Abstract
Gastrointestinal dysmotility in systemic sclerosis (SSc) is associated with autoantibodies against muscarinic-3 receptor (M3-R). We investigated the temporal course of the site of action of these autoantibodies at the myenteric neurons (MN) vs. the smooth muscle (SM) M3-R in relation to disease duration, and determined the role of intravenous immunoglobulin (IVIG) in reversing these changes. Immunoglobulins purified from SSc patients (SScIgG) were used to assess their differential binding to MN and SM (from rat colon) employing immunohistochemistry (IHC). Effect of SScIgG on neural and direct muscle contraction was determined by cholinergic nerve stimulation and bethanechol-induced SM contraction. Effects of IVIG and its antigen-binding fragment F(ab′)2 on SScIgG binding were studied by enzyme-linked immunosorbent assay (ELISA) of rat colonic longitudinal SM myenteric plexus (LSMMP) lysate and to second extracellular loop peptide of M3-R (M3-RL2). SScIgG from all patients demonstrated significantly higher binding to MN than to SM. With progression of SSc duration, binding at MN and SM increased in a linear fashion with a correlation coefficient of 0.696 and 0.726, respectively (P < 0.05). SScIgG-mediated attenuation of neural and direct SM contraction also increased with disease duration. ELISA analysis revealed that IVIG and F(ab′)2 significantly reduced SScIgG binding to LSMMP lysate and M3-RL2. Dysmotility in SSc occurs sequentially, beginning with SScIgG-induced blockage of cholinergic neurotransmission (neuropathy), which progresses to inhibition of acetylcholine action at the SM cell (myopathy). IVIG reverses this cholinergic dysfunction at the neural and myogenic receptors by anti-idiotypic neutralization of SScIgG.
Keywords: scleroderma autoantibodies, muscarinic receptor, smooth muscle, myenteric neuron
systemic sclerosis (SSc) is a systemic autoimmune disease characterized by skin and internal organ fibrosis, vasculopathy, and immune dysregulation. Among the target organs affected by SSc, the gastrointestinal tract (GIT) is the most commonly affected internal organ. While dysmotility accounts for the vast majority of SSc-associated GIT symptoms, its pathogenesis is poorly understood (22, 26).
Recent advances in SSc pathogenesis have implicated immune dysregulation, vascular dysfunction, and fibrosis as the unifying mechanism of internal organ involvement (10). Among other factors, the lack of appropriate animal models reproducing gastrointestinal manifestations of SSc has limited our understanding of the pathophysiological mechanism of dysmotility and has also hampered the development of new therapies (28).
Humoral immunity dysregulation has been recognized to play an important role in SSc pathogenesis. However, despite the fact that autoantibodies are present in more than 95% of patients with SSc, they were traditionally considered to be nonpathogenic. It is now hypothesized that anti-endothelial, anti-fibroblast, anti-MMP, and anti-fibrillin antibodies may be directly pathogenic in SSc (17). It has recently been demonstrated that IgG isolated from sera of SSc patients targets vascular smooth muscle cells and may be responsible for pulmonary hypertension (4). Similarly, research in the last decade has shown that gastrointestinal dysmotility in SSc may in part be related to functional autoantibodies (8, 11).
Earlier studies from our laboratory have demonstrated that gastrointestinal dysmotility in SSc is associated with circulating autoantibodies against the muscarinic-3 receptor (M3-R) (24, 25). These autoantibodies inhibited the contraction of smooth muscle cells (SMC) directly stimulated with a cholinergic agent and also blocked indirect muscle response induced by electric field neural stimulation suggesting cholinergic blockade by M3-R inactivation at neural and muscular levels. Of significant interest, the neural and myogenic effects of these autoantibodies were reproducibly abrogated by intravenous immunoglobulin (IVIG) strongly suggesting that the antibody could be removed from the receptor or could be neutralized in vitro (24, 25).
None of the earlier studies, however, examined the temporal sequence of neurogenic or myogenic site involvement, or investigated whether this involvement correlates with duration or severity of gastrointestinal SSc. Although treatment with IVIG has been studied in tight skin mouse models and in patients with cutaneous manifestations of SSc (21, 28), there are no data to indicate whether IVIG would be able to restore gastrointestinal dysfunction in SSc patients at different stages of the disease.
In this study, we tested the hypothesis that IgG from sclerodoma patients (SScIgG) initially leads to neuropathy via inhibition of M3-R at the myenteric cholinergic neurons (MCN) which progresses to myopathy by inhibition of M3-R at the gastrointestinal SMC in the advanced stages of SSc.
The aims of the present study were 1) to investigate the effect of SScIgG at different stages of SSc (defined by duration of disease) by comparison of their sites of action at the MCN vs. the SMC; and 2) to determine the role of IVIG in reversing SSc gastrointestinal manifestations, and identify its mechanism of action.
MATERIALS AND METHODS
Subjects
Ten patients meeting the 2013 American College of Rheumatology criteria for the classification of SSc were selected retrospectively (29). Patients were included in the study if they had documented gastrointestinal symptoms attributable to SSc (determined by the UCLA SCTC GIT 2.0) along with typical esophageal manometric abnormalities. Patients on immunosuppressive and disease modifying drugs were excluded from the study. Patients were divided into two groups based on duration of SSc (skin manifestation was used to define the disease onset date). Patients with disease duration ≤ 15 years (180 mo) were placed in group I, while those in group II had a disease duration ≥ 16 years (192 mo).
