Abstract
The impairment of vasodilator nitric oxide (NO) production is well accepted as a typical marker of endothelial dysfunction in vascular diseases, including in the pathophysiology of pulmonary arterial hypertension (PAH), but the molecular mechanisms accounting for loss of NO production are unknown. We hypothesized that low NO production by pulmonary arterial endothelial cells in PAH is due to inactivation of NO synthase (eNOS) by aberrant phosphorylation of the protein. To test the hypothesis, we evaluated eNOS levels, dimerization, and phosphorylation in the vascular endothelial cells and lungs of patients with PAH compared with controls. In mechanistic studies, eNOS activity in endothelial cells in PAH lungs was found to be inhibited due to phosphorylation at T495. Evidence pointed to greater phosphorylation/activation of protein kinase C (PKC) α and its greater association with eNOS as the source of greater phosphorylation at T495. The presence of greater amounts of pT495-eNOS in plexiform lesions in lungs of patients with PAH confirmed the pathobiological mechanism in vivo. Transfection of the activating mutation of eNOS (T495A/S1177D) restored NO production in PAH cells. Pharmacological blockade of PKC activity by β-blocker also restored NO formation by PAH cells, identifying one mechanism by which β-blockers may benefit PAH and cardiovascular diseases through recovery of endothelial functions.
Keywords: endothelial cells, nitric oxide, protein kinases
the hallmark of endothelial vascular dysfunction in pulmonary arterial hypertension (PAH) is the impaired production of nitric oxide (NO) by endothelial NO synthase (eNOS) (13, 22, 29, 55). As a potent pulmonary vasodilator, NO has been mechanistically linked to PAH pathogenesis (5, 12, 29, 49, 50, 55). Although impairment of NO production is well accepted in the pathophysiology of PAH, mechanisms underlying the loss of eNOS activity remain unknown. Pulmonary artery endothelial cells (PAEC) derived from PAH lungs have lower levels of NO production, but the levels of eNOS protein are similar among PAH and control endothelial cells (37, 55). Activation of eNOS requires dimerization of the enzyme along with cofactors including tetrahydrobiopterin and calmodulin (51), but the dominant control of eNOS activity occurs via phosphorylation of specific residues within the enzyme (6, 18, 27, 39, 40). Agonists such as bradykinin (BK) lead to signal transduction events that result in the phosphorylation of the serine 1177 (S1177) in the reductase domain that releases the enzyme from auto inhibition. In contrast, phosphorylation of threonine 495 (T495) negatively regulates NO production by mechanisms that are not fully understood (6, 18, 27, 39, 40) (Fig. 1A). Thus eNOS activity is controlled by a balance of kinase-phosphatase pathways (1, 3, 8, 14, 17, 18, 27, 40). Here, we hypothesized that loss of eNOS activity in PAH may be caused by abnormalities in enzyme phosphorylation. Aberrant T495 eNOS phosphorylation was found in cells and tissues of lungs from patients with PAH, and in mechanistic studies, protein kinase C (PKC) and eNOS-associated PKC activity were found to be higher in PAH than controls. Recovery of eNOS activity was accomplished genetically in PAH endothelial cells via transfection of eNOS phospho-mutants and pharmacologically via inhibition of PKC activity by β-adrenergic receptor blockade.
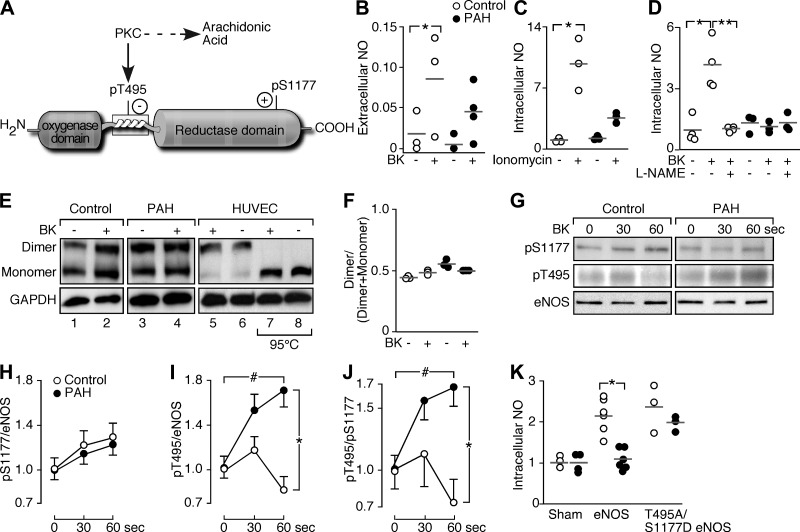
Fig. 1.
