Abstract
Ventilator-induced lung injury (VILI) is associated with activated inflammatory signaling, such as cytokine production by endothelial and epithelial cells and macrophages, although the precise mechanisms of inflammatory activation induced by VILI-relevant cyclic stretch (CS) amplitude remain poorly understood. We show that exposure of human pulmonary endothelial cells (EC) to chronic CS at 18% linear distension (18% CS), but not at physiologically relevant 5% CS, induces “EC-activated phenotype,” which is characterized by time-dependent increase in ICAM1 and VCAM1 expression. A preconditioning of 18% CS also increased in a time-dependent fashion the release of soluble ICAM1 (sICAM1) and IL-8. Investigation of potential signaling mechanisms of CS-induced EC inflammatory activation showed that 18% CS, but not 5% CS, induced time-dependent upregulation of VEGF receptor 2 (VEGFR2), as monitored by increased protein expression and VEGFR2 tyrosine phosphorylation. Both CS-induced VEGFR2 expression and tyrosine phosphorylation were abrogated by cotreatment with reactive oxygen species inhibitor, N-acetyl cysteine. Molecular inhibition of VEGFR2 expression by gene-specific siRNA or treatment with VEGFR2 pharmacological inhibitor SU-1498 attenuated CS-induced activation of ICAM1 and VCAM1 expression and sICAM1 release. Chronic EC preconditioning at 18% CS augmented EC inflammation and barrier-disruptive response induced by proinflammatory cytokine TNF-α. This effect of chronic 18% CS preconditioning was attenuated by siRNA-induced VEGFR2 knockdown. This study demonstrates for the first time a VEGFR2-dependent mechanism of EC inflammatory activation induced by pathological CS. We conclude that, despite the recognized role of VEGF as a prosurvival and angiogenic factor, excessive activation of VEGFR2 signaling by high-tidal-volume lung mechanical ventilation may contribute to ventilator-induced (biotrauma) lung inflammation and barrier dysfunction by augmenting cell response to VILI-associated inflammatory mediators.
Keywords: permeability, inflammation, cytoskeleton, lung endothelium, cyclic stretch
nearly all patients with acute respiratory distress syndrome (ARDS) require mechanical ventilation and are therefore at risk for ventilator-induced lung injury (VILI). VILI in these conditions may develop as a result of the uneven distribution of mechanical distension, leading to overdistension of certain lung segments. Mechanical stress produced by mechanical ventilation leads to the upregulation of an inflammatory response (21). Increased cytokine production is a well-recognized prognostic factor of acute lung injury (ALI)/ARDS severity (4). Existing experimental models of lung pathological mechanical stimulation recapitulate increased inflammatory cytokine production (14, 24, 27), neutrophil adhesion to pulmonary endothelial cells (ECs) (30), and apoptosis (22, 36) observed in a clinical setting of VILI/ARDS. Acute exposure of pulmonary ECs to pathologic cyclic stretch (CS) in vitro also causes rapid but transient elevation of EC permeability and cytoskeletal remodeling (9, 18). High-magnitude CS also potentiates barrier-disruptive effects of agonists via synergistic stimulation of the RhoA pathway of actomyosin-driven cell contraction and disassembly of cell-cell junction complexes, leading to EC barrier dysfunction (8).
TNF-α is a proinflammatory cytokine produced by activated leukocytes and lung alveolar epithelial cells and ECs during the course of VILI (4). TNF-α directly contributes to the generation of permeability edema by stimulating RhoA- and p38 MAP kinase- mediated pathways of lung vascular endothelial hyperpermeability (47) and inducing cell apoptosis (6, 23).
Along with activation of cytokines, increased levels of VEGF have been detected in pulmonary circulation in animal models (15) and in patients with ARDS at different stages of ALI (29, 33, 40). Activation of VEGF receptor 2 (VEGFR2) regulates vascular permeability to water and proteins, and VEGF overexpression in the lungs as well as injection of purified VEGF increases endothelial permeability in vivo (25, 35). VEGFR2 activation is mediated by tyrosine phosphorylation via ligand-dependent and ligand-independent mechanisms, which stimulates VEGFR2 receptor kinase activity and triggers downstream signaling pathways (31). Whether VEGFR2 signaling is involved in CS-induced inflammatory response remains unknown. This study tested the effects of 18% CS on VEGFR2 expression and activation and evaluated the role of VEGFR2 as a potential mechanism of CS-induced EC inflammatory activation.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary artery endothelial cells (HPAEC), human lung microvascular cells (HLMVEC) and cell culture basal medium (EBM-2) with growth supplements were obtained from Lonza (Allendale, NJ), cultured according to the manufacturer's protocol, and used at passages 5–7. VEGFR2 inhibitor SU1498 was obtained from EMD Millipore (Billerica, MA). Human TNF-α was obtained from R&D Systems (Minneapolis, MN). GEF-H1, VEGFR2, and phospho-VEGFR2 antibodies were purchased from Cell Signaling (Beverly, MA); ICAM1 and VCAM1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). All reagents for immunofluorescence staining were purchased from Molecular Probes (Eugene, OR). Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO).
Cell culture under CS.
