vitro with serum from mice exposed to LA, mesothelial cells upregulated collagen matrix production. This effect was lost when the serum was cleared of IgG using protein G beads, implicating IgG autoantibodies. Using the peritoneal cavity as a surrogate for the pleural cavity, groups of naïve (non-asbestos-exposed) mice were injected intraperitoneally with 1) control serum, 2) one dose of serum from LA-exposed mice (LA serum), 3) two doses of LA serum, or 4) two doses of LA serum cleared of IgG. After 1 mo, analysis of collagen in peritoneal walls using two-photon confocal microscopy (SHG analysis) and a hydroxyproline assay demonstrated significant increases in collagen by LA serum but not control or cleared serum. These data support the hypothesis that MCAA in LA-exposed mice induce fibrotic responses in vivo, demonstrating that an autoimmune component may be contributing to the progressive pleural fibrosis seen in LA-exposed patients.
Keywords: amphibole asbestos, autoimmunity, pleural/peritoneal fibrosis
vermiculite is a micaceous mineral that has many uses, most commonly as insulation. From the early 1900s to the 1970s, the largest producer of vermiculite in the United States was a mine near the small town of Libby, MT. The vermiculite mined there and shipped throughout the country was contaminated with a combination of asbestiform minerals in the amphibole family (16). Over the years, residents in and around the town of Libby were exposed to the contaminated vermiculite, which was later linked to profound health problems, including respiratory disease and systemic autoimmune disease (1, 12, 24).
Asbestos-related diseases noted in the Libby population include both malignant (mesothelioma) and nonmalignant pleural disease (1, 19, 31), in addition to classical pulmonary fibrosis termed asbestosis (11, 15, 19). However, pleural disease is the most prevalent disease in Libby, causing significant morbidity and mortality (19, 32). Interestingly, an increased risk for systemic autoimmune diseases, specifically systemic lupus erythematosus, rheumatoid arthritis, and systemic sclerosis, has also been reported (18). This was further supported by reports of an increased frequency of positive antinuclear autoantibody (ANA) tests in this population and other cohorts exposed to amphibole asbestos in particular (24, 25). Further examination of potential health effects of the Libby amphibole (LA) revealed that it could induce autoantibodies in mice (and rats) (23, 26) while chrysotile asbestos exposure did not (7). These data strongly support the ability of LA to induce autoimmune responses in exposed individuals, with some evidence suggesting this immune dysfunction contributes to the incidence of pulmonary disease (14, 24).
Studies were undertaken to attempt to understand the mechanisms whereby autoantibodies could play a role in the predominant nonmalignant pulmonary diseases seen in Libby. This led to the discovery of tissue-specific autoantibodies in addition to the ANA, including anti-fibroblast antibodies in LA-exposed mice (22) and mesothelial cell autoantibodies (MCAA) in a LA-exposed cohort (14). These autoantibodies were subsequently shown to induce collagen production in cultured fibroblasts and mesothelial cells, respectively (22, 28), suggesting that the LA-induced autoantibodies could contribute to the fibrotic process. The public health significance of this was further highlighted by evidence that the LA nonmalignant pleural disease was more severe and progressive than what is classically described for most occupational (chrysotile) asbestos exposures and that the radiographic lesions were clearly associated with pulmonary function decline (2, 12, 30).
The current study was undertaken in a mouse model to specifically demonstrate the ability of the autoantibodies to induce collagen production in serous membranes in vivo, providing a novel immunological explanation for this uniquely severe and progressive pleural disease, now being termed “LA Disease.” The approach was to 1) demonstrate the presence of MCAA in serum of mice exposed to LA, 2) demonstrate the ability of these antibodies to induce collagen production in vitro, and 3) demonstrate the ability of the antibodies to induce collagen production in peritoneal walls following intraperitoneal instillation in mice never exposed directly to the asbestos fibers.
MATERIALS AND METHODS
Mice.
The Idaho State University Institutional Animal Care and Use Committee approved all experiments on mice [Institutional Animal Care and Use Committee (IACUC) protocol no. 692]. The mice used were wild-type C57BL/6 (Jackson Laboratories, Bar Harbor, ME) raised in-house in the Idaho State University Animal Care Facility. These mice were housed under specific pathogen-free conditions with a 12:12-h light-dark cycle, constant temperature (22°C) and humidity (45%), and ad libitum access to standard rodent chow and filtered water.
Collection and culture of primary mouse mesothelial cells.
Mice were killed by CO2 asphyxiation. The abdominal skin was removed to reveal the peritoneum. Following peritoneal wash with warmed sterile PBS, the peritoneal wall was cut away from the right and left side of the mouse. Each wall was placed inside down immediately in a six-well cell culture plate (Costar Corning, Corning, NY) containing 3 ml/well RPMI medium (Mediatech, Manassas, VA) supplemented with 10% FBS (Atlanta Biologics, Lawrenceville, GA), insulin, transferrin, sodium selenite, and ampicillin B, penicillin/streptomycin (all from Sigma, St. Louis, MO). The walls were flattened with a sterile microscope slide cut to fit into the culture plate wells. The plates were placed in a humidified CO2 incubator kept at 37°C, replacing media every 2 days until cell growth was observed on the bottom of the wells.
After colonies of cells had begun to grow on the plate, the tissue was removed, and cells were fed fresh media every 4 days. Cells were allowed to grow to confluency, which took 2–3 wk. At this time the wells were scanned for colonies of cell morphology that matched those of a commercially supplied mesothelial cell line (Met5A; ATCC, Manassas, VA). The locations of colonies were identified by placing a blue mark just to the right of a colony of interest, and images were captured at ×200 with a Leica DMIL inverted microscope using a Leica DFC295 digital camera (Leica Microsystems, Buffalo Grove, IL).
