Abstract
Leptin signals energy sufficiency to the reproductive hypothalamic-pituitary-gonadal (HPG) axis. Studies using genetic models have demonstrated that hypothalamic neurons are major players mediating these effects. Leptin receptor (LepR) is also expressed in the pituitary gland and in the gonads, but the physiological effects of leptin in these sites are still unclear. Female mice with selective deletion of LepR in a subset of gonadotropes show normal pubertal development but impaired fertility. Conditional deletion approaches, however, often result in redundancy or developmental adaptations, which may compromise the assessment of leptin's action in gonadotropes for pubertal maturation. To circumvent these issues, we adopted a complementary genetic approach and assessed if selective reexpression of LepR only in gonadotropes is sufficient to enable puberty and improve fertility of LepR null female mice. We initially assessed the colocalization of gonadotropin-releasing hormone receptor (GnRHR) and LepR in the HPG axis using GnRHR-IRES-Cre (GRIC) and LepR-Cre reporter (tdTomato or enhanced green fluorescent protein) mice. We found that GRIC and leptin-induced phosphorylation of STAT3 are expressed in distinct hypothalamic neurons. Whereas LepR-Cre was observed in theca cells, GRIC expression was rarely found in the ovarian parenchyma. In contrast, a subpopulation of gonadotropes expressed the LepR-Cre reporter gene (tdTomato). We then crossed the GRIC mice with the LepR null reactivable (LepRloxTB) mice. These mice showed an increase in FSH levels, but they remained in a prepubertal state. Together with previous findings, our data indicate that leptin-selective action in gonadotropes serves a role in adult reproductive physiology but is not sufficient to allow pubertal maturation in mice.
Keywords: gonadotropes, HPG axis, obesity, puberty, sexual maturation
the adipocyte-derived hormone leptin signals energy sufficiency to the hypothalamic-pituitary-gonadal (HPG) axis and acts as a permissive factor for reproductive function (3, 7, 8, 20, 23, 39). Humans and mice with a loss-of-function mutation in the leptin (LEP/Lep) or leptin receptor (LEPR/Lepr) genes are obese, infertile, and remain in a sexually immature state (12, 24, 38, 44, 54). Administration of leptin to leptin-deficient subjects increases gonadotropin secretion and restores fertility (3, 8, 23). Leptin binds to cognate receptors (LepR) expressed in multiple tissues, including every node of the HPG axis, i.e., the hypothalamus, the anterior pituitary gland, and the gonads (1, 21, 42, 45, 53). Thus leptin might control reproductive physiology by acting at different levels of the HPG axis. However, studies using genetically modified mouse models have implicated the brain as the major player. Expression of LepR only in the brain of mice otherwise null for LepR is sufficient to restore reproductive function in males and females (16). Vice versa, deletion of LepR from hypothalamic neurons precludes sexual maturation and reproductive function (11, 40, 55). These observations initially diminished the interest in the physiological actions of leptin outside of the brain. However, recent findings showing that lack of leptin action only in LH-β+-gonadotropes also impairs fertility have reinvigorated the field. Female mice with deletion of LepR in luteinizing hormone β-subunit expressing cells (LH-β, which is exclusively expressed in a subset of gonadotropes) display subfertility demonstrated by increased time to pregnancy and decreased litter size (1).
Because reproduction is essential for species survival, multiple parallel systems may be in place to ensure reproductive success. In this regard, it is possible that the reduction, but not loss, of reproductive fitness in gonadotrope-specific LepR null mice (1) may have been obscured by the existence of redundant pathways or developmental adaptations. This is, in fact, a recurrent observation in the reproductive field. For example, global deletion of highly relevant genes in reproductive function results in small or undetectable phenotypes (34, 37, 46, 52). In the attempt to circumvent these issues and gain insights into leptin's physiological action in reproductive physiology, we adopted an alternative genetic approach. We used LepR null reactivable (LepRloxTB) mice to conditionally reexpress endogenous LepR in gonadotropes (5, 13). The LepRloxTB mice have a phenotype reminiscent of that of the LepR-deficient (db/db) mouse: they are obese, diabetic and infertile. Males and females remain in a prepubertal state, and no sexual maturation or reproductive capacity is attained (13). Using the Cre-loxP system, we reexpressed endogenous LepRs selectively in gonadotropes using the gonadotropin-releasing hormone (GnRH) receptor (GnRHR)-internal ribosome entry site (IRES)-Cre (GRIC) mouse model (50). We assessed whether leptin action only in those cells is sufficient to induce puberty and improve fertility in otherwise infertile LepR null female mice.
METHODS
Animals.
The GnRHR-IRES-Cre (GRIC) (50), the LepR-Cre (JAX mice, stock no. 008320) (17), the LepRloxTB (kindly provided by Dr. Elmquist, UTSW Medical Center, Dallas, TX, available in JAX mice, stock no. 018989) (5), the R26 tdTomato (JAX mice, stock no. 007914) (36), the R26 enhanced green fluorescent protein (eGFP) (JAX mice, stock no. 004077) (25, 26) and db/db (JAX mice, stock no. 000697) mice were kept in the University of Michigan animal facility in a light- (12 h on/off) and temperature-controlled (21–23°C) environment with free access to water and food. Mice were fed phytoestrogen-reduced Harlan diet 2016 (16% protein/4% fat), except during breeding when mice were fed higher protein and fat phytoestrogen-reduced Harlan diet 2019 (19% protein/8% fat) (Teklad Global Rodent diet, Harlan Laboratories). All procedures and experiments were carried out in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Committee on Use and Care of Animals (Animal Protocol no. 04380).
The GRIC mouse is a knock-in strain that coexpresses Cre-recombinase with the Gnrhr gene. The LepR-Cre line is also a knock-in strain with an IRES-Cre sequence inserted immediately 3′ of the stop codon of the Lepr gene. The LepRloxTB mice have a loxP-flanked transcription-blocking (TB) cassette inserted between exons 16 and 17 of the Lepr gene, allowing the generation of mice lacking the long isoform of LepR in a conditional Cre recombinase-dependent approach. The ROSA26 (R26)-tdTomato and R26-eGFP mice carry targeted mutations of the R26 locus with a loxP-flanked TB cassette preventing the expression of CAG promoter-driven tdTomato or eGFP reporters, respectively. Cre-mediated excision of the loxP-flanked TB cassette allows expression of the red fluorescent protein variant tdTomato or of the eGFP.
