Abstract
Keratins (K) are intermediate filament proteins important in protection from stress. The roles of keratins in the intestine are not clear, but K8 knockout (K8−/−) mice develop a Th2-type colonic inflammation, epithelial hyperproliferation, and mild diarrhea caused by a keratin level-dependent decrease in short-circuit current and net sodium and chloride absorption in the distal colon. The lack of K8 leads to mistargeting or altered levels of membrane proteins in colonocytes; however, the main transporter responsible for the keratin-related ion transport defect is unknown. We here analyzed protein and mRNA levels of candidate ion transporters CFTR, PAT-1, NHE-3, and DRA in ileum, cecum, and proximal and distal colon. Although no differences were observed for CFTR, PAT-1, or NHE-3, DRA mRNA levels were decreased by three- to fourfold and DRA protein was almost entirely lost in K8−/− cecum and proximal and distal colon compared with K8+/+, whereas the levels in ileum were normal. In K8+/− mice, DRA mRNA levels were unaltered, while decreased DRA protein levels were detected in the proximal colon. Immunofluorescence staining confirmed the loss of DRA in K8−/− distal colon, while K8+/− displayed a similar but more patchy apical DRA distribution compared with K8+/+. DRA was similarly decreased when K8 was knocked down in Caco-2 cells, confirming that K8 levels modulate DRA levels in an inflammation-independent manner. Taken together, the loss of DRA in the K8−/− mouse colon and cecum explains the dramatic chloride transport defect and diarrheal phenotype after K8 inactivation and identifies K8 as a novel regulator of DRA.
Keywords: keratin, epithelium, colon, DRA, ion transport, chloride, sodium
keratins (K) are cytoskeletal intermediate filament proteins of epithelial cells and perform crucial functions of cytoprotection by participating in many cellular events such as cell polarity, migration, signaling, organelle organization, or susceptibility to apoptosis (6, 24, 27, 35, 40, 41). This is evident from the fact that keratin mutations predispose to or cause human diseases, such as skin and liver diseases, which are often phenocopied in mouse models (13, 23, 38). In intestinal epithelial cells, simple epithelial keratins are composed of obligate heteropolymers of a type I (K18–K20 and K23) and a type II (K7 and K8) intermediate filament (7, 23). The role of keratins in intestinal diseases is not clear, although some inflammatory bowel disease (IBD) patients have been identified with K8 mutations (25, 37). Also, impaired barrier function has been reported in HT-29 cells with K8 mutations (47). Evidence supporting a role for keratins in colonic health is manifested in the K8 knockout (K8−/−) mouse, which develops an early Th2-type colitis including epithelial hyperproliferation, decreased apoptosis, altered energy metabolism, and mistargeting of membrane proteins leading to mild diarrhea (2, 3, 11, 12, 14, 39). Ussing chamber experiments with K8−/− distal colon showed a decrease in short-circuit current associated with net chloride (Cl−) secretion and decreased net sodium (Na+) and chloride (Cl−) absorption, which could explain the diarrhea. However, K8−/− tight junction permeability and paracellular transport remained unaltered compared with K8+/+ (39). The K8−/− colon also exhibits significantly increased levels, but impaired targeting, of the anion exchanger 1/2 (AE1/2). The epithelial sodium transporter ENaCγ exhibited altered membrane distribution, although the protein levels in K8−/− were the same as in K8+/+ colon (39). Although K8−/− small intestine appeared normal compared with K8−/− colon, in which colitis is observed, there was a loss in the polarized expression of Cl− transporter cystic fibrosis transmembrane conductance regulator (CFTR) in the villi (but not the crypts) (2). Interestingly, K8+/− distal colon that expresses ∼50% less keratins compared with K8+/+ has an intermediate ion transport, short-circuit current, and proliferation phenotype, but no inflammation (3, 39). K8+/− mice are, however, more sensitive to dextran sulfate sodium (DSS)-induced colitis, indicating that a certain amount of keratins are needed for protection from intestinal stress (3).
The coupled operation of Na+/H+ exchanger-3 (NHE3 or SLC9A3) and Cl−/HCO3− exchanger downregulated in adenoma (DRA or SLC26A3) favors electroneutral NaCl absorption in the intestine (17). NHE3 is the major transporter for Na+ absorption in the ileum and colon and loss of functional NHE3 transporter results in altered intestinal Na+ and water absorption as seen in IBD patients and NHE3 knockout mice (9, 36). Double-knockout mice lacking IL-10 and NHE3 suffer from severe colitis, reflecting the need of NHE3 in maintaining intestinal integrity (21). In this regard, DRA and putative anion transporter 1 (PAT-1) are the two main candidates involved in the apical Cl−/HCO3− exchange. However, DRA plays a major role in the apical Cl−/HCO3− exchange process based on its implication in congenital chloride diarrhea (CLD), which is a genetic disorder characterized by voluminous diarrhea (22). The important role played by DRA in apical Cl−/HCO3− exchange is supported by the finding that DRA knockout mice, but not PAT-1 knockout mice, develop a severe intestinal Cl− absorption deficiency and diarrheal phenotype similar to CLD (42, 45). Impaired DRA expression and function have also been reported in diarrheal disorders associated with IBD or pathogenic infections (10, 45).