Medical records for all participants were obtained to verify the diagnosis and to characterize the disease. We collected the following information about SSc patients: date of first diagnosis, extent of skin involvement (limited vs. diffuse), medication regimen, SSc specific autoantibody profile, gastrointestinal symptoms, esophageal manometry findings and UCLA SCTC GIT 2.0 score (18). The study was approved by the University's Institutional Review Board.
Isolation and Purification of IgGs from SSc Patients and Normal Volunteers
Written informed consent was obtained for drawing blood samples. Total IgGs were purified from plasma of the 10 SSc patients (SScIgGs) and 2 normal volunteers (NIgGs) by the use of biocompatible polypropylene columns packed with 5 ml of protein G-Sepharose from genetically modified protein G lacking albumin affinity and with high binding capacity for human IgG (HI-trap protein G HP-GE Healthcare, Pittsburgh, PA) (13).
Experiments Using Intact Rat Colon Smooth Muscle (SM) Strips
Colonic smooth muscle strip preparations and isometric tension recording.
Male Sprague-Dawley rats (300–350 g) were euthanized by decapitation, and the lower portion of the colon was removed surgically and transferred to oxygenated (95% O2 + 5% CO2) Krebs physiological solution (KPS) at 37°C. The composition of KPS (in mmol/l) was as follows: 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.1 glucose. The SM strips (∼0.5 mm thick and 7 mm long) were prepared from the circular SM layer of the colon.
The SM strips were then transferred to 2-ml organ baths containing oxygenated KPS. Isometric tension was monitored by use of force transducers (FORT10, WPI, Sarasota, FL) and Chart 4.1.2 via PowerLab/8SP data-acquisition system (AD Instruments, Colorado Springs, CO). Each SM strip was initially stretched to a tension of 1.0 g followed by 90 min of equilibration. The basal tone in each SM strip was determined at the end of the experiment by the administration of Ca2+-free (0Ca2+) KPS. The experimental protocol of the study was approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University in accordance with the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
Direct muscle stimulation with bethanechol (BeCh).
The responses of the colonic SM strips to BeCh (10−7 to 10−3 M) were quantified before and after NIgG (1 mg/ml), SScIgG (1 mg/ml), IVIG (10 mg/ml), and IVIG (10 mg/ml) + SScIgG (1 mg/ml) after incubation in the muscle bath for 15 min. To determine the selectivity of the action of SScIgG on M3-R inactivation, we compared the effects of K+ depolarization by KCl (2.5 to 40 mM). These contractile responses were calculated and plotted as percentage of maximal contraction by 10−4 M BeCh.
Cholinergic nerve stimulation experiments.
To determine the effects of cholinergic nerve stimulation, electrical field stimulation (EFS; 10 V, 0.5–20 Hz, 4-s train, each pulse of 0.5 ms) was delivered using a Grass stimulator (model S88; Grass Instruments, Co, Quincy, MA). The EFS responses (percentage maximal increase in the basal activity) were quantified before and after NIgG (1 mg/ml), SScIgG (1 mg/ml), IVIG (10 mg/ml), and IVIG (10 mg/ml) + SScIgG (1 mg/ml).
Acetylcholine (ACh) measurements.
To measure the release of ACh in response to EFS, we determined the effect of 30-s train of EFS (10 V, 5 Hz, each pulse of 0.5-ms duration). The muscle bath perfusates were collected in the basal state, and before and after NIgG (1 mg/ml), SScIgG (1 mg/ml), IVIG (10 mg/ml), and IVIG (10 mg/ml) + SScIgG (1 mg/ml). ACh measurements were made using the choline/ACh quantification kit (BioVision, Milpitas, CA) following the manufacturer's instructions. Data were quantified using fluorometric (Fluorometer Optima Micropipette Reader; BMG Labtech, Ortenberg, Germany) analysis with MARS software (Cary, NC).
Immunohistochemistry (IHC) of Rat Colon
Whole mount and sections.
Rats were euthanized by decapitation and colon was removed immediately and cleaned with 4% paraformaldehyde and kept in it overnight. Paraformaldehyde was then replaced with 70% ethanol and tissues were sent for embedding and sectioning to Thomas Jefferson University Histopathology Core facility. Sections were deparaffinized by keeping them for 30 min at 70°C and washed two times (10 min each wash) in xylene. Sections were hydrated in 2 changes of 100% ethanol for 5 min each, followed by 2 changes of 95% ethanol, 1 change of 80% ethanol, and 2 changes of 70% ethanol for 3 min each. Sections were then rinsed in 2 changes of distilled water for 5 min each. Antigen retrieval was done in Tris-buffered saline containing Tween-20 (0.05 M TBS, 0.05% Tween-20, pH 9.0) in a pressure cooker for 30 min. Slides were washed twice with distilled water and stained with cuprolinic blue for 30 min (15). Slides were again washed with distilled water twice and blocked with donkey serum in PBST for 1 h. SScIgGs and Anti-M3-R against rat were added on the sections in PBST and kept in humidified chamber at 4°C for overnight incubation. Then, the slides were washed with PBST and stained with anti-human IgG and Anti-goat IgG in PBST buffer and incubated at room temperature (RT) for 1 h and washed three times with PBST and fixed with Vectashield mounting media (Burlingame, CA) and viewed under confocal microscope (Carl Zeiss, Germany) as well as an Evos fluorescent microscope (Life technologies), and photographs were taken and analyzed with ImageJ2 (NIH).
Immunofluorescence intensity calculation.