All mice were tail-genotyped before and after experiments by extracting DNA (RED Extract-N-Amp Tissue PCR Kit catalog no. XNAT, Sigma, St. Louis, MO) and performing PCR. The primers used for genotyping are described in Table 1. The db/db mice were directly purchased from JAX mice and were not genotyped.
Table 1.
List of primers used for genotyping of mouse models
| Mouse | Forward | Reverse | Source |
|---|---|---|---|
| GRIC | 5′CTTTCCTAAACCCGTGCTTC 3′ | 5′TGATCTAAGGAGGAAGTAAAGAATCC 3′ | Wen at al. (50) |
| LepR-Cre | 5′TGCTTCTGTCCGTTTGCCGGT 3′ | 5′GTGAAACAGCATTGCTGTCAC 3′ | stock no. 08320* |
| R26-tdTom | 5′CTGTTCCTGTACGGCATGG 3′ | 5′GGCATTAAAGCAGCGTATCC 3′ | stock no. 07914* |
| R26-eGFP | 5′AAGTTCATCTGCACCACCG 3′ | 5′TCCTTGAAGAAGATGGTGCG 3′ | stock no. 04077* |
| LepRloxTB | 5′CAGTCTGGACCGAAGGTGTT 3′ | 5′TAGGGCCAAACCCACATTTA 3′ | stock no. 18989* |
Commercially available in JAX mice.
Perfusion and histology.
Mice were deeply anesthetized with isoflurane (Fluriso, Vet One, Boise, ID) and perfused intracardially with 10% buffered formalin (Sigma). Brains, pituitary gland, uterus, and ovaries were dissected. Brains were sectioned on a freezing microtome (30-μm sections, 5 series) in the frontal plane. Pituitary glands and ovaries were sectioned using a cryostat (14-μm sections, 3 series) or microtome (5-μm sections). The DsRed (tdTomato) was assessed in series of brain, pituitary gland, and ovary sections from GRIC and LepR-Cre reporter mice. The visualization of eGFP was performed after an amplification step using immunohistochemistry.
Leptin administration.
To evaluate the colocalization of LepR and GnRHR, a group of GRIC-tdTomato female mice (n = 4) was fasted overnight and received intraperitoneal leptin [5 μg/g, purchased from Dr. A. F. Parlow, Harbor-UCLA Medical Center, Torrance, CA, through the National Hormone and Peptide Program (NHPP)] to assess leptin-induced phosphorylation of STAT3 immunoreactivity (pSTAT3-ir), as described before (18, 19). Two hours after leptin treatment, mice were perfused, and brains and pituitary glands were dissected and sectioned using a freezing microtome (brains) or a cryostat (pituitary glands). Brain and pituitary gland sections were incubated in antisera against pSTAT3 raised in rabbit (1:2,000, Cell Signaling, Danvers, MA) at 4°C for 48–72 h. Sections were then incubated in biotin-conjugated donkey anti-rabbit secondary antisera (1:1,000, for 1 h; Jackson Laboratories, Bar Harbor, ME) followed by an avidin-biotin complex (1:500 for 1 h; Vector Laboratories, Burlingame, CA) and finally subjected to an immunoperoxidase reaction using 0.05% diaminobenzidine and 0.05% nickel sulfate as chromogens.
Immunofluorescence.
To assess the colocalization of LepR in gonadotropes (GnRHR expressing cells), the pituitary glands from LepR-Cre reporter mice (tdTomato, n = 4 males and n = 4 females) were sectioned on a cryostat (14-μm sections), mounted onto slides, and subjected to immunofluorescence analysis for LH-β (purchased from Dr. A. F. Parlow through the NHPP). Sections of the pituitary gland were rinsed in 0.1 M PBS, pH 7.4, and incubated overnight in anti-LH-β primary antisera raised in guinea pig (1:2,000). On the following day, sections were incubated in biotinylated secondary donkey anti-guinea pig antisera (1:1,000, Jackson Laboratories) for 1 h, followed by streptavidin AlexaFluor-488 (1:500, Invitrogen, Carlsbad, CA) for 1 h. Tissue was rinsed in PBS, dried at room temperature, and coverslipped with Fluoromount (EMS, Hatfield, PA).
Distribution of GRIC-eGFP was assessed in the pituitary glands and ovaries. Tissues were sectioned on a cryostat (14-μm sections), mounted onto slides, and submitted to immunofluorescence for GFP (antisera generated in chicken, Aves Laboratories, Tigard, OR). Slides were rinsed in PBS and incubated overnight in anti-GFP (1:5,000). On the following day, sections were incubated with AlexaFluor-488 conjugated secondary donkey anti-chicken antisera (1:500) for 1 h. Tissue was rinsed in PBS, dried at room temperature, and coverslipped with Vectashield antifade mounting medium with 4,6-diamidino-2-phenylindole (Vector Laboratories).
Endogenous reexpression of LepR in GnRHR cells.
Mice with expression of LepR only in GnRHR cells were generated by crossing the LepRloxTB with GRIC mice. Mice homozygous for LeprloxTB allele (null for LepR long form) are obese, diabetic, and infertile. Hemizygous (LepRloxTB/+) male mice were bred with GRIC females to generate our breeders. Female GRIC+/− LeprloxTB/+ mice were crossed with male GRIC+/+ LeprloxTB/+ mice to avoid germ-line activation of Cre recombinase by the male breeder (48). Genotypes of the resulting progeny were produced at the expected Mendelian ratio. Groups were divided into wild-type (control with no GRIC or LepRloxTB allele), LepRloxTB (control homozygous for LepRloxTB allele and no GRIC allele) and GRIC LepRloxTB (experimental mice). At the end of the experiment, genotypes were confirmed, and hypothalami, pituitary glands, and tail tips were processed with the Sigma kit and LepRloxTB primers to assess Cre activity and DNA recombination (defined by adding the pDis Reac primer, 5′ CCC AAG GCC ATA CAA GTG TT 3′, band size = 600 bp).
