Abstract
Microsomal prostaglandin E synthase-1 (mPGES-1) is the terminal enzyme for the synthesis of prostaglandin E2 (PGE2), a proproliferative and antiapoptotic lipid molecule important for tissue regeneration and injury repair. In this study, we developed transgenic (Tg) mice with targeted expression of mPGES-1 in the liver to assess Fas-induced hepatocyte apoptosis and acute liver injury. Compared with wild-type (WT) mice, the mPGES-1 Tg mice showed less liver hemorrhage, lower serum alanine transaminase (ALT) and aspartate transaminase (AST) levels, less hepatic necrosis/apoptosis, and lower level of caspase cascade activation after intraperitoneal injection of the anti-Fas antibody Jo2. Western blotting analysis revealed increased expression and activation of the serine/threonine kinase Akt and associated antiapoptotic molecules in the liver tissues of Jo2-treated mPGES-1 Tg mice. Pretreatment with the mPGES-1 inhibitor (MF63) or the Akt inhibitor (Akt inhibitor V) restored the susceptibility of the mPGES-1 Tg mice to Fas-induced liver injury. Our findings provide novel evidence that mPGES-1 prevents Fas-induced liver injury through activation of Akt and related signaling and suggest that induction of mPGES-1 or treatment with PGE2 may represent important therapeutic strategy for the prevention and treatment of Fas-associated liver injuries.
Keywords: mPGES-1, PGE2, Jo2, Akt, liver injury
prostaglandin e2 (PGE2) is a biologically active lipid mediator implicated in a variety of physiological and pathophysiological processes, including cell proliferation, apoptosis, inflammation, and carcinogenesis, among others (35, 47, 48, 50). The synthesis of PGE2 in human cells is controlled by several key enzymes including cyclooxygenases (COXs) that catalyze the formation of endoperoxide prostaglandin H2 (PGH2) from arachidonic acid (AA) and microsomal prostaglandin E synthase (mPGES) that isomerizes PGH2 to PGE2 (12, 27, 28, 35, 38, 39, 41, 45).
In the liver, the production of PGE2 is markedly enhanced by upregulation of two inducible enzymes, cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1), in response to various proinflammatory stimuli (1, 5, 30, 35). Studies have shown that PGE2 production via overexpression of COX-2 accelerates endotoxin-induced acute liver inflammation and tissue damage (10), potentiates liver regeneration following partial hepatectomy (2), and promotes hepatocellular cancer cell growth and invasiveness in vitro/vivo (51).
Similar to COX-2, mPGES-1 is a cytokine-inducible enzyme critically involved in pain, inflammation, and carcinogenesis. Notably, mPGES-1 is functionally coupled with COX-2, coordinately regulating PGE2 synthesis. However, the potential role of mPGES-1 in modulation of tissue and cell functions is far less recognized compared with COX-2. While recent studies from our laboratory have shown an important role of mPGES1 in liver cancers (21, 44), it remains unknown whether mPGES-1 is implicated in other aspects of liver pathobiology.
Fas is a cell surface protein that belongs to the tumor necrosis factor receptors superfamily (15, 17, 31, 42). In the liver, Fas-induced hepatocyte apoptosis is implicated in the pathogenesis of diverse liver diseases, including hepatitis B and C infections, alcoholic and nonalcoholic liver steatohepatitis, cholestatic liver injury, and hepatocarcinogenesis (6, 8, 14, 18, 22, 25, 26, 32, 37, 46, 49). Thus Fas-induced hepatocyte apoptosis and liver injury have broad implications in various acute and chronic liver diseases.
The goal of the current study is to investigate the potential role of mPGES-1 in Fas-induced hepatocyte apoptosis and acute liver injury. We generated transgenic mice with target expression of mPGES-1 in the liver (mPGES-1 Tg); the produced mice were subjected to intraperitoneal injection of the Fas antibody Jo2. Our data showed that mPGES-1 overexpression prevents Fas-induced hepatocyte apoptosis and liver injury through activation of Akt and related signaling molecules. Consistent with these findings, we observed that inhibition of mPGES-1 or Akt restored the sensitivity of the mPGES-1 Tg mice to Jo2-induced liver injury. Our findings reveal a novel cross talk between mPGES-1 and Akt signaling pathways in the liver, which is important for protection against Fas-induced hepatocyte apoptosis.
MATERIALS AND METHODS
Animal studies.
Transgenic mice with expression of mPGES-1 gene in hepatocytes were developed by pronuclear injection of a mPGES-1 transgene construct into fertilized mouse eggs of B6D2F1 background at the single cell stage. Specifically, human mPGES-1 cDNA was ligated to mouse albumin promoter/enhancer and the construct was microinjected into the pronuclei during the window of time the eggs were visible within the protoplasm. The injected eggs were then transferred into the oviducts of pseudopregnant foster mice. The pups born to the foster mothers with genomic integration of the injected DNA were identified by using tail DNA samples and become transgenic founder mice. The founder was backcrossed with the C57BL/6 wild-type (WT) mice for more than five consecutive generations to produce incipient congenic mPGES-1 Tg mice (B6, ALB-hu-mPGES-1). All the animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Tulane University.
To develop Fas-induced liver injury, male mice at the age of 8–10 wk were injected intraperitoneally with 0.5 μg/g body weight the anti-Fas antibody Jo2 (BD Bioscience, Franklin Lakes, NJ; Jo2 was dissolved in sterile 1× Dulbecco's phosphate-buffered saline). After Jo2 injection, the mice were followed for 12 h for survival analysis or killed at 5 h to obtain blood and liver tissue samples. For inhibitor treatment, mice were injected intraperitoneally with Akt inhibitor V (1 μg/g body wt; Merck Millipore, Billerica, MA) at 2 h before Jo2 injection or were administrated via oral gavage with mPGES-1 inhibitor MF63 (50 μg/g body wt, twice every 12 h; Abmole Bioscience, Houston, TX) before Jo2 injection.
Alanine aminotransferase/aspartate aminotransferase analysis.
Blood samples were centrifuged at 3,000 rpm for 15 min to obtain serum. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured with an automatic analyzer at the Department of Clinical Chemistry, Tulane University Hospital.
Immunohistochemical procedure.
Liver tissues were fixed in 10% buffered formalin and embedded in paraffin. Sections (4 μm) were deparaffinized and processed for hematoxylin-eosin (H&E) staining and immunohistochemistry. Antibodies were diluted in 1× PBS containing 4% horse serum, 0.2% Triton-X100, and 0.4 mg/ml methiolate. mPGES-1 antibody (1:200; Novus Biologicals, Littleton, CO) was used to detect mPGES-1 expression. Cleaved caspase-3 antibody (1:200; Biocare Medical, Pike Lane Concord, CA) was used to detect apoptosis; Ki67 antibody (1:500; Abcam, Cambridge, UK) was used to detect cell proliferation. Horseradish peroxidase-conjugated goat anti-rabbit IgG was used as secondary antibody. Signals were visualized using 0.2 mg/ml diaminobenzidine, 0.01% hydrogen peroxide in 0.1 M phosphate buffer.
Caspases activity analysis.
Liver protein extracts were prepared as previous described (20). Caspase-3/7, caspase-8, and caspase-9 activities were measured with Caspase-Glo Assay kit (Promega, Madison, WI). The caspase activities were expressed as fold changes over the control (corresponding WT mice).
Isolation and culture of primary mouse liver cells.
Hepatocytes were isolated by a two-step collagenase perfusion technique as described previously (9). Collagen I-coated plates and dishes were purchased form BD Biosciences (San Jose, CA). Then, 1 × 106, 3 × 106, or 2.5 × 104 hepatocytes were plated onto collagen-coated 6-well plates, 10-cm dishes, or 96-well plates, respectively. Hepatocytes were maintained in Williams' medium E medium (Invitrogen) supplemented with Hepatocyte Maintenance Supplement Pack (Invitrogen), 10% fetal calf serum (Sigma), 2 mM l-glutamine (Invitrogen), and antibiotics (Invitrogen).
Primary Kupffer cells were isolated and assessed as described previously (16). For treatment with hepatocyte conditioned medium (CM), the CM was collected from hepatocyte cultures and filtered through a 0.22-μm sterile filter; the CM was then diluted 1:1 (vol/vol) with Dulbecco's modified Eagle's medium containing 5% FBS before addition to cultured Kupffer cells.
Hepatic stellate cells were isolated by collagenase digestion and differential centrifugation on Opti-Prep (Sigma)-based density gradient as previously described (16, 24).
Prostaglandin E assay.
Isolated hepatocytes were cultured in serum-free medium with or without supplementation of AA (10 μM) and/or the mPGES-1 inhibitor MF63 (1 μM; Cayman, Ann Arbor, MI; 1 μM) for 6 h. Before culture medium was collected, cells were stimulated with calcium ionophore A23187 (10 μM) for 10 min. Prostaglandin E concentration in culture medium was analyzed according to instruction of the Prostaglandin E2 ELISA kit (Abcam).
Western blotting.
Liver tissue samples or isolated liver cells were homogenized and lysed by NP-40 lysis buffer. All lysis buffers were prepared with the protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Cellular proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked by PBS-T (0.5% Tween 20 in PBS) containing 5% nonfat milk for 1 h at room temperature and then incubated with individual primary antibodies in PBS-T containing 5% nonfat milk for 2–5 h at room temperature with the dilutions specified by the manufacturers. Following three washes with PBS-T, the membranes were incubated with IRDye 680LT/IRDye 800CW secondary antibodies (LI-COR Biosciences, Lincoln, NE) in PBS-T for 1 h at room temperature. The membranes were then washed with PBS-T and the protein bands were visualized by using the ODYSSEY infrared imaging system (LI-COR Biosciences).
Real-time PCR.
Total RNA was extracted from either liver tissue samples or isolated Kupffer cells by using the TRIzol Reagent (Life Technology, Grand Island, NY). The levels of mRNA were quantified by using RT2 SYBR Green qPCR kit (Qiagen, Germantown, MD); GAPDH is used as internal control. The primers used are listed in Table 1.
Table 1.
Primers used in this study
| Primer Name | Sequence 5′ to 3′ |
|---|---|
| m-IL1β | |
| Forward | ACTACAGGCTCCGAGATGAA |
| Reverse | TGGGTCCGACAGCACGAGGC |
| m-IL6 | |
| Forward | CCTAGTGCGTTATGCCTAAGCA |
| Reverse | CCACAG TGAGGAATGTCCACAA |
| m-TNF-α | |
| Forward | CCTGGCCAACGGCATGGATC |
| Reverse | CGGCTGGCACCACTCGTTGG |
| m-MCP-1 | |
| Forward | ACCACAGTCCATGCCATCAC |
| Reverse | TTGAGGTGGTTGTGGAAAAG |
| m-COX-1 | |
| Forward | AGGAGATGGCTGCTGAGTTGG |
| Reverse | AATCTGACTTTCTGAGTTGCC |
| m-COX-2 | |
| Forward | ACACACTCTATCACT GGCACC |
| Reverse | TTCAGGGAGAAGCGT TTGC |
| m-cPGES | |
| Forward | GGTAGAGACCGCCGGAGT |
| Reverse | TCGTACCACTTTGCAGAAGCA |
| h-mPGES-1 | |
| Forward | CATCCTAAAGCATACGGGTCC |
| Reverse | GCTGGTCTTGCCATTCCTG |
| m-mPGES-2 | |
| Forward | GCTGGGGCTGTACCACAC |
| Reverse | GATTCACCTCCACCACCTGA |
| m-15-PGDH | |
| Forward | GTTCGTCCAGTGTGATGTGG |
| Reverse | CCTTCACCTCCGTTTTGCTT |
MCP-1, monocyte chemoattractant protein-1; COX, cyclooxygenase; mPGES and cPGES, mitochondrial and cystolic prostaglandin E synthase; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase.
Statistical analysis.
Data are presented as means ± SE. Differences between two groups were determined by a two-tailed Student's t-test. Kaplan-Meier survival analysis was used for mortality analysis. P < 0.05 was considered to be statistically significant.
RESULTS
Generation of mPGES-1 transgenic mice.
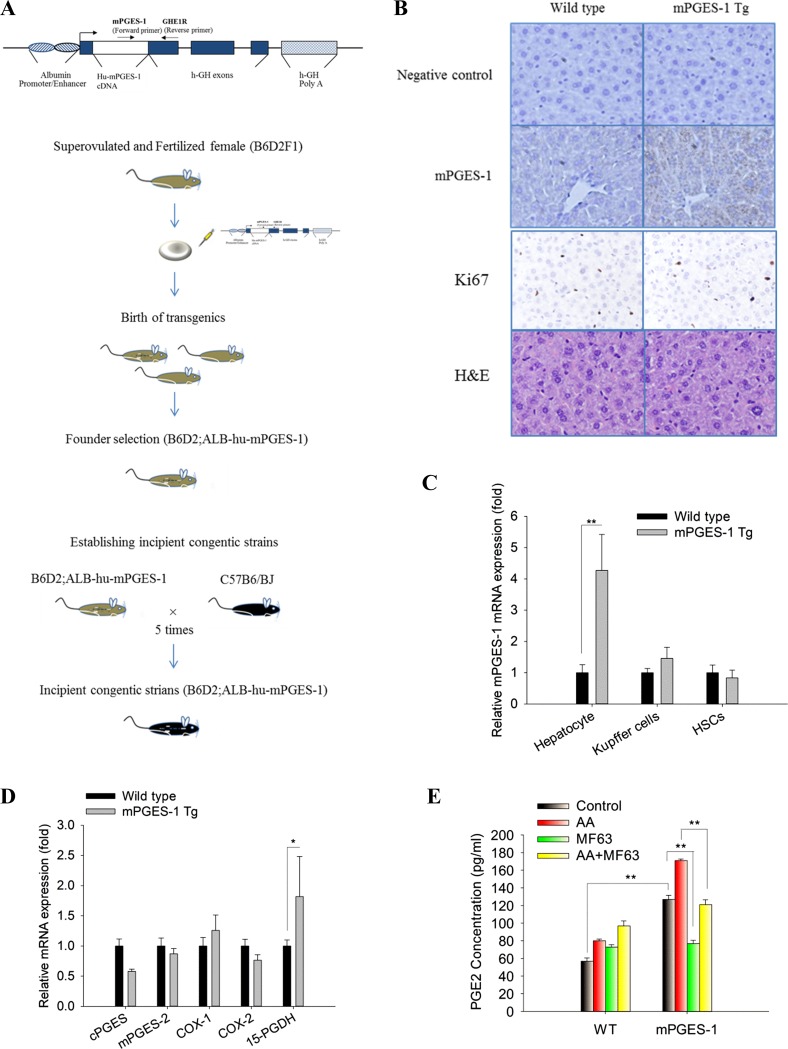
We generated transgenic mice (Tg) with targeted expression of mPGES-1 in the liver by pronuclear injection of a mPGES-1 transgene construct (under the control of albumin promoter/enhancer) into fertilized mouse eggs at the single cell stage (Fig. 1A). Successful hepatocyte expression of mPGES-1 from the produced transgenic mice was confirmed by immunostaining of the liver tissues (Fig. 1B) and by quantitative RT-PCR analysis in isolated hepatocytes (Fig. 1C).
Fig. 1.
Development of liver specific microsomal prostaglandin E synthase-1 (mPGES-1) transgenic (Tg) mice. A: schematic presentation of the strategy to develop mPGES-1 transgenic mice as described in materials and methods. B: representative images of liver tissue sections stained by h-mPGES-1 immunohistochemical (IHC), Ki67 IHC, and hematoxylin-eosin (H&E; ×400). C: mRNA levels of mPGES-1 in hepatocytes, Kupffer cells, and hepatic stellate cells. The results are expressed as mean ± SE of fold changes over wild-type (WT) group. **P < 0.01. D: mRNA levels of other eicosanoid-forming enzymes [cystolic (C)PGES, mPGES-2, cyclooxygenase (COX)-1, COX-2, and 15-hydroxyprostaglandin dehydrogenase (15-PGDH)] in liver tissue homogenates. The results are expressed as mean ± SE of fold changes over WT group. *P < 0.05. E: PGE2 concentration in the media from cultured hepatocytes isolated from WT and mPGES-1 Tg mice. The hepatocytes were incubated with or without the arachidonic acid substrate (10 μM) and treated with or without the mPGES-1 inhibitor MF63 (1 μM). The data are expressed as mean ± SE. **P < 0.01.
The mPGES-1 Tg mice developed normally with no significant alteration of hepatocyte proliferation (Fig. 1B, Ki67 stain) and showed no histological abnormality (Fig. 1B, H&E stain) under normal housing conditions. The mPGES-1 Tg mice showed slightly higher body weight compared with the WT mice, although the liver-to-body weight ratio between the mPGES-1 Tg and WT mice was not statistically different (Table 2). The expression levels of other eicosanoid-forming enzymes in mPGES-1 Tg livers slightly differ from the WT controls [mPGES-1 Tg mice show slight decrease of cystolic (c)PGES and modest increase of 15-hydroxyprostaglandin dehydrogenase (15-PGDH); Fig. 1D].
Table 2.
Overexpression of mPGES-1 in mice liver increases body weight
| Wild Type | mPGES-1 Tg | |
|---|---|---|
| Body weight, g | 24.46 ± 0.26 | 25.76 ± 0.41* |
| Liver weight, g | 1.23 ± 0.03 | 1.27 ± 0.11 |
| Liver/body weight ratio (%) | 5.02 ± 0.11 | 4.93 ± 0.26 |
Values are expressed as mean ± SD from 5 male mice (8 wk).
P < 0.01.
Consistent with the enzymatic action of mPGES-1 for PGE2 synthesis, hepatocytes isolated from the mPGES-1 Tg mice showed a higher level of PGE2 production compared with WT hepatocytes; this effect was augmented when the hepatocytes were incubated with AA, the substrate for PG synthesis. Conversely, the production of PGE2 in the mPGES-1 Tg hepatocytes was decreased by treatment with the mPGES-1 inhibitor MF63 (Fig. 1E). These findings demonstrate that the mPGES-1 Tg mice express functional mPGES-1 in hepatocytes.
Hepatic overexpression of mPGES-1 protects mice against Fas-induced liver injury.
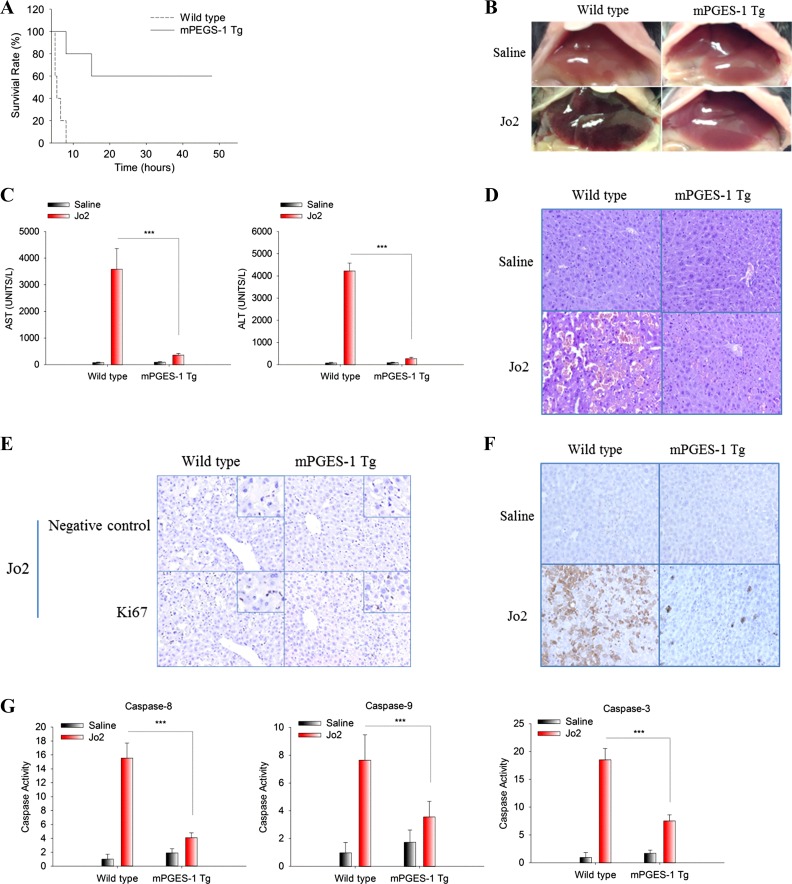
To investigate the effect of mPGES-1 in Fas-induced liver injury, mPGES-1 Tg mice and their age/sex-matched WT mice were intraperitoneally injected with a single dose of Fas monoclonal antibody Jo2 and the animals were closely monitored for mortality. We observed that the mPGES-1 Tg mice had a higher survival rate (60%; monitored for at least 48 h) compared with the WT mice (0% survival rate; all WT mice died by 8 h; P = 0.004, Kaplan-Meier survival analysis; Fig. 2A).
Fig. 2.
Hepatic overexpression of mPGES-1 protects mice against Fas-induced liver injury. mPGES-1 Tg mice and their age/sex-matched WT mice were intraperitoneally injected with a single dose of purified hamster anti-mouse Fas monoclonal antibody Jo2 (0.5 μg/g body wt). Following Jo2 injection, the mice were closely monitored for survival (n = 5). Separate groups of mice were killed at 5 h after Jo2 injection to collect serum and liver tissue samples (n = 3). A: survival curve of mice after Jo2 injection (the mice were closely monitored for at least 48 h). B: representative gross images of livers from different groups of mice. C: serum alanine transaminase (ALT) and aspartate transaminase (AST) levels. The data are expressed as mean ± SE from three mice per group. *** P < 0.001. D: representative microscopic images of liver tissue sections (H&E stain, ×200). E: representative images of IHC stain for Ki67 in liver tissues (×200, insets at higher magnification); note the positive Ki67 stain in sinusoidal leukocytes, but negative Ki67 stain in hepatocytes. F: representative images of IHC stain for cleaved caspase-3 in liver tissues (×200). G: caspase-9, -8, and -3 activities in liver tissue homogenates. The results are presented as mean ± SE of fold changes over saline-treated WT group. *** P < 0.001.
On the basis of the survival curve, additional groups of animals were killed at 5 h after Jo2 injection to collect blood and liver tissues for evaluation of liver injury. Upon Jo2 treatment, mPGES-1 Tg mice exhibited less liver injury, as evidenced by less hemorrhagic appearance under gross examination (Fig. 2B), lower serum ALT and aspartate AST levels (Fig. 2C), less liver tissue injury under histological examination (H&E staining; Fig. 2D), and lower level of apoptosis as determined by immunostaining for cleaved caspase-3 (Fig. 2F), caspase activity assays (Fig. 2G) and poly(ADP-ribose) polymerase (PARP) cleavage (see Fig. 4A). Immunohistochemical analysis for Ki67 showed that mPGES-1 overexpression did not significantly alter hepatocyte proliferation at this time frame (Fig. 2E). These results demonstrate that hepatic overexpression of mPGES-1 protects mice against Fas-induced hepatocyte apoptosis and liver injury. Our data support that the protective effect of mPGES-1 is mediated predominantly through enhancement of hepatocyte survival rather than through regulation of hepatocyte proliferation/regeneration.
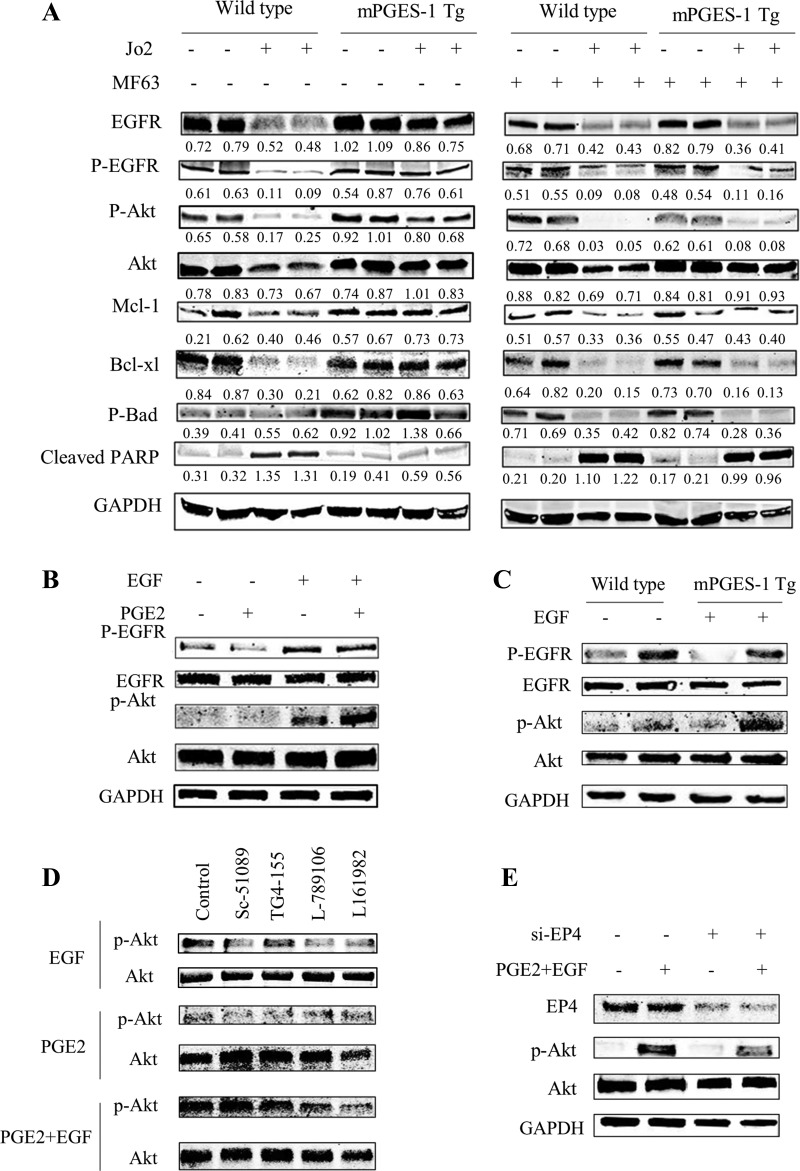
Fig. 4.
Western blotting analysis in liver tissues and hepatocytes. A: levels of EGFR, Akt, and related molecules in liver tissue homogenates. Equal amounts of the liver tissue proteins were subjected to SDS-PAGE and Western blotting analysis to determine the levels of EGFR, p-EGFR, Akt, p-Akt, Mcl-1, Bcl-xl, p-Bad, and cleaved poly(ADP-ribose) polymerase (PARP). GAPDH was measured as the loading control. Quantified band intensity was expressed as ratio over GAPDH. B: Western blotting for EGFR, p-EGFR, Akt, and p-Akt in cultured WT primary hepatocyte incubated with PGE2 (1 μM) for 6 h followed by EGF (10 nM) stimulation for 10 min. C: Western blotting for EGFR, p-EGFR, Akt, and p-Akt in WT and mPGES-1 Tg hepatocytes treated with or without EGF (10 nM for 10 min). D: Western blotting for Akt and p-Akt in cultured WT primary hepatocytes treated with indicated EP receptor antagonists. The cells were incubated with PGE2 and indicated EP receptor antagonists (Sc-51089, EP1 inhibitor; TG4-155, EP2 inhibitor; L-789106, EP3 receptor; L161982, EP4 receptor) for 6 h (with or without 10 nM EGF stimulation for 10 min). E: Western blotting for Akt and p-Akt in WT hepatocytes with siRNA knockdown of EP4. The cells were transfected with scramble control or si-EP4; 48 h later the cells were incubated with PGE2 (1 μM) for 6 h followed by EGF (10 nM) stimulation for 10 min.
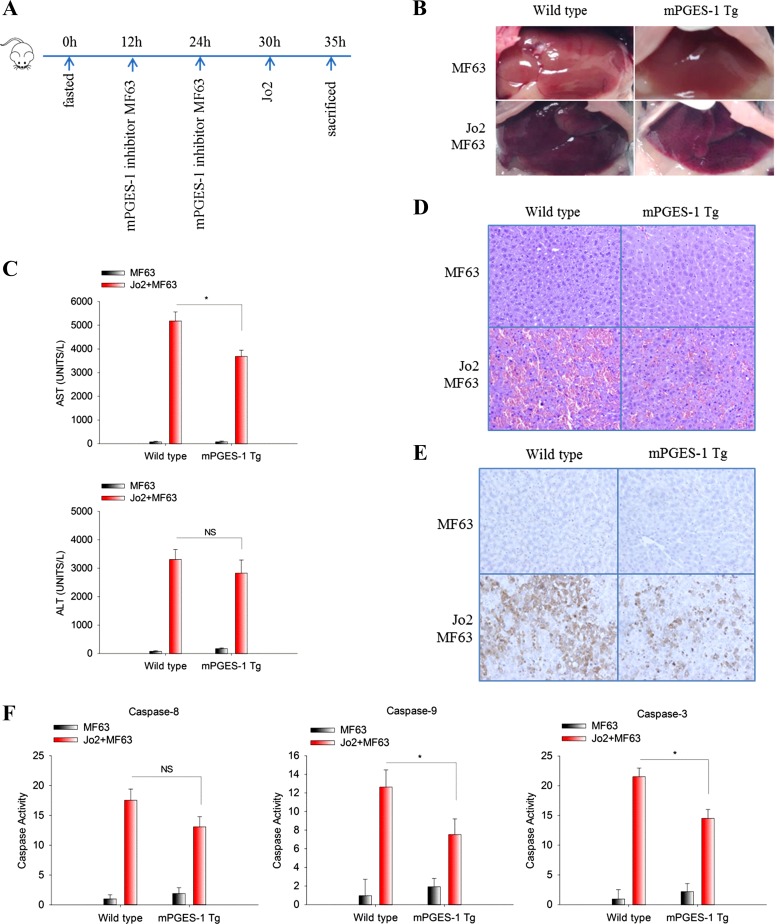
We next utilized a complementary pharmacological approach to further determine the role of mPGES-1 in Fas-induced liver injury. Specifically, the mice were pretreated with MF63, a pharmacological inhibitor of mPGES-1, before Jo2 injection. For this protocol, two doses of MF63 were administrated via oral gavage (every 12 h) before Jo2 injection and the mice were killed 5 h after Jo2 injection (outlined in Fig. 3A). We observed that MF63 pretreatment partially reversed the resistance of mPGES-1 Tg mice to Jo2-induced liver injury (Fig. 3, B–F). These findings further support the role of mPGES-1 in modulation of Fas-induced hepatocyte apoptosis and liver injury.
Fig. 3.
The mPGES1 inhibitor MF63 restores the susceptibility of mPGES-1 Tg mice to Fas-induced liver injury. mPGES-1 Tg mice and their age/sex-matched WT controls (n = 3) were fasted overnight. The mPGES1 inhibitor MF63 (50 μg/g body wt) was administrated via oral gavage (twice every 12 h) before Jo2 (0.5 μg/g body wt) injection. Mice were killed at 5 h after Jo2 injection to collect serum and liver tissue samples. A: timeline of the experiments. B: representative gross images of livers from different groups of mice. C: serum ALT and AST levels. The data are expressed as mean ± SE from 3 mice per group, *P < 0.05; NS, not significant. D: representative microscopic images of liver tissue sections (H&E stain, ×200). E: representative images of IHC stain for cleaved caspase-3 in liver tissues (×200). F: caspase-9, -8, and -3 activities in liver tissue homogenates. The results are expressed as mean ± SE of fold changes over MF63-treated WT group, *P < 0.05; NS, not significant.
Hepatic overexpression of mPGES-1 enhances EGFR/Akt signaling.
PGE2 is known to activate EGFR/Akt cascade and enhance hepatic cell survival (4, 51). Accordingly, activation of EGFR/Akt is able to induce the expression of several antiapoptotic molecules, including Bcl-xl and Mcl-1 (7, 36, 40). In our system, we postulate that mPGES-1 may render hepatocytes resistant to apoptosis via activation of the EGFR/Akt pathway. To evaluate for this possibility, we performed Western blotting analysis to determine the levels of EGFR/Akt and associated apoptosis-regulatory molecules. Under baseline condition (i.e., without Jo2 treatment), the mPGES-1 Tg and WT livers showed similar levels of EGFR expression/phosphorylation (Fig. 4A). Following Jo2 treatment, while the levels of EGFR and p-EGFR in the WT livers became decreased, the mPGES-1 Tg livers showed sustained EGFR expression and phosphorylation. Consistent with the activation of Akt by EGFR, the levels of hepatic p-Akt and associated antiapoptotic molecules (Mcl-1, Bcl-xl, and p-Bad) in Jo2-treated mPGES-1 Tg mice were higher compared with Jo2-treated WT mice (Fig. 4A). The above effects were attenuated by pretreatment with the mPGES-1 inhibitor MF63 in Jo2-treated mPGES-1 Tg mice (Fig. 4A). These findings indicate an important role of EGFR/Akt and associated apoptosis-regulatory molecules in mPGES-1-mediated protection against liver injury.
We next isolated primary hepatocytes to further evaluate PGE2 interaction with EGFR/Akt signaling. In cultured hepatocytes, PGE2 has been reported to enhance EGF-induced EGFR/Akt phosphorylation, although PGE2 treatment alone is insufficient to induce Akt phosphorylation under the in vitro cell culture condition (4). Similarly, in our system, we observed that PGE2 treatment of cultured primary hepatocytes enhanced EGF-induced Akt phosphorylation, while PGE2 treatment alone did not significantly alter Akt phosphorylation (Fig. 4B). Our data showed that EGF induced more Akt phosphorylation in mPGES-1 Tg hepatocytes compared with WT hepatocytes (Fig. 4C); these findings suggest that mPGES-1-derived PGE2 enhances EGF-induced Akt phosphorylation in primary hepatocytes.
PGE2 elicits cellular function via binding to its membrane receptors (EP1 through EP4). To assess the role of EP receptor subtypes that mediate PGE2 actions in hepatocytes, we pretreated hepatocytes with different EP receptor antagonists and observed that the EP4 receptor antagonist L161982 inhibited PGE2/EGF-induced Akt phosphorylation, whereas the antagonists for EP1 (Sc-51089), EP2 (TG4-155), and EP3 (L-789106) exhibited no significant effect (Fig. 4D). The involvement of EP4 receptor was further substantiated by the observation that siRNA knockdown of EP4 in hepatocytes prevented PGE2/EGF-induced Akt phosphorylation (Fig. 4E). Taken together, our findings suggest that EP4 plays a leading role in PGE2/EGFR-induced Akt phosphorylation in primary hepatocytes. Thus it is conceivable that mPGES-1-mediated PGE2 production may enhance EGFR/Akt signaling through EP4 receptor in hepatocytes and this mechanism may be important for prevention of Fas-induced liver injury.
Inhibition of Akt reverses mPGES-1-mediated resistance to Fas-induced liver injury.
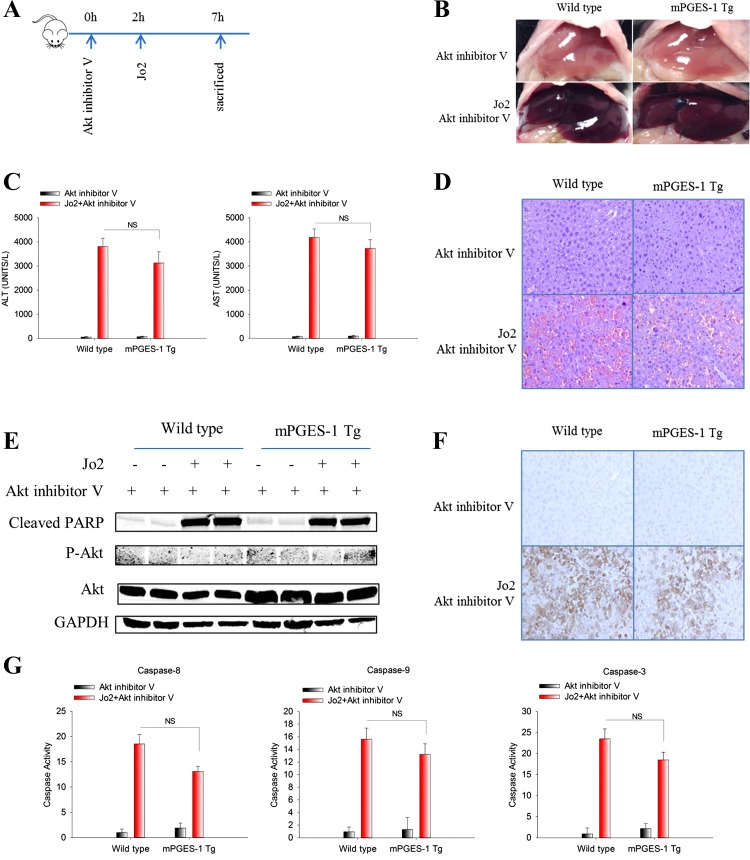
To further determine the role of Akt in mPGES-1-mediated resistance to Fas-induced liver injury, we employed a pharmacological inhibitor of Akt in our system. Specifically, Akt inhibitor V was intraperitoneally injected to WT and mPGES-1 Tg mice 2 h prior to Jo2 injection (outlined in Fig. 5A); 5 h after Jo2 injection the blood and liver tissues were collected to determine the extent of liver injury. We observed that pretreatment with Akt inhibitor V reversed the resistance of mPGES-1 Tg mice to Fas-induced liver injury (Fig. 5, B–G; the efficacy of Akt inhibition is indicated by the fact that Akt inhibitor V treatment abolished the phosphorylation of Akt in the liver tissues). Collectively, our findings support an important role of Akt in mPGES-1-mediated protection against Fas-induced liver injury.
Fig. 5.
Akt inhibitor V restores the susceptibility of mPGES-1 Tg mice to Fas-induced liver injury. mPGES-1 Tg and their age/sex-matched WT mice (n = 3) were intraperitoneally injected with a single dose of Akt inhibitor V (1 μg/g body wt) 2 h before Jo2 (0.5 ug/g body wt) injection. Mice were killed at 5 h after Jo2 injection to collect serum and liver tissue samples. A: timeline of the experiments. B: representative gross images of the livers from different groups of mice. C: serum ALT and AST levels. The data are expressed as mean ± SE from 3 mice per group. D: representative microscopic images of the liver sections (H&E stain, ×200). E: Western blotting for cleaved PARP, p-Akt, and Akt in liver tissue homogenates. GAPDH was used as loading control. F: representative images of IHC stain for cleaved caspase-3 in liver tissues (×200). G: caspase-9, -8, and -3 activities in liver tissue homogenates. The results are expressed as mean ± SE of fold changes over Akt inhibitor V-treated WT group.
The effect of hepatic mPGES-1 on inflammatory cytokines.
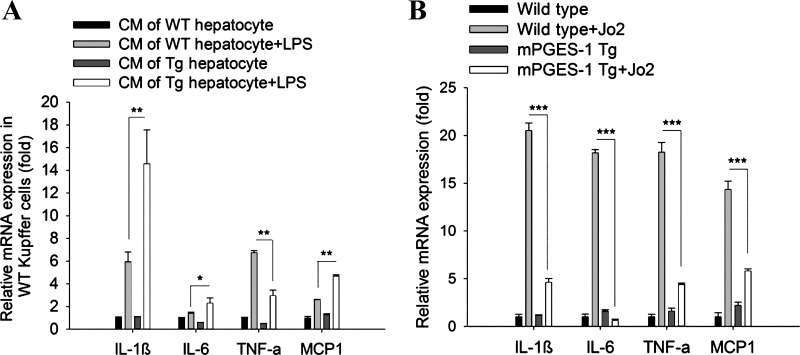
PGE2 is a well-known inflammatory mediator that can regulate cytokine production in the liver. To evaluate the effect of mPGES-1 overexpression on inflammatory cytokine production, we employed an indirect hepatocyte-Kupffer cell coculture system, in which the CM of WT and mPGES-1 Tg hepatocytes was incubated with WT Kupffer cells (LPS was added to induce cytokine production). In this system, we observed that the CM of mPGES-1 Tg hepatocytes increased the expression of IL-1β, IL-6, and monocyte chemoattractant protein-1 (MCP-1), but decreased the expression of TNF-α in Kupffer cells (Fig. 6A). In parallel, we also measured the expression of these inflammatory cytokines in the liver tissues harvested form WT and mPGES-1 Tg mice (with or without Jo2 treatment); we observed that the levels of TNF-α, IL-6, L-1β, and MCP-1 in the liver tissues from Jo2 treated mPGES-1 Tg mice were lower compared with the liver tissues from Jo2-treated WT mice (Fig. 6B). These findings suggest that hepatocyte mPGES-1 can modulate cytokine production under specific conditions.
Fig. 6.
The effect of hepatic mPGES-1 on inflammatory cytokine expression. A: mRNA levels of proinflammatory cytokines [IL-1β, IL-6, TNF-α, and monocyte chemoattractant protein-1 (MCP-1)] in WT Kupffer cells incubated with hepatocyte CM (with or without LPS stimulation) for 4 h. The data are expressed as mean ± SE of fold changes (*P < 0.05; **P < 0.01). B: mRNA levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α, and MCP-1) in liver tissue homogenates from WT and mPGES-1 Tg mice treated with or without Jo2. The results are expressed as mean ± SE of fold changes (***P < 0.001).
DISCUSSION
In the current study, we developed a novel transgenic mouse model with targeted overexpression of mPGES-1 in the liver and the produced animals were utilized to assess Fas-induced hepatocyte apoptosis and acute liver injury. We observe that the mPGES-1 Tg mice protect against Fas-induced hepatocyte apoptosis and liver injury (based on gross examination of the livers, histological evaluation of the liver tissues, assessment of serum transaminases levels, and caspase activity assays). These results are further corroborated by the finding that inhibition of mPGES-1 by its pharmacological inhibitor MF63 restored the susceptibility of mPGES-1 Tg mice to Jo2-induced liver injury.
Our findings suggest that hepatocyte mPGES-1 confers resistance to Fas-induced liver injury through activation of the Akt signaling cascade. This assertion is supported by the following observations: 1) under Jo2 treatment, the mPGES-1 Tg mice showed increased Akt activation as well as increased expression of Akt upstream activator (EGFR) and Akt downstream antiapoptotic molecules (Mcl-1, Bcl-xl, and p-Bad); 2) treatment of the mPGES-1 Tg mice with the mPGES-1 inhibitor MF63 reduced the level of Akt in hepatocytes and liver tissues; and 3) treatment of mPGES-1 Tg mice with Akt inhibitor V restored the sensitivity of mPGES-1 Tg mice to Jo2-induced liver injury. Our data suggest mPGES-1-mediated upregulation of EGFR expression for activation of Akt and related antiapoptotic molecules; this statement is consistent with the previous study that PGE2 induces EGFR expression and Akt activation in hepatocytes (4).
PGE2 is known to play a proliferative and antiapoptotic role in hepatocytes (11, 13, 33) and has been well documented to confer protection against liver damage in animal experiments (29, 34, 43). Accordingly, transgenic mice with hepatic overexpression of COX-2 are resistant to Fas-induced liver injury (3, 19, 23). However, a limitation of the COX-2 transgenic mouse model relates to the fact that COX-2 mediates the synthesis of various prostanoids, including prostacyclin (PGI2), thromboxane A2 (TXA2), and other prostanoids, in addition to PGE2; as such, the possibility of contribution from other prostanoids could not be conclusively excluded for the COX-2 transgenic model. This drawback is avoided in the current study by liver-specific expression of mPGES-1, the terminal synthase of PGE2. We observe that the level of PGE2 production in the mPGES-1 Tg mice is not as high as in the liver-specific COX-2 transgenic mice as described previously (3, 19, 52). This phenomenon may be explained by the limited availability of the substrate for mPGES-1 (PGH2 derived from cyclooxygenases), given that the cyclooxygenase isotype COX-1, but not COX-2, is constitutively expressed in the hepatocytes and that COX-1 exhibits modest enzymatic activity compared with COX-2.
Since PGE2 is an active inflammatory mediator that is known to modulate cytokine production in the liver, we examined the effect of mPGES-1-derived PGE2 on the expression of several key inflammatory cytokines by analyzing the liver tissue samples and by employing the hepatocyte-Kupffer cell coculture system. For the liver tissue sample analysis, we observed that mPGES-1 overexpression inhibited the expression of TNF-α IL-6, L-1β, and MCP-1 in Jo2-treated mice. For the hepatocyte-Kupffer cell coculture studies, our data showed that mPGES-1 overexpression in hepatocytes increased the expression of IL-1β, IL-6, and MCP-1 but decreased the expression of TNF-α in Kupffer cells. While the experimental conditions for the above-indicated assay systems are different and the results cannot be directly compared, our findings from these studies suggest that hepatocyte mPGES-1 is able to modulate cytokine production. Given that different cytokines are known to mediate various effects in the liver (e.g., activation of further immune response by IL-1β/MCP-1, stimulation of hepatocyte proliferation by IL-6, and induction of hepatocyte apoptosis by TNF-α), it is reasonable to speculate that mPGES-1-derived PGE2 might influence certain aspects of hepatic injury and inflammation, at least in part, through regulation of cytokine production. However, the exact role of inflammatory cytokines in mPGES-1-mediated protection against Fas-induced liver injury remains to be further determined.
In summary, this study describes a novel transgenic mouse model with targeted overexpression of mPGES-1 in the liver. We provide the first evidence that mPGES-1 overexpression in the liver prevents Fas-induced hepatocyte apoptosis and liver injury through activation of EGFR/Akt and downstream antiapoptotic molecules. Further studies are warranted to evaluate whether induction of mPGES-1 expression or treatment with PGE2 analog could be developed as a new therapeutic strategy for effective prevention and treatment of Fas-associated liver injuries.
GRANTS
This work was supported by National Cancer Institute Grants R01-CA-102325 and R01-CA-106280.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.Y., C.H., and T.W. conception and design of research; L.Y. and W.C. performed experiments; L.Y. analyzed data; L.Y. interpreted results of experiments; L.Y. prepared figures; L.Y. drafted manuscript; L.Y. and T.W. edited and revised manuscript; T.W. approved final version of manuscript.
REFERENCES
- 1.Bezugla Y, Kolada A, Kamionka S, Bernard B, Scheibe R, Dieter P. COX-1 and COX-2 contribute differentially to the LPS-induced release of PGE2 and TxA2 in liver macrophages. Prostaglandins Other Lipid Mediat 79: 93–100, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Casado M, Callejas NA, Rodrigo J, Zhao X, Dey SK, Bosca L, Martin-Sanz P. Contribution of cyclooxygenase 2 to liver regeneration after partial hepatectomy. FASEB J 15: 2016–2018, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Casado M, Molla B, Roy R, Fernandez-Martinez A, Cucarella C, Mayoral R, Bosca L, Martin-Sanz P. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology 45: 631–638, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Dajani OF, Meisdalen K, Guren TK, Aasrum M, Tveteraas IH, Lilleby P, Thoresen GH, Sandnes D, Christoffersen T. Prostaglandin E2 upregulates EGF-stimulated signaling in mitogenic pathways involving Akt and ERK in hepatocytes. J Cell Physiol 214: 371–380, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Dieter P, Scheibe R, Bezugla Y, Matthe E, Schuch S, Treffkorn L, Bernard B, Kamionka S, Kolada A. The regulatory role of prostaglandin E2 in liver (patho) physiology is controlled at its site of synthesis and its action on the receptors. Comp Hepatol 3, Suppl 1: S35, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125: 437–443, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Fleischer B, Schulze-Bergkamen H, Schuchmann M, Weber A, Biesterfeld S, Muller M, Krammer PH, Galle PR. Mcl-1 is an anti-apoptotic factor for human hepatocellular carcinoma. Int J Oncol 28: 25–32, 2006. [PubMed] [Google Scholar]
- 8.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut 54: 1024–1033, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han C, Bowen WC, Li G, Demetris AJ, Michalopoulos GK, Wu T. Cytosolic phospholipase A2alpha and peroxisome proliferator-activated receptor gamma signaling pathway counteracts transforming growth factor beta-mediated inhibition of primary and transformed hepatocyte growth. Hepatology 52: 644–655, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han C, Li G, Lim K, DeFrances MC, Gandhi CR, Wu T. Transgenic expression of cyclooxygenase-2 in hepatocytes accelerates endotoxin-induced acute liver failure. J Immunol 181: 8027–8035, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto N, Watanabe T, Ikeda Y, Yamada H, Taniguchi S, Mitsui H, Kurokawa K. Prostaglandins induce proliferation of rat hepatocytes through a prostaglandin E2 receptor EP3 subtype. Am J Physiol Gastrointest Liver Physiol 272: G597–G604, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Kang YJ, Mbonye UR, DeLong CJ, Wada M, Smith WL. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res 46: 108–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura M, Osumi S, Ogihara M. Prostaglandin E(2) [EP(1)] receptor agonist-induced DNA synthesis and proliferation in primary cultures of adult rat hepatocytes: the involvement of TGF-alpha. Endocrinology 142: 4428–4440, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med 3: 409–413, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Krammer PH. CD95's deadly mission in the immune system. Nature 407: 789–795, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Kwon H, Song K, Han C, Chen W, Wang Y, Dash S, Lim K, Wu T. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease (NAFLD). Hepatology 63: 1155–1169, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ 19: 36–41, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med 165: 657–663, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Han C, Xu L, Lim K, Isse K, Wu T. Cyclooxygenase-2 prevents fas-induced liver injury through up-regulation of epidermal growth factor receptor. Hepatology 50: 834–843, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, Collins RD, Hawiger J. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem 279: 48434–48442, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lu D, Han C, Wu T. Microsomal prostaglandin E synthase-1 inhibits PTEN and promotes experimental cholangiocarcinogenesis and tumor progression. Gastroenterology 140: 2084–2094, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43: S31–44, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Mayoral R, Molla B, Flores JM, Bosca L, Casado M, Martin-Sanz P. Constitutive expression of cyclo-oxygenase 2 transgene in hepatocytes protects against liver injury. Biochem J 416: 337–346, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Mederacke I, Dapito DH, Affo S, Uchinami H, Schwabe RF. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc 10: 305–315, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minana JB, Gomez-Cambronero L, Lloret A, Pallardo FV, Del Olmo J, Escudero A, Rodrigo JM, Pelliin A, Vina JR, Vina J, Sastre J. Mitochondrial oxidative stress and CD95 ligand: a dual mechanism for hepatocyte apoptosis in chronic alcoholism. Hepatology 35: 1205–1214, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 117: 669–677, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Murakami M, Kudo I. Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr Pharm Des 12: 943–954, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh S, Kudo I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem 275: 32783–32792, 2000. [DOI] [PubMed] [Google Scholar]
- 29.North TE, Babu IR, Vedder LM, Lord AM, Wishnok JS, Tannenbaum SR, Zon LI, Goessling W. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc Natl Acad Sci USA 107: 17315–17320, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119: 229–240, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, Wallach D, Wiltrout RH, Zornig M, Lynch DH. The CD95 receptor: apoptosis revisited. Cell 129: 447–450, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Pianko S, Patella S, Ostapowicz G, Desmond P, Sievert W. Fas-mediated hepatocyte apoptosis is increased by hepatitis C virus infection and alcohol consumption, and may be associated with hepatic fibrosis: mechanisms of liver cell injury in chronic hepatitis C virus infection. J Viral Hepat 8: 406–413, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Refsnes M, Thoresen GH, Dajani OF, Christoffersen T. Stimulation of hepatocyte DNA synthesis by prostaglandin E2 and prostaglandin F2 alpha: additivity with the effect of norepinephrine, and synergism with epidermal growth factor. J Cell Physiol 159: 35–40, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Rincon-Sanchez AR, Covarrubias A, Rivas-Estilla AM, Pedraza-Chaverri J, Cruz C, Islas-Carbajal MC, Panduro A, Estanes A, Armendariz-Borunda J. PGE2 alleviates kidney and liver damage, decreases plasma renin activity and acute phase response in cirrhotic rats with acute liver damage. Exp Toxicol Pathol 56: 291–303, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev 59: 207–224, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, Zink D, Buchler P, Muller M, Krammer PH. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology 39: 645–654, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Sharma AD, Narain N, Handel EM, Iken M, Singhal N, Cathomen T, Manns MP, Scholer HR, Ott M, Cantz T. MicroRNA-221 regulates FAS-induced fulminant liver failure. Hepatology 53: 1651–1661, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69: 145–182, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest 107: 1491–1495, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010: 215158, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stichtenoth DO, Thorén S, Bian H, Peters-Golden M, Jakobsson PJ, Crofford LJ. Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J Immunol 167: 469–474, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity 30: 180–192, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takano M, Nishimura H, Kimura Y, Washizu J, Mokuno Y, Nimura Y, Yoshikai Y. Prostaglandin E2 protects against liver injury after Escherichia coli infection but hampers the resolution of the infection in mice. J Immunol 161: 3019–3025, 1998. [PubMed] [Google Scholar]
- 44.Takii YA, Fujioka H, Nakamura M, Komori A, Ito M, Taniguchi K, Daikoku M, Meda Y, Ohata K, Yano K, Shimoda S, Yatsuhashi H, Ishibashi H, Migita K. Expression of microsomal prostaglandin E synthase-1 in human hepatocelluar carcinoma. Liver Int 27: 989–996, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Thoren S, Weinander R, Saha S, Jegerschold C, Pettersson PL, Samuelsson B, Hebert H, Hamberg M, Morgenstern R, Jakobsson PJ. Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination. J Biol Chem 278: 22199–22209, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Valva P, Casciato P, Lezama C, Galoppo M, Gadano A, Galdame O, Galoppo MC, Mullen E, De Matteo E, Preciado MV. Serum apoptosis markers related to liver damage in chronic hepatitis C: sFas as a marker of advanced fibrosis in children and adults while M30 of severe steatosis only in children. PLoS One 8: e53519, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 10: 181–193, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29: 781–788, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Na B, Ou JH, Pulliam L, Yen TS. Hepatitis B virus alters the antioxidant system in transgenic mice and sensitizes hepatocytes to Fas signaling. PLoS One 7: e36818, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu T. Cyclooxygenase-2 and prostaglandin signaling in cholangiocarcinoma. Biochim Biophys Acta 1755: 135–150, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Wu T. Cyclooxygenase-2 in hepatocellular carcinoma. Cancer Treat Rev 32: 28–44, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Hui AY, Chu ES, Cheng AS, Go MY, Chan HL, Leung WK, Cheung KF, Ching AK, Chui YL, Chan KK, Sung JJ. Expression of a cyclo-oxygenase-2 transgene in murine liver causes hepatitis. Gut 56: 991–999, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]