Abstract
High-fat-diet (HFD) consumption is associated with colon cancer risk. However, little is known about how the lipid composition of a HFD can influence prooncogenic processes. We examined the effects of three HFDs differing in the percentage of total calories from saturated fat (SF) (6, 12, and 24% of total caloric intake), but identical in total fat (40%), and a commercially available Western diet (26 and 41% saturated and total fat, respectively) on colon cancer development using the azoxymethane (AOM)/dextran sulfate sodium (DSS) murine model. A second dose-response experiment was performed using diets supplemented with the saturated-fatty-acid (SFA)-rich coconut oil. In experiment 1, we found an inverse association between SF content and tumor burden. Furthermore, increased SF content was associated with reduced inflammation, increased apoptosis, and decreased proliferation. The second dose-response experiment was performed to test whether this effect may be attributed to the SF content of the diets. Consistent with the initial experiment, we found that high SF content was protective, at least in male mice; there was a decrease in mortality in mice consuming the highest concentration of SFAs. To explore a potential mechanism for these findings, we examined colonic mucin 2 (Muc2) protein content and found that the HFDs with the highest SF content had the greatest concentration of Muc2. Our data suggest that high dietary SF is protective in the AOM/DSS model of colon cancer, which may be due, at least in part, to the ability of SF to maintain intestinal barrier integrity through increased colonic Muc2.
Keywords: high-fat diet, inflammation, mucin 2
the etiology of colon cancer encompasses the interaction between genetic and environmental factors. Interestingly, however, colon cancer is considered to be an environmental disease given that the majority of cases are likely preventable through lifestyle changes (22). Recently, obesity has emerged as a leading environmental risk factor for colon cancer. This is of concern given that excessive consumption of energy-dense food along with physical inactivity has driven obesity levels to 35% in the United States (33). Similarly, high-fat-diet (HFD) intake strongly influences the risk for colon cancer. In fact, it is thought that changes in food habits may reduce up to 70% of this cancer burden (49). Thus, understanding the relationship between these lifestyle factors and colon cancer risk is of critical public health importance.
Animal studies have generally supported the hypothesis that both HFD consumption and obesity are associated with colon cancer risk. The majority of studies have used either the Apcmin/+ mouse model of intestinal tumorigenesis or chemically induced models, namely azoxymethane (AOM) or a combination of AOM and dextran sulfate sodium (AOM/DSS). For instance, we have reported that consumption of a HFD was associated with an increase in the number of large polyps in the Apcmin/+ mouse (12). However, given that Apcmin/+ mice develop cachexia at ∼13 wk of age, the duration of HFD feedings was limited to 8 wk (4–12 wk of age), greatly restricting our ability to develop an obese phenotype. Similarly, the majority of the studies using chemically induced models initiate colon cancer at the beginning of a HFD regime; given the relatively short duration of these models, it is difficult to determine whether altered tumorigenesis is driven by the HFD itself or an obese phenotype.
The relationship between HFD and colon cancer risk is further complicated by the composition of the HFD used. The intake of total dietary fat and fatty acid (FA) composition can alter pathways that are known to affect colon cancer development independent of obesity. For example, we recently examined the influence of three HFDs (40% of total calories from fat) differing in the percentage of total calories from saturated fat (SF) (6, 12, and 24%) on body composition, macrophage behavior, inflammation, and metabolic dysfunction in mice and found marked differences across the diets (13, 15). However, there is currently little evidence on the impact of dietary total fat or FA composition on colon cancer. Furthermore, the use of inappropriate control diets has limited the conclusiveness of the findings from the available literature.
The purpose of this study was to determine the effects of three HFDs differing in the percentage of total calories from SF (6, 12, and 24% of total caloric intake), but identical in total fat (40%), on colon cancer development using the chemically induced AOM/DSS model. We also sought to examine changes in body composition, metabolic outcomes, and adipose tissue inflammation to begin to differentiate between HFD and obesity effects. We used a control diet that was similar to the AIN-76A diet but modified so that the polyunsaturated fatty acid:monounsaturated fatty acid (PUFA:MUFA) and omega-6 fatty acid:omega-3 fatty acid were identical to that of the HFDs. Furthermore, we included a commercially available Western diet as a comparison for the custom HFDs that we generated. Unexpectedly, we found an inverse association between SF content and tumor burden. To test the hypothesis that this may be attributed to the saturated fatty acid (SFA) content of the diets, we performed an additional dose-response experiment and found that increasing dietary SFA content was protective in the AOM/DSS model of colon cancer.
METHODS
Animals.
For all experiments, male and female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were cared for in the animal facility at the University of South Carolina. For experiment 1, only male mice were used, whereas, both male and female mice were used in experiment 2. Mice were housed five per cage, maintained on a 12:12-h light-dark cycle in a low-stress environment (22°C, 50% humidity, low noise), and given food and water ad libitum. The maintenance diet during habituation for all mice was a “standard” chow diet (Harlan Teklad Rodent Diet, no. 8604). All methods were in accordance with the American Association for Laboratory Animal Science, and the Institutional Animal Care and Usage Committee of the University of South Carolina approved all experiments.
Experiment 1 diet protocol.
At 10 wk of age, male mice were randomly assigned to one of six treatment groups (n = 10/group): a control group (AIN-76A Mod Noncancer) and five AOM/DSS groups (AIN-76A Mod, 6% SF, 12% SF, 24% SF, and Western diet). Of these treatment groups, the AIN-76A Mod Noncancer and AIN-76A Mod groups consumed a low-fat control diet, whereas the remaining four groups consumed one of four HFDs. The control diet (AIN-76A Mod) was modified from the original AIN-76A diet commonly used in nutrition studies to match the MUFA:PUFA and omega-6:omega-3 of the HFD. Three of the HFDs were original purified diets created by our team in which the percentage of total calories from saturated fat differed (6, 12, and 24% of total caloric intake), but calories from total fat (40%) did not. Additionally, the PUFA:MUFA and the omega-6:omega-3 fatty acids were identical and were designed to be similar to the standard American diet (19, 39). The remaining purified HFD used was a commercially available “Western diet.” All diets were manufactured by BioServ (Frenchtown, NJ) and are described in detail in Table 1.
Table 1.