The ovaries of wild-type, LepRloxTB and GRIC LepRloxTB mice were placed in 10% formalin (Sigma), stored at 4°C and submitted to the Unit for Laboratory Animal Medicine in vivo animal core of the University of Michigan for the standard paraffin embedding procedure and hematoxylin and eosin staining (5-μm sections). The presence of corpora lutea and follicles in different stages was evaluated. The uteri were weighed immediately after euthanasia, placed in 10% formalin, and photographed without further preparation.
Body weight and reproductive phenotyping.
Mice were weighed weekly between 5 and 15 wk of age. They were monitored for puberty onset [vaginal opening (VO)] and sexual maturation (until 10 mo of age). Females were further tested for fertility by breeding with sexually experienced males. Three trials of fertility testing were performed (n = 3–4/genotype). Each trial was set up to include three to four cages of mice. Each cage housed one LepRloxTB female, one GRIC LepRloxTB female, and one wild-type female for 4–6 wk. Fertility was assessed by the presence of live pups. The estrous cycles of wild-type females were monitored by vaginal cytology (6), and mice were euthanized during the diestrus phase. At the time of euthanasia, mutant mice were also assessed for uterus weight, and ovaries were collected to evaluate the presence of corpora lutea.
Quantitative PCR.
Adult wild-type (in diestrus), LepRloxTB and GRIC LepRloxTB female mice were deeply anesthetized with isoflurane and euthanized by decapitation. The hypothalamus and pituitary gland were rapidly dissected and frozen on dry ice. Hypothalamic blocks were limited by an incision 1 mm anterior to the optic chiasm and another immediately posterior to the mammillary bodies. Lateral limits were defined by the optic tract, and superior limits were defined by the dorsal tip of the third ventricle (14). Tissues were stored at −80°C until quantitative PCR (qPCR) was performed. Tissue was homogenized using Qiazol, and total RNA was isolated using the miRNeasy minikit (cat no. 217004, Qiagen, Germantown, MD). Samples were then quantified using an Epoch reader (Biotek, Suwanee, GA). Total RNA (500–750 ng) was treated with DNase (Qiagen catalog no. 79254) and reverse-transcribed into cDNA. The qPCR was performed using Integrated DNA Technologies PrimeTime qPCR Assays and TaqMan technology in a CFX96 (C1000, BioRad, Hercules, CA). Primers and references are described in Table 2. Changes in expression of the following genes were assessed: Gnrh1 and Gnrhr in the hypothalamus, and Gnrhr, Lhb, Fshb (follicle stimulating hormone), and Cga (glycoprotein hormone α-polypeptide) in the pituitary gland. The ribosomal protein S29 (Rps29 gene, a component of the ribosome 40S subunit) and β-actin were used as housekeeping genes (29). The qPCR data were analyzed by the ΔΔCT method.
Table 2.
List of primer/probe pairs used for qPCR studies
| Gene | Sequence | Company | Reference |
|---|---|---|---|
| β Actin | F primer: 5′ GAT TAC TGC TCT GGC TCC TAG 3′ | IDT | Assay no. Mm.PT.39a.22214843.g |
| R primer: 5′ GAC TCA TCG TAC TCC TGC TTG 3′ | |||
| Probe: 5′ CTG GCC TCA /ZEN/CTG TCC ACC TTC C 3′ | |||
| GnRH | F primer: 5′ GGG AAA GAG AAA CAC TGA ACA C 3′ | IDT | Custom |
| R primer: 5′ AGT ACA TTC GAA GTG CTG GG 3′ | |||
| Probe: 5′ AGT GGG CAA GGA GGT GGA TCA AAT 3′ | |||
| GnRH R | F primer: 5′ TCA GCA TTG TCT TTG CAG GA 3′ | IDT | Assay no. Mm.PT.45.16240237 |
| R primer: 5′ TCA CAC ATT GCG AGA AGA CTG 3′ | |||
| Probe: 5′ TGA TCT ACC TAG CAG ACG GCT CTG G 3′ | |||
| RPS29 | F primer: 5′ TGA AGG CAA GAT GGG TCA C 3′ | IDT | Custom |
| R primer: 5′ GCA CAT GTT CAG CCC GTA TT 3′ | |||
| Probe: 5′ AGT CAC CCA CGG AAG TTC GG 3′ | |||
| LHβ | F primer: 5′ CCA GTC TGC ATC ACC TTC AC 3′ | IDT | Assay # Mm.PT.45.5612498 |
| R primer: 5′ GAG GCA CAG GAG GCA AAG 3′ | |||
| Probe: 5′ AGT ACT CGG ACC ATG CTA GGA CAG T 3′ | |||
| FSHβ | F primer: 5′ TTC AGC TTT CCC CAG AAG AG 3′ | IDT | Assay no. Mm.PT.45.17694677 |
| R primer: 5′ TCC AGC ACC AGA ATA AGA TGC 3′ | |||
| Probe: 5′ AGC TAC GTC CTG TGC AGT CAG C 3′ | |||
| CGA | F primer: 5′ AGC ATG ACC AGA ATG ACA GC 3′ | IDT | Assay no. Mm.PT.58.31855537 |
| R primer: 5′ CCT CAG ATC GAC AAT CAC CTG 3′ | |||
| Probe: 5′ CCC TCA AAA AGT CCA GAG CTT GCA GA 3′ |
F, forward; R, reverse.
Hormone profiles.
The LH and FSH levels were assessed from plasma samples taken from the trunk blood at the time of death. After 45-min incubation at room temperature, the blood samples were centrifuged at 1,000 g for 20 min at 4°C. Serum samples were collected and stored at −20°C. Analyses of LH and FSH serum levels were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA) using the EMD Millipore mouse/rat LH/FSH multiplex assay. The detection limits were 0.24 ng/ml for LH and 2.4 ng/ml for FSH.