Immunofluorescence intensity (IFI) was calculated by using ImageJ2 (NIH). Areas of interest from the images were selected corresponding to the myenteric plexus and smooth muscle, and intensity surface plots were plotted and intensity per unit area was calculated by dividing total intensity by total area selected. For colocalization studies Pearson coefficient of colocalization was calculated by using Col2 plugins and plotted as bar graph in prism software.
Isolation of longitudinal smooth muscle-myenteric plexus (LSMMP) layer.
Rat colon was removed proximal to the sigmoid colon and divided into 3- to 4-cm segments. The lumen of these segments was flushed with KPS. These segments were then stretched over a glass rod and the mesentery carefully removed. The longitudinal muscle layer with adherent myenteric plexus was separated from the underlying circular muscle layer by gently stroking tangentially away from the mesenteric attachment with a cotton-tipped swab and peeling away the mucosal, submucosal and circular SM layers (2). LSMMP was used for IHC, and its lysates prepared under fresh conditions were used for ELISA.
Enzyme-linked immunosorbent assay (ELISA).
To determine the binding affinity of SScIgG to the M3-R we performed ELISA studies with M3-RL2 synthetic peptide and LSMMP lysate; 400 μg/ml rat colonic LSMMP lysate and 400 pg/ml of a peptide corresponding to the M3-RL2 (KRTVPPGECFIQFLSEPTITFGTAI, amino acids 213–237) were separately dissolved in carbonate buffer and adsorbed onto separate multiwell plates used for ELISA. Between 5 and 40 nM each of SScIgG, IVIG, antigen binding fragment of IVIG [F(ab′)2] and SScIgG + IVIG were added to these multiwell plates and incubated at 37°C for 1 h. Plates were washed three times with DPBST and incubated for 1 h at RT with anti-human-HRP-conjugated secondary antibodies for SScIgG, or IVIG, washed three times with DPBST, and 100 μl of TMB substrate was added and kept for 15 min at RT. Then, 100 μl of Stop solution was added to each well, and absorbance was recorded at 450 nm with ELISA reader.
Drugs and Chemicals
Bethanechol, KCl, and M3-R antibody were purchased from Sigma Aldrich (St. Louis, MO). M3-R loop-2 (M3-RL2) was purchased from Peptide 2.0 (Chantilly, VA). IVIG and its pepsin-derived antigen-binding fragment F(ab′)2 were prepared as previously described (16) and were obtained from CSL Behring (King of Prussia, PA).
Statistical Analysis
Data are presented as means ± SE of multiple experiments. P values < 0.05 were considered statistically significant. The concentration-response curves were fitted by nonlinear regression, and comparisons were made using unpaired Student's t-test, or ANOVA, using the computer software Prism (GraphPad Software, San Diego, CA). Pearson's coefficient was determined to assess correlation between binding and disease duration.
RESULTS
Characteristics of Study Participants
All patients were females aged between 38 and 73 yr (Table 1). Among all patients, 6 had diffuse cutaneous involvement, and 4 had limited disease. The mean ± SD disease duration of patients in group I was 141.6 ± 35.4 mo, while that of patients in group II was 290.4 ± 95.4 mo. Their gastrointestinal symptom score as determined by the UCLA SCTC GIT 2.0 questionnaire ranged from 0.75 to 15.1. No significant correlation was found among various SSc specific autoantibodies and gastrointestinal disease.
Table 1.
Baseline characteristics of patients
| Patient | Age, yr | Disease Duration, mo | SSc Subtype | UCLA GIT 2.0 |
|---|---|---|---|---|
| 1 | 71 | 108 | Limited | 3.47 |
| 2 | 73 | 120 | Limited | 2.3 |
| 3 | 55 | 120 | Limited | 1.85 |
| 4 | 38 | 180 | Diffuse | 2.74 |
| 5 | 64 | 180 | Diffuse | 2.04 |
| 6 | 49 | 192 | Diffuse | 0.75 |
| 7 | 62 | 240 | Limited | 2.25 |
| 8 | 61 | 240 | Diffuse | 15.1 |
| 9 | 67 | 360 | Diffuse | 2.49 |
| 10 | 64 | 420 | Diffuse | 4.72 |
Binding of SScIgG to Myenteric Plexus (MP) and Smooth Muscle (SM)
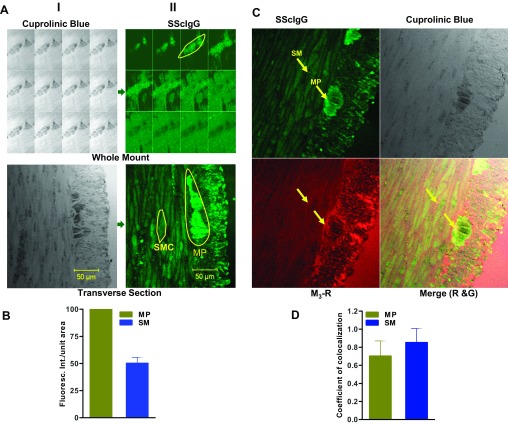
Myenteric plexus in whole mount rat colonic sections was identified by selective staining with neuronal dye cuprolinic blue. SScIgG from all ten patients demonstrated positive staining with rat colonic myenteric plexus and smooth muscle (Fig. 1A). Immunofluorescence intensity (IFI) analysis revealed that the binding intensity of SScIgG to myenteric plexus was significantly higher (IFI of SScIgG bound to MP was considered as 100%) in contrast to smooth muscle immunofluorescence intensity, which was <50% (Fig. 1B; P < 0.05; n = 3). These data reveal that SScIgG binds at two sites in the rat colon: myenteric plexus and the smooth muscle, but with higher binding intensity to the former.
