Abstract
The production of prostaglandin E2 (PGE2) increases dramatically during pneumococcal pneumonia, and this lipid mediator impairs alveolar macrophage (AM)-mediated innate immune responses. Microsomal prostaglandin E synthase-1 (mPGES-1) is a key enzyme involved in the synthesis of PGE2, and its expression is enhanced during bacterial infections. Genetic deletion of mPGES-1 in mice results in diminished PGE2 production and elevated levels of other prostaglandins after infection. Since PGE2 plays an important immunoregulatory role during bacterial pneumonia we assessed the impact of mPGES-1 deletion in the host defense against pneumococcal pneumonia in vivo and in AMs in vitro. Wild-type (WT) and mPGES-1 knockout (KO) mice were challenged with Streptococcus pneumoniae via the intratracheal route. Compared with WT animals, we observed reduced survival and increased lung and spleen bacterial burdens in mPGES-1 KO mice 24 and 48 h after S. pneumoniae infection. While we found modest differences between WT and mPGES-1 KO mice in pulmonary cytokines, AMs from mPGES-1 KO mice exhibited defective killing of ingested bacteria in vitro that was associated with diminished inducible nitric oxide synthase expression and reduced nitric oxide (NO) synthesis. Treatment of AMs from mPGES-1 KO mice with an NO donor restored bacterial killing in vitro. These results suggest that mPGES-1 plays a critical role in bacterial pneumonia and that genetic ablation of this enzyme results in diminished pulmonary host defense in vivo and in vitro. These results suggest that specific inhibition of PGE2 synthesis by targeting mPGES-1 may weaken host defense against bacterial infections.
Keywords: bacterial pneumonia, lung, host defense, Streptococcus pneumoniae, microsomal prostaglandin E synthase, prostaglandins
pneumonia is the leading cause of death from infectious disease globally and the eighth leading cause of death in the US (30). There are four million cases of community-acquired pneumonia in the US annually, resulting in more hospitalizations than any other condition except childbirth (30, 35). Streptococcus pneumoniae is the most common cause of community-acquired pneumonia, and treatment of this condition has become more difficult because of the increased resistance of S. pneumoniae to routinely prescribed antibiotics (12). Although there have been significant improvements in medical care, mortality from bacteremic pneumococcal pneumonia has been unchanged during the past 50 years (17). Therefore, increasing our understanding of the host response to S. pneumoniae infection is essential in devising new therapeutic strategies in the treatment of pneumococcal pneumonia.
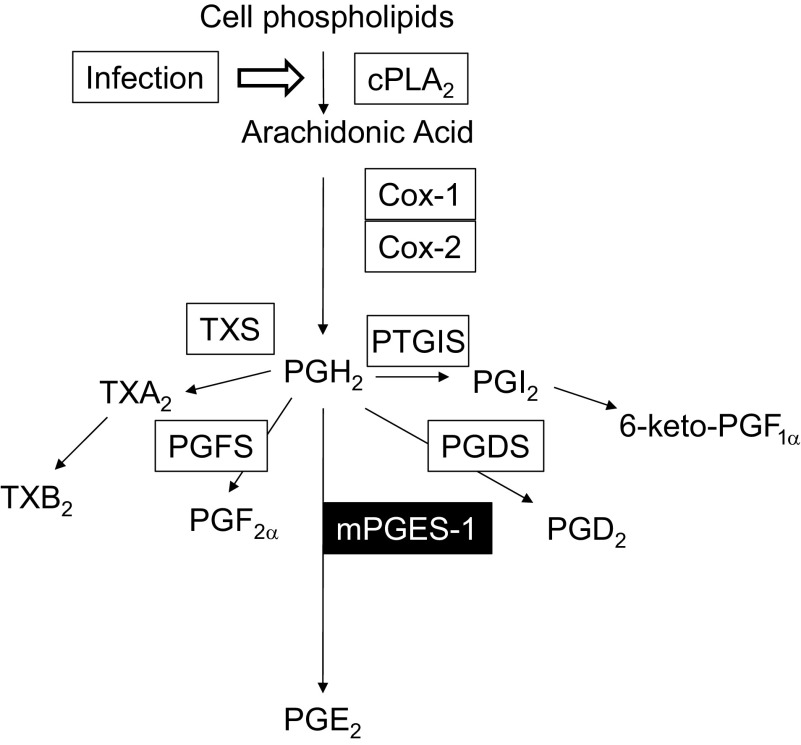
S. pneumoniae serotype 3, a frequent cause of pneumococcal pneumonia, is a heavily encapsulated strain that induces a profound inflammatory response in the lungs of the infected host that is characterized by elevated levels of proinflammatory cytokines and lipid mediators and the recruitment of leukocytes (14, 19, 20, 34). Among the lipid mediators known to regulate the immune response during pneumococcal pneumonia is prostaglandin E2 (PGE2), which increases dramatically after infection (14). PGE2 synthesis begins with the liberation of arachidonic acid (AA) by cytosolic phospholipase A2 from tissue phospholipids in response to bacterial and viral infections of the lung as well as other proinflammatory stimuli (Fig. 1) (11, 14, 25, 42). AA undergoes oxygenation and peroxidation reactions that are mediated by prostaglandin H synthase enzymes 1 and 2 (also known as cyclooxygenase or COX-1 and COX-2), resulting in the formation of prostaglandin H2 (PGH2). PGH2 does not accumulate in cells but is rapidly converted by specific terminal synthase enzymes that form the prostanoids, PGD2, PGE2, PGF2α, PGI2 (prostacyclin), and thromboxane (TXA2). Microsomal prostaglandin synthase-1 (mPGES-1) is an inducible enzyme that converts PGH2 to PGE2. PGI2 and TXA2 are unstable and are rapidly converted to 6-keto-PGF1α and TXB2, respectively (40).
Fig. 1.