Since the roles of keratins in ion transport and intestinal diarrhea are still unclear, we have in the present study evaluated the possible role of keratins in intestinal ion transport processes. Our findings demonstrate that ablation of K8 leads to an almost complete downregulation of DRA in transgenic mice. Consistent with our in vivo data, in vitro studies also demonstrate that DRA expression was significantly reduced by K8 knockdown in Caco-2 cells.
MATERIALS AND METHODS
Mice.
K8−/−, K8+/−, and K8+/+ mice in the FVB/n background were generated by breeding of K8+/− mice and genotyped as previously described (4, 39). For collection of samples, age- and sex-matched mice were euthanized by CO2 inhalation and colons were excised and further processed as described below. All animal experiments were approved by the Animal Experimental Board in Finland and conformed to the legal acts, regulations, and requirements set by the European Union concerning protection of animals used for research.
RNA extraction and quantitative real-time PCR.
Total RNA was extracted from K8+/+, K8+/−, and K8−/− mouse intestinal samples (ileum, cecum, and proximal and distal colon) by using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Equal amounts of RNA from K8+/+, K8+/−, and K8−/− were reverse transcribed and amplified in a one-step reaction using Brilliant SYBR Green qRT-PCR Master Mix Kit (Agilent Technologies, Santa Clara, CA). Gene specific primers used are listed in Table 1. Gene expression levels were normalized to GAPDH (19).
Table 1.
Primers used for real-time PCR
| Gene | Species | Primer Sequence |
|---|---|---|
| DRA | Mouse | (F) 5′-TGGTGGGAGTTGTCGTTACA-3′ |
| (R) 5′-CCCAGGAGCAACTGAATGAT-3′ | ||
| PAT-1 | Mouse | (F) 5′-GAAATGGAGCTGCAGAGGA-3′ |
| (R) 5′-GCT GGAGCAGAAGAGAATGG-3′ | ||
| NHE-3 | Mouse | (F) 5′-GGCCTTCATTCGCTCCCCAAG-3′ |
| (R) 5-ATGCTTGTACTCCTGCCGAGG-3′ | ||
| GAPDH | Mouse | (F) 5′-TGTGTCCGTCGTGGATCTGA-3′ |
| (R) 5′-CCTGCTTCACCACCTTCTTGAT-3′ | ||
| CFTR | Mouse | (F) 5′-CTGGACCACACCAATTTTGAGG-3′ |
| (R) 5′-GCGTGGATAAGCTGGGGAT-3′ |
Tissue lysates and Western blotting.
Tissue lysates were prepared from mucosal scrapings of the colon (14) and total protein was extracted by using RIPA lysis buffer (Cell Signaling, Danvers, MA) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN). A bullet blender was used to homogenize mucosa followed by sonication (three pulses for 20 s each). The cell debris was removed by centrifuging the lysates at 13,000 rpm for 7 min at 4°C. The Bradford method was used to determine the protein concentration in the samples. Equal amounts (75 μg/sample) of tissue lysates were solubilized in SDS-gel loading buffer and boiled for 5 min. Lysates were run on 7.5–10% SDS-polyacrylamide gels and blotted to a nitrocellulose or polyvinylidene fluoride membrane after electrophoretic separation. After 1 h of incubation in blocking buffer (1× PBS and 5% nonfat dry milk), the membranes were probed with affinity-purified rabbit anti-DRA antibody. The affinity-purified DRA antibody was raised against the COOH-terminal amino acid (745–764) sequence INTNGGLRNRVYEPVETKF of SLC26A3 (accession number: BC025671) at Research Resource Center, University of Illinois at Chicago (28). Other primary antibodies used were rat anti-K8 antibody (Troma I, Developmental Studies Hybridoma Bank, University of Iowa), or rabbit-GAPDH antibody (Sigma and Cell Signaling). The membranes were incubated with the antibodies in 1× PBS and 2.5% nonfat dry milk overnight at 4°C. Next, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody for 1 h at room temperature, washed for 30 min, and detected by the ECL (enhanced chemiluminescence, Bio-Rad, Hercules, CA) detection method (28).
Immunofluorescence staining.
Colon samples were fresh frozen and embedded in Optimal Cutting Temperature compound (OCT) (Sakura Finetek, Alphen aan den Rijn, The Netherlands). Sections of colonic tissue from K8−/−, K8+/−, and K8+/+ mice were fixed with 4% paraformaldehyde in PBS (pH 8.5), for 15 min at room temperature and permeabilized by using 0.5% Nonidet P-40 in PBS for 5 min, followed by blocking in 2.5% NGS (normal goat serum) for 120 min at room temperature. Sections were then incubated with 1% NGS containing anti-DRA (1:100) or NHE3 (1:300, acquired from Dr. Jaleh Malakooti, University of Illinois at Chicago) and anti-villin (1:100, Abcam, Cambridge, MA) antibodies for 60 min. After washing with 1% NGS, sections were incubated with secondary antibodies, Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) and Alexa Fluor 568-conjugated goat anti-mouse IgG (Invitrogen) for 60 min and then mounted with Slowfade Gold antifade with DAPI reagent (Invitrogen) under coverslips. Edges of the coverslip were sealed with quick-dry nail enamel. Sections were imaged on a Carl Zeiss LSM 510 laser-scanning confocal microscope with a ×20 water immersion objective.