Fig. 1.
A: (i) Identification of myenteric neuronal plexus (MP) by cuprolinic blue staining on whole mount z-stack and transverse section (ii) staining of MP and smooth muscle(SM) by SScIgG; Anti human FITC-conjugated-antibody was used against SScIgG, which gives green florescence. B: bar graph of IFI showing that the binding intensity of SScIgG to MP is significantly higher compared with SM (P < 0.05; n = 3). C: immunohistochemical colocalization of SScIgG (FITC-conjugated; green) and M3-R (TR-conjugated; red). Arrows indicate colocalization of both probes on SM and MP (P < 0.05; n = 3). D: bar graph data show significant coefficient of colocalization of SScIgG with the M3-R at SM and MP (P < 0.05; n = 3).
Colocalization of SScIgG and M3-R on MP and SM
Data show that SScIgG binds specifically to the M3-R on rat colonic SM and myenteric plexus (Fig. 1C). IFI analysis showed a significant colocalization of M3-R and SScIgG binding at the colonic myenteric plexus and smooth muscle (correlation coefficient 0.70 and 0.85, respectively) (Fig. 1D; P < 0.05; n = 3). This binding of SScIgG at the same position corresponding with M3-R antibody binding suggests that SScIgG binds at the M3-R on both the smooth muscle and myenteric plexus.
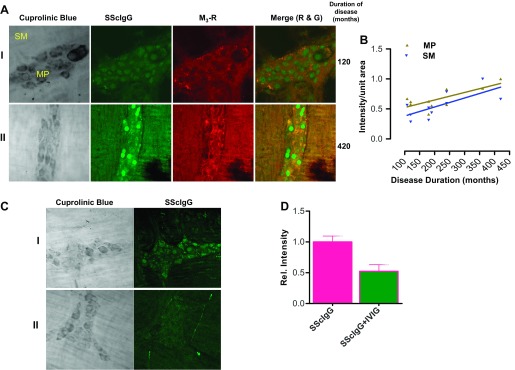
Disease Duration-Dependent Binding of SScIgG at MP and SM
Data reveal that IgG from a patient in group I (disease duration: 120 mo) had significantly higher binding at myenteric plexus compared with binding at the smooth muscle (IFI 0.36 vs. 0.27, respectively) (Fig. 2A; P < 0. 05; n = 3). In contrast, IgG from a patient in group II (disease duration: 420 mo) revealed significantly higher binding at both the myenteric plexus and smooth muscle (IFI 0.53 vs. 0.50, respectively), with the binding intensity at the latter almost approaching the binding intensity at the myenteric plexus (Fig. 2A; P < 0.05; n = 3).
Fig. 2.
A: (i) SScIgG from a patient with early disease duration (120 mo) had significantly higher binding at the MP compared with the SM (P < 0.05; n = 3). (ii) SScIgG from a patient with advanced SSc duration (420 mo) shows progression of binding intensity at the MP and SM (P < 0.05; n = 3). B: correlation between SScIgG binding intensity at SM and MP with disease duration shows that binding intensity increases in linear fashion with disease duration (P < 0.05). C: SScIgG binds to the colonic smooth muscle and myenteric plexus (i). This binding is significantly attenuated by pretreatment of SScIgG with IVIG (ii) (P < 0.05; n = 3). D: bar graph showing the relative IFI of SScIgG binding and its attenuation by IVIG (P < 0.05; n = 3).
The IFI of SScIgG binding, at myenteric plexus and smooth muscle from all patients when plotted against duration of disease, revealed a strong positive correlation coefficient of R = 0.7, P < 0.05 and R = 0.8, P < 0.05, respectively (Fig. 2B). Data suggest that binding of SScIgG at myenteric plexus and smooth muscle increases with the duration of disease in a linear fashion accounting for the progressive nature of the disease.
Effect of IVIG on SScIgG Binding
IVIG reversed the binding of SScIgG at both the myenteric plexus and smooth muscle as evidenced by the decrease in IFI (Fig. 2, C and D; P < 0.05; n = 3). This reversal effect was consistently observed with SScIgG from all ten patients.
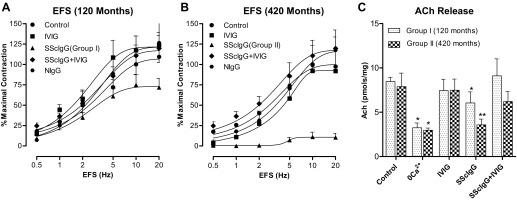
Effect of SScIgG on Cholinergic Nerve Stimulation in Intact Rat Colonic SM
EFS caused a frequency-dependent increase in the contraction of the colonic smooth muscle. The data further revealed that SScIgG (1 mg/ml) and not IVIG (10 mg/ml) by itself, caused inhibition of EFS-induced smooth muscle contraction. Moreover SScIgG from a patient in group II (disease duration: 420 mo) nearly obliterated the EFS-induced response to 80%, compared with the effect of the SScIgG from a patient in group I (disease duration: 120 mo) which caused only 30% inhibition of EFS response (Fig. 3, A and B; P < 0.05; n = 4). This effect of SScIgG was partially reversed when it was administered premixed with IVIG (Fig. 3, A and B; P < 0.05; n = 4).
Fig. 3.
