Abstract
During embryonic development, environmental perturbations can affect organisms' developing phenotype, a process known as developmental plasticity. Resulting phenotypic changes can occur during discrete, critical windows of development. Critical windows are periods when developing embryos are most susceptible to these perturbations. We have previously documented that hypoxia reduces embryo size and increases relative heart mass in American alligator, and this study identified critical windows when hypoxia altered morphological, cardiovascular function and cardiac gene expression of alligator embryos. We hypothesized that incubation in hypoxia (10% O2) would increase relative cardiac size due to cardiac enlargement rather than suppression of somatic growth. We exposed alligator embryos to hypoxia during discrete incubation periods to target windows where the embryonic phenotype is altered. Hypoxia affected heart growth between 20 and 40% of embryonic incubation, whereas somatic growth was affected between 70 and 90% of incubation. Arterial pressure was depressed by hypoxic exposure during 50–70% of incubation, whereas heart rate was depressed in embryos exposed to hypoxia during a period spanning 70–90% of incubation. Expression of Vegf and PdgfB was increased in certain hypoxia-exposed embryo treatment groups, and hypoxia toward the end of incubation altered β-adrenergic tone for arterial pressure and heart rate. It is well known that hypoxia exposure can alter embryonic development, and in the present study, we have identified brief, discrete windows that alter the morphology, cardiovascular physiology, and gene expression in embryonic American alligator.
Keywords: critical windows, embryo, hypoxia, reptile, gene expression
developmental plasticity is an important trait during vertebrate maturation, resulting in phenotypic modifications to the organism (4, 8, 14, 19, 30, 40, 55, 65). Developmental phenotypic plasticity has been extensively studied for disease states in humans (3, 4, 31), and impacts of the developmental environment in nonmammalian species that routinely experience fluctuations in abiotic factors are a burgeoning area of study. Investigations of terrestrial egg-laying species have shown that the developmental environment has important effects on organismal phenotype (20–22, 24, 32–34, 43, 63, 65, 66, 68). Certain species exhibit increased susceptibility to environmental perturbation during discrete developmental periods, and application of stress during these “critical windows” may alter phenotype of the resulting offspring (9, 10, 65). Changes in gene expression often dictate the morphological and functional phenotype of the individual. Therefore, for species that experience natural fluctuations in abiotic factors, fluctuations in the developmental environment during critical windows can result in phenotypic change. Reptilian embryos encounter variation in several abiotic factors (1, 11, 45, 48, 51, 53, 56) and receive limited, if any, parental care after oviposition. The developmental environment could have profound impacts on the phenotype of reptilian hatchlings, juveniles, and adults (70).
Chronic hypoxic exposure (10% O2) from 20% to 90% of embryonic incubation reduces embryonic mass and increases relative heart mass in American alligator (Alligator mississippiensis) and the common snapping turtle (Chelydra serpentina) (14, 25, 27). Hypoxic incubation in both species alters baseline arterial pressure and heart rate (14, 23, 26, 27), and both species exhibit plasticity in the maturation of cardiovascular regulatory mechanisms in response to developmental hypoxia (23, 26, 27). We recently identified a critical period in snapping turtle development, from 50 to 70% of embryonic incubation, during which hypoxia produces an enlarged heart (65). Two explanations were postulated: 1) either the relative increase in heart mass represents the capacity to maintain growth of the heart in low O2, while embryonic somatic growth is suppressed, or 2) low O2 directly elicits a response from the developing cardiac tissue (65). Alligator embryos have a lengthier embryonic development period than snapping turtles and do not increase metabolic rate substantially until 70% of incubation; therefore, a window affecting heart size may occur at a later time point (52, 66).
The purpose of this study was to identify critical windows when hypoxia alters cardiac gene expression, cardiovascular function, and morphological phenotypes of American alligator embryos. These critical windows could have pronounced impacts on the embryonic, juvenile, and adult cardiovascular phenotype, as in endotherms (36, 37). We hypothesized that incubation in 10% O2 increases relative cardiac size, which is due to cardiac enlargement and not suppression in somatic growth. We also hypothesized that hypoxic heart tissue would show altered expression of genes controlling metabolism, cell growth and proliferation, and cardiac contractile proteins, and that hypoxic somatic tissue would show altered expression of genes controlling metabolism and cell growth and proliferation. Further, we hypothesized that a 10% O2 critical window producing hypotensive embryos spans from 70% to 90% of incubation in alligators. This window coincides with the greatest rate of embryonic growth in American alligators (14, 18), the timeframe when arterial pressure increases (14), and the onset of baroreflex function in embryonic alligators (17). We tested these hypotheses by shifting embryonic alligators between normoxia (21% O2) and hypoxia (10% O2) at multiple points of incubation. All morphological and physiological phenotypic assessments were conducted at 90% of incubation. Our findings show that alteration of cardiovascular phenotype in American alligator embryos requires relatively brief exposures to reduced environmental O2.
MATERIALS AND METHODS
Alligator embryo acquisition and incubation.