Endothelial NO synthase (eNOS) activity in pulmonary arterial hypertension (PAH). A: eNOS phosphorylation sites. Phosphorylation of T495 (pT495) by protein kinase C (PKC) blocks eNOS activity. PKC generates arachidonic acid (ARA). B: bradykinin (BK, 1 μM) increases extracellular NO [nitrite (μM)] in control PAEC (n = 3; *P = 0.04), but not significantly in PAH PAEC (n = 4; P = 0.14). C: ionomycin (100 μM) increases intracellular NO (relative fluorescence) in control (n = 3; *P = 0.0001) but not PAH PAEC (n = 3; P = 0.1). D: l-NG-nitroarginine methyl ester (l-NAME; 3 mM) blocks NO induced by BK (n = 4; *P = 0.0003; **P = 0.0003). Intracellular NO does not change under any conditions in PAH PAEC (n = 3; P = 0.8). E: dimeric and monomeric eNOS with BK (1 μM) or untreated (lanes 1−6, at 4°C). Human umbilical vein endothelial cell (HUVEC) eNOS was in the dimeric state at 4°C but monomeric when heated at 95°C (HUVEC, lanes 7 and 8). F: dimer/(dimer + monomer) is similar among control (n = 3) and PAH PAEC (n = 3; P = 0.3). G: eNOS phosphorylation with BK. H–J: although there was a tendency to see a very small and transient increase of pT495 in control PAEC at 30 s, BK does not lead to a significant induction of T495 phosphorylation in control cells (n = 3, P > 0.05) but induces T495 phosphorylation in PAH (n = 3) (ANOVA #P = 0.01, *P = 0.02). Ratio of pT495 to pS1177 in PAH PAEC increases with BK (ANOVA #P = 0.03) and is higher than control by 60 s (*P = 0.01). K: intracellular NO in control (n = 6) and PAH PAEC (n = 6) transfected with wild-type eNOS or T495A/S1177D mutant. Control PAEC had greater NO production with wild-type (P = 0.001) or T495A/S1177D eNOS mutant (P = 0.001) in the absence of agonist induction, but PAH cells increased NO only with T495A/S1177D transfection (P = 0.0003). Intracellular NO in PAH PAEC with wild-type eNOS transfection was lower than control (*P = 0.0007) but increased to control levels when transfected with T495A/S1177D eNOS (P = 0.3). Data are shown as means ± SE.
MATERIALS AND METHODS
Human samples.
Human lung tissues were obtained either from unused, explanted control donor lungs or lungs explanted from PAH patients undergoing lung transplantation (9, 55). Lungs and cells were derived from eight individuals with idiopathic PAH undergoing lung transplantation (age 47 ± 3 yr; mean pulmonary artery pressures 56 ± 5 mmHg; all white; 5 women) and from control lungs donated, but not used in lung transplantation, from 11 individuals (age 41 ± 6 yr; all white; 6 women). The study was approved by the Institutional Review Board of the Cleveland Clinic, and written, informed consent was obtained from individuals.
Pulmonary artery endothelial cell cultures and treatments.
Primary PAEC were isolated and cultured identically as previously described from six unused explanted PAH lungs or three healthy control lungs (9, 55), and five control PAEC were purchased from Lonza (Walkersville, MD). All PAEC were used in passage 5–7. Briefly, pulmonary arteries to the third and fourth branches were dissected from explanted pulmonary hypertension lungs and donor lungs not used for transplant. The arteries were placed in 10–15 ml PBS containing 2 mg/ml type II collagenase (Worthington Biochem, Lakewood, NJ) and kept at 37°C and 5% CO2 with 90% humidity for 20 min. The arteries were massaged with a spatula followed by a gentle shake to detach the PAEC. Cells were passaged at 70–80% confluency, and primary cultures of passages 6–8 were used in experiments. Human umbilical vein endothelial cells, only shown as positive control for dimer-monomer distribution analysis, and PAEC were grown in complete endothelial growth medium (EBM-2, Lonza) on fibronectin-coated plates. Serum-containing medium was replaced by serum-free medium 2 h prior to stimulation of cells in all experiments. Cells were treated with BK (1–5 μM) in the presence of 10 μM l-arginine or with ionomycin (100 μM) in the presence or absence of β-blocker (propranolol, 100 μM).
Generation of mutants.
The human eNOS was cloned into the mammalian expression vector pCDNA3.1 using HindIII and XbaI restriction enzyme cloning sites. Human eNOS double mutant (T495A/S1177D) was prepared by site-directed mutagenesis of the pCDNA3.1 vector containing the cDNA of the human eNOS full-length enzyme construct (26). First, we mutated the T495 to alanine, and then the T495A mutant eNOS was used as a template to mutate S1177 to aspartate. Oligonucleotides for site-directed mutagenesis were obtained from Integrated DNA Technologies (Coralville, IA) (Table 1). Site directed-mutagenesis was performed with the QuikChange XL mutagenesis kit (Stratagene, La Jolla, CA). Mutations were confirmed by DNA sequencing at the Cleveland Clinic Genomics Core Facility.
Table 1.
Primers for site-directed mutagenesis
| Primer | Sequence |
|---|---|
| T495A_Forward | 5′-ACCAGGAAGAAGGCCTTTAAAGAAGTGGCC-3′ |
| T495A_Reverse | 5′-GGCCACTTCTTTAAAGGCCTTCTTCCTGGT-3′ |
| S1177D_Forward | 5′-ATACGCACCCAGGACTTTTCCTTGCAGGAG-3′ |
| S1177D_Reverse | 5′-CTCCTGCAAGGAAAAGTCCTGGGTGCGTAT-3′ |
Mutated bases are boldface.
Transfection of PAEC by wild-type eNOS or T495A/S1177D double mutants.