Diet composition
| Saturated Fat Diet |
|||||
|---|---|---|---|---|---|
| AIN-76A Modified | 6% | 12% | 24% | Western Diet | |
| Ingredient, g/kg | |||||
| Casein | 200 | 165 | 165 | 165 | 195 |
| dl-Methionine | 3 | 3 | 3 | 3 | 3 |
| Lard | 0 | 3.3 | 35.4 | 68.6 | 0 |
| Coconut oil | 0 | 1 | 30 | 96.7 | 0 |
| Corn oil | 15.6 | 62.5 | 49.9 | 25.2 | 10 |
| Soybean oil | 3.6 | 14.1 | 9.3 | 2.5 | 0 |
| Olive oil | 30.9 | 122.1 | 78.4 | 10 | 0 |
| Anhydrous milkfat | 0 | 0 | 0 | 0 | 200 |
| Corn starch | 80 | 50 | 50 | 50 | 50 |
| Maltodextrin | 100 | 100 | 100 | 100 | 100 |
| Sucrose | 469.5 | 381.5 | 381.5 | 381.5 | 339.5 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| Vitamin mix (AIN-76A) | 10 | 10 | 10 | 10 | 10 |
| Mineral mix (AIN-76A) | 35 | 35 | 35 | 35 | 35 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2 |
| Energy, kcal/g | 3.79 | 4.57 | 4.57 | 4.57 | 4.57 |
| Energy, %kcal | |||||
| Carbohydrate | 68.7 | 47 | 47 | 47 | 43 |
| Fat | 12.2 | 40 | 40 | 40 | 41 |
| Protein | 19.1 | 13 | 13 | 13 | 16 |
| Fatty acid profile, g/kg | |||||
| Butyric acid (C4:0) | 0 | 0 | 0 | 0 | 6.5 |
| Caproic acid (C6:0) | 0 | 0 | 0 | 0 | 3.8 |
| Caprylic acid (C8:0) | 0 | 0.075 | 2.3 | 7.3 | 2.2 |
| Capric acid (C10:0) | 0 | 0.063 | 1.8 | 5.9 | 5 |
| Lauric acid (C12:0) | 0 | 0.45 | 13.5 | 43.3 | 5.6 |
| Myristic acid (C14:0) | 0.004 | 0.23 | 5.5 | 17.1 | 20 |
| Palmitic acid (C16:0) | 5.5 | 22.7 | 26 | 28.3 | 53.4 |
| Palmitoleic acid (C16:1) | 0.4 | 1.7 | 2 | 2 | 4.5 |
| Stearic acid (C18:0) | 1.05 | 4.6 | 8.5 | 12.7 | 24.3 |
| Oleic acid (C18:1) | 27.1 | 108.7 | 88 | 48.5 | 52.7 |
| Linoleic acid (C18:2) | 13.2 | 52.9 | 43.2 | 24.5 | 9.9 |
| α-Linolenic acid (C18:3) | 0.66 | 2.6 | 2.2 | 1.2 | 3 |
| Total calories from SFAs, % | 1.7 | 6 | 12 | 24 | 26 |
| Total calories from SCFAs (C4:0), % | 0 | 0 | 0 | 0 | 1.4 |
| Total calories from MCFAs (C6:0-C12:0), % | — | 0.1 | 3.6 | 11.8 | 7.9 |
| Total calories from LCSFAs (C14:0-C18:0), % | 1.7 | 5.9 | 8.4 | 12.2 | 16.7 |
| Total calories from LCFAs (C14:0-C-18:3), % | 10.5 | 39.9 | 36.4 | 28.2 | 31.7 |
| Total calories from USFAs, % | 10.5 | 34 | 28 | 16 | 15 |
| Total calories from MUFAs, % | 7 | 22.6 | 18.6 | 10.6 | 12.3 |
| Total calories from PUFAs, % | 3.5 | 11.4 | 9.4 | 5.4 | 2.7 |
| Total calories from n-3 FAs, % | 0.16 | 0.53 | 0.45 | 0.25 | 0.6 |
| Total calories from n-6 FAs, % | 3.2 | 10.8 | 8.9 | 5.1 | 2.1 |
| Cholesterol, mg/kg | 0 | 3 | 34 | 65 | 1.5 |
| Ratio MUFA:PUFA | 2:1 | 2:1 | 2:1 | 2:1 | 4.6:1 |
| Ratio n-6:n-3 FA | 20:1 | 20:1 | 20:1 | 20:1 | 3.5:1 |
Diet composition of treatment diets from experiment 1.
SFAs, saturated fatty acids; MCFAs, medium-chain fatty acids; LCSFAs, long-chain saturated fatty acids; LCFA, long-chain fatty acid; USFAs, unsaturated fatty acids; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids; FAs, fatty acids.
Experiment 1 AOM/DSS protocol.
Concomitant with diet administration, at 10 wk of age (baseline week 0) mice received either an intraperitoneal injection of the carcinogen, AOM (10 mg/kg) (Sigma, St. Louis, MO), diluted in PBS or PBS alone (AIN-76A Mod Noncancer). Mice receiving the AOM injection were subject to three cycles of DSS (36–50 kDa) (MP Biomedical, Solon, OH)-supplemented water at final concentrations of 2, 1, and 1% at weeks 1, 4, and 7, respectively. Each DSS cycle lasted for a 1-wk period. Mice were killed 2 wk after the last day of DSS consumption, for a total of 10 wk of treatment. Body weights and symptom scores were determined biweekly while food and water intake was measured weekly. Calculation of symptom score was performed as previously described (10), taking into account body weight loss, stool consistency, and rectal bleeding. Mean symptom score throughout the study was calculated by the following equation: (sum of all symptom scores/no. of symptom score measurements performed over the course of the study) = mean symptom score.
“Hemoccult” tape with developer (Beckman Coulter, Brea, CA) was used to assess rectal bleeding.
Body composition.
Body composition for the mice from experiment 1 was assessed at baseline and at the end of the experiment (10 wk of treatment) using dual-energy X-ray absorptiometry (DEXA) (Lunar PIXImus, Madison, WI).
Glucose metabolism.
After 9.5 wk of treatment, blood samples were collected from the tip of the tail after a 5-h fast for the mice consuming the AIN-76A Mod Noncancer, AIN-76A Mod, and Western diet. Glucose metabolism data were analyzed for the Western diet over the other HFDs, since Western diet-fed mice displayed the heaviest body weight and fat mass (as assessed by DEXA) of all the HFD groups. Blood glucose concentrations were determined in whole blood using a glucometer (Bayer Contour, Michawaka, IN). Collected blood was centrifuged at 4,000 revolutions/min for 10 min at 4°C. Plasma was separated into aliquots and stored at −80°C until analysis. Plasma insulin concentrations were determined by a commercially available ELISA kit (Mercodia, Uppsala, Sweden). Insulin resistance was estimated by the homeostatic model assessment (HOMA) index according to the following formula: insulin resistance index = fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5 (31).
Tissue collection.
After 10 wk of treatment, mice were killed for tumor count and tissue collection. Tissues were removed, weighed, and immediately snap-frozen in liquid nitrogen and stored at −80°C or fixed in 10% formalin until analysis. The colon was carefully dissected distal to the cecum and proximal to the anus. Mesentary adipose tissue was removed with tweezers. The colon was flushed with PBS, opened longitudinally, and flattened with a cotton swab. Colon length and width were measured using calipers, and colon weight was also recorded. Tumors were counted under a dissecting microscope and were categorized according to size (>3, 2–3, and <2 mm). A 5-mm piece of the distal portion of the colon was cut and fixed in 10% buffered formalin (Fisher Scientific, Pittsburg, PA) for 24 h for immunohistochemical analysis. The remaining colon was cut in half and snap-frozen for RNA and protein analyses.
Hematoxylin and eosin staining and F4/80 immunohistochemistry.
Hematoxylin and eosin staining of epididymal adipose tissue and colons were performed as previously described (14). Immunohistochemistry for adipose tissue F4/80 (AbD-Serotec) was performed as described by the manufacturer's instructions.
Gene expression.
Quantification of colonic expression of TNF-α, monocyte chemoattractant protein-1 (MCP-1), F4/80, IL-6, IL-10, mucin (Muc) 2, Muc6, Muc5ac, tight junction protein 1 (TJP1), and occludin as well epididymal adipose tissue gene expression for TNF-α, F4/80, and CD11c (Applied Biosystems, Foster City, CA) were performed as previously described (13). Briefly, RNA was extracted using TRIzol reagent (Life Technologies, GIBCO-BRL, Carlsbad, CA) and chloroform procedures. Because DSS has been shown to inhibit polymerase and reverse transcriptase activity, lithium chloride was used to purify the RNA as described in detail by Viennois et al. (45). Quantitative RT-PCR analysis was carried out as per the manufacturer's instructions (Applied Biosystems) using TaqMan Gene Expression Assays using 18S rRNA as an internal control.
Western blotting.
Briefly, colon was homogenized in Mueller Buffer containing a cocktail protease inhibitor (Sigma Aldrich) (7). Total protein concentrations were determined by the Bradford method. Equal amounts of crude protein homogenates were fractioned on a hand-casted 6.5–18% SDS-polyacrylamide gel and electrophoretically transferred to a polyvinylidene difluoride membrane using a Monster Genie Blotter (IDEA Scientific, Minneapolis, MN). Membranes were stained with a Ponceau S solution to verify equal protein loading and transfer efficiency. Western blots were performed as previously described using the primary antibody, Muc2 (Abcam, Cambridge, MA), phosphorylated (p)-NF-κB p65 (S536), total NF-κB p65, p-STAT-3 (Y705), total STAT3, p-ERK (Thr202/Tyr204), total ERK, p-p38 (Thr180/Tyr182), total p38, cleaved caspase 3 (Asp175), and proliferating cell nuclear antigen (PCNA) (Cell Signaling, Danvers, MA) (14). β-Actin and α-tubulin were used for loading controls depending on the molecular weight of the target antibody. Densitometry of Muc2, given its large molecular weight (540 kDa) and the fact that all proteins below ≈100 kDa were run off the gel, was normalized to the Ponceau S stain as previously described (37).