American alligator (Alligator mississippiensis) eggs were collected from Rockefeller Wildlife Refuge in Grand Chenier, LA, and transported to the University of North Texas (UNT), Denton, TX, during June 2013 and 2014. Egg mass was determined to the nearest milligram using an analytical balance (Mettler Toledo, XS204, Columbus, OH). Two alligator embryos from each clutch were staged after collection each year to determine approximate age of each clutch in accordance with previously published methods (72-day incubation period at 30°C; 14, 28). Six clutches where used in 2013 (80 eggs), and eight clutches were used in 2014 (42 eggs). Each year, equal numbers of eggs from each clutch were randomly distributed to plastic containers (2.5 liter Ziploc Container, SC Johnson, Racine, WI) and buried to the midpoint of the egg in a bed of moist vermiculite mixed in a 1:1 ratio of vermiculite:water. Water content of the vermiculite was maintained by weighing the box two to three times weekly and adding water as needed to keep the mass constant, as previously described (14). Eggs were incubated at 30°C to ensure all embryos developed as females (28, 29).
Incubation in experimental conditions began at ∼20% of embryonic development when containers were sealed inside large Ziploc® bags with two holes that allowed parallel inflow and outflow of gas. Oxygen mixtures were made using compressed N2 and room air (10% O2) connected to two rotameters (Sho-Rate Brooks Instruments Division, Hatfield, PA) or room air (21% O2). Gas mixtures passed through a H2O-bubbler to ensure adequate water saturation of ≥80–95% relative humidity. Gas composition was monitored continuously with an oxygen analyzer (S-3AI, Applied Electrochemistry, IL) connected to a PowerLab 16/35 data recording system connected to a computer running LabChart Pro software (v 7.2 ADInstruments, Colorado Springs, CO), and data were recorded at 10 Hz, as previously described (23).
Experimental shifts during incubation.
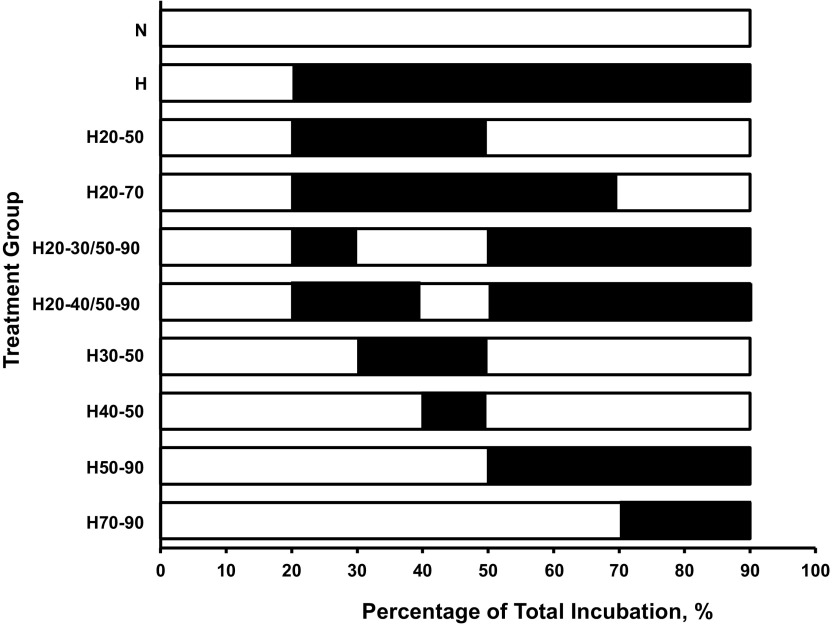
During the 2013 and 2014 summer research season, eggs from each clutch were divided into groups subjected to different gas exposure manipulations (Fig. 1). During each year, two groups were continuously incubated in either 21% O2 (N, for normoxia) or 10% O2 (H, for hypoxia). All clutches within a year were represented in each oxygen exposure protocol. Designations for each group are as follows: 1) N: a group incubated in 21% O2 from 20% to 90% of incubation; 2) H: a group incubated in 10% O2 from 20% to 90% of incubation; 3) H50-90: a group incubated in 10% O2 from 50 to 90% of incubation (2013); 4) H20-50: a group incubated in 10% O2 from 20 to 50% of incubation (2013); 5) H70-90: a group incubated in 10% O2 from 70 to 90% of incubation (2013); 6) H20-70: a group incubated in 10% O2 from 20 to 70% of incubation (2013); 7) H30-50: a group incubated in 10% O2 from 30 to 50% of incubation (2014); 8) H20-30/50-90: a group incubated in 10% O2 from 20 to 30% and from 50 to 90% of incubation (2014); 9) H40-50: a group incubated in 10% O2 from 40 to 50% of incubation (2014), and 10) H20-40/50-90: a group incubated in 10% O2 from 20 to 40% and from 50 to 90% of incubation (2014).
Fig. 1.