CS experiments were performed as previously described (5, 38) using a FX-4000T Flexcell Tension Plus system (Flexcell International, McKeesport, PA) equipped with a 25-mm BioFlex Loading station designed to provide uniform radial and circumferential strain across a membrane surface along all radii. Each BioFlex membrane is stretched over the post when under vacuum pressure, creating a single-plane uniformly stretched circle. The radial and circumferential strain was experimentally determined by the vendor (Flexcell International). After 72 h of culture, cells were exposed to high-magnitude (18% linear elongation, sinusoidal wave, 25 cycles/min) or physiologically relevant (5% linear elongation) CS stretch to recapitulate the mechanical stresses experienced by the alveolar endothelium at high- and low-tidal-volume mechanical ventilation (5, 8, 44). Control BioFlex plates with static EC culture were placed in the same cell culture incubator and processed similarly to CS-preconditioned cells. At the end of experiment, cell lysates were collected for Western blot analysis, or CS-exposed endothelial monolayers were fixed and used for immunofluorescence staining.
siRNA transfection.
To deplete endogenous VEGFR2, Stealth Select siRNA sets were used. Predesigned human siRNAs of standard purity were ordered from Invitrogen (Carlsbad, CA), and transfection of ECs with siRNA was performed as previously described (39). After 48–72 h of transfection, cells were used for experiments or harvested for Western blot verification of specific protein depletion. Nonspecific, nontargeting siRNA (Dharmacon, Lafayette, CO) was used as a control treatment.
Immunoblotting.
Confluent HPAECs exposed to 18% CS, 5% CS, or static conditions were treated with vehicle of inflammatory agonists, and Western blot analysis of total cell lysates was performed with the primary antibody of interest followed by incubation with secondary horseradish peroxidase-conjugated antibody, as described elsewhere (10).
Immunofluorescence.
Endothelial monolayers grown on BioFlex plates were exposed to CS and subjected to immunofluorescence staining as described previously (9). Membranes with attached cells were mounted on 4 × 4 cm rectangular coverslips and analyzed using a Nikon video imaging system (Nikon Instech, Tokyo, Japan). Images were processed with Image J software (National Institutes of Health, Bethesda, MD) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software. Quantitative analysis of paracellular gap formation was performed as previously described (5, 7, 13). The 16-bit images were analyzed using MetaVue 4.6 software (Universal Imaging, Downington, PA). The gap formation was expressed as a ratio of the gap area to the area of the whole image. The values were statistically processed using Sigma Plot 7.1 (SPSS Science, Chicago, IL) software. For each experimental condition, at least 10 microscopic fields in each independent experiment were analyzed.
Measurement of IL-8 and soluble ICAM1.
For IL-8 and soluble ICAM1 (sICAM1) measurements in preconditioned medium of human pulmonary EC cultures, supernatants from treated ECs were collected and centrifuged to remove debris. IL-8 and sICAM1 levels were determined by ELISA (R&D Systems) following the manufacturer's protocol. Absorbance was read at 450 nm within 30 min in a microplate reader (Thermomax; Molecular Devices, Menlo Park, CA).
Statistical analysis.
Results are expressed as means ± SD. The normal distribution was evaluated before statistical analysis using the Kolmogorov-Smirnov test. Experimental samples were compared with controls by unpaired Student's t-test. For multiple-group comparisons, a one-way ANOVA and post hoc multiple-comparison tests were used. If the distribution was not normal, nonparametric Mann-Whitney Wilcoxon test was used instead of ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Chronic 18% CS augmented TNF-α-induced EC barrier dysfunction and IL-8 production.
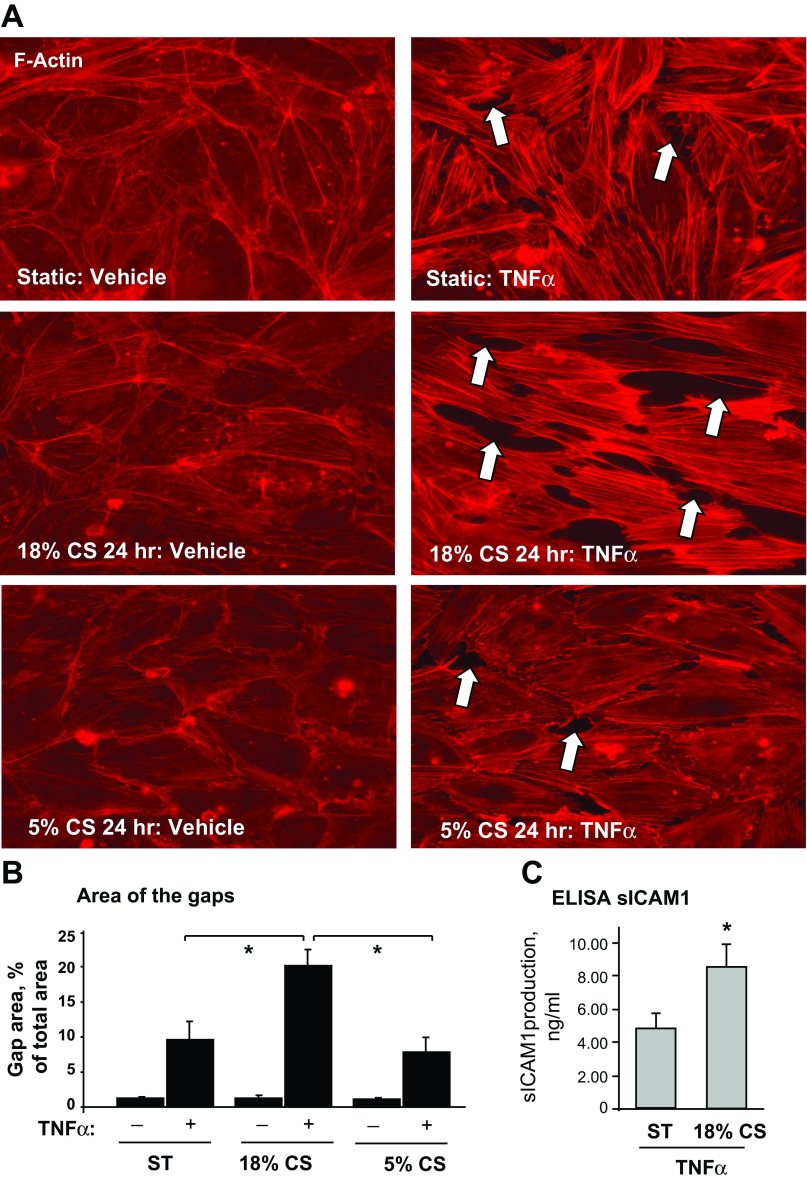
We examined the effects of chronic 18% CS and 5% CS on EC barrier failure induced by TNF-α. Human pulmonary ECs grown to confluence on Flexcell plates were exposed to 18% CS, 5% CS, or static conditions for 24 h and treated with vehicle or TNF-α immediately after CS was stopped. Cytoskeletal remodeling was monitored by immunofluorescence staining for F-actin. Increased EC monolayer disruption in 18% CS-preconditioned EC monolayers was manifested by more pronounced formation of actin stress fibers and intercellular gaps, compared with TNF-α-stimulated cells under 5% CS or static conditions (Fig. 1A). Quantitative image analysis of control and stimulated EC monolayers shows that EC exposure to 18% CS enhanced TNF-α-induced paracellular gap formation compared with cells exposed to static conditions or 5% CS (Fig. 1B). CS preconditioning also augmented production of soluble ICAM1 (sICAM1) by pulmonary EC in response to TNF-α challenge (Fig. 1C). These data suggest that chronic preconditioning at 18% CS exacerbates barrier-disruptive effects of TNF-α.