Prostanoid biosynthesis begins with the activation of cytosolic phospholipase A2 (cPLA2) after infection. After liberation from tissue phospholipids, arachidonic acid (AA) is oxygenated by cyclooxygenase enzymes (COX-1 and COX-2), resulting in the formation of prostaglandin H2 (PGH2). Subsequent metabolism by terminal synthase enzymes thromboxane synthase (TXS), prostaglandin F synthase (PGFS), microsomal prostaglandin E2 synthase-1 (mPGES-1), prostaglandin D2 synthase (PGDS), and prostaglandin I2 synthase (PTGIS) converts PGH2 to thromboxane (TXA2), prostaglandin F2α (PGF2α), prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), and prostacyclin (PGI2), respectively. TXB2 and 6-keto-PGF1α are stable metabolites of TXA2 and PGI2, respectively.
The contribution of PGE2 to the innate immune response during infection is complex and mediated by four distinct G protein-coupled E prostanoid receptors (EP1–EP4) (8). Acting through EP2 and EP4, PGE2 activates Gαs-coupled increases in adenosine 3′,5′-cyclic monophosphate (cAMP), which generally suppress pulmonary host defense in vivo and leukocyte antibacterial functions in vitro (1, 2, 6, 26, 36, 38). In contrast, PGE2-mediated EP3 receptor activation results in more complex intracellular signaling events involving Gαi/o-protein-mediated suppression of cAMP and an influx of Ca2+ (29). Unexpectedly, EP3 receptor deletion was found to be protective against pneumococcal pneumonia in vivo, and leukocytes from EP3 knockout (KO) animals exhibit enhanced bactericidal functions (4).
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat PGE2-dependent fever and pain associated with a variety of medical conditions, including pneumonia. However, these agents inhibit biosynthesis of other COX-derived prostanoids as well, and adverse effects such as myocardial infarction and excessive bleeding are attributed to inhibition of the generation of PGI2 and TXA2, respectively. Since mPGES-1 is a major contributor to PGE2 biosynthesis during inflammation, novel pharmacological agents have been developed to selectively inhibit the production of PGE2 and thus avoid the adverse consequences of nonselective prostanoid inhibition (43). This new generation of anti-inflammatory drugs may be a promising treatment for chronic inflammatory disorders. However, this approach may have unintended consequences for host defense against infection.
Previous reports have demonstrated that mPGES-1 KO mice were highly susceptible to Mycobacterium tuberculosis in vivo, and the lack of alveolar macrophage (AM)-derived PGE2 enhanced M. tuberculosis replication in vitro (10). In contrast, genetic or pharmacological ablation of mPGES-1 protected mice against infection from the influenza A virus (11). In the later model, AM-derived PGE2 enhanced viral replication by inhibiting type I interferon production. In the present study, we report that mPGES-1 KO mice exhibit greater susceptibility to pneumococcal pneumonia due to impaired killing of ingested bacteria and reduced nitric oxide (NO) production by AMs.
MATERIALS AND METHODS
Animals.
Mice with a targeted deletion of both alleles of the Ptges encoding mPGES-1 were generated as previously reported (23) Eight- to twelve-week-old female mPGES-1 KO mice (mPGES-1−/−) were bred on a C57BL/6 background. Genotypes of mouse strains were confirmed by tail-snip DNA PCR analyses. Age-matched, female C57BL/6 wild-type (WT) animals (mPGES-1+/+ mice) were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were treated according to National Institutes of Health guidelines for the use of experimental animals with the approval of the University of Michigan Committee for the Use and Care of Animals.
Murine model of pneumococcal pneumonia.
S. pneumoniae serotype 3, 6303 (American Type Culture Collection, Manassas, VA) and the D39 strain of S. pneumoniae serotype 2 were grown to mid-log phase in Todd Hewitt broth (THB) with 0.5% yeast extract (Difco, Detroit, MI), washed in PBS, and serially diluted in sterile PBS. After anesthesia with ketamine (80 mg/kg) and xylazine (10 mg/kg) delivered via an intraperitoneal injection, a midline incision was made to expose the trachea, a 30-μl inoculum containing 50,000 colony forming units (CFUs) of S. pneumoniae was administered via the trachea with a 26-gauge needle, and the wound was closed with surgical glue (Vetbond, 3M, St. Paul, MN). Animals were observed for survival. In a separate group of mice, lung and spleen homogenates were prepared from mice harvested 24 h and 48 h (24 h only for D39 strain of S. pneumoniae) after infection following euthanasia with CO2 asphyxiation. Serial dilutions of tissue homogenates were plated on blood agar, and CFUs were calculated as previously described (33).
Quantification of leukocyte counts in bronchoalveolar lavage fluid and peripheral blood.
After euthanasia, lungs were removed and bronchoalveolar lavage was performed at 24 and 48 h after infection with 2 ml of lavage buffer (HEPES-buffered saline solution) as previously described (33). Leukocytes were enumerated with a hemocytometer, spun onto glass slides with a cytocentrifuge, and stained with a modified Wright-Giemsa stain (American Scientific Products, McGaw Park, IL). The total and differential counts were determined as previously described (18). The total number and differential counts were determined in peripheral blood obtained by cardiac puncture with a Hemavet cell analyzer (Drew Scientific) operated by the University of Michigan Unit for Laboratory Animal Medicine Animal Diagnostic Laboratory.
Quantification of cytokines and eicosanoids in lung tissue.
TNF-α, IL-6, IL-10, IL-17, and macrophage inflammatory protein 2 (MIP-2) were measured in lung homogenates by ELISA (R&D DuoSet, R&D Systems, Minneapolis, MN). ELISAs were performed by the University of Michigan Cancer Center Cellular Immunology Core. PGE2, TXB2, and 6-keto-PGF1α were assessed with their respective enzyme immunoassay kits (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
AM isolation and culture.
AMs were obtained by lavage from WT and mPGES-1 KO mice as previously described (33). Briefly, AMs were recovered by washing the lungs removed from euthanized mice ex vivo with HEPES-buffered lavage fluid. The cells were resuspended in RPMI 1640 (Invitrogen, Carlsbad, CA) at a final concentration of 1 × 106 cells/ml; 105 cells were adhered to tissue culture plates for 1 h at 37°C in a 5% CO2 incubator. After adherence, the cell culture medium was replaced with warm RPMI containing 10% fetal calf serum (FCS) (Invitrogen) and 1% penicillin-streptomycin-amphotericin B (Invitrogen). On the following day, the culture medium was removed and the AMs were washed with warmed medium to remove nonadherent cells.