Experiment 2 diet protocol.
Ten-week-old male and female mice were randomly assigned to one of four treatment groups (n = 10/group) whose group name corresponded to the type of diet consumed: a control group [low-fat-diet (LFD) Noncancer] and three AOM/DSS groups [LFD, medium-fat-diet (MFD), and HFD]. Each treatment group consumed one of three liquid diets, each differing in the percentage of kilocalories from fat. The liquid diets were chosen for this experiment to be able to simultaneously and accurately monitor water, food, and DSS intake over the course of the experiment. Hence, the liquid diet served as both the food and water source for the mice and was made available to the mice using “liquid diet feeding tubes” (BioServ) (which allows for a precise assessment of liquid intake). The LFD Noncancer and LFD groups consumed a low-fat liquid diet consisting of 3.9% of total calories from fat, whereas the MFD and HFD groups consumed liquid diets consisting of medium (19.9% of total calories from fat) and high (33.2% of total kcal from fat) levels of fat, respectively. It was not possible to completely remove fat from the diet, since dietary essential FAs are necessary for proper physiology. Thus, each diet contained a basal level of 3.9 g/l of soybean oil, an oil rich in essential FAs. Because our tumor burden data from experiment 1 suggested that SFAs may protect against colon carcinogenesis, we supplemented the liquid diets with varying levels of coconut oil as the sole source of fat (besides the basal level of soybean oil) to test this hypothesis. Coconut oil was chosen since it is the naturally occurring oil richest in SFAs. No coconut oil was added to the LFD, whereas the MFD and HFD were supplemented with the oil at a density of 18 and 36 g/l, respectively. All diets were manufactured by BioServ and are described in detail in Table 2.
Table 2.
Liquid diet composition
| Low-Fat Diet | Medium-Fat Diet | High-Fat Diet | |
|---|---|---|---|
| Ingredient, g/l | |||
| Casein hydrolysate | 52 | 52 | 52 |
| dl-Methionine | 0.8 | 0.8 | 0.8 |
| Soybean oil | 3.9 | 3.9 | 3.9 |
| Coconut oil | 0 | 18.1 | 36.2 |
| Cellulose | 12.9 | 12.9 | 12.9 |
| Maltodextrin | 12.4 | 12.4 | 12.4 |
| Sucrose | 5.0 | 5.0 | 5.0 |
| Corn syrup solids | 155.2 | 137.1 | 119.0 |
| Vitamin mix | 2.6 | 2.6 | 2.6 |
| Mineral mix | 9.1 | 9.1 | 9.1 |
| Choline bitartrate | 0.52 | 0.52 | 0.52 |
| Suspending aids | 4.5 | 4.5 | 4.5 |
| Sodium aaccharin | 0.1 | 0.1 | 0.1 |
| Energy, kcal/l | 901 | 994 | 1,088 |
| Energy, %kcal | |||
| Carbohydrate | 75.3 | 61.2 | 49.6 |
| Fat | 3.9 | 19.9 | 33.2 |
| Protein | 20.8 | 18.9 | 17.2 |
| Fatty acid profile, g/l | |||
| Butyric acid (C4:0) | 0 | 0 | 0 |
| Caproic acid (C6:0) | 0 | 0.1 | 0.2 |
| Caprylic acid (C8:0) | 0 | 1.4 | 2.7 |
| Capric acid (C10:0) | 0 | 1.1 | 2.2 |
| Lauric acid (C12:0) | 0 | 8.1 | 16.2 |
| Myristic acid (C14:0) | 0 | 3.0 | 6.1 |
| Palmitic acid (C16:0) | 0.4 | 1.9 | 3.4 |
| Palmitoleic acid (C16:1) | 0 | 0 | 0 |
| Stearic acid (C18:0) | 0.2 | 0.7 | 1.2 |
| Oleic acid (C18:1) | 0.9 | 1.9 | 3.0 |
| Linoleic acid (C18:2) | 2.0 | 2.3 | 2.6 |
| α-Linolenic acid (C18:3) | 0.3 | 0.3 | 0.3 |
| Total calories from SFAs, % | 0.6 | 15.6 | 28.0 |
| Total calories from SCFAs (C4:0) | 0 | 0 | 0 |
| Total calories from MCFAs (C6:0-C12:0), % | 0 | 10.2 | 18.7 |
| Total calories from LCSFAs (C14:0-C18:0), % | 0.6 | 5.4 | 9.4 |
| Total calories from LCFAs (C14:0-C-18:3), % | 3.9 | 9.7 | 14.5 |
| Total calories from USFAs, % | 3.3 | 4.3 | 5.2 |
| Total calories from MUFAs, % | 0.9 | 1.8 | 2.6 |
| Total calories from PUFAs, % | 2.4 | 2.5 | 2.5 |
| Total calories from n-3 FAs, % | 0.3 | 0.3 | 0.3 |
| Total calories from n-6 FAs, % | 2.1 | 2.2 | 2.3 |
| Cholesterol, g/l | 0 | 0 | 0 |
| Ratio MUFA:PUFA | 0.4:1 | 0.7:1 | 1:1 |
| Ratio n-6:n-3 FA | 6.7:1 | 7.7:1 | 8.7:1 |
Diet composition of treatment liquid diets from experiment 2.
SCFA, short-chain fatty acid.
Experiment 2 AOM/DSS protocol.
Mice were acclimated to the liquid diet for 1 wk (from 10 to 11 wk of age) before initiation of the AOM/DSS protocol. During the final 3 days of the week-long acclimation period, daily liquid food consumption was monitored to determine the average liquid food intake per mouse, which was calculated based on the following formula: (no. of ml of diet consumed for each cage)/(no. of mice in each cage) = no. of ml consumed/mouse.
For both male and female mice, average liquid food intake for each diet consumed during this acclimation period was ∼13 ml of liquid diet, per mouse, per day. After the acclimation period, mice were started on the same AOM/DSS protocol as in experiment 1 (baseline week 0). However, because mice consumed roughly 13 ml of liquid diet/day compared with mice in experiment 1, who consumed roughly 3.5 ml of water/day, the percentage of DSS in the liquid diet was scaled down to match the gram intake of DSS per day of the mice in experiment 1. Thus, in each of the three DSS cycles, the percentage of DSS in the liquid diet was lowered from 2, 1, and 1% in experiment 1 to 0.54, 0.27, 0.27% in experiment 2, for cycles 1, 2, and 3, respectively. Liquid diet consumption along with any incidences of mortality (the primary outcome of the study) was monitored daily, and diets were made fresh each day throughout the experiment. While our initial design included measurement of symptom severity score and tumor burden, given the large mortality rate among the LFD and MFD groups (60%), we instead focused on mortality as our primary outcome. The small remaining sample size, as well as the fact that our findings would be underestimated given that the surviving mice in the LFD and MFD groups are likely to be those with lower symptom scores and tumor burden, hampered the generation of any reliable data on these outcomes.
It should be noted that the DSS used in both experiments were from the same manufacturing lot.
Statistical analyses.
All data (except for mortality data) were analyzed using a one-way ANOVA through the use of commercial software (SigmaStat; SPSS, Chicago, IL). A Student-Newman-Keuls test was used for all post hoc analyses. A Pearson correlation test was used for all correlational analyses. The AIN76A-Mod Noncancer group was not included in these correlational analyses. Any data that were not normally distributed or did not display equal variance were logarithmically transformed so that these criterion were met. If these parameters were not met even after logarithmic transformation, the data were subject to a nonparametic multiple-comparisons test (Dunn's test). Differences in mortality were determined using a Lifetest Survival Analysis Program. Statistical significance was set with an alpha value of P ≤ 0.05. Data are presented as means ± SE.
RESULTS
Diets influence body weights and AOM/DSS-associated symptoms of mice.
