Abstract
Treatment with the antileukemic agent asparaginase can induce acute pancreatitis, but the pathophysiology remains obscure. In the liver of mice, eukaryotic initiation factor 2 (eIF2) kinase general control nonderepressible 2 (GCN2) is essential for mitigating metabolic stress caused by asparaginase. We determined the consequences of asparaginase treatment on the pancreata of wild-type (WT, GCN2-intact) and GCN2-deleted (ΔGcn2) mice. Mean pancreas weights in ΔGcn2 mice treated with asparaginase for 8 days were increased (P < 0.05) above all other groups. Histological examination revealed acinar cell swelling and altered staining of zymogen granules in ΔGcn2, but not WT, mice. Oil Red O staining and measurement of pancreas triglycerides excluded lipid accumulation as a contributor to acini appearance. Instead, transmission electron microscopy revealed dilatation of the endoplasmic reticulum (ER) and accumulation of autophagic vacuoles in the pancreas of ΔGcn2 mice treated with asparaginase. Consistent with the idea that loss of GCN2 in a pancreas exposed to asparaginase induced ER stress, phosphorylation of protein kinase R-like ER kinase (PERK) and its substrate eIF2 was increased in the pancreas of asparaginase-treated ΔGcn2 mice. In addition, mRNA expression of PERK target genes, activating transcription factors 4, 3, and 6 (Atf4, Atf3, and Atf6), fibroblast growth factor 21 (Fgf21), heat shock 70-kDa protein 5 (Hspa5), and spliced Xbp1 (sXbp1), as well as pancreas mass, was elevated in the pancreas of asparaginase-treated ΔGcn2 mice. Furthermore, genetic markers of oxidative stress [sirtuin (Sirt1)], inflammation [tumor necrosis factor-α (Tnfα)], and pancreatic injury [pancreatitis-associated protein (Pap)] were elevated in asparaginase-treated ΔGcn2, but not WT, mice. These data indicate that loss of GCN2 predisposes the exocrine pancreas to a maladaptive ER stress response and autophagy during asparaginase treatment and represent a genetic basis for development of asparaginase-associated pancreatitis.
Keywords: amino acid response, eukaryotic initiation factor 2, endoplasmic reticulum stress, protein kinase R-like ER kinase, unfolded protein response
acute lymphoblastic leukemia is the most common form of childhood cancer (23). Among the standard chemotherapies used to induce remission, asparaginase stands out as responsible for a spectrum of life-threatening toxicities that include anaphylaxis, immunosuppression, hepatotoxicity, coagulopathy, thrombosis, and pancreatitis (10, 13, 22). While different forms of the drug offer multiple options for patients who experience allergic reactions, all forms are associated with metabolic complications that are difficult to predict, yet intensify with age (1, 2, 13, 16, 22, 28, 31, 50, 52, 62).
Asparaginase-associated pancreatitis (AAP) is a major toxicity in the treatment of childhood acute lymphoblastic leukemia (ALL) and is among the most common reasons for termination of treatment (11, 43, 44). Although this complication is long-recognized, the mechanism precipitating this event remains unknown (31, 41, 45). Development of pancreatitis results in termination of chemotherapy, allowing for cancer progression; consequently, patients diagnosed with ALL who develop AAP are at greater risk for death (28, 44, 61). Genetic predispositions are hypothesized to play a role (5, 28, 41), but evidence to establish causation is lacking. A deeper understanding of the molecular basis for AAP will provide an important guide to improve patient treatment through the development of evidence-based guidelines for prevention and management.
Administration of bacterial asparaginase breaks down circulating asparagine and glutamine, depriving the leukemic cells of amino acids needed for tumor growth (10, 14, 47). The amino acid depletion also instigates a cellular stress response in nontumor tissues that we and others have previously described (4, 9, 29, 46, 55, 64, 65). In the liver and spleen, asparaginase corresponds with increased phosphorylation of eukaryotic initiation factor 2 (eIF2) by the general control nonderepressible 2 (GCN2) protein kinase (46). Phosphorylation of eIF2 by GCN2 activates an integrated stress response (ISR), which simultaneously decreases general protein synthesis while favoring mRNA translation of genes encoding transcription factors such as activating transcription factor (ATF) 4 (20). ATF4 and other transcription factors then bind C/EBP-ATF response elements [CARE, also known as amino acid response elements (AARE)] in the promoters of target genes, thereby altering the transcriptome (59). This homeostatic alteration in gene expression to amino acid supply is referred to as the amino acid response (AAR) (30, 57). Activation of the AAR by GCN2 is essential for adaptation and survival during asparaginase treatment. In the absence of GCN2, failure to induce the AAR augments immunosuppression (8) and hepatotoxicity (64, 65). These disease outcomes are in agreement with human genetic studies that report a correlation between single-nucleotide polymorphisms (SNPs) in the AAR genes asparagine synthetase (ASNS) and ATF5 and worse treatment outcome to asparaginase (6, 49).
The role of the GCN2/eIF2/ATF4 pathway within the pancreas during asparaginase treatment is understudied. Our laboratory previously reported the effects of acute asparaginase treatment in mouse pancreas and found no change in eIF2 phosphorylation or altered gene expression following a single injection (9, 46). Pancreatic acinar cells possess an abundance of endoplasmic reticulum (ER) due to its function as an exocrine gland and highly express another eIF2 kinase (56), protein kinase R-like ER kinase (PERK), which is essential for viability of acinar cells (26). High protein turnover in the pancreas makes it susceptible to dysregulation of ER homeostasis, which can result in an accumulation of misfolded proteins in the organelle. A homeostatic response to this condition in endocrine and exocrine pancreas is called the unfolded protein response (UPR) (42). Upon UPR activation, PERK functions in conjunction with other ER transmembrane proteins such as inositol-requiring enzyme 1 (IRE1) and ATF6 to remediate and regain ER homeostasis (48). Based on our previous findings in the liver, along with reports showing how GCN2 and PERK can play auxiliary roles to each other (19), we hypothesized that, during longer-term asparaginase treatment, a lack of GCN2 function would trigger a maladaptive UPR in the pancreas, compromising organ health. Here, we reveal how loss of GCN2 function negatively impacts the pancreas during asparaginase treatment at the anatomic, biochemical, molecular, and ultrastructural levels. These data identify a novel genetic target and cellular pathway to pursue in understanding genetic predisposition to onset of AAP.
MATERIALS AND METHODS
Materials.
Phosphorylated (Ser51) eIF2 (catalog no. 3597), phosphorylated (Thr980) PERK (catalog no. 3179), and GAPDH (14C10; catalog no. 2118) were purchased from Cell Signaling Technology (Beverly, MA) and total eIF2α (catalog no. sc-11386) from Santa Cruz Biotechnology (Dallas, TX). A colorimetric triglyceride quantification kit was purchased from BioVision (Mountain View, CA), an amylase activity assay kit from Sigma-Aldrich (St. Louis, MO), the Trevigen TACS 2 TdT-Blue Label In Situ Apoptosis Detection Kit from R & D Systems, the RNeasy Plus Mini Kit from Qiagen, and a high-capacity cDNA reverse transcription kit from Applied Biosystems (Foster City, CA).