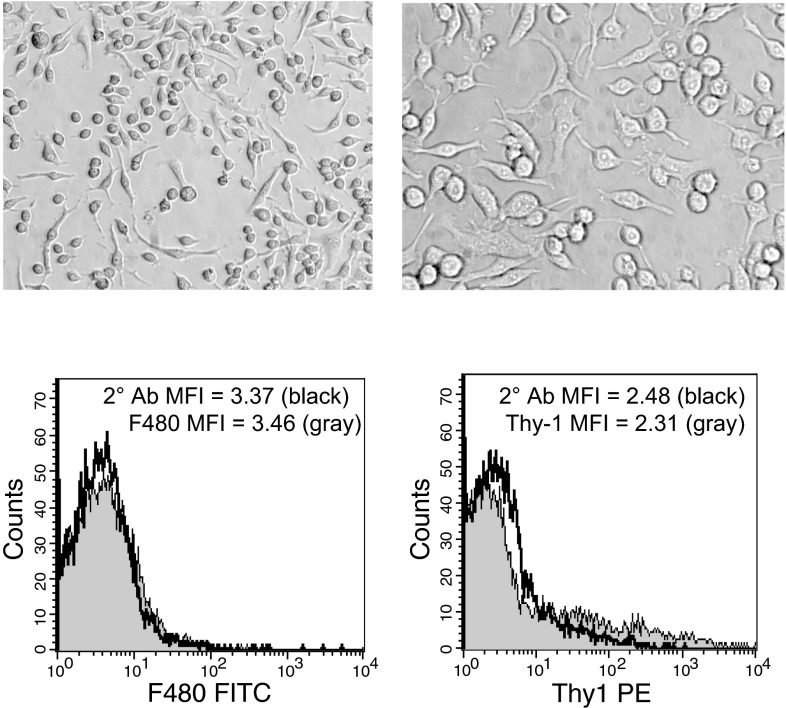
Colonies of interest were scraped with a fine point of a disposable pipette tip. Cells were suspended in media via pipetting, transferred to a T-25 flask (Costar Corning), and grown to confluency. Flow cytometric analysis was used to confirm that cultures were not contaminated by macrophage or fibroblast cells. Briefly, cells were stained with antibodies against macrophage (anti-F480; Serotec, Raleigh, NC) and fibroblast-specific markers [anti-mouse Pan-Reticular Fibroblast Marker (Cedarlane, Hornby, Ontario); anti-CD90.2 (Thy-1) (eBioscience, San Diego, CA)] and then stained with appropriate fluorescence-conjugated secondary antibodies (Invitrogen, Eugene, OR). Cell staining was assessed via flow cytometry (FACS Calibur; BD Biosciences) using staining with secondary antibodies only as background on gated live cell populations. Cells were determined to be primary mesothelial cells if they showed mesothelial cell morphology (Fig. 1) and lacked both fibroblast- and macrophage-specific markers. Cells were only used for the studies from flasks showing no difference in median fluorescence intensity between the secondary antibody-only staining and the specific macrophage or fibroblast markers. Representative histograms are shown in Fig. 1.
Fig. 1.
Images of primary mouse mesothelial cells in culture. Images were taken with a Leica DFC295 digital camera attached to a Leica DMIL inverted microscope. Left, ×200 magnification; right, ×400 magnification. These cultures were shown to be negative for markers of macrophages (bottom left) and fibroblasts (bottom right) by flow cytometry. MFI, median fluorescence intensity; 2° Ab, secondary antibody only.
Intratracheal instillations.
Mice were exposed to LA (U.S. Geological Survey) or saline by intratracheal injection of fibers suspended in sterile 1× PBS to a final concentration of 1 mg/ml, as previously described (23). Mice were injected in the peritoneum with a mixture of 100 mg/kg ketamine and 1 mg/kg butorphanol (MWI Veterinary Supply, Boise, ID) for sedation, and then their necks were wiped down with ethanol wipes and shaved. An incision exposed the trachea, and 30 μl of the 1 mg/ml LA fiber suspension or vehicle were injected down the trachea. The incision was closed using VetBond adhesive and cleaned with betadyne. Mice recovered from the procedure in a warmed cage before being returned to normal caging. At the end of the 7-mo period, mice were killed by CO2 asphyxiation (according to IACUC guidelines), and blood was harvested by cardiac puncture, allowed to clot, and then centrifuged for serum collection.
Detection of mouse MCAA in asbestos-exposed animals.
A cell-based ELISA was performed as previously described (14) to test for the binding of serum antibodies to mesothelial cells. Briefly, mouse primary mesothelial cells were seeded at confluency on 96-well plates, attached overnight, and fixed in 1% paraformaldehyde. Following washing with PBS-Tween (0.05%), cells were blocked with 5% nonfat dry milk/PBS and then exposed to pooled sera from each of the treatment groups diluted in 3% BSA/PBS (1:100). Following a 2-h incubation with serum, cells were washed and blocked a second time. The secondary antibody horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Invitrogen) was applied at a dilution of 1:1,000 in 3% BSA/PBS and incubated for 1 h. Excess antibody was removed, and plates were developed using TMB reagent (Thermo Scientific) followed by 50 μl 1 M HCl. Plates were analyzed at 450 nm on a microtiter plate reader (BioTek Instruments, Winooski, VT). Correction for nonspecific secondary antibody binding was performed on a plate-to-plate basis by subtracting the mean optical density (OD) for the secondary antibody only control wells from the mean OD of each sample. Samples were determined to be MCAA-positive (MCAA+) if the corrected OD was at least three SDs above the mean OD for control (normal, untreated) mouse serum or serum cleared of IgG (below).
Protein G clearance of IgG antibodies from mouse sera.