PAEC were cultured to 70% confluency and transfected according to recommendations in the Optimized Protocol Nucleofection (Lonza). Briefly, 5×105 PAEC were transfected with 1 μg DNA (wild-type or double mutant). For optimal conditions, posttransfection PAEC were plated in 12-well plates at a density of 2.5×105 or in a 96-well plate at a density of 0.8×105. PAEC were analyzed 24–48 h after transfection for NO generation.
Measurement of extracellular nitrite accumulation in cell culture supernatant.
NO production was determined from the level of nitrite accumulation in the culture media measured by chemiluminescence method using Sievers 280i Nitric Oxide Analyzer (GE Analytical Instruments, Boulder, CO) (55). Briefly, argon was bubbled through 7 ml of glacial acetic acid in the purge vessel, and 0.05 g of potassium iodide was dissolved in 1 ml of deionized water and then added to the acetic acid. A silicone/Teflon septum was used to close the purge vessel. Samples were injected with a syringe through the septum. Amount of nitrite present in the samples was analyzed by measuring the area under curve of the signal generated. All samples were measured in duplicate and the nitrite concentrations were determined by interpolation using authentic standards of nitrite.
Measurement of intracellular NO.
Intracellular NO generation was monitored with DAF-FM diacetate (4-amino 5-methylamino-2′,7′-difluorofluorescein diacetate, Life Technologies, Grand Island, NY), a cell-permeable fluorescent dye that forms a fluorescent benzotriazole upon reaction with NO. Inside the cell, DAF-FM diacetate is deacetylated by intracellular esterase to generate DAF-FM, which reacts with NO rapidly. PAEC were serum starved for 2 h, followed by incubation with 10 μM of DAF-FM diacetate for 1 h at 37°C. Cells were rinsed with PBS and placed into the Flex Station 3 (Molecular Devices, Sunnyvale, CA) for fluorometric analysis in presence of BK or ionomycin. A duplicate set of cells was pretreated with l-NG-nitroarginine methyl ester (l-NAME, 3 mM), an eNOS inhibitor, for 30 min prior to addition of BK or ionomycin, to validate specificity of the fluorescent signal. Change in fluorescence was measured at excitation and emission wavelengths of 495 and 515 nm, respectively. Relative fluorescence for each treatment was measured over a 10-min time period.
Sample preparation for dimer-monomer distribution assay and Western analysis.
Cells were lysed in modified RIPA buffer containing Tris·HCl (pH 7.5; 50 mM), NaCl (150 mM), EDTA (1 mM), EGTA (1 mM), NaF (20 mM), Na4P2O7 (2.5 mM), Na3VO4 (1 mM), leupeptin (1 μg/ml), pepstatin A (1 μg/ml), aprotinin (2 μg/ml), PMSF (1 mM), Nonidet P-40 (1% vol/vol), 0.05% SDS, and 10% glycerol, and left on ice for 10 min (20). Lysed cells were centrifuged at 14,000 rpm for 15 min. Cell supernatant was either maintained at 4°C for dimer-monomer distribution analysis or processed at 95°C for immunoprecipitation and/or Western blot analysis.
PAEC cell lysates were separated on 4–15% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membrane for Western blots (20). The protein-containing blots were blocked at room temperature, followed by overnight primary antibody at 4°C. The blots were probed with a peroxidase-conjugated secondary antibody, and signal was by enhanced chemiluminescent system (ECL; Amersham Bioscience, Piscataway, NJ). The primary antibodies were monoclonal anti-eNOS (BD Laboratory, Franklin Lakes, NJ), polyclonal anti-phospho-eNOS (pS1177, pT495, Cell Signaling, Danvers, MA), monoclonal anti-GAPDH (Fitzgerald, North Acton, MA), polyclonal anti-PKCα (Santa Cruz Biotechnology), and polyclonal anti-pan-phospho-PKC (βllSer660; Cell Signaling). For all phospho-specific Western blots (either phospho-eNOS or pan-phospho-PKC), blocking and primary incubation were done in 2% BSA; other Western analyses were performed with blocking in 5% milk.
Immunoprecipitation and Western blot analyses.
Proteins in cell supernatant were incubated overnight at 4°C with polyclonal anti-eNOS antibody (Santa Cruz Biotechnology, Dallas, TX), followed by protein G-Sepharose incubation at 4°C for 2 h (20). The proteins captured by the beads were extracted in denaturing-nonreducing buffer, analyzed for anti-phospho-eNOS immunoreactivity and reblotted with monoclonal anti-eNOS antibody.
Immunohistochemical analyses.
Immunohistochemistry in lung tissues was performed on a Ventana Benchmark XT automated immunostainer (Roche Diagnostics, Indianapolis, IN) utilizing a Ventana iVIEW DAB Detection kit with protease 2 retrieval for 8 min. For immunohistochemical analyses, polyclonal anti-eNOS (1:30; Santa Cruz Biotechnology), polyclonal anti-phospho-eNOS (pS1177, pT495, 1:50; Cell Signaling), and monoclonal anti-CD31 (1:30; DAKO, Carpinteria, CA) were used. Slides were counterstained with Hematoxylin II, dehydrated, cleared, and permanently mounted for viewing.
PKCα activity assay.