Recovery of glycogen-elicited peritoneal neutrophils.
Five hours after intraperitoneal injection of a 1% glycogen solution, peritoneal neutrophils (PMNs) were recovered by peritoneal lavage as described previously (28). With this procedure, >90% of the cells recovered from mice were identified as PMNs (22). The PMNs were pelleted by centrifugation, resuspended in ice-cold RPMI 1640, and adhered to 96-well tissue culture plates at a concentration of 105 cells/well at 37°C in 5% CO2 in air. One hour later, the medium was replaced with warm RPMI 1640, and the cells were assessed for phagocytosis and killing of S. pneumoniae.
AM stimulation for prostanoid and cysteinyl leukotriene synthesis.
AMs (1 × 105/well) were stimulated with heat-killed S. pneumoniae (HK-S.p) at a multiplicity of infection of 50:1 (HK-S.p:AMs) for 2 h. Cell culture medium was recovered and stored at −70°C. PGE2, TXB2, 6-keto-PGF1α, and cysteinyl leukotrienes were measured with enzyme immunoassay kits as described above. For cytokine synthesis, AMs (1 × 105/well) were stimulated with 10 μg/ml of lipoteichoic acid (LTA) from Staphylococcus aureus (Sigma-Aldrich) for 24 h. Cell culture medium was recovered and stored at −70°C. IL-6, IL-10, and TNF-α were measured by ELISA as described above.
Tetrazolium dye reduction assay of S. pneumoniae killing.
The ability of bacteria to survive within AMs was quantified with a tetrazolium dye reduction assay as described elsewhere (31, 32, 37). In this assay, bacterial growth is determined colorimetrically based on the ability of live bacteria to convert MTT to a purple formazan salt that absorbs light at 595 nm (A595). Briefly, 105 AMs were adhered in duplicate 96-well tissue culture plates for 1 h and cultured overnight with RPMI 1640 and 10% FCS. On the following day, S. pneumoniae was suspended in RMPI 1640 and opsonized with 10% normal rat serum by incubating this suspension on a rotating platform for 30 min at 37°C. The macrophages were then infected with opsonized S. pneumoniae (5 × 106 CFUs; multiplicity of infection, 50:1) and incubated for 60 min at 37°C to allow phagocytosis to occur. After 60 min, both plates of macrophages were washed with warmed HBSS to remove unbound bacteria. One plate was transferred to a refrigerator (4°C), and the other plate was incubated for an additional 90 min at 37°C. At the end of 90 min, the medium was removed from each plate and the AMs were lysed with 5% saponin in THB. After 1 min, THB was added to each well and both plates were incubated for 4 h at 37°C to allow the bacteria to grow. At the end of incubation tetrazolium dye (5 mg/ml in RPMI 1640) (Sigma) was added to each well, and this was incubated for 30 min for the development of the purple reduction product. The intensity of the purple color was quantified with a SpectraMax Plus plate reader (Molecular Devices, Sunnyvale, CA) at A595, and this was directly proportional to the number of intracellular bacteria ingested by the AMs (31). In some of these experiments, AMs were cultured with or without indomethacin (10 μM), PGE2 (1 μM; Cayman Chemical), the TXA2 receptor (TP receptor) agonist U-46619 (100 nM; Cayman Chemical), the TP receptor antagonist S18886 (100 nM; Cayman Chemical), or the nitric oxide donor S-nitroso N-acetyl-penicillamine (SNAP) (1 µM; Cayman Chemical) during the killing assay. Results were expressed as percent killing of ingested bacteria: %killing of ingested bacteria = 100 × [A595 (phagocytosis plate) − A595 (killing plate)/A595 (phagocytosis plate)].
Fluorometric assay of alveolar macrophage phagocytosis.
The ability of AMs to phagocytose S. pneumoniae was assessed with a previously published protocol for determining the ingestion of fluorescent, FITC-labeled bacteria (33). Briefly, heat-killed S. pneumoniae were labeled with FITC, as previously described (FITCS. pneumoniae) (5). A total of 105 murine AMs were seeded in replicates of 8 in 384-well tissue culture plates with opaque sides and optically clear bottoms (Costar, Corning Life Sciences). On the following day, FITCS. pneumoniae were opsonized with 10% normal rat serum. AMs were then infected with FITCS. pneumoniae with a multiplicity of infection of 150:1 for 60 min to allow phagocytosis to occur. Trypan blue was added to quench extracellular fluorescence, and the plates were read by a SpectraMax Gemini EM fluorometer 485 ex/535 em (Molecular Devices). The phagocytic index was calculated, as previously described, in relative fluorescence units (5).
Assessment of AM nitric oxide and reactive oxygen intermediates.
AMs were adhered to 96-well plates at a concentration of 105 cells/well and cultured for 24 h with DMEM (high glucose; 4.5 g/l) supplemented with 1% sodium pyruvate (Invitrogen) containing 10% FCS and penicillin-streptomycin, with or without 10 μg/ml LTA from S. aureus (Sigma-Aldrich) and 100 ng/ml IFN-γ (R&D Systems). In some of these experiments, AMs were incubated with the live D39 strain of S. pneumoniae for 2.5 h at a multiplicity of infection of 50:1. NO production was determined by measuring stable nitrite concentrations with a modified Griess reaction according to the manufacturer's instructions (Cayman Chemical).
For reactive oxygen intermediates (ROIs), AMs were adhered to 384-well plates at 1.25 × 105 cells/well and cultured overnight in RPMI 1640 containing 10% FCS with 1% penicillin-streptomycin-amphotericin B (Invitrogen). On the next day, the medium was replaced with PBS containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate, and the cells were cultured for 1 h. The medium was then replaced with warmed HBSS with calcium and magnesium, and the cells were stimulated with heat-killed S. pneumoniae with a multiplicity of infection of 50:1. ROI production was assessed every 30 min for 2.5 h by measuring fluorescence with a SpectraMax Gemini XS fluorometer (Molecular Devices) with excitation/emission setting at 493/522 nm.