Mouse serum was cleared of MCAA by removal of all IgG antibody using protein G beads (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. Briefly, pooled MCAA+ serum was diluted 1:3 in binding buffer (0.1 M sodium phosphate, 0.15 sodium chloride, pH 7.4), mixed with protein G beads at a 1:1 ratio, and incubated on a rotator overnight at 4°C. The suspension was then centrifuged at 200 g for 5 min, and a visible pellet was observed. Supernatant was concentrated back to original volume using an Amicon Ultra 10K Centrifugal Filter (Millipore, Austin, TX) and centrifuging at 14,000 g until original volume was reached. Retained suspension was transferred to a clean tube and stored at −20°C until use. Cleared serum was separated by SDS-PAGE to confirm IgG heavy and light chain bands were no longer present.
In vitro collagen assay.
Primary mouse mesothelial cells were seeded in a 96-well plate to confluency and allowed to attach to the plate overnight at 37°C. Wells were treated with 2 μl/well of either pooled MCAA+ serum from mice exposed to LA (LA serum), the same serum cleared of all IgG (cleared serum), pooled serum from mice exposed to saline (saline serum), or 1× PBS as a negative control. Cells were then allowed to incubate for 4 days. Wells were rinsed with 200 μl of PBS-Tween (0.05%) four times for 4 min each time. Wells were then blocked for 1 h with 5% nonfat dry milk, and then 100 μl of rat anti-collagen I antibody (Abcam, Cambridge, MA) were applied at a dilution of 1:1,000 in 3% BSA/PBS and incubated overnight at 4°C. Wells were washed and blocked as before, and 100 μl of anti-rat HRP-conjugated secondary antibody (Invitrogen) were applied at a dilution of 1:1,000 in 3% BSA/PBS and incubated overnight. Excess antibody was removed, and plates were developed using TMB reagent followed by 50 μl 1 M HCl. Plates were analyzed at 450 nm on a microtiter plate reader.
Intraperitoneal serum injections.
Pooled LA serum was diluted 1:20 in 1× PBS. The mice were instilled intraperitoneally using 23-gauge needles, with serum diluted to give ∼150 μg IgG in 300 μl sterile saline. Four mice were used for each treatment group as follows: saline serum, LA serum, and cleared serum. Second injections (doses) were given 2 wk after the first injection in an attempt to simulate a subacute immune response. Mice were killed 1 mo after the second injection.
Peritoneal wash and tissue harvest.
After death, mouse abdomens were cleaned with ethanol, and a small incision was made from lower to upper abdomen with care taken to not cut the peritoneal wall. Skin was physically removed from the base of the abdomen to the rib cage to expose the peritoneal cavity. Peritoneal washes were performed by injecting 5 ml of warmed sterile PBS using a 23-gauge needle, and, after the mouse was briefly rocked, 4 ml of fluid were removed with an 18-gauge needle. The fluid was centrifuged to pellet the cells (for flow cytometry, below), and supernatant was collected for soluble collagen assay. Use of equivalent wash volumes allowed standardization of soluble collagen (below) from mouse to mouse. Peritoneal walls were cut at midline, removed, and stored at 4°C in six-well plates submerged in 2 ml of HistoChoice fixative (Amresco, Solon, OH) with a slide holding them flat.
Flow cytometry: Peritoneal cell populations.
Pelleted cells from the serum-instilled mice were washed in PBS and then suspended in 100 μl of PBS with 3% BSA for staining, using the following combination of antibodies (all from BD Biosciences, San Jose, CA): CD19-PE, IgM-PerCP-Cy5.5, and CD5-APC. Cell populations were analyzed using the FACS Calibur flow cytometer, gating out cell debris, red blood cells, and doublets, as follows: macrophages, granulocytes, and lymphocytes by forward scatter × side scatter; B cells = CD19 positive; T cells = CD5 positive; B1a B cells = CD5 and IgM positive (21). All data are given as percent of the parent population, either percent of all peritoneal cells or percent of lymphocytes.
ANA assay.
Serum collected from the serum-instilled mice was assayed for ANA using commercial HEp2 ANA slides from ImmunoConcepts (Sacramento, CA). Serum was diluted 1:80 in PBS/BSA, and 20 μl were placed on each well of the slides. Slides were incubated, washed, and stained with anti-mouse IgG conjugated to AlexaFluor488 (Life Technologies). All slides were blinded and evaluated by two readers using the same settings on a Leica DMR fluorescence microscope using the ×40 objective. Background percent positive in mice has previously been shown to be around 15–25% (7, 23).
Sircol collagen assay.
The concentrations of soluble collagen in peritoneal washes from mice treated intraperitoneally for 1 wk with LA serum or saline serum as described above were determined using a Sircol Collagen Assay (Biocolor, Carrickfergus, North Ireland) according to the manufacturer's instructions. Following collagen extraction from peritoneal wash fluid and addition of the Sircol dye, samples were analyzed using a microtiter plate reader with absorbance measured at 562 nm. Mean OD of samples were normalized to reagent blanks.
Multiphoton confocal microscopy.
Collagen deposition was quantified by second-harmonic generation imaging microscopy (20) on an Olympus multiphoton confocal microscope and using MetaMorph image analysis software (Molecular Devices, Sunnyvale, CA). Peritoneal walls were pinned to a wax substrate and submerged in 1× PBS during imaging. All software settings were held constant. Each half of a peritoneum was imaged four times at random fields, and the mean two-photon excited fluorescence intensity (collagen density) of each image was recorded. The mean intensity for each half of the peritoneum was calculated and then averaged with the other half to give an overall reading for each mouse. A mean for each treatment group was calculated using average intensities for each mouse in the treatment group.
QuickZyme hydroxyproline assay.