Quantification, data analyses, and production of digital images.
Sections of brain, pituitary gland, and ovary were analyzed using an Axio Imager M2 microscope (Carl Zeiss, Jena, Germany). The distribution of single-labeled (pSTAT3-ir+ or tdTomato+) and dual-labeled (pSTAT3-ir+ and tdTomato+) neurons was assessed in the entire hypothalamus. Quantification of dual-labeled neurons (LepR-Cre tdTomato+ and LH-β-ir+) and the percentage of colocalization were determined in one representative section of the anterior pituitary gland. Distribution of GFP-ir (GRIC-eGFP mice) and tdTomato (LepR-tdTomato mice) was analyzed in the entire ovary. Statistical analysis was performed using GraphPad Prism 6 software. Comparison among groups was determined by one-way ANOVA, applying Geisser-Greenhouse correction for data variability followed by the Tukey post hoc multiple comparison test. Data are presented as means ± SE and α values (P < 0.05) were considered significant. Photomicrographs were produced by capturing images with a digital camera (Axiocam, Zeiss) mounted directly on the microscope using the Zen software. Adobe Photoshop CS6 image-editing software was used to integrate photomicrographs into plates. Only sharpness, contrast, and brightness were adjusted.
RESULTS
LepR expression in GnRHR expressing cells.
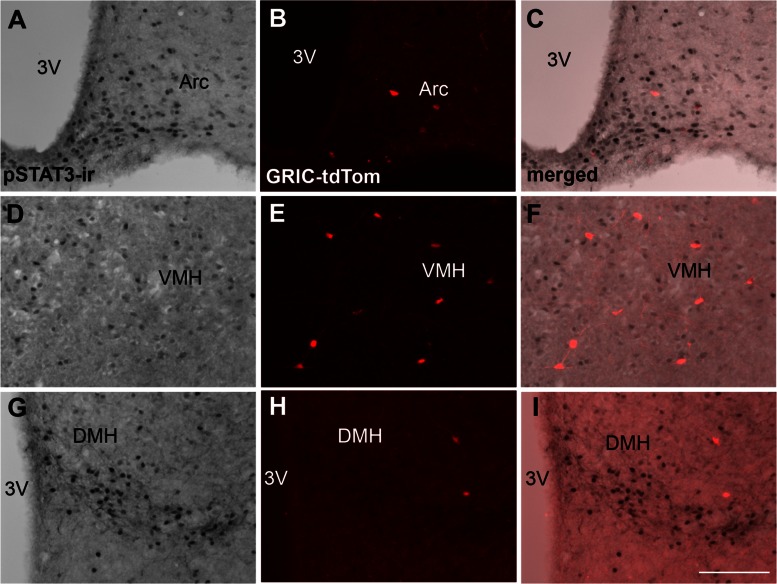
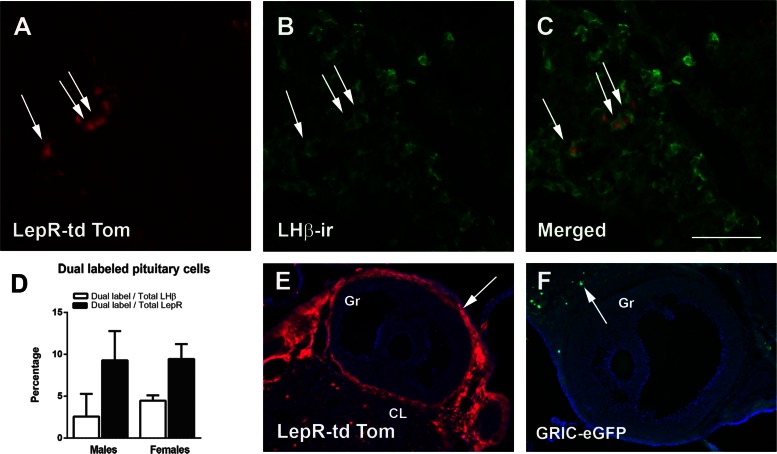
To determine the cell population and degree of colocalization of LepR and GnRHR, we evaluated the distribution of leptin-induced pSTAT3-ir in the hypothalamus and in the pituitary gland of GRIC reporter mice (tdTomato). In the hypothalamus, very few GRIC cells were observed in areas that express LepR or leptin-induced pSTAT3, in agreement with a previous report (49). Small numbers of GRIC-tdTomato neurons were found in the arcuate, ventromedial, and dorsomedial nuclei. Virtually no leptin-induced pSTAT3-ir and GRIC colocalization was observed in the adult female hypothalamus (n = 3, Fig. 1). Because we were unable to detect reliable leptin-induced pSTAT3-ir in sections from the pituitary gland, we used the LepR-Cre reporter mice (tdTomato) to map the degree of colocalization of LepR in gonadotropes (LH-β immunoreactive cells). We observed that ∼10% of LepR-Cre cells coexpress LH-β immunoreactivity and ∼5% of LH-β immunoreactive cells exhibited LepR-Cre activity (n = 3 males and n = 4 females, Fig. 2, A–D) in both males and females. In addition, ovarian theca cells express LepR (n = 5, Fig. 2E), but GRIC-reporter genes (tdTom or eGFP, n = 3), were sporadically observed in only a few cells of the ovarian parenchyma (Fig. 2F).
Fig. 1.
LepR and GnRHR (GRIC-reporter gene) are expressed in different hypothalamic cell populations. Bright-field and fluorescent images show the distribution of leptin-induced STAT3 phosphorylation (pSTAT3-ir) and GnRHR (GRIC-tdTomato) in the arcuate nucleus (Arc; A–C), in the ventromedial nucleus of the hypothalamus (VMH; D–E), and in the dorsomedial nucleus of the hypothalamus (DMH; G–I). 3V, Third ventricle. Scale bar: 200 μm.
Fig. 2.