Analysis of inducible nitric oxide synthase gene expression.
RNA was isolated from AMs cultured with LTA and IFN-γ, as mentioned above, with TRIzol according to the manufacturer's protocol (Invitrogen). Quantitative real-time PCR was performed as previously described (21) to quantify expression of inducible nitric oxide synthase (iNOS), normalizing mRNA levels to GAPDH. Primers for murine iNOS included Forward: 5′-CCCTCCTGATCTTGTGTTGGA-3′ and Reverse: 5′-CAACCCGAGCTCCTGGAA-3′. Primers for murine GAPDH included Forward: 5′-TGCACCACCAACTGCTTAG-3′ and Reverse: 5′-GGATGCAGGGATGATGTTC-3′.
Statistical analyses.
Statistical analyses were conducted with Prism 6.0 software (GraphPad Software, La Jolla, CA). Differences in survival were evaluated with a log rank (Mantel-Cox) test. Where appropriate, mean values were compared with a paired Student t-test or a one-way analysis of variance (ANOVA) followed by Bonferroni correction. Differences were considered significant if P < 0.05. Unless otherwise stated, all experiments were performed on at least three separate occasions. Data are presented as means ± SE unless otherwise noted.
RESULTS
Reduced survival and defective S. pneumoniae clearance in mPGES-1 KO mice.
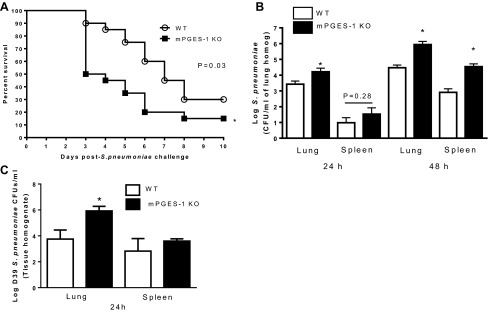
We have previously reported that mice lacking PGE2 receptors EP2 and EP3 are protected against pneumococcal pneumonia (1, 4). In contrast, as shown in Fig. 2A, a dramatic difference in survival was apparent by 3 days after infection, with survival of 90% of the WT and only 50% of the mPGES-1 KO mice after infection. Thereafter, progressive mortality occurred in parallel in both groups at all time points; the KO mice exhibited significantly more lethality. Bacterial burdens in the lungs were increased in mPGES-1 KO mice, 1 log higher than in WT mice at 24 h and 1.5 log higher than in WT mice at 48 h after S. pneumoniae infection (Fig. 2B). Spleen bacterial counts were also greater in mPGES-1 KO mice at 24 and 48 h after infection (Fig. 2B), suggesting increased dissemination of bacteria from lungs to peripheral blood in mPGES-1 KO mice. Likewise, we also observed higher bacterial burdens in the lungs of mPGES-1 KO mice 24 h after infection with D39 S. pneumoniae serotype (Fig. 2C). These data suggest that mPGES-1 plays an essential role in host defense against the highly invasive serotype 3 6303 and D39 serotype 2, another representative strain of S. pneumoniae.
Fig. 2.
Reduced survival and increased pulmonary and spleen bacterial burdens in mPGES-1 KO mice after S. pneumoniae infection. A: wild-type (WT) and mPGES-1 KO mice were infected with 5 × 104 CFUs of the serotype 3 6303 strain of S. pneumoniae, and survival was monitored for 10 days. B: in a separate group of mice, bacterial burdens were quantified in lungs and spleens harvested 24 and 48 h after infection. C: WT and mPGES-1 KO mice were infected with 50,000 CFUs of the D39 serotype 2 strain of S. pneumoniae, and lung and spleen bacterial burdens were assessed 24 h later. Survival curves, representing n = 20 from 4 separate experiments, were evaluated with a log-rank test. *P < 0.05 comparing WT to KO by Student's t-test. Bars represent means ± SE of n = 5–11 mice per group from 3 separate experiments.
No differences in leukocyte recruitment in mPGES-1 KO mice after S. pneumoniae challenge.
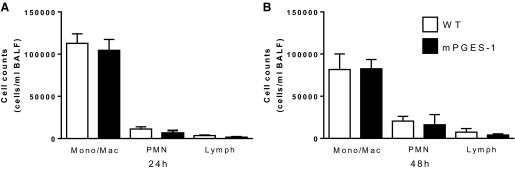
We previously observed enhanced recruitment of PMNs to the lungs of EP2 KO mice following infection with S. pneumoniae (1). To determine whether mPGES-1 affects the recruitment of leukocytes to the air space during pneumococcal pneumonia, we recovered leukocytes from the bronchoalveolar lavage fluid (BALF) after infection. As shown in Fig. 3, we did not observe any differences between WT and KO mice in the number or types of leukocytes recovered at 24 h or 48 h after infection. We also assessed blood leukocyte counts after infection with S. pneumoniae but did not find any differences between WT and mPGES-1 KO mice in either total or differential counts (data not shown). These results suggest that the impairment in bacterial clearance observed in mPGES-1 KO mice was not due to differences in leukocyte recruitment to the lungs after infection.
Fig. 3.
Leukocyte counts in bronchoalveolar lavage fluid recovered from mice after S. pneumoniae challenge. WT and mPGES-1 KO mice were infected with 5 × 104 CFUs of S. pneumoniae. Twenty-four (A) and forty-eight (B) hours later, leukocytes were recovered by bronchoalveolar lavage, and monocyte/macrophage (Mono/Mac), neutrophil (PMN), and lymphocyte (lymph) counts were determined. Bars represent means ± SE of n = 8–15 mice per group from 3 separate experiments.
Redistribution of lung prostanoid production in mPGES-1 KO mice after infection and in AMs stimulated in vitro.