The amount of collagen in the peritoneal walls of mice treated with saline serum and LA serum was determined by using QuickZyme Total Collagen Assay (QuickZyme Biosciences, Leiden, The Netherlands) according to the manufacturer's instructions. Three same-sized tissue punches were obtained from three peritoneal walls of separate mice of the same treatment type, either LA or saline serum. Tissue punches were hydrolyzed in 6 M HCl at 95°C, and then the hydroxyproline was oxidized. After the hydroxyproline residues were stained with QuickZyme stain, absorbance was measured at 540 nm on the microtiter plate reader.
Statistical analyses.
All graphs are representative of at least two experiments. Overall statistical differences were assessed by one-way ANOVA using StatPlus (StatPlus Software, Walnut, CA). Two-tailed t-tests were used to assess differences between two means, using Excel or StatPlus. Statistical significance was defined as P values <0.05. Data are graphed with error bars indicating SE.
RESULTS
Confirmation of the presence of MCAA in serum from LA-exposed mice.
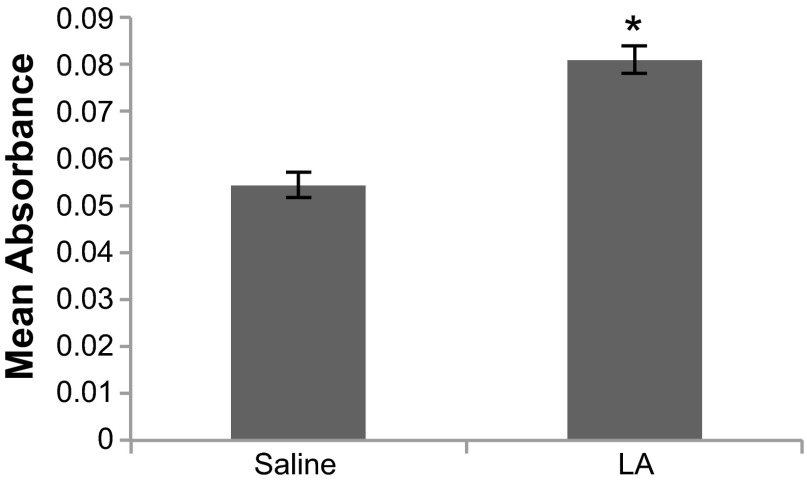
We hypothesized that MCAA would lead to increased collagen deposition in the peritoneal walls of mice exposed to the LA serum. To test the hypothesis, confirmation of the presence of MCAA in LA-treated mice was required. Mouse serum was screened for MCAA using cell-based ELISA on primary mouse mesothelial cells identified using morphological and flow cytometric analyses. Cell exposure to pooled serum from mice instilled intratracheally with LA fibers resulted in an increased OD (absorbance) compared with sera from mice instilled with saline (Fig. 2), thus confirming the presence of MCAA in sera of LA-exposed mice. In the same cell-based ELISA strategy but using individual serum samples to detect binding to mesothelial cells, 63.6% (7 of 11 mice) of the LA-exposed mice were positive for MCAA, whereas none of the saline-exposed mice were positive (P = 0.03 by Fisher's Exact Test).
Fig. 2.
Binding ELISA with mouse mesothelial cells to demonstrate the presence of mesothelial cell autoantibodies (MCAA). Sera were pooled from mice exposed it to saline only or to 30 μg Libby Amphibole (LA) in sterile saline for 7 mo. Mouse primary mesothelial cells were plated in 96-well plates and then stained for MCAA binding using the pooled serum as the primary antibody and anti-mouse IgG-horseradish peroxidase (HRP) for the secondary antibody. n = 3 Wells/treatment group. *P < 0.05 by 2-tailed t-test.
Collagen deposition induced by MCAA in vivo.
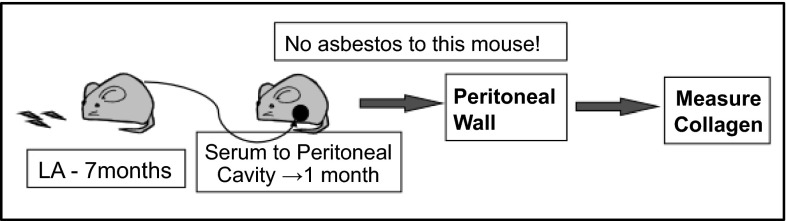
To examine the contribution of MCAA to the fibrotic process, we injected MCAA+ sera in the peritoneal cavities of naïve (non-asbestos-exposed) mice, as shown in Fig. 3. This approach allowed us to determine the fibrotic effects of autoantibodies alone, in the absence of asbestos fibers.
Fig. 3.
Schematic of experimental design. Autoantibodies were produced in mice exposed to LA for 7 mo. Serum from those mice, or mice treated with sterile saline only, was transferred to the peritoneal cavity of naïve mice. After 1 wk and 1 mo, the peritoneal walls were harvested and analyzed for collagen production.