PKCα and eNOS were immunoprecipitated from cell lysates (200 μg) by using anti-PKCα (1:100; Santa Cruz Biotechnology) and anti-eNOS (1:40; Santa Cruz Biotechnology) along with protein G-Sepharose. Following immunoprecipitation at 4°C for 12 h, the beads were washed and resuspended in assay dilution buffer (20 mM MOPS, pH 7.2, 25 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 1 mM DTT, 1 mM CaCl2). The beads were washed once with assay dilution buffer and the immunoprecipitate beads were subjected to radioactive PKC kinase activity assay as per manufacturer's protocol (PKC activity assay kit, Millipore, Billerica, MA); 10 μCi of γ-32[P]-ATP was added to each reaction and the tubes were incubated at 30°C for 10 min. Following the reaction, the tubes were centrifuged at 6,000 rpm for 1 min and 25 μl of supernatant was aliquoted on to P81 cellulose paper. The P81 cellulose paper was washed three times with 0.75% phosphoric acid and subjected to scintillation counting.
ARA release.
Arachidonic acid (ARA) release in cell supernatant was measured as previously described (46). Briefly, the PAEC from control or PAH subjects were labeled with [3H]arachidonate (0.2 Ci/well) for 16 h. Cells were then washed three times and incubated with 500 μl of DMEM containing 2 mg/ml BSA. Medium was removed and centrifuged at 800 g. Radioactivity was determined in a Packard β-counter after addition of 2 ml of Ecolite scintillation fluid.
Statistical analysis.
Data are shown as means ± SE. Comparisons were performed by ANOVA or Student's t-test. The study was approved by the Institutional Review Board of the Cleveland Clinic.
RESULTS
Deficient NO production in PAH.
PAH and control PAEC at baseline unstimulated conditions in culture had low levels of NO production. Agonist-induced or calcium ionophore-triggered extracellular NO production, measured in the form of nitrite (μM) in culture supernatant, by PAH PAEC is defective (Fig. 1B). BK (1 μM) induced NO production in control PAEC (n = 3; P = 0.04), but not in PAH (n = 4; P = 0.14). Intracellular NO, measured as relative fluorescent emission of DAF-FM within cells, increased significantly in control PAEC with 100 μM ionomycin (Fig. 1C; P = 0.0001) or with 5 μM BK (Fig. 1D; P = 0.0003). BK induction of intracellular NO was blocked by pretreatment of cells with NOS inhibitor (3 mM l-NAME), demonstrating the NO specificity of the measure (Fig. 1D; P = 0.0003). In contrast, ionomycin and BK did not increase intracellular NO in PAH (Fig. 1, C and D). To our knowledge, this is the first evidence that intracellular NO production is defective in PAH, indicating that abnormality of NO production is not simply related to abnormal metabolism of the NO products.
eNOS and dimer/monomer distribution.
Consistent with our previous work (54), and again here, eNOS levels in PAH endothelial cells were similar to controls. PAEC had very low to undetectable levels of inducible NO synthase (iNOS) and neuronal NO synthase (nNOS) by Western blot analyses (data not shown). However, since eNOS is active as a homodimer (10, 23), dimerization of eNOS was evaluated as a mechanism accounting for decreased NO production in PAH. Human umbilical vein endothelial cells had eNOS mainly in the homodimer state, and eNOS disassociated fully to monomeric forms after lysates were heated at 95°C for 5 min (Fig. 1E). PAEC had nearly equal portions of dimeric and monomeric eNOS (Fig. 1E), and the distribution of dimeric and monomeric eNOS in PAH was similar to control cells unstimulated or treated with BK (Fig. 1F; P = 0.3), indicating that dimerization or cofactors required for dimerization were not a limitation for eNOS activity.
Abnormalities of eNOS phosphorylation in PAH.
Studies have identified phosphorylation of S1177 (pS1177) and T495 as pivotal sites for regulating eNOS activity (Fig. 1A). Control and PAH PAEC (n = 3 each) had increase of pS1177 eNOS as early as 30 s after BK treatment (Fig. 1, G–J). Control cells had decrease of T495 phosphorylation that was maximal at 1 min, but PAH cells had increase of pT495 (ANOVA P = 0.01) (Fig. 1, G–J), resulting in increased proportion of inhibitory pT495 to pS1177 in PAH PAEC after BK (Fig. 1J; ANOVA P = 0.03). In contrast, the pT495/pS1177 ratio in control cells decreased and was lower than found in PAH PAEC after 1 min of BK (Fig. 1J; P = 0.01). To confirm the importance of pT495 and pS1177 in the loss of eNOS activity/NO production in PAH, control (n = 6) and PAH PAEC (n = 6) were transfected with wild-type eNOS, or eNOS with the T495 mutated to alanine as a dephospho-mimic (T495A) and S1177 mutated to aspartic acid (S1177D) as a phospho-mimic. Intracellular NO was measured at baseline-unstimulated conditions in transfected cells. Control PAEC had significant increase in intracellular NO upon transfection with the wild-type (P = 0.001) or T495A/S1177D eNOS (P = 0.001) (Fig. 1K). PAH PAEC transfected with wild-type eNOS did not have an increase of intracellular NO, but, when transfected with the T495A/S1177D eNOS, intracellular NO increased to levels similar to control cells (Fig. 1K; P = 0.0003), indicating that the double-activating mutation of T495A/S1177D recovers NO production in PAH.