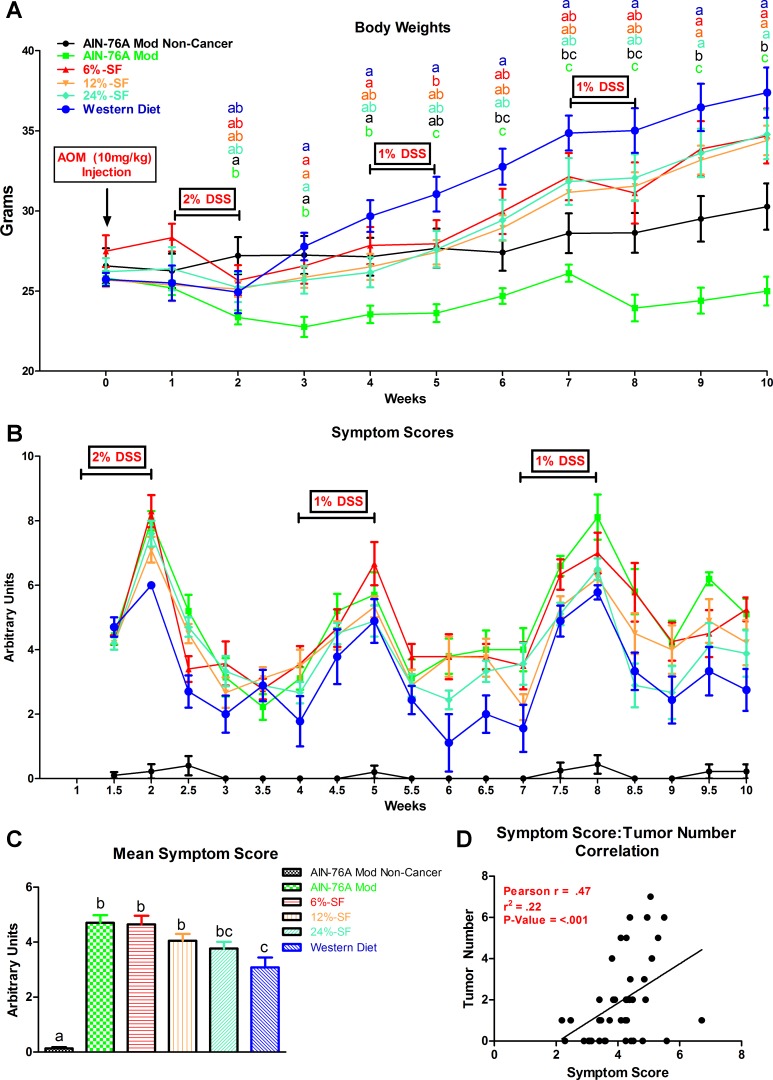
Body weights and symptom scores over the course of experiment 1 are presented in Fig. 1. Mean symptom scores over the course of the study are presented in Fig. 1C. In general, over the course of the study, the AIN-76A Mod group displayed significantly less body weight than all other groups (P ≤ 0.05). At the end of the study, consumption of each of the four HFDs led to increased body weight relative to the AIN-76A Mod Noncancer and AIN-76A Mod groups (P ≤ 0.05).
Fig. 1.
Influence of the azoxymethane (AOM)/dextran sulfate sodium (DSS) model on body weights (A), symptom scores (B), mean symptom score data over the course of the study (C), and symptom score-tumor number correlational analysis (D) (n = 8–10/group). Groups not sharing a common letter differ significantly from one another (P ≤ 0.05).
With respect to symptom score, consumption of the Western diet resulted in the lowest mean symptom score of all AOM/DSS-treated groups (P ≤ 0.05) (Fig. 1C). All other cancer-induced groups displayed significantly higher mean symptom scores, except for the 24% SF group (P ≤ 0.05). Symptom score was positively correlated with tumor number (P ≤ 0.05) (Fig. 1D). It should be noted that there was no difference in water intake across AOM/DSS groups over the course of the experiment.
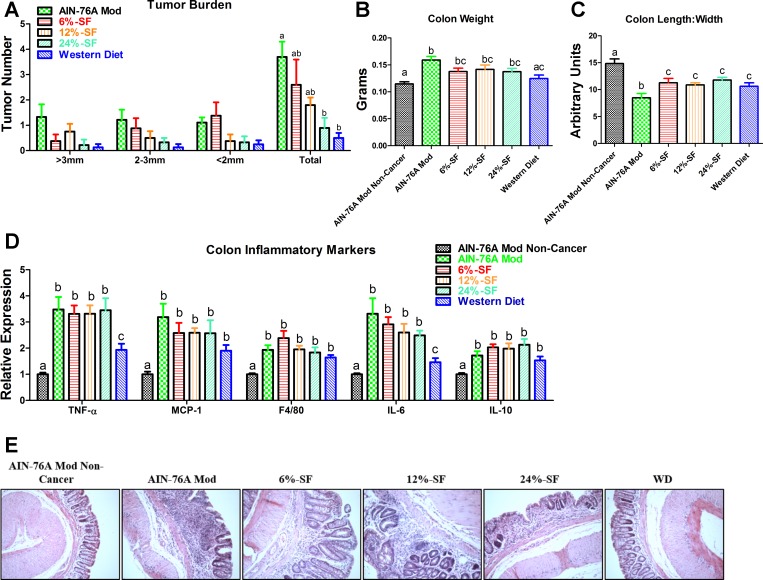
Tumor burden is lowest in mice consuming diets of the highest SF content.
Although there were no significant differences among groups with respect to tumor size, both the 24% SF and Western diet groups exhibited a lower tumor burden than the AIN-76A Mod group (P ≤ 0.05) (Fig. 2A). Additionally, consumption of the Western diet resulted in a colon weight similar to the AIN-76A Mod Noncancer group, whereas consumption of all other diets increased colon weight relative to this group (P ≤ 0.05) (Fig. 2B). However, the AIN-76A Mod diet was the only diet to significantly increase colon weight relative to the Western diet (P ≤ 0.05). All diets decreased the colon length-to-width ratio relative to the AIN-76A Mod Noncancer group (P ≤ 0.05) (Fig. 2C). However, among the AOM/DSS-treated groups, the AIN-76A Mod diet resulted in the smallest colon length-to-width ratio (P ≤ 0.05).
Fig. 2.
Tumor and colon inflammatory data from experiment 1. Shown are tumor burden (A), colon weight (B), colon length-to-width ratio (C), colon inflammatory markers (D), and representative colonic hematoxylin and eosin (H&E) images (E) (×20) of each treatment group (n = 8–10/group). SF, saturated fat; WD, Western diet. Groups not sharing a common letter differ significantly from one another (P ≤ 0.05).
Colitis development is attenuated in mice consuming the western diet.
With regard to colon inflammatory markers, all AOM/DSS-treated groups increased colon gene expression of TNF-α, MCP-1, F4/80, IL-6, and IL-10 relative to the AIN-76A Mod Noncancer group (P ≤ 0.05) (Fig. 2D). However, among the cancer-induced groups, colon gene expression of TNF-α and IL-6 was found to be downregulated in the Western diet group compared with all other groups. Histologically, dysregulation of crypts and villi was evident in all AOM/DSS-treated groups except for the Western diet in which there was minimal existence of colon inflammation or structural damage, similar to the AIN-76A Mod group (Fig. 2E).
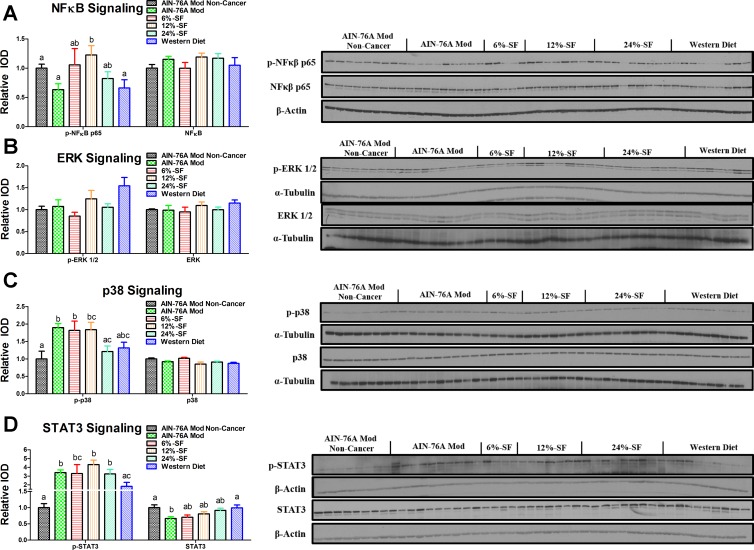
Dietary saturated fat content impacts NF-κB, STAT3, and p38 activation.