Leptin receptor (LepR) is expressed in a subpopulation of cells expressing GnRHR in the pituitary gland. A–C: fluorescent images showing that subsets of LepR-expressing cells (LepR-Cre tdTomato; A) display LH-β immunoreactivity (LH-β-ir; B) in males (arrows indicate dual-labeled cells). C: merged. D: bar graphs showing the percentage of colocalization between LepR-tdTom and LH-β-ir in the pituitary gland of male and female mice (n = 3 males and n = 5 females). E and F: fluorescent images showing the distribution of LepR-reporter gene (tdTom; E) (arrows mark these cells) and GRIC-reporter gene (eGFP; F) (arrow indicates a cell in the parenchyma) in the mouse ovary. CL, corpus luteum; Gr, granulosa layer. Scale bar: 100 μm in A–C and 200 μm in E and F.
GRIC-LepRloxTB mice show no improvement of the metabolic and the reproductive phenotypes.
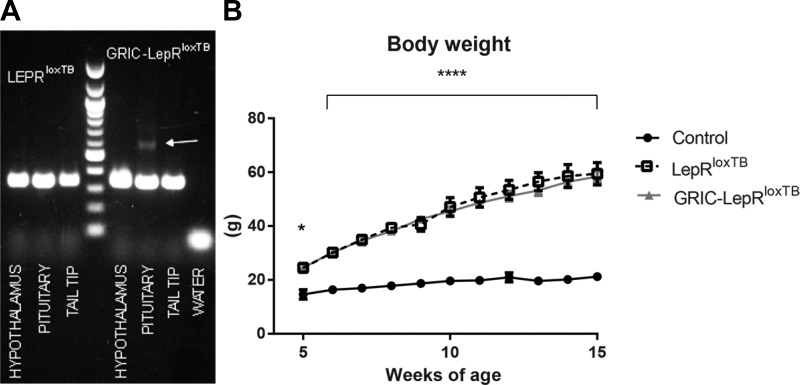
To generate mice with endogenous reexpression of LepR selectively in GnRHR cells, we crossed the GRIC mouse with the LepRloxTB mouse previously described (5, 13, 48, 50). For validation of the mouse model and Cre-induced DNA recombination, DNA was extracted from hypothalamus, pituitary gland, and tail tips, and then DNA recombination was tested using LepRloxTB primers. Only the pituitary gland showed a detectable band indicating successful genomic recombination (Fig. 3A).
Fig. 3.
Reexpression of LepR selectively in GnRHR cells causes no change in body weight of LepRloxTB mice. A: agarose gel demonstrating Cre-induced DNA recombination (higher band) of LepRloxTB in the pituitary (but not in the hypothalamus and tail) of GRIC LepRloxTB mice (n = 2). B: graph showing the progression of body weight of wild-type, LepRloxTB, and GRIC LepRloxTB female mice (n = 3–8 for each time point). *P < 0.05 and ****P < 0.0001 per one-way ANOVA, Tukey's post hoc multiple-comparison test.
Because lack of leptin signaling in gonadotropes only had a minor impact on male fertility (1) and because of germ line transgene activation in GRIC males, we focused our studies on female physiology. Three cohorts of GRIC-LepRloxTB (n = 7) animals were evaluated and compared with LepRloxTB (n = 17) and wild-type (n = 18) littermates. Due to leptin's role in the regulation of body weight and the deleterious effects of metabolic dysfunction in reproductive control (20, 22, 30, 35), we evaluated changes in body weight of GRIC-LepRloxTB mice compared with LepRloxTB and wild-type control littermates, from 5 to 15 wk of age. No difference between LepRloxTB and GRIC LepRloxTB mice was observed, but a similar increase in body weight over time for these two models compared with wild-type littermates was apparent (Fig. 3B).
Mice were weaned at 3 wk of age (postnatal day 21) and were monitored for VO as an external estrogen-dependent marker for puberty onset. At 4 wk of age (postnatal day 28), all wild-type littermate controls had shown VOs (n = 18), whereas LepRloxTB (n = 17) and GRIC-LepRloxTB (n = 7) mice never did achieve a developed opening up to 10 mo of age. Wild-type littermate controls displayed normal estrous cycles (data not shown), but we were unable to evaluate any change in vaginal cytology in LepRloxTB or GRIC-LepRloxTB mice due to the lack of VO. Because male odorants can induce sexual maturation in female rodents (47), we further characterized the reproductive phenotype of the females housed with a male of proven fertility. After 4–6 wk of mating, wild types (n = 10) were each able to become pregnant and produce a litter of live pups, but none of the LepRloxTB (n = 10) nor the GRIC-LepRloxTB (n = 7) animals showed any signs of pregnancy.
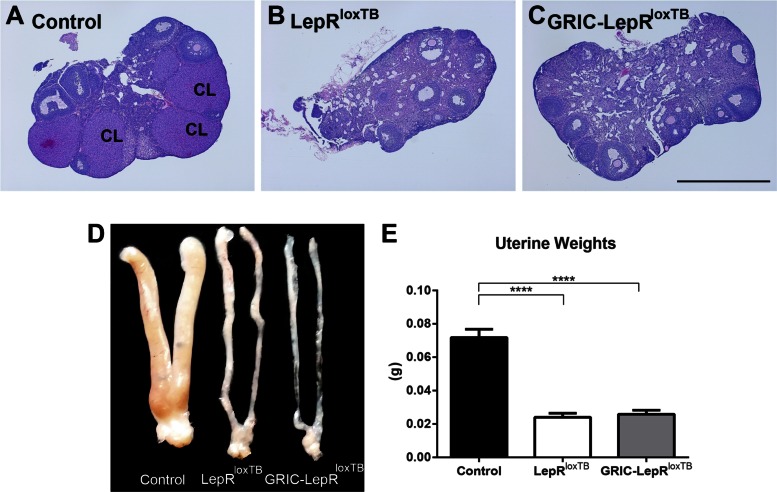
The ovaries of the LepRloxTB and GRIC-LepRloxTB mice contained follicles in different developmental stages, but no corpora lutea, indicating lack of ovulation. We also noticed higher numbers of atretic follicles in LepRloxTB and GRIC-LepRloxTB ovaries compared with wild type (Fig. 4, A–C). The uteri of LepRloxTB and GRIC-LepRloxTB mice were poorly developed (Fig. 4D), and their weights were significantly smaller than wild-type littermate controls at similar ages (20–30 wk old, Fig. 4E).