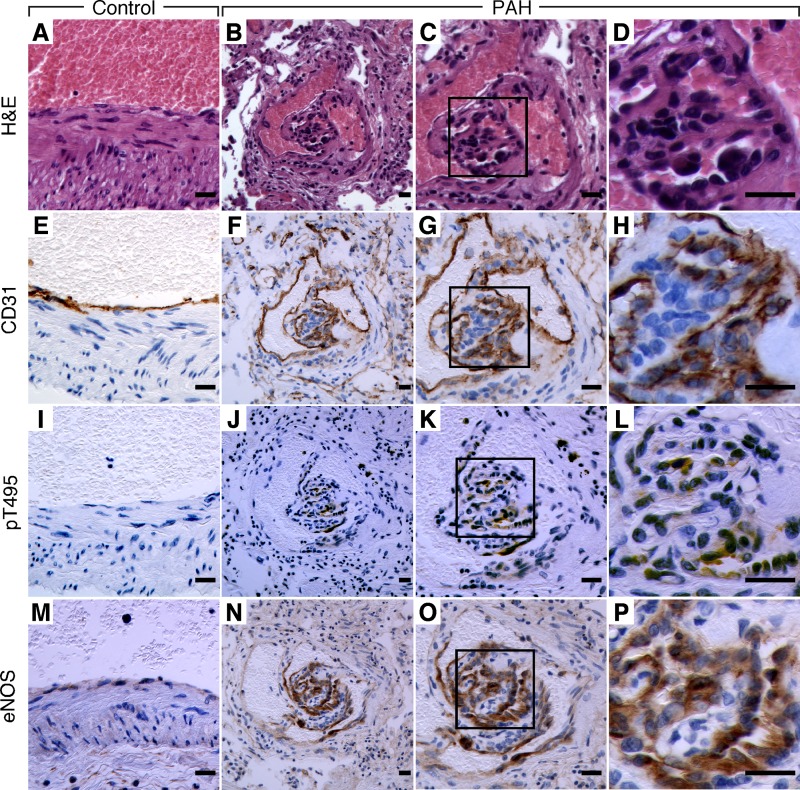
pT495 eNOS expression in PAH plexiform lesions.
Plexiform lesions are a well-established pathological hallmark of PAH and have a distinct cellular composition prominent for endothelial cells. All PAH plexiform lesions of seven sections from two PAH lungs were positive for T495 eNOS (Fig. 2, J–L). Control lungs (n = 3) had no evidence of pT495 eNOS positivity in vascular cells or other cells within the lung (Fig. 2I). All endothelial cells in control (Fig. 2M) and PAH lungs (Fig. 2, N–P) were strongly and equivalently positive for eNOS. Positive CD31 staining confirmed endothelial cells in the control pulmonary arteries (Fig. 2E) and PAH plexiform lesions (Fig. 2, F–H). These data support that PAH endothelial cells in vitro and in lesional tissue in vivo hold similar pathological eNOS phosphorylation that leads to loss of NOS activity.
Fig. 2.
Immunohistochemical analyses of pT495 eNOS in PAH. Sequential lung sections for hematoxylin and eosin (H&E; A–D), or immunostaining for CD31 (E–H), pT495 eNOS (I–L), or total eNOS (M–P). D, H, L, and P are high-power views of C, G, K and O, respectively. CD31 (brown) confirms the endothelial nature of cells in control artery of image E and PAH plexiform lesion of images F–H. pT495 eNOS expression (brown) is only present in endothelial cells found in PAH plexiform lesions (boxed area) of images J–L. High magnification of pT495 eNOS staining in L from inset of K shows positivity in endothelial cells in plexiform lesions. Control pulmonary artery has no detectable pT495 eNOS in I. Positive eNOS staining in endothelial cells of control pulmonary artery (M) and PAH plexiform lesion (N–P). Scale bar: 20 μm. Images representative of 7 sections from 2 PAH and 3 sections from 3 control lungs.
PKC activity associated with eNOS in PAH.
