Abstract
In pancreatic acinar cells, the Src family of kinases (SFK) is involved in the activation of several signaling cascades that are implicated in mediating cellular processes (growth, cytoskeletal changes, apoptosis). However, the role of SFKs in various physiological responses such as enzyme secretion or in pathophysiological processes such as acute pancreatitis is either controversial, unknown, or incompletely understood. To address this, in this study, we investigated the role/mechanisms of SFKs in acute pancreatitis and enzyme release. Enzyme secretion was studied in rat dispersed pancreatic acini, in vitro acute-pancreatitis-like changes induced by supramaximal COOH-terminal octapeptide of cholecystokinin (CCK). SFK involvement assessed using the chemical SFK inhibitor (PP2) with its inactive control, 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3), under experimental conditions, markedly inhibiting SFK activation. In CCK-stimulated pancreatic acinar cells, activation occurred of trypsinogen, various MAP kinases (p42/44, JNK), transcription factors (signal transducer and activator of transcription-3, nuclear factor-κB, activator protein-1), caspases (3, 8, and 9) inducing apoptosis, LDH release reflective of necrosis, and various chemokines secreted (monocyte chemotactic protein-1, macrophage inflammatory protein-1α, regulated on activation, normal T cell expressed and secreted). All were inhibited by PP2, not by PP3, except caspase activation leading to apoptosis, which was increased, and trypsin activation, which was unaffected, as was CCK-induced amylase release. These results demonstrate SFK activation is playing a dual role in acute pancreatitis, inhibiting apoptosis and promoting necrosis as well as chemokine/cytokine release inducing inflammation, leading to more severe disease, as well as not affecting secretion. Thus, our studies indicate that SFK is a key mediator of inflammation and pancreatic acinar cell death in acute pancreatitis, suggesting it could be a potential therapeutic target in acute pancreatitis.
Keywords: pancreatic acini, carboxy-terminal octapeptide of cholecystokinin, signaling, chemokines, caspases
the src family of kinases (SFKs) plays important roles in many cells mediating both physiological (endocytosis, secretion, growth, apoptosis, regulation of cytoskeletal changes) and pathophysiological (neoplastic initiation/growth, response to injury, cell death) responses (1).
Of the nine subtypes of SFKs, various studies report pancreatic acinar cells possess a number of SFK members (Yes, Lyn, pp60Src) that can be activated by various pancreatic secretagogues and growth factors (55, 57, 68). In general, while the role of SFKs in pancreatic cancer has been extensively studied (40), there is only limited data on their roles in other pancreatic acinar pathophysiological conditions such as pancreatitis. Similarly, in terms of physiological responses of pancreatic acinar cells, while the signaling cascades of activation of SFKs have been well studied (55, 57, 68), in such physiological responses as enzyme secretion, various studies report divergent results for the effects of SFK activation (36, 42, 84). These results are potentially important for a number of reasons.
Acute pancreatitis can vary from a mild disease to a severe disease in which the pancreas digests itself and its surroundings (6, 56). Despite the increasing incidence of this serious disorder, there is a lack of therapies directed to its molecular pathogenesis (5). Numerous in vitro and in vivo models of acute pancreatitis have been developed that are providing important insights into the pathogenesis of this disease (37). These studies in general provide evidence that an important initiating event is the premature activation of trypsin in the pancreas, as well as an important early role for activation of nuclear factor-κB (NF-κB) resulting in stimulation of proinflammatory cytokines/chemokines with resultant stimulation of an inflammatory response that can progress to cell necrosis (8, 22, 44, 56). From these studies a number of cellular signaling cascades have been identified that are reported to play important roles in either the activation or maintenance of these pathological processes that can contribute to the initiation as well as the maintenance and even progression or severity of the acute pancreatitis (5, 30, 56). Recent studies using gene expression in acute pancreatitis have called attention to the central role of SFKs in interacting with and activation of a number of these signaling cascades (30, 38, 43). This conclusion is supported by studies showing inhibition of SFKs can lead to amelioration of acinar cell injury in models of pancreatitis, reduced extent of inflammation, and the generation of some inflammatory mediators (48, 62). However, similar to its unclear role in the physiological process of secretion, the mechanism and role of SFK-induced changes in acute pancreatitis, such that its inhibition ameliorates the disease, is not clear. In the present study, to address both of these issues, we used conditions that result in marked SFK inhibition (51) to examine the effect of SFK on enzyme secretion, and a number of signaling cascades known to be activated in acute pancreatitis and mediate many of the cellular changes, including activation of chemokines/cytokines [NF-κB, macrophage inflammatory protein-2 (MIP-2), monocyte chemotactic protein-1 (MCP-1), MIP-1α, regulated on activation, normal T cell expressed and secreted (RANTES)], signal transducer and activator of transcription-3 (STAT-3), activator protein-1, stimulation of caspases (3, 8, 9) stimulating apoptosis, and stimulation of necrosis, using a well-established in vitro CCK-induced model of pancreatitis-like cell damage (37).
MATERIALS AND METHODS
Materials
Male Sprague-Dawley rats (150–250 g) were obtained from the Small Animals Section, Veterinary Resources Branch, National Institutes of Health (Bethesda, MD). Rabbit anti-phospho-Src family (Tyr416), rabbit anti (Thr202/Tyr204)-p42/44, antirabbit (Thr183/Tyr185) JNK, antirabbit anti-Src family, antirabbit anti-p42/44, antirabbit anti-JNK, and nonfat dry milk were purchased from Cell Signaling Technology (Beverly, MA). Antirabbit horseradish peroxidase-conjugated antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Tris·HCl pH 8.0 and 7.5, was from Mediatech (Herndon, VA). 2-Mercaptoethanol, protein assay solution, sodium lauryl sulfate (SDS), and Tris/glycine/SDS (10×) were from Bio-Rad Laboratories (Hercules, CA). MgCl2, CaCl2, Tris·HCl, 1 M, pH 7.5, and Tris/glycine buffer (10×) were from Quality Biological (Gaithersburg, MD). Minimal essential media (MEM) vitamin solution, 100× amino acids, Dulbecco's phosphate-buffered saline (DPBS), glutamine (200 mM), Tris-glycine gels, l-glutamine, Waymouth's MB 752/1 medium, fetal bovine serum (FBS) and RANTES, and MIP-2 Rat ELISA kits were from Invitrogen (Carlsbad, CA). COOH-terminal octapeptide of cholecystokinin (CCK) was from Bachem Bioscience (King of Prussia, PA). 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) and 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3) were from Calbiochem (La Jolla, CA). Dimethyl sulfoxide (DMSO), l-glutamic acid, glucose, fumaric acid, pyruvic acid, trypsin inhibitor, HEPES, Tween 20, Triton X-100, phenylmethanesulfonyl fluoride (PMSF), ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), sucrose, sodium orthovanadate, and sodium azide were from Sigma-Aldrich (St. Louis, MO). Rat MCP-1 ELISA, albumin standard, and Super Signal West (Pico, Dura) chemiluminescent substrate were from Pierce (Rockford, IL). Protease inhibitor tablets were from Roche (Basel, Switzerland). Purified collagenase (type CLSPA) was from Worthington Biochemicals (Freehold, NJ). Nitrocellulose membranes were from Schleicher and Schuell Bioscience (Keene, NH). Biocoat collagen I Cellware 60-mm dishes were from Becton-Dickinsen Labware (Bedford, MA). Albumin bovine fraction V was from MP Biomedical (Solon, OH). NaCl, KCl, and NaH2PO4 were from Mallinckrodt (Paris, KY). Rat MIP-1α ELISA Kit was from Genprice (Santa Clara, CA). Cytotox 96 Non Radioactive Cytotoxicity Assay was from Promega (Madison, WI). The Phadebas Amylase test was from Magle Life Science (Lund, Sweden). Caspase fluorogenic substrates, Ac-IETD-AMC, Ac-DEVD-AMC, Ac-LEHD-AMC, trypsin fluorogenic substrate, Boc-Gln-Ala-Arg-AMC, and 7-amino-4-methylcoumarin (AMC) calibration standard were from Enzo Life Sciences (Farmingdale, NY). Nuclear protein extraction kit and ELISA-based TransAM for STAT-3 and NF-κB p65 assay kits were from Active Motif (Carlsbad, CA). The TF-Detect activator protein (AP)-1/c-Jun Activity Assay Kit was from GeneCopoeia (Rockville, MD).
Methods
Tissue preparation.
All animal experiments were approved by the Animal Ethics Committee of the National Institutes of Health and carried out in accordance with the International Guiding Principles for Animal Research. Pancreatic acini were obtained by collagenase digestion as previously described (65, 68, 79). Standard incubation solution contained 25.5 mM HEPES (pH 7.45), 98 mM NaCl, 6 mM KCl, 2.5 mM NaH2PO4, 5 mM sodium pyruvate, 5 mM sodium glutamate, 5 mM sodium fumarate, 11.5 mM glucose, 0.5 mM CaCl2, 1 mM MgCl2, 1 mM glutamine, 1% (wt/vol) albumin, 0.01% (wt/vol) trypsin inhibitor, 1% (vol/vol) vitamin mixture, and 1% (vol/vol) amino acid mixture.
Acini stimulation.
After collagenase digestion, dispersed acini were preincubated in standard incubation solution for 2 h at 37°C with or without inhibitors as described previously (54, 79). After preincubation, 1-ml aliquots of dispersed acini were incubated at 37°C with or without stimulants at the required incubation times. Supernatants were collected for the required measurements, and cells were lysed in lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium azide, 1 mM EGTA, 0.4 mM EDTA, 0.2 mM sodium orthovanadate, 1 mM PMSF, and one protease inhibitor tablet/10 ml). After sonication, lysates were centrifuged at 10,000 g for 15 min at 4°C, and protein concentration was measured using the Bio-Rad protein assay reagent.
Western blotting.
Western blotting was performed as described previously (21). The intensity of the protein bands was measured using Kodak ID Image Analysis, which were assessed in the linear detection range. When reprobing was necessary, membranes were incubated in stripping buffer (Pierce) for 30 min at room temperature, washed two times for 10 min in washing buffer, blocked for 1 h in blocking buffer at room temperature, and reprobed as described above. SFK activation was assessed by determining Y416 Src phosphorylation, a well-known activation site for Src (64).
Nuclear extract preparation.
Nuclear extracts were prepared by employing a kit (Active Motif) and following the manufacturer's instructions. Nuclear extracts were prepared from pancreatic acinar cells isolated following the protocol described in Tissue preparation.
SFK action on STAT-3, NF-κB, and AP-1 DNA-binding activity.