During bacterial pneumonia, PGE2 biosynthesis is dramatically increased in the lung (1). As expected, PGE2 concentrations in lung homogenates of S. pneumoniae-infected mPGES-1 KO mice were dramatically reduced at both 24 and 48 h after infection (Fig. 4A). Since the substrate for mPGES-1, PGH2, can be metabolized by other prostanoid and thromboxane synthase enzymes, we assessed the levels of pulmonary TXB2 and 6-keto-PGF1α. We observed a modest increase in TXB2 at 24 h and a nonsignificant increase at 48 h after infection. Similarly, 6-keto-PGF1α concentrations increased slightly in mPGES-1 KO mice compared with WT mice after infection (Fig. 4B), although these differences were not statistically significant. We observed a similar response, including an 80% reduction in PGE2 production, increased TXB2 production, and no differences in 6-keto-PGF1α production, in in vitro S. pneumoniae-challenged AMs obtained from mPGES-1 KO compared with WT mice (Fig. 4C). We also assessed cysteinyl leukotrienes known to play a protective role in pneumococcal pneumonia but observed no differences in concentrations of this lipid mediator in cell culture media after stimulation of WT and KO AMs in vitro (data not shown). These results demonstrate that PGE2 production is dramatically reduced and there is a redistribution of substrate resulting in an increased production of alternate prostanoids in AMs and in the lungs of mPGES-1 KO mice during S. pneumoniae infection.
Fig. 4.
Redistribution of prostanoids in mPGES-1 KO mice after S. pneumoniae infection. WT and mPGES-1 KO mice were infected with 5 × 104 CFUs of S. pneumoniae. A and B: lung homogenates were assayed for PGE2 (A) and TXB2 and 6-keto-PGF1α (B). AMs obtained from WT and mPGES-1 KO mice were infected with S. pneumoniae. C: supernatants harvested at 2 h were assayed for PGE2, TXB2, and 6-keto-PGF1α. Bars represent means ± SE of n = 3–10 mice per group from 2 separate experiments, and AM stimulation in vitro was done with n = 3 with 3–5 replicates per experiment. *P <0.05 compared with WT by Student's t-test.
Effects of mPGES-1 deficiency on cytokine production during S. pneumoniae infection.
Because PGE2 inhibits the synthesis of TNF-α and other cytokines that play an important role in the innate immune response to bacterial pneumonia (39), we measured cytokine production in lungs of WT and KO mice after infection. There were no differences between WT and KO mice in concentrations of IL-6, IL-10, IL-12 p40, IL-17, MIP-2, or TNF-α 24 h after infection (Fig. 5A). We did observe higher MIP-2 levels in mPGES-1 KO mice compared with WT mice 48 h after infection, but there were no other differences in cytokine concentrations at that time point (Fig. 5B). After in vitro infection of AMs from WT and KO mice, we detected increased concentrations of TNF-α and IL-6 in the supernatants of AMs from KO mice, although these effects were modest in magnitude (Fig. 5C). IL-10 concentrations in supernatants of infected AMs from WT and KO mice were below the limit of detection (data not shown). Thus the substantial reduction in PGE2 during pneumococcal pneumonia in mPGES-1 KO mice had very limited effects on cytokine production in vivo and in vitro.
Fig. 5.
Cytokine production in mPGES-1 KO mice after S. pneumoniae infection. WT and mPGES-1 KO mice were infected with S. pneumoniae. A and B: lung homogenates were assayed for IL-6, IL-10, IL-17, MIP-2, TNF-α, and IL-12 p40 by ELISA. Bars represent means ± SE of n = 5–10 mice per group from 2 separate experiments. C: alveolar macrophages (AMs) were stimulated with lipoteichoic acid (LTA, 10 μg/ml) for 24 h, and concentrations of IL-6, IL-10, and TNF-α were measured in supernatants by ELISA. Bars represent means ± SE of n = 3. *P <0.05 compared with WT by Student's t-test.
Impaired killing of S. pneumoniae in AMs from mPGES-1 KO mice associated with reduced NO synthesis.
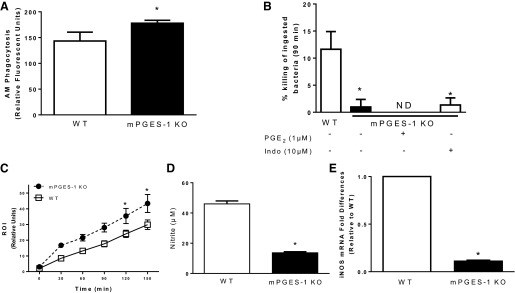
The AM plays a critical role in the defense against bacterial colonization of the alveolar space during the early stages of infection, and the ability of these cells to phagocytose and kill bacteria is regulated by prostanoids produced in the lung during bacterial pneumonia (1, 2, 4). Since we have previously observed that COX inhibitors increase while exogenous PGE2 decreases bacterial phagocytosis in AMs (2, 3, 5), we compared the ability of AMs from WT and mPGES-1 KO mice to phagocytose serum-opsonized, FITC-labeled S. pneumoniae. As shown in Fig. 6A, we observed greater bacterial phagocytosis by AMs from mPGES-1 KO mice than AMs from WT mice. We also examined the bactericidal function of AMs from WT and mPGES-1 KO mice. As shown in Fig. 6B, the ability of AMs from mPGES-1 KO mice to kill ingested S. pneumoniae was substantially impaired. Decreased killing by AMs from KO mice was not restored when AMs were cultured with PGE2 overnight and during the duration of the killing experiment. To determine whether the impairment in bacterial killing was due to a redistribution of eicosanoids in AMs from mPGES-1 KO mice we cultured cells overnight and during the killing assay with indomethacin, but this did not affect the bactericidal response (Fig. 6B). Pretreatment of AMs from WT and mPGES-1 KO mice with TP receptor agonist (U-46619) or TP receptor antagonist (S18886) had no effect on bacterial phagocytosis or killing (data not shown). In addition, there were no differences in phagocytosis or killing of S. pneumoniae in PMNs recovered from WT and mPGES-1 KO mice (data not shown).
Fig. 6.