Consumption of the 12% SF diet increased p-NF-κB compared with the AIN-76A Mod Noncancer, AIN-76A Mod, and Western diet groups (P ≤ 0.05) (Fig. 3A). There were no differences in ERK activation among any of the groups (Fig. 3B). All cancer groups, except for the 24% SF and Western diet groups, increased p38 activation compared with the AIN-76A Mod Noncancer group (P ≤ 0.05) (Fig. 3C). Among the cancer groups, the 24% SF group trended to, or did exhibit, lower p38 activation compared with the 12% SF (P = 0.08) and AIN-76A Mod and 6% SF groups, respectively (P ≤ 0.05). Additionally, the Western diet group trended (P = 0.07) to display lower p38 phosphorylation compared with the AIN-76A Mod group. Regarding STAT3 activation, all cancer groups, except for the Western diet group, increased STAT3 phosphorylation compared with the AIN-76A Mod Noncancer group (P ≤ 0.05) (Fig. 3D). Among the cancer groups, the Western diet group displayed significantly less STAT3 activation compared with all other groups except for the 6% SF diet group (P ≤ 0.05). The AIN-76A Mod also displayed significantly less total STAT3 compared with the AIN-76A Mod Noncancer and Western diet groups (P ≤ 0.05) (Fig. 3D).
Fig. 3.
Activation of proteins regulating carcinogenesis. Shown are representative Western blots of colonic phosphorylated (p)-NF-κB p65 and total NF-κB (A), p-ERK ½ and total ERK ½ (B), p-p38 and total p38 (C), and p-STAT-3 and total STAT3 (D) (n = 4–9/group). IOD, intensity of densitometry. Groups not sharing a common letter differ significantly from one another (P ≤ 0.05).
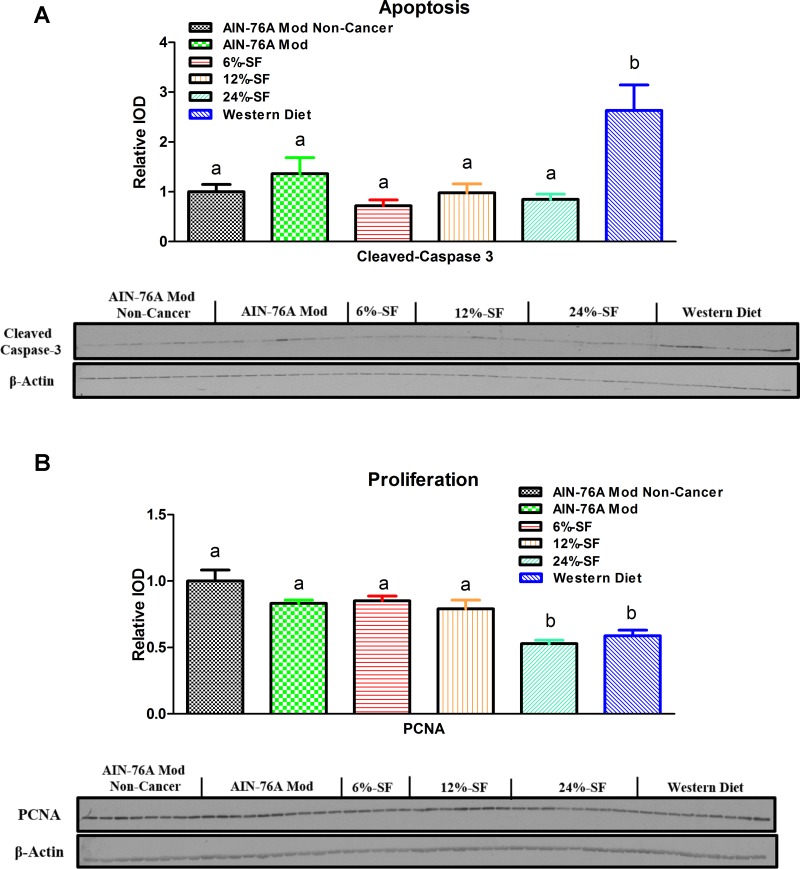
Proliferation and apoptosis is influenced by high dietary SF content.
Cleaved caspase 3, a marker of apoptosis, was found to be increased in the Western diet groups compared with all other groups (P ≤ 0.05) (Fig. 4A). PCNA, a marker of proliferation, was found to be decreased in the 24% SF and Western diet groups compared with all other groups (P ≤ 0.05) (Fig. 4B). The 12% SF group also trended (P = 0.09) to display a significantly lower amount of PCNA compared with the AIN-76A Mod Noncancer group.
Fig. 4.
Apoptosis and proliferation. Shown are representative Western blots of colonic cleaved caspase 3 (A) and proliferating cell nuclear antigen (PCNA, B) (n = 5–9/group). Groups not sharing a common letter differ significantly from one another (P ≤ 0.05).
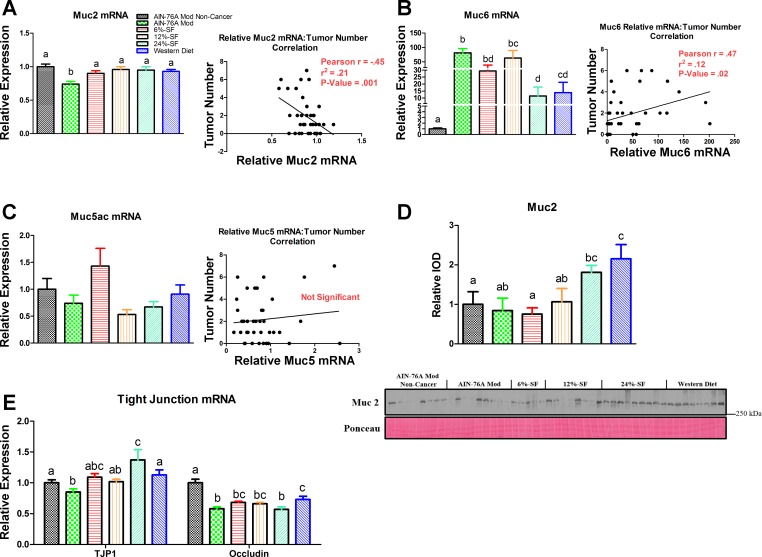
Dietary SF impacts colonic mucin and tight junction expression and Muc2 protein content.
Muc2 gene expression was found to be significantly lower in the AIN-76A Mod group compared with all other groups and was negatively associated with tumor burden (P ≤ 0.05) (Fig. 5A). Muc6 mRNA was increased in all cancer groups compared with the AIN-76A Mod Noncancer group, but the 24% SF and Western diet groups trended to (P < 0.1) or did display significantly less Muc6 gene expression compared with all other cancer groups (P ≤ 0.05) (Fig. 5B). Overall, Muc6 gene expression was positively associated with tumor number (P ≤ 0.05) (Fig. 5B). No differences in Muc5ac gene expression were found among any of the groups, nor was there any correlation with tumor number (Fig. 5C).
Fig. 5.
Gut barrier integrity. Colonic mRNA expression and correlation to tumor number of mucin (Muc) 2 (A), Muc6 (B), and Muc5ac (C) (n = 8–10/group), as well as representative Western blot of colonic Muc2 (D) (n = 5–9/group) and colonic mRNA expression of tight junction protein 1 (TJP1) and occludin (E) (n = 8–10/group). Groups not sharing a common letter differ significantly from one another (P ≤ 0.05).
Colonic Muc2 protein was found to be significantly increased in the Western diet group compared with all other groups except for the 24% SF group (P ≤ 0.05) (Fig. 5D). The 24% SF group also trended to (P < 0.1) or did significantly (P ≤ 0.05) display higher amounts of colonic Muc2 compared with the AIN-76A Mod and 6% SF and the AIN-76A Mod Noncancer groups, respectively.
With respect to gene expression of TJP1, the 24% SF group exhibited increased TJP1 mRNA content compared with all other groups except for the 6% SF group (P ≤ 0.05) (Fig. 5E). The AIN-76A Mod group, on the other hand, trended to, or did display, significantly less mRNA content compared with the 6% SF (P = 0.06), and the AIN-76A Mod Noncancer, 24% SF, and Western diet groups (P ≤ 0.05) (Fig. 5E). Occludin mRNA content was found to be decreased in all cancer groups compared with the AIN-76A Mod Noncancer group (P ≤ 0.05) (Fig. 5E). Among the cancer groups, the Western diet group displayed increased mRNA expression compared with both the AIN-76A Mod and 24% SF diet groups, whereas the 12% SF group trended to P = 0.07 compared with the AIN-76A Mod group.
HFDs increase adiposity but do not substantially produce a proinflammatory environment in epididymal adipose tissue nor produce insulin resistance.
Because there was no statistically significant difference in any body composition measurements (lean and fat mass and body fat percent) at the start of the study, across any of the groups, only terminal body composition data are presented in Fig. 6A. Lean mass of the AIN-76A Mod group was significantly lower compared with all other groups except compared with the 6% SF group (P ≤ 0.05). In terms of overall fat mass and body fat percent, the AIN-76A Mod group displayed the lowest values compared with all other groups, whereas all HFDs increased body fat levels relative to the AIN-76A Mod and AIN-76A Mod Noncancer groups (P ≤ 0.05). Additionally, among the HFD groups, the consumption of the Western diet produced the greatest accretion of fat mass than any other HFD (P ≤ 0.05). With regard to visceral fat accumulation, the AIN-76A Mod group displayed lower amounts of epididymal, mesentery, retroperitoneal, and total visceral adipose tissue compared with the AIN-76A Mod Noncancer group (P ≤ 0.05) (Fig. 6B). On the other hand, all HFD groups increased all visceral adipose tissue weights relative to the AIN-76A Mod Noncancer group (P ≤ 0.05).
Fig. 6.
Adipose tissue outcomes from experiment 1. Shown are body composition (A), visceral adipose tissue (B), adipose tissue inflammatory markers (C), glucose metabolism (E), and representative adipose tissue H&E images (×20) and F4/80 immunohistochemistry (D) (×40) of each treatment group (n = 10/group). Groups not sharing a common letter differ significantly from one another (P ≤ 0.05).
Overall, the adipose tissue of all HFD groups displayed minimal levels of inflammation as evidenced by gene expression analysis of inflammatory markers (Fig. 6C) and tissue staining (Fig. 6D). Specifically, with respect to gene expression of adipose tissue inflammatory markers, only the 6% SF diet increased TNF-α gene expression relative to the AIN-76A Mod and AIN-76A Mod Noncancer groups, whereas all HFDs increased its expression compared with the AIN-76A Mod group only (P ≤ 0.05) (Fig. 6C). Similarly, with respect to F4/80 gene expression, only the 6% SF increased F4/80 expression relative to the AIN-76A Mod and AIN-76A Mod Noncancer groups (P ≤ 0.05); however, no other differences in F4/80 gene expression among the groups were found. CD11c gene expression was upregulated with the consumption of all HFDs relative to the AIN-76A Mod and AIN-76A Mod Noncancer groups (P ≤ 0.05). Among the HFD groups, the 6% SF group displayed a significant increase in CD11c mRNA content relative to the 12% SF diet (P ≤ 0.05). Histologically, although minimal, there was some evidence of F4/80-positive crown-like structures in the adipose tissue of each of the HFD groups (Fig. 6D).
We chose to assess insulin resistance using the HOMA index for the AIN-76A Mod Noncancer and AIN-76A Mod groups as well as the group within the HFD groups that produced the greatest body weight and adiposity (the Western diet). No significant difference was found with respect to fasting blood glucose, insulin, or HOMA index between any of these three groups (Fig. 6E).
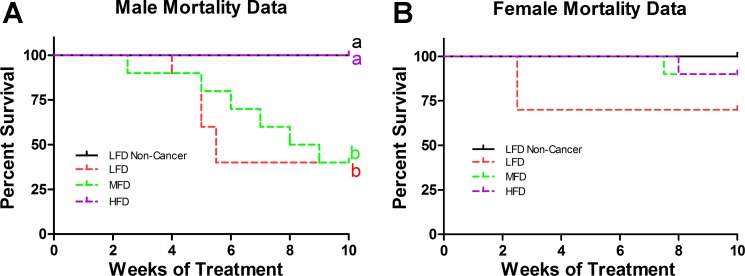
Lipid content of liquid diet influences mortality rate.
Male and female mortality data are presented in Fig. 7, A and B, respectively. There was no difference in liquid diet intake among any of the AOM/DSS-treated groups. For the males, both the LFD and MFD treatments resulted in a similar degree of mortality (60%) that was significantly greater than the HFD and LFD Noncancer treatments (0%) (P ≤ 0.05). With respect to the females, there was no statistically significant difference in mortality across any of the treatment groups. As in experiment 1, the general trend implicates a protective effect of the HFD on symptom score and tumor burden compared with the LFD and MFD groups. However, given that the majority (60%) of mice from each of the LFD and MFDs groups died, we do not have sufficient power to reach statistical significance. Furthermore, we believe that reporting any data on symptom scores or tumor burden would be misleading. It is likely that the surviving 40% of mice in the LFD and MFD groups are those with lower symptom scores and tumor burden, which would skew the results in favor of a reduced tumorigenesis in these groups, and thus the comparative benefits of the HFD treatment would likely be underestimated.
Fig. 7.
Mortality data of males (A) and females (B) from experiment 2 (n = 10/group). Groups not sharing a common letter differ significantly from one another (P ≤ 0.05).
DISCUSSION
Both epidemiological and animal research suggests that consumption of a HFD is positively associated with colon cancer risk (1, 22). However, the factors linking HFD and colon cancer are still poorly understood. Given this, through the employment of the commonly used AOM/DSS colon cancer model (9), we sought to determine if HFD does, in fact, influence colon cancer development, and whether or not the lipid composition of a HFD has any effect on prooncogenic processes.
Surprisingly, in our initial experiment we found no effect for any one of four HFDs to promote tumorigenesis more so than a low-fat control diet (AIN-76A Mod). In fact, consumption of all HFDs produced a significantly higher colon length-to-width ratio, and the two HFDs composed of the highest amount of SFAs (24% SF and Western diet) led to significantly less tumor burdens than the AIN-76A Mod. To substantiate this outcome, we performed a second experiment using the AOM/DSS model in which we provided mice with a liquid diet comprised of varying levels (0, 18, and 36 g/l) of the SFA-rich coconut oil as the primary fat source. In agreement with our first experiment, the liquid diet with the greatest amount of SFAs produced significantly less mortality (0% mortality) than the two diets comprised of less SFAs (60% mortality each) within male mice.
Previous work examining the effect of HFD consumption on colon cancer progression using the AOM/DSS model is scarce and conflicting. A study performed by Park et al. found that HFD (45% kcal from fat)-induced obesity in male A/J mice facilitated tumor development (34). Contrary to this and consistent with our findings, Boddicker et al. showed that a high-fat lard-based diet (60% kcal from fat) significantly decreased total colonic lesions compared with a LFD in male C57BL/6 mice (5). Of further interest is the study of Laroui et al., whose findings suggest that DSS is responsible for colitis development by forming “nanolipocomplexes” with MCFAs in the colon (27). Other studies have also shown that DSS treatment paired with HFD consumption produces a more aggressive form of colitis than DSS coupled with a LFD (17, 29, 40, 41). The data from our study contradict the findings of Laroui et al., since we show, in two separate experiments, that the diets with the greatest content of SFAs, MCFAs in particular, result in the smallest tumor burden and lowest mortality rate.
The discrepancies in the results between Laroui et al. and our experiments may be explained by the fact that we used DSS in combination with AOM instead of DSS alone. However, we believe if FAs and DSS do form complexes with one another, it is unlikely that the administration of AOM would influence this interaction. The inconsistencies between studies may also be explained by other factors such as the use of different diets, differences in manufacturer, lot, and molecular weight of the DSS, duration of DSS exposure, mouse strain and sex, as well as dissimilar gut microbiomes of the animals used in each study. In fact, because of the finicky nature of the DSS model, a review article has been written describing the model's “Traps and Tricks” (36).
Interestingly, we found that the tumor burden data were negatively associated with the SFA content of the diets (1.7, 6, 12, 24, and 26% of total caloric intake for the AIN-76A Mod, 6% SF, 12% SF, 24% SF, and Western diet, respectively). To attempt to explain this outcome, we examined the gene expression of several inflammatory mediators, the protein content of several well-characterized proteins known to impact carcinogenesis, as well as markers of apoptosis and proliferation.
Similar to exhibiting a lower tumor number, the Western diet displayed decreased STAT3 activation and lower IL-6 and TNF-α mRNA expression compared with all other groups. Additionally, the Western diet group did not significantly result in increased p38 activation as exhibited by the AIN-76A Mod, 6% SF, and 12% SF groups. The IL-6-STAT3 pathway has been shown to be a key regulator of colon cancer development (46), and it has recently been shown that p38 is required for cancer cell survival (20), and can serve as an activator of STAT3 (21). Thus, it is not surprising that the decreased p38 activity was associated with less STAT3 activation and proliferation in the Western diet group. Similarly, the 24% SF diet also displayed less p38 activation and exhibited less proliferation. A surprising finding was that the Western diet exhibited increased apoptosis compared with all other groups. A plausible explanation for this outcome may be explained by the greater content of butyric acid (C4:0) in the Western diet, which was not found in any other diet used in this study. Butyric acid is thought to be an anticarcinogenic agent, largely because of its ability to induce apoptosis (18). Although NF-κB and ERK signaling has been implicated as playing a significant role in carcinogenesis (16, 48), we found the activation of these proteins to be minimally changed or unchanged across groups at the particular time of death in our study. While collectively all of these factors may have resulted in the decreased tumor burden in the 24% SF and Western diet groups, we sought to explore potential upstream mechanisms that may have contributed to these effects.
It has recently been shown in both in vivo and in vitro models that SFAs can increase the production of Muc2, leading to a strengthening of the intestinal barrier (3). Thus, we examined colonic Muc2 gene expression and protein content and found that only the AIN-76A Mod group significantly exhibited decreased Muc2 mRNA expression, and the diets with the highest amount of SFAs (24% SF and Western diet) either trended to P < 0.1 or did result in significant increases in Muc2. Muc2, produced by goblet cells, is critical for maintaining the protective mucosal layer overlaying the intestines. Our data support this, since we found a negative correlation between Muc2 mRNA content and tumor number. This mucosal layer prevents the influx of various substances, including antigens, carcinogens, and other foreign molecules, in the lumen of the intestines. In fact, mice deficient in Muc2 spontaneously develop colorectal tumors (42, 44). Additionally, in humans, modulations to Muc2 may influence cancer development, since loss of Muc2 expression is associated with colon carcinogenesis (32). It should be pointed out that both the consumption of the 24% SF and Western diets increased colonic Muc2 protein content more so than the AIN-76A Mod Noncancer group, which was not different from the AIN-76A Mod, suggesting that the consumption of this amount of SFAs was able to increase colonic Muc2 independent of cancer. Thus, it is very plausible that the elevated Muc2 production observed following consumption of the 24% SF and Western diets elicited an increase in the intestinal mucosal layer, ultimately leading to protection against colon carcinogenesis in the AOM/DSS model. However, given the complexity of the gut, it is possible that other factors may also be playing a role in this process.
To further examine the influence of dietary composition on various secreted mucins, we examined mRNA expression of Muc6 and Muc5ac. Muc6 has been shown to be upregulated in human colorectal carcinomas (35). In support of this, we found colonic Muc6 expression to be positively correlated with tumorigenesis. Furthermore, we found up to a 200-fold increase in its expression in some of the AOM/DSS-treated mice, suggesting that this mucin's expression is dramatically upregulated, more so than other genes examined, in this particular model of colon cancer. Muc5ac has also been linked to colon carcinogenesis (47). However, we found no correlation between its expression and tumor number in our model.
Because tight junction barrier function has been linked to cancer metastasis, we examined the mRNA expression of two tight junction proteins, TJP1 and occludin (30). In both genes analyzed, we found lower expression in the AIN-76A Mod group compared with both the 24% SF and Western diets (TJP1), or the Western diet alone (occludin), suggesting that tight junction integrity may have been more compromised in the AIN-76A Mod group (the group with the most tumors) compared with the HFDs with the highest SF content (groups with the least tumor burden). Although more research is needed to determine the impact that differing dietary FA species has on tight junction integrity, it has been shown that dietary SFs can maintain gut-barrier integrity better than unsaturated fatty acids (26).
Based off of our initial findings from experiment 1, we performed a second experiment to determine if the SFA content of the diet was influencing tumorigenesis. When initiating experiment 2 with the liquid diets, the intention was to perform symptom score, tumor burden, colon inflammatory analyses, etc., similar to what was performed in experiment 1. However, we unexpectedly found that the combination of the liquid LFD and MFDs was extremely deadly in male C57BL/6 mice (60% mortality) (likely because of the fact that, compared with solid food, the liquid-based meal was more readily absorbed and digested, which enhanced the efficacy of the DSS to induce its deleterious effects). We believe that reporting data other than mortality would be misleading, since it would be difficult to interpret the results given that so many mice had died from each of these groups. Nonetheless, we believe the dramatic difference in mortality rate between the LFD (0.6% of total kcal from SF) and MFD (15% of total kcal from SF) vs. the HFD (28% of total kcal from SF) is convincing enough to suggest that a diet composed of more SFAs is protective against mortality in the AOM/DSS model. Our results further suggest that high levels of dietary SF, ≥24% of total calories from SF, are necessary to induce significant changes in Muc2 protein levels and protect against carcinogenesis. In experiment 1, neither the 6% SF nor 12% SF diets resulted in any significant changes in Muc2 content nor decreased tumorigenesis compared with the AIN-76A Mod group, and, in experiment 2, only the HFD composed of 28% of total kilocalories from SF minimized the mortality rate.
Because Laroui et al. performed their experiment using female C57BL/6 mice (27), we decided to include female C57BL/6 in experiment 2 to see if sex influenced mortality. We found that this was the case, since there was no significant difference in mortality among any of the female treatment groups. Interestingly, this finding was consistent with that of Boddicker et al. who also showed that male mice consuming a HFD displayed less total colon lesions than LFD-fed male mice after AOM/DSS administration, but the same was not true within female mice (5). Thus, it seems that there does exist a sex effect with respect to AOM/DSS and diet interaction and its influence on mortality, which may result from differences in sex hormones (2).
It should be noted that our results suggest that the AOM/DSS model may not be an ideal model for examining the effect of HFD-induced obesity on colon cancer progression, since we believe that this model does not appropriately allow for the development of an obese phenotype. Despite the fact that each of the HFDs increased fat mass, body fat percentage, and total visceral fat mass compared with the AIN-76A Mod Noncancer and AIN-76A Mod diets, minimal adipose tissue inflammation was detected, and there was no evidence of insulin resistance for the group with the highest adiposity (Western diet), two characteristics not only commonly linked to obesity but also believed to be the driving force behind the increased cancer risk associated with obesity (28). Ideally, an obese phenotype would be developed before cancer induction. However, this is problematic with the AOM/DSS model, since HFD-induced obesity has been shown to decrease water intake (the vehicle for DSS) in diet-induced obese mice compared with LFD mice (24). Additionally, the appropriate dose of AOM to administer is of concern, since AOM has been shown to dose dependently influence tumorigenesis (4). Usually AOM is given per kilogram of body weight, however, because obese mice weigh significantly more (≈20 grams) than their LFD-fed control counterparts, the question that arises is whether to administer AOM per body weight or at an equal dose across all groups. Additionally, the gene expression of the metabolic activator of AOM, cytochrome P-450 CYP2E, which resides in the liver and activates AOM before its delivery to the colon, has been shown to be modulated in an obese setting (6, 8, 23), further bringing into question the reliability of the model.