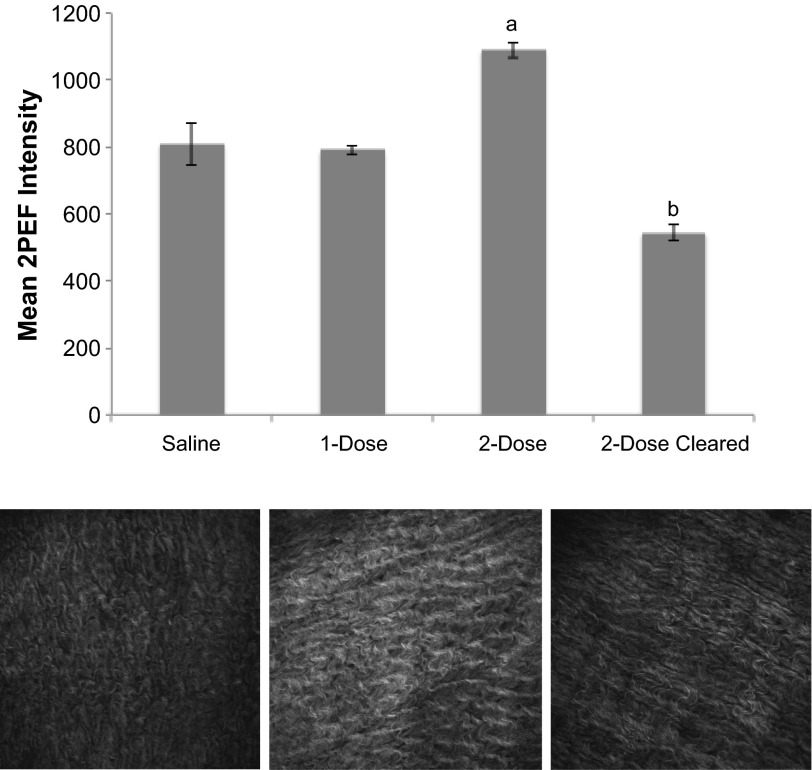
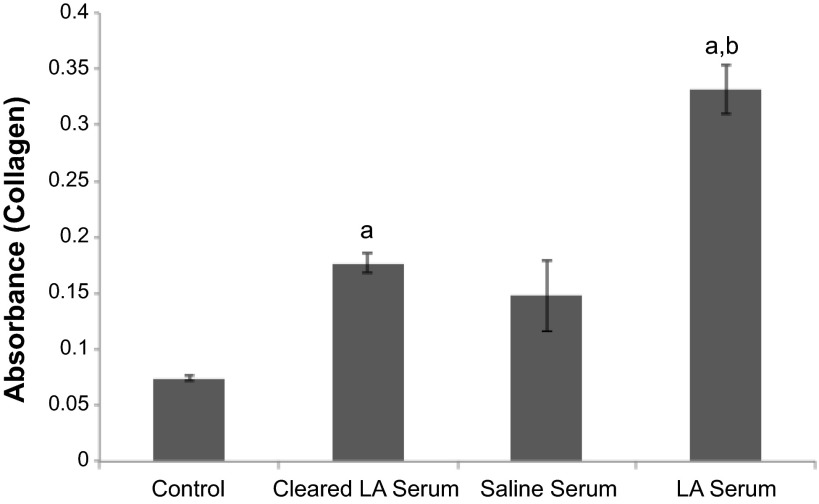
Following exposure to LA serum, the amount of collagen in the mouse peritoneal tissue was quantified. Due to collagen's fibril structure, it is detectable by second harmonic generation imaging (20), allowing us to selectively image the collagen in the peritoneum and deduce the relative amount of collagen in exposed mice (Fig. 4). To simulate an ongoing autoimmune response where the MCAA were present in the peritoneal cavity for an extended period, one group of mice received a second injection (dose) of LA serum 2 wk after the first dose. To rule out the possibility that serum components other than MCAA were contributing to collagen deposition, the last group of mice was exposed to sera cleared of all IgG, since previous isotyping data suggested that MCAA is an IgG-type antibody (27). Mice treated with two doses of LA serum had significantly more collagen in their peritoneal walls at 1 mo postinstillation than mice treated with two doses of serum from mice exposed to saline (saline serum) or to a single dose of LA serum (Fig. 4).
Fig. 4.
Collagen deposition in mouse peritoneal cavity as assessed by multiphoton microscopy. Top, collagen density in peritoneal walls of mice treated for 1 mo with 2 doses of LA serum was significantly higher than that of mice treated with only 1 dose of LA serum or with 2 doses of saline serum. This increase was lost after clearing the LA serum of IgG (cleared). Mean 2-photon excited fluorescence intensity (2PEF) was determined for each mouse individually at 8 randomly chosen fields (640 × 640 μm) on the peritoneal tissue. Intensity data were then pooled within treatment groups (n = 4 mice/treatment) to give an overall collagen density. Significance was determined by 1-way ANOVA, aP < 0.05 compared with saline and 1-dose treatments and bP < 0.01 compared with 2-dose treatment of LA serum. Bottom, representative images (individual fields of 640 × 640 μm) of peritoneal walls obtained by multiphoton microscopy. Mice were exposed to saline serum (left), LA serum (middle), or cleared serum (right), and collagen density was assessed as described above. Mean intensity values (shown here in white/gray with white being most dense) were significantly higher in peritoneal tissue of mice exposed to LA serum (middle) than to saline serum or cleared serum.