Downregulation of K8 in Caco-2 cells.
siRNA targeting K8 was done as described previously (14). Briefly, K8 siRNA [5′-GCCUCCUUCAUAGACAAGGUA(dTdT)-3′] was synthesized by Eurofins Genomics (Ebersberg, Germany) and transfected into Caco-2 cells using Lipofectamine 2000 (Invitrogen). A nontarget scrambled siRNA based on the K8 siRNA sequence [5′-GUCGUAUAUGACACCGUACCA(dTdT)-3] (Eurofins Genomics) was used as a negative control. Caco-2 cells were plated on 24-well plates and grown to 30–50% confluency, the cells were washed once with sterile PBS, and 500 μl of growth medium was added to each well. For each transfection sample, 50 pmol of siRNA was diluted in 50 μl of Opti-MEM (Gibco, Thermo Fisher Scientific, Waltham MA) and 1.5 μl Lipofectamine 2000 (Thermo Fisher Scientific) was diluted in 50 μl of Opti-MEM. Each solution was mixed gently and incubated for 5 min, after which the diluted siRNA was mixed with the diluted Lipofectamine 2000 and incubated for 20 min. After the incubation, 100 μl of the siRNA-Lipofectamine 2000 complexes were added to each well. After 72 h, the cells were harvested and used for Western blot analysis as described above.
Statistical analysis.
Results are expressed as means ± SE of three to five independent experiments. Student's t-test or one-way ANOVA with Tukey's test was used for statistical analysis. P < 0.05 or less was considered statistically significant.
RESULTS
Decreased DRA mRNA levels in K8−/− colon.
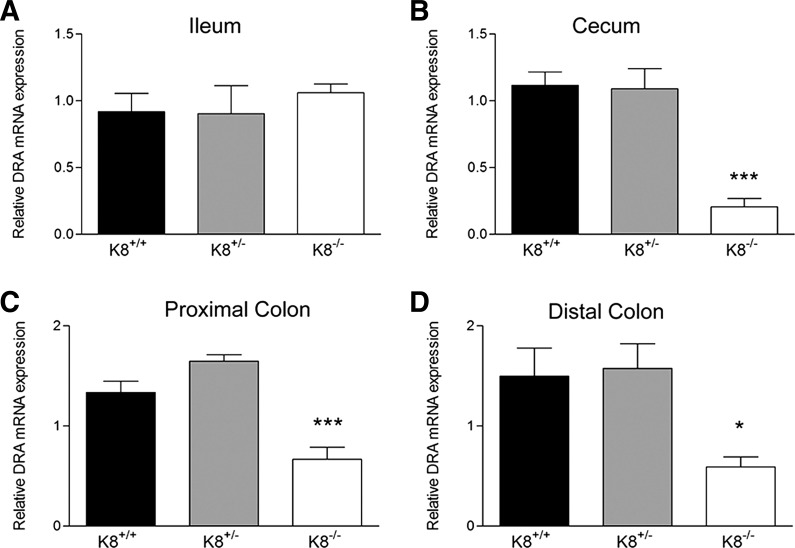
Since K8−/− and K8+/− distal colon display a keratin level-dependent (3) decreased Na+ and Cl− absorption (39), we set out to analyze the molecular basis of the transporter defects responsible for this phenotype. In epithelial scrapings from K8−/−, K8+/−, and K8+/+ ileum, cecum, and proximal colon and distal colon, the mRNA levels of CFTR, PAT-1, NHE3, and DRA were determined. No major differences were observed in mRNA levels of CFTR, PAT-1, and NHE-3 (Table 2) in cecum, ileum, and colon scrapings in K8−/− or K8+/− mice compared with K8+/+. However, DRA mRNA levels were decreased two- to fourfold in K8−/− cecum and proximal and distal colon compared with K8+/+, whereas DRA mRNA levels were unaltered in K8−/− ileum and throughout the K8+/− colon (Fig. 1).
Table 2.
mRNA levels of ion transporters in the intestine
| Proximal Colon |
Distal Colon |
||||||
|---|---|---|---|---|---|---|---|
| Transporter | n | K8+/+ | K8+/− | K8−/− | K8+/+ | K8+/− | K8−/− |
| CFTR | 4 | 0.80 ± 0.11 | 1.10 ± 0.01* | 0.96 ± 0.05 | 1.30 ± 0.26 | 2.31 ± 0.41 | 1.56 ± 0.19 |
| NHE3 | 4 | 1.26 ± 0.15 | 1.07 ± 0.13 | 1.65 ± 0.19 | 1.23 ± 0.34 | 1.53 ± 0.39 | 1.70 ± 0.24 |
| PAT-1 | 4 | 1.11 ± 0.09 | 0.93 ± 0.01 | 0.73 ± 0.14 | ND | ND | ND |
| Cecum |
Ileum |
||||||
|---|---|---|---|---|---|---|---|
| Transporter | n | K8+/+ | K8+/− | K8−/− | K8+/+ | K8+/− | K8−/− |
| CFTR | 4 | 1.00 ± 0.07 | 0.93 ± 0.22 | 0.43 ± 0.12 | 0.84 ± 0.20 | 0.80 ± 0.15 | 1.00 ± 0.11 |
| NHE3 | 4 | 1.20 ± 0.26 | 1.30 ± 0.26 | 0.49 ± 0.20 | 1.07 ± 0.10 | 0.81 ± 0.12 | 0.70 ± 0.04 |
| PAT-1 | 4 | ND | ND | ND | 0.90 ± 0.10 | 1.20 ± 0.33 | 0.74 ± 0.12 |
Values are means ± SE.