Animal care and experimental design.
The animal procedures and experimental protocols were approved by the Institutional Animal Care and Use Committees at Rutgers, The State University of New Jersey. Before and throughout the experiment unless otherwise denoted, mice were maintained in plastic cages with corn cob bedding; tap water and commercial pelleted diet (5001 Laboratory Rodent Diet, Lab Diet) were freely provided. Eight-week-old male and female Gcn2+/+ (WT) and Gcn2−/− (ΔGcn2) mice on a C57BL/6J genetic background for 10 generations were assigned in equal numbers (n = 12 per group) to receive eight daily injections of native Escherichia coli l-asparaginase (Elspar, Merck) in phosphate-buffered saline (PBS) at 0 or 3.0 IU/g body wt ip. Before injections, asparaginase activity was determined using the Nesslerization technique to detect the level of ammonia as previously described (46). Treatment groups were defined as follows: WT + PBS (WP), WT + asparaginase (WA), ΔGcn2 + PBS (GP), and ΔGcn2 + asparaginase (GA). Animals received injections at the same time each day and were euthanized ∼8 h after the final injection. Body weight was recorded each day throughout the experiment, including the day of euthanasia. In some experiments, food intake was measured daily, and all mice were pair-fed to GA mice. Mice were euthanized by decapitation, and serum was collected from the trunk blood of the animal. Tissues were rapidly dissected, rinsed in PBS, and weighed. One portion of the pancreas was processed immediately for RNA isolation, another portion was fixed for histological and ultrastructural evaluation, and a final portion was quickly frozen in liquid nitrogen for protein expression and other biochemical analyses.
Biochemical analyses.
Serum amylase concentrations were measured in mouse serum by ELISA using a commercial kit. Triglyceride concentrations were measured from frozen prepared pancreas tissue lysates (∼20 mg) using a commercial kit as previously described (65). Circulating amino acid concentrations were measured in the serum of mice killed 8 h after the last injection of asparaginase or saline excipient. Serum samples (60 μl) were mixed with 180 μl of 0.1% formic acid in methanol and then filtered using Captiva nondrip lipid filtration tubes (catalog no. A5400635, Agilent Technologies, Wilmington, DE). Collected eluent (20 ul) was spiked with internal standard (100 pmol/ml final concentration) before analysis by high-performance liquid chromatography (HPLC). Amino acids were derivatized with o-phthalaldehyde/9-fluorenyl-methyl chloroformate using an automated liquid sampler attached to an Agilent 1260 HPLC with a quaternary pump, fluorescence detector, and multiple-wavelength detector. Amino acid standards were prepared and liquid chromatography was conducted exactly as described in Agilent application notes 5990-4547EN using a ZORBAX Eclipse Plus C18 rapid-resolution high-definition, 2.1 × 100-mm, 1.8-μm particle-size column (catalog no. 959758-902, Agilent Technologies).
Histological and ultrastructural examination.
Tissues fixed in 4% paraformaldehyde were frozen and then sectioned (10 μm) using a cryostat. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays were performed using frozen sections as previously described (64). Frozen sections were also stained with Oil Red O to visualize lipid content (38, 64). Paraffin-embedded liver specimens were sectioned (6 μm) and stained with hematoxylin and eosin to visualize general histology and identify histopathological features under light microscopy. A subset of pancreata were fixed in 2.5% glutaraldehyde, postfixed in buffered 1% osmium tetroxide, and stained en bloc with uranyl acetate. After dehydration in a graded series of ethanol and propylene oxide, samples were embedded in epoxy resin in the usual manner. Ultrathin sections were stained with uranyl acetate and lead citrate and visualized and imaged using a Philips CM12 electron microscope.
Immunoblots.
Frozen pancreas tissue (∼20 mg) was homogenized in 10 volumes per gram of a RIPA-buffered solution containing 50 mM Tris·HCl (pH 7.9), 150 mM sodium chloride, 1% Nonidet P-40, 0.1% SDS, 100 mM sodium fluoride, 17.5 mM β-glycerophosphate, 0.5% sodium deoxycholate, and 10% glycerol supplemented with EDTA-free protease inhibitor cocktail. Tissue lysates were processed for SDS-PAGE and immunoblot analysis as previously described (60). Protein expression was visualized using enhanced chemiluminescence, and signal intensities were digitally captured using a FluorChem M multiplex imager (ProteinSimple) and band densities were quantified using imaging software.
mRNA measurements.
Total RNA was extracted from fresh mouse pancreas tissue immediately following dissection. The ratio of absorbance at 260 nm to absorbance at 280 nm (A260/280) was 1.8–2.0 following RNA clean-up. Changes in gene expression were determined by reverse transcription to generate cDNAs, followed by quantitative PCR using TaqMan reagents and the StepOnePlus Real-Time PCR System (Applied Biosystems). Levels of mRNAs were measured in triplicate and normalized against Actb (β-actin) mRNA. Fold change in gene expression was calculated using the comparative cycle threshold (Ct) method (9). Data are expressed as fold change compared with the experimental control (WP) group.
Statistics.
Results were analyzed using the STATISTICA statistical software package (StatSoft, Tulsa, OK). Data are reported as means ± SE for 8–12 per group. Differences between treatment groups were analyzed by two-way ANOVA, with mouse strain and drug treatment as independent variables. Data sets that failed homoscedasticity by Bartlett's test were logarithmically transformed before ANOVA. When significant main or interaction effects were detected by ANOVA, differences among group means were evaluated using Tukey's post hoc test. Pearson's one-tailed correlation coefficient was calculated to evaluate the variance in gene expression within the GA group according to pancreas mass. The level of significance was set at P < 0.05 for all statistical tests.
RESULTS
Asparaginase-treated ΔGcn2 mice develop increased pancreas mass, but not elevated serum amylase or lipid accumulation in exocrine tissue.
Asparaginase reduces food intake and body weight in mice (65). To control for differences in food intake, mice were pair-fed to the lowest intake (GA group). We found that all mice lost body weight; however, mice treated with asparaginase (WA and GA groups) lost more body weight than mice injected with saline excipient (Fig. 1A), indicating a negative effect of asparaginase on body weight independent of energy intake. Serum amino acid profiles showed that asparaginase treatment also significantly reduced circulating concentrations of asparagine and glutamine at 8 h after the final injection (Table 1). Furthermore, asparaginase increased circulating concentrations of serine, glycine, threonine, methionine, arginine, alanine, lysine, leucine, isoleucine, and valine in both mouse strains. A main effect of strain was also evident for threonine, alanine, lysine, leucine, isoleucine, and valine.