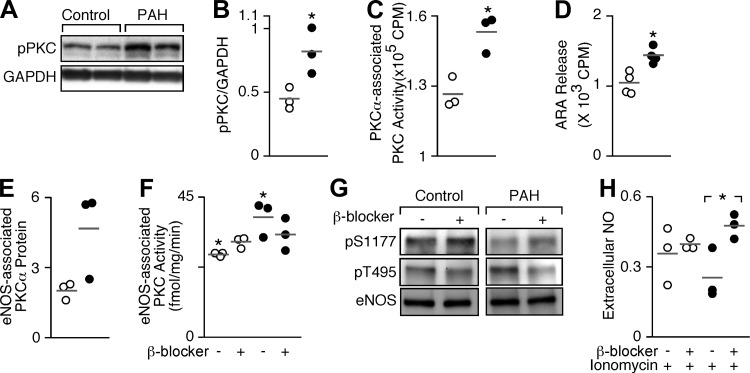
Numerous in vitro biochemical studies identify that PKC phosphorylates T495 and hinders eNOS activity and NO release (16, 17, 38). Thus the abundance of phosphorylated PKC was evaluated in PAH PAEC (Fig. 3). The active phosphorylated form of PKC was significantly higher in PAH PAEC (n = 3) compared with control (n = 3, P = 0.03) (Fig. 3, A and B). Likewise, PKCα activity (P = 0.01) (Fig. 3C) was greater in PAH compared with control cells at basal conditions, indicating more activation of PKC in PAH, i.e., more phosphorylated PKCα. In addition to its effects on eNOS, PKC activates cytosolic phospholipase A2, which results in ARA formation, which is frequently used as a simple quantitative measure of PKC activity in cells. The basal ARA release was higher in PAH compared with control cells (P = 0.005) (Fig. 3D). To evaluate eNOS-associated PKCα activity, eNOS protein was immunoprecipitated from control and PAH PAEC. Greater amounts of PKCα protein and activity coimmunoprecipitated with eNOS from PAH cells compared with controls (P = 0.006) (Fig. 3, E and F). Altogether these data indicate that PKCα is activated and associated with eNOS to a greater degree in PAH than in controls. Phosphatases are also important regulators of eNOS, i.e., protein phosphatase 1 (PP1) dephosphorylates T495 in agonist-stimulated endothelial cells, and PP2A dephosphorylates S1177 (40). Here, PP1 and PP2 phosphatases were similarly expressed among control and PAH PAEC (all P > 0.1, data not shown).
Fig. 3.
PKC activity and association with eNOS. A: total phospho-PKC (pPKC) is greater in PAH PAEC compared with control PAEC at baseline. GAPDH is loading control. B: densitometric quantitation of pPKC/GAPDH is significantly higher in PAH PAEC (n = 3) than in control (n = 3, *P = 0.03). C: PKCα-associated PKC activity is increased in PAH (n = 3) compared with control PAEC (n = 3, *P = 0.01). D: ARA release as a measure of PKC activity is greater from PAH (n = 4) than control PAEC (n = 4, *P = 0.005). E: eNOS-associated PKCα protein in PAH (n = 3) compared with control PAEC (n = 3, P = 0.07). F: eNOS-associated PKC activity in PAH PAEC (n = 3) is greater than control PAEC (n = 3) (*P = 0.006). β-Blocker (propranolol, 100 μM) decreases eNOS-associated PKC activity in PAH but not in control PAEC (ANOVA, P = 0.03). G: pT495 decreases in PAH PAEC upon β-blocker treatment (propranolol, 100 μM) (n = 3). H: extracellular NO [measured as nitrite (μM)] in response to ionomycin (100 μM) in control (n = 3) and PAH PAEC (n = 3) pretreated with β-blocker (100 μM). In contrast to control, extracellular NO in PAH PAEC increases significantly with β-blocker (*P = 0.03). Data are shown as means ± SE.
Pharmacological inhibition of PKC recovers eNOS activity.
Perros et al. (45) recently proposed β-adrenergic receptor as a novel target for PAH therapy. According to the study, β-adrenergic receptor improves endothelial dysfunction and pulmonary remodeling in PAH patients. β-Adrenergic receptor blockers are also potent PKC inhibitors (48). In experimental models of pulmonary hypertension, β-blockers reverse vascular remodeling and improve right ventricular function, although the mechanisms of effect are unknown (2). To test whether β-blocker might block PKC activity and restore NO production in PAH, cells were treated with propranolol (100 μM). The eNOS-associated PKC activity consistently decreased in all PAH PAEC (n = 3), but not in controls (n = 3) (ANOVA P = 0.03) (Fig. 3F). Concurrently, pT495 eNOS abundance decreased in PAH with β-blocker (Fig. 3G). NO production with ionomycin by control cells was greater than PAH, but β-blocker treatment recovered NO production by PAH cells in response to ionomycin (P = 0.03) so that levels were similar to those produced by ionomycin-treated control cells (Fig. 3H).
DISCUSSION
This study identifies that NO production by eNOS in endothelial cells in PAH lungs is impaired due to eNOS phosphorylation at T495. Evidence points to greater activation and association of PKCα with eNOS as the source of greater T495 phosphorylation. Pulmonary and total body NO are lower in PAH patients compared with healthy controls (22, 29, 36, 44, 54). NO, a potent gaseous vasodilator, is produced by NO synthases, which include nNOS, iNOS, and eNOS (51). eNOS is the primary form of NOS in endothelial cells (25, 31, 42), and studies of mice genetically deficient in NOS identify eNOS as the primary regulator of basal lung vessel tone (5, 12, 13, 49, 50). Prior study suggested diminished expression of eNOS in PAH lungs (21), but other work showed that eNOS expression was increased in the PAH plexiform endothelial lesions (37). Here, PAEC derived from PAH lungs have similar eNOS expression as cells from control lungs, which is consistent to prior report that eNOS levels are similar between PAH and controls PAEC (54).