Effects of mPGES-1 deficiency on alveolar macrophage (AM) bactericidal function: phagocytosis (A), killing (B), reactive oxygen intermediate (ROI; C), nitrite production (D), and inducible nitric oxide synthase (iNOS) expression (E) in AMs from WT and mPGES-1 KO mice. A: phagocytosis of S. pneumoniae was assessed in AMs from WT and mPGES-1 KO mice. B: intracellular killing of S. pneumoniae was assessed in AMs from WT and mPGES-1 KO, mPGES-1 KO with PGE2 (1 μM), or mPGES-1 KO with indomethacin (10 μM) where indicated. C: ROI production was assessed in AMs stimulated with heat-killed S. pneumoniae. D: nitrite production was assessed in AMs stimulated with lipoteichoic acid (LTA, 10 μg/ml) and IFN-γ (100 ng/ml) for 24 h. E: quantitative real-time PCR was used to quantify mRNA levels of inducible nitric oxide synthase (iNOS); data were normalized to GAPDH and expressed relative to WT. Bars represent means ± SE of n = 3–7 experiments with at least 5 replicates per experiment. *P <0.05 compared with WT by ANOVA. ND, none detected.
To assess bactericidal mechanisms in AMs, we measured ROI production in AMs after stimulation with heat-killed S. pneumoniae. Consistent with our previous observation that PGE2 acutely suppresses ROI synthesis in AMs in vitro (38), AMs from mPGES-1 KO mice produced higher levels of ROI than AMs from WT mice (Fig. 6C). Since PGE2 enhances NO synthesis in AMs (16), we measured NO production by WT and KO AMs after overnight stimulation with LTA and IFN-γ. Nitrite levels were substantially lower in the supernatants of AMs from mPGES-1 KO mice (Fig. 6D). Consistent with this response, we also assessed NO synthesis in AMs treated with live S. pneumoniae in vitro and observed reduced nitrite synthesis in AMs from mPGES-1 KO mice (1.2 ± 0.01 μM vs. 0.68 ± 0.01 μM). Pretreatment of AMs from WT or mPGES-1 KO mice with either the TP receptor agonist U-44619 or the antagonist S18886 did not affect NO synthesis. In addition, iNOS mRNA levels were lower in AMs from mPGES-1 KO mice compared with AMs from WT mice after stimulation with LTA and IFN-γ (Fig. 6E). In total, these results demonstrate that the impaired killing of ingested S. pneumoniae in AMs from mPGES-1 KO mice was associated with impairment in NO synthesis, a consequence of reduced iNOS expression.
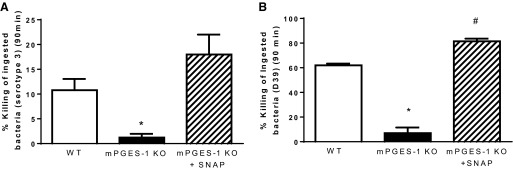
NO donor restores killing of S. pneumoniae in AMs from mPGES-1 KO mice.
Since we observed an impairment in bacterial killing in AMs from mPGES-1 KO mice that was associated with reduced NO synthesis, we added the NO donor S-nitroso-N-acetyl-penicillamine to cells from KO animals to determine whether this would restore bactericidal function. As shown in Fig. 7, the addition of exogenous SNAP restored killing of S. pneumoniae serotype 3 (Fig. 7A) and the D39 strain of S. pneumoniae (Fig. 7B) in AMs from mPGES-1 KO mice. These results provide functional evidence that diminished NO synthesis contributed to the impairment in bacterial killing in AMs from mPGES-1 KO mice.
Fig. 7.
The NO donor SNAP restores bacterial killing in AMs from mPGES-1 KO mice. Intracellular killing of S. pneumoniae serotype 3 (A) or the D39 strain of S. pneumoniae (B) was assessed in AMs from WT mice, mPGES-1 KO mice, and mPGES-1 KO mice treated with SNAP (1 μM). Bars represent means ± SE of n = 3–4 experiments with at least 5 replicates per experiment. *P <0.05 compared with WT and mPGES-1 KO + SNAP, #P < 0.05 vs. WT by ANOVA.
DISCUSSION
In this study we compared the responses of WT and mPGES-1 KO mice after an intratracheal challenge with the clinically relevant pathogen S. pneumoniae, the most common cause of community-acquired pneumonia. As expected, ablation of mPGES-1 resulted in a dramatic reduction in PGE2 synthesis in the lungs after infection in vivo and by AMs in vitro. PGE2 has been shown to suppress innate immune responses against bacterial infection, and blocking the synthesis of all cyclooxygenase products with NSAIDs or blocking EP-mediated intracellular signaling events by using EP-deficient animals and pharmacological receptor antagonists has been reported to improve bacterial clearance in vivo and AM phagocytosis and killing of bacteria in vitro (1, 2, 4, 9, 13, 26, 36, 38, 41). Likewise, increased production of PGE2 has also been associated with a suppression of host defense against bacterial pneumonia (6, 21). These immunosuppressive effects of PGE2 are thought to play an important role in the resolution of infection and protection of the host from collateral damage caused by an overexuberant inflammatory response. In contrast to these findings, we unexpectedly observed that reduced PGE2 synthesis in mPGES-1 KO mice was associated with impaired pulmonary host defense against an intratracheal challenge with two different strains of S. pneumoniae.
Our observation that mPGES-1 KO mice were more susceptible to pneumococcal pneumonia was similar to the report by Chen et al. (10), who found that these animals exhibited greater pulmonary M. tuberculosis burdens after infection. In that study, PGE2 protected macrophages from mitochondrial inner membrane perturbation and necrosis induced by a virulent strain of M. tuberculosis (10). This response, the induction of mitochondrial permeability transition, led to mitochondrial damage and cellular necrosis and ultimately permitted M. tuberculosis to evade AM-mediated killing. While we did not measure cell death in our studies, it is possible that the observed defects in host defense against S. pneumoniae in AMs from mPGES-1 KO mice were related to necrotic cell death as a consequence of reduced NO synthesis (23).