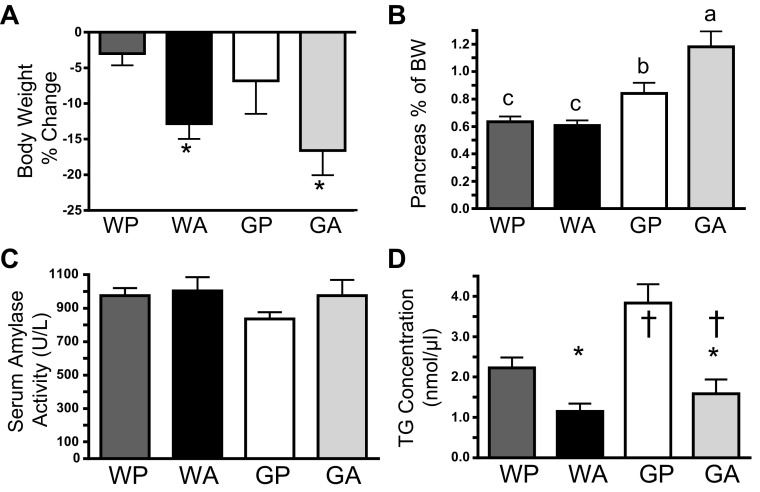
Fig. 1.
Increased pancreas mass in general control nonderepressible 2 (GCN2)-depleted (ΔGcn2) mice following 8 daily injections of asparaginase does not correspond with elevated serum amylase or triglyceride accumulation in pancreatic acini. A: percent change in body weight in pair-fed wild-type (WT) and ΔGcn2 mice. *P < 0.05 for main effect of drug. B: change in pancreas mass relative to body weight (BW). Means not sharing a common letter are different, reflecting a significant strain × drug interaction (P < 0.05). C: serum amylase was similar among treatment groups. D: triglyceride (TG) concentrations in the pancreas were higher in ΔGcn2 mice and reduced by asparaginase. *P < 0.05 for main effect of drug. †P < 0.05 for main effect of strain. Values are means ± SE (n = 8–12 per group). Treatment groups were defined as follows: WT + PBS (WP), WT + asparaginase (WA), ΔGcn2 + PBS (GP), and ΔGcn2 + asparaginase (GA).
Table 1.
Serum amino acid profiles in WT and ΔGcn2 mice following eight daily injections of asparaginase or saline excipient
| WP | WA | GP | GA | |
|---|---|---|---|---|
| Aspartic acid | 44.2 ± 6.2 | 36.4 ± 8.6 | 34.6 ± 5.0 | 59.1 ± 9.5 |
| Asparagine† | 48.9 ± 4.8 | BDL | 43.3 ± 2.8 | BDL |
| Glutamic acid† ‡ | 64.7 ± 4.2d | 546.7 ± 56.8b | 137.5 ± 33.8c | 811.6 ± 123.7a |
| Glutamine† | 476.1 ± 20.3 | 148.3 ± 22.7 | 403.8 ± 18.5 | 132.3 ± 25.5 |
| Serine† | 123.1 ± 5.8 | 185.5 ± 17.5 | 117.8 ± 10.7 | 191.3 ± 29.8 |
| Glycine† | 279.6 ± 12.3 | 349.2 ± 25.1 | 222.7 ± 13.6 | 460.7 ± 94.4 |
| Threonine† ‡ | 148.6 ± 6.8 | 269.7 ± 29.3 | 107.7 ± 8.6 | 181.6 ± 30.1 |
| Methionine† | 39.9 ± 2.6 | 64.5 ± 5.4 | 35.7 ± 4.1 | 52.3 ± 15.4 |
| Cystine | 18.1 ± 1.4b | 21.4 ± 2.2b | 19.6 ± 1.5b | 30.2 ± 5.1a |
| Arginine† | 109.6 ± 7.9 | 130.1 ± 25.3 | 100.0 ± 6.2 | 139.5 ± 16.7 |
| Alanine† ‡ | 304.9 ± 21.6 | 484.8 ± 41.5 | 317.9 ± 32.6 | 623.1 ± 105.1 |
| Valine† ‡ | 219.9 ± 15.2 | 271.1 ± 32.0 | 165.0 ± 15.6 | 229.4 ± 29.8 |
| Isoleucine† ‡ | 85.1 ± 6.2 | 101.1 ± 13.5 | 63.2 ± 6.2 | 88.9 ± 12.2 |
| Leucine† ‡ | 135.4 ± 10.9 | 163.9 ± 20.9 | 98.1 ± 9.4 | 155.3 ± 20.2 |
| Lysine† ‡ | 188.5 ± 6.2 | 264.4 ± 26.1 | 143.0 ± 9.0 | 248.0 ± 45.5 |
| Tryptophan | 113.5 ± 5.0 | 120.6 ± 10.6 | 92.8 ± 4.8 | 107.4 ± 11.6 |
| Histidine | 49.1 ± 2.8 | 42.0 ± 3.7 | 57.9 ± 7.3 | 58.7 ± 5.9 |
| Proline | 292.9 ± 29.5 | 364.8 ± 102.4 | 241.0 ± 32.1 | 280.1 ± 56.9 |
Values (means ± SE) are μmol/l (n = 8–12 per group). Serum was collected from the trunk blood of mice 8 h after the final injection. WT, wild-type; ΔGcn2, general control nonderepressible 2-depleted; BDL, below instrument detection limit; WP, WT + PBS; WA, WT + asparaginase; GP, ΔGcn2 + PBS; GA, ΔGcn2 + asparaginase. Means not sharing a common letter are different (P < 0.05).
P < 0.05 for main effect of drug.
P < 0.05 for main effect of strain.
After eight daily injections of asparaginase, pancreas weight was significantly greater for ΔGcn2 than WT mice, with GA mice having the largest mass (Fig. 1B). Expansion in mass was noted during tissue dissection to be localized mostly to the duodenal portion of the pancreas. A recognized feature of pancreatitis is an increase in pancreas mass (25). To assess pancreatic injury, serum amylase was measured; this biochemical marker showed little change among treatment groups (Fig. 1C). To determine if increased pancreas mass corresponded with lipid accumulation, biochemical analyses of pancreatic triglyceride concentrations were conducted alongside Oil Red O staining to visualize neutral lipids. Imaging of Oil Red O-stained sections from WP and GP mice revealed low endogenous lipid accumulation, consistent with a previous report (38), and sections from asparaginase-treated mice showed no staining (Fig. 2B). Reduced levels of triglycerides upon asparaginase treatment supported the histological findings and revealed that asparaginase lowered lipid content in the pancreas (Fig. 1D).
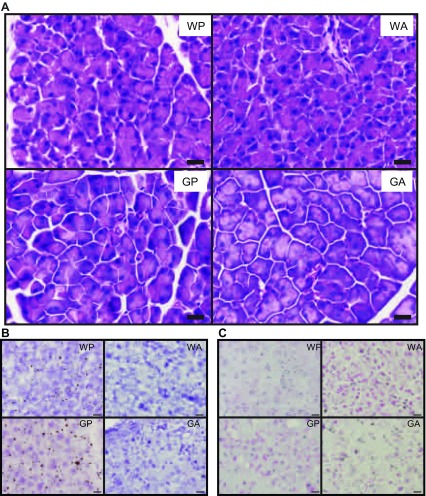
Fig. 2.
Histological features of WT and ΔGcn2 mice following 8 daily injections of asparaginase or saline excipient. A: hematoxylin-eosin-stained pancreatic acinar tissue sections show basally located nuclei and weak staining of zymogen granules in GA mice. Scale bars = 16 μm. B: Oil Red O shows faint staining of neutral lipids in saline-treated (WP and GP) mice that is even fainter in asparaginase-treated (WA and GA) mice. Scale bars = 16 μm. C: TUNEL assay shows broad, diffuse staining of cells across sections from all treatment groups. Scale bars = 16 μm. Images represent visual features identified in sections from 3 mice per treatment group. Treatment groups were defined as follows: WT + PBS (WP), WT + asparaginase (WA), ΔGcn2 + PBS (GP), and ΔGcn2 + asparaginase (GA). Treatment groups were defined as follows: WT + PBS (WP), WT + asparaginase (WA), ΔGcn2 + PBS (GP), and ΔGcn2 + asparaginase (GA).
Induced ER stress and autophagy in ΔGcn2 mice treated with asparaginase.
Hematoxylin-eosin staining was utilized to visualize morphology of pancreatic acini (Fig. 2A). Examination of hematoxylin-eosin-stained pancreas sections by light microscopy showed GP acini to be wedge-shaped and arranged in grape-like clusters with basally located nuclei. Acinar cells appeared to be slightly swollen in ΔGcn2 compared with WT pancreas, with these features being more prominent in GA mice. Altered staining of acini was also apparent, with sections from GA pancreata showing broad areas of pale intracellular staining. To determine if differences in hematoxylin-eosin staining corresponded with cell death, a TUNEL assay was performed to assess DNA fragmentation. Contrary to our expectation, TUNEL assay showed no obvious differences among treatment groups (Fig. 2C).
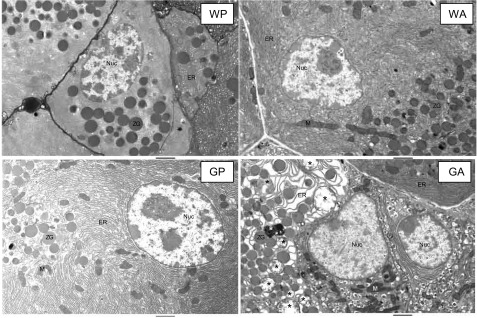
We next carried out transmission electron microscopy to visualize the cellular features in the pancreas in more detail (Fig. 3). In both saline-treated groups (WP and GP), the ER appeared neatly stacked and was interspersed with mitochondria, while zymogen granules localized to the apical pole. In the WA group, there were no obvious changes in the appearance of the ER, mitochondria, nuclei, and zymogen granules. By contrast, morphological and histological features in GA acini were notably heterogeneous, with normal-looking cells adjacent to cells that displayed severely disorganized and distended ER and shrunken mitochondria. An accumulation of large vacuolar structures, some surrounding zymogen granules and cellular organelles, was also evident: some consisted of a single membrane and were quite large, whereas others were whorl-like in appearance or contained multiple membranes filled with electron-dense material. Autophagy is a central component of an activated integrated stress response (32) but, during acute pancreatitis, shows inefficient progression and resolution, resulting in vacuole accumulation that mediates inflammation and acinar cell necrosis (17, 18). In the current investigation the appearance of numerous cytoplasmic vacuoles before nuclear alteration suggests autophagic cell death, a major form of physiological cell death associated with onset of acute pancreatitis (17, 18). Amassing of these intercellular features in the absence of increased serum amylase and apoptotic nuclei suggested that GA mice were in the early stages of AAP.
Fig. 3.
Electron micrographs illustrate in detail acinar cells of WT and ΔGcn2 mice following 8 daily injections of asparaginase or saline excipient. Images of sections from WP, WA, and GP mice show abundant endoplasmic reticulum (ER) densely arranged in neat stacks and interspersed with mitochondria and zymogen granules. Sections from GA mice show swollen ER, shrunken mitochondria, and an accumulation of single- and double-membrane vesicles and whorl-like structures, some containing granular inclusions. ∗, Some of the more prominent vacuolar features. M, mitochondria; Nuc, nucleus; ZG, zymogen granules. Scale bars = 2 μm. Treatment groups were defined as follows: WT + PBS (WP), WT + asparaginase (WA), ΔGcn2 + PBS (GP), and ΔGcn2 + asparaginase (GA).
Asparaginase activated a maladaptive UPR in the pancreas of ΔGcn2 mice.
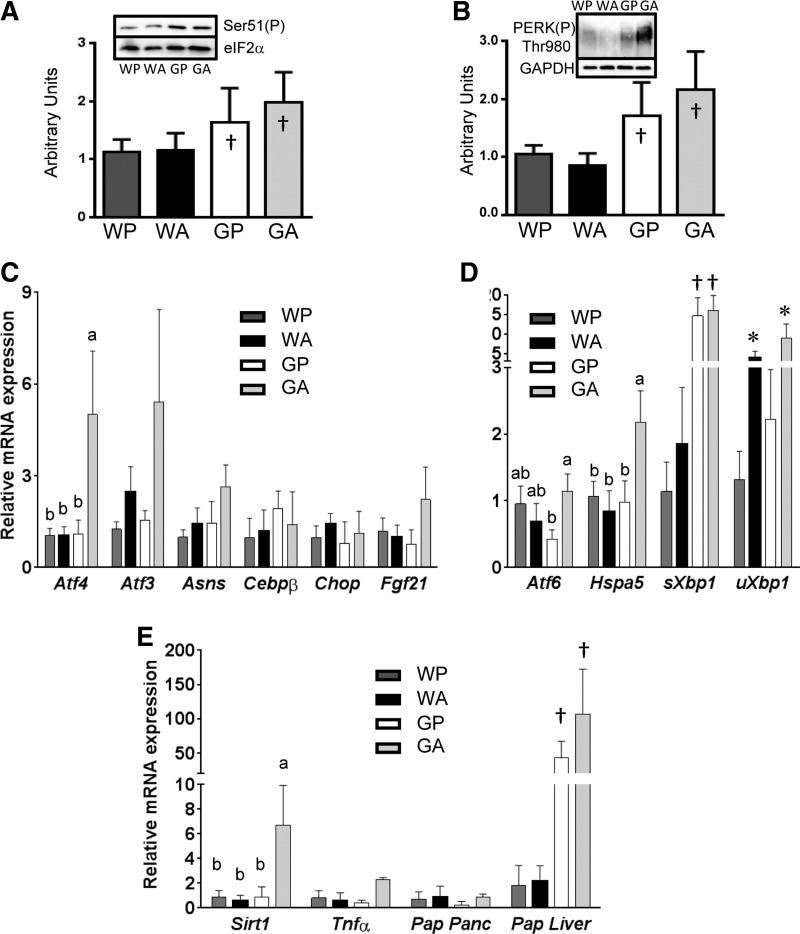
Activation of the UPR serves to help the cell regain homeostasis during environmental stress (63). To determine if the cellular pathology visualized corresponded with induction of the UPR, biochemical evaluation of eIF2 and PERK phosphorylation in tissue lysates was undertaken. Previously we reported that phosphorylation of eIF2α did not change in the pancreas following a single injection of asparaginase (9, 46). The tissue homogenization buffer used in those studies lacked detergent and, thus, excluded proteins associated with cellular membranes. Because our electron micrographs suggested induction of ER stress and autophagy, tissue samples in the current study were homogenized in RIPA lysis buffer to capture PERK and other proteins associated with the ER. Immunoblot analysis showed that phosphorylation of eIF2α was unchanged by asparaginase in WT mice but was elevated in the pancreas of ΔGcn2 mice (Fig. 4A). Phosphorylation of PERK, a measure of activation by ER stress, was also significantly induced within the pancreas of ΔGcn2 mice (Fig. 4B). Mean responses were highest in GA mice, which showed twofold increases in phosphorylation of eIF2α and PERK compared with WP controls.
Fig. 4.
Activation of the amino acid response (AAR) and unfolded protein response (UPR) in asparaginase-treated ΔGcn2 mice. A: phosphorylation (Ser51) of eukaryotic initiation factor 2α (eIF2α) in whole pancreas by immunblot. B: phosphorylation (Thr980) of protein kinase R-like ER kinase (PERK) in whole pancreas by immunoblot. C: RT-quantitative PCR (qPCR) was used to calculate mRNA expression of AAR genes activating transcription factor (ATF) 4 (Atf4), ATF3 (Atf3), asparagine synthetase (Asns), CCAAT/enhancer-binding protein-β (Cebpb), C/EBP homologous protein (Chop), and fibroblast growth factor 21 (Fgf21) in freshly isolated whole pancreas. D: RT-qPCR was used to calculate mRNA expression of UPR genes Atf6, heat shock 70-kDa protein 5 (Hsp5a), spliced Xbp1 (sXbp1), and unspliced Xbp1 (uXbp1) in freshly isolated whole pancreas. E: RT-qPCR was used to calculate mRNA expression of a biomarker of oxidative stress [sirtuin (Sirt1)], inflammation [TNFα (Tnfα)], and tissue injury [pancreatitis-associated protein (Pap) in pancreas (Panc) and liver]. Means not sharing the same letter(s) are different (P < 0.05). *P < 0.05 for main effect of drug. †P < 0.05 for main effect of strain. Values are means ± SE (n = 8–12 per group). Treatment groups were defined as follows: WT + PBS (WP), WT + asparaginase (WA), ΔGcn2 + PBS (GP), and ΔGcn2 + asparaginase (GA).
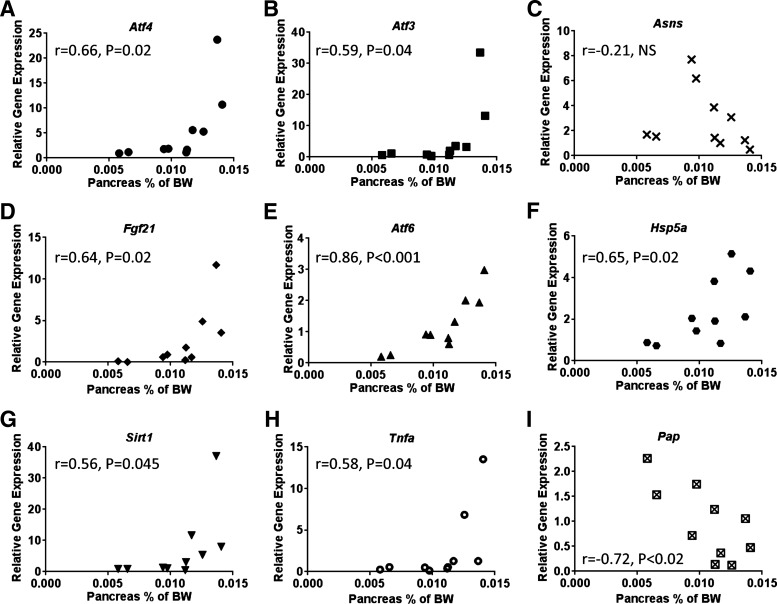
Activation of the AAR occurs in part via ATF4-driven transcriptional control (30). Atf4 mRNA expression in the pancreas of WA mice did not differ from that in the pancreas of WP mice, and the ATF4 gene targets Asns, Atf3, and fibroblast growth factor 21 (Fgf21) were unchanged (Fig. 4C). In contrast, gene expression levels of Atf4 in the pancreas of ΔGcn2 mice were significantly (∼5-fold) elevated by asparaginase treatment. This increase corresponded with higher mean expression levels of several ATF4 target genes (Atf3, Asns, and Fgf21) in GA mice, but sample variation prevented statistical significance, despite logarithmic transformation to achieve homoscedasticity. Further examination into the basis for the variance in the GA group revealed that the expression levels of some AAR genes correlated with increased pancreas weight. Specifically, GA animals with higher pancreas weight relative to body weight expressed higher mRNA levels of Atf4, Atf3, and Fgf21 (Fig. 5, A–D). On the other hand, not all genes with CARE/AARE cis elements, such as C/EBP homologous protein (Chop) and CCAAT/enhancer-binding protein-β (Cebpb), which remained similar among the four treatment groups, were altered by drug and/or strain (Fig. 4C). Furthermore, even though mean Asns levels were highest in GA mice, these levels did not correlate with pancreas mass (Fig. 5C).
Fig. 5.
Pearson correlation calculations comparing gene expression of Atf4 (A), Atf3 (B), Asns (C), Fgf21 (D), Atf6 (E), Hsp5a (F), Sirt1 (G), Tnfa (H), and Pap (I) in the pancreas of GA mice with GA pancreas weight. Pancreas weight is expressed as a proportion of body weight. Correlation coefficient (r) of each comparison is indicated with the corresponding P value.
Sustained activation of the PERK pathway can instigate a maladaptive UPR, contributing to the etiology of disease (63). To evaluate induction of the other branches of the UPR, we next measured the mRNA levels of the UPR targets Atf6, heat shock 70-kDa protein 5 (Hspa5), and spliced and unspliced Xbp1 (Fig. 4D). Similar to ATF4, mean expression levels of Atf6 and Hspa5 were highest in GA mice. In addition, a main effect of strain on spliced Xbp1 mRNA was present in combination with a main effect of drug on the unspliced Xbp1 gene transcript. The Xbp1 combined results suggest an increase in Xbp1 mRNA splicing in GA pancreata. Furthermore, comparison of UPR gene expression with pancreas weight revealed a significant correlation of mRNA levels of Atf6 and Hspa5 to pancreas weight (Fig. 5, E and F). This distribution of effect in GA mice is consistent with a model in which onset of AAP is dynamic, in agreement with our previous report showing onset of morbidity between days 8 and 12 (65). To examine this last point further, genetic markers of oxidative stress [sirtuin (Sirt1)], inflammation [tumor necrosis factor-α (Tnfα)], and pancreatic injury [pancreatitis-associated protein (Pap)] were also assessed (Fig. 4E). Similar to markers of AAR and UPR activation, no changes in gene expression were noted in WA mice, and mean responses were highest in GA mice. Again, comparison of gene expression in GA mice with pancreas weight showed that, similar to the AAR and UPR, larger pancreata displayed higher expression levels of Sirt1 and Tnfa transcripts (Fig. 5, G and H). Thus the preponderance of oxidative stress and inflammation among animals is suggested to correlate with increased pancreas mass. Contrary to our expectation, pancreatic expression of Pap did not share this same relationship and, instead, was inversely correlated with organ mass. However, examination of Pap in the livers of these mice revealed increased mRNA expression in ΔGcn2 mice, with the highest level of expression in GA mice (Fig. 4E). These data, in combination with our previous reports (64, 65), suggest that pancreatic deterioration was occurring along with a maladaptive UPR. These findings suggest that asparaginase treatment of the ΔGcn2 mice can aggravate induction of the PERK branch of the UPR, along with increased pancreas mass.
DISCUSSION
Acute pancreatitis is a well-known, but little understood, complication of asparaginase treatment. Here, we show that deletion of Gcn2 instigates a maladaptive response to repeated doses of asparaginase in the pancreas of mice, predisposing to AAP. The molecular mechanism of this maladaptive response is rooted in heightened PERK phosphorylation under basal conditions, which evolves into an exaggerated UPR and autophagy in pancreatic acini. Asparaginase-treated ΔGcn2 mice displayed increased pancreas mass, which then showed significant induction of key UPR markers. Interestingly, recognized biomarkers of asparagine depletion (Asns) and pancreatic injury (serum amylase, TUNEL-positive nuclei, and expression of Pap) were not useful in reflecting the degree of pancreatic stress in the mouse. Instead, biomarkers of inflammation (Tnfa) and oxidative stress (Sirt1), but not lipotoxicity, corresponded with hyperactivation of the UPR in the exocrine pancreas of GA mice. These latter findings were substantiated by histochemical and ultrastructural evidence of edema, ER stress, and cytoplasmic vacuole accumulation in GA acini. These data collectively suggest that a compromised GCN2-driven ISR/AAR primes the pancreas toward ER stress during asparaginase treatment, resulting in injury and, eventually, AAP.
The phenotypic heterogeneity in GA pancreata is likely a consequence of the choice to end the study before GA mice became uniformly moribund. Previously, we showed that WT mice can tolerate up to 14 daily injections of asparaginase without adverse events, whereas ΔGcn2 mice become progressively ill following 6 daily injections and require euthanasia following 8–12 daily injections of asparaginase (64, 65). On the basis of this time course, the absence of changes in serum amylase alongside molecular and histological variability within GA pancreata at day 8 is consistent with an early stage of acute edematous pancreatitis. It is perplexing that eIF2 phosphorylation in the pancreas is insensitive to asparaginase acutely (9, 46) and chronically (Fig. 4C). The specific activity of ASNS in mouse tissues is highest in the pancreas, with exocrine cells contributing >99% of the activity to provide asparagine for important pancreatic proteins, namely, digestive enzymes that contain asparaginyl residues (39). However, the pancreas does not contribute to circulating concentrations of asparagine (40), suggesting that the pancreas may be insensitive to and/or buffered from changes in serum asparagine. We speculate that eIF2 phosphorylation is briefly activated in response to glutamine depletion, but the study end point missed the window of detection. This idea is consistent with our previous report showing increased Asns mRNA expression at 6 h after injection of asparaginase, despite no change in eIF2 phosphorylation (46). The current study also points to basal activation of UPR components as early predisposing factors to developing AAP. Future acute time-course studies are warranted to assess if circulating levels of amino acids can reflect UPR activation and injury in WT and ΔGcn2 mice by asparaginase.
Acute pancreatitis occurs when premature activation of pancreatic zymogens instigates pancreatic autodigestion (7). Premature activation can occur when the expression level of trypsinogen exceeds that of the proteinase inhibitor α1-antitrypsin, allowing for autoactivation into trypsin. Pancreatic α1-antitrypsin is a member of the serpin (serine proteinase) superfamily of proteins, which are involved in regulation of a multitude of cellular processes that include digestion, coagulation, complement activation, fibrinolysis, inflammation, and tissue remodeling (24). In the liver of WT mice, antithrombin and α1-antitrypsin are susceptible to protein misfolding and aggregation during asparaginase treatment, resulting in a transient state of ER stress described as a “temporary conformational disease” (21) that resolves with continued treatment over 6–14 days (64, 65). In contrast, asparaginase-treated ΔGcn2 mice do not demonstrate recovery of serpin function and, instead, show reduced hepatic levels of antithrombin III in concert with altered hemostasis (65). When these findings are applied to the pancreas, one possible mechanism by which ΔGcn2 predisposes to AAP is via reduced expression of α1-antitrypsin and other serpins, promoting premature zymogen activation.
Exocrine pancreas acinar cells have the highest protein production capacity of any mammalian organ, necessitating a highly developed ER to properly synthesize, fold, and secrete a large client load of digestive enzymes (42). ER stress is documented in multiple forms of pancreatitis (27, 33, 34, 36, 37, 54) and, when self-limiting in action, the UPR plays an adaptive role, facilitating cellular protection to environmental stressors (35). On the other hand, more severe forms of pancreatic disease show exaggerated and/or unremitting UPR activation, corresponding with oxidative and inflammatory stress and cell death (51). These studies indicate that, while an adaptive UPR helps resolve cell stress, a maladaptive UPR drives cell fate toward death pathways. It is hypothesized that when key factors necessary to support an adaptive UPR are lacking, the exocrine cell is unable to manage the client load during environmental stressors, resulting in dysfunction and cellular pathology (42). The current results support this notion and suggest that, during the strain of amino acid depletion by asparaginase, a key determinant is the function of GCN2. It is suggested that diminishment of the GCN2 pathway can render a patient susceptible to the secondary ER stress, which, in combination with asparaginase treatment, can trigger maladaptive responses and, eventually, complications of the pancreas.
GCN2 is conserved from yeast to humans. Under conditions of balanced amino acid nutrition, whole body deletion of Gcn2 in mice is nonlethal and asymptomatic, without effect on growth or reproduction (67). However, single or imbalanced removal of amino acids from the diet results in rapid deterioration of health in organisms lacking GCN2 (3). Because the US diet is nutritionally replete with protein, alterations in GCN2 function are likely to be masked. Small genetic changes in EIF2AK4 are widely present in the population, with >6,500 SNPs listed in the National Center for Biotechnology Information SNP database. At least one identified SNP is associated with ethnicity (66), and several others correspond with pathogenic diseases such as schizophrenia and pulmonary hypertension (12, 15). Considering that two genes regulated by GCN2 activity, namely, ATF5 and ASNS, are identified as harboring SNPs that influence asparaginase treatment success (6, 49), we suggest that polymorphisms in EIF2AK4, alone or in combination with other AAR genes, may promote ER stress and instigate maladaptive outcomes during asparaginase treatment.
A unique feature of the current study paradigm is the use of asparaginase as a single agent and in a nontumor environment, so results are not confounded by tumor biology or drug interactions. This is important to identify the mechanism by which asparaginase by itself can become toxic in humans and other species such as dogs (53, 58). With the understanding that asparaginase is rarely used as a single agent, future studies examining asparaginase in combination with the standard cocktail of chemotherapies [especially steroids that are also associated with pancreatitis (31)] are warranted. Future studies are also needed to resolve whether differential outcomes in the pancreas require pancreatic GCN2 or are secondary to GCN2 action in another tissue or tissues. Tissue-specific ΔGcn2 animal and/or tumor models will be useful in further assessing how the timing or dose influences onset of AAP and other toxicities. Finally, a more detailed exploration over time into the relationship between the UPR, autophagy, and cell death should reveal additional markers that can better predict the onset of AAP. A deeper understanding of the nature of genetic variations in the GCN2-driven AAR and their relationship to treatment success in patients diagnosed with ALL will lead to novel screening tools and other evidence-based treatment options.
GRANTS
This work was supported by National Institutes of Health Grant HD070487 (T. G. Anthony).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.P.-W., E.T.M., Y.W., W.G.M., and T.G.A. performed the experiments; L.P.-W., E.T.M., Y.W., and T.G.A. analyzed the data; L.P.-W. and T.G.A. interpreted the results of the experiments; L.P.-W. and T.G.A. prepared the figures; L.P.-W. and T.G.A. drafted the manuscript; L.P.-W., W.G.M., R.C.W., and T.G.A. edited and the revised manuscript; L.P.-W., E.T.M., Y.W., W.G.M., R.C.W., and T.G.A. approved the final version of the manuscript; T.G.A. developed the concept and designed the research.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expert technical assistance of Inna Nikonorova and Rana J. T. Al Baghdadi.
REFERENCES
- 1.Aldoss I, Douer D, Behrendt CE, Chaudhary P, Mohrbacher A, Vrona J, Pullarkat V. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol 96: 375–380, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez OA, Zimmerman G. Pegaspargase-induced pancreatitis. Med Pediatr Oncol 34: 200–205, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 279: 36553–36561, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian MN, Butterworth EA, Kilberg MS. Asparagine synthetase: regulation by cell stress and involvement in tumor biology. Am J Physiol Endocrinol Metab 304: E789–E799, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerji J. Asparaginase treatment side-effects may be due to genes with homopolymeric Asn codons. Int J Mol Med 36: 607–626, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Tanfous M, Sharif-Askari B, Ceppi F, Laaribi H, Gagne V, Rousseau J, Labuda M, Silverman LB, Sallan SE, Neuberg D, Kutok JL, Sinnett D, Laverdiere C, Krajinovic M. Polymorphisms of asparaginase pathway and asparaginase-related complications in children with acute lymphoblastic leukemia. Clin Cancer Res 21: 329–334, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 286: G189–G196, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bunpo P, Cundiff JK, Reinert RB, Wek RC, Aldrich CJ, Anthony TG. The eIF2 kinase GCN2 is essential for the murine immune system to adapt to amino acid deprivation by asparaginase. J Nutr 140: 2020–2027, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunpo P, Dudley A, Cundiff JK, Cavener DR, Wek RC, Anthony TG. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent l-asparaginase. J Biol Chem 284: 32742–32749, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairo MS. Adverse reactions of l-asparaginase. Am J Pediatr Hematol Oncol 4: 335–339, 1982. [PubMed] [Google Scholar]
- 11.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 119: 34–43, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drago A, Giegling I, Schäfer M, Hartmann AM, Konte B, Friedl M, Serretti A, Rujescu D. Genome-wide association study supports the role of the immunological system and of the neurodevelopmental processes in response to haloperidol treatment. Pharmacogenet Genomics 24: 314–319, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol 7: 600–606, 2009. [PubMed] [Google Scholar]
- 14.El-Nagga NEA, El-Ewasy SM, El-Shweihy NM. Microbial l-asparaginase as a potential therapeutic agent for the treatment of acute lymphoblastic leukemia: the pros and cons. Int J Pharmacol 10: 182–199, 2014. [Google Scholar]
- 15.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, Chelghoum N, Coulet F, Bonnet D, Dorfmüller P, Fadel E, Sitbon O, Simonneau G, Tregouët DA, Humbert M, Soubrier F. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 46: 65–69, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Figueiredo L, Cole PD, Drachtman RA. Asparaginase Erwinia chrysanthemi as a component of a multi-agent chemotherapeutic regimen for the treatment of patients with acute lymphoblastic leukemia who have developed hypersensitivity to E. coli-derived asparaginase. Expert Rev Hematol 9: 227–234, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol 303: G993–G1003, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, Gukovskaya AS. Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol 27 Suppl 2: 27–32, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA. PERK and GCN2 contribute to eIF2α phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell 16: 5493–5501, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Espinosa D, Minano A, Martinez C, Perez-Ceballos E, Heras I, Fuster JL, Vicente V, Corral J. l-Asparaginase-induced antithrombin type I deficiency: implications for conformational diseases. Am J Pathol 169: 142–153, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma 57: 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med 373: 1541–1552, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Huntington JA. Serpin structure, function and dysfunction. J Thromb Haemost 9 Suppl 1: 26–34, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Hyun JJ, Lee HS. Experimental models of pancreatitis. Clin Endosc 47: 212–216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2α kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol 8: 38, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji B, Chen X, Misek D, Kuick R, Hanash S, Ernst S, Najarian R, Logsdon C. Pancreatic gene expression during the initiation of acute pancreatitis: identification of EGR-1 as a key regulator. Physiol Genomics 14: 59–72, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kearney SL, Dahlberg SE, Levy DE, Voss SD, Sallan SE, Silverman LB. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer 53: 162–167, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilberg MS, Balasubramanian M, Fu L, Shan J. The transcription factor network associated with the amino acid response in mammalian cells. Adv Nutr 3: 295–306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20: 436–443, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knoderer HM, Robarge J, Flockhart DA. Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer 49: 634–639, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 40: 280–293, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubisch CH, Logsdon CD. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 292: G1804–G1812, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, Gorelick FS, Pandol SJ. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 140: 987–997, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lugea A, Waldron RT, Pandol SJ. Pancreatic adaptive responses in alcohol abuse: role of the unfolded protein response. Pancreatology 15: S1–S5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malo A, Kruger B, Goke B, Kubisch CH. 4-Phenylbutyric acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Pancreas 42: 92–101, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Malo A, Kruger B, Seyhun E, Schafer C, Hoffmann RT, Goke B, Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 299: G877–G886, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 8: 1149–1154, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Milman HA, Cooney DA. The distribution of l-asparagine synthetase in the principal organs of several mammalian and avian species. Biochem J 142: 27–35, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milman HA, Cooney DA, Young DM. Role of pancreatic l-asparagine synthetase in homeostasis of l-asparagine. Am J Physiol Endocrinol Metab Gastrointest Physiol 236: E746–E753, 1979. [DOI] [PubMed] [Google Scholar]
- 41.Muwakkit S, Saab R, Yazbeck N, Samia L, Abboud MR. l-Asparaginase-induced pancreatitis in children with acute lymphoblastic leukemia: is allopurinol protective? Pediatr Hematol Oncol 27: 496–501, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Pandol SJ, Gorelick FS, Lugea A. Environmental and genetic stressors and the unfolded protein response in exocrine pancreatic function—a hypothesis. Front Physiol 2: 8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raja RA, Schmiegelow K, Albertsen BK, Prunsild K, Zeller B, Vaitkeviciene G, Abrahamsson J, Heyman M, Taskinen M, Harila-Saari A, Kanerva J, Frandsen TL. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the NOPHO ALL2008 protocol. Br J Haematol 165: 126–133, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Raja RA, Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol 159: 18–27, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Raja RA, Schmiegelow K, Henriksen BM, Leth Frandsen T. Serial ultrasound monitoring for early recognition of asparaginase associated pancreatitis in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol 32: 474–481, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Reinert RB, Oberle LM, Wek SA, Bunpo P, Wang XP, Mileva I, Goodwin LO, Aldrich CJ, Durden DL, McNurlan MA, Wek RC, Anthony TG. Role of glutamine depletion in directing tissue-specific nutrient stress responses to l-asparaginase. J Biol Chem 281: 31222–31233, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Richards NG, Kilberg MS. Asparagine synthetase chemotherapy. Annu Rev Biochem 75: 629–654, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ron D, Harding HP. Protein-folding homeostasis in the endoplasmic reticulum and nutritional regulation. Cold Spring Harb Perspect Biol 4: a103177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rousseau J, Gagne V, Labuda M, Beaubois C, Sinnett D, Laverdiere C, Moghrabi A, Sallan SE, Silverman LB, Neuberg D, Kutok JL, Krajinovic M. ATF5 polymorphisms influence ATF function and response to treatment in children with childhood acute lymphoblastic leukemia. Blood 118: 5883–5890, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rytting ME. Role of l-asparaginase in acute lymphoblastic leukemia: focus on adult patients. Blood Lymph Cancer Targets Ther 2: 117–124, 2012. [Google Scholar]
- 51.Sah RP, Garg SK, Dixit AK, Dudeja V, Dawra RK, Saluja AK. Endoplasmic reticulum stress is chronically activated in chronic pancreatitis. J Biol Chem 289: 27551–27561, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samarasinghe S, Dhir S, Slack J, Iyer P, Wade R, Clack R, Vora A, Goulden N. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol 162: 710–713, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Schleis SE, Rizzo SA, Phillips JC, LeBlanc AK. Asparaginase-associated pancreatitis in a dog. Can Vet J 52: 1009–1012, 2011. [PMC free article] [PubMed] [Google Scholar]
- 54.Seyhun E, Malo A, Schafer C, Moskaluk CA, Hoffmann RT, Goke B, Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 301: G773–G782, 2011. [DOI] [PubMed] [Google Scholar]
- 55.Shan J, Fu L, Balasubramanian MN, Anthony T, Kilberg MS. ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. J Biol Chem 287: 36393–36403, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18: 7499–7509, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siu F, Bain PJ, LeBlanc-Chaffin R, Chen H, Kilberg MS. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem 277: 24120–24127, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Smallwood TL, Small GW, Suter SE, Richards KL. Expression of asparagine synthetase predicts in vitro response to l-asparaginase in canine lymphoid cell lines. Leuk Lymphoma 55: 1357–1365, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem 283: 35106–35117, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, Wek RC. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell 22: 4390–4405, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Treepongkaruna S, Thongpak N, Pakakasama S, Pienvichit P, Sirachainan N, Hongeng S. Acute pancreatitis in children with acute lymphoblastic leukemia after chemotherapy. J Pediatr Hematol Oncol 31: 812–815, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Vrooman LM, Kirov I, Dreyer ZE, Kelly M, Hijiya N, Brown P, Drachtman RA, Messinger YH, Ritchey AK, Hale GA, Maloney K, Lu Y, Plourde PV, Silverman LB. Activity and toxicity of intravenous Erwinia asparaginase following allergy to E. coli-derived asparaginase in children and adolescents with acute lymphoblastic leukemia. Pediatr Blood Cancer 63: 228–233, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529: 326–335, 2016. [DOI] [PubMed] [Google Scholar]
- 64.Wilson GJ, Bunpo P, Cundiff JK, Wek RC, Anthony TG. The eukaryotic initiation factor 2 kinase GCN2 protects against hepatotoxicity during asparaginase treatment. Am J Physiol Endocrinol Metab 305: E1124–E1133, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson GJ, Lennox BA, She P, Mirek ET, Al Baghdadi RJ, Fusakio ME, Dixon JL, Henderson GC, Wek RC, Anthony TG. GCN2 is required to increase fibroblast growth factor 21 and maintain hepatic triglyceride homeostasis during asparaginase treatment. Am J Physiol Endocrinol Metab 308: E283–E293, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang F, Chen XD, Tan LJ, Shen J, Li DY, Zhang F, Sha BY, Deng HW. Genome wide association study: searching for genes underlying body mass index in the Chinese. Biomed Environ Sci 27: 360–370, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 22: 6681–6688, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]