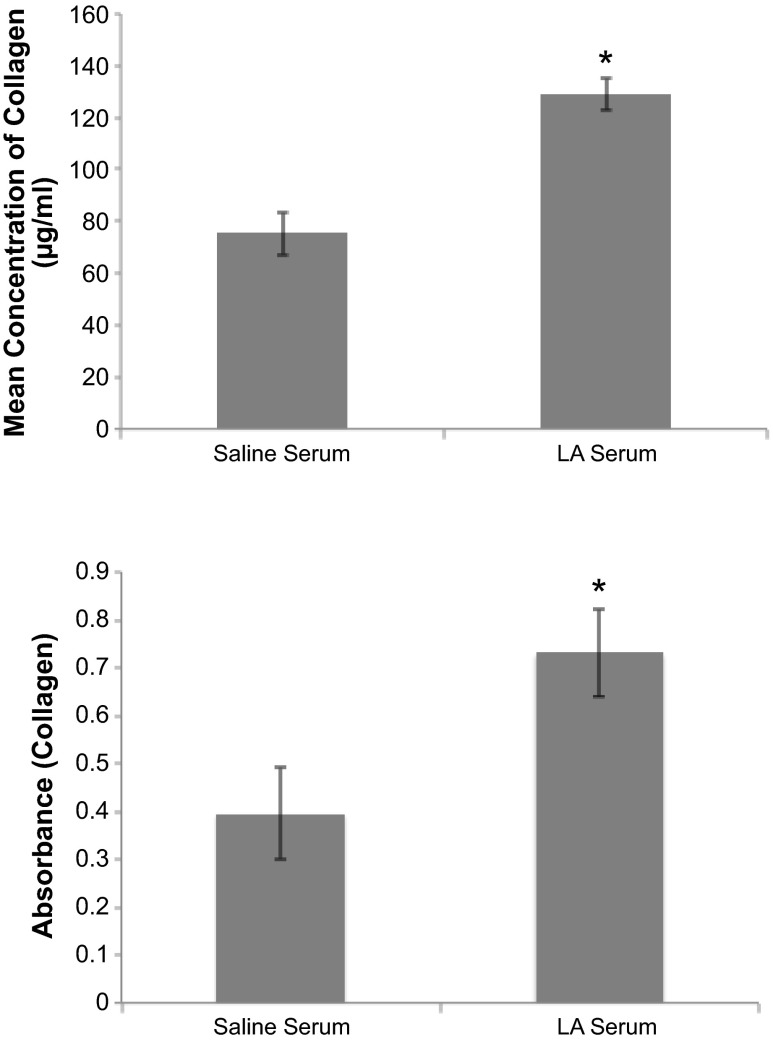
Data from QuickZyme collagen assay confirmed multiphoton analysis. Peritoneal walls of mice that received two intraperitoneal injections of LA serum contained significantly more deposited collagen than mice that received intraperitoneal injections of saline serum (Fig. 5, top). To further confirm collagen synthesis in vivo, 1 wk after exposure to intraperitoneal serum, the presence of soluble collagen in peritoneal washes was measured. Exposure to LA serum resulted in a significant increase in soluble collagen compared with saline serum exposure (Fig. 5, bottom).
Fig. 5.
Collagen production in response to instillation of serum in the peritoneal cavity of mice was measured. Top, the QuickZyme collagen assay measured collagen in tissue punches from the peritoneal walls after 1 mo exposure. N = 4 mice, *P < 0.05. Bottom, soluble collagen was measured in peritoneal washes of mice treated ip for 1 wk using a Sircol (picrosirius red) soluble collagen assay as described in materials and methods. n = 4 Mice, *P < 0.05.
Collagen production by primary mouse mesothelial cells in vitro.
A cell-based ELISA was conducted to determine whether or not primary mouse mesothelial cells could be stimulated by the MCAA to produce collagen in vitro. Figure 6 shows that cells treated with LA serum produce significantly more collagen than the cells treated with saline serum and that this effect is lost when the LA serum is cleared of IgG. These results in the in vitro model are consistent with our findings in the in vivo model.
Fig. 6.
In vitro collagen production by cultured mouse mesothelial cells induced by treatment with serum autoantibodies was measured using a cell-based ELISA; mouse primary mesothelial cells were plated in 96-well plates, treated for 4 days with pooled serum, and then stained with anti-collagen 1 antibody and HRP-conjugated secondary antibody. Control, untreated cells (media only). n = 5 Wells/treatment, aP < 0.01 compared with control and bP < 0.01 compared with cleared and saline serum.
Lack of inflammatory or autoantibody responses after peritoneal instillations.
To test whether there was an inflammatory or autoantibody response to the intraperitoneally instilled serum, serum ANA and peritoneal white blood cell populations were tested in the mice 1 mo after instillation of two doses of saline, LA serum, or cleared serum. There were no significant differences in any of the cell types or the frequency of positive ANA tests (Table 1). Similar results were seen at 1 wk and 3 mo (data not shown).
Table 1.
Autoantibody response
| Cell Type | Saline Serum, % (SD) | LA Serum, % (SD) | Cleared Serum, % (SD) | P Value (1-way ANOVA) |
|---|---|---|---|---|
| Macrophagea | 55.4 (6.0) | 62.9 (5.3) | 59.9 (3.4) | 0.12 |
| Granulocytea | 0.48 (0.46) | 0.48 (0.4) | 0.67 (0.2) | 0.62 |
| Lymphocytea | 27.1 (9.1) | 25.7 (8.3) | 31.0 (6.3) | 0.56 |
| Bb | 79.2 (1.7) | 80.8 (3.3) | 80.9 (7.4) | 0.85 |
| Tb | 14.3 (3.1) | 15.9 (4.3) | 17.2 (7.2) | 0.72 |
| B1a Bb | 28.5 (10) | 26.7 (8.2) | 30.5 (8.1) | 0.79 |
| ANA positive, % | 25 | 25 | No data |
ANA, antinuclear autoantibody.
Percent of total peritoneal wash cells, based on forward × side scatter.
Percent of peritoneal lymphocytes, based on staining with CD19 (B cells), CD5 (T cells), CD5 + IgM.
DISCUSSION
The predominant disease resulting from LA exposure is pleural fibrosis that differs from typical pleural fibrosis in its severe and progressive nature (1, 2, 19). Multiple fibrotic diseases have been shown to have an autoimmune component, and a systemic autoimmune component of this LA disease has also been described (5, 9, 13, 25). While the mechanism behind this phenomenon is not completely understood, the link between autoantibodies and fibrosis is supported by a growing literature. Scleroderma is a systemic autoimmune disease involving fibrosis of connective tissue in various areas of the human body (8). The severity of the disease correlates with the presence of autoantibodies, but their relevance remains unclear (8, 9). Additionally, there is a correlation between endothelial cell autoantibodies and pulmonary fibrosis with several mechanisms suggested (5, 13). Similarly, our previous work identified cell-specific autoantibodies present in mice and people exposed to LA asbestos and demonstrated potential mechanisms by which these antibodies may contribute to pleural fibrosis using in vitro models (14, 22, 28). However, this current study is the first to demonstrate an in vivo role for these autoantibodies in driving specific events of fibrosis.