Although epidemiological literature suggests a potential link between SF-rich animal lard consumption and colon cancer risk, this association is not very sound (43). This is largely due to the fact that other factors such as the means of processing and cooking meat, particularly red meat, have also been shown to be potential risk factors for colon cancer development (11, 25, 38). Thus, as with any nonmechanistic investigations, determining whether the potential increased colon cancer risk is due to dietary fat content or the means by which fat-rich foods are processed and cooked is not possible. Thus, it is evident that more studies are needed to better understand the impact that different species of FAs, SFAs in particular, have on colon cancer initiation and development.
In conclusion, the combined results from our experiments suggest that high dietary SF content is protective against colon carcinogenesis induced by the AOM/DSS model, which was associated with reduced inflammation, an increase in apoptosis, and a decrease in proliferation. This may be regulated, at least in part, through the ability of SF to increase Muc2 production. Future studies using various controlled diets are necessary to determine if these findings are substantiated in other models of colon cancer.
GRANTS
This work was supported by grants from the National Cancer Institute (R21-CA-167058, R21-CA-175636, and K01-AT-007824 to E. A. Murphy) and the University of South Carolina (Advanced Support Programs for Innovative Research Excellence to E. A. Murphy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.T.E., K.T.V., J.L.M., T.L.C., M.N., P.S.N., J.M.D., and E.A.M. conception and design of research; R.T.E., K.T.V., J.L.M., and T.L.C. performed experiments; R.T.E. analyzed data; R.T.E., K.T.V., J.L.M., T.L.C., and E.A.M. interpreted results of experiments; R.T.E. prepared figures; R.T.E. and E.A.M. drafted manuscript; R.T.E., K.T.V., J.L.M., T.L.C., M.N., P.S.N., J.M.D., and E.A.M. edited and revised manuscript; R.T.E., K.T.V., J.L.M., T.L.C., M.N., P.S.N., J.M.D., and E.A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kei Lam, Mike Gore, and Joey Farrow of the University of South Carolina School of Medicine Machine Shop for technical support and Dr. Jaime Lecker (BioServ) for help with diet creation.
REFERENCES
- 1.Aleksandrova K, Nimptsch K, Pischon T. Obesity and colorectal cancer. Front Biosci (Elite Ed) 5: 61–77, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: estrogen pathway in colorectal cancer. Clin Cancer Res 19: 5842–5848, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit B, Bruno J, Kayal F, Estienne M, Debard C, Ducroc R, Plaisancie P. Saturated and unsaturated fatty acids differently modulate colonic goblet cells in vitro and in rat pups. J Nutr 145: 1754–1762, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Bissahoyo A, Pearsall RS, Hanlon K, Amann V, Hicks D, Godfrey VL, Threadgill DW. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice: effects of dose, route, and diet. Toxicol Sci 88: 340–345, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Boddicker RL, Whitley E, Birt DF, Spurlock ME. Early lesion formation in colorectal carcinogenesis is associated with adiponectin status whereas neoplastic lesions are associated with diet and sex in C57BL/6J mice. Nutr Cancer 63: 1297–1306, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bu SY, Kwon H, Sung MK. Supplementation of seaweeds extracts suppresses azoxymethane-induced aberrant DNA methylation in colon and liver of ICR mice. J Cancer Prev 19: 216–223, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carson JA, Lee WJ, McClung J, Hand GA. Steroid receptor concentration in aged rat hindlimb muscle: effect of anabolic steroid administration. J Appl Physiol 93: 242–250, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther 8: 1313–1317, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin 28: 1450–1459, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993. [PubMed] [Google Scholar]
- 11.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res 63: 2358–2360, 2003. [PubMed] [Google Scholar]
- 12.Day SD, Enos RT, McClellan JL, Steiner JL, Velazquez KT, Murphy EA. Linking inflammation to tumorigenesis in a mouse model of high-fat-diet-enhanced colon cancer. Cytokine 64: 454–462, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA, Murphy EA. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res 54: 152–163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enos RT, Velazquez KT, McClellan JL, Cranford TL, Walla MD, Murphy EA. Lowering the dietary omega-6: omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model. Nutr Res 35: 449–459, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enos RT, Velazquez KT, Murphy EA. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J Nutr Biochem 25: 600–612, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol 6: 322–327, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gabele E, Dostert K, Hofmann C, Wiest R, Scholmerich J, Hellerbrand C, Obermeier F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol 55: 1391–1399, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab 14: 994–1008, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Grotto D, Zied E. The Standard American Diet and its relationship to the health status of Americans. Nutr Clin Pract 25: 603–612, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Gupta J, del Barco Barrantes I, Igea A, Sakellariou S, Pateras IS, Gorgoulis VG, Nebreda AR. Dual function of p38alpha MAPK in colon cancer: suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 25: 484–500, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Gupta J, Nebreda AR. Roles of p38alpha mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J 282: 1841–1857, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 22: 191–197, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irizar A, Barnett CR, Flatt PR, Ioannides C. Defective expression of cytochrome P450 proteins in the liver of the genetically obese Zucker rat. Eur J Pharmacol 293: 385–393, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AW. Dietary manipulations influence sucrose acceptance in diet induced obese mice. Appetite 58: 215–221, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Joosen AM, Kuhnle GG, Aspinall SM, Barrow TM, Lecommandeur E, Azqueta A, Collins AR, Bingham SA. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis 30: 1402–1407, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res 36: 835–846, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One 7: e32084, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louie SM, Roberts LS, Nomura DK. Mechanisms linking obesity and cancer. Biochim Biophys Acta 1831: 1499–1508, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma X, Torbenson M, Hamad AR, Soloski MJ, Li Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol 151: 130–138, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta 1788: 872–891, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 32.Mizoshita T, Tsukamoto T, Inada KI, Hirano N, Tajika M, Nakamura T, Ban H, Tatematsu M. Loss of MUC2 expression correlates with progression along the adenoma-carcinoma sequence pathway as well as de novo carcinogenesis in the colon. Histol Histopathol 22: 251–260, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. J Am Med Assoc 311: 806–814, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SY, Kim JS, Seo YR, Sung MK. Effects of diet-induced obesity on colitis-associated colon tumor formation in A/J mice. Int J Obes (Lond) 36: 273–280, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Percinel S, Savas B, Ensari A, Kuzu I, Kuzu MA, Bektas M, Cetinkaya H, Kursun N. Mucins in the colorectal neoplastic spectrum with reference to conventional and serrated adenomas. Turk J Gastroenterol 18: 230–238, 2007. [PubMed] [Google Scholar]
- 36.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol 2012: 718617, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer 60: 131–144, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 233: 674–688, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira LG, Leonel AJ, Aguilar EC, Batista NV, Alves AC, Coimbra CC, Ferreira AV, de Faria AM, Cara DC, Alvarez Leite JI. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis 10: 204, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Logt EM, Blokzijl T, van der Meer R, Faber KN, Dijkstra G. Westernized high-fat diet accelerates weight loss in dextran sulfate sodium-induced colitis in mice, which is further aggravated by supplementation of heme. J Nutr Biochem 24: 1159–1165, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract 27: 613–623, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295: 1726–1729, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Viennois E, Chen F, Laroui H, Baker MT, Merlin D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes 6: 360, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldner MJ, Foersch S, Neurath MF. Interleukin-6–a key regulator of colorectal cancer development. Int J Biol Sci 8: 1248–1253, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh MD, Clendenning M, Williamson E, Pearson SA, Walters RJ, Nagler B, Packenas D, Win AK, Hopper JL, Jenkins MA, Haydon AM, Rosty C, English DR, Giles GG, McGuckin MA, Young JP, Buchanan DD. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod Pathol 26: 1642–1656, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol 6: 327–334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willett WC. Diet and cancer: an evolving picture. J Am Med Assoc 293: 233–234, 2005. [DOI] [PubMed] [Google Scholar]