Fig. 4.
Reexpression of LepR selectively in GnRHR cells causes no improvement of the reproductive phenotype of LepRloxTB mice. A–C: bright-field images showing hematoxylin and eosin stained sections of the ovary of controls in diestrus (A), LepRloxTB (B), and GRIC LepRloxTB (C) mice. Note the presence of corpus luteum (CL) only in the ovary of the control mouse. D: digital image showing the uterine morphology of controls in diestrus, LepRloxTB, and GRIC LepRloxTB mice. E: bar graphs comparing uterus weight of controls in diestrus (n = 11), LepRloxTB (n = 11), and GRIC LepRloxTB (n = 5) adult mice. No difference between uterus weight of LepRloxTB and GRIC LepRloxTB mice was observed. ****P < 0.001 compared with wild-type control mice per one-way ANOVA, Tukey's post hoc multiple comparison test. Scale bar: 1 mm.
FSH-β expression and FSH levels are increased in GRIC-LepRloxTB mice.
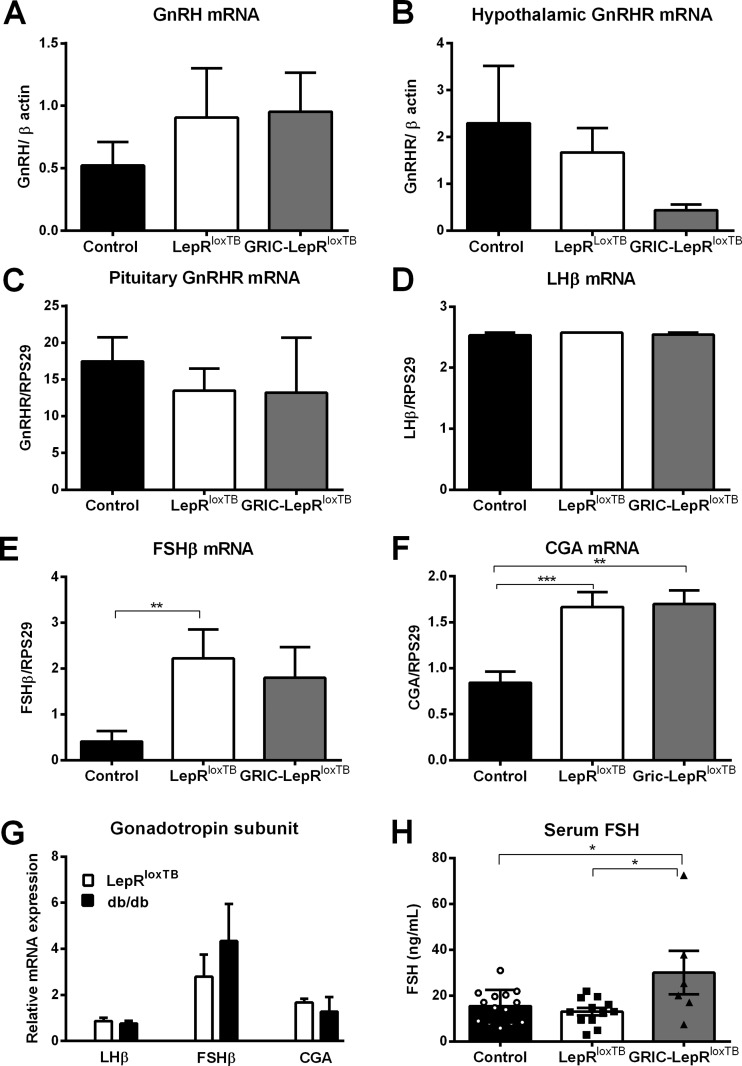
No differences in hypothalamic GnRH and GnRHR mRNAs, as well as pituitary gland GnRHR and LH-β mRNAs expression, were observed among the genotypes (Fig. 5, A–D). However, we found that FSH-β and CGA mRNAs were increased in LepRloxTB and in GRIC-LepRloxTB mice compared with wild types (Fig. 5, E and F). No difference was observed between LepRloxTB and GRIC-LepRloxTB mice. The potential effect of the loxTB allele in these findings was evaluated by comparing the expression of gonadotrope subunits between LepRloxTB and db/db female mice. No difference in Lhb, Fshb, and Cga expression was observed between both genotypes (Fig. 5G).
Fig. 5.
Increased FSH levels following endogenous reexpression of LepR in GnRHR cells. A and B: expression of GnRH (A) and GnRHR (B) in the hypothalamus of diestrus control (n = 7), LepRloxTB (n = 6), and GRIC LepRloxTB (n = 4) mice. C–F: expression of GnRHR (C), LH-β (D), FSH-β (E), and CGA mRNAs (F) in the pituitary gland of diestrus wild-type (n = 7), LepRloxTB (n = 6), and GRIC LepRloxTB (n = 4) mice. G: expression of LH-β, FSH-β, and CGA mRNAs in the pituitary gland of LepRloxTB (n = 7) and db/db (n = 5) mice. All data were normalized to the housekeeping gene RPS29. H: serum levels of FSH in diestrus wild-type littermates (n = 14), LepRloxTB (n = 12), and GRIC LepRloxTB (n = 6) mice. Increase in FSH levels was observed in GRIC LepRloxTB female mice compared with littermate controls in diestrus and LepRloxTB female mice. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with wild-type control mice per one-way ANOVA, Tukey's post hoc multiple-comparison test.
Serum LH levels were very low in all groups (mostly below the detection limit of the multiplex assay, i.e., 0.24 ng/ml), whereas higher circulating levels of FSH were observed in GRIC-LepRloxTB compared with wild-type and LepRloxTB littermates (Fig. 5H).
DISCUSSION
In this study, we used a combination of different mouse models to gain insight into the role of leptin action in gonadotropes. Using Cre-mediated recombination as a surrogate marker for LepR expression, we found that a subpopulation of gonadotropes expresses LepR. In ovaries, LepR is expressed in theca cells, whereas GRIC was rarely observed in cells of the ovarian parenchyma. No coexpression of leptin-induced pSTAT3 and GRIC was noticed in hypothalamic neurons. We found that endogenous reexpression of LepR selectively in gonadotropes (GRIC-LepRloxTB mice) was not sufficient to induce puberty and improve fertility of female mice. However, interestingly, GRIC-LepRloxTB mice have increased FSH levels.
The use of mouse models to interrogate complex physiological systems has become an important experimental approach in basic sciences. It has been particularly useful in areas in which standard methodologies have come across inherent difficulties. The physiology of the mouse pituitary gland is an example due to its size, anatomical location, and volume of hormone release. Because of these issues, the role of leptin direct action on gonadotropes has been difficult to determine.
Recent studies using mouse genetics have suggested that leptin signaling in gonadotropes is required for normal reproductive function. Conditional deletion of LepR in cells expressing LH-β delayed first pregnancy and decreased the number of pups per litter (1). The mechanisms underlying these effects are not completely understood, but the authors propose that the lack of leptin signaling altered GnRH binding sites and secretion of pituitary hormones. Among these hormones, a significant decrease in FSH levels and Fshb expression in pituitary gland was reported (1). Changes in growth or energy homeostasis were unremarkable. In agreement and as predicted from these findings, our mutant mice showed no change in body weight, discouraging any further evaluation of their metabolic phenotype. Because lack of leptin signaling in gonadotropes caused only minor impact on male fertility (1), and the production of the conditional gene reexpression requires a laborious breeding strategy due to male germ-line transgene activation in GRIC mouse, we focused our studies on female physiology.
The infertility of the leptin signaling-deficient mice has puzzled investigators for decades. Studies focusing on different reproductive organs indicate that the leptin-deficient ob/ob mice have normal development of the HPG axis until puberty, when sexual maturation is arrested. The ovarian morphology of juvenile ob/ob animals is comparable to that of juvenile wild-type mice, and gametogenesis can be induced following gonadotropin administration (32, 41). Reproductive hormones remain low throughout life, but ob/ob mice can ovulate and become fertile if levels of gonadotropins and sex steroids are maintained in physiological ranges (31). Castration increases gonadotropin secretion, although at a lower magnitude compared with controls, and the negative feedback action of sex steroids is exacerbated, also consistent with a prepubertal condition (33, 43).
Previous studies have shown that the leptin-deficient ob/ob mice have decreased circulating levels of gonadotropins and reduced pituitary content of LH. However, pituitary FSH content is increased in obese models (4, 43). In our mutant mice, we found that pituitary Lhb expression is not different from control or LepRloxTB mice, but Fshb and Cga expression is increased in LepRloxTB and in GRIC-LepRloxTB females compared with controls in diestrus. Because the difference in Fshb and Cga expression between LepRloxTB and GRIC-LepRloxTB is not different, the role of restoration of LepR in transcript regulation is unlikely.
A dynamic change in leptin and LepR across the estrous cycle has been demonstrated (27). At midcycle or on estrogen stimulation, leptin rises, and leptin signaling is potentiated (15, 27). This effect appears to be correlated with the metabolic effects of estrogen in female rodents (2, 9, 10, 28). However, it is possible that the increase in leptin signaling at midcycle also has a physiological effect in reproductive function. It may amplify the LH surge and the FSH rise preceding ovulation. Using our genetic approach, we were unable to test this hypothesis due to lack of pubertal development. However, the increase in FSH levels in the GRIC-LepRloxTB mice suggests that leptin action in gonadotropes may potentiate gonadotropin release (1). Previous studies have shown the majority of isolated gonadotropes express leptin-induced pSTAT3, and that mice with lack of leptin signaling in gonadotropes have decreased FSH levels (1). Using the reporter mice, we found that only a small subset of gonadotropes express LepR. The use of different approaches may explain the differences between the studies. Nevertheless, the results are complementary and reinforce the findings that leptin action in gonadotropes exerts a primary effect in adult, not prepubertal, mice and has a role in FSH secretion (1). Further studies using different models will be necessary to validate our findings and assess the mechanisms associated with the increase in FSH levels.
Little is known about the pituitary gland physiology of leptin signaling-deficient (ob/ob or db/db) mice. Initial studies have indicated that the GnRH action to stimulate FSH secretion is amplified in the pituitary of juvenile ob/ob mice, whereas its effect to induce LH secretion is blunted compared with controls (51). This may be due to the higher FSH content and expression and lower LH content in obese leptin signaling-deficient mice (43). Because previous studies have suggested that leptin action in gonadotropes may increase gonadotropin release by increasing GnRH binding sites, it is possible that the restoration of LepR in gonadotropes of the GRIC-LepRloxTB mice has facilitated FSH release observed as increased FSH levels. Higher FSH, but not LH, secretion was observed, likely due to the low GnRH availability in LepR null mice. Again, additional studies are warranted to test this model.
Perspectives and Significance
Our studies indicate that endogenous reexpression of LepR in a subpopulation of gonadotropes increases FSH secretion. However, this increase was isolated and not sufficient to induce puberty and improve the infertility phenotype of the LepRloxTB mice. Together with previous findings, our data indicate that leptin-selective action in gonadotropes has a role in adult reproductive physiology but is not sufficient to allow pubertal maturation in mice. Further studies will be necessary to determine the mechanisms by which leptin can directly modulate gonadotropin secretion.
GRANTS
This work was supported by the National Institute of Child Health and Human Development (R01-HD-069702 to C. F. Elias; HD-28934 for L. L. Burger) and by the Deutsche Forschungsgemeinschaft (SFB894 to U. Boehm).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.J.A. and B.C.B. performed experiments; S.J.A., D.G.-G., B.C.B., U.B., and C.F.E. analyzed data; S.J.A., D.G.-G., B.C.B., L.L.B., U.B., and C.F.E. interpreted results of experiments; S.J.A. prepared figures; S.J.A., D.G.-G., B.C.B., L.L.B., U.B., and C.F.E. edited and revised manuscript; S.J.A., D.G.-G., B.C.B., L.L.B., U.B., and C.F.E. approved final version of manuscript; C.F.E. conception and design of research; C.F.E. drafted manuscript.
ACKNOWLEDGMENTS
We thank the Unit for Laboratory Animal Medicine from the University of Michigan for the expert technical assistance with ovary histology, and the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for hormone assays. The University of Virginia Ligand Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD-28934. We also thank Hosung Sim and Lei Lei from the Department of Cell and Developmental Biology, University of Michigan, for insightful discussions.
REFERENCES
- 1.Akhter N, CarlLee T, Syed MM, Odle AK, Cozart MA, Haney AC, Allensworth-James ML, Benes H, Childs GV. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology 155: 4027–4042, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361: 1251–1263, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology 137: 3144–3147, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Batt RAL, Everard DM, Gillies G, Wilkinson M, Wilson CA, Yeo TA. Investigation into the hypogonadism of the obese mouse (genotype ob/ob). J Reprod Fertil 64: 363–371, 1982. [DOI] [PubMed] [Google Scholar]
- 5.Berglund ED, Vianna CR, Donato J Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest 122: 1000–1009, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caligioni CS. Assessing reproductive status/stages in mice. In: Current Protocols in Neuroscience. Hoboken, NJ: Wiley, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin- gonadal axis. Pituitary 4: 87–92, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12: 318–320, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Chu SC, Chou YC, Liu JY, Chen CH, Shyu JC, Chou FP. Fluctuation of serum leptin level in rats after ovariectomy and the influence of estrogen supplement. Life Sci 64: 2299–2306, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55: 978–987, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14: 141–148, 1978. [DOI] [PubMed] [Google Scholar]
- 13.Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, Vianna C, Elias CF. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One 8: e58698, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173: 37–56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Biasi SN, Apfelbaum LI, Apfelbaum ME. In vitro effect of leptin on LH release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur J Endocrinol 145: 659–665, 2001. [DOI] [PubMed] [Google Scholar]
- 16.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC Jr. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115: 3484–3493, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist LW, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291: 2608–2613, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Donato J Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci 29: 5240–5250, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato J Jr, Cavalcante JC, Silva RJ, Teixeira AS, Bittencourt JC, Elias CF. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol Behav 99: 67–77, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci 70: 841–862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998. [PubMed] [Google Scholar]
- 22.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493: 63–71, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110: 1093–1103, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356: 237–247, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frazao R, Cravo RM, Donato J Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor alpha. J Neurosci 33: 2807–2820, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frazao R, Lemko HM, da Silva RP, Ratra DV, Lee CE, Williams KW, Zigman JM, Elias CF. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab 306: E606–E614, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fungfuang W, Nakada T, Nakao N, Terada M, Yokosuka M, Gizurarson S, Hau J, Moon C, Saito TR. Serum leptin concentrations, leptin mRNA expression, and food intake during the estrous cycle in rats. Lab Anim Res 29: 1–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fungfuang W, Terada M, Komatsu N, Moon C, Saito TR. Effects of estrogen on food intake, serum leptin levels and leptin mRNA expression in adipose tissue of female rats. Lab Anim Res 29: 168–173, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci 34: 15060–15069, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294: E827–E832, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hummel KP. Transplantation of ovaries of the obese mouse. Anat Rec 128: 569, 1957. [Google Scholar]
- 32.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950. [DOI] [PubMed] [Google Scholar]
- 33.Lane PW. The pituitary-gonad response of genetically obese mice in parabiosis with thin and obese siblings. Endocrinology 65: 863–868, 1959. [DOI] [PubMed] [Google Scholar]
- 34.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1/mice exhibit more variable hypogonadism than Gpr54/mice. Endocrinology 148: 4927–4936, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Louis GW, Myers MG Jr. The role of leptin in the regulation of neuroendocrine function and CNS development. Rev Endocr Metab Disord 8: 85–94, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci 14: 704–710, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril 77: 433–444, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150: 2805–2812, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Runner MN, Gates A. Sterile, obese mothers. J Hered 45: 51–55, 1954. [Google Scholar]
- 42.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology 98: 1359–1364, 1976. [DOI] [PubMed] [Google Scholar]
- 44.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Tena-Sempere M, Pinilla L, Gonzalez LC, Dieguez C, Casanueva FF, Aguilar E. Leptin inhibits testosterone secretion from adult rat testis in vitro. J Endocrinol 161: 211–218, 1999. [DOI] [PubMed] [Google Scholar]
- 46.True C, Nasrin Alam S, Cox K, Chan YM, Seminara SB. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology 156: 1386–1397, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenbergh JG. Coordination of social signals and ovarian function during sexual development. J Anim Sci 67: 1841–1847, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A 107: 16372–16377, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen S, Götze IN, Mai O, Schauer C, Leinders-Zufall T, Boehm U. Genetic identification of GnRH receptor neurons: a new model for studying neural circuits underlying reproductive physiology in the mouse brain. Endocrinology 152: 1515–1526, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology 149: 2701–2711, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson M, Moger WH. Transpubertal modulation of pituitary and testicular function in the ob/ob mouse. Horm Res 14: 95–103, 1981. [DOI] [PubMed] [Google Scholar]
- 52.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 153: 1498–1508, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology 65: 223–228, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue (see comments). Nature 372: 425–432, 1994. [Erratum. Nature 374 (Mar): 479, 1995.] [DOI] [PubMed] [Google Scholar]
- 55.Zuure WA, Roberts AL, Quennell JH, Anderson GM. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J Neurosci 33: 17874–17883, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]