Our findings support the existence of a novel mechanism by which MCAA may induce or exacerbate fibrosis. Using primary mesothelial cell cultures, we established the presence of MCAA in sera of mice exposed intratracheally to LA fibers and demonstrated that these antibodies induce collagen deposition in vitro. This effect was lost upon clearance of IgG, suggesting that the antibodies, and not another component of serum, were responsible for the observed increase in collagen. Because excessive collagen deposition is characteristic of multiple fibrotic diseases (29), these results suggest a mechanism by which LA-induced MCAA drive fibrosis and corroborate our previous findings (28).
We found that LA-associated antibodies induced collagen deposition in vivo as well. To delineate the antibody effects from those of the fibers, we collected serum from LA-exposed mice (“LA serum”) and injected it in the peritoneal cavities of naïve mice. Because both the peritoneum and pleura are lined with mesothelial cells, and it is difficult to both inject into the murine pleural space and dissect out the pleural tissue for postmortem analysis, we used the peritoneum as a surrogate. This approach was based on previous studies that have shown this to be reasonable in terms of induction of mesothelioma (10, 17). A single installation of LA serum did not significantly increase collagen deposition throughout the peritoneal tissue when compared with a single instillation of saline serum. In contrast, two consecutive LA serum instillations 2 wk apart resulted in uniform and significant increases in collagen detection in all mice, as assessed by both multiphoton confocal microscopy and hydroxyproline assay. These effects were lost upon clearance of IgG from the serum, again suggesting that it is the autoantibodies driving this fibrotic response.
To further confirm that the observed increases in collagen were due to autoantibody presence and not to systemic or local immune responses, we assessed mouse serum for the presence of ANA and assessed pleural wash fluid for the presence of immune cells, in accordance with previously described protocols (7, 21). At 1 mo after intraperitoneal instillation of saline serum or LA serum, there were no significant changes in serum ANA in recipient mice, suggesting little or no contribution of systemic immune responses to the observed increases in peritoneal collagen deposition. Additionally, we detected no differences in the relative frequencies of macrophages, granulocytes, total lymphocytes, or lymphocyte subsets (B cells, T cells, or B1a B cells) in peritoneal wash fluid from mice exposed to different serum treatments, further negating the contribution of a general immunological response in these results. While the inflammatory cytokine transforming growth factor (TGF)-β has been shown to mediate fibrogenic responses by inducing epithelial-mesenchymal transition (EMT) (3, 6), previous work from our laboratory has shown that MCAA binding to mesothelial cells induces collagen deposition in the absence of EMT or increases in serum TGF-β (28). These results, coupled with the fact that collagen deposition was lost upon clearance of all IgG-type antibodies from LA serum, led us to conclude that it is the antibodies and not any additional serum component mediating the observed responses.
By exposing naïve mice only to serum containing LA-induced autoantibodies and not to the fibers themselves, we were able to demonstrate a role for autoantibodies alone in driving fibrosis; to our knowledge, this is the first time such a distinction has been made. If this new mechanism of autoantibody-mediated fibrosis is confirmed to be responsible for LA disease, it could in fact be responsible for other types of fibrosis not yet understood.
Further studies need to be conducted to completely characterize this novel mechanism by which MCAAs induce collagen deposition and to demonstrate a causative link between MCAA and pleural fibrosis in humans. In an early study of the Libby cohort, we detected MCAA in 18.5% of subjects (14), but, more recently using a larger cohort (n = 313) and including more samples with longer time since exposure, we have detected MCAA in 31% of the subjects (26). In the current study, we found that over 60% of LA-exposed mice were positive for MCAA, using our cut-off system of three SDs above the mean of a control set of sera. However, there may be low levels of MCAA even in subjects and mice that simply do not reach this level of binding in our cell-based ELISA, so are not detected as positive. It is possible that there are different types of MCAA that are responsible for the effects seen, and work is underway in our laboratory to identify specific protein targets for LA-induced MCAA, which will allow us to specifically detect those antibodies and determine potential mechanisms that link them to collagen deposition. The intracellular mechanism responsible for the increase in collagen deposition needs to be characterized to better understand the nature of the disease. The insight gained from this study reinforces the autoimmune component behind the disease and helps illuminate new potential treatment approaches for this devastating disease.
GRANTS
This work was supported by National Institutes of Health Grants R15-ES-21884-01 and INBRE P20-GM-103408.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
J.G., K.S., R.H., and J.C.P. conception and design of research; J.G., K.S., C.D., M.A., R.H., and T.H. performed experiments; J.G., K.S., C.D., M.A., R.H., T.H., and J.C.P. analyzed data; J.G., K.S., R.H., and J.C.P. interpreted results of experiments; J.G. and J.C.P. prepared figures; J.G. drafted manuscript; J.G., K.S., and J.C.P. edited and revised manuscript; J.G., K.S., C.D., M.A., R.H., T.H., and J.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the intellectual contributions of the Libby Epidemiology Research Program (LERP): Icahn School of Medicine at Mt. Sinai, including Drs. Raja Flores and Jaime Szeinuk, rheumatology consultant Dr. Roger Diegel (Kalispell, MT), and the Center for Asbestos Related Diseases, Dr. Brad Black. We also acknowledge the Idaho State University Molecular Research Core Facility, which supports the instrumentation that was essential for this project.