Pancreatic acinar cells were preincubated with 0.1% DMSO or 10 μM PP2 or PP3 (final DMSO 0.1%) and then incubated with 100 nM CCK for 2 h as previously described (62). The binding of STAT-3, NF-κB, and AP-1 to DNA was measured in nuclear extracts with three different ELISA-based TransAM assay kits for detecting STAT-3, NF-κB p65, and AP-1 c-jun, respectively. These assays use multiwell plates coated with an unlabeled oligonucleotide containing the consensus binding site for STAT-3 (5′-TTCCCGGAA-3′), NF-κB (5′-GGGACTTTCC-3′), and AP-1 (5′-TGAGTCA-3′). Nuclear proteins (5 μg) were added to each well and incubated for 1 h at room temperature to allow the nuclear transcription factors to bind their respective consensus sequence. Subsequently, by using the respective antibodies for STAT-3, NF-κB, and p65/AP-1 c-jun subunit, the complexes of STAT-3, NF-κB, and AP-1 c-jun bound to their respective oligonucleotide were detected. Addition of the secondary antibody conjugated to horseradish peroxidase provided a sensitive colorimetric readout that was quantified by spectrophotometry. Absorbance was read at 450 nm within 5 min by using a 96-well microplate reader (Tecan, San José, CA). The wild-type consensus oligonucleotides were provided as competitors for STAT-3, NF-κB, and AP-1 binding to monitor the specificity of each assay. Results were expressed as fold increase over the control group.
Chemokine detection.
Pancreatic acinar cells were preincubated with 0.1% DMSO or 10 μM PP2 or PP3 (final DMSO 0.1%) and then incubated with 100 nM CCK for 30 min (RANTES) or 2 h for the rest of the chemokines. The choice of these incubation times was based on previous studies (62, 87). Cell supernatants were assayed for MCP-1, MIP-1α, MIP-2, and RANTES using a sandwich ELISA, according to the manufacturer's instructions. In general, samples were incubated on ELISA plates, together with a primary biotinylated antibody, and then washed and incubated with streptavidin bound to horseradish peroxidase. After a further wash, tetramethylbenzidine was added for color development, and the reaction was terminated with a commercial stop solution. Absorbance was read at 450 nm by using a 96-well microplate reader (Tecan).
Lactate dehydrogenase assay.
The assay was performed by using the commercially available Cytotox 96 nonradioactive cytotoxicity assay kit according to the manufacturer's instructions (Promega) and based on previous studies in pancreatic acinar cells (87). In brief, acinar cells (50,000/ml) were pretreated with 10 μM PP2 or PP3 for 1 h followed by stimulation with 0.3 or 100 nM CCK for a further 2 h. Samples were collected and centrifuged at 30 g for 30 s. A 120-μl aliquot of medium was then removed to measure lactate dehydrogenase (LDH) release from the cells. A manufacturer-provided lysis reagent was then added to the remaining 380 μl of cells and medium to determine total LDH. Both cell and medium samples were assayed. The results were expressed as the percent of cellular LDH released in the medium during the incubation.
Measurement of caspase activities.
Caspase activities were measured as previously described (23). We used a fluorogenic assay with substrates specific for caspase-3 (Ac-DEVD-AMC), caspase-8 (Ac-IETD-AMC), or caspase-9 (Ac-LEHD-AMC) as described previously (45, 76). Pancreatic acinar cells were lysed in buffer containing 50 mM Tris·HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium azide, 1 mM EGTA, 0.4 mM EDTA, 0.2 mM sodium orthovanadate, 1 mM PMSF, and one protease inhibitor tablet/10 ml and centrifuged for 15 min at 15,000 g, and supernatants were collected. Proteolytic reactions were carried out at 37°C in a buffer containing 25 mM HEPES (pH 7.5), 10% sucrose, 0.1% CHAPS, and 10 mM dithiothreitol, using specific substrates for each caspase. Cleavage of these substrates releases AMC, which emits a fluorescence signal with excitation at 380 nm and emission at 440 nm. Fluorescence was calibrated using a standard curve for AMC. The data are expressed as picomoles of AMC per milligram of protein per minute.
Trypsin activity assay.
Trypsin activity was measured using a fluorogenic assay with a substrate specific for trypsin (Boc-Glu-Ala-Arg-AMC) as described previously (35). After the cells were treated with the various agents, they were washed with ice-cold PBS, lysed in 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, pH 7.0 (containing 250 mM sucrose, 5 mM MOPS, and 1 mM MgSO4), and centrifuged for 15 min at 15,000 g, and supernatants, with the addition of the substrate, were used for the assay. Trypsin activity was measured fluorometrically in 800 μg of cell lysate from each sample, applying the same principle used for determining the activity of the caspases with excitation at 380 nm and emission at 440 nm. Fluorescence was calibrated using a standard curve with trypsin. To compare values between different treatments, the data were expressed as the percentage of maximal activity obtained when acini were incubated with 100 nM CCK for 20 min.
Amylase release.
Amylase release was measured using the procedure published previously (68, 81). Amylase activity was determined after a 30-min incubation using the Phadebas reagent and was expressed as percentage of the total cellular amylase released in the extracellular medium during the incubation.
Statistical Analysis
All experiments were performed at least four times. Data are presented as means ± SE and were analyzed using the one-way ANOVA with Dunnett's or Bonferroni multiple test as posttests using the GraphPad 5.0 software. P values <0.05 were considered significant.
RESULTS
Assessment of SFK Inhibition by PP2 in Pancreatic Acinar Cells
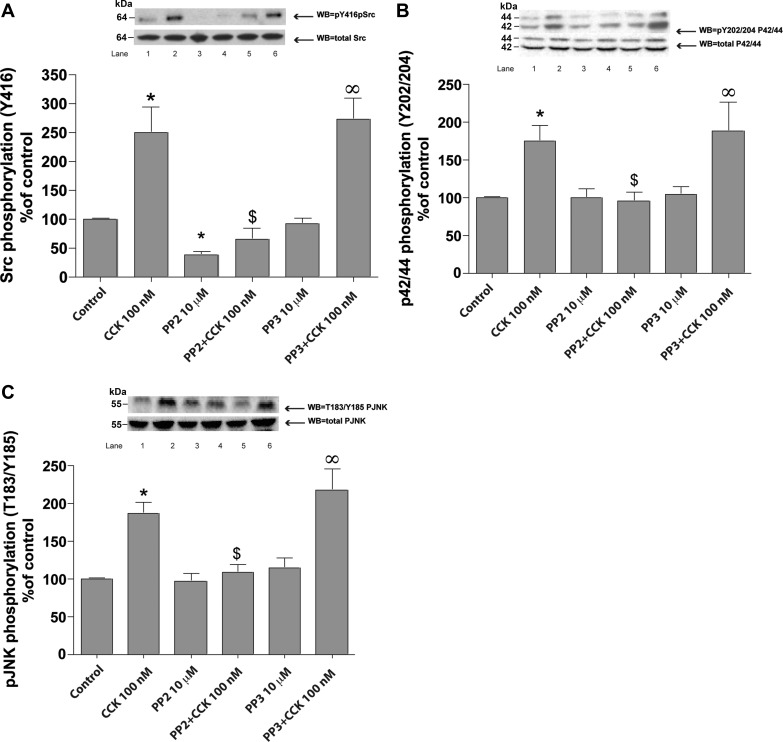
The supraphysiological 100 nM concentration of CCK (Fig. 1A) induced activation of SFK (250 ± 45% of basal, P < 0.01) after 2 h of incubation. Basal SFK activity (Fig. 1A and Table 1) was significantly reduced after 1 h preincubation with 10 μM PP2 (−62 ± 6%Δ of basal, P < 0.001) (Fig. 1A), whereas it was unaffected by 10 μM of the inactive analog PP3 (Fig. 1A). In addition, PP2 (10 μM) inhibited by >85% stimulation of SFK induced by 2 h incubation with 100 nM CCK (Fig. 1) while PP3 had no effect on CCK-induced SFK stimulation.
Fig. 1.
Effect of 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) and 4-amino-7-phenylpyrazol[3,4-d]pyrimidine (PP3) on COOH-terminal octapeptide of cholecystokinin (CCK)-mediated stimulation of the Src family of kinases (SFKs), p42/44, and JNK in pancreatic acinar cells. Rat pancreatic acinar cells were pretreated with no additions or with PP2 (10 μM) or PP3 (10 μM) for 1 h and then incubated with no additions (control) (all with final-0.1% DMSO) or with 100 nM CCK for 2 h and then lysed. Whole cell lysates were submitted to SDS-PAGE and transferred to nitrocellulose membranes. To determine SFK (A), p42/44 (B), and JNK (C) activity, we assessed the phosphorylation of Y416 Src, Y202/204 p42/44, and p-T183/Y185 JNK that correlate with their activity. Membranes were analyzed using anti-pY416 Src, anti-pY202/204 p42/44, and anti-p-T183/Y185 JNK antibodies. Total Src, JNK, and p42/44 antibodies were used to verify loading of equal amounts of protein. The bands were visualized using chemoluminescence, and quantification of phosphorylation was assessed using scanning densitometry. Top, a representative experiment of 3 others; bottom, each set is the mean of 5 experiments. Lanes in top are in the same order as shown for the means. *P < 0.05 vs. control, ∞P < 0.05 vs. PP3 alone, and $P < 0.05 comparing stimulants (CCK) vs. stimulants preincubated with PP2 or PP3, respectively.
Table 1.
SFK role in some physiological and pathophysiological functions in rat pancreatic acini
| Variable | Yes | No |
|---|---|---|
| Signaling cascade activation | ||
| Stimulated by 100 nM CCKa | SFK, p42/44, JNK | |
| Basal inhibition by PP2a | SFK | p42/44, JNK |
| PP2 inhibition of CCK-induced stimulationa | SFK, p42/44, JNK | |
| Transcription factor activation | ||
| 100 nM CCK inducedb | STAT3, NF-κB, and AP-1 | |
| PP2 inhibition of basalb | STAT3, NF-κB, AP-1 | |
| PP2 inhibition of CCK inducedb | STAT3, NF-κB, and AP-1 | |
| Chemokine release | ||
| Stimulated by 100 nM CCKc,d | MCP-1, MIP-1α, RANTES | MIP-2. |
| Basal inhibition by PP2c,d | MIP-1, MCP-1 | MIP-1-α, RANTES |
| PP2 inhibition of CCK-induced stimulationc,d | MCP-1, MIP-1α, RANTES | MIP-2 |
| LDH release | ||
| 100 nM CCK inducede | Stimulation of 237 ± 32% over control | |
| PP2 inhibition of basale | No effect vs. control | |
| PP2 inhibition of CCK inducede | −59 ± 7%Δ of CCK alone | |
| Cell death caspases activity | ||
| 100 nM CCKf | Caspase-3, -8, and -9 | |
| PP2 stimulation of basalf | Caspase-3, -8, and -9 | |
| PP2 inhibition of CCK-induced LDH releasef | Caspase-3, -8, and -9 | |
| Trypsinogen activationg | Stimulated by 100 nM CCK | Not inhibited by PP2 |
| Amylase releaseh | Stimulated by CCK at 0.01, 0.1, and 100 nM | Not inhibited by PP2 at any concentration |
SFK, Src family of kinases; JNK, c-Jun-NH2-terminal kinase; CCK, COOH-terminal octapeptide of cholecystokinin; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; STAT3, signal transducer and activator of transcription-3; AP-1, activator protein-1; MCP-1, monocyte chemotactic protein-1; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T cell expressed and secreted.