Periods of exposure to 10% O2 in the different treatment groups of American alligator embryos. In all cases, the open bar segment represents the period of incubation in normoxic conditions, while the solid bar represents the exposure to 10% O2. Groups were either maintained in normoxic conditions (N), 10% O2 conditions (H), or shifted as follows: from 21 to 10% O2 at 50% of incubation (H50-90), from 10% to 21% O2 at 50% of incubation (H20-50), from 21 to 10% O2 at 70% of incubation (H70-90), from 10% to 21% O2 at 70% of incubation (H20-70), from N to H at 30% and back to N at 50% of incubation (H30-50), moved from H to N at 30% of incubation and back to H at 50% of incubation (H20-30/50-90), from N to H at 7 of incubation and back to N at 50% of incubation (H40-50) incubation, and moved from H to N at 30% of incubation and back to H at 50% of incubation (H20-40/50-90).
2013 surgical instrumentation only.
Eggs were removed from O2 treatments for study at 90% of incubation (∼65 days), corresponding to stage 25 of development (28). In normoxia, eggs were first candled to identify a tertiary chorioallantoic membrane (CAM) artery, placed in a temperature-controlled surgical chamber (21% O2, 30°C), and ∼1 cm2 of the eggshell removed under a dissection microscope (Leica MZ6; Leica Microsystems, Waukegan, IL). Heat-pulled polyethylene (PE)-50 tubing filled with heparinized saline solution (0.9% NaCl and 50 IU/ml heparin) was then inserted into the CAM artery, as previously described (13, 16).
Following catheterization, embryos were transferred to a water-jacketed, six-chamber stainless-steel experimental apparatus (∼700 cm3 per chamber, one embryo per chamber) and allowed to recover until cardiovascular parameters stabilized (at least 60 min). Chamber temperature was maintained at 30°C using a recirculating constant temperature water bath (VWR International, LLC, West Chester, PA). Each chamber had a lid with small holes, allowing the catheter and airlines to enter the chamber. All incoming air was humidified by passing through a bubbler and warmed by traversing 2 m of copper tubing in contact with the warmed stainless-steel apparatus encased by 7 cm of polystyrene. Warmed, humidified room air was pumped into each chamber at 400 ml/min. Each arterial catheter was attached to a disposable pressure transducer (ADInstruments, model no. MLT0699) 1–3 cm above the egg. The transducer was connected to an amplifier (Quad Bridge Amp, ADinstruments), and the pressure signal acquired at 40 Hz using a PowerLab 16/35 data recording system (ADInstruments) and LabChart Pro software (v 7.2.5, ADInstruments). Pressure transducers were calibrated prior to the study with a vertical column of saline, and heart rate (fH) was calculated on the basis of the arterial pulse frequency.
All embryos received an initial injection of 150 μl of heparinized saline (0.9% NaCl, 50 IU/ml) to determine mean arterial pressure (Pm) and fH responses to the injection volume.
2013 Pm and fH response to cholinergic and adrenergic receptor blockade.
Following the recovery period, the Pm and fH response to cholinergic and adrenergic blockade was determined, as previously described (23). After a second saline flush, each embryo received serial injections of the cholinergic antagonist atropine (3 mg/kg), the β-adrenergic antagonist propranolol (3 mg/kg) and the α-adrenergic antagonist phentolamine (3 mg/kg) with a 30–60-min recovery period between individual injections to ensure maximal responses, as previously reported (14, 23). Sample sizes for the treatment groups are provided in figure captions for each drug injection. Decreases in sample sizes reflect embryos that displaced the catheter.
2013 and 2014 animal euthanasia and wet masses.
At the completion of each study, all embryos were euthanized with an overdose of isoflurane (Isoflo; Abbott Laboratories, North Chicago, IL). Wet mass of the whole embryo (excluding embryonic membranes), heart (combined atria and ventricular mass), yolk, liver, lungs, kidneys, and the whole rear right limb were determined to the nearest milligram using an analytical balance (Mettler Toledo XS204). All tissues were then flash frozen with liquid N2 and stored at −80°C for gene expression studies. Embryo stage was verified upon death to ensure normoxic and hypoxic embryos were at the same developmental stage. A subset of the stored tissue was used for gene expression studies. All studies were carried out according to an approved animal care protocol: UNT Institutional Animal Care and Use Committee nos. 11-007 and 1403-04.
2013 quantitative real-time PCR.
We used real-time PCR to measure mRNA expression for the following genes in heart tissue at 90% of development: 18S ribosomal RNA (18S rRNA), glyceraldehyde 3-phosphate dehydrogenase (Gapdh), hypoxia-inducible factor 1, alpha subunit (Hif1α), lactate dehydrogenase (Ldha), ryanodine receptor 2 (Ryr2), vascular endothelial growth factor (Vegf), platelet-derived growth factor B chain (PdgfB), myosin, heavy chain 6, cardiac muscle, alpha (Myh6), cardiac myosin binding protein-C (Mybpc3), phospholamban (Pln), insulin-like growth factor 2 (Igf2), and titin (Ttn). Whole limb was used to determine mRNA expression for the following genes at 90% of development: 18S rRNA, Gapdh, hypoxia-inducible factor 1, alpha subunit (Hif1α), Ldha, Vegf, PdgfB, