Abstract
Obesity and Type 2 diabetes are major worldwide public health issues today. A relationship between total fat intake and obesity has been found. In addition, the mechanisms of long-term and excessive high-fat diet (HFD) intake in the development of obesity still need to be elucidated. The ventromedial hypothalamus (VMH) is a major site involved in the regulation of glucose and energy homeostasis where “metabolic sensing neurons” integrate metabolic signals from the periphery. Among these signals, fatty acids (FA) modulate the activity of VMH neurons using the FA translocator/CD36, which plays a critical role in the regulation of energy and glucose homeostasis. During low-fat diet (LFD) intake, FA are oxidized by VMH astrocytes to fuel their ongoing metabolic needs. However, HFD intake causes VMH astrocytes to use FA to generate ketone bodies. We postulate that these astrocyte-derived ketone bodies are exported to neurons where they produce excess ATP and reactive oxygen species, which override CD36-mediated FA sensing and act as a signal to decrease short-term food intake. On a HFD, VMH astrocyte-produced ketones reduce elevated caloric intake to LFD levels after 3 days in rats genetically predisposed to resist (DR) diet-induced obesity (DIO), but not leptin-resistant DIO rats. This suggests that, while VMH ketone production on a HFD can contribute to protection from obesity, the inherent leptin resistance overrides this inhibitory action of ketone bodies on food intake. Thus, astrocytes and neurons form a tight metabolic unit that is able to monitor circulating nutrients to alter food intake and energy homeostasis.
Keywords: astrocytes, feeding, hypothalamus, ketone bodies, metabolic sensing
obesity and type 2 diabetes mellitus have increased drastically in prevalence around the world. Of particular concern is the associated “metabolic syndrome” (hypertension, dyslipidemia, diabetes), which increases the morbidity and mortality of obese individuals. Obesity and diabetes have a major impact upon, and are affected by, the overall regulation of energy and glucose homeostasis. The origins of the obesity epidemic are complex, but commonly cited factors include the consumption of large quantities of highly palatable, energy-dense food, especially those rich in fat (13, 27). Indeed, a correlation between total fat intake and the development of obesity has been demonstrated (27, 30, 77). However, the effects and underlying mechanisms by which chronic and excessive high-fat diet (HFD) intake contributes to the development of obesity are still poorly understood.
Hunger and satiety are two important mechanisms involved in body weight regulation. The brain's actions to regulate these conditions are influenced by nutrients, hormones, peptides and other metabolically related signaling molecules, which cross the blood-brain barrier to alter the activity of specific “metabolic sensing” neurons scattered across diverse anatomical locations in the brain. While the mature human brain weighs only 2–3% of the total body weight (34), neurons themselves store very little fuel and, thus, depend upon the continuous exogenous supply of glucose as its primary metabolic substrate for the majority of the brain's energy requirements (90, 100).
Although much attention has been given to metabolic sensing neurons as regulators of energy and glucose homeostasis, astrocytes, which provide metabolic support for neurons (23, 109), have received much less attention. Astrocyte foot processes directly abut brain microvessels and, thus, are the first cells encountered by nutrients entering the brain (1, 105). This makes them a major site for nutrient uptake, storage, and processing. Aside from their critical roles in maintaining neuronal transmission (5, 66, 85, 88, 89), astrocytes have several important metabolic functions. These include the storage of glycogen and the production of lactate as important substrates for neuronal metabolism, especially during enhanced neuronal activity (86, 87). Especially in the ventromedial hypothalamus [VMH = ventromedial (VMN) + arcuate (ARC) nuclei], astrocytes also produce ketone bodies from free fatty acids (FA) (50, 51). Unlike lactate production, which occurs as a continuous process (9, 86), astrocyte ketone production occurs predominantly when blood FA levels rise as a result of dietary intake (50, 51). While both neuronal glucose and fatty acid sensing have been reviewed extensively in the past, this review will focus specifically on the newly recognized role of local production of ketones by VMH astrocytes as regulators of food intake during intake of HFD. We also provide novel hypotheses regarding the ways in which astrocytes can regulate FA turnover in the VMH and mechanisms by which astrocyte-produced ketones override normal neuronal FA sensing to regulate feeding.
Astrocytes: the Major Source of Brain FA Oxidation and Ketone Body Production
During energy deficits, blood glucose levels decline due to rapid depletion of glycogen stores in the liver and muscle (20, 75, 97). Once glycogen stores are depleted, lipolysis is stimulated in adipose tissue with the release of nonesterified FA. These FA are then converted by the liver into ketone bodies [β-hydroxybutyrate (βOHB) and acetoacetate] through mitochondrial β-oxidation and ketogenesis (95). Recent studies (7, 43) demonstrated that the intestines also produce significant quantities of ketones, which can stimulate local visceral afferents regulating feeding. Both liver- and gut-derived ketones, as well as free FA, can be also transported into the brain to serve as an alternate energy source when glycogen stores are severely depleted, and blood glucose levels decline during fasting (95). However, when energy intake is sufficient to provide surplus amounts of FA, hypothalamic astrocytes also produce ketone bodies from FA (10, 28, 33, 50). Other areas of the brain can also produce ketone bodies but in less abundance (35).
Astrocytes are the major site of FA oxidation and the only source of ketone body production in the brain (10, 24, 25). When glucose is limiting, astrocytes utilize FA as their major source of ATP production (25). These FA directly enter the mitochondria via CPT1, where they undergo β-oxidation (25, 91). In the presence of excess FA and reduced dietary glucose, hypothalamic astrocytes produce ketone bodies (10, 24, 25, 50). The ketogenic pathways used by hepatocytes and astrocytes are almost identical. As shown in vitro, both cell types prefer FA to glucose as their primary metabolic fuel and produce ketone bodies (25, 33, 69). When excess FA are present, ketone bodies are produced using the rate-limiting enzymes 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase and HMG-CoA lyase (36, 37). During intake of a low-fat diet (LFD), after a 24-h fast, we have shown (50) that VMH ketone body levels are ∼20 μM, whereas the FFA levels are ∼37 μM in the presence of ∼350 μM and ∼300 μM serum ketone and FFA levels, respectively. However, when fed a high-fat diet comprising 60% fat (HFD) for several days on a 3 h/day restricted intake schedule, VMH FA levels average only ∼22.5 μM, which is actually lower than levels seen during low-fat dietary intake (∼35 μM) (50). On the other hand, VMH ketone levels spike to ∼100 μM for 1 h during the first 1½ to 2½ h after onset of HFD feeding (50). We postulate (and our preliminary data suggest) that the lower VMH FA and the spike of ketone levels during HFD intake are due to a more avid uptake of FA into astrocytes, which have been primed to produce ketone bodies by the chronic exposure to excess dietary FA. Another possibility is that differences in extracellular FA are caused by altered transport of FA across the blood-brain barrier due to differential regulation of FA transporters on cerebral microvessels during intake of LFD vs. HFD. Once FA enter astrocytes from the extracellular space, they produce ketone bodies that are transported out of the astrocytes via monocarboxylate transporter 1 (MCT1) and are then taken up into neurons by MCT2 (6, 23, 25, 99). In neurons, ketone bodies are then metabolized in the mitochondria to produce ATP and reactive oxygen species (ROS) through the tricarboxylic acid cycle using 3-hydroxybutyrate dehydrogenase, oxoacid CoA-transferase, and acetyl-coenzyme A acetyltransferase (14, 70).
Metabolic-Sensing Neurons
The regulation of food intake and energy homeostasis is under the control of specialized “metabolic sensing” neurons that use various metabolic substrates, hormones, peptides, and neurotransmitters to alter their activity (4, 11, 16, 39, 40, 44, 61, 67, 73, 82, 106). These metabolic sensing neurons are located in the VMH, as well as multiple other brain sites, which, collectively, form a distributed network that monitors and regulates the metabolic status of the body (54, 63). The concept of metabolic sensing neurons arose from Jean Mayer's first proposal in the 1950s that there were specialized glucosensing neurons in the VMH (68). He postulated that such neurons sensed changes in glucose oxidation as a means of regulating their activity and the control of feeding. But it was not until 1964 that Oomura et al. (81) and Anand et al. (4) actually identified and physiologically characterized such glucosensing neurons. Whereas glucosensing neurons, like all neurons, use glucose as their primary fuel, they also have a variety of specialized mechanisms by which they translate alterations in ambient extracellular glucose levels into changes in membrane potential, neuronal activity, and neurotransmitter and peptide release (21, 22, 41, 42, 53, 59–61, 94, 102).
Glucosensing neurons are also sensitive to many other nutrients, such as FA, lactate, and ketone bodies (21, 41, 42, 48, 52, 102), as well as peripheral hormones such as leptin (38, 39, 103), insulin (96, 104, 107, 108), and ghrelin (17); thus, the term “metabolic sensing” neurons. Glucose-excited neurons increase, whereas glucose-inhibited neurons decrease their firing rate as ambient glucose levels rise (93). During the diurnal cycle, brain glucose levels vary from ∼10–20% of blood levels. After an overnight fast, VMH levels are ∼0.5–0.7 mM and after a full meal, they rise to 2.0–2.5 mM (22, 71, 98, 101). Glucose-excited neurons are activated at 2.5 mM and inhibited at 0.5 mM VMH glucose levels, while glucose-inhibited neurons are excited and inhibited at 0.5 mM and 2.5 mM glucose, respectively (41, 42, 93).
Metabolic sensing neurons also possess specialized pathways by which they use FA as signaling molecules to regulate their activity (52). Nanomolar concentrations of oleic acid either excite or inhibit 70% of dissociated VMN neurons (52). A subset of these FA-sensing neurons responds to both glucose and FA, often with opposing effects; i.e., when glucose excites, FA often inhibit these neurons (52). Since fasting is associated with low glucose and high blood FA levels, while feeding is associated with raised glucose and FA levels, these specialized FA- and glucosensing neurons should be able to differentiate between the fasted and fed states. Although 20 to 40% of VMH neuronal FA sensing is due to the metabolism of FA through β-oxidation and the formation of ROS and ATP (52), we have shown that 60–80% of VMH neuronal FA-sensing neurons use the FA translocator/receptor FAT/CD36 (CD36) to regulate their FA sensing (48, 52, 76). CD36 is a member of class B scavenger receptor proteins that displays preferential binding to long-chain fatty acids (LCFA) (8, 26). Depleting CD36 in VMH neurons in vivo increases food intake, body weight, and fat mass and leads to insulin resistance in lean and obese rats. It also shifts the deposition of fat from visceral to subcutaneous depots. This demonstrates that VMH CD36-mediated FA sensing plays an important role in the regulation of energy and glucose homeostasis and fat deposition in rats (47, 48).
Regulation of Food Intake by Astrocyte-Derived Ketone Bodies
Manipulating brain FA oxidation can alter food intake (2, 18, 19, 74, 76, 78, 91). Indeed, hypothalamic levels of LCFA-CoAs can be increased by enhancing esterification of circulating or central lipids (45, 79) and/or by the local inhibition of lipid oxidation (78). These interventions also result in marked inhibition of eating and liver glucose fluxes (3, 45, 78, 79, 91). These observations suggest that inhibiting FA synthase and stimulating the entry of FA into the mitochondria through CPT1 can reduce food intake and body weight in rodents. However, the majority of such studies have failed to address the issue of which cells were affected by these manipulations of FA oxidation.
Given their prominent role in both FA oxidation and ketone production in the brain (25), our studies (50, 51) suggest that it is likely that such manipulations affect mainly astrocytes rather than neurons. These studies focused on the VMH because of its high concentration of metabolic sensing neurons (80, 83, 101), and its well-established role in the control of ingestive behavior (15, 64, 67, 72). To understand the role of VMH astrocyte-derived ketone bodies in the regulation of food intake, we developed novel methods to simultaneously monitor food intake, VMH and blood FA, and ketone body levels. In an initial set of studies, lean rats were trained to eat all of their daily calories on a 13.5% LFD or 60% HFD for 3 h/day. On testing day, they were assessed for continuous food intake, serum and VMH ketone or FFA levels over a 6-h period after dark onset (50). Although rats fed LFD and HFD ate the same amount over the first 3 h, those on HFD ate only 50% as much and consumed fewer meals during the second 3-h period after dark onset. Unexpectedly, extracellular VMH FA levels were lower in HFD than LFD rats during the entire 6-h period (50). Given these somewhat paradoxical findings, we postulated that astrocytes from rats fed HFD had increased their FA uptake at a rate that was greater than the influx of FA from blood; i.e., there was a greater flux into astrocytes leading to lowered extracellular FA levels (Fig. 1).
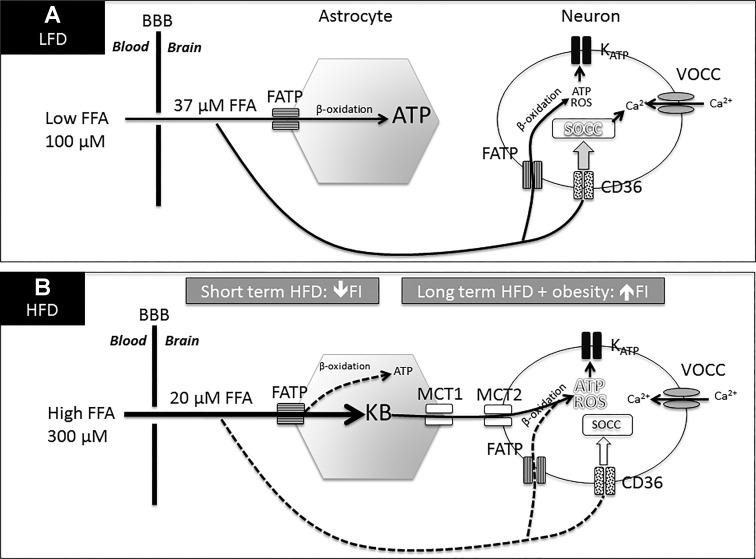
Fig. 1.
Hypothetical model for the interaction of CD36-mediated neuronal fatty acid (FA) sensing and astrocyte-produced ketone bodies. A: during low-fat diet (LFD) intake, FA entering the ventromedial hypothalamus (VMH) from the blood across the blood-brain barrier (BBB) are taken up and oxidized by astrocytes to produce ATP to fuel their ongoing metabolic needs. Long-chain fatty acids (LCFA) bind to CD36 as a FA “receptor” on metabolic sensing neurons to excite or inhibit them by activating store-operated Ca2+ channels (SOCC). On a LFD, VMH metabolic sensing neurons are either excited or inhibited by FA, partially dependent upon ambient glucose levels. B: after continued HFD intake, extracellular FA levels are decreased (vs. LFD in A) because flux into VMH astrocytes is increased since they have been “primed” to rapidly take up and utilize extracellular FA to generate ketone bodies (KB). These are then exported via MCT1 [or MCT4 (65)], taken up by neurons via MCT2 and utilized to produce excess ATP and ROS, which override CD36-mediated FA sensing by activating or inhibiting the KATP channel. LCFA levels were derived by zero net flux determinations and probe efficiency calculations (50). In the setting of long-term HFD, intake and the development of obesity and elevated leptin levels, ketone bodies, and FA play a secondary role in the control of food intake due to the development of leptin resistance, which promotes continued hyperphagia on the HFD.
While reduced transport of FA transport across the blood-brain barrier in rats fed a HFD could also contribute to these lower extracellular FA levels, the fact that VMH ketone levels spiked during the first 1 h after dark onset in HFD-fed rats suggests that increased FA uptake by astrocytes was the primary mechanism underlying lowered extracellular FA levels (51). The mechanism by which this early spike in VMH ketones might decrease food intake 3–6 h later has yet to be elucidated but might be explained by altered gene transcription or translation or the secretion of anorexic gut hormones. Regardless of the cause, we confirmed that this initial spike in VMH astrocyte ketone production was responsible for the delayed reduction in food intake since local inhibition of VMH ketone production during the last 2 h prior to dark onset fully restored the intake of HFD to that of LFD during the second 3-h epoch after feeding onset (50). To understand how such local VMH ketone production might alter the activity of VMH neurons responsible for mediating food intake, we assessed the effects of FA and ketones on dissociated VMH neurons using calcium imaging as a surrogate for changes in neuronal activity (52). We found that ketone bodies override normal FA sensing in many of these neurons, primarily by exciting neurons that are either activated or inhibited by FA. We postulate, but have not proven, that this predominantly excitatory effect of excess ketone bodies is due to the overproduction of ATP and ROS in neuronal mitochondria that overrides the CD36-mediated FA-sensing mechanism (Fig. 1).
However, our original method of assessing the effects of LFD vs. HFD on VMH FA levels, ketone production, and food intake were relatively artificial given the utilization of a highly restricted feeding regimen. Thus, we turned to a model of normal spontaneous diurnal feeding in rats selectively bred to develop diet-induced obesity (DIO) or to be diet-resistant (DR) when fed a moderate-fat (31%), high-energy (HE) diet. DIO rats are larger but not fatter than DR rats when fed a low-fat chow diet but rapidly become hyperphagic, obese, and insulin-resistant when fed an HE diet (57, 62, 92). Importantly, the DIO phenotype is inherited as a polygenic trait (55, 58, 92). We previously showed (58) that, when DIO and DR rats are switched from chow to HE diet, both become hyperphagic for 3 days. However, after 3 days on HE diet, DR rats reduce their intake to chow-fed levels, while DIO rats remain hyperphagic for an additional 6–8 wk, despite their early, marked and persistent increase in leptin levels (32). Importantly, DIO rats have defective VMN neuronal FA sensing while on a LFD (53), while 3 days of an HE diet intake markedly alters their VMN neuronal responses to both FA (47) and ketone bodies (51), but does not affect neurons in DR rats.
In fact, on day 3 of the HE diet, DIO and DR rats have comparable VMH ketone levels over the entire 6-h period after dark-onset feeding. However, only DR rats reduce their intake over this 6-h period, as well as the entire 24 h of day 3 (51). In keeping with the hypothesis that raised VMH ketone levels are responsible for the decreased day 3 intake of HE diet intake by DR rats, local inhibition of their VMH astrocyte ketone production for 2 h prior to dark-onset feeding completely reversed their reduced 6-h food intake and substantially increased their overall intake over the entire 24 h (51). Since DR rats take 3 days to reduce their intake of the HE diet, we postulate that it takes this long for the elevated fat content of the HE diet to prime VMH astrocytes to make ketone bodies in both DIO and DR rats. In fact, our unpublished data do suggest that DR VMH ketone levels are not elevated on day 2 of HE diet intake but, when VMH ketone levels are elevated to day 3 levels on day 2, DR rats reduce their intake to chow-fed levels. We further postulate that, since both DIO and DR rats have similarly elevated VMH ketone levels on day 3 but only DR rats respond to these elevated levels by reducing their food intake at that time, DR FA-sensing neurons are more sensitive to the overriding effects of ketones on normal FA sensing. Taken together, our data strongly support a role for elevated VMH ketone body levels in the reduction in food intake of both outbred rats fed 60% fat, 6% sucrose diet for 2 wk, and DR rats fed 31.5% fat, and 25% sucrose HE diet for 3 days. Such data suggest that defective VMH neuronal FA and ketone sensing might contribute to the persistent hyperphagia of DIO rats on HE diet (Fig. 1). However, the additional resistance to the markedly increased levels of the catabolic hormone leptin that occurs on day 3 of HE diet intake (58) is likely to be a major cause of the persistent hyperphagia and development of obesity in DIO rats on such diets (84). In other words, FA and ketone sensing by VMH neurons are likely to be important mediators of food intake and energy homeostasis in outbred and DR rats, but they play a less important role in regulating these processes in DIO rats, which are leptin resistant from an early age (12, 32, 58). Thus, while the short-term inhibitory effects of HFD on feeding appear to rely on an interplay between neuronal FA and ketone sensing, the long-term hyperphagia of animals and humans ingesting HFD may be due to factors such as the development of leptin resistance as obesity progressively develops.
Perspectives and Significance
The chronic overconsumption of a palatable, HFD contributes to the excess caloric intake that leads to the development of obesity in many individuals. However, some animals reduce their intake of HFD and are obesity-resistant depending upon dietary content, intake schedule, and genetic background. Specialized hypothalamic metabolic sensing neurons monitor changes in ambient brain levels of substrates, such as glucose, FA, and ketone bodies by using them as signaling molecules that alter their activity (11, 16, 29, 40, 41, 44, 46, 48, 50). The effect of ketone bodies on VMH neuronal FA sensing to alter short-term food intake differs as a function of both diet and genetic background. Outbred rats fed a LFD exhibit little increase in VMH ketone bodies during ingestion and have a consistent level of food intake, which maintains a stable weight. When those same rats are fed a HFD on a restricted schedule, their VMH ketone bodies spike transiently during early intake, and this leads to reduced intake after a 3- to 6-h delay. Because rats fed 3 h per day on HFD maintain the same caloric intake and body weight as those on LFD, this suggests that monitoring both VMH FA and ketone levels might be an important factor in preventing them from overeating and becoming obese.
Genetically predisposed, selectively bred DIO rats are inherently leptin-resistant before they become obese (12, 31, 56) and have abnormal VMH neuronal FA sensing after exposure to HFD (51, 53). Whereas DR rats respond to elevated VMH ketone levels that arise after 3 days on HE diet by reducing their energy intake to their LFD levels, DIO rats fail to respond to these same ketone elevations, remain hyperphagic, and become obese. This is presumably due to a combination of defective FA and ketone sensing and their inherent leptin resistance (58). Thus, although several issues relating to FA and ketone regulation of both short- and long-term control of food intake remain to be elucidated, the monitoring of VMH FA and the production of ketones by VMH astrocytes appear to be important regulators of food intake and energy homeostasis in leptin-responsive DR rats, but not in leptin-resistant DIO rats in the presence of HFD.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.F. conception and design of research; C.L.F. interpreted results of experiments; C.L.F. prepared figures; C.L.F. drafted manuscript; C.L.F. and B.E.L. approved final version of manuscript; B.E.L. edited and revised manuscript.
ACKNOWLEDGMENTS
This work was supported by the Research Service of the Department of Veterans Affairs and by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant NIDDK-DK RO1-53181).
REFERENCES
- 1.Abbott NJ, Revest PA, Romero IA. Astrocyte-endothelial interaction: physiology and pathology. Neuropathol Appl Neurobiol 18: 424–433, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Aja S, Landree LE, Kleman AM, Medghalchi SM, Vadlamudi A, McFadden JM, Aplasca A, Hyun J, Plummer E, Daniels K, Kemm M, Townsend CA, Thupari JN, Kuhajda FP, Moran TH, Ronnett GV. Pharmacological stimulation of brain carnitinepalmitoyl-transferase-1 decreases food Intake and body weight. Am J Physiol Regul Integr Comp Physiol 294: R352–R361, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Aja S, McFadden J, Aplasca A, Plummer E, Hyun J, Medghalchi SM, Vadlamudi A, Thupari JN, Townsend CA, Kuhajda FP, Moran TH, Ronnett GV. Intracerebroventricular administration of either C89b, a stimulator of carnitinepalmitoyl-transferase-1 (CPT-1s), or cerulenin, an inhibitor of fatty acid synthase (FASi), reduces food intake and body weight in mice. Appetite 46: 338, 2006. [Google Scholar]
- 4.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamus feeding centers: effect of glucose. Am J Physiol 207: 1146–1154, 1964. [DOI] [PubMed] [Google Scholar]
- 5.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 81: 728–739, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem 56: 1376–1386, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Azari EK, Ramachandran D, Weibel S, Arnold M, Romano A, Gaetani S, Langhans W, Mansouri A. Vagal afferents are not necessary for the satiety effect of the gut lipid messenger oleoylethanolamide. Am J Physiol Regul Integr Comp Physiol 307: R167–R178, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol 153: 75–81, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14: 724–738, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Blazquez C, Sanchez C, Daza A, Galve-Roperh I, Guzman M. The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. J Neurochem 72: 1759–1768, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res 209: 1–12, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7: 179–185, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav 83: 549–555, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Brekke E, Morken TS, Sonnewald U. Glucose metabolism and astrocyte-neuron interactions in the neonatal brain. Neurochem Int 82: 33–41, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Campfield A, Brandon P, Smith F. On-line continuous measurement of blood glucose and meal pattern in free feeding rats: The role of glucose in meal initiation. Brain Res Bull 14: 605–616, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Carneiro L, Geller S, Fioramonti X, Hebert A, Repond C, Leloup C, Pellerin L. Evidence for hypothalamic ketone body sensing: impact on food intake and peripheral metabolic responses in mice. Am J Physiol Endocrinol Metab 310: E103–E115, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Ge Y, Jiang Z, Liu C, Depoorter I, Peeters T. Effects of ghrelin on hypothalamic glucose responding neurons in rats. Brain Res 1055: 131–136, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ. Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Diabetes 51: 3196–3201, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Cruciani-Guglielmacci C, Marsollier N, Guissard C, Lorsignol A, Penicaud L, Magnan C. Short-term increase in brain FFA reduces food intake. Diabetes 57: A84, 2008. [Google Scholar]
- 20.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin NA 88: 787–835, ix, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose excited and glucose inhibited central neurons. Diabetes 51: 2056–2065, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci 29: 7015–7022, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmond J. Energy metabolism in developing brain cells. Can J Physiol Pharmacol 70 Suppl: S118–S129, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Edmond J, Higa TA, Korsak RA, Bergner EA, Lee WN. Fatty acid transport and utilization for the developing brain. J Neurochem 70: 1227–1234, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18: 551–561, 1987. [DOI] [PubMed] [Google Scholar]
- 26.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem 283: 12,949–12,959, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Ericson U, Hellstrand S, Brunkwall L, Schulz CA, Sonestedt E, Wallstrom P, Gullberg B, Wirfalt E, Orho-Melander M. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am J Clin Nutr 101: 1065–1080, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, Dhenain M, Deglon N, Hantraye P, Pellerin L, Bonvento G. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci 27: 7094–7104, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fioramonti X, Lorsignol A, Taupignon A, Penicaud L. A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes 53: 2767–2775, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Golay A, Bobbioni E. The role of dietary fat in obesity. Int J Obes Relat Metab Disord 21 Suppl 3: S2–S11, 1997. [PubMed] [Google Scholar]
- 31.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol 291: R768–R778, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 292: R1782–R1791, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Guzman M, Blazquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids 70: 287–292, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann P, Ramseier A, Gudat F, Mihatsch MJ, Polasek W. [Normal weight of the brain in adults in relation to age, sex, body height and weight]. Der Pathologe 15: 165–170, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins RA, Biebuyck JF. Ketone bodies are selectively used by individual brain regions. Science 205: 325–327, 1979. [DOI] [PubMed] [Google Scholar]
- 36.Hegardt FG. Regulation of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene expression in liver and intestine from the rat. Biochem Soc Trans 23: 486–490, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Hegardt FG. Transcriptional regulation of mitochondrial HMG-CoA synthase in the control of ketogenesis. Biochimie 80: 803–806, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Irani BG, Le Foll C, Dunn-Meynell A, Levin BE. Effects of leptin on rat ventromedial hypothalamic neurons. Endocrinology 149: 5146–5154, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irani BG, Le Foll C, Dunn-Meynell AA, Levin BE. Ventromedial nucleus neurons are less sensitive to leptin excitation in rats bred to develop diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 296: R521–R527, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jo YH, Su Y, Gutierrez-Juarez R, Chua S Jr. Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J Neurophysiol 101: 2305–2316, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Kang L, Routh VH, Kuzhikandathil EV, Gaspers L, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Karimian Azari E, Leitner C, Jaggi T, Langhans W, Mansouri A. Possible role of intestinal fatty acid oxidation in the eating-inhibitory effect of the PPAR-α agonist Wy-14643 in high-fat diet fed rats. PloS One 8: e74869, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes 60: 107–113, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320–327, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Lamy CM, Sanno H, Labouebe G, Picard A, Magnan C, Chatton JY, Thorens B. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab 19: 527–538, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Le Foll C, Dunn-Meynell A, Levin BE. Role of FAT/CD36 in fatty acid sensing, energy and glucose homeostasis regulation in DIO and DR rats. Am J Physiol Regul Integr Comp Physiol 308: R188–R198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Foll C, Dunn-Meynell A, Musatov S, Magnan C, Levin BE. FAT/CD36: a major regulator of neuronal fatty acid sensing and energy homeostasis in rats and mice. Diabetes 62: 2709–2716, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes 63: 1259–1269, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Role of VMH ketone bodies in adjusting caloric intake to increased dietary fat content in DIO and DR rats. Am J Physiol Regul Integr Comp Physiol 308: R872–R878, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE. Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am J Physiol Regul Integr Comp Physiol 297: R655–R664, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE. Effects of maternal genotype and diet on offspring glucose and fatty acid sensing ventromedial hypothalamic nucleus neurons. Am J Physiol Regul Integr Comp Physiol 297: R1351–R1357, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levin BE. Metabolic sensors: viewing glucosensing neurons from a broader perspective. Physiol Behav 76: 397–401, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Levin BE, Dunn-Meynell AA. Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul Integr Comp Physiol 278: R231–R237, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Levin BE, and Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol Endocrinol Metab 285: E949–E957, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol Regul Integr Comp Physiol 276: R1223–R1231, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucosensing and the KATP channel. Nat Neurosci 4: 459–460, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Levin BE, Kang L, Sanders NM, Dunn-Meynell AA. Role of neuronal glucosensing in the regulation of energy homeostasis. Diabetes 55 Suppl 2: S122–S130, 2006. [Google Scholar]
- 62.Levin BE, Keesey RE. Defense of differing body weight set-points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998. [DOI] [PubMed] [Google Scholar]
- 63.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152: 2552–2557, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes 53: 2521–2528, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Liu B, Niu L, Shen MZ, Gao L, Wang C, Li J, Song LJ, Tao Y, Meng Q, Yang QL, Gao GD, Zhang H. Decreased astroglial monocarboxylate transporter 4 expression in temporal lobe epilepsy. Mol Neurobiol 50: 327–338, 2014. [DOI] [PubMed] [Google Scholar]
- 66.Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol Sci 14: 177–182, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med 249: 13–16, 1953. [DOI] [PubMed] [Google Scholar]
- 68.Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and lipostatic hypothesis. Ann NY Acad Sci 63: 15–43, 1955. [DOI] [PubMed] [Google Scholar]
- 69.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 49: 395–420, 1980. [DOI] [PubMed] [Google Scholar]
- 70.McKenna MC. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem Res 37: 2613–2626, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: effects of microdialysis flow rate, strain, and age. J Neurochem 72: 785–790, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Melanson KJ, Westerterp-Plantenga MS, Campfield LA, Saris WH. Blood glucose and meal patterns in time-blinded males, after aspartame, carbohydrate, and fat consumption, in relation to sweetness perception. Br J Nutr 82: 437–446, 1999. [PubMed] [Google Scholar]
- 73.Migrenne S, Cruciani-Guglielmacci C, Kang L, Wang R, Rouch C, Lefevre AL, Ktorza A, Routh VH, Levin BE, Magnan C. Fatty acid signaling in the hypothalamus and the neural control of insulin secretion. Diabetes 55 Suppl 2: S139–S144, 2006. [Google Scholar]
- 74.Migrenne S, Magnan C, Cruciani-Guglielmacci C. Fatty acid sensing and nervous control of energy homeostasis. Diabetes Metab 33: 177–182, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr 3: 286–294, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moulle VS, Le Foll C, Philippe E, Kassis N, Rouch C, Marsollier N, Bui LC, Guissard C, Dairou J, Lorsignol A, Penicaud L, Levin BE, Cruciani-Guglielmacci C, Magnan C. Fatty acid transporter CD36 mediates hypothalamic effect of fatty acids on food intake in rats. PloS One 8: e74021, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munsters MJ, Saris WH. Body weight regulation and obesity: dietary strategies to improve the metabolic profile. Annu Rev Food Science Technol 5: 39–51, 2014. [DOI] [PubMed] [Google Scholar]
- 78.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9: 756–761, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Oomura Y. Glucose as a regulator of neuronal activity. In: Advances in Metabolic Disorders, edited by Szabo AJ. New York: Academic Press, 1983, p. 31–65. [DOI] [PubMed] [Google Scholar]
- 81.Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal activities of the ventromedial and lateral hypothalamic areas of cats. Science 143: 484–485, 1964. [DOI] [PubMed] [Google Scholar]
- 82.Oomura Y, Nakamura T, Sugimori M, Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav 14: 483–486, 1975. [DOI] [PubMed] [Google Scholar]
- 83.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurons of the rat hypothalamus. Nature 222: 282–284, 1969. [DOI] [PubMed] [Google Scholar]
- 84.Park S, da Kim S, Daily JW. Central infusion of ketone bodies modulates body weight and hepatic insulin sensitivity by modifying hypothalamic leptin and insulin signaling pathways in type 2 diabetic rats. Brain Res 1401: 95–103, 2011. [DOI] [PubMed] [Google Scholar]
- 85.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci 91: 10,625–10,629, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32: 1152–1166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 20: 291–299, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32: 421–431, 2009. [DOI] [PubMed] [Google Scholar]
- 89.Perea G, Sur M, Araque A. Neuron-glia networks: integral gear of brain function. Front Cell Neurosci 8: 378, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. The selfish brain: competition for energy resources. Neurosci Biobehav Rev 28: 143–180, 2004. [DOI] [PubMed] [Google Scholar]
- 91.Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116: 1081–1091, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively-bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 285: R610–R618, 2003. [DOI] [PubMed] [Google Scholar]
- 93.Routh VH, McArdle JJ, Levin BE. Phosphorylation modulates the activity of the ATP-sensitive K+ channel in the ventromedial hypothalamic nucleus. Brain Res 778: 107–119, 1997. [DOI] [PubMed] [Google Scholar]
- 94.Routh VH, Song Z, Liu X. The role of glucosensing neurons in the detection of hypoglycemia. Diabetes Technol Ther 6: 413–421, 2004. [DOI] [PubMed] [Google Scholar]
- 95.Rui L. Energy metabolism in the liver. Compr Physiol 4: 177–197, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 97.Sharabi K, Tavares CD, Rines AK, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med 46: 21–33, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silver IA, Erecinska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol 79: 1733–1745, 1998. [DOI] [PubMed] [Google Scholar]
- 99.Simpson JL. Genetics of diabetes mellitus (DM) and anomalies in offspring of diabetic mothers. Semin Perinatol 2: 383–394, 1978. [PubMed] [Google Scholar]
- 100.Sokoloff L, Reivich M, Kennedy C, DesRosiers MH, Patlak CS, Pettigrew O, Sakaruda O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 23: 897–916, 1977. [DOI] [PubMed] [Google Scholar]
- 101.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus (VMN). Diabetes 50: 2673–2681, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 54: 15–22, 2005. [DOI] [PubMed] [Google Scholar]
- 103.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521–525, 1997. [DOI] [PubMed] [Google Scholar]
- 104.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci 16: 877–885, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J Neurophysiol 95: 1491–1498, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53: 1959–1965, 2004. [DOI] [PubMed] [Google Scholar]
- 108.Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282: 503–505, 1979.116135 [Google Scholar]
- 109.Yi CX, Habegger KM, Chowen JA, Stern J, Tschop MH. A role for astrocytes in the central control of metabolism. Neuroendocrinology 93: 143–149, 2011. [DOI] [PubMed] [Google Scholar]