Results are calculated from the data shown in Fig. 1.
Concentration and incubation times are reported in Fig. 2.
Concentration and incubation times are reported in Fig. 3.
Concentration and incubation times are reported in Fig. 4.
Concentration and incubation times are reported in Fig. 5.
Concentration and incubation times are reported in Fig. 6.
Concentration and incubation times are reported in Fig. 7.
Concentration and incubation times are reported in Tables 2 and 3.
Involvement of SFK in CCK-Induced MAPKs in Pancreatic Acinar Cells
After 2 h of incubation, the supraphysiological 100 nM concentration of CCK induced activation of p42/44 (75 ± 9% over basal, P < 0.05) and JNK (87 ± 7% over basal, P < 0.01). Basal p42/44 and JNK kinase activity was not affected either by PP2 or by the inactive analog PP3 (Fig. 1, B and C, and Table 1). However, PP2 (10 μM) inhibited stimulation of p42/44 (Fig. 1B) and JNK (Fig. 1C) induced by 2 h incubation with 100 nM CCK.
Involvement of SFK in CCK-Induced Activation of STAT-3, NF-κB, and AP-1 in Pancreatic Acinar Cells
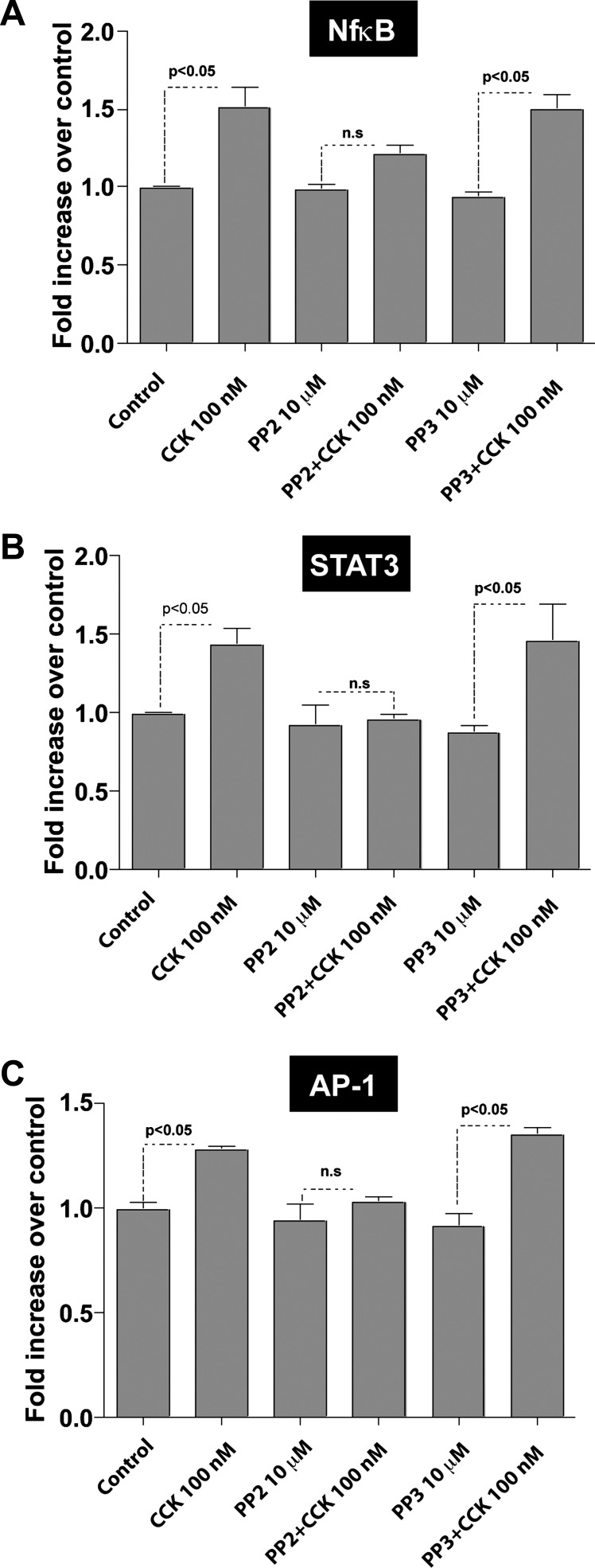
Cells were incubated for 1 h with 0.1% DMSO, 10 μM PP2, or its inactive analog PP3 (final DMSO 0.1%) followed by stimulation with 100 nM CCK for 2 h. Incubation with 100 nM CCK led to an increase in the activity of NF-κB (1.57 ± 0.09 times basal, P < 0.05), STAT-3 (1.43 ± 0.12 times basal, P < 0.05), and AP-1 (1.28 ± 0.02 times basal, P < 0.05) (Fig. 2 and Table 1). Preincubation with PP2 did not reduce the basal activation values of these nuclear transcription factors. However, PP2 inhibited the CCK-induced DNA-binding activity of NF-κB (0.77 ± 0.09 times CCK alone, P < 0.05), STAT-3 (0.68 ± 0.04 times CCK alone, P < 0.05), and AP-1 (0.80 ± 0.01 times CCK alone, P < 0.05), whereas PP3 had no effect in their CCK-mediated activation.
Fig. 2.
Effect of PP2 and PP3 on CCK-mediated stimulation of nuclear transcription factors [nuclear factor-κB (NF-κB, A), signal transducer and activator of transcription (STAT)-3 (B), and activator protein (AP-1, C)] in pancreatic acinar cells. Freshly isolated pancreatic acini were preincubated with no additions or with PP2 (10 μM) or PP3 (10 μM) followed by stimulation with 100 nM CCK for 2 h. The nuclear extracts were used for STAT-3, NF-κB, and AP-1 DNA-binding assays that were carried out as described in materials and methods. ns, Not significant. The results are means ± SE from 5 independent experiments.
Role of SFK in CCK-Mediated Production of CC Chemokines in Pancreatic Acinar Cells
Incubation with supraphysiological concentrations of CCK, a model of in vitro pancreatitis-like damage (23), is reported to stimulate chemokine release (4, 6, 23, 45, 76). We examined the role of SFK in mediating this effect by incubating acini with 100 nM CCK under conditions that resulted in >85% SFK inhibition induced by CCK (Fig. 1).
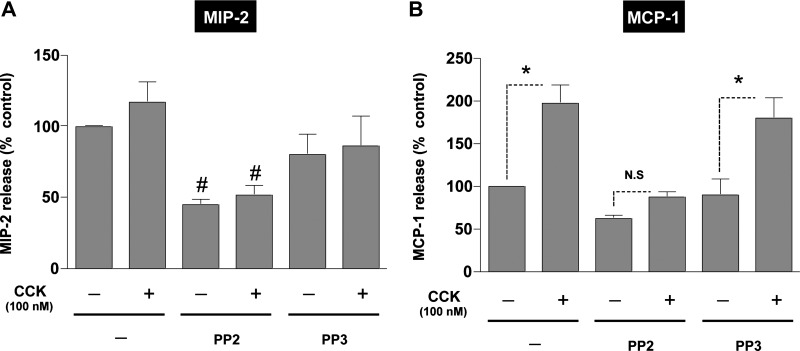
Acini were pretreated with either PP2 or its negative control PP3 (both at 10 μM) for 1 h followed by stimulation of 100 nM CCK (Fig. 3). The release of the chemokines, MIP-2, MCP-1, MIP-1α, and RANTES, was determined in the cell culture medium by specific ELISAs. The basal absolute values for chemokine release in the pancreatic acinar were 0.75 ± 0.21 ng/ml for MIP-2, 0.62 ± 0.11 ng/ml for MCP-1, 73.4 ± 12.9 pg/ml for MIP-1α, and 1.91 ± 0.36 ng/ml for RANTES. Pretreatment with PP2 (Fig. 3) markedly decreased the basal release of MIP-2 (45 ± 4% of control, P < 0.05, n = 4) (Fig. 3A) and MCP-1 (62 ± 4% of control, P < 0.05, n = 4) (Fig. 3B); however, there was no effect on the basal release of MIP-1α (10 ± 2% over control, n = 4) (Fig. 4A) or the basal release of RANTES (28 ± 3% over control, n = 4) (Fig. 4B). Preincubation with PP3 did not affect the basal levels of any of these chemokines (Figs. 3 and 4 and Table 1).
Fig. 3.
Effect of PP2 and PP3 on CCK-mediated secretion of macrophage inflammatory protein (MIP)-2 or monocyte chemotactic protein-1 (MCP-1) in pancreatic acinar cells. Freshly isolated rat pancreatic acini were preincubated with either 10 μM PP2 or PP3 for 1 h followed by stimulation with 100 nM CCK for 2 h as described above. The supernatant was used to measure MIP-2 (A) and MCP-1 (B) levels by ELISA as described in materials and methods. Results shown are means ± SE of 4 independent experiments. The basal absolute values for chemokine release in the pancreatic acinar were 0.75 ± 0.21 ng/ml for MIP-2 and 0.62 ± 0.11 ng/ml for MCP-1. *P < 0.05 for CCK, PP2-CCK, or PP3-CCK compared with control, PP2, or PP3 alone, respectively. #P < 0.05, PP2 preincubated cells vs. control.
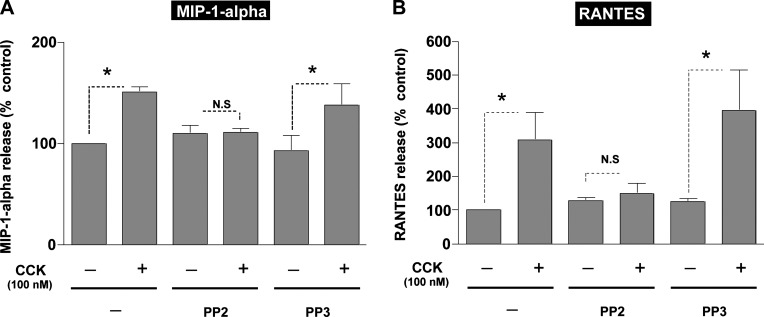
Fig. 4.
Effect of PP2 and PP3 on CCK-mediated secretion of MIP-1α or regulated on activation, normal T cell expressed and secreted (RANTES) in pancreatic acinar cells. Freshly isolated rat pancreatic acini were preincubated with either 10 μM PP2 or PP3 for 1 h followed by stimulation with 100 nM CCK for 2 h (MIP-1α) or 30 min (RANTES). The supernatant was used to measure MIP-1α (A) and RANTES (B) levels by ELISA as described in materials and methods. Results shown are means ± SE of 4 independent experiments. The basal absolute values for chemokine release in the pancreatic acinar were 73.4 ± 12.9 pg/ml for MIP-1-α and 1.91 ± 0.36 ng/ml for RANTES. *P < 0.05 for CCK, PP2-CCK, or PP3-CCK compared with control, PP2, or PP3 alone, respectively.
Treatment of the control pancreatic acini with 100 nM CCK resulted in a significant increase in the release of the three CC chemokines (Figs. 3 and 4 and Table 1) MCP-1 (97 ± 10% over control, P < 0.01, n = 4) (Fig. 3B), MIP-1α (50 ± 2% over control, P < 0.05, n = 4) (Fig. 4A), and RANTES (208 ± 53% over control, n = 4) (Fig. 4B). The data suggested a possible increase in the secretion of the CXC chemokine MIP-2 (17 ± 2% over control, n = 4) (Fig. 3A), but it did not reach significance (P = 0.29).
Treatment with PP2 (10 μM) inhibited markedly 100 nM CCK-stimulated release of three of the four chemokines (Figs. 3 and 4). Specifically, in the presence of PP2, 100 nM CCK did not induce stimulation in MIP-1α (3 ± 1% over PP2 control) (Fig. 4A) and RANTES (14 ± 2% over PP2 control) (Fig. 4B). Preincubation with PP2 also inhibited the CCK-induced stimulation of MCP-1 (40 ± 1% over PP2 control) (Fig. 3A).
PP3 preincubation did not affect CCK stimulation of the release of MCP-1 (123 ± 24% over PP3 control, P < 0.05) (Fig. 3B), MIP-1α (50 ± 2% over PP3 control, P < 0.05) (Fig. 4A), or RANTES (204 ± 53% over PP3 control, P < 0.05) (Fig. 4B).
SFK Regulates Cell Death Pathways in Pancreatic Acinar Cells Promoting Cell Necrosis and Inhibiting Apoptosis
Supramaximal CCK concentrations, used in in vitro models of pancreatitis, are reported to activate cell death pathways stimulating apoptosis and necrosis (90). We used our experimental conditions of inhibition of SFKs (Fig. 1) to investigate its role in mediating CCK-stimulated cell death pathways involving cell necrosis or apoptosis in pancreatic acinar cells. Cells (pretreated or not with PP2/PP3) were incubated with or without 100 nM CCK, and necrosis was assessed by measuring LDH release in the extracellular medium as well as assessing apoptosis by measuring caspase activity in the cell lysates (Figs. 5 and 6).
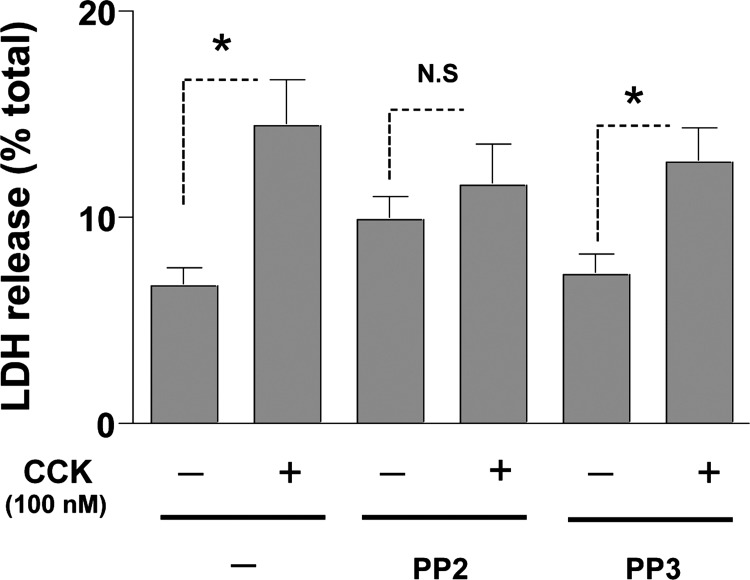
Fig. 5.
Effect of inhibition of SFK on CCK-mediated lactate dehydrogenase (LDH) release in pancreatic acini. Freshly isolated rat pancreatic acini were preincubated with either 10 μM PP2 or PP3 for 1 h followed by stimulation with 100 nM CCK for 2 h. Acinar cell necrosis was measured by the percentage of total LDH released in the medium, as described in materials and methods. Results are representative of 4 independent (n = 4) experiments. Results shown are means ± SE. *P < 0.05 comparing control, PP2, or PP3 alone vs. CCK, PP2-CCK, or PP3-CCK, respectively.
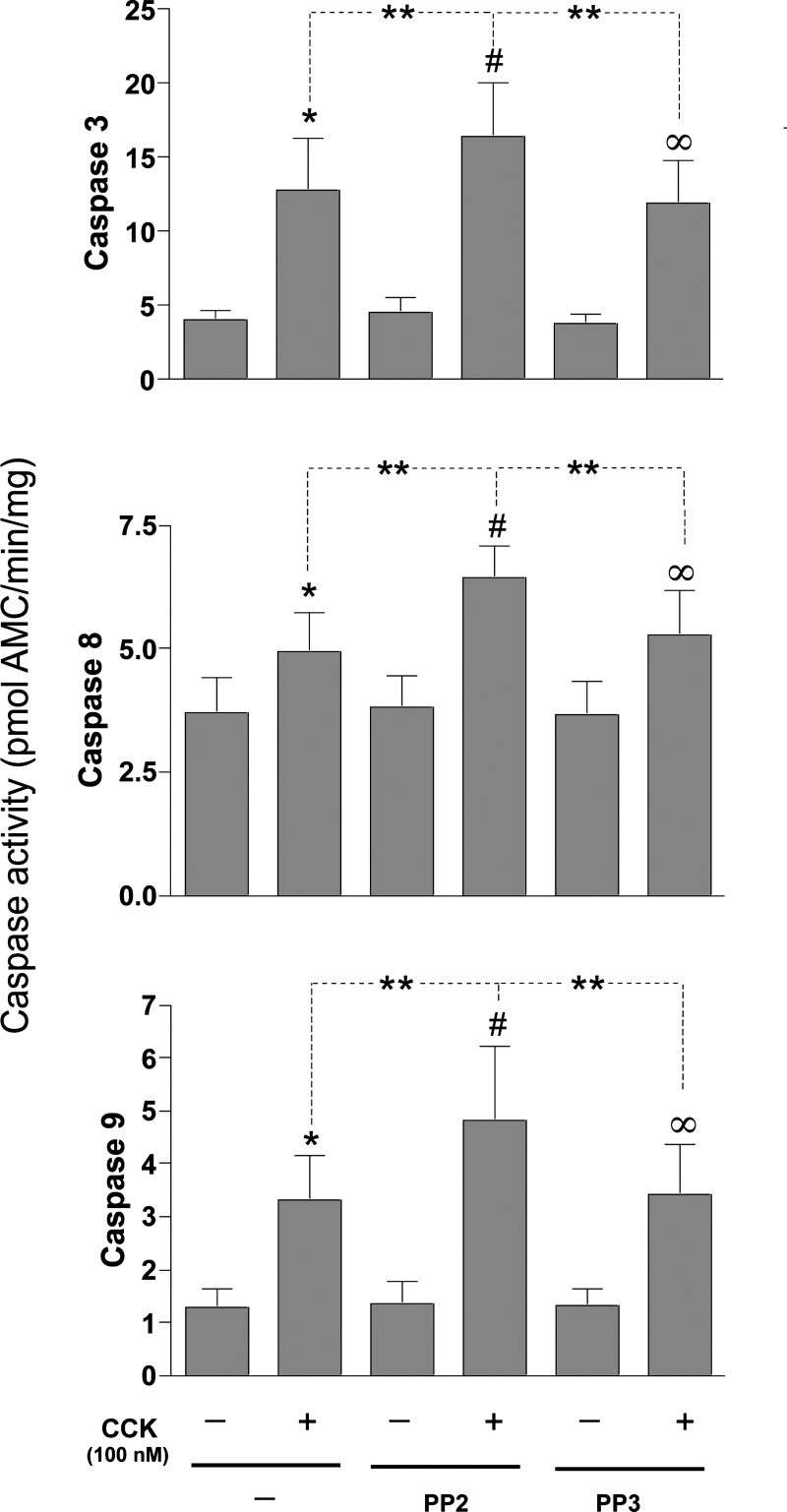
Fig. 6.
Effect of inhibition of SFK on CCK-mediated caspase activation in pancreatic acini. Freshly isolated rat pancreatic acini were preincubated with either 10 μM PP2 or PP3 for 1 h followed by stimulation with 100 nM CCK for 4 h. Caspase-3, -8, and -9 activities were measured as described in materials and methods in the lysates of isolated pancreatic acini. Results shown are means ± SE of 4 independent experiments. *P < 0.05 vs. control, #P < 0.05 vs. PP2 alone, ∞P < 0.05 vs. PP3 alone, and **P < 0.05 comparing CCK alone vs. PP2-CCK.
Cells incubated with 100 nM CCK demonstrated increased LDH release (Fig. 5). Specifically, cells incubated with no stimulant released 8.3 ± 1.8 of total LDH (Fig. 4), whereas the cells incubated with CCK showed a higher LDH release of 14.4 ± 2.2 of total LDH, representing an increase of 73 ± 14% over control (P < 0.05). Neither PP2 (39 ± 5% of control) nor PP3 (28 ± 7% of control) had a significant effect on the basal level of LDH released from the cells (Fig. 5). However, pretreatment of the acinar cells with PP2 significantly reduced the effect of the supramaximal dose of CCK on LDH release to 40 ± 3% over the PP2 control, resulting in a reduction of 45 ± 2% of the stimulation induced by CCK alone (P < 0.05 vs. CCK-treated cells, n = 4) (Fig. 5). In contrast, the inactive analog PP3 had no effect on CCK-mediated stimulation of LDH release (81 ± 2%, P < 0.05 over PP3 control; not significant vs. CCK-treated cells, n = 4) (Fig. 5).
Apoptosis in pancreatic acinar cells is mediated principally by activation of caspases (6, 45, 56). Treatment of pancreatic acini with CCK (100 nM) stimulated activation of caspases-3 (208.1 ± 28.9%), -8 (37.5 ± 3.5%), and -9 (168.2 ± 18.8%) in pancreatic acinar cells (Fig. 6, A–C). However, in the acini incubated with CCK, preincubation with PP2 resulted in a proapoptotic effect manifested by an enhanced activation of each of these caspases (3, 8, and 9) induced by a supramaximal concentration of CCK. Specifically, in the presence of PP2 there was a 261.6 ± 13.2% increase in caspase-3 activity, 188.6 ± 19.2% increase in caspase-8, and 255.5 ± 14.2% increase with caspase-9 (P < 0.005, Fig. 6, A–C). In contrast, the inactive analog PP3 had no effect (Fig. 6, A–C). These results demonstrated that SFK regulates cell death in pancreatic acinar cells by promoting cell necrosis while suppressing caspase activation.
Effects of SFK Inhibition on Amylase Release
In the literature, confusing results are reported for the role of SFKs in CCK-mediated enzyme secretion (36, 42, 84). To study the effect of SFK activation in the stimulation of amylase release in rat pancreatic acinar cells, isolated acini were preincubated with either no additions, with 10 μM PP2 or with PP3, and then subsequently incubated with CCK at either a low (0.01 nM), maximal (0.1 nM), or supramaximal (100 nM) concentration (Table 2). Each CCK concentration resulted in a significant increase in enzyme secretion (Table 2). The different CCK concentrations resulted in a biphasic effect on CCK-stimulated enzyme secretion, as previously reported in numerous studies (36, 42, 84), with 0.01 nM CCK causing a 37.7 ± 1.1% increase in amylase release, 0.1 nM a 103.3 ± 4.9% increase, and with 10 nM CCK 70.5 ± 1.6%, demonstrating a supramaximal inhibition of 31.1 ± 3.6% with respect to the maximal stimulation of amylase release (Table 2). At each of these CCK concentrations neither 10 μM PP2, nor 10 μM PP3, its inactive analog, had any effect on stimulated enzyme secretion (Table 2). To further explore if a possible effect might be unmasked using different PP2 concentrations, we performed additional studies using three different concentrations of PP2 (10, 100, and 1,000 nM) and 1,000 nM PP3 followed by incubation with CCK at either maximal (0.1 nM) or supramaximal (100 nM) concentration (Table 3). Preincubation with the SFK inhibitor or its inactive analog at any of the three concentrations did not produce any modification of the known biphasic stimulation of the amylase secretion by CCK (Table 3).
Table 2.
Effect of Src inhibition on amylase secretion induced by different CCK concentrations
| Amylase Secretion, %total |
|||
|---|---|---|---|
| Secretagogue | No PP2/PP3 | PP2 (10 μM) | PP3 (10 μM) |
| None | 6.1 ± 0.6 | 5.2 ± 1.2 | 5.9 ± 0.5 |
| CCK, nM | |||
| 0.01 | 8.4 ± 1.1 | 8.9 ± 0.4 | 8.3 ± 0.8 |
| 0.1 | 12.4 ± 1.2 | 13.9 ± 0.5 | 13.3 ± 0.7 |
| 100 | 10.4 ± 0.4 | 10.7 ± 0.6 | 10.5 ± 0.4 |
Pancreatic acini were incubated with no additions, and three different concentrations of CCK (0.01, 0.1, and 100 nM) alone or with the active SFK inhibitor PP2 (10 μM) or its inactive analog PP3 (10 μM). PP3, 4-amino-7-phenylpyrazol[3,4-d]pyrimidine.
Table 3.
Effect of Src inhibition on amylase secretion by different PP2 concentrations
| Amylase Secretion, %total |
|||||
|---|---|---|---|---|---|
| Secretagogue | No PP2/PP3 | PP2 (10 nM) | PP2 (100 nM) | PP2 (1 μM) | PP3 (1 μM) |
| None | 6.0 ± 0.8 | 6.7 ± 0.7 | 6.1 ± 0.9 | 7.2 ± 0.5 | 7.1 ± 0.6 |
| CCK, nM | |||||
| 0.1 | 13.6 ± 1.7 | 14.7 ± 1.3 | 14.5 ± 1.2 | 15.1 ± 1.0 | 14.4 ± 1.1 |
| 100 | 11.3 ± 2.1 | 12.2 ± 1.4 | 11.7 ± 1.8 | 12.3 ± 1.0 | 11.4 ± 1.2 |
Pancreatic acini were incubated with no additions and three different concentrations of PP2 (10 nM, 100 nM, and 1 μM) alone or with CCK (0.1 and 100 nM). Amylase release, expressed, as %cellular total amylase was determined after 30 min incubation. Results are averages of 5 experiments.
Effects of SFK Inhibition in Trypsin Activation
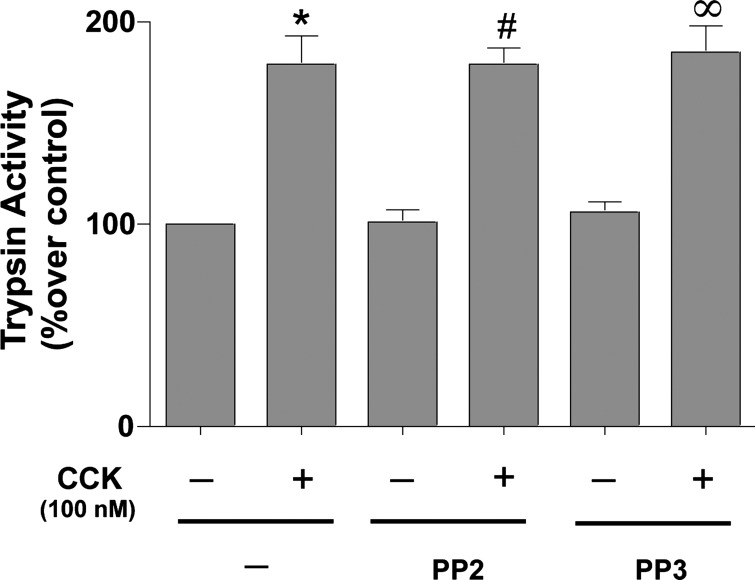
CCK (100 nM) stimulated trypsin activity (79 ± 6%, P < 0.05). Neither preincubation with 10 μM PP2 (78 ± 4% over PP2 control), nor its inactive analog PP3 (84 ± 6% over PP3 control), reduced CCK-induced trypsin activity (Fig. 7 and Table 1).
Fig. 7.
Effect of inhibition of SFK on CCK-mediated trypsin activation in pancreatic acini. Freshly isolated rat pancreatic acini were preincubated with either 10 μM PP2 or PP3 for 1 h followed by stimulation with 100 nM CCK for 20 min. Trypsin activity was measured as described in materials and methods in the lysates of isolated pancreatic acini. Results shown are means of 4 independent experiments. *P < 0.05 vs. control, #P < 0.05 vs. PP2 alone, and ∞P < 0.05 vs. PP3 alone.
DISCUSSION
Acute pancreatitis is an acute inflammatory process of the pancreas (37, 56). The major pathophysiological processes underlying acute pancreatitis are inflammation, edema, and necrosis of pancreatic tissue as well as inflammation and injury of extrapancreatic organs (56). Acinar cell injury is important in the first stages of pancreatitis development (5). This fact and the lack of therapies directed to the molecular pathogenesis of pancreatitis (5) make very relevant the understanding of the physiology and pathophysiology of pancreatic acinar cells. This could lead to new promising targets for the development of new approaches to understand and treat this disease. Various studies have demonstrated that, in acute pancreatitis, a number of cellular signaling cascades are activated and may play important roles in onset, progression, and severity of disease and thus be possible targets for therapy (5, 30, 56). These signaling cascades include: activation of ERK kinases (MAPK, p38, JNK), PKCs (PKC-δ, PKC-ε, PKC-ζ), PKD, PKA, JAK-STAT signaling, and NF-κB (5, 30, 56). A number of recent studies, including gene expression profiling studies in acute pancreatitis, have called attention to the central role of SFKs in interacting with and activation of a number of these signaling cascades (30, 38, 43).
In various tissues, SFKs mediate secretion, proliferation, growth, protein synthesis, membrane recycling, inflammatory responses, and cell death, and, in pancreatic acinar cells, SFKs are involved in numerous cellular signaling cascades mediating different physiological/pathophysiological responses, including in acute pancreatitis (4, 6, 23, 36, 42, 45, 50, 51, 55, 76, 80, 84). However, the role of SFKs in CCK mediation of a number of acinar cell physiological/pathophysiological responses, including in pancreatitis, are not clear, with studies either providing no data or conflicting results in many cases. In a recent study (51) using both chemical inhibition and dominant negative/positive adenoviral expression to alter SFK activity in pancreatic acini, we were able to completely explore the cellular cascades that SFKs interact with in pancreatic acini, and we set conditions that ensure at least an 85–90% inhibition of SFKs. In the present work we used a similar approach using chemical inhibition of SFK activation to study the role of SFK in a well-established in vitro model of pancreatitis-like cell damage (37, 56), as well as in pancreatic secretion, a physiological acinar cell response in which there are contradictory reports of the role of SFKs (36, 42, 84).
To carry out the present study, it was first essential to establish incubation conditions that allowed prolonged inhibition of SFK activity for a number of reasons. In our previous study (51) assessing the effect of the widely used Src inhibitor PP2 and its inactive control PP3 (2, 3) on acinar cell signaling cascades, the incubation periods were short (<10 min), whereas studies using in vitro CCK-induced rat models of pancreatitis generally require an incubation with supramaximal concentrations of CCK for 1–3 h (23, 37, 37, 70). The importance of this point is illustrated by our findings from the short-term previous study with SFK's role in CCK-mediated activation of JNK and p42/44 kinases. In the previous short-term study (51), while demonstrating CCK activation of p42/44 and JNK, as reported by others (13, 17), neither the chemical SFK inhibitor PP2 nor the dominant negative inhibitory adenoviral construct inhibited CCK activation of p42/44 or JNK (51) even though they inhibited CCK-mediated activation of SFKs by >90%. While this result suggests activation of SFKs may not be important in CCK-mediated activation of p42/44 and JNK, it does not completely rule out its possible importance in CCK-mediated or other models of acute pancreatitis, which almost universally report p42/44 and JNK activation (13, 14, 30, 31, 43, 47, 62), because the experimental conditions are different, with longer preincubation times with CCK in the experimental models. Our results in this study demonstrate that, under the experimental conditions used with the prolonged CCK incubations required to induce experimental pancreatitis, activation of SFKs was inhibited by >85%, and not only were JNK and p42/44 activated, both occurred in a Src-dependent manner in that both were inhibited by PP2, but not the inactive control PP3. There could be some concern about the specificity of PP2 for inhibiting Src (51). However, in a previous study (51) we fully addressed this concern by doing incubations in parallel with two different types of adenovirus (dominant negative and wild type). Those experiments showed similar results to those performed with PP2. The results from this previous study (51), added to the fact that in the present study we included the inactive control PP3, support our conclusion that the observed changes we see with PP2 are reflecting changes in Src activity. Unfortunately, the execution of adenovirus experiments for the present study has many technical difficulties because the long incubation times required are incompatible with measuring the secretory function, since the cells lose a lot of functionality. These findings established that the experimental conditions used were suitable to study the role of SFKs on cellular processes related to onset of experimental acute pancreatitis.
Our results with prolonged CCK incubations required to induce experimental pancreatitis demonstrating that p42/p44 activation was Src dependent are similar to the effect of substance P in pancreatic acinar cells (62), bradykinin in trabecular meshwork cells (85), urocortin in mouse myocytes (91), or angiotensin II in vascular smooth cells (88), which all stimulate Src-dependent p42/44 activation. However, they are in contrast to our own previous observation with short CCK incubation times (49) and also with results in vascular smooth cells stimulated by endothelin-1 (ET-1), which activates ERKs in a Src-independent way (88). Our result that JNK activation by CCK under these prolonged incubation conditions was affected by Src inhibition is also in contrast to our own previous observation during short-term incubations (49) and to angiotensin II stimulation of JNK in vascular smooth muscle cells (18). On the other hand, our results are similar to JNK activation induced by sphingosine 1-phosphate or ET-1 in smooth muscle cells, each of which is dependent on Src (18, 88), and also to JNK activation by substance P in pancreatic acinar cells (62). These results demonstrate that, not only does the role of SFK in mediating activation of different MAPKs differ in different cells activated by various stimuli, but also even within the same cell type depending on the different incubation conditions.
Previous studies have demonstrated in several models of acute pancreatitis, including CCK-induced, that there is activation of several important transcriptional factors such as NF-κB (25, 27, 30, 56, 62), STAT-3 (9, 33, 62, 89), and AP-1 (26, 33, 62). Our results support the conclusion that, in pancreatic acinar cells, the CCK-induced activation of NF-κB, STAT-3, and AP-1 is Src-dependent. These results are consistent with activation of these transcriptional factors by other stimulants like somatostatin (39), hydrogen sulfide (78), and substance P (62), which are also Src-dependent. There are no previous studies on the role of Src on activation of these transcription factors in CCK-induced pancreatitis, but our results demonstrating that their activation is Src dependent are consistent with studies in substance P-induced acute pancreatitis reporting that activation of a number of these transcription factors is Src-dependent (62, 78).
During acute pancreatitis, chemokine/cytokine production occurs (5, 28, 56, 63, 67, 71, 78), and a study with a substance P mouse model of acute pancreatitis has reported it is Src-dependent (62). In other studies in different models of experimental pancreatitis, chemokine/cytokine production is reported to require activation of p42/44, JNK, NF-κB, and AP-1 (33, 60–63). In the present study, we demonstrate that, in CCK-induced acute pancreatitis, not only does the stimulation of p42/44, JNK, NF-κB, and AP-1 occur in a Src-dependent manner but the release of chemokines is also affected by Src activation. Specifically, we found that, in the pancreatic acini preincubated with a supraphysiological dose of CCK, there was stimulation of the release of the chemokines MCP-1, MIP-1α, and RANTES, whereas MIP-2 release was unaffected. Our results differ from those in a previous study reporting MIP-2 transcription was upregulated in pancreatic acinar cells exposed to supraphysiological concentrations of CCK (53); however, they are in agreement with other studies reporting CCK stimulates/induces MCP-1 (58, 87) and RANTES (87). These results demonstrate for the first time that activation of SFKs in a CCK model of acute pancreatitis-like cell damage is needed for chemokine production. This result is similar to findings in a study (62) with substance P-stimulated release of the chemokines MCP-1 and MIP-1α in a mouse pancreatitis model, which was found to be Src-dependent. However, our results differ from findings in the same study (62) with release of the chemokine MIP-2, which was also reported to be Src-dependent in mouse pancreatic acini (62), whereas we saw no Src effect.
During acute pancreatitis, cell death can occur both with apoptosis and necrosis (7, 34, 45, 56). It is increasingly recognized that caspases play an essential role in mediating apoptosis, but also protect from necrosis, as well as decreasing the severity of pancreatitis induced by supraphysiological concentrations of CCK and other causes (6, 24, 45, 56, 76, 90). Similar to other studies using similar experimental conditions (23, 23, 45, 90), our results also support the conclusion that apoptosis is stimulated in CCK-induced experimental pancreatitis, due in part to activation of both initiator and effector caspases-3, -8, and -9 (4, 6, 23, 45, 76, 90). Before this study the role of activation of SFK in CCK-mediated caspase activation had not been investigated. Our results demonstrate that Src activation has an important effect on CCK activation of caspases. In our study SFK inhibition enhanced the activation of caspases-3, -8, and -9 mediated by CCK, leading to the conclusion that SFK activation during CCK initiated pancreatitis and has a restraining effect on activation of these caspases and stimulation of apoptosis. It would be expected that this change would support the development of more severe forms of pancreatitis with necrosis because of the suppression of the well-described protective effect of caspase activation by stimulating apoptosis and decreasing necrosis (7, 23, 34, 45). This novel Src-mediated inhibitory apoptotic effect of CCK has not been previously described in pancreatic acini; however, it is similar to previous studies demonstrating an inhibitory role of SFKs upon caspase activation in a number of other cells (10, 12, 16, 19, 52, 74, 77). Nonetheless, our results differ from findings in cerebral cortex or in hepatoma cells where Src activation has either a stimulatory effect on caspases (15) or did not have any effect on their activity (16).
To provide support for our proposal that SFK activation may be leading to the development of more severe forms of pancreatitis by supporting necrosis and restraining apoptosis, we assessed CCK-mediated LDH release because LDH release in the extracellular medium has been frequently used as a measure of necrosis in isolated pancreatic acini in numerous studies of pancreatitis (23, 24, 45, 76). Similar to others we found that, without SFK inhibitors present, supramaximal CCK concentrations inducing in vitro pancreatitis in the acinar cells increased the LDH release from these cells, supporting the conclusion that, in our study, similar to others, some necrosis is occurring (23, 37, 76, 83, 90). Whereas SFK inhibition had no effect on basal LDH release, it significantly reduced LDH release stimulated by supramaximal CCK concentrations. These results provide additional support for the conclusion that SFKs are a having a novel dual role in cell death during CCK-induced pancreatitis, in that, SFK activation inhibits apoptosis and increases necrosis, with the final effect being that this dual role shifts the balance to a more severe form of pancreatitis.
In CCK-induced in vitro pancreatitis, as well as in other forms of pancreatitis, premature intracellular activation of the digestive enzyme trypsinogen in the pancreatic acinar cells is considered to be an important early event (22, 32, 37, 56, 67, 75). Similar to other studies (27, 29, 41, 66, 82), we found that a supramaximal CCK concentration induced trypsin activation in pancreatic acinar cells. However, inhibition of SFK activation had no effect on CCK-induced trypsin activation. This result is in contrast to results from other studies in pancreatic acinar cells in which pancreatitis-like changes induced by other methods, including treatment with taurolithocholic acid 3-sulfate (43) or pervanadate (48), were reported to require SFKs for trypsin activation. At first sight it might seem inconsistent that activation of SFKs in our model is proposed to be inducing a more severe form of in vitro pancreatitis yet not affecting one of the most important initiating events, which is the activation of trypsin. A number of recent studies have provided evidence that, in CCK-induced pancreatitis as well as pancreatitis due to other causes, the activation of trypsin and the stimulation of NF-κB resulting in the generation of chemokines and cytokines are independent processes (27, 29, 32). Our finding that inhibition of Src activation in CCK-mediated experimental pancreatitis inhibited NF-κB and chemokine activation without affecting the trypsin activation supports the separate pathogenesis of these two processes. The activation of NF-κB and the subsequent stimulation of proinflammatory chemokines/cytokines has been shown to play an important role in mediating many of the features of acute pancreatitis due to CCK and other causes, including necrosis and inflammation (11, 25, 46, 59). Therefore, the ability of SFK inhibitors to prevent activation of the NF-κB cascade could be an important therapeutic approach, possibly resulting in decreased development of more severe forms of acute pancreatitis.
Previous studies report conflicting effects of SFK activation on CCK-induced amylase release from pancreatic acinar cells (36, 42, 84). Because CCK-stimulated enzyme secretion is mediated by activation of both high and low CCKAR affinity states that have different signaling cascades (65, 69, 86), to fully address the question of the possible involvement of SFKs in CCK-mediated enzyme secretion, we assessed whether amylase release caused by either physiological, maximal, or supraphysiological concentrations of CCK was Src-dependent. As previously observed (36, 42, 65, 84), we found CCK-stimulated amylase secretion in a biphasic fashion, with supramaximal concentrations causing decreasing stimulation. However, inhibiting CCK-stimulated SFK activation almost completely, or to various degrees, did not alter CCK-stimulated amylase secretion by any of the CCK concentrations examined. From this study we conclude that CCK-induced amylase secretion at either physiological, maximal, or supraphysiological concentrations is not SFK-dependent. This result differs from the SFK dependence of β-adrenergic agents to stimulate secretion from isolated rat salivary gland cells (73) or growth hormone to stimulate insulin release from pancreatic islets (92).