EFS-induced contraction of the rat colonic smooth muscle. SScIgG from a patient in group II (disease duration 420 mo) (B) causes more marked attenuation of EFS response than SScIgG from a patient in group I (disease duration 120 mo) (A) (P < 0.05, n = 4). Attenuation of these EFS responses is reversed by IVIG to values not significantly different from those obtained in the control and IVIG-alone experiments (P > 0.05). C: values of ACh release following EFS (10 V, 5 Hz, 0.5 ms, 30-s train) in the colonic SM strips used for the experiments for SScIgGs from group I vs. group II patients. Data show that EFS causes a significant (P < 0.05, n = 4) increase in ACh release that is mitigated significantly (*P < 0.05, n = 4) by 0 Ca2+ and SScIgG. The effects of SScIgG are reversed by IVIG pretreatment to values not significantly different from those obtained in control and IVIG-alone experiments (P > 0.05). NIgG, IgGs from normal subjects.
These findings signify that with progression of disease duration, greater proportion of IgG molecules within the SScIgG pool recognizes the target epitope (M3-R) leading to progression of cholinergic neuropathy.
Effect of SScIgG on EFS-Evoked ACh Release in Intact Rat Colonic SM
Measurement of ACh release from colonic smooth muscle stimulation revealed that in control experiments (in the presence of normal IgG) EFS (10 V, 5 Hz, 0.5 ms, 30-s train) caused a significant increase in ACh release, which was attenuated by 0Ca2+ but remained unaffected by IVIG. In addition, SScIgG caused a significant decrease (*P < 0.05; Fig. 3C) in ACh release. Notably SScIgG from patients in group II caused further significant decrease (*P < 0.05; Fig. 3C) in ACh release compared with SScIgG from patients in Group I, validating the progression of cholinergic neuropathy with advancement of disease duration. The suppressant effect of SScIgG (from patients in both groups) on ACh release was reversed by IVIG (Fig. 3C; P < 0.05), which by itself had no significant effects on basal release of ACh. These data suggest that IVIG could potentially reverse SScIgG-mediated cholinergic dysfunction.
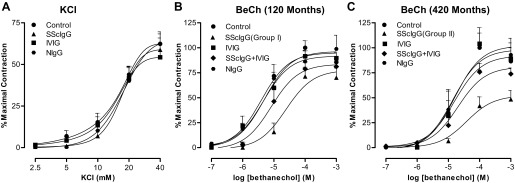
Effect of SScIgG on Direct Colonic Smooth Muscle Contraction by Bethanechol (BeCh)
BeCh caused a concentration-dependent increase in contraction of the colon smooth muscle strips that was attenuated by SScIgG. KCl also caused concentration-dependent increase in colon smooth muscle contraction that was not affected by SScIgG (Fig. 4, A and B; P < 0.05; n = 4) suggesting the selectivity of the suppressant effects of SScIgG on M3-R activation in the colon.
Fig. 4.
A: SScIgG or IVIG have no significant effect (P > 0.05, n = 4) on KCl-induced colonic smooth muscle contraction. SScIgG from a patient in group II (disease duration 420 mo) (C) causes more marked attenuation of M3-R activation by BeCh than SScIgG from a patient in group I (disease duration 120 mo) (B) (P < 0.05, n = 4). Attenuation of these BeCh responses is reversed by IVIG to values not significantly different from control (NIgG), and pooled human IgG (IVIG)-alone experiments (P > 0.05).
In these experiments the effect of SScIgG (1 mg/ml) from a patient in group I (disease duration: 120 mo) was compared with that of SScIgG (1 mg/ml) from a patient in group II (disease duration: 420 mo). SScIgG from the former patient with disease of less duration attenuated BeCh response to only 70% of control (Fig. 4B; P < 0.05; n = 4) whereas SScIgG from patient in group II led to much greater attenuation of BeCh response to 50% of maximal contraction (Fig. 4C; P < 0.05; n = 4). [Maximal contraction with BeCh (10−4 mol/l) was regarded as 100% contraction.]
The attenuation of smooth muscle contraction induced by SScIgG from both patients was significantly reversed by pretreatment of the smooth muscle strips with 10 mg/ml IVIG (Fig. 4, B and C; P < 0.05, n = 4). IVIG by itself had no significant effect on BeCh-induced smooth muscle contraction.
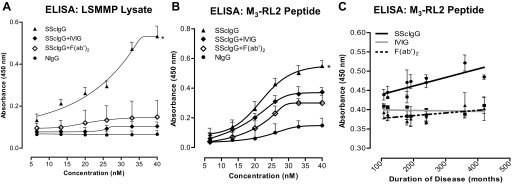
Assessment of SScIgG Binding to M3-R and Influence of Complete IVIG And F(ab′)2 Using Synthetic M3-RL2 Peptide and LSMMP Lysate
In these experiments, we used multiwell plates (used for ELISA) preadsorbed with 400 pg/ml of M3-RL2 or 400 μg/ml of LSMMP lysate and determined the OD after incubation with varying concentrations of SScIgGs. Data show that SScIgG (and not NIgG) binds to LSMMP lysate significantly in a concentration-dependent manner with the maximal OD at 40 nM of SScIgG as 0.532 (Fig. 5A; *P < 0.05; n = 4). Pretreatment of the SScIgG with IVIG (SScIgG + IVIG) and its F(ab′)2 fragment [SScIgG+ F(ab′)2] caused a significant decrease in the binding of the SScIgG to the LSMMP lysate as reflected by a decrease in the OD (to 0.148 and 0.104, respectively) (Fig. 5A; P < 0.05; n = 4). These data suggest that the LSMMP has M3-R domains that bind selectively with the SScIgGs.
Fig. 5.
A: ELISA binding studies for LSMMP lysate. OD concentration curves show that SScIgG (but not NIgG) bind with M3-R on LSMMP lysate in a concentration-dependent manner (*P < 0.05; n = 4). IVIG and F(ab′)2 significantly decrease this binding (P < 0.05; n = 4). B: similarly, ELISA binding studies for synthetic M3-RL2 peptide show that SScIgG (but not NIgG) bind with M3-RL2 in a concentration-dependent manner (*P < 0.05; n = 4). IVIG and F(ab′)2 significantly decrease this binding (P < 0.05; n = 4). C: graph showing significant linear correlation of binding intensity of SScIgG (40 nM) to synthetic M3-RL2 peptide with disease duration and its inhibition by IVIG and F(ab′)2 (P < 0.05).
ELISA with M3-RL2 revealed similar results that showed a concentration-dependent increase in M3-R binding with SScIgG (Fig. 5B; *P < 0.05; n = 4) and not with NIgG (P > 0.05). Maximal OD at 40 nM of SScIgG was 0.54 (Fig. 5B; P < 0.05; n = 4). Pretreatment of the SScIgG with IVIG (SScIgG+ IVIG) and its F(ab′)2 fragment [SScIgG+ F(ab′)2] caused a significant and concentration-dependent decrease in the OD (P < 0.05; n = 4).
When the OD obtained with SScIgG (40 nM) binding to M3-RL2 from all patients was plotted against their duration of disease a selective and disease duration-dependent increase in the binding was observed. This binding was attenuated significantly by IVIG and also by its antigen-binding fragment F(ab′)2 suggesting the presence of anti-idiotypic antibodies in IVIG that neutralize the pathological activity of SScIgGs (Fig. 5C; P < 0.05).
DISCUSSION
In continuation from our previous work on the pathogenesis of gastrointestinal dysmotility in SSc, the current study demonstrates 1) SScIgGs from patients with disease duration of less than 15 years display high affinity inhibition of M3-R at myenteric cholinergic neurons (MCN); 2) further progression of disease duration leads to combined inhibition of M3-R at the MCN and smooth muscle (SM); and 3) IVIG attenuates the binding of these autoantibodies at the MCN and SM receptors by anti-idiotypic neutralization.
Evidence for neuropathy in gastrointestinal SSc comes from two landmark studies in the 1970s. First, Cohen et al. (5) demonstrated that the esophageal smooth muscle responded to direct acting agent methacholine but not to indirectly acting edrophonium. (Edrophonium increases the levels of ACh by preventing its breakdown by acetylcholinesterase whereas methacholine, similar to ACh, is a muscarinic receptor agonist.) Subsequently, DiMarino et al. (7) showed that duodenal myoelectric activity was intact but there was an abnormality in intestinal activation by mechanical and hormonal stimuli in SSc patients. These data collectively suggest an early neuropathic phase characterized by dysregulation of ACh release from the myenteric cholinergic neurons. Further evidence of neuropathy in SSc is supported by studies demonstrating impaired anal sensation and rectoanal inhibitory reflex in this subset of patients (14).
Multiple mechanisms were proposed to explain the mechanism of neuropathy in SSc but none could gather much scientific attention until Goldblatt et al. (11) demonstrated that sera of SSc patients contained antibodies that could inhibit cholinergic-mediated contraction of the mouse colon. The strongest evidence for autoantibody-mediated dysmotility can be drawn from a study that showed that SSc patients develop manometric abnormalities in the absence of histopathological changes in the esophageal musculature (27). Studies from our laboratory confirmed subsequently that autoantibodies isolated from SSc patients inhibit direct and indirect (neurally mediated) rat colonic and internal anal sphincter smooth muscle contraction (24).
The present studies provide evidence in favor of the temporal course of SScIgG binding to the neural and myogenic M3-R. Immunohistochemical data reveal that binding of SScIgG at the myenteric cholinergic neurons was higher in patients early in the course of their disease and further increased over time. Most notably, binding to the SM increased over time as well. These data demonstrate a positive correlation between the binding intensity of SScIgG to the myenteric neurons and smooth muscle with disease duration. Thus, greater the duration of the disease, the greater is the binding accounting for the progressive nature of GIT involvement in SSc. Similar results were shown in a study wherein esophageal stiffness and impaired muscle function on manometry correlated with disease duration (12).
Results of functional studies further reiterate the higher affinity of SScIgG to the MCN earlier and later to smooth muscle with progression of the disease. This progressive effect of SScIgGs at the M3-R located on myenteric cholinergic neurons was shown by the marked decline in EFS-induced colonic smooth muscle contraction and actual release of ACh following pretreatment of the smooth muscle strips with SScIgG from patients in group II compared with patients from group I. More importantly, SScIgGs from patients with significantly advanced duration of disease in group II inhibited BeCh-induced contraction of the rat colonic SM to a greater extent compared with group I, suggesting the targeting of smooth muscle M3-R (myopathy) with disease progression.
M3-R-specific ELISA studies revealed that SScIgGs cause a selective and disease duration-dependent increase in the binding to the M3-RL2 peptide and whole M3-R in LSMMP lysates. Although the exact reason for such an increase in affinity of SScIgGs toward MCN as well as SM (observed during ELISA, and IFI studies as discussed above) is not known, a few possibilities exist: Firstly, a change in the epitope of M3-R that is recognized by SScIgG (owing to structural or anatomical changes in the target tissues possibly caused by edema or inflammation) as SSc progresses. Secondly, a phenomenon of affinity maturation of the antibody response (6, 9, 20), with temporal progression of SSc. This issue, however, merits further investigation.
Present studies provide further evidence that IVIG reverses SScIgG-induced M3-R inactivation at both the neural and myogenic sites. Evidence for the effects of IVIG comes first from IHC studies showing significant decrease in M3-R binding intensity following SScIgG, at both the myenteric cholinergic neurons and smooth muscle. Second, IVIG reverses both the SScIgG-induced attenuation of BeCh and EFS-stimulated colonic muscle contraction. Finally, IVIG attenuates SScIgG-induced increase in binding intensity to the M3-R when multiwell plates are preadsorbed with LSMMP lysate or M3-RL2 peptide.
In our previous study we had demonstrated that IVIG produces its effect by directly competing with pathogenic SScIgGs (24, 25). The three main mechanisms that are likely to involve direct competition with pathologic autoantibodies are anti-idiotypic binding, FcRn saturation, and complement scavenging (3, 23). Our findings that F(ab′)2 fragments of the IVIG have the same effect as IVIG in vitro (ELISA binding studies to the M3-RL2 peptide and whole M3-R in LSMMP) suggest the presence of anti-idiotypic antibodies in IVIG that block the activity of pathogenic SScIgGs (3).
Currently the treatment of gastrointestinal disease in SSc is symptomatic and ineffective (19). In this regard IVIG offers hope for treatment of SSc-associated gastrointestinal motility disorders. A recent study focusing on the effect of IVIG in SSc patients reported no significant worsening in gastrointestinal symptom score at 1 yr follow up as a secondary end point (21). However, the exact mechanism, and the role of other pathways in the therapeutic efficiency and efficacy of IVIG, remain to be determined.
SSc is a heterogeneous disease in which internal organ involvement and outcomes vary considerably from patient to patient. There is a subset of patients who develop rapidly progressive debilitating gastrointestinal symptoms within a few years of diagnosis. Whether this subset of patients exhibit neuropathy followed by myopathy, remains to be determined. Moreover, autoantibody-associated dysmotility does not explain the mechanism of fibrosis seen in the gastrointestinal tract and most importantly does not correlate with the symptomatology of gastrointestinal disease (determined by the UCLA SCTC GIT 2.0). Another limitation of the present study is sample size in terms of limited number of patients. Despite these limitations, the results of our study are novel and should be followed up with a multicenter study involving a large number of SSc patients at different stages of the disease.
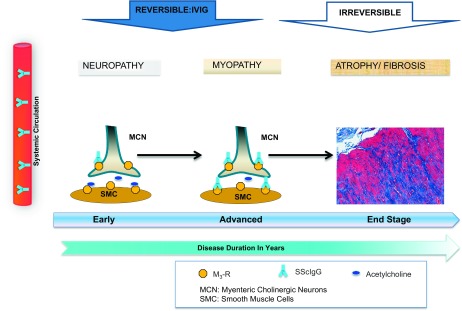
In conclusion we propose that the pathophysiological changes of the gut in SSc occur in a staged process beginning with neuropathy and progressing to myopathy (see Fig. 6). Initially, circulating M3-R autoantibodies block cholinergic neurotransmission via inhibition of ACh release at the MCN (neuropathic damage) and later lead to myopathy via inhibition of ACh action at the gastrointestinal smooth muscle cell proper. We further suggest that SSc-associated intestinal dysfunction at both the neuropathic and myopathic stages may be potentially reversible with IVIG.
Fig. 6.
Proposed pathogenesis of gastrointestinal dysmotility in SSc suggests that SScIgGs initially block cholinergic neurotransmission by inhibition of ACh release by the MCN (neuropathy). With progression of the disease, SScIgGs lead to additional myopathy via inhibition of ACh action at the gastrointestinal smooth muscle proper (myopathy). This is followed by the last stage of intestinal fibrosis by yet unknown mechanisms. Dysmotility at the neuropathic and myopathic stages may be potentially reversible with IVIG, before smooth muscle fibrosis/atrophy ensue.
GRANTS
The work was supported by Grant Number RO1-DK-035385 from the National Institutes of Diabetes and Digestive and Kidney Diseases, an industrial support from CSL Behring, King of Prussia, PA, and an institutional grant from Thomas Jefferson University. E. S. Blomain is the recipient of the Ruth L. Kirschstein National Research Service Award for Individual Predoctoral MD/PhD Fellows (1 F30 CA180500) from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.K., J.S., R.K., F.M., S.A.J., A.J.D., S.C., and S.R. conception and design of research; S.K., J.S., and E.S.B. performed experiments, analyzed data, and prepared figures; S.K., J.S., R.K., F.M., S.C., and S.R. drafted manuscript; S.K., J.S., R.K., F.M., S.A.J., E.S.B., A.J.D., S.C., and S.R. interpreted results, edited, revised, and approved final version of manuscript.