eNOS is a multidomain enzyme consisting of an NH2-terminal oxygenase domain and a COOH-terminal reductase domain, which are connected by a calmodulin binding domain. Activity requires the presence of heme in the protein and availability of substrate arginine and cofactors including tetrahydrobiopterin, calmodulin, flavin mononucleotide (FMN) FAD, and NADPH (7, 47, 51, 52). The enzyme dimerizes to form homodimer in the presence of heme and tetrahydroobiopterin and subsequently binds substrate and cofactors to produce NO (10, 23, 52). Here, the dimeric distribution of eNOS in PAH was similar to controls, indicating that the enzyme has a sufficient supply of heme and tetrahydrobiopterin. Increased activity of arginases, enzymes that convert arginine to ornithine and urea, may compete with eNOS for substrate (35, 53). Arginase I is expressed in the liver and contributes to the majority of the body's total arginase activity, whereas arginase II is present in most tissues, including the lungs (54). Increased arginase I expression is implicated in the development of PAH in sickle cell disease (43), whereas increased arginase II is found in pulmonary endothelial cells of patients with idiopathic PAH (54). Stable isotopic studies confirm that there is increased breakdown of arginine by arginase in PAH patients, which affects NO synthesis (30). Although the effect of arginine substrate availability was not investigated in this study, all experiments were performed in conditions of excess arginine to avoid substrate limitations.
More recent studies show that regulation of dimeric eNOS activity is primarily through autoinhibition via interaction of autoinhibitory control elements (ACE) in the reductase domain with an autoinhibitory domain of the enzyme. Phosphorylation of specific amino acids in eNOS controls the autoinhibition of the enzyme. Certain phosphorylation events enhance interaction of the ACEs with the autoinhibitory domain and thus prevent enzyme activity, whereas other phosphorylation events release the ACEs from the autoinhibitory domain, enabling calmodulin to bind to the enzyme and NO to be synthesized (19, 24, 34). Phosphorylation is controlled by intracellular kinase-phosphatase pathways that are activated by extracellular agonists, such as BK, endothelin-1, and VEGF (1, 11, 18). The main phosphorylation activation, which releases the enzyme from autoinhibition, is phosphorylation of the S1177. This allows calmodulin binding and is required for synthesis of NO (1, 11, 18). The phosphorylation of T495 of eNOS inhibits activity by promoting autoinhibition via ACEs and preventing calmodulin binding (18, 33, 41).
PKC is one of the key kinases that regulate eNOS activity (28, 38). Here, eNOS-associated basal PKC activity is higher in PAH compared with control, suggesting the mechanism of eNOS inhibition. Abundance of phosphorylated PKC supports that PKC activity is upregulated in PAH. Arachidonic acid formation, an indirect measure of PKC activation, is also increased. Although not evaluated here, increasing PKC phosphorylation of T495 eNOS has previously been shown (6) to lead to eNOS uncoupling, i.e., loss of NO production and greater superoxide generation. β-Adrenergic receptors, the most well-studied transmembrane G protein-coupled receptors, also play an important role in regulation of eNOS activity (3, 15, 32). β-Blockers have been shown to inhibit PKC (4, 48). The ability to block PKC activity by β-blocker and restore NO formation by PAH cells indicates one mechanism by which β-blockers may benefit PAH. Although not currently recommended in guidelines for care of PAH patients, β-adrenergic receptor blockade reverses RV remodeling and improves RV function in experimental pulmonary hypertension (2), which together with other reports of benefits of β-blockade on endothelial dysfunction (45) support a rationale to consider treatment of PAH with β-blocker.
One limitation of this study is that use of primary cells from PAH patients led to a limited number of biological replicates. However, all PAH samples consistently exhibited T495 aberrant phosphorylation in multiple experimental conditions. Pharmacological PKC inhibitors may also have off-target effects that limit conclusions about kinase-specific effects. The phospho- and dephospho-mutant eNOS provide confidence that aberrant phosphorylation of the enzyme is a fundamental mechanism underlying the loss of eNOS function in the PAH. Thus altogether the data indicate that the lower level of NO production in PAH is due to phosphorylation inactivation of eNOS in pulmonary arterial endothelial cells. These findings provide a rationale to investigate therapies targeting kinase pathways in PAH.
GRANTS
This study was supported by HL60917 and HL115008 from National Heart, Lung, and Blood Institute and in part by the National Center for Advancing Translational Sciences UL1TR000439.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.G. and S.C.E. conception and design of research; S.G., M.K.G., W.X., D.A.M., A.J.J., S.A.C., M.M.H., and J.Y. performed experiments; S.G. and A.J.J. analyzed data; S.G., D.J.S., P.P., and S.V.N.P. interpreted results of experiments; S.G., W.X., A.J.J., and S.C.E. prepared figures; S.G. drafted manuscript; S.G. and S.C.E. edited and revised manuscript; S.C.E. approved final version of manuscript.
ACKNOWLEDGMENTS
Thanks to J. Lang and D. Schumick for artwork.