Fig. 1.
Effects of chronic cyclic stretch (CS) on TNF-α-induced endothelial cell (EC) barrier dysfunction. Human pulmonary artery ECs (HPAECs) grown on Flexcell plates were subjected to 18% CS, 5% CS, or static conditions (ST) for 24 h followed by TNF-α (20 ng/ml, 4 h) stimulation. A: cytoskeletal remodeling in static and stretched ECs was examined by immunofluorescence staining for F-actin; paracellular gaps are marked by arrows. B: quantitative analysis of paracellular gap formation in control and TNF-α-stimulated HPAECs with or without 5% CS or 18% CS preconditioning. Data are expressed as means ± SD; n = 3, *P < 0.05 vs. static. C: soluble ICAM1 (sICAM1) content in preconditioned media was determined using an ELISA assay; n = 4, *P < 0.05 vs. static.
Chronic 18% CS induced expression of EC adhesion molecules and IL-8 production.
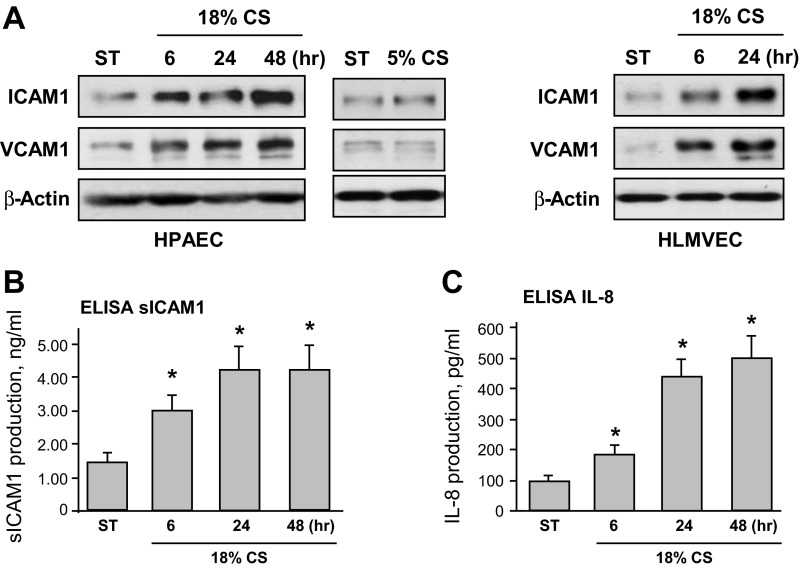
To examine effects of chronic high-magnitude CS preconditioning on endothelial inflammatory activation, we first evaluated the induction of cell adhesion molecules ICAM1 and VCAM1 in HPAECs exposed to 18% CS, which was compared with cells exposed to physiologically relevant 5% CS or static conditions. Western blot analysis of ICAM1 and VCAM1 protein expression showed a pronounced, time-dependent increase in ICAM1 and VCAM1 protein levels in cells exposed to 18% CS but not to 5% CS or cells under static conditions (Fig. 2A, left). Because phenotypic differences between macro- and microvascular endothelium are well recognized (19), we next performed comparative analysis of 18% CS-induced ICAM1 induction in HPAECs and HLMVECs. The results (Fig. 2A, right) show similar levels of 18% CS upregulation of ICAM1 expression in HLMVEC. EC exposure to 18% CS also caused accumulation of soluble ICAM1 and IL-8 in the conditioned medium (Fig. 2, B and C). Maximal expression levels of all markers of EC inflammatory activation were observed after 48 h of 18% CS stimulation.
Fig. 2.
Effects of chronic CS on EC inflammatory activation. HPAECs were subjected to 18% CS for 6, 24, or 48 h, exposed to 5% CS for 24 h, or left under static conditions. In addition, human lung microvascular ECs (HLMVECs) were exposed to 18% CS for 6 or 24 h. A: time-dependent expression of ICAM1 and VCAM1 was monitored by immunoblotting with corresponding antibodies. Equal protein loading was confirmed by determination of β-actin content in total cell lysates. Results are representative of 3 independent experiments. B and C: production of soluble ICAM1 (B) or IL-8 (C) was evaluated by ELISA assay. Data are expressed as means ± SD; n = 3, *P < 0.05 vs. static.