REFERENCES
- 1.Antao VC, Larson TC, Horton DK. Libby vermiculite exposure and risk of developing asbestos-related lung and pleural diseases. Curr Opin Pulm Med 18: 161–167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black B, Szeinuk J, Whitehouse AC, Levin SM, Henschke CI, Yankelevitz DF, Flores RM. Rapid progression of pleural disease due to exposure to Libby amphibole: “not your grandfather's asbestos related disease.” Am J Ind Med 57: 1197–1206, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Broaddus VC, Everitt JI, Black B, Kane AB. Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J Toxicol Environ Health B Crit Rev 14: 153–178, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JM, Pfau JC, Holian A. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal Toxicol 16: 133–139, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Corallo C, Franci B, Lucani B, Montella A, Chirico C, Gonnelli S, Nuti R, Giordano N. From microvasculature to fibroblasts: Contribution of anti-endothelial cell antibodies in systemic sclerosis. Int J Immunopathol Pharmacol 28: 93–103, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Decologne N, Kolb M, Margetts PJ, Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P, Bonniaud P. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J Immunol 179: 6043–6051, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Ferro A, Zebedeo CN, Davis C, Ng KW, Pfau JC. Amphibole, but not chrysotile, asbestos induces anti-nuclear autoantibodies and IL-17 in C57BL/6 mice. J Immunotoxicol doi. 10.3109/1547691X.2013.847510: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med 360: 1989–2003, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa M. B lymphocytes: shedding new light on the pathogenesis of systemic sclerosis. J Dermatol 37: 3–10, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Kane AB. Animal models of malignant mesothelioma. Inhal Toxicol 18: 1001–1004, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Larson TC, Antao VC, Bove FJ. Vermiculite worker mortality: estimated effects of occupational exposure to Libby amphibole. J Occupat Environ Med 52: 555–560, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Larson TC, Lewin M, Gottschall EB, Antao VC, Kapil V, Rose CS. Associations between radiographic findings and spirometry in a community exposed to Libby amphibole. Occupat Environ Med 69: 361–366, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Lewandowska K, Ciurzynski M, Gorska E, Bienias P, Irzyk K, Siwicka M, Zycinska K, Pruszczyk P, Demkow U. Antiendothelial cells antibodies in patients with systemic sclerosis in relation to pulmonary hypertension and lung fibrosis. Advan Exp Med Biol 756: 147–153, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Marchand LS, St-Hilaire S, Putnam EA, Serve KM, Pfau JC. Mesothelial cell and anti-nuclear autoantibodies associated with pleural abnormalities in an asbestos exposed population of Libby MT. Toxicol Lett 208: 168–173, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald JC, Harris J, Armstrong B. Mortality in a cohort of vermiculite miners exposed to fibrous amphibole in Libby, Montana. Occupat Environ Med 61: 363–366, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meeker GP, Bern AM, Brownfield IK, Lowers HA, Sutley SJ, Hoefen TM, Vance JS. The composition and morphology of amphiboles from the Rainy Creek Complex, Near Libby, Montana. Am Mineraologist 88: 1955–1969, 2003. [Google Scholar]
- 17.Moalli PA, MacDonald JL, Goodglick LA, Kane AB. Acute injury and regeneration of the mesothelium in response to asbestos fibers. Am J Pathol 128: 426–445, 1987. [PMC free article] [PubMed] [Google Scholar]
- 18.Noonan CW, Pfau JC, Larson TC, Spence MR. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environ Health Perspect 114: 1243–1247, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peipins LA, Lewin M, Campolucci S, Lybarger JA, Miller A, Middleton D, Weis C, Spence M, Black B, Kapil V. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ Health Perspect 111: 1753–1759, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pena AM, Fabre A, Debarre D, Marchal-Somme J, Crestani B, Martin JL, Beaurepaire E, Schanne-Klein MC. Three-dimensional investigation and scoring of extracellular matrix remodeling during lung fibrosis using multiphoton microscopy. Microscopy Res Tech 70: 162–170, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Pfau JC, Hurley K, Peterson C, Coker L, Fowers C, Marcum R. Activation and trafficking of peritoneal B1a B-cells in response to amphibole asbestos. J Immunotoxicol 11: 90–98, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Pfau JC, Li S, Holland S, Sentissi JJ. Alteration of fibroblast phenotype by asbestos-induced autoantibodies. J Immunotoxicol 8: 159–169, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfau JC, Sentissi JJ, Li S, Calderon-Garciduenas L, Brown JM, Blake DJ. Asbestos-induced autoimmunity in C57BL/6 mice. J Immunotoxicol 5: 129–137, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Pfau JC, Sentissi JJ, Weller G, Putnam EA. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environ Health Perspect 113: 25–30, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfau JC, Serve KM, Noonan CW. Autoimmunity and asbestos exposure. Autoimmune Dis 2014: 782045, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfau JC, Serve KM, Woods L, Noonan CW. Asbestos exposure and autoimmunity. In: Current Topics in Environmental Health and Preventive Medicine: Biological Effects of Fibrous and Particulate Substances. New York, NY: Elsevier, 2015, chapt. 10, p. 181–194. [Google Scholar]
- 27.Salazar KD, Copeland CB, Wood CE, Schmid JE, Luebke RW. Evaluation of anti-nuclear antibodies and kidney pathology in Lewis rats following exposure to Libby amphibole asbestos. J Immunotoxicol 10: 329–333, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Serve KM, Black B, Szeinuk J, Pfau JC. Asbestos-associated mesothelial cell autoantibodies promote collagen deposition in vitro. Inhal Toxicol 25: 774–784, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoulders MD, Raines RT. Collagen structure and stability. Ann Rev Biochem 78: 929–958, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitehouse AC. Asbestos-related pleural disease due to tremolite associated with progressive loss of lung function: serial observations in 123 miners, family members, and residents of Libby, Montana. Am J Ind Med 46: 219–225, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Whitehouse AC, Black CB, Heppe MS, Ruckdeschel J, Levin SM. Environmental exposure to Libby Asbestos and mesotheliomas. Am J Ind Med 51: 877–880, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Winters CA, Hill WG, Rowse K, Black B, Kuntz SW, Weinert C. Descriptive analysis of the respiratory health status of persons exposed to Libby amphibole asbestos. Br Med J 2: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]