The present study has a number of potential shortcomings. In the present study the specific signaling cascades activating Src and activated by Src kinases, which mediated the changes in pancreatitis described above with supramaximal CCK treatment, were not studied. In pancreatic acinar cells, Src activation can occur through a wide range of signaling cascades and with CCK involve primarily activation of PKCs and changes in cytosolic calcium (48, 55, 68). Once activated under physiological conditions, Src is involved in pancreatic acinar cells in a wide range of signaling cascades, including activation of focal adhesion kinases (p125FAK, PYK-2), adaptor proteins (paxillin, p130Cas), Shc, and Akt (51). In pathophysiological conditions such as pancreatitis induced by supramaximal CCK, activation of PKCs, PKD, and NF-κB is particularly important (56, 90). Which of these signaling Src specifically affects cascade activation to induce the changes described in this study is currently unclear. It is also difficult to tell which of the Src isoforms (Yes, Lyn, Fyn, or pp60Src) are involved in this regulation. In previous studies we demonstrated that Yes (68) and Lyn (55) are the Src isoforms expressed in pancreatic acini. Although some studies have described pp60Src and/or Fyn expression in pancreatic acini (20, 55, 72), we did not detect either pp60Src or Fyn in pancreatic acini. As a conclusion, and based on our previous results, we can conclude that the effects observed here are likely due to Yes and Lyn. However, at present, there are no tools to specifically inhibit the different Src isoforms, and, moreover, rat pancreatic acini cannot be cultured for a time long enough to perform small-interfering RNA experiments. Because the Src isoforms are difficult to discriminate on the basis of molecular weight, the question of which Src isoform is more important in mediating the pancreatitis-like changes shown in this study remains unanswered. Last, in the present study, we did not examine the effect of Src inhibition on morphological changes of CCK-induced pancreatitis-like changes. Numerous studies have demonstrated that Src inhibition largely reverses pancreatitis-associated morphological changes whether induced by CCK, substance P, pervanadate, or by tumor necrosis factor-α (42, 48, 72).
In conclusion, using conditions in which SFK activation was inhibited, we demonstrated that SFKs were important in regulating a number of key mediators of its ability to induce acute pancreatitis, including release of chemokines, mediators of apoptosis (caspase-3, -8, and -9), and severity of necrosis. Our results demonstrate that CCK-stimulated Src activation has a novel dual role in pancreatitis by increasing necrosis while at the same time inhibiting caspase activation, which inhibits proapoptotic pathways, with the result that it shifts the balance to a more severe pancreatitis. Furthermore, our approach helps resolve the conflicting reports of SFKs role in mediating CCK-stimulated enzyme secretion by showing no effect with physiological, maximal, or supraphysiological CCK concentrations with different degrees of Src activation. These findings, in addition to providing a more complete assessment of the role of SFK activation by CCK in mediating the cellular cascades important for transduction of its physiological and pathophysiological effects, also provide evidence that the use of Src kinase inhibitors may be a useful therapeutic approach in acute pancreatitis to ameliorate its course.
GRANTS
This work was partially supported by intramural funds of the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.N.-B., I.R.-x., and R.T.J. conception and design of research; B.N.-B., I.R.-x., and R.T.J. performed experiments; B.N.-B., I.R.-x., and R.T.J. analyzed data; B.N.-B., I.R.-x., and R.T.J. interpreted results of experiments; B.N.-B., I.R.-x., and R.T.J. drafted manuscript; B.N.-B., I.R.-x., and R.T.J. edited and revised manuscript; B.N.-B., I.R.-x., and R.T.J. approved final version of manuscript; I.R.-x. and R.T.J. prepared figures.
REFERENCES
- 1.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia 12: 599–607, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beil M, Leser J, Lutz MP, Gukovskaya A, Seufferlein T, Lynch G, Pandol SJ, Adler G. Caspase 8-mediated cleavage of plectin precedes F-actin breakdown in acinar cells during pancreatitis. Am J Physiol Gastrointest Liver Physiol 282: G450–G460, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy 1: 343–351, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J Cell Mol Med 8: 402–409, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 286: G189–G196, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology 5: 132–144, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, Huang L, Zhang Y, Qiao M, Yao W, Yuan Y. The antagonist of the JAK-1/STAT-1 signaling pathway improves the severity of cerulein-stimulated pancreatic injury via inhibition of NF-kappaB activity. Int J Mol Med 27: 731–738, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol Cancer Ther 4: 217–224, 2005. [PubMed] [Google Scholar]
- 11.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology 122: 448–457, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Cursi S, Rufini A, Stagni V, Condo I, Matafora V, Bachi A, Bonifazi AP, Coppola L, Superti-Furga G, Testi R, Barila D. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J 25: 1895–1905, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabrowski A, Grady T, Logsdon CD, Williams JA. Jun kinases are rapidly activated by cholecystokinin in rat pancreas both in vitro and in vivo. J Biol Chem 271: 5686–5690, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Dabrowski A, Tribillo I, Dabrowska MI, Wereszczynska-Siemiatkowska U, Gabryelewicz A. Activation of mitogen-activated protein kinases in different models of pancreatic acinar cell damage. Z Gastroenterol 38: 469–481, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Delivoria-Papadopoulos M. Mechanism of caspase-9 activation during hypoxia in the cerebral cortex of newborn piglets: the role of Src kinase. Neurosci Lett 523: 19–23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Toni EN, Kuntzen C, Gerbes AL, Thasler WE, Sonuc N, Mucha SR, Camaj P, Bruns C, Goke B, Eichhorst ST. P60-c-src suppresses apoptosis through inhibition of caspase 8 activation in hepatoma cells, but not in primary hepatocytes. J Hepatol 46: 682–691, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Duan RD, Williams JA. Cholecystokinin rapidly activates mitogen-activated protein kinase in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 267: G401–G408, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Duru EA, Fu Y, Davies MG. SRC regulates sphingosine-1-phosphate mediated smooth muscle cell migration. J Surg Res 175: 30–34, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eguchi R, Kubo S, Takeda H, Ohta T, Tabata C, Ogawa H, Nakano T, Fujimori Y. Deficiency of Fyn protein is prerequisite for apoptosis induced by Src family kinase inhibitors in human mesothelioma cells. Carcinogenesis 33: 969–975, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Freedman SD, Katz MH, Parker EM, Gelrud A. Endocytosis at the apical plasma membrane of pancreatic acinar cells is regulated by tyrosine kinases. Am J Physiol Cell Physiol 276: C306–C311, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Garcia LJ, Rosado JA, Gonzalez A, Jensen RT. Cholecystokinin-stimulated tyrosine phosphorylation of p125FAK and paxillin is mediated by phospholipase C-dependent and -independent mechanisms and requires the integrity of the actin cytoskeleton and participation of p21rho. Biochem J 327: 461–472, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorelick FS, Otani T. Mechanisms of intracellular zymogen activation. Baillieres Best Pract Res Clin Gastroenterol 13: 227–240, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J Biol Chem 277: 22595–22604, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology 4: 567–586, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-κB activation is associated with hormone-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 275: G1402–G1414, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Guo L, Sans MD, Hou Y, Ernst SA, Williams JA. c-Jun/AP-1 is required for CCK-induced pancreatic acinar cell dedifferentiation and DNA synthesis in vitro. Am J Physiol Gastrointest Liver Physiol 302: G1381–G1396, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-κB in rat pancreatic acinar cells. Am J Physiol Cell Physiol 280: C465–C472, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Han B, Logsdon CD. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-κB activation. Am J Physiol Cell Physiol 277: C74–C82, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun 280: 388–395, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Xia W, Lu M, Gao B, Qiao X, Sun B, Zhang W, Zhang Y, Xue D. Role of kinase epidermal growth factor receptor and SRC in the caerulein-induced acute pancreatitis in mice. Pancreas 44: 152–157, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Irrera N, Bitto A, Interdonato M, Squadrito F, Altavilla D. Evidence for a role of mitogen-activated protein kinases in the treatment of experimental acute pancreatitis. World J Gastroenterol 20: 16535–16543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji B, Gaiser S, Chen X, Ernst SA, Logsdon CD. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem 284: 17488–17498, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ju KD, Yu JH, Kim H, Kim KH. Role of mitogen-activated protein kinases, NF-kappaB, and AP-1 on cerulein-induced IL-8 expression in pancreatic acinar cells. Ann NY Acad Sci 1090: 368–374, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol Cell Physiol 269: C1295–C1304, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Kawabata S, Miura T, Morita T, Kato H, Fujikawa K, Iwanaga S, Takada K, Kimura T, Sakakibara S. Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem 172: 17–25, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Kim M, Nozu F, Kusama K, Imawari M. Cholecystokinin stimulates the recruitment of the Src-RhoA-phosphoinositide 3-kinase pathway by Vav-2 downstream of G(alpha13) in pancreatic acini. Biochem Biophys Res Commun 339: 271–276, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology 144: 1180–1193, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Ma B, Lu M, Qiao X, Sun B, Zhang W, Xue D. Construction of network for protein kinases that play a role in acute pancreatitis. Pancreas 42: 607–613, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Liu AM, Wong YH. Activation of nuclear factor κB by somatostatin type 2 receptor in pancreatic acinar AR42J cells involves Gα14 and multiple signaling components: a mechanism requiring protein kinase C, calmodulin-dependent kinase II, ERK, and c-Src. J Biol Chem 280: 34617–34625, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Liu ST, Pham H, Pandol SJ, Ptasznik A. Src as the link between inflammation and cancer. Front Physiol 4: 416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Yuan J, Tan T, Jia W, Lugea A, Mareninova O, Waldron RT, Pandol SJ. Genetic inhibition of protein kinase Cepsilon attenuates necrosis in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol 307: G550–G563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch G, Kohler S, Leser J, Beil M, Garcia-Marin LJ, Lutz MP. The tyrosine kinase Yes regulates actin structure and secretion during pancreatic acinar cell damage in rats. Pflugers Arch 447: 445–451, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Ma B, Wu L, Lu M, Gao B, Qiao X, Sun B, Xue D, Zhang W. Differentially expressed kinase genes associated with trypsinogen activation in rat pancreatic acinar cells treated with taurolithocholic acid 3-sulfate. Mol Med Rep 7: 1591–1596, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Malo A, Kruger B, Seyhun E, Schafer C, Hoffmann RT, Goke B, Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 299: G877–G886, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem 281: 3370–3381, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Mayer J, Rau B, Schoenberg MH, Beger HG. Mechanism and role of trypsinogen activation in acute pancreatitis. Hepatogastroenterology 46: 2757–2763, 1999. [PubMed] [Google Scholar]