P <0.05; ND, not determined.
Fig. 1.
Decreased DRA mRNA levels in colon and cecum but not ileum of K8−/− mice. The mRNA levels of DRA were determined from mucosal scrapings isolated from ileum (A), cecum (B), and proximal (C) and distal colon (D) of K8+/+, K8+/−, and K8−/− mice (n = 4). DRA mRNA levels were decreased 2- to 4-fold in K8−/− cecum and proximal and distal colon compared with K8+/+ (*P < 0.05, ***P < 0.001).
K8−/− mice have lower levels of DRA protein.
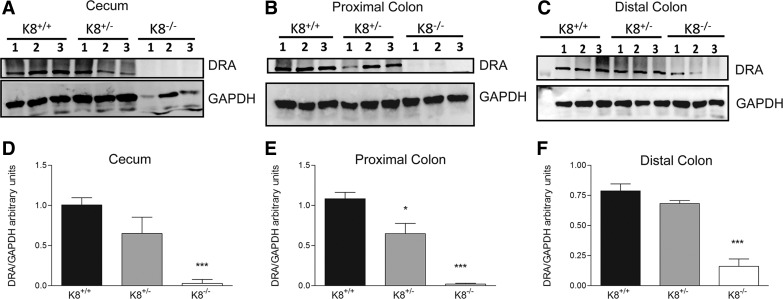
To analyze the consequences of decreased DRA mRNA in K8−/− mice, Western blot analysis was performed on colon and cecum samples from K8+/+, K8+/−, and K8−/− mice. The results showed that DRA protein levels were nearly completely abolished in K8−/− cecum and proximal colon (Fig. 2, A, B, D, and E) and significantly decreased in the distal colon (Fig. 2, C and F). Interestingly, in the K8+/− colon DRA protein levels were significantly reduced in the proximal colon while cecum and distal colon showed a trend of decrease, which did not reach significance compared with K8+/+ (Fig. 2). Together, these findings suggest an involvement of K8 in regulation of DRA mRNA and protein expression.
Fig. 2.
Significant loss of DRA protein levels in the cecum, proximal and distal colon of K8−/− mice. Western blot showed prominent DRA downregulation in cecum (A) and proximal (B) and distal colon (C). DRA levels were quantified and normalized to the housekeeping gene GAPDH (D–F). DRA levels were significantly lower in K8+/− proximal colon and almost absent in K8−/− colon and cecum compared with K8+/+ mice (n = 3; *P < 0.05, ***P < 0.001). Mouse K8 genotype was determined by PCR (not shown).
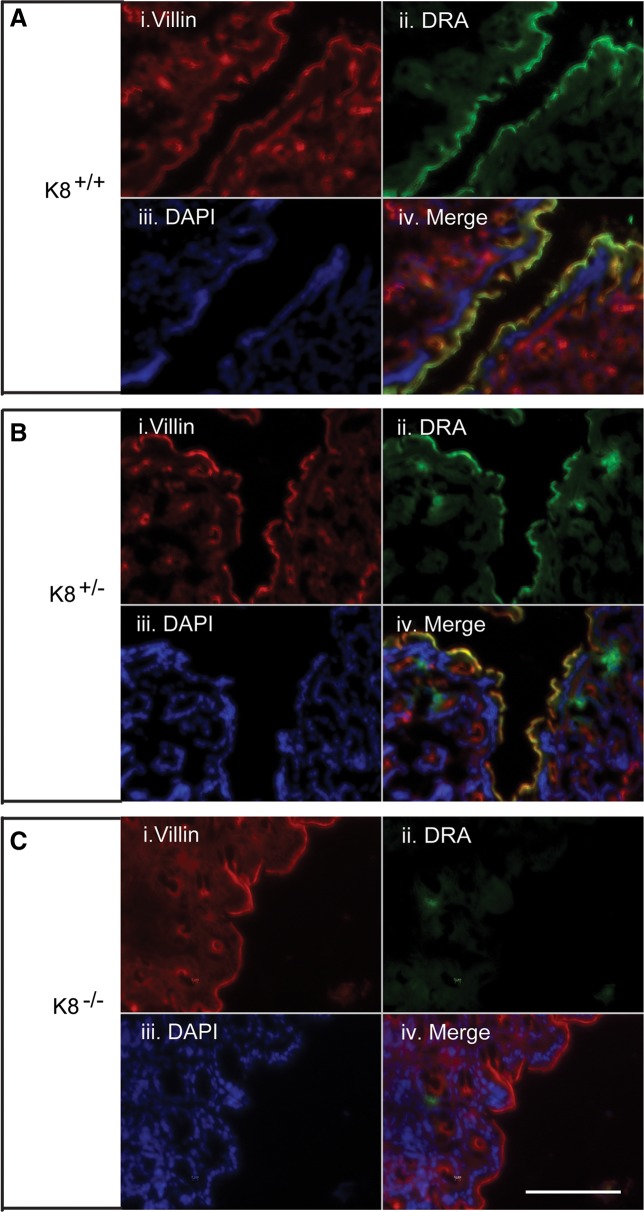
DRA is almost absent in K8−/− colonic crypts compared with the baseline apical distribution in K8+/+ mice.