Chronic 18% CS stimulated expression and sustained activation of VEGFR2.
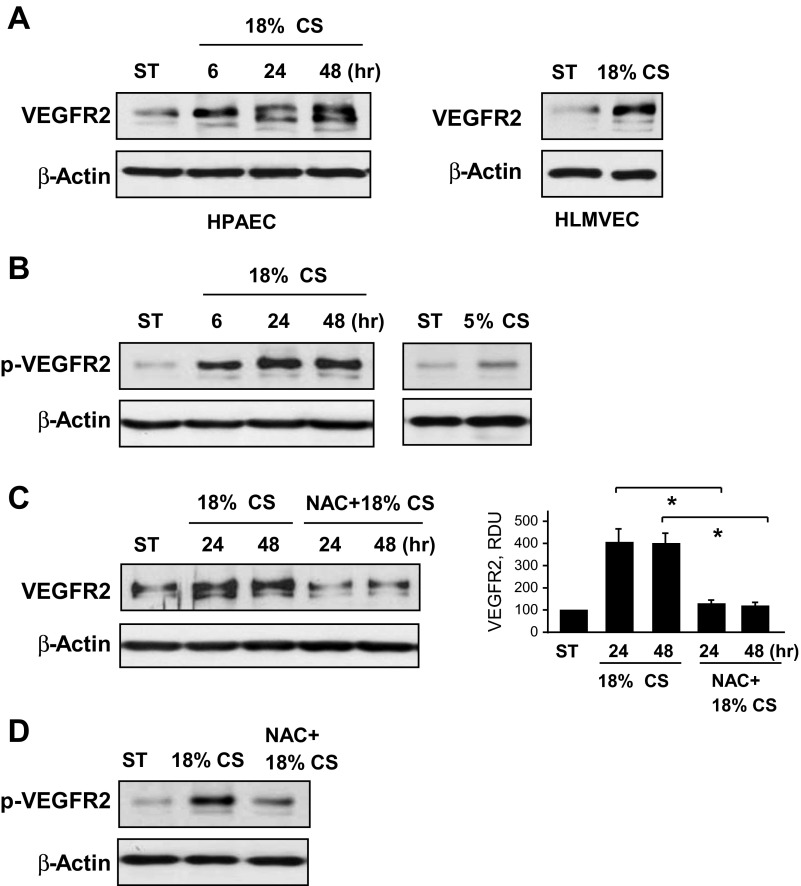
VEGFR2 regulates many EC functions, including angiogenesis, regulation of permeability, migration, proliferation, and EC survival (31). The next experiments tested whether 18% CS stimulates VEGFR2 expression in human pulmonary ECs. Cells were exposed to 18% CS for different periods of time. A noticeable increase in VEGFR2 protein level was observed in human pulmonary ECs after 6 h of mechanical stimulation and reached maximal levels after 48 h of CS (Fig. 3A, left). Similar effects of CS-preconditioned VEGFR2 expression were observed in microvascular ECs (Fig. 3A, right). Importantly, besides stimulation of protein expression, 18% CS also caused sustained activation of VEGFR2, reflected by increased VEGFR2 tyrosine phosphorylation detected by immunoblotting of cell lysates with phosphotyrosine-specific VEGFR2 antibody (Fig. 3B, left). In contrast, EC chronic exposure to 5% CS (24 h) did not elevate VEGFR2 phosphorylation to the levels observed in ECs exposed to 18% CS for the same time period (Fig. 3B, right). Induction of VEGFR2 expression and VEGFR2 phosphorylation induced by 18% CS were abrogated by pretreatment with inhibitor of reactive oxygen species (ROS), N-acetyl cysteine (Fig. 3, C and D).
Fig. 3.
Effects of chronic CS on expression and activation of VEGF receptor 2 (VEGFR2). HPAECs were subjected to 18% CS for 6, 24, or 48 h, stretched at 5% for 24 h, or left under static conditions. HLMVECs were exposed to 18% CS for 24 h. A: time-dependent VEGFR2 expression was monitored by immunoblotting with corresponding antibodies. Equal protein loading was confirmed by determination of β-actin content in total cell lysates. B: CS-induced VEGFR2 tyrosine phosphorylation in ECs exposed to 18% CS and 5% CS was monitored by immunoblotting with phospho-VEGFR2 antibody. C and D: HPAECs grown on Flexcell plates were subjected to CS with or without N-acetyl cysteine (NAC) (1 mM, 30 min) pretreatment. CS-induced VEGFR2 expression was assessed by Western blot analysis. Data are expressed as means ± SD; n = 3, *P < 0.05 vs. 18% CS (C). HPAECs pretreated with NAC or vehicle were exposed to 18% CS (6 h) or static conditions. VEGFR2 tyrosine phosphorylation was monitored by immunoblotting with phospho-VEGFR2 antibody (D). Equal protein loading was confirmed by normalization to β-actin content in the total cell lysates. Results are representative of 3 independent experiments.
CS-induced activation of ICAM1 and VCAM1 expression is mediated by activation of VEGFR2.