In contrast to bacterial and mycobacterial infections, mPGES-1 KO mice and WT mice treated with an mPGES-1 inhibitor produced higher levels of type I IFNs (IFN-β), which was associated with improved viral clearance and enhanced survival after influenza A infection (11). PGE2 also delays T-cell recruitment and the antigen-specific T-cell response in the lungs after influenza A infection (11). However, there were no differences between WT and mPGES-1 KO mice in viral pathogenesis after infection with mouse adenovirus type 1 (25). These differences in host defense against different types of infectious agents suggest that PGE2 plays distinctly different roles in the setting of bacterial, mycobacterial, and viral infections.
Exogenous administration of PGE2 to cultured AMs inhibits bactericidal functions by enhancing Gαs protein-mediated increases in cAMP, subsequently activating protein kinase A (PKA) and EPAC and downregulating the respiratory burst, which leads to reduced phagocytosis and bactericidal activity (3, 38). Genetic ablation and pharmacological inhibition of the EP2 receptor enhances AM bactericidal functions in vitro (1, 6, 26). Consistent with these results, we observed modestly enhanced phagocytosis and ROI generation in AMs from mPGES-1 KO mice. While ROI production during pneumococcal pneumonia is not required for bacterial clearance, the absence of iNOS diminishes pulmonary bacterial clearance (24). A mechanism by which PGE2 mediates increased NO synthesis was previously described by Kim et al. (16). In that study, endogenous PGE2 production increased after TLR2 or TLR4 activation, leading to EP2-mediated increases in intracellular cAMP, AKAP10-anchored PKA-I activation, and subsequent induction of iNOS and NO synthesis. Consistent with that mechanism, we found that bacterial killing, iNOS expression, and NO synthesis were reduced in AMs from mPGES-1 KO mice.
As previously described in other contexts (10, 11, 15), mPGES-1 deletion was associated with a redistribution of prostanoid and thromboxane production after S. pneumoniae infection in vivo and in vitro. The increased production of the stable metabolites TXB2 and 6-keto-PGF1α likely reflects shunting of the intermediate metabolite, PGH2, and subsequent metabolism by TXS and PTGIS, enzymes that are selectively expressed in the lung and in AMs. It is unlikely that elevated levels of TXA2 or PGI2 contributed to the observed impairments in host defense in mPGES-1 KO mice, since indomethacin (which would inhibit production of all COX products, preventing overproduction of TXA2 and PGI2) and the TP receptor antagonist S18886 did not restore bacterial killing in AMs from these animals. This suggests that enhanced production of alternate prostanoids was not likely to be responsible for the observed defect in AM killing. It is worth noting that bone marrow-derived dendritic cells from mPGES-1 KO mice produce more PGD2 than cells from WT mice (27). However, we did not assess the levels of PGD2 in our experiments, since macrophages produce very low levels of this prostanoid, about 30- to 50-fold less than PGE2, in response to TLR agonists (10, 15). Finally, it may be possible that an increase in other eicosanoids or lipoxins resulting from shunting, not inhibited by indomethacin, were responsible for the observed host defense defects in mPGES-1 KO mice (7).
Exogenous administration of PGE2 substantially reduces production of TNF-α and increases production of IL-6 and IL-10 by AMs stimulated with LPS (16). In the present study, we observed only modest increases in IL-6 and TNF-α production by AMs from mPGES-1 KO mice and very little difference in lung cytokine production in mPGES-1 KO mice despite dramatically less PGE2 production in vitro and in vivo. In contrast, McCarthy et al. reported less virus-induced production of IFN-γ, CXCL1, and CCL5 in mPGES-1 KO mice compared with WT mice 7 days after an intranasal challenge with murine adenovirus, suggesting that PGE2 may play a distinctly different role in the setting of viral infection (25).
In summary, these data provide the first evidence that ablation of mPGES-1 impairs host defense against S. pneumoniae, the most common cause of community-acquired pneumonia. They also suggest that caution should be used as novel pharmacological mPGES-1 inhibitors are developed as treatment for patients with chronic inflammatory conditions. As is the case with S. pneumoniae infection in mPGES-1-deficient mice, selective pharmacological inhibition of PGE2 synthesis has the potential to make patients more susceptible to bacterial pneumonia.
GRANTS
Support for this work was provided by a grant from the Flight Attendants Medical Research Institute (FAMRI) (CIA-103071, P. Mancuso and D. M. Aronoff), Institutional Training Grant T32 ES007062 (E. O'Brien), and National Institute of Allergy and Infectious Diseases Grant R01 AI-083334 (J. B. Weinberg and D. M. Aronoff).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.D., E.O., A.A., M.C.P., L.W., and P.M. performed experiments; J.M.D., E.O., and P.M. analyzed data; J.M.D., J.B.W., D.M.A., M.P.-G., and P.M. interpreted results of experiments; J.M.D. and P.M. drafted manuscript; J.M.D., J.B.W., E.O., D.M.A., L.J.C., M.P.-G., L.W., and P.M. edited and revised manuscript; J.M.D., J.B.W., E.O., A.A., M.C.P., D.M.A., L.J.C., M.P.-G., L.W., and P.M. approved final version of manuscript; J.B.W., D.M.A., M.P.-G., and P.M. conception and design of research; P.M. prepared figures.
ACKNOWLEDGMENTS
The authors thank Kristin Angle, Michael Carnegie, Michael LaFramboise, and Marisa Mead for their help with some of the experiments and Suzanne Dawid, who provided the D39 stain of S. pneumoniae serotype 2.