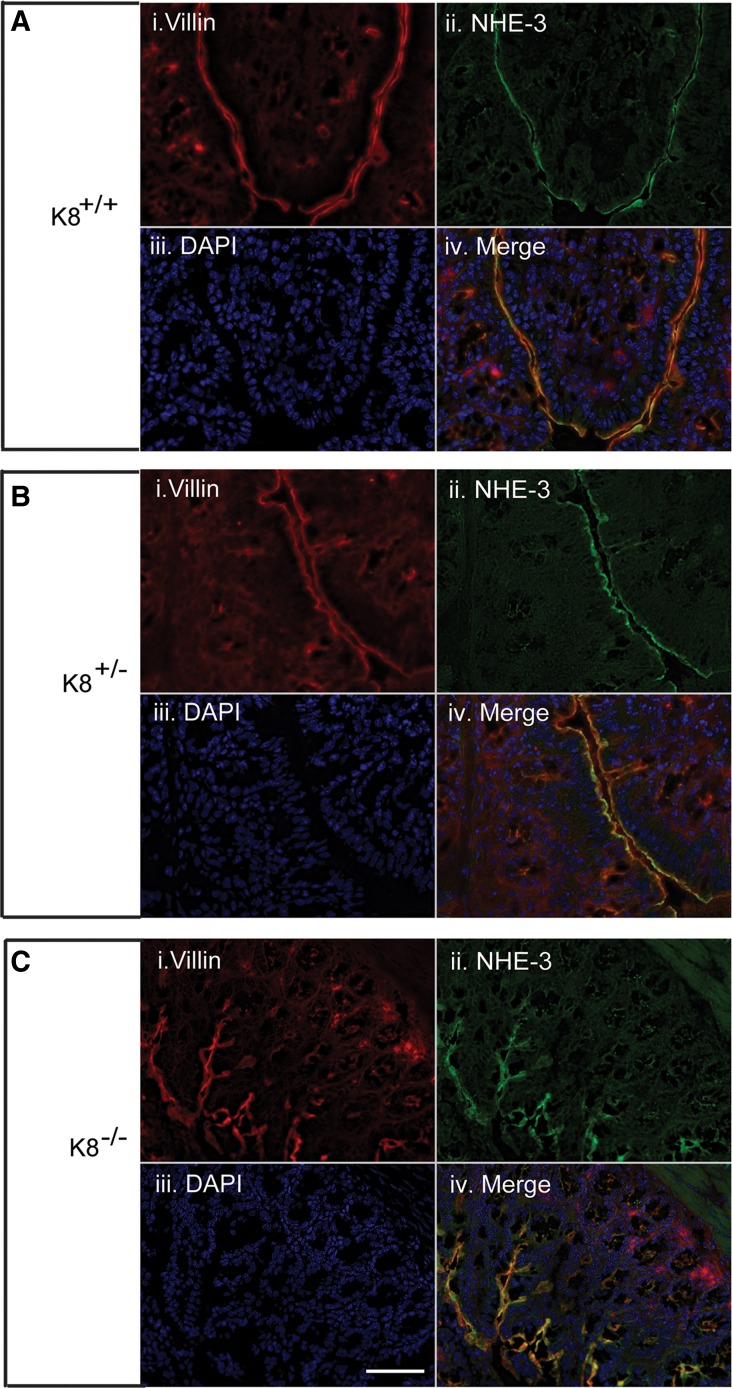
To analyze the cellular location of colonic DRA as a function of K8, immunofluorescence staining and confocal microscopy were performed. DRA was almost abolished in the K8−/− distal colon apical membrane compared with K8+/− and K8+/+ mice, while the microvillar apical membrane marker villin was normally distributed after K8 inactivation (Fig. 3). Interestingly, K8+/− distal colon showed patchy apical staining for DRA (Fig. 3). No differences were seen in NHE3 distribution in colonic crypts of K8−/− mice compared with K8+/+ mice (Fig. 4). Since the K8−/− phenotype starts very early with hyperproliferation 1–2 days after birth, and obvious inflammation by 2 wk (39), we attempted to analyze DRA levels in 1- to 2-day-old mice by immunostaining to ask whether the decrease of DRA in adult mice is a consequence of decreased K8 levels or due to inflammation. Interestingly, DRA was not yet expressed at significant level at this young age and could, thus, not be detected in the mouse colon (not shown).
Fig. 3.
Immunofluorescence staining of distal colon shows the dramatic loss of DRA in K8−/−, but not in K8+/− mice. Distal colon cryosections fixed with 4% paraformaldehyde in PBS for 15 min at room temperature were stained with DRA. The results show absence of DRA in K8−/− mice (C) compared with normal DRA staining in K8+/+ mice (A). K8+/− mice showed patchy DRA staining (B). DRA was abolished in the K8−/− distal colon apical membrane compared with K8+/− and K8+/+ mice, whereas apical villin was normally distributed. DAPI was used to stain nuclei. Scale bar 100 μm.
Fig. 4.
Immunofluorescence staining of proximal colon shows the normal levels and distribution of NHE-3 in K8+/− and K8−/− mice. Proximal colon cryosections from K8−/−, K8+/−, and K8+/+ mice were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and permeabilized with 0.5% Nonidet P-40 in PBS for 5 min, followed by blocking in 2.5% NGS (normal goat serum) for 120 min at room temperature. There was no difference in NHE-3 staining and apical villin was not altered in K8+/+ (A), K8+/− (B), and K8−/− (C) mice. DAPI was used to stain nuclei. Scale bar 50 μm.