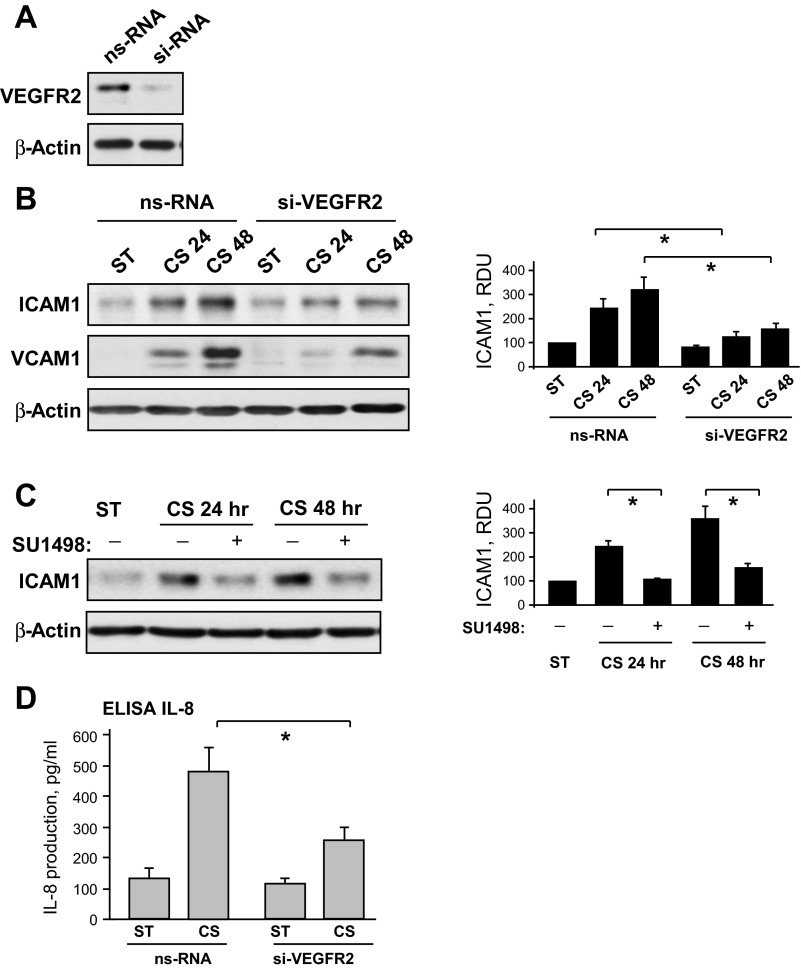
Involvement of VEGFR2 signaling in CS-induced ICAM1 and VCAM1 expression was tested using siRNA-induced VEGFR2 knockdown (Fig. 4A). In these experiments, cell treatment with VEGFR2-specific siRNA almost completely abolished 18% CS-induced increase in VCAM1 and ICAM1 protein expression by pulmonary ECs (Fig. 4B). In addition to VEGFR2 knockdown, inhibition of VEGFR2 tyrosine kinase activity by pharmacological inhibitor SU-1498 also abolished 18% CS-induced increases in the ICAM1 expression (Fig. 4B). VEGFR2 depletion also significantly attenuated production of IL-8 induced by chronic 18% CS (Fig. 4C).
Fig. 4.
Role of VEGFR2 activation in EC inflammatory activation caused by chronic CS. A: human pulmonary ECs were transfected with VEGFR2-specific or nonspecific siRNA. siRNA-induced protein knockdown was confirmed by Western blot. B: HPAECs transfected with VEGFR2-specific or nonspecific siRNA were subjected to 18% CS for indicated periods of time. Control cells were left under static conditions. Effect of VEGFR2 knockdown on CS-induced ICAM1 and VCAM1 expression was analyzed in control and stretched cells. siRNA-induced VEGFR2 depletion was confirmed by Western blot. β-Actin was used as a normalization control; n = 3; *P < 0.05 vs. nonspecific RNA (ns-RNA). C: cells were treated with vehicle or 5 μM SU-1498 for 30 min before exposure to CS. ICAM1 expression was analyzed by Western blot with corresponding antibody. β-Actin was used as a normalization control; n = 3; *P < 0.05 vs. 18% CS. D: effect of VEGFR2 knockdown on 18% CS-induced IL-8 production was evaluated by ELISA assay. Data are expressed as means ± SD; n = 4, *P < 0.05 vs. ns-RNA.