REFERENCES
- 1.Aronoff DM, Bergin IL, Lewis C, Goel D, O'Brien E, Peters-Golden M, Mancuso P. E-prostanoid 2 receptor signaling suppresses lung innate immunity against Streptococcus pneumoniae. Prostaglandins Other Lipid Mediat 98: 23–30, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 173: 559–565, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 174: 595–599, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff DM, Lewis C, Serezani CH, Eaton KA, Goel D, Phipps JC, Peters-Golden M, Mancuso P. E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection. J Immunol 183: 2642–2649, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arredouani M, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol 35: 474–478, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballinger MN, Aronoff DM, McMillan TR, Cooke KR, Olkiewicz K, Toews GB, Peters-Golden M, Moore BB. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol 177: 5499–5508, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Behar SM, Martin CJ, Booty MG, Nishimura T, Zhao X, Gan HX, Divangahi M, Remold HG. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol 4: 279–287, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Butler RR, Wise WC, Halushka PV, Cook JA. Gentamicin and indomethacin in the treatment of septic shock: effects on prostacyclin and thromboxane A2 production. J Pharmacol Exp Ther 225: 94–101, 1983. [PubMed] [Google Scholar]
- 10.Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, Remold HG. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med 205: 2791–2801, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulombe F, Jaworska J, Verway M, Tzelepis F, Massoud A, Gillard J, Wong G, Kobinger G, Xing Z, Couture C, Joubert P, Fritz JH, Powell WS, Divangahi M. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity 40: 554–568, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Feldman C, Anderson R. Antibiotic resistance of pathogens causing community-acquired pneumonia. Semin Respir Crit Care Med 33: 232–243, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Goldmann O, Hertzen E, Hecht A, Schmidt H, Lehne S, Norrby-Teglund A, Medina E. Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes. J Immunol 185: 2372–2381, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Hsu A, Aronoff D, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol 150: 332–339, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idborg H, Olsson P, Leclerc P, Raouf J, Jakobsson PJ, Korotkova M. Effects of mPGES-1 deletion on eicosanoid and fatty acid profiles in mice. Prostaglandins Other Lipid Mediat 107: 18–25, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Serezani CH, Okunishi K, Zaslona Z, Aronoff DM, Peters-Golden M. Distinct protein kinase A anchoring proteins direct prostaglandin E2 modulation of Toll-like receptor signaling in alveolar macrophages. J Biol Chem 286: 8875–8883, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig E, Bonanni P, Rohde G, Sayiner A, Torres A. The remaining challenges of pneumococcal disease in adults. Eur Respir Rev 21: 57–65, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol 168: 4018–4024, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Mancuso P, Huffnagle GB, Olszewski MA, Phipps J, Peters-Golden M. Leptin corrects host defense defects following acute starvation in murine pneumococcal pneumonia. Am J Respir Crit Care Med 173: 212–218, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Mancuso P, Lewis C, Serezani CH, Goel D, Peters-Golden M. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect Immun 78: 2264–2271, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancuso P, Myers MG, Goel D, Serezani CH, O'Brien E, Goldberg J, Aronoff DM, Peters-Golden M. Ablation of leptin receptor-mediated ERK activation impairs host defense against Gram-negative pneumonia. J Immunol 189: 867–875, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancuso P, Nana-Sinkam P, Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun 69: 2011–2016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J 18: 1126–1128, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Marriott HM, Hellewell PG, Whyte MKB, Dockrell DH. Contrasting roles for reactive oxygen species and nitric oxide in the innate response to pulmonary infection with Streptococcus pneumoniae. Vaccine 25: 2485–2490, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy MK, Levine RE, Procario MC, McDonnell PJ, Zhu L, Mancuso P, Crofford LJ, Aronoff DM, Weinberg JB. Prostaglandin E2 induction during mouse adenovirus type 1 respiratory infection regulates inflammatory mediator generation but does not affect viral pathogenesis. PLoS One 8: , 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med 206: 61–68, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monrad SU, Kojima F, Kapoor M, Kuan EL, Sarkar S, Randolph GJ, Crofford LJ. Genetic deletion of mPGES-1 abolishes PGE2 production in murine dendritic cells and alters the cytokine profile, but does not affect maturation or migration. Prostaglandins Leukot Essent Fatty Acids 84: 113–121, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore SI, Huffnagle GB, Chen GH, White ES, Mancuso P. Leptin modulates neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun 71: 4182–4185, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto K, Shirata N, Taketomi Y, Tsuchiya S, Segi-Nishida E, Inazumi T, Kabashima K, Tanaka S, Murakami M, Narumiya S, Sugimoto Y. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J Immunol 192: 1130–1137, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 371: 1619–1628, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Ofek I, Mesika A, Kalina M, Keisari Y, Podschun R, Sahly H, Chang D, McGregor D, Crouch E. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect Immun 69: 24–33, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods 82: 131–140, 1985. [DOI] [PubMed] [Google Scholar]
- 33.Phipps JC, Aronoff DM, Curtis JL, Goel D, O'Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect Immun 78: 1214–1220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-κB RelA during pneumococcal pneumonia. J Immunol 178: 1896–1903, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez JA, Anzueto AR. Changing needs of community-acquired pneumonia. J Antimicrob Chemother 66: iii3–iii9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadikot RT, Zeng H, Azim AC, Joo M, Dey SK, Breyer RM, Peebles RS, Blackwell TS, Christman JW. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol 37: 1001–1009, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Serezani C, Aronoff D, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 106: 1067–1075, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol 37: 562–570, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinomiya S, Naraba H, Ueno A, Utsunomiya I, Maruyama T, Ohuchida S, Ushikubi F, Yuki K, Narumiya S, Sugimoto Y, Ichikawa A, Oh-ishi S. Regulation of TNFα and interleukin-10 production by prostaglandins I2 and E2: studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochem Pharmacol 61: 1153–1160, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev 111: 5821–5865, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stables MJ, Newson J, Ayoub SS, Brown J, Hyams CJ, Gilroy DW. Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria. Blood 116: 2950–2959, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White KE, Ding Q, Moore BB, Peters-Golden M, Ware LB, Matthay MA, Olman MA. Prostaglandin E2 mediates IL-1β-related fibroblast mitogenic effects in acute lung injury through differential utilization of prostanoid receptors. J Immunol 180: 637–646, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu D, Rowland SE, Clark P, Giroux A, Côté B, Guiral S, Salem M, Ducharme Y, Friesen RW, Méthot N, Mancini J, Audoly L, Riendeau D. MF63 [2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)-isophthalonitrile], a selective microsomal prostaglandin E synthase-1 inhibitor, relieves pyresis and pain in preclinical models of inflammation. J Pharmacol Exp Ther 326: 754–763, 2008. [DOI] [PubMed] [Google Scholar]