Abstract
Heat stress causes morbidity and mortality in humans and animals and threatens food security by limiting livestock productivity. Inflammatory signaling may contribute to heat stress-mediated skeletal muscle dysfunction. Previously, we discovered increased circulating endotoxin and intramuscular oxidative stress and TNF-α protein abundance, but not inflammatory signaling following 24 and 72 h of heat stress. Thus the purpose of this investigation was to clarify the role of inflammatory signaling in heat-stressed skeletal muscle. Crossbred gilts (n = 8/group) were assigned to either thermal neutral (24°C), heat stress (37°C), or pair-fed thermal neutral (24°C) conditions for 12 h. Following treatment, animals were euthanized, and the semitendinosus red (STR) and white (STW) were recovered. Heat stress did not alter inflammatory signaling in STW. In STR, relative heat shock protein abundance was similar between groups, as was nuclear content of heat shock factor 1. In whole homogenate, relative abundance of the NF-κB activator inhibitory κB kinase-α was increased by heat stress, although abundance of NF-κB was similar between groups. Relative abundance of phosphorylated NF-κB was increased by heat stress in nuclear fractions. Activator protein-1 (AP-1) signaling was similar between groups. While there were few differences in transcript expression between thermal neutral and heat stress, 80 and 56% of measured transcripts driven by NF-κB or AP-1, respectively, were increased by heat stress compared with pair-fed thermal neutral. Heat stress also caused a reduction in IL-6 transcript and relative protein abundance. These data demonstrate that short-term heat stress causes inflammatory signaling through NF-κB in oxidative, but not glycolytic, skeletal muscle.
Keywords: hyperthermia, inflammation, heat stroke, NF-κB, AP-1
heat-related complications continue to be a major human health concern and lead to potentially life-threatening conditions, ranging from heat exhaustion to heat stroke and death (28). These may also lead to longer term health problems, including cardiovascular (14, 48) and kidney disease (13). These detrimental health effects were made clear during a 2006 heat wave, where there were 655 heat-related deaths in California, as well as 1,620 more hospitalizations, and more than 16,000 additional emergency room visits during a 2-wk period at an estimated cost of approximately $5.4 billion (16). Furthermore, nearly 50,000 Europeans died because of heat stress in 2003, and, in 2015, ∼1,800 Indians and 1,200 Pakistani died during a heat wave. In addition to human health concerns, heat stress also negatively affects animal production and welfare. For example, the US swine industry loses approximately $1 billion annually due to heat stress (1, 43). While there are numerous factors contributing to these economic losses, a significant component results from a reduction in efficient growth and a failure to maximize lean tissue accretion (2). Despite the broad negative impacts of heat stress, the cellular and molecular changes induced by heat stress in skeletal muscle are not well characterized. This mechanistic understanding is a prerequisite for developing effective countermeasures and therapeutic interventions in both animal agriculture and human medicine.
The detrimental effects of heat stress are in stark contrast to acute exposures to heat, termed therapeutic hyperthermia. Indeed, 30 min of heat exposure have been shown to decrease muscle atrophy (26, 40), augment regrowth (41, 47), and maintain insulin sensitivity in skeletal muscle (10, 11). Moreover, studies intending to model heat stroke generally include heat exposure of <2 h and components of exercise and have distinct outcomes different from prolonged heat exposure (4, 29, 53). Inflammation appears to be a central component in these studies, where changes in circulating cytokines, as well as alterations in skeletal muscle, indicate interleukin-6 (IL-6) is a key factor in this response (51). In addition, changes appear to be due to activator protein 1 (AP-1), but not nuclear factor-κB (NF-kB) signaling (44, 50, 51).
Heat stress is unique in that exposure to heat is longer than 2 h and does not include a deliberate exercise component. Under these conditions, 12 h of heat stress increased circulating endotoxin (i.e., lipopolysaccharide) and decreased circulating tumor necrosis factor-α (TNF-α) in pigs (34). Findings of increased circulating endotoxin were confirmed following longer term heat stress in multiple species (8, 17, 31). Interestingly, intramuscular TNF-α protein abundance was increased following 24 h of heat stress, raising the possibility of TNF-α migration from the vasculature into tissues (25). Increased oxidative stress was also detected in these muscles (25). Increased circulating endotoxin, intramuscular TNF-α, or oxidative stress would ostensibly initiate an inflammatory response via NF-κB (24). Additionally, the proinflammatory c-Fos/c-Jun heterodimer, AP-1, has been implicated during heat stroke in skeletal muscle (49). Despite this compelling rationale to expect increased inflammatory signaling following heat stress, our laboratory was previously unable to detect increased inflammatory signaling in intestinal tissue (32) and in oxidative or glycolytic skeletal muscle following 24 or 72 h of heat stress (25). Our laboratory's previous findings of increased circulating endotoxin following 12 h of heat stress (34) serves to provide a plausible mechanism triggering this inflammatory response. The purpose of this investigation was to clarify the role of inflammatory signaling in heat-stressed skeletal muscle with a focus on short-term heat stress. We hypothesized that short-duration heat stress would induce inflammatory signaling in oxidative and glycolytic skeletal muscle.
MATERIALS AND METHODS
Animal treatments.
All procedures were reviewed and approved by the Iowa State University Institutional Animal Care and Use Committee. A detailed approach and data from these animals have been previously published (3, 30, 34). Briefly, 24 individually penned crossbred gilts [65 ± 3 kg body weight (BW), mean ± SE] were selected by BW and were allocated to one of three treatments: 1) thermal neutral ad libitum (24°C, 40% humidity, n = 8); 2) heat stress ad libitum conditions (37°C, 40% humidity, n = 8); or 3) pair-fed thermal neutral (feed intake matched to heat stress counterparts and reared in thermal neutral conditions, n = 8). The pair-fed thermal neutral group is an energetic control and was included because an immediate effect of heat stress is reduced feed intake (36). Therefore, to differentiate between direct and indirect effects of heat stress, we used both thermal neutral (ad libitum access to food) and pair-fed thermal neutral (reduced access to food) models to eliminate the potentially confounding effects of different feed intake. All pigs had ad libitum access to water and were fed the same corn- and soybean-based diet throughout the study period. Animals were monitored, and rectal temperature, respiratory rate (breaths per minute), and feed intake were recorded for every 2 h throughout the study period. BW was recorded at the beginning and end of the treatment. Following a 12-h application of the environmental heat treatment, gilts were killed using barbiturate overdose (Fatal Plus dosed at 1 ml/4.5 kg BW). The semitendinosus was collected and divided into red (STR) and white (STW) portions. Muscle samples were frozen in liquid nitrogen for further analyses.
QRT-PCR.
STR and STW were powdered on dry ice. For mRNA extraction, 50 mg of muscle (n = 8/group) were homogenized in Trizol (Invitrogen, Carlsbad, CA). Insoluble materials were removed from the homogenate by centrifugation at 1,500 g for 10 min at 4°C. Supernatant was mixed with one volume of ethanol and applied to a Direct-zol Mini Prep column (Zymo, Irvine, CA) and then treated with DNase to prevent DNA contamination. RNA concentration was determined using an ND-1000 Spectrophotometer (λ = 260/280 nm; NanoDrop Technologies, Wilmington, DE). Synthesis of 1 μg of RNA to cDNA was carried out using QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA). To assess transcript expression, we used Fluidigm protocol B (Fast Gene Expression for Analysis Using EvaGreen) and Eppendorf PCR Master Cycler (Quantitect SYBR Green PCR kit: Qiagen) for subsequent measures targeting cytokine expression. For the Fluidigm protocol, a pool of all primers (Table 1) and PerfeCTa master mix (Quanta Biosciences, Gaithersburg, MD) were used to preamplify 62.5 ng of cDNA from each sample. Preamplified samples were treated with exonuclease 1 (New England BioLabs, Ipswich, MA) and diluted 1:10 using EvaGreen supermix (Bio-Rad, Hercules, CA) and DNA binding dye (Fluidigm, South San Francisco, CA). Forward and reverse primers were prepared using loading agent (Fluidigm). Once Dynamic Array 48.48 IFC (Fluidigm) was primed, samples and primers were loaded using IFC controller MX (Fluidigm). After loading, the array was transferred to the BioMark HD (Fluidigm) and run using a GE 48 × 48 PCR+Melt plate v2.pcl program. For the Eppendorf PCR master cycler, we synthesized new primers for cytokines and used the following program: 15-min hold at 95°C and 45 cycles of denaturing at 95°C for 15 s, annealing at 58°C for 15 s, and extension at 72°C for 20 s at which point data were acquired. Data were analyzed using the ΔΔCT method with 18S as our reference gene (CTsample − CT18S = ΔCT; ΔCTsample − ΔCThighest|ΔCT|value = ΔΔCT). All statistical analyses were performed on the ΔCT values, and data are reported as fold change (2ΔΔCT).
Table 1.
Sequences of primer pairs used for QRT-PCR
| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | aaacggctaccacatccaag | tcgcggaaggatttaaagtg |
| BNIP3 | cgcacacagtgttggagaaa | tccctcctcctctccatgtaa |
| BTG2 | ttttcagcggggctctcc | agcccttggatggcttttca |
| CAT | acaaagacctgcggaccaa | agcttcccgttgccatca |
| CST3 | acaactgtcccttccccaac | ttccaggggacggtgtaaac |
| DDIT4L | agttgctagaccgtagcttcc | ttgggttcagggacaacgtaa |
| EIF4EBP1 | tggagtgtcggaactcacct | atcacccacagggctggt |
| FBXO32 | gaaggacatgctgaacagcaaa | agtacttcctttgtgaacatagatcca |
| FLOT1 | gctatcatggcccacatgac | tctgaggaggccactttgaa |
| GHR | gagcccatttgcatgtgaa | caccgttagcccaaatattcc |
| GNB2L1 | ttctcacccaacagcagcaa | gcttgcagttagccaagttcc |
| HBXIP | ttggagcagcacttggag | cccagattgagtccttgtgaa |
| HNRPDL | cggattgagcccagatacttca | ggctcttcgtctgtgtacgtaa |
| HSPA2 | acgacaaaggtcgtctaagca | ggcctcatcttccgacttgta |
| HSPA9 | acaggaacaccaccattcca | ctccacttgagtctgtccatca |
| IGFBP3 | cagcgctacaaggtcgacta | gtctcgcgcttggactca |
| IL-1β | ccaaagagggacatggagaa | ttatatcttggcgccctttg |
| IL-2 | tgcagctcttgtgttgcatt | agcatcctggagagatcagc |
| IL-6 | aggaacccagctatgaactcc | agtagccatcaccagaagca |
| IL-8 | gaaatcacaggtgcccagt | tgcaagttgaggcaagaaga |
| IL-10 | tgtgccctatggtgttcaac | ctttgtcacactccggaagc |
| IL-15 | cagaagcaacctggcagcacg | acgcgtaactccacgagaaagca |
| LIPE | ctggatgtgcacttctggaaa | gccgatgccatgtttgcta |
| MCT4 | gtgtgtgtgaatcgctttgg | gaccccagtggtgaggtaga |
| PHB2 | agtgtggtggccaagttca | gtcagctcccttcggatcaa |
| RAB3D | agcgagttcgaaggcagaa | tggagggtgggctggaa |
| RNF4 | gccttggaggcagaacctata | accacgggctctaaagattca |
| SOD1 | atggtgggccaaaggatca | gatgtacacagtggccacac |
| SP1 | agaggcataaacgcacacac | atgctttgacaggtggtcac |
| TET2 | gcctcagcacgtacaaaaca | ctggtcctgaaagtcgcaaaa |
| TNFα | ctggccccttgagcatca | gggcttatctgaggtttgagac |
| TPX2 | gaaggcacaaacttggaagca | ttggacgagccttgaagca |
Immunochemistry.
Fifty milligrams of STR and STW muscle (n = 8/group) were powdered on dry ice and homogenized in 1.5 ml of protein extraction buffer (10 mM sodium phosphate, pH 7.0, and 2% SDS). To obtain the whole homogenate, the samples were then centrifuged at 1,000 g for 15 min at 4°C, and the supernatant collected. Protein concentration was measured using a BCA Microplate Protein Assay Kit (Pierce, Rockford, IL), according to the manufacturer's instructions.
Nuclear fraction protein was isolated using a NE-PER Nuclear and Cytoplasmic Extraction Kit, according to the manufacturer's instructions (Thermo Scientific, Rockford, IL). Briefly, 20 mg of STR muscle (n = 8/group) were powdered on dry ice and homogenized with 200 μl of cytoplasmic extraction reagent I (CERI). Pellets were then harvested using 11 μl of cytoplasmic extraction reagent II by centrifugation and suspended in 100 μl of nuclear extraction reagent. Samples were vortexed for 15 s every 10 min, for a total of 40 min and then centrifuged at 1,500 g for 10 min. The resultant supernatant contained the protein found in the nuclear fraction, and the protein concentration was measured using a BCA kit.
Homogenates were diluted to 4 mg/ml in loading buffer (62.5 mM Tris, pH 6.8, 1.0% SDS, 0.01% bromophenol blue, 15.0% glycerol, and 5% β-mercaptoethanol). Forty micrograms of protein were loaded into each well in 4–20% precast gradient gel (Bio-Rad, Hercules, CA), and proteins were separated at room temperature for 10 min at 60 V, followed by 60 min at 120 V. Afterward, proteins were transferred (60 min, 100 V) to a nitrocellulose membrane (Bio-Rad, Hercules, CA) with a pore diameter of 0.2 μm. Membranes were blocked for 1 h in 5% milk in Tris-buffered saline containing 0.2% Tween 20 (TTBS). Membranes with protein from whole homogenate were incubated with each of the following: heat shock protein (HSP) 72 (primary 1:1,000, cat. no. NB120-2788, Novus Biological, Littleton, CO; secondary 1:5,000), HSP27 (primary 1:1,000, cat. no. ADI-SPA-800, ENZO Life Sciences, Farmingdale, NY; secondary 1:2,000), phospho-HSP27 (primary 1:1,000, cat. no. 2406, Cell Signaling Technology, Danvers, MA; secondary 1:1,500), HSP90 (primary 1:1,000, cat. no. 4877, Cell Signaling Technology; secondary 1:1,500), TNF-α (primary 1:400, cat. no. ab6671, Abcam, Cambridge, MA; secondary 1:5,000), NF-κB (primary 1:5,000, cat. no. ab7970, Abcam, secondary 1:5,000), phospho-NF-κB p65 (primary 1:1,000, cat. no. MA5-15160, Thermo Scientific, Rockford, IL; secondary 1:2,000), inhibitory κB kinase-α (IKB-α) (primary 1:1,000, cat. no. SC-371, Santa Cruz Biotechnology, Dallas, TX; secondary 1:2,000), inhibitory κB kinase-α (IKK-α) (primary 1:1,000, cat. no. SAB4500258, Sigma Aldrich, St. Louis, MO; secondary 1:5,000), SAPK/JNK (primary 1:1,000, cat. no. 9252s, Cell Signaling Technology, secondary 1:2,000) or phospho-SAPK/JNK (primary 1:1,000, cat. no. 4668s, Cell Signaling Technology; secondary 1:2,000), IL-1β (primary 1:1,000, cat. no. 12703, Cell Signaling Technology; secondary 1:1,000), IL-2 (primary 1:1,000, cat. no. 12703, Cell Signaling Technology; secondary 1:1,000), IL-6 (primary 1:1,000, cat. no. ab6672, Abcam; secondary 1:2,000) overnight at 4°C. Membranes with protein from the nuclear fraction were incubated with antibodies to NF-κB (primary 1:5,000, cat. no. ab7970, Abcam; secondary 1:5,000), phospho-NF-κB p65 (primary 1:1,000, cat. no. 3033, Cell Signaling Technology; secondary 1:2,000), AP-1 (primary 1:1,000, cat. no. A5968, Sigma Aldrich; secondary 1:3,000), heat shock factor (HSF)-1 (primary 1:1,000, cat. no. 4356; Cell Signaling Technology; secondary 1:3,000) or phospho-HSF-1 (primary 1:5,000; cat. no. ab76076, Abcam; secondary 1:3,000) overnight at 4°C.
Following three washes in TTBS, membranes were incubated with species-specific secondary antibodies (in dilutions as mentioned above, anti-rabbit: cat. no. 7074, anti-mouse: cat. no. 7076, Cell Signaling Technology) for 1 h at room temperature. Membranes were washed three times in TTBS and incubated in enhanced chemiluminescence detection substrate (ECL Plus, GE Healthcare Bio-Sciences, Pittsburgh, PA) for 5 min, and then membranes were exposed to X-ray film. Densitometry of the appropriate bands was performed using Carestream 5.0 molecular imaging software (Carestream Health, New Haven, CT). Optical density was normalized to thermal neutral values. To confirm equal loading, all membranes were stained with Ponceau S, and the resultant images were captured and lane optical densities quantified. In all instances, resultant Ponceau S signal was similar between groups.
Statistics.
To determine the effect of heat stress, data from thermal neutral, heat stress, and pair-fed thermal neutral animals were compared using one-way ANOVA followed by Newman-Keuls post hoc test (GraphPad Prism, version 5.04). Statistical significance was set at P < 0.05. Values are reported as means ± SE, unless otherwise noted.
RESULTS
Our laboratory has previously reported the phenotype of 12-h heat-stress pigs and has published successful application of a heat load and heating curve (34). The rectal temperatures in thermal neutral and pair-fed thermal neutral were 39.2 ± 0.12 and 39.1 ± 0.12°C, respectively, and in heat-stressed pigs were 41.5 ± 0.13°C (P < 0.05) (34). In addition, respiratory rate in thermal neutral and pair-fed thermal neutral were 42.18 ± 7.16 and 40.25 ± 6.85 breaths/min, respectively, and in heat stress was 142.24 ± 6.94 breaths/min (P < 0.05) (34). Over the course of the 12-h treatment period, heat-stress pigs consumed 0.11 ± 0.03 kg feed, and pair-fed thermal neutral pigs consumed 0.18 ± 0.04 kg feed, while thermal neutral pigs consumed 1.00 ± 0.14 kg feed (P < 0.05). Hematocrit and hemoglobin were within normal limits following 12 h of heat stress, indicating animals were similarly hydrated (34).
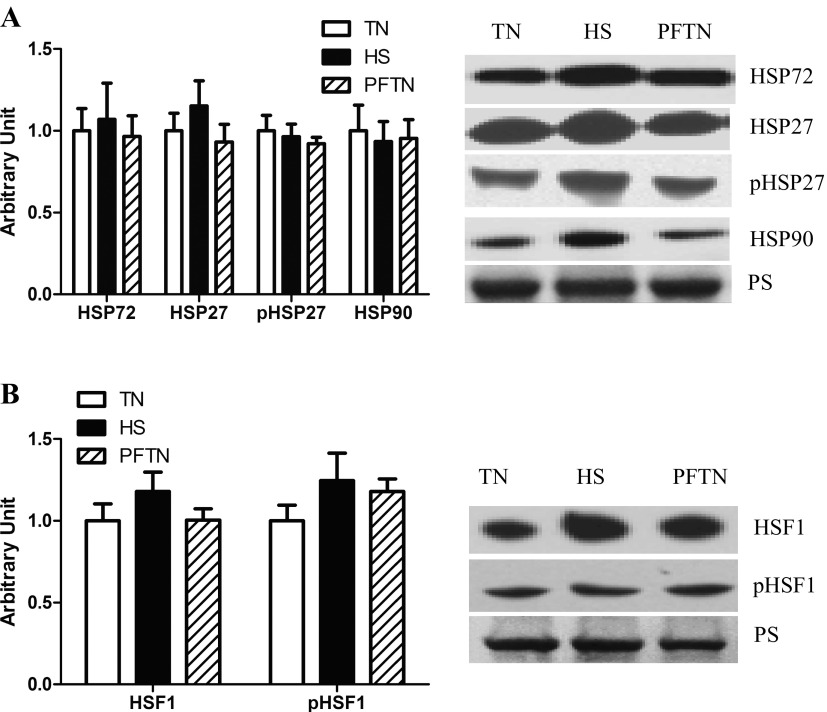
To determine the extent to which heat stress led to a heat shock response, we measured protein abundance of HSP72, HSP27, and HSP90. In oxidative muscle, we could not detect differences in the relative abundance of HSP72, HSP27, phospho-HSP27, and HSP90 compared with thermal neutral and pair-fed thermal neutral animals (Fig. 1A). HSF plays an important role in the regulation of HSP abundance; thus we measured HSF-1 in the nuclear fraction and found that relative abundance of HSF-1 and phospho-HSF-1 protein abundance was similar between groups (Fig. 1B).
Fig. 1.
Effect of heat stress on heat shock responses in semitendinosus red. Following 12 h of heat stress, HSP72, HSP27, pHSP27, and HSP90 protein abundance in whole homogenate (A) and HSF-1 or phosphorylated HSF-1 protein abundance in nuclear fraction (B) were measured by Western blot. Representative blots are included. Ponceau S stain (PS) was used as a loading control. Values are means ± SE; n = 8/group. TN, thermal neutral; HS, heat stress; PFTN, pair-fed thermal neutral.
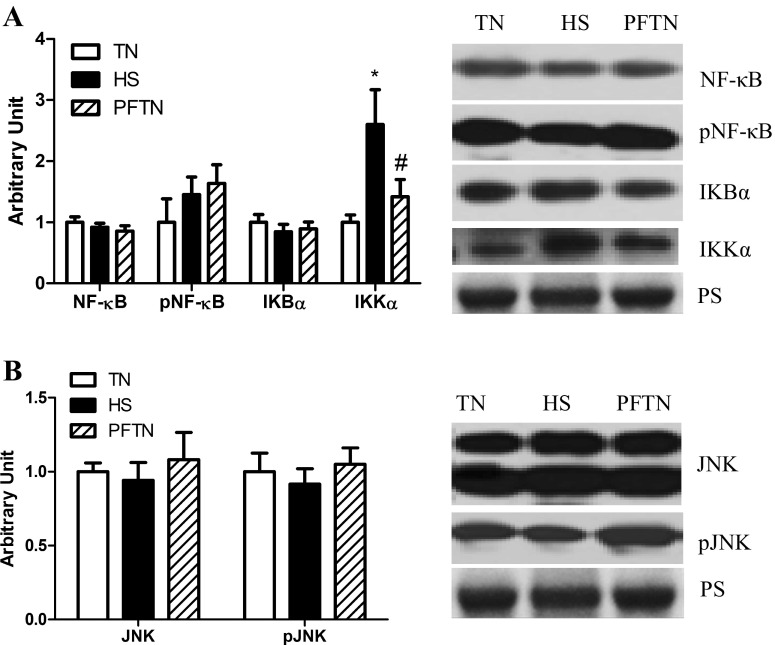
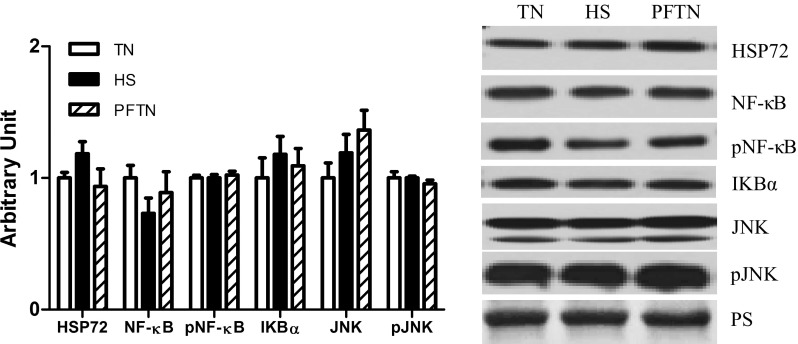
Next, we measured markers of NF-κB and AP-1 pathway activation. Relative abundance of these measures was similar between both thermal neutral groups for all measures, despite differences in feed intake. In oxidative muscle, relative protein abundance of NF-κB, phospho-NF-κB, and the NF-κB inhibitor IKB-α were similar between groups. However, in heat-stressed animals, the NF-κB activator IKK-α was increased by 159% compared with thermal neutral pigs and 118% compared with pair-fed thermal neutral pigs (Fig. 2A). Because there is previous evidence of increased AP-1-mediated inflammatory signaling during heat stroke (21, 49), we also measured abundance of the upstream activator of AP-1, MAPK c-Jun kinase (JNK), and discovered that relative protein abundance of total and phospho-JNK were similar between groups (Fig. 2B).
Fig. 2.
Effect of heat stress on inflammatory signaling in semitendinosus red (STR) whole homogenate. Following 12 h of heat stress, TNF-α, NF-κB pathway (A), and JNK (B) pathway protein expression for STR were measured by Western blot in whole homogenate. Representative blots are included. Ponceau S stain (PS) was used as a loading control. Values are means ± SE; n = 8/group. *Significantly different from TN. #Significantly different from HS. TN, thermal neutral; HS, heat stress; PFTN, pair-fed thermal neutral.
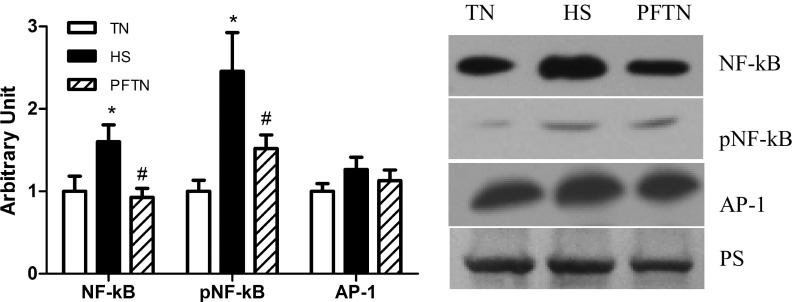
To further investigate the potential for inflammatory signaling in heat-stressed skeletal muscle, we isolated nuclear fractions so that the abundance of transcription factors within the nuclei could be quantified. Heat stress increased nuclear NF-κB by 60 and 74% compared with thermal neutral and pair-fed thermal neutral, respectively (P < 0.05). In addition, phospho-NF-κB also increased by 140 and 62% compared with thermal neutral and pair-fed thermal neutral, respectively (P < 0.05; Fig. 3). However, nuclear abundance of AP-1 was similar between groups (Fig. 3).
Fig. 3.
The relative abundance of transcription factors in semitendinosus red (STR) nuclear fraction. In the STR transcription factor for NF-κB and AP-1 pathway, protein abundance was measured by Western blot in the nuclear fraction. Sample blots are included. Ponceau S stain (PS) was used as a loading control. Values are means ± SE; n = 8/group. *Significantly different from TN. #Significantly different from HS. TN, thermal neutral; HS, heat stress; PFTN, pair-fed thermal neutral.
To more thoroughly explore the role of NF-κB and AP-1-altered downstream gene regulation, we measured expression of transcripts driven by NF-κB or by AP-1 signaling via QRT-PCR (n = 8/group). While there were few differences in transcript expression between thermal neutral and heat stress in oxidative muscle, 80% (P < 0.05) of transcripts driven by NF-κB (90% at P < 0.1; Table 2) and 56% (P < 0.05) of transcripts driven by AP-1 (81% at P < 0.1; Table 3) were increased in heat-stressed pigs compared with the pair-fed thermal neutral pigs. Of interest, restricted food intake caused a broad numerical reduction in the expression of nearly all transcripts measured (thermal neutral vs. pair-fed thermal neutral); however, these generally failed to reach statistical significance (Tables 2 and 3).
Table 2.
Relative abundance of NF-κB-regulated transcript expressions in STR and STW
| STR |
STW |
|||||||
|---|---|---|---|---|---|---|---|---|
| Gene Name | Description | Function | TN | HS | PFTN | TN | HS | PFTN |
| BTG2 | B-cell translocation gene 2 | Antiproliferative | 1.00 ± 0.31 | 2.00 ± 0.87 | 0.49 ± 0.18# | 1.00 ± 0.31 | 1.24 ± 0.58 | 1.04 ± 0.42 |
| CAT | Catalase | Antioxidant | 1.00 ± 0.36 | 1.60 ± 0.47 | 0.55 ± 0.28 | 1.00 ± 0.25 | 0.62 ± 0.20 | 0.77 ± 0.18 |
| EIF4EBP1 | Eukaryotic translation initiation factor 4E binding protein 1 | Translation repression | 1.00 ± 0.26 | 2.21 ± 0.54 | 0.50 ± 0.18# | 1.00 ± 0.22 | 0.84 ± 0.21 | 0.97 ± 0.19 |
| GNB2L1 | Guanine nucleotide binding protein (G protein), β polypeptide 2-like | Translational repression | 1.00 ± 0.25 | 1.85 ± 0.37 | 0.53 ± 0.23# | 1.00 ± 0.24 | 0.70 ± 0.19 | 0.85 ± 0.17 |
| PHB2 | Prohibitin 2 | Transcriptional repression | 1.00 ± 0.31 | 2.07 ± 0.47 | 0.53 ± 0.21# | 1.00 ± 0.25 | 0.66 ± 0.19 | 0.99 ± 0.14 |
| IGFBP3 | Insulin-like growth factor-binding protein 3 | Insulin signaling | 1.00 ± 0.33 | 2.78 ± 0.57* | 0.44 ± 0.12# | 1.00 ± 0.25 | 1.02 ± 0.28 | 1.02 ± 0.20 |
| LIPE | Lipase, hormone-sensitive | Converts cholesteryl esters to free cholesterol | 1.00 ± 0.30 | 3.30 ± 1.09 | 0.63 ± 0.22# | 1.00 ± 0.22 | 0.91 ± 0.40 | 1.13 ± 0.45 |
| RNF4 | E3 ubiquitin ligase | Mobilize the stored fats; protein modification; protein ubiquitination | 1.00 ± 0.26 | 1.98 ± 0.50 | 0.66 ± 0.24# | 1.00 ± 0.22 | 0.84 ± 0.23 | 1.11 ± 0.19 |
| TPX2 | Targeting protein for Xklp2 | Spindle assembly factor | 1.00 ± 0.31 | 1.98 ± 0.38 | 0.67 ± 0.33# | 1.00 ± 0.24 | 0.83 ± 0.23 | 1.10 ± 0.29 |
Values are fold change ± SE; n = 8/group. The transcript expressions were measured using QRT-PCR with Fluidigm dynamic array for NF-κB-driven transcript in semitendinosus red (STR) and semitendinosus white (STW).
TN, thermal neutral; HS, heat stress; PFTN, pair fed thermal neutral.
Significantly different from TN (P < 0.05).
Significantly different from HS (P < 0.05). Gene function is based on the Gene cards.
Table 3.
Relative abundance of AP-1-regulated transcript expressions in STR and in STW
| Gene Names | Description | Function | STR |
STW |
||||
|---|---|---|---|---|---|---|---|---|
| TN | HS | PFTN | TN | HS | PFTN | |||
| BNIP3 | BCL2/adenovirus E1B 19 kDa interacting protein 3 | Apoptosis | 1.00 ± 0.20 | 2.27 ± 0.66 | 0.48 ± 0.13# | 1.00 ± 0.32 | 1.14 ± 0.50 | 1.24 ± 0.35 |
| DDIT4L | DNA damage-inducible transcript 4-like protein | Inhibits cell growth | 1.00 ± 0.37 | 5.45 ± 1.72 | 0.49 ± 0.24# | 1.00 ± 0.24 | 1.96 ± 0.23* | 1.31 ± 0.17 |
| FBXO32 | F-box only protein 32 | Muscle atrophy, proteasomal degradation | 1.00 ± 0.81 | 6.84 ± 2.43 | 0.58 ± 0.38# | 1.00 ± 0.46 | 2.99 ± 0.75* | 1.53 ± 0.48 |
| FLOT1 | Flotillin 1 | Scaffolding protein | 1.00 ± 0.32 | 1.95 ± 0.82 | 0.46 ± 0.18 | 1.00 ± 0.34 | 1.05 ± 0.30 | 1.09 ± 0.31 |
| GHR | Growth hormone receptor | Modulator/inhibitor of GH signaling | 1.00 ± 0.33 | 2.50 ± 0.51 | 1.51 ± 0.24# | 1.00 ± 0.28 | 0.80 ± 0.22 | 0.84 ± 0.18 |
| CST3 | Cystatin C | Proteinase inhibitor | 1.00 ± 0.25 | 1.52 ± 0.30 | 0.56 ± 0.20# | 1.00 ± 0.33 | 0.52 ± 0.11 | 0.67 ± 0.11 |
| HBXIP | Hepatitis B virus X-interacting protein | Cell proliferation | 1.00 ± 0.26 | 1.78 ± 0.33 | 0.51 ± 0.21# | 1.00 ± 0.19 | 0.67 ± 0.19 | 0.93 ± 0.11 |
| MCT4 | Proton-linked monocarboxylate transporter | Cell proliferation | 1.00 ± 0.06 | 0.82 ± 0.25 | 0.41 ± 0.10* | 1.00 ± 0.35 | 0.73 ± 0.32 | 0.83 ± 0.20 |
| HNRPDL | Heterogeneous nuclear ribonucleoprotein d-like | Transcriptional regulator | 1.00 ± 0.36 | 1.35 ± 0.26 | 0.52 ± 0.23 | 1.00 ± 0.23 | 0.69 ± 0.16 | 1.01 ± 0.07 |
| HSPA2 | Heat shock 70-kDa protein 2 | Molecular chaperone | 1.00 ± 0.26 | 2.04 ± 0.90 | 0.62 ± 0.30 | 1.00 ± 0.31 | 0.72 ± 0.20 | 0.74 ± 0.16 |
| HSPA9 | Heat shock 70-kDa protein 9 | Molecular chaperone | 1.00 ± 0.24 | 2.50 ± 0.49 | 0.58 ± 0.22# | 1.00 ± 0.25 | 0.87 ± 0.21 | 1.04 ± 0.14 |
| RAB3D | Ras-related protein Rab-3D | Protein transport | 1.00 ± 0.25 | 7.13 ± 5.78 | 0.64 ± 0.21 | 1.00 ± 0.19 | 0.80 ± 0.26 | 0.74 ± 0.10 |
| SOD1 | Superoxide dismutase 1 | Antioxidant | 1.00 ± 0.25 | 1.88 ± 0.28 | 0.57 ± 0.22# | 1.00 ± 0.23 | 0.99 ± 0.23 | 1.00 ± 0.11 |
| SP1 | Sp1 transcription factor | Transcription factor | 1.00 ± 0.27 | 2.12 ± 0.65 | 0.60 ± 0.18 | 1.00 ± 0.21 | 0.99 ± 0.21 | 1.18 ± 0.12 |
| TET2 | Tet methylcytosine dioxygenase 2 | DNA demethylation | 1.00 ± 0.29 | 1.92 ± 0.48 | 0.53 ± 0.19# | 1.00 ± 0.16 | 0.81 ± 0.26 | 0.83 ± 0.17 |
Values are fold change ± SE; n = 8/group. The transcript expressions were measured using QRT-PCR with Fluidigm dynamic array for AP-1-related transcripts in semitendinosus red (STR) and semitendinosus white (STW) muscle.
Significantly different from TN (P < 0.05).
Significantly different from HS (P < 0.05). Gene function is based on the Gene cards. TN, thermal neutral; HS, heat stress; PFTN, pair-fed thermal neutral.
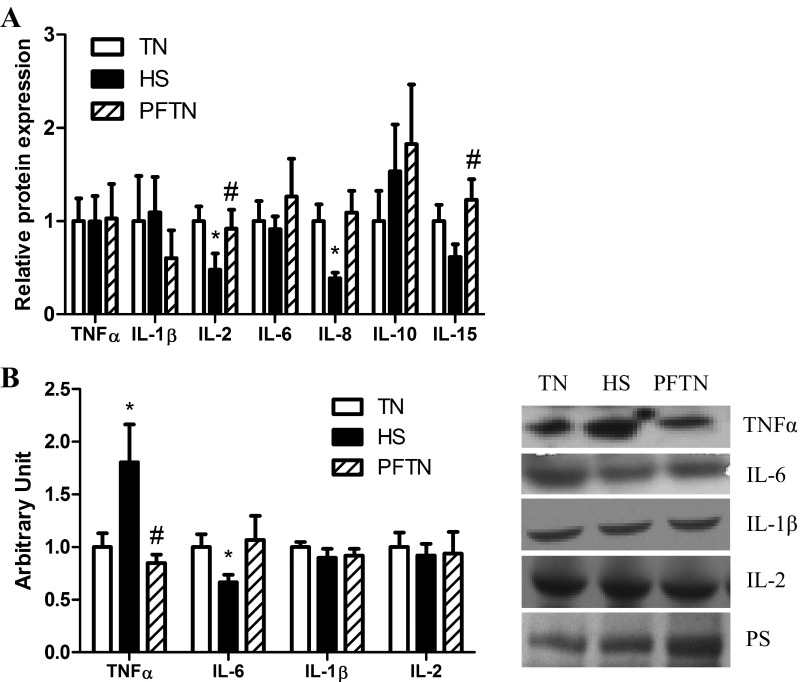
Since increased circulating endotoxin was previously found in these animals (34), we also measured transcript expression and relative protein abundance of cytokines in muscle. Heat stress increased transcript expression of IL-1β by 520% (P < 0.05) compared with thermal neutral and by 127% (P < 0.05) compared with pair-fed thermal neutral. IL-15 was increased by 332% (P < 0.05) compared with thermal neutral and 57% (P < 0.05) compared with pair-fed thermal neutral. Heat stress decreased TNF-α by 72% (P < 0.05) compared with thermal neutral and 105% (P < 0.05) compared with pair-fed thermal neutral; IL-2 (P < 0.05) by 99% compared with thermal neutral; IL-10 by 109% (P < 0.05) compared with pair-fed thermal neutral; and IL-6 by 84% (P < 0.05) compared with thermal neutral and 86% (P < 0.05) compared with pair-fed thermal neutral (Fig. 4A). Despite reduction in transcript expression, relative protein abundance of TNF-α was increased 80% (P < 0.05) by heat stress compared with thermal neutral and by 96% compared with pair-fed thermal neutral. Consistent with transcript changes, heat stress decreased (P < 0.05) IL-6 protein abundance by 40% compared with pair-fed thermal neutral. Relative protein abundance of IL-1β and IL-2 was similar between groups (Fig. 4B).
Fig. 4.
Effect of heat stress on inflammatory cytokines in semitendinosus red (STR). A: following 12 h of heat stress, transcript expression for inflammatory cytokines was measured by QRT-PCR in STR. Values are fold change ± SE; n = 8/group. B: relative protein abundance was measured by Western blot in whole homogenate. Sample blots are also included. Ponceau S stain (PS) was used as a loading control. Values are means ± SE; n = 8/group. *Significantly different from TN. #Significantly different from HS. TN, thermal neutral; HS, heat stress; PFTN, pair-fed thermal neutral.
In glycolytic muscle, relative protein abundance of all measures was similar between groups (Fig. 5). Transcript expression of NF-κB- and AP-1-driven genes was largely unchanged by heat stress or reduced food intake (Tables 2 and 3). However, DDIT4L and FBXO32 mRNA expression was increased by heat stress compared with thermal neutral (P < 0.05; Tables 2 and 3).
Fig. 5.
Effect of heat stress on inflammatory signaling in semitendinosus white whole homogenate. Relative protein abundance was measured by Western blot in whole homogenate. Sample blots are also included. Ponceau S stain (PS) was used as a loading control. Values are means ± SE; n = 8/group. TN, thermal neutral; HS, heat stress; PFTN, pair-fed thermal neutral.
DISCUSSION
Prolonged exposure to elevated heat loads can result in heat stress, which is detrimental to human (23) and animal health and performance (1, 43). Aside from cooling and rehydration, there is no other consistently applied protocol available to mitigate heat stress-mediated injuries (15). Our laboratory's previous work demonstrated that heat stress increased oxidative stress but not inflammatory signaling in porcine skeletal muscle after 1 day of heat exposure (25). Given the strong rationale to suspect inflammatory signaling, including increased free radical injury, TNF-α, and circulating endotoxin, in this investigation we hypothesized that heat stress would increase inflammatory signaling in skeletal muscle. We found that 12 h of heat stress increased NF-κB signaling but not AP-1 signaling. In addition, heat stress led to increased expression of transcripts driven by NF-κB and AP-1 signaling, as well as inflammatory cytokines.
Increased circulating endotoxin (31) and intramuscular TNF-α (25) could serve as triggers of an inflammatory response. Previously, our laboratory reported increased endotoxin and decreased TNF-α in blood following 12 h of heat stress (34). Here, we report increased intramuscular TNF-α protein abundance with discordant transcript expression, supporting our laboratory's previous observations following 24 h of heat stress (25, 32). These data support our laboratory's previous speculation (25) that circulating TNF-α may migrate from the vasculature into skeletal muscle. To determine the extent to which inflammatory signaling contributed to the altered intracellular environment, we measured NF-κB pathway activation. While NF-κB abundance in whole homogenate was similar between groups, relative abundance of IKK-α was increased following 12 h of heat stress. IKK-α is essential for the phosphorylation of IkB and translocation of NF-κB to the nucleus (37, 46). In addition to increased nuclear NF-κB content, we also discovered that expression of transcripts driven by NF-κB was increased. Of note, these changes were predominantly found between the pair-fed thermal neutral and the heat stress groups, indicating that these are heat stress-induced changes independent from decreased food intake. Supporting this, numerical changes between thermal neutral and heat stress were of the same direction but smaller magnitude. Increased NF-κB signaling may be due to increased intestinal permeability, as heat stress of >6 h results in leaky gut (33, 34), whereas heat stress for 2 and 4 h was insufficient to increase gut permeability in pigs (33). The role of heat stress-mediated inflammatory signaling following 2–6 h of heat stress exposure is unknown, but is currently being explored by our group. Consistent with an interaction of intestinal permeability and NF-κB signaling, preliminary findings are supportive of NK-κB pathway activation at only the 6-h time point in oxidative skeletal muscle (unpublished observations). When modeling heat stroke using a heating paradigm of ∼2 h, NF-kB signaling does not appear to contribute to muscle dysfunction; however, AP-1 appears to play a major role (50).
Our findings of increased expression of AP-1-driven transcripts following 12 h of heat stress were surprising, as measures of AP-1 pathway signaling and nuclear abundance of AP-1 were similar between groups. It seems likely that elevated transcript expression is a remnant of previous pathway activation. Such a hypothesis is not without precedent, as studies focused on heat stroke have found increased AP-1 activation in both soleus and diaphragm in mice (49).
Apart from inflammation, increased NF-κB- and AP-1-driven transcripts regulate a variety of cellular functions. Apoptosis signaling was increased by heat stress, suggesting an interaction of heat stress and apoptosis in skeletal muscle. In support of this, previous work demonstrated that heat stress causes apoptosis in umbilical vein endothelial cell (9) and in swine skeletal muscle satellite cells (7).
In addition to transcripts driven by either NF-κB or AP-1, we also specifically measured transcript expression and relative protein abundance of several cytokines. Consistent with our hypothesis of a proinflammatory environment IL-1β transcript expression was increased, along with several inflammatory cytokines; however, these changes did not lead to increased protein abundance. Of particular interest was the reduction in IL-6 transcript and relative protein abundance following heat stress compared with thermal neutral animals. It has been previously established that, during heat stroke, IL-6 is exported from skeletal muscle (50, 51) and provides protection to other organ systems (12, 18–20, 27, 35). Indeed, failure to adequately maintain increased IL-6 production may be a key factor that distinguishes heat stroke from heat stress and may also help to explain the multiorgan dysfunctions that occur during heat stress. Furthermore, the reduction in IL-6 may be related to elevated TNF-α, as TNF-α is inversely correlated with IL-6 (39, 51).
These data provide an initial description of the chronology of heat stress-mediated inflammatory signaling in skeletal muscle. Together with our previous data, it appears that inflammatory signaling is increased following 12 h of heat stress and then returns to baseline following 24 h of heat stress. Elevated abundance of HSP would provide a simple means by which to inhibit inflammatory signaling (42); however, HSP72 and other HSPs (HSP27, phospho-HSP27, and HSP90) were similar between groups. In a proteomics experiment making use of the same tissues as in this investigation, our laboratory found a modest (∼10%) HSP response, including increased expression of mitochondrial HSP70, HSP27, HSP20, and α-B-crystallin (3). Several of these proteins can blunt AP-1 inflammatory signaling, among other functions (5, 6, 45). This raises the possibility of a differential capacity of HSPs to squelch inflammatory pathway activities, such that AP-1 signaling is more sensitive to HSPs than is NF-κB.
The lack of heat stress-mediated changes in glycolytic (STW) muscle was striking, although it is in agreement with our laboratory's previous observations following 24 and 72 h of heat stress (25). A proteomic assessment of these tissues, however, detected some modest heat stress-mediated alterations in protein abundance related primarily to HSP expression, metabolism, and antioxidants (3). Given the widespread changes seen in STR, but not STW, the mitochondria are implicated as central figures in heat stress-mediated cellular dysfunction. Further implicating mitochondria is the metabolic shift away from oxidative phosphorylation, as pyruvate entry into the mitochondria appears blunted during heat stress (1, 3, 52). It is unclear if mitochondria are a proximal or distal cause of heat stress-mediated cellular dysfunction, as mitochondrial malfunction would be expected following oxidative stress or loss of Ca2+ homeostasis, which have been reported or may be anticipated during heat stress (22, 25, 38).
Perspective and Significance
In summary, following 12 h of heat stress, NF-κB signaling was enhanced in oxidative skeletal muscle, as indicated by increased abundance of NF-κB activator IKK-α, increased NF-κB abundance in the nucleus, increased expression of NF-κB-driven transcripts, and increased expression of inflammatory cytokines. Elevated expression of AP-1-driven transcripts is likely due to the previous activation of AP-1 signaling that has subsided or was effectively inhibited following 12 h of heat stress. As we were unable to detect elevated AP-1 content in the nuclear fraction, our expectation is that AP-1-mediated transcript expression will soon cease. Likewise, given our findings following 24 h of heat stress, we also expect that NF-κB signaling will also be inactivated. In conclusion, 12-h heat stress caused inflammatory signaling in oxidative muscles. These data also confirm our laboratory's previous results that oxidative muscle is more sensitive to heat stress than is glycolytic muscle.
GRANTS
This work was supported by US Department of Agriculture Grants 2014-67015-21627 (J. T. Selsby) and 2011–6700330007 (L. H. Baumgard).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.G., S.C.P., N.K.G., L.H.B., R.P.R., and J.T.S. conception and design of research; S.G., C.R., K.H., S.C.P., N.K.G., L.H.B., R.P.R., and J.T.S. performed experiments; S.G., C.R., and J.T.S. analyzed data; S.G. and J.T.S. interpreted results of experiments; S.G. and J.T.S. prepared figures; S.G. and J.T.S. drafted manuscript; S.G., C.R., K.H., S.C.P., N.K.G., L.H.B., R.P.R., and J.T.S. edited and revised manuscript; S.G., C.R., K.H., S.C.P., N.K.G., L.H.B., R.P.R., and J.T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jermilia Charles, Shannon Cruzen, and Martin Curry for technical assistance.
REFERENCES
- 1.Baumgard LH, Rhoads RP. Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci 1: 311–337, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Collin A, van Milgen J, Dubois S, Noblet J. Effect of high temperature and feeding level on energy utilization in piglets. J Anim Sci 79: 1849–1857, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Cruzen SM, Pearce SC, Baumgard LH, Gabler NK, Huff-Lonergan E, Lonergan SM. Proteomic changes to the sarcoplasmic fraction of predominantly red or white muscle following acute heat stress. J Proteomics 128: 141–153, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Diaz PT, Brownstein E, Clanton TL. Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol 77: 2434–2439, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Fan GC, Yuan Q, Song G, Wang Y, Chen G, Qian J, Zhou X, Lee YJ, Ashraf M, Kranias EG. Small heat-shock protein Hsp20 attenuates β-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res 99: 1233–1242, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Gabai VL, Meriin AB, Yaglom JA, Volloch VZ, Sherman MY. Role of Hsp70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett 438: 1–4, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Gao CQ, Zhao YL, Li HC, Sui WG, Yan HC, Wang XQ. Heat stress inhibits proliferation, promotes growth, and induces apoptosis in cultured Lantang swine skeletal muscle satellite cells. J Zhejiang Univ Sci B 16: 549–559, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gathiram P, Wells MT, Brock-Utne JG, Gaffin SL. Antilipopolysaccharide improves survival in primates subjected to heat stroke. Circ Shock 23: 157–164, 1987. [PubMed] [Google Scholar]
- 9.Gu ZT, Wang H, Li L, Liu YS, Deng XB, Huo SF, Yuan FF, Liu ZF, Tong HS, Su L. Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci Rep 4: 4469, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58: 567–578, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol 110: 451–457, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammami MM, Bouchama A, Al-Sedairy S, Shail E, Alohaly Y, Mohamed GED. Concentrations of soluble tumor necrosis factor and interleukin-6 receptors in heatstroke and heatstress. Crit Care Med 25: 1314–1319, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Hansen AL, Bi P, Ryan P, Nitschke M, Pisaniello D, Tucker G. The effect of heat waves on hospital admissions for renal disease in a temperate city of Australia. Int J Epidemiol 37: 1359–1365, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Huynen MM, Martens P, Schram D, Weijenberg MP, Kunst AE. The impact of heat waves and cold spells on mortality rates in the Dutch population. Environ Health Perspect 109: 463–470, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilbourn EM. Heat waves and hot environments. In: The Public Health Consequences of Disasters, edited by Noji E. New York: Oxford University Press, 1997, p. 245–269. [Google Scholar]
- 16.Knowlton K, Rotkin-Ellman M, Geballe L, Max W, Solomon GM. Six climate change-related events in the united states accounted for about $14 billion in lost lives and health costs. Health Aff (Millwood) 30: 2167–2176, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci 87: E101–E108, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Leon LR. Heat stroke and cytokines. Prog Brain Res 162: 481–524, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J Appl Physiol 100: 1400–1409, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Leon LR, DuBose DA, Mason CW. Heat stress induces a biphasic thermoregulatory response in mice. Am J Physiol Regul Integr Comp Physiol 288: R197–R204, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol 109: 1980–1988, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Lepock JR, Rodahl AM, Zhang C, Heynen ML, Waters B, Cheng KH. Thermal denaturation of the Ca2+-ATPase of sarcoplasmic reticulum reveals two thermodynamically independent domains. Biochemistry (Mosc) 29: 681–689, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Medina-Ramón M, Schwartz J. Temperature, temperature extremes, and mortality: a study of acclimatisation and effect modification in 50 US cities. Occup Environ Med 64: 827–833, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor κB activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A 101: 5634–5639, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montilla SIR, Johnson TP, Pearce SC, Gardan-Salmon D, Gabler NK, Ross JW, Rhoads RP, Baumgard LH, Lonergan SM, Selsby JT. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature (Austin) 1: 42–50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol 88: 359–363, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Novosad VL, Richards JL, Phillips NA, King MA, Clanton TL. Regional susceptibility to stress-induced intestinal injury in the mouse. Am J Physiol Gastrointest Liver Physiol 305: G418–G426, 2013. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill MS, Ebi KL. Temperature extremes and health: impacts of climate variability and change in the United States. J Occup Environ Med 51: 13–25, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Oliver SR, Wright VP, Parinandi N, Clanton TL. Thermal tolerance of contractile function in oxidative skeletal muscle: no protection by antioxidants and reduced tolerance with eicosanoid enzyme inhibition. Am J Physiol Regul Integr Comp Physiol 295: R1695–R1705, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce SC, Lonergan SM, Huff-Lonergan E, Baumgard LH, Gabler NK. Acute heat stress and reduced nutrient intake alter intestinal proteomic profile and gene expression in pigs. PLoS One 10: e0143099, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Baumgard LH, Gabler NK. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J Anim Sci 90, Suppl 4: 257–259, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 8: e70215, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearce SC, Sanz-Fernandez MV, Hollis JH, Baumgard LH, Gabler NK. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J Anim Sci 92: 5444–5454, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Pearce SC, Sanz Fernandez MV, Torrison J, Wilson ME, Baumgard LH, Gabler NK. Dietary organic zinc attenuates heat stress-induced changes in pig intestinal integrity and metabolism. J Anim Sci 93: 4702–4713, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Phillips NA, Welc SS, Wallet SM, King MA, Clanton TL. Protection of intestinal injury during heat stroke in mice by interleukin-6 pretreatment. J Physiol 593: 739–753, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhoads ML, Rhoads RP, VanBaale MJ, Collier RJ, Sanders SR, Weber WJ, Crooker BA, Baumgard LH. Effects of heat stress and plane of nutrition on lactating Holstein cows. I. Production, metabolism, and aspects of circulating somatotropin 1. J Dairy Sci 92: 1986–1997, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Rothwarf DM, Karin M. The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. Science STKE 1999: re1, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Schertzer JD, Green HJ, Tupling AR. Thermal instability of rat muscle sarcoplasmic reticulum Ca2+-ATPase function. Am J Physiol Endocrinol Metab 283: E722–E728, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL- 6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75: 40–47, 1990. [PubMed] [Google Scholar]
- 40.Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289: R134–R139, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol 102: 1702–1707, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Sheppard PW, Sun X, Khammash M, Giffard RG. Overexpression of heat shock protein 72 attenuates NF-κB activation using a combination of regulatory mechanisms in microglia. PLoS Comput Biol 10: e1003471, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St. Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci 86, Suppl: E52–E77, 2003. [Google Scholar]
- 44.Starkie RL, Hargreaves M, Rolland J, Febbraio MA. Heat stress, cytokines, and the immune response to exercise. Brain Behav Immun 19: 404–412, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, Vosler P, Zhang L, Signore A, Graham SH, Chen J. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci 28: 13038–13055, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science 259: 1912–1915, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi K, Hatade T, Wakamiya S, Fujita N, Arakawa T, Miki A. Heat stress promotes skeletal muscle regeneration after crush injury in rats. Acta Histochem 116: 327–334, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Tian Z, Li S, Zhang J, Guo Y. The characteristic of heat wave effects on coronary heart disease mortality in Beijing, China: a time series study. PLoS One 8: e77321, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welc SS, Clanton TL, Dineen SM, Leon LR. Heat stroke activates a stress-induced cytokine response in skeletal muscle. J Appl Physiol 115: 1126–1137, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Welc SS, Judge AR, Clanton TL. Skeletal muscle interleukin-6 regulation in hyperthermia. Am J Physiol Cell Physiol 305: C406–C413, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Welc SS, Phillips NA, Oca-Cossio J, Wallet SM, Chen DL, Clanton TL. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Cell Physiol 303: C455–C466, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao L, McMillan RP, Xie G, Zhang Z, Baumgard LH, El-Kadi S, Selsby JT, Ross JW, Gabler NK, Hulver M, Rhoads RP. Effect of heat stress on pig skeletal muscle metabolism. FASEB J 29: 7557, 2015. [Google Scholar]
- 53.Zuo L, Christofi FL, Wright VP, Liu CY, Merola AJ, Berliner LJ, Clanton TL. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol 279: C1058–C1066, 2000. [DOI] [PubMed] [Google Scholar]