Downregulation of K8 by siRNA in Caco-2 cells similarly decreases DRA levels.
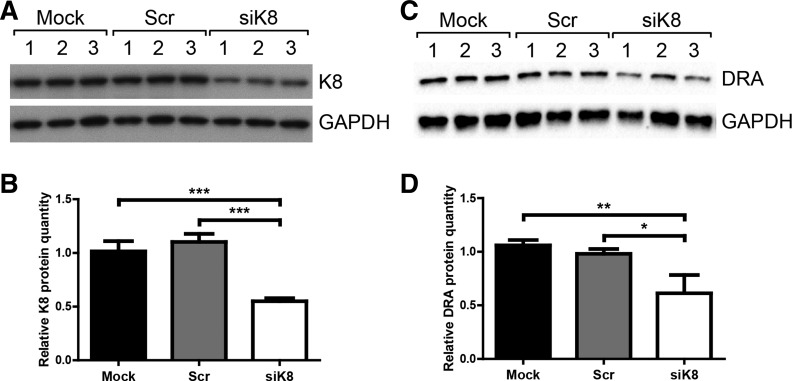
Since K8−/− mice exhibit inflammation in the colon and DRA expression has been shown to be reduced during inflammation (18, 34, 44, 46), we tested whether the reduced DRA levels in K8−/− mice were secondary to inflammation or a direct consequence of K8 loss. For this purpose, K8 was silenced in Caco-2 cells by use of siRNA, followed by evaluation of DRA expression. The K8 siRNA treatment led to a 50% decrease in K8 levels (Fig. 5, A and B) and a concomitant 50% decrease in DRA expression (Fig. 5, C and D), whereas no significant difference was seen between mock and scramble siRNA samples. These data suggest that keratins modulate DRA expression in an inflammation-independent manner.
Fig. 5.
Reduced DRA expression in K8-silenced Caco-2 cells. Caco-2 cells were mock-transfected (treated with transfection reagents only; Mock 1–3; n = 3) or transfected with scrambled control siRNA (Scr 1–3; n = 3) or K8 siRNA (siK8 1–3; n = 3) and total cell lysates normalized by protein assay. The same samples were evaluated by Western blot for K8 (A) and DRA (C), and GAPDH was detected as loading control for each blot (A and C). Densitometry analysis of the Western blot data was used to quantify DRA, K8, and GAPDH levels, and the level of K8 knockdown and DRA levels are indicated as relative protein quantity normalized to the GAPDH signal (B and D). The results were analyzed by 1-way ANOVA and Tukey's multiple-comparison test (*P < 0.05, **P < 0.01; ***P < 0.001).
DISCUSSION
Although keratins play an important role in cellular signaling (24, 26, 27), their potential role in modulation of intestinal ion transport has not been investigated at the molecular level. In this communication, we have examined in detail the main transporters responsible for colonic Na+ and Cl− transport, and found that K8 inactivation in vivo in mice lead to a dramatic downregulation of the Cl−/HCO3− exchanger DRA mRNA and protein levels in the entire colon and cecum, but not in ileum. In vitro studies with Caco-2 cells treated with K8 siRNA also showed a similar keratin level-dependent decrease in DRA levels, confirming a causal link between K8 levels and DRA expression.
The Na+/H+ exchanger 3 (NHE3) and the Cl−/HCO3− exchanger DRA together facilitate intestinal electroneutral NaCl absorption (20). In the present study no difference in NHE3 mRNA or protein levels and protein localization were observed, suggesting that the previously described mislocalization of the Na+ transporter ENaCγ (39) is the main reason behind the Na+ uptake defect after K8 inactivation. Since DRA is a central intestinal Cl−/HCO3− transporter, the nearly complete loss of DRA in the K8−/− colon described here, together with the upregulation and mistargeting of AE1/2 (39), explains the decreased Cl− uptake. No major differences in mRNA levels for PAT-1 or CFTR were observed; however, a role for K8/K18 in CFTR function cannot be ruled out, since K18 was recently found to bind CFTR and regulate CFTR localization to the membrane (8). Indeed, in K18−/− (8) and K8−/− (2) small intestine villi (but not crypts), CFTR surface expression was reduced. However, in K8−/− mice, colonic crypt CFTR had normal apical levels and localization pattern (2), suggesting a cell type-specific regulation of CFTR by intermediate filaments (16).
K8+/− mice, which have a significant and intermediate lower Cl− and Na+ uptake in the distal colon in Ussing chambers studies (39), have normal DRA mRNA levels but on average less (and intermediate levels between K8+/+ and K8−/−) DRA protein in the colon. The reduced DRA protein levels in K8+/− mice reached significance only in the proximal colon, where the K8−/−-mediated effect is also more dramatic compared with the distal colon. DRA mRNA and protein levels can be upregulated in response to anti-inflammatory agents, such as butyrate and probiotics (Lactobacillus acidophilus, Bifidobacterium sp.) (1, 19, 29), suggesting that the microflora, which is more abundant in the proximal colon, may have played a role. To this end, broad-spectrum antibiotic treatment normalizes the K8−/− colitis (11). K8−/− mice also have a defect in butyrate uptake from the colon due to decreased levels of the monocarboxylate transporter isoform 1 (MCT1) that transports butyrate into colonic epithelial cells (14), which is interesting considering the fact that butyrate is known to upregulate DRA levels in the colon (1).