REFERENCES
- 1.Baleva M, Nikolov K. The role of intravenous immunoglobulin preparations in the treatment of systemic sclerosis. Int J Rheumatol 2011: 1–4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett DM, Maguma HT, Taylor DA. Time course of altered sensitivity to inhibitory and excitatory agonist responses in the longitudinal muscle-myenteric plexus and analgesia in the Guinea pig after chronic morphine treatment. Front Pharmacol 2: 88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger M, McCallus DE, Lin CSY. Rapid and reversible responses to IVIG in autoimmune neuromuscular diseases suggest mechanisms of action involving competition with functionally important autoantibodies. J Peripher Nerv Syst 18: 275–296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussone G, Tamby MC, Calzas C, Kherbeck N, Sahbatou Y, Sanson C, Ghazal K, Dib H, Weksler BB, Broussard C, Verrecchia F, Yaici A, Witko-Sarsat V, Simonneau G, Guillevin L, Humbert M, Mouthon L. IgG from patients with pulmonary arterial hypertension and/or systemic sclerosis binds to vascular smooth muscle cells and induces cell contraction. Ann Rheum Dis 71: 596–605, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Fisher R, Lipshutz W, Turner R, Myers A, Schumacher R. The pathogenesis of esophageal dysfunction in scleroderma and Raynauds's disease. J Clin Invest 51: 2663–2668, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins AM, Jackson KJ. A temporal model of human IgE and IgG antibody function. Front Immunol 4: 235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMarino AJ, Carlson G, Myers A, Schumaker HR, Cohen S. Duodenal myoelectric activity in scleroderma. Abnormal responses to mechanical and hormonal stimuli. N Engl J Med 289: 1220–1223, 1973. [DOI] [PubMed] [Google Scholar]
- 8.Eaker EY, Kuldau JG, Verne GN, Ross SO, Sallustio JE. Myenteric neuronal antibodies in scleroderma: passive transfer evokes alterations in intestinal myoelectric activity in a rat model. J Lab Clin Med 133: 551–556, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Eisen HN. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res 2: 381–392, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Gabrielli A, Avvedimento EV, Krieg T. Mechanisms of disease: Scleroderma. N Engl J Med 360: 1989–2003, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Goldblatt F, Gordon TP, Waterman SA. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology 123: 1144–1150, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Gregersen H, Villadsen GE, Liao D. Mechanical characteristics of distension-evoked peristaltic contractions in the esophagus of systemic sclerosis patients. Dig Dis Sci 56: 3559–3568, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Grodzki AC, Berenstein E. Antibody purification: affinity chromatography-protein A and protein G sepharose. Methods Mol Biol 588: 41, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Heyt GJ, Oh MK, Alemzadeh N, Rivera S, Jimenez SA, Rattan S, Cohen S, DiMarino AJ. Impaired rectoanal inhibitory response in scleroderma (systemic sclerosis): an association with fecal incontinence. Dig Dis Sci 49: 1040–1045, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Holst MC, Powley TL. Cuprolinic blue (quinolinic phthalocyanine) counterstaining of enteric neuron for peroxidase immunocytochemistry. J Neurosci Methods 62: 121–127, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Kasermann F, Boerema DJ, Ruegsegger M, Hofmann A, Wymann S, Zuercher AW, Miescher S. Analysis and functional consequences of increased Fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS One 7: e37243, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser C, Fritzler MJ. Autoantibodies in systemic sclerosis: unanswered questions. Front Immunol 6: 167, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna D, Hays RD, Maranian P, Seibold JR, Impens A, Mayes MD, Clements PJ, Getzug T, Fathi N, Bechtel A, Furst DE. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum 61: 1257–1263, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby DF, Chatterjee S. Evaluation and management of gastrointestinal manifestations in scleroderma. Curr Opin Rheumatol 26: 621–629, 2014. [DOI] [PubMed] [Google Scholar]
- 20 .Pauyo T, Hilinski GJ, Chiu PT, Hansen DE, Choi YJ, Ratner DI, Shah-Mahoney N, Southern CA, O'Hara PB. Genetic and fluorescence studies of affinity maturation in related antibodies. Mol Immunol 43: 812–821, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Poelman CL, Hummers LK, Wigley FM, Anderson C, Boin F, Shah AA. Intravenous immunoglobulin may be an effective therapy for refractory, active diffuse cutaneous systemic sclerosis. J Rheumatol 42: 236–242, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savarino E, Mei F, Parodi A, Ghio M, Furnari M, Gentile A, Berdini M, Di SA, Bendia E, Bonazzi P, Scarpellini E, Laterza L, Savarino V, Gasbarrini A. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology (Oxford) 52: 1095–1100, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 13: 176–189, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Singh J, Cohen S, Mehendiratta V, Mendoza F, Jimenez SA, DiMarino AJ, Rattan S. Effects of scleroderma antibodies and pooled human immunoglobulin on anal sphincter and colonic smooth muscle function. Gastroenterology 143: 1308–1318, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh J, Mehendiratta V, Del Galdo F, Jimenez SA, Cohen S, DiMarino AJ, Rattan S. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 297: G1206–G1213, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjögren RW. Gastrointestinal features of scleroderma. Curr Opin Rheumatol Curr Opin Rheumatol 8: 569–575, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Teacy WL, Baggentoss AH, Slocumb CH, Code CF. Scleroderma of the esophagus. A correlation of histologic and physiologic findings. Ann Intern Med 59: 351–356, 1963. [DOI] [PubMed] [Google Scholar]
- 28.Thoua NM, Derrett-Smith EC, Khan K, Dooley A, Shi-Wen X, Denton CP. Gut fibrosis with altered colonic contractility in a mouse model of scleroderma. Rheumatology (Oxford) 51: 1989–1998, 2012. [DOI] [PubMed] [Google Scholar]
- 29.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr, Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Muller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Csuka ME, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE. 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 65: 2737–2747, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]