- 47.Minutoli L, Altavilla D, Marini H, Passaniti M, Bitto A, Seminara P, Venuti FS, Famulari C, Macri A, Versaci A, Squadrito F. Protective effects of SP600125 a new inhibitor of c-jun N-terminal kinase (JNK) and extracellular-regulated kinase (ERK1/2) in an experimental model of cerulein-induced pancreatitis. Life Sci 75: 2853–2866, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Mishra V, Cline R, Noel P, Karlsson J, Baty CJ, Orlichenko L, Patel K, Trivedi RN, Husain SZ, Acharya C, Durgampudi C, Stolz DB, Navina S, Singh VP. Src dependent pancreatic acinar injury can be initiated independent of an increase in cytosolic calcium. PLoS ONE 8: e66471, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J 374: 145–155, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagaraj NS, Smith JJ, Revetta F, Washington MK, Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther 9: 2322–2332, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuche-Berenguer B, Moreno P, Jensen RT. Elucidation of the Roles of the Src kinases in pancreatic acinar cell signaling. J Cell Biochem 116: 22–36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto W, Okamoto I, Yoshida T, Okamoto K, Takezawa K, Hatashita E, Yamada Y, Kuwata K, Arao T, Yanagihara K, Fukuoka M, Nishio K, Nakagawa K. Identification of c-Src as a potential therapeutic target for gastric cancer and of MET activation as a cause of resistance to c-Src inhibition. Mol Cancer Ther 9: 1188–1197, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Orlichenko LS, Behari J, Yeh TH, Liu S, Stolz DB, Saluja AK, Singh VP. Transcriptional regulation of CXC-ELR chemokines KC and MIP-2 in mouse pancreatic acini. Am J Physiol Gastrointest Liver Physiol 299: G867–G876, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pace A, Garcia-Marin LJ, Tapia JA, Bragado MJ, Jensen RT. Phosphospecific site tyrosine phosphorylation of p125FAK and proline-rich kinase 2 is differentially regulated by cholecystokinin receptor A activation in pancreatic acini. J Biol Chem 278: 19008–19016, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Pace A, Tapia JA, Garcia-Marin LJ, Jensen RT. The Src family kinase, Lyn, is activated in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors which stimulate its association with numerous other signaling molecules. Biochim Biophys Acta 1763: 356–365, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Pandol SJ, Saluja AK, Imrie Cw, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 132: 1127–1151, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Parker EM, Zaman MM, Freedman SD. GP2, A GPI-anchored protein in the apical plasma membrane of the pancreatic acinar cell, co-immunoprecipitates with src kinases and caveolin. Pancreas 21: 219–225, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Petrella C, Agostini S, Alema' GS, Casolini P, Carpino F, Giuli C, Improta G, Linari G, Petrozza V, Broccardo M. Cannabinoid agonist WIN55,212 in vitro inhibits interleukin-6 (IL-6) and monocyte chemo-attractant protein-1 (MCP-1) release by rat pancreatic acini and in vivo induces dual effects on the course of acute pancreatitis. Neurogastroenterol Motil 22: 1248–56, e323, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Rakonczay Z Jr, Jarmay K, Kaszaki J, Mandi Y, Duda E, Hegyi P, Boros I, Lonovics J, Takacs T. NF-kappaB activation is detrimental in arginine-induced acute pancreatitis. Free Rad Biol Med 34: 696–709, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Ramnath RD, Sun J, Adhikari S, Bhatia M. Effect of mitogen-activated protein kinases on chemokine synthesis induced by substance P in mouse pancreatic acinar cells. J Cell Mol Med 11: 1326–1341, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramnath RD, Sun J, Adhikari S, Zhi L, Bhatia M. Role of PKC-δ on substance P-induced chemokine synthesis in pancreatic acinar cells. Am J Physiol Cell Physiol 294: C683–C692, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Ramnath RD, Sun J, Bhatia M. Involvement of SRC family kinases in substance P-induced chemokine production in mouse pancreatic acinar cells and its significance in acute pancreatitis. J Pharmacol Exp Ther 329: 418–428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramnath RD, Sun J, Bhatia M. PKC delta mediates pro-inflammatory responses in a mouse model of caerulein-induced acute pancreatitis. J Mol Med 88: 1055–1063, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun 331: 1–14, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Rowley WH, Sato S, Huang SC, Collado-Escobar DM, Beaven MA, Wang LH, Martinez J, Gardner JD, Jensen RT. Cholecystokinin-induced formation of inositol phosphates in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 259: G655–G665, 1990. [DOI] [PubMed] [Google Scholar]
- 66.Saluja AK, Donovan EA, Yamanaka K, Yamaguchi Y, Hofbauer B, Steer ML. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology 113: 304–310, 1997. [DOI] [PubMed] [Google Scholar]
- 67.Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol 69: 249–269, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Sancho V, Nuche-Berenguer B, Jensen RT. The Src kinase Yes is activated in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters, but not pancreatic growth factors, which stimulate its association with numerous other signaling molecules. Biochim Biophys Acta 1823: 1285–1294, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato S, Stark HA, Martinez J, Beaven MA, Jensen RT, Gardner JD. Receptor occupation, calcium mobilization and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol Gastrointest Liver Physiol 257: G202–G209, 1989. [DOI] [PubMed] [Google Scholar]
- 70.Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR Jr, Shimosegawa T, Pandol SJ. PKC delta and epsilon regulate NF-κB activation induced by cholecystokinin and TNF-α in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 287: G582–G591, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Shanmugam MK, Bhatia M. The role of pro-inflammatory molecules and pharmacological agents in acute pancreatitis and sepsis. Inflamm Allergy Drug Targets 9: 20–31, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Singh VP, McNiven MA. Src-mediated cortactin phosphorylation regulates actin localization and injurious blebbing in acinar cells. Mol Biol Cell 19: 2339–2347, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slomiany BL, Slomiany A. Salivary phospholipid secretion in response to beta-adrenergic stimulation is mediated by Src kinase-dependent epidermal growth factor receptor transactivation. Biochem Biophys Res Commun 318: 247–252, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Slomiany BL, Slomiany A. Ghrelin protection against lipopolysaccharide-induced gastric mucosal cell apoptosis involves constitutive nitric oxide synthase-mediated caspase-3 S-nitrosylation. Mediators Inflamm 2010: 280464, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steer ML. Early events in acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol 13: 213–225, 1999. [DOI] [PubMed] [Google Scholar]
- 76.Sung KF, Odinokova IV, Mareninova OA, Rakonczay Z Jr, Hegyi P, Pandol SJ, Gukovsky I, Gukovskaya AS. Prosurvival Bcl-2 proteins stabilize pancreatic mitochondria and protect against necrosis in experimental pancreatitis. Exp Cell Res 315: 1975–1989, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takadera T, Fujibayashi M, Koriyama Y, Kato S. Apoptosis induced by SRC-family tyrosine kinase inhibitors in cultured rat cortical cells. Neurotox Res 21: 309–316, 2012. [DOI] [PubMed] [Google Scholar]
- 78.Tamizhselvi R, Koh YH, Sun J, Zhang H, Bhatia M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway. Exp Cell Res 316: 1625–1636, 2010. [DOI] [PubMed] [Google Scholar]
- 79.Tapia JA, Ferris HA, Jensen RT, Marin LJ. Cholecystokinin activates PYK2/CAKβ, by a phospholipase C-dependent mechanism, and its association with the mitogen-activated protein kinase signaling pathway in pancreatic acinar cells. J Biol Chem 274: 31261–31271, 1999. [DOI] [PubMed] [Google Scholar]
- 80.Tapia JA, Garcia-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C-delta activation, tyrosine phosphorylation and translocation is mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem 12: 35220–35230, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Tapia JA, Jensen RT, Garcia-Marin LJ. Rottlerin inhibits stimulated enzymatic secretion and several intracellular signaling transduction pathways in pancreatic acinar cells by a non-PKC-delta-dependent mechanism. Biochim Biophys Acta 1763: 25–38, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve J Jr, Pandol SJ, Gorelick FS. The novel protein kinase C isoforms-δ and -ε modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 294: G1344–G1353, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thrower EC, Yuan J, Usmani A, Liu Y, Jones C, Minervini SN, Alexandre M, Pandol SJ, Guha S. A novel protein kinase D inhibitor attenuates early events of experimental pancreatitis in isolated rat acini. Am J Physiol Gastrointest Liver Physiol 300: G120–G129, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsunoda Y, Yoshida H, Africa L, Steil GJ, Owyang C. Src kinase pathways in extracellular Ca2+-dependent pancreatic enzyme secretion. Biochem Biophys Res Commun 227: 876–884, 1996. [DOI] [PubMed] [Google Scholar]
- 85.Webb JG, Yang X, Crosson CE. Bradykinin activation of extracellular signal-regulated kinases in human trabecular meshwork cells. Exp Eye Res 92: 495–501, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams JA, Sans MD, Tashiro M, Schafer C, Bragado MJ, Dabrowski A. Cholecystokinin activates a variety of intracellular signal transduction mechanisms in rodent pancreatic acinar cells. Pharmacol Toxicol 91: 297–303, 2002. [DOI] [PubMed] [Google Scholar]
- 87.Yang BM, Demaine AG, Kingsnorth A. Chemokines MCP-1 and RANTES in isolated rat pancreatic acinar cells treated with CCK and ethanol in vitro. Pancreas 21: 22–31, 2000. [DOI] [PubMed] [Google Scholar]
- 88.Yogi A, Callera GE, Montezano AC, Aranha AB, Tostes RC, Schiffrin EL, Touyz RM. Endothelin-1, but not Ang II, activates MAP kinases through c-Src-independent Ras-Raf-dependent pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 27: 1960–1967, 2007. [DOI] [PubMed] [Google Scholar]
- 89.Yu JH, Kim H. Role of janus kinase/signal transducers and activators of transcription in the pathogenesis of pancreatitis and pancreatic cancer. Gut Liver 6: 417–422, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan J, Liu Y, Tan T, Guha S, Gukovsky I, Gukovskaya A, Pandol SJ. Protein kinase d regulates cell death pathways in experimental pancreatitis. Front Physiol 3: 60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yuan Z, McCauley R, Chen-Scarabelli C, Abounit K, Stephanou A, Barry SP, Knight R, Saravolatz SF, Saravolatz LD, Ulgen BO, Scarabelli GM, Faggian G, Mazzucco A, Saravolatz L, Scarabelli TM. Activation of Src protein tyrosine kinase plays an essential role in urocortin-mediated cardioprotection. Mol Cell Endocrinol 325: 1–7, 2010. [DOI] [PubMed] [Google Scholar]
- 92.Zhang F, Zhang Q, Tengholm A, Sjoholm A. Involvement of JAK2 and Src kinase tyrosine phosphorylation in human growth hormone-stimulated increases in cytosolic free Ca2+ and insulin secretion. Am J Physiol Cell Physiol 291: C466–C475, 2006. [DOI] [PubMed] [Google Scholar]