DRA is downregulated in inflammation as shown in ulcerative colitis patients, IL-10 knockout mice (18, 46), and mice treated with DSS (34, 44). This effect is partly mediated by the proinflammatory cytokines IL-1β and IFN-γ (30, 31, 46). We therefore cannot rule out that the decrease in DRA in K8−/− could be at least in part a consequence of colitis and epithelial hyperproliferation (4, 11, 39), even if we have shown that some ion transporter abnormalities and membrane protein mistargeting already occur 1–2 days after birth, when the hyperproliferation and inflammation are not yet present (39). Since there is a significant ion transport defect and mild DRA phenotype in K8+/− mice, which clearly do not have inflammation but have lower keratin levels (3), these data suggest that the keratin-DRA link is not entirely related to, or a consequence of, inflammation. In support of this argument is the fact that there is a high correlation between downregulation of DRA and knockdown of K8 in Caco-2 cells transfected with K8 siRNA. Interestingly, DRA knockout mice and K8−/− mice have a very similar colonic phenotype (33) with increased epithelial proliferation, altered ion transport, and diarrhea (4, 39). Furthermore, DRA knockout mice (45) and K8+/− mice (3) are both more susceptible to DSS-induced colitis than their respective wild-type mice. In this regard, although a few patients with keratin mutations and IBD have been described, this association is not as clear as the well-studied association of keratin variants with liver disease, such that 12% of liver disease patients harbor keratin mutations (23, 37, 38). However, our findings warrant further studies to examine the role of keratins in IBD and associated diarrhea.
The disruption of microtubules and epithelial cell polarity as a result of keratin knockdown could be one potential mechanism for DRA downregulation (2, 10, 15, 32). Since the downregulation of DRA is so dramatic after K8 inactivation, one could envision that keratins participate in regulation of translation or transcription of DRA. In this regard, keratins are being touted to have an emerging role in gene expression (5) and translation (27) since mice without the entire type II keratin cluster, which includes the K8 gene, exhibit a major defect in protein synthesis (43).
In conclusion, our present study demonstrates a novel link between K8 and DRA expression in the large intestine that appears to underlie the diarrheal phenotype. Earlier studies have shown that K8−/− mice have decreased NaCl absorption but no change in paracellular permeability and ENaCγ mistargeting (39). In addition, our present study shows that NHE3 expression or targeting was not altered. This clearly demonstrates that DRA downregulation, secondary to K8 inactivation, is central to the diarrheal phenotype observed in these mice.
GRANTS
Our work was financially supported by the Academy of Finland no. 140759/12616, Sigrid Juselius Foundation, EU FP7 IRG KIFREO, Åbo Akademi University (ÅAU) Center of Excellence of Cell Stress and Molecular Aging, Liv och Hälsa Foundation, and the COST Action BM1002 Nanonet (to D. M. Toivola); Turku Doctoral Programme in Molecular Biosciences at ÅAU (M. N. Asghar, J. H. Nyström); Turku Doctoral Program of Biomedical Sciences and ÅAU (M. N. Asghar); Swedish Cultural Foundation; and Makarna Agneta and Carl-Erik Olins Foundation (J. H. Nyström). This work was also supported by Department of Veterans Affairs Merit Award no. BX002011 (P. K. Dudeja) and the National Institute of Diabetes and Digestive and Kidney Diseases grants DK54016, DK81858, and DK92441 (P. K. Dudeja).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. conception and design of research; M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. performed experiments; M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. analyzed data; M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. interpreted results of experiments; M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. prepared figures; M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. drafted manuscript; M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. edited and revised manuscript; M.N.A., S.P., J.H.N., A.N.A., P.K.D., and D.M.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the personnel at the Central Animal Laboratory at the University of Turku, and The Cell Imaging Core at Turku Centre for Biotechnology, students and members of the Toivola laboratory for skillful technical assistance, Héléne Baribault (Amgen) for providing the K8−/− mouse strain, Jonas Silvander for analysis of young mice, and Amika Singla for initiating this collaboration.