REFERENCES
- 1.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem 278: 14841–14849, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 182: 652–660, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol 285: C499–C508, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborti S, Roy S, Chowdhury A, Mandal A, Chakraborti T. Role of PKCalpha-p38 MAPK-Gialpha axis in peroxynitrite-mediated inhibition of beta-adrenergic response in pulmonary artery smooth muscle cells. Cell Signal 25: 512–526, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Champion HC, Bivalacqua TJ, Greenberg SS, Giles TD, Hyman AL, Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc Natl Acad Sci USA 99: 13248–13253, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen F, Kumar S, Yu Y, Aggarwal S, Gross C, Wang Y, Chakraborty T, Verin AD, Catravas JD, Lucas R, Black SM, Fulton DJ. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+ -toxins. PLoS One 9: e99823, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen PF, Tsai AL, Berka V, Wu KK. Mutation of Glu-361 in human endothelial nitric-oxide synthase selectively abolishes l-arginine binding without perturbing the behavior of heme and other redox centers. J Biol Chem 272: 6114–6118, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Comhair SA, Xu W, Mavrakis L, Aldred MA, Asosingh K, Erzurum SC. Human primary lung endothelial cells in culture. Am J Respir Cell Mol Biol 46: 723–730, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, Tainer JA. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science 279: 2121–2126, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Fagan KA, Fouty BW, Tyler RC, Morris KG Jr, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagan KA, McMurtry I, Rodman DM. Nitric oxide synthase in pulmonary hypertension: lessons from knockout mice. Physiol Res 49: 539–548, 2000. [PubMed] [Google Scholar]
- 14.Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res 93: 907–916, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Ferro A, Queen LR, Priest RM, Xu B, Ritter JM, Poston L, Ward JP. Activation of nitric oxide synthase by beta 2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol 126: 1872–1880, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res 43: 532–541, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, Zheng L, Kaveti S, Kinter M, Hazen SL, Erzurum SC. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol 176: 5587–5597, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333: 214–221, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Girgis RE, Champion HC, Diette GB, Johns RA, Permutt S, Sylvester JT. Decreased exhaled nitric oxide in pulmonary arterial hypertension: response to bosentan therapy. Am J Respir Crit Care Med 172: 352–357, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol 16: 515–523, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem 275: 22268–22272, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA 92: 7809–7813, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque MM, Panda K, Tejero J, Aulak KS, Fadlalla MA, Mustovich AT, Stuehr DJ. A connecting hinge represses the activity of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 9254–9259, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 276: 16587–16591, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Hirata K, Kuroda R, Sakoda T, Katayama M, Inoue N, Suematsu M, Kawashima S, Yokoyama M. Inhibition of endothelial nitric oxide synthase activity by protein kinase C. Hypertension 25: 180–185, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 158: 917–923, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Kao CC, Wedes SH, Hsu JW, Bohren KM, Comhair SA, Jahoor F, Erzurum SC. Arginine metabolic endotypes in pulmonary arterial hypertension. Pulm Circ 5: 124–134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol 9: 371–377, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Kou R, Michel T. Epinephrine regulation of the endothelial nitric-oxide synthase: roles of RAC1 and beta3-adrenergic receptors in endothelial NO signaling. J Biol Chem 282: 32719–32729, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of l-arginine metabolism to efficient nitric oxide production. J Biol Chem 278: 44719–44726, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Hughes TE, Sessa WC. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J Cell Biol 137: 1525–1535, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maarsingh H, Pera T, Meurs H. Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch Pharmacol 378: 171–184, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado RF, Londhe Nerkar MV, Dweik RA, Hammel J, Janocha A, Pyle J, Laskowski D, Jennings C, Arroliga AC, Erzurum SC. Nitric oxide and pulmonary arterial pressures in pulmonary hypertension. Free Radic Biol Med 37: 1010–1017, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, Polak JM. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol 185: 313–318, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Matsubara M, Hayashi N, Jing T, Titani K. Regulation of endothelial nitric oxide synthase by protein kinase C. J Biochem 133: 773–781, 2003. [DOI] [PubMed] [Google Scholar]
- 39.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276: 17625–17628, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem 277: 42344–42351, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294: 81–90, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving epoprostenol therapy. Lung 179: 233–243, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Perros F, Ranchoux B, Izikki M, Bentebbal S, Happe C, Antigny F, Jourdon P, Dorfmuller P, Lecerf F, Fadel E, Simonneau G, Humbert M, Bogaard HJ, Eddahibi S. Nebivolol for improving endothelial dysfunction, pulmonary vascular remodeling, and right heart function in pulmonary hypertension. J Am Coll Cardiol 65: 668–680, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Prado GN, Taylor L, Polgar P. Effects of intracellular tyrosine residue mutation and carboxyl terminus truncation on signal transduction and internalization of the rat bradykinin B2 receptor. J Biol Chem 272: 14638–14642, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Sessa WC, Harrison JK, Barber CM, Zeng D, Durieux ME, D'Angelo DD, Lynch KR, Peach MJ. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem 267: 15274–15276, 1992. [PubMed] [Google Scholar]
- 48.Sozzani S, Agwu DE, McCall CE, O'Flaherty JT, Schmitt JD, Kent JD, McPhail LC. Propranolol, a phosphatidate phosphohydrolase inhibitor, also inhibits protein kinase C. J Biol Chem 267: 20481–20488, 1992. [PubMed] [Google Scholar]
- 49.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res 81: 34–41, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101: 2468–2477, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta 1411: 217–230, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Wang ZQ, Tejero J, Wei CC, Haque MM, Santolini J, Fadlalla M, Biswas A, Stuehr DJ. Arg375 tunes tetrahydrobiopterin functions and modulates catalysis by inducible nitric oxide synthase. J Inorg Biochem 108: 203–215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA 104: 1342–1347, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]