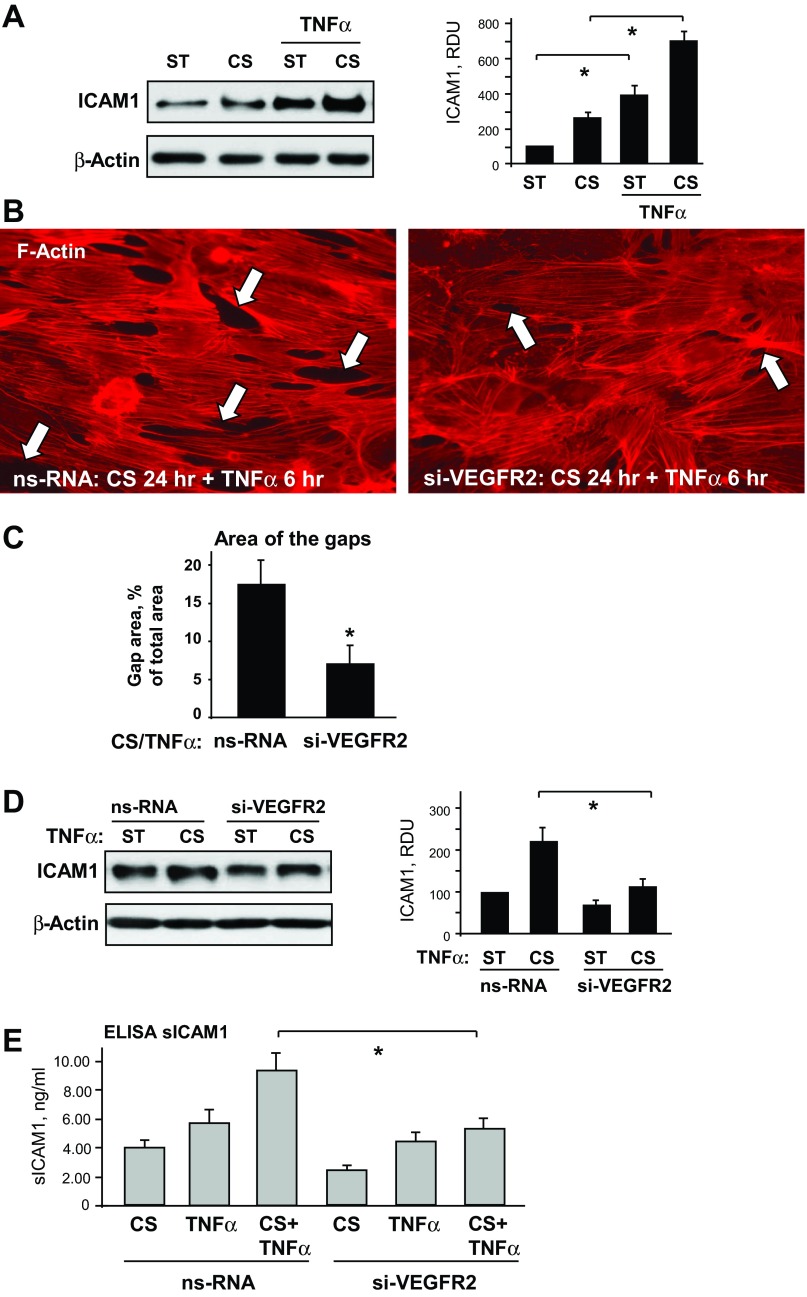
Role of VEGFR2 signaling in the two-hit model of EC dysfunction caused by chronic 18% CS and TNF-α.
The effects of VEGFR2 knockdown on EC inflammation and permeability induced by chronic CS were further tested in the two-hit model of EC dysfunction caused by 18% CS and TNF-α challenge. 18% CS significantly potentiated TNF-α-induced stimulation of ICAM1 expression, which is an inflammatory marker (Fig. 5A). This functional EC response was accompanied by pronounced remodeling of actin cytoskeleton and formation of paracellular gaps (Fig. 5, B and C). VEGFR2 knockdown attenuated F-actin stress fiber formation and significantly reduced the area of paracellular gaps in EC monolayers exposed to chronic 18% CS and stimulated with TNF-α (Fig. 5, B and C). VEGFR2 knockdown also suppressed ICAM1 protein expression (Fig. 5D) and decreased the release of soluble ICAM1 in the conditioned medium (Fig. 5E) in EC monolayers exposed to combined stimulation with 18% CS and TNF-α.
Fig. 5.
Role of VEGFR2 signaling in the 2-hit model of EC dysfunction. A: HPAECs were subjected to 18% CS for 24 h followed by TNF-α (20 ng/ml, 4 h) stimulation. Control cells were left under static conditions. ICAM1 expression was analyzed in control and stretched cells. β-Actin was used as a normalization control; n = 3; *P < 0.05 vs. static. B and C: ECs were transfected with VEGFR2-specific RNA or ns-RNA, followed by application of 18% CS for 24 h and TNF-α stimulation. Cytoskeletal remodeling in control and stretched ECs was examined by immunofluorescence staining for F-actin; paracellular gaps are marked by arrows (B). Quantitative analysis was performed of paracellular gap formation in control and VEGFR2-depleted ECs. Data are expressed as means ± SD; n = 3, *P < 0.05 vs. ns-RNA (C). D: effect of VEGFR2 knockdown on CS-induced ICAM1 expression was analyzed in static and stretched cells (18% CS, 24 h) stimulated with TNF-α (20 ng/ml, 4 h). β-Actin was used as a normalization control; n = 3; *P < 0.05 vs. ns-RNA. E: sICAM1 content in the culture media from control and VEGFR2-depleted cells was determined in stretch (18% CS, 24 h) and/or TNF-α-stimulated samples (20 ng/ml, 4 h) using ELISA assay; n = 4, *P < 0.05 vs. ns-RNA.
DISCUSSION
The pathological mechanical forces experienced by lung tissue during mechanical ventilation at high tidal volume dramatically alter pulmonary endothelial responses to bioactive molecules and may further propagate VILI and pulmonary edema (34, 43, 45). Pulmonary ECs exposed to VILI-relevant high-magnitude CS acquire activated phenotype and become more susceptible to inflammatory insults. The mechanism of such stretch-induced EC sensitization remained unclear. This study shows for the first time the stimulation of VEGFR2 expression and signaling in pulmonary ECs exposed to high-magnitude CS as a novel mechanism contributing to the pulmonary EC inflammatory response to chronic CS. We report an unexpected effect of CS-induced VEGFR2 activation on the expression of proinflammatory adhesive molecules and cytokine production. This conclusion is supported by experiments with VEGFR2 knockdown, which dramatically attenuated CS-induced expression of ICAM1 and VCAM1.
The exact mechanisms how VEGFR2 is stimulated by biomechanical signals in vivo remain unclear. Our unpublished results show that CS-induced VEGFR2 autophosphorylation may be mediated by CS-induced uncoupling from VE-cadherin, which relieves the inhibitory effect of VE-cadherin and leads to VEGFR2 activation. Activated VEGFR2 then may phosphorylate VE-cadherin, which stimulates disassembly of VE-cadherin-containing adherens junctions and causes VE-cadherin internalization (17). This signaling loop may represent a vicious circle of VEGFR2-VE-cadherin-induced EC permeability. Indeed, our unpublished data show dissociation of VEGFR2 from VE-cadherin in CS-stimulated ECs. CS-induced, time-dependent phosphorylation of VE-cadherin and VEGFR2 may be driven by this mechanism of VEGFR2 activation.
Our data also show that CS-induced VEGFR2 activation is dependent on CS magnitude and may also be attenuated by ROS inhibition. These results suggest a ROS-dependent mechanism of VEGFR2 activation by pathological CS. Indeed, high-magnitude CS is known to elevate ROS-activating systems such as NADPH oxidase (20) and xanthine oxidoreductase (1). NADPH oxidase-dependent inflammatory gene expression was described in other models of inflammatory activation associated with oxidative stress. For example, VEGFR2 transactivation mediated inflammatory EC response to proinflammatory lipid oxidation products (26), whereas VEGFR2 inhibitors and siRNA-induced VEGFR2 knockdown decreased the transcription of IL–8, (26, 48). These findings suggest a potential mechanism of elevated expression of inflammatory molecules in the context of pathologic CS, which includes high-magnitude CS-induced activation of ROS production (by NADPH oxidase or xanthine oxidoreductase), leading to stimulation of VEGFR2 expression, VEGFR2 transactivation, and VEGFR2-mediated facilitation of expression of inflammatory molecules in the context of elevated CS.
The use of macrovascular ECs in this study was based on higher efficiency of HPAEC attachment to elastic substrates during CS exposure. Without a doubt, functional and phenotypic heterogeneity is a fundamental property of ECs from different organs and vascular beds (2). For example, direct activation of store-operated channel Ca2+ entry increased extra-alveolar, but not alveolar, EC permeability (16). However, in our experiments, 18% CS caused similar effects on stimulation of ICAM1 and VCAM1 expression (Fig. 2A) and VEGFR2 activation (Fig. 3A) in both macro- and microvascular ECs. Comparative analysis of cell monolayers exposed to 18% CS, the magnitude relevant to CS experienced by alveolar endothelium, was also performed in previous studies and showed similar effects developed by human lung micro- and macrovascular ECs in response to CS (12). Both stretch preconditioned EC types developed increased permeability response to thrombin, which was linked to increased Rho activation and remained even 16 h after EC replating onto nonelastic substrates. Long-term preconditioning at 18% CS (72 h) induced similar changes in signaling and contractile protein expression in the pulmonary microvascular and macrovascular ECs (12). These results suggest that HPAECs recapitulate major functional and gene expression responses of microvascular ECs, when both cell types are exposed to similar levels of mechanical stimulation. It is, however, conceivable that, because of anatomical constrains, ECs lining larger-caliber lung vessels may not experience the same levels of mechanical stretch in situ as lung parenchyma and capillary endothelium adjacent to alveoli in mechanically ventilated lungs. Altogether, these results may reflect a significant level of phenotypic plasticity of the lung vascular endothelium. Nevertheless, a more detailed characterization of endothelial heterogeneity in the lung is important for deeper understanding of the mechanisms of inflammation and vascular dysfunction in VILI/ARDS settings.
VILI develops in association with elevated circulating barrier-active molecules such as TNF-α (4). The results of this study show synergistic effects of pathological CS on TNF-α-induced inflammatory signaling in pulmonary ECs. Chronic CS also exacerbated TNF-α-induced stress fiber formation and disruption of the EC monolayer, effects that were abolished by knockdown of VEGFR2. The mechanism of TNF-α-induced EC permeability involves activation of Rho signaling, leading to Rho-dependent stress fiber formation, phosphorylation of myosin light chains, actomyosin contractility, and disruption of cell-cell junctions (32). Activation of VEGFR2 also triggers a Rho pathway of EC permeability (3, 41). Thus CS-induced stimulation of VEGFR2 signaling may underlie the potentiation of TNF-α-induced EC barrier disruption.
Of note, the same Rho activation may also contribute to the increased inflammatory response by induction of ICAM1, VCAM1, and IL-8 expression because Rho may additionally stimulate NFκB inflammatory signaling triggered by canonical Toll-like receptors or cytokine receptors (28, 37).
The results of this study suggest that potentiating effects of pathological stretch and inflammatory effects triggered by a wide range of circulating molecules including TNF-α, IL-6, and others may be blunted by pharmacological or molecular inhibition of VEGFR2 signaling. Such a generalized approach of VEGFR2 inhibition-dependent modulation of inflammation may be more beneficial than pinpointed inhibition of specific inflammatory mediators. For example, despite well-documented involvement of inflammatory mediators such as IL-6 and TNF-α in the mechanisms of VILI-associated inflammation and EC barrier dysfunction, these and other cytokines may be critical for lung response in different settings of ALI. For example, injection of IL-6-blocking antibodies in mice after VILI significantly increased rates of bronchoalveolar lavage albumin flux (46), whereas IL-6 generated by mast cells improved survival during sepsis by enhancing neutrophil killing of bacteria (42). It is also important to note that inflammatory and barrier-disruptive mechanisms are only induced by high-magnitude CS and not activated by physiologically relevant CS magnitudes (8, 11, 30), which did not affect VEGFR2 signaling (this study, data not shown). Instead, exposure to physiologically relevant CS amplitudes (5% CS) enhanced pulmonary EC barrier properties (5).
In conclusion, this study shows a new mechanism of augmentation of EC inflammation and barrier dysfunction by chronic high-magnitude CS through CS-induced expression and activation of VEGFR2 signaling. Inhibition of VEGFR2 attenuated parameters of EC inflammation caused by CS alone and significantly downregulated inflammation in the more clinically relevant two-hit model of EC exposed to chronic CS and TNF-α. Further validation of this mechanism in animal models may lead to new insight in therapeutic treatment of patients with ARDS under mechanical ventilation by pharmacological modulation of VEGFR2 signaling.
GRANTS
This work was supported by grants HL87823, HL076259, and HL107920 from the National Heart, Lung, and Blood Institutes and GM114171 from the National Institute of General Medical Sciences and by training grant T32 HL007605 for J. J. O’Donnell III.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.G., S.S., Y.T., and J.J.O. performed experiments; G.G., S.S., Y.T., J.J.O., K.G.B., and A.A.B. analyzed data; G.G., S.S., Y.T., J.J.O., and A.A.B. prepared figures; G.G. and A.A.B. drafted manuscript; G.G., S.S., Y.T., J.J.O., K.G.B., and A.A.B. approved final version of manuscript; J.J.O., K.G.B., and A.A.B. edited and revised manuscript; K.G.B. and A.A.B. conception and design of research; K.G.B. and A.A.B. interpreted results of experiments.
REFERENCES
- 1.Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE 3rd, Kayyali US, Garcia JG, Tuder RM, Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291: L345–L353, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2: a006429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 103: 40–55, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Belperio JA, Keane MP, Lynch JP 3rd, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med 27: 350–364, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Birukov KG, Zebda N, Birukova AA. Barrier enhancing signals in pulmonary edema. Compr Physiol 3: 429–484, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Birukova AA, Birukov KG, Smurova K, Adyshev DM, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol 298: L837–L848, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-β-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 293: L199–L211, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birukova AA, Rios A, Birukov KG. Long-term cyclic stretch controls pulmonary endothelial permeability at translational and post-translational levels. Exp Cell Res 314: 3466–3477, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: Role of Rho-dependent mechanisms. J Cell Physiol 201: 55–70, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Epshtein Y, Ni X, Dull RO, Cress AE, Garcia JG, Jacobson JR. Role of integrin beta4 in lung endothelial cell inflammatory responses to mechanical stress. Sci Rep 5: 16529, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med 167: 1627–1632, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid Redox Signal 11: 765–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111: 1853–1865, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Gawlak G, Tian Y, O'Donnell JJ 3rd, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J 28: 3249–3260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res 68: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92: e80–e86, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Halbertsma FJ, Vaneker M, Scheffer GJ, van der Hoeven JG. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med 63: 382–392, 2005. [PubMed] [Google Scholar]
- 22.Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am J Respir Cell Mol Biol 30: 396–402, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Hirase T, Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am J Physiol Heart Circ Physiol 302: H499–H505, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki M, Ito S, Morioka M, Iwata S, Numaguchi Y, Ishii M, Kondo M, Kume H, Naruse K, Sokabe M, Hasegawa Y. Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem Biophys Res Commun 389: 531–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 22: 657–664, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic Biol Med 47: 145–151, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letsiou E, Rizzo AN, Sammani S, Naureckas P, Jacobson JR, Garcia JG, Dudek SM. Differential and opposing effects of imatinib on LPS- and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 308: L259–L269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J Immunol 182: 3522–3529, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax 61: 621–626, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meliton AY, Munoz NM, Meliton LN, Birukova AA, Leff AR, Birukov KG. Mechanical induction of group V phospholipase A(2) causes lung inflammation and acute lung injury. Am J Physiol Lung Cell Mol Physiol 304: L689–L700, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-α-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 280: L1168–L1178, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Quesnel C, Marchand-Adam S, Fabre A, Marchal-Somme J, Philip I, Lasocki S, Lecon V, Crestani B, Dehoux M. Regulation of hepatocyte growth factor secretion by fibroblasts in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 294: L334–L343, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282: 54–61, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 108: 2369–2379, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Esteban J, Wang Y, Cicchiello LA, Rubin LP. Cyclic mechanical stretch inhibits cell proliferation and induces apoptosis in fetal rat lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 282: L448–L456, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Shibolet O, Giallourakis C, Rosenberg I, Mueller T, Xavier RJ, Podolsky DK. AKAP13, a RhoA GTPase-specific guanine exchange factor, is a novel regulator of TLR2 signaling. J Biol Chem 282: 35308–35317, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 304: 40–49, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res 104: 978–986, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern JB, Fierobe L, Paugam C, Rolland C, Dehoux M, Petiet A, Dombret MC, Mantz J, Aubier M, Crestani B. Keratinocyte growth factor and hepatocyte growth factor in bronchoalveolar lavage fluid in acute respiratory distress syndrome patients. Crit Care Med 28: 2326–2333, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 13: 237–247, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol 181: 5598–5605, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99: 944–952, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol 86: 2026–2033, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Villar J, Flores C, Mendez-Alvarez S. Genetic susceptibility to acute lung injury. Crit Care Med 31: S272–S275, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, Frank JA. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol 182: 8056–8062, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xing J, Birukova AA. ANP attenuates inflammatory signaling and Rho pathway of lung endothelial permeability induced by LPS and TNFalpha. Microvasc Res 79: 26–62, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimman A, Mouillesseaux KP, Le T, Gharavi NM, Ryvkin A, Graeber TG, Chen TT, Watson AD, Berliner JA. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arterioscler Thromb Vasc Biol 27: 332–338, 2007. [DOI] [PubMed] [Google Scholar]