REFERENCES
- 1.Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am J Physiol Gastrointest Liver Physiol 293: G923–G934, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Ameen NA, Figueroa Y, Salas PJ. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J Cell Sci 114: 563–575, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Asghar MN, Silvander JS, Helenius TO, Lahdeniemi IA, Alam C, Fortelius LE, Holmsten RO, Toivola DM. The amount of keratins matters for stress protection of the colonic epithelium. PLoS One 10: e0127436, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev 8: 2964–2973, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chung BM, Arutyunov A, Ilagan E, Yao N, Wills-Karp M, Coulombe PA. Regulation of C-X-C chemokine gene expression by keratin 17 and hnRNP K in skin tumor keratinocytes. J Cell Biol 208: 613–627, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol 25: 600–612, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 14: 110–122, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Duan Y, Sun Y, Zhang F, Zhang WK, Wang D, Wang Y, Cao X, Hu W, Xie C, Cuppoletti J, Magin TM, Wang H, Wu Z, Li N, Huang P. Keratin K18 increases cystic fibrosis transmembrane conductance regulator (CFTR) surface expression by binding to its C-terminal hydrophobic patch. J Biol Chem 287: 40547–40559, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habtezion A, Toivola DM, Asghar MN, Kronmal GS, Brooks JD, Butcher EC, Omary MB. Absence of keratin 8 confers a paradoxical microflora-dependent resistance to apoptosis in the colon. Proc Natl Acad Sci USA 108: 1445–1450, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habtezion A, Toivola DM, Butcher EC, Omary MB. Keratin-8-deficient mice develop chronic spontaneous Th2 colitis amenable to antibiotic treatment. J Cell Sci 118: 1971–1980, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Haines RL, Lane EB. Keratins and disease at a glance. J Cell Sci 125: 3923–3928, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Helenius TO, Misiorek JO, Nystrom JH, Fortelius LE, Habtezion A, Liao J, Asghar MN, Zhang H, Azhar S, Omary MB, Toivola DM. Keratin 8 absence down-regulates colonocyte HMGCS2 and modulates colonic ketogenesis and energy metabolism. Mol Biol Cell 26: 2298–2310, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer D, Jons T, Kraemer J, Drenckhahn D. From cytoskeleton to polarity and chemoreception in the gut epithelium. Ann NY Acad Sci 859: 75–84, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Jakab RL, Collaco AM, Ameen NA. Characterization of CFTR High Expresser cells in the intestine. Am J Physiol Gastrointest Liver Physiol 305: G453–G465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol 73: 261–281, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, Alrefai WA, Gill RK, Dudeja PK. Probiotic Bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol 307: C1084–C1092, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamprecht G, Hsieh CJ, Lissner S, Nold L, Heil A, Gaco V, Schafer J, Turner JR, Gregor M. Intestinal anion exchanger down-regulated in adenoma (DRA) is inhibited by intracellular calcium. J Biol Chem 284: 19744–19753, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larmonier CB, Laubitz D, Thurston RD, Bucknam AL, Hill FM, Midura-Kiela M, Ramalingam R, Kiela PR, Ghishan FK. NHE3 modulates the severity of colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol 300: G998–G1009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makela S, Kere J, Holmberg C, Hoglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat 20: 425–438, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Omary MB. “IF-pathies”: a broad spectrum of intermediate filament-associated diseases. J Clin Invest 119: 1756–1762, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest 119: 1794–1805, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens DW, Lane EB. Keratin mutations and intestinal pathology. J Pathol 204: 377–385, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms. Sci STKE 2006: pe53, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Pan X, Hobbs RP, Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol 25: 47–56, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priyamvada S, Anbazhagan AN, Gujral T, Borthakur A, Saksena S, Gill RK, Alrefai WA, Dudeja PK. All-trans-retinoic acid increases SLC26A3 DRA (down-regulated in adenoma) expression in intestinal epithelial cells via HNF-1beta. J Biol Chem 290: 15066–15077, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saksena S, Dwivedi A, Singla A, Gill RK, Tyagi S, Borthakur A, Alrefai WA, Ramaswamy K, Dudeja PK. Characterization of the 5′-flanking region and regulation of expression of human anion exchanger SLC26A6. J Cell Biochem 105: 454–466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saksena S, Tyagi S, Goyal S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Stimulation of apical Cl−/HCO3−(OH−) exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent. Am J Physiol Gastrointest Liver Physiol 299: G1334–G1343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salas PJ. Insoluble gamma-tubulin-containing structures are anchored to the apical network of intermediate filaments in polarized CACO-2 epithelial cells. J Cell Biol 146: 645–658, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol 307: G623–G631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol 15: 163–177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan S, Alex P, Dassopoulos T, Zachos NC, Iacobuzio-Donahue C, Donowitz M, Brant SR, Cuffari C, Harris ML, Datta LW, Conklin L, Chen Y, Li X. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis 15: 261–274, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao GZ, Strnad P, Zhou Q, Kamal A, Zhang L, Madani ND, Kugathasan S, Brant SR, Cho JH, Omary MB, Duerr RH. Analysis of keratin polypeptides 8 and 19 variants in inflammatory bowel disease. Clin Gastroenterol Hepatol 5: 857–864, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Toivola DM, Boor P, Alam C, Strnad P. Keratins in health and disease. Curr Opin Cell Biol 32: 73–81, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Toivola DM, Krishnan S, Binder HJ, Singh SK, Omary MB. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J Cell Biol 164: 911–921, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toivola DM, Strnad P, Habtezion A, Omary MB. Intermediate filaments take the heat as stress proteins. Trends Cell Biol 20: 79–91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol 15: 608–617, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Vijayaraj P, Kroger C, Reuter U, Windoffer R, Leube RE, Magin TM. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol 187: 175–184, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao F, Juric M, Li J, Riederer B, Yeruva S, Singh AK, Zheng L, Glage S, Kollias G, Dudeja P, Tian DA, Xu G, Zhu J, Bachmann O, Seidler U. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3− secretion in murine ileocolonic inflammation. Inflamm Bowel Dis 18: 101–111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao F, Yu Q, Li J, Johansson ME, Singh AK, Xia W, Riederer B, Engelhardt R, Montrose M, Soleimani M, Tian DA, Xu G, Hansson GC, Seidler U. Slc26a3 deficiency is associated with loss of colonic HCO3 − secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol (Oxf) 211: 161–175, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, Silberg DG, Antalis TM, Schweinfest CW, Wu GD. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 275: G1445–G1453, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Zupancic T, Stojan J, Lane EB, Komel R, Bedina-Zavec A, Liovic M. Intestinal cell barrier function in vitro is severely compromised by keratin 8 and 18 mutations identified in patients with inflammatory bowel disease. PLoS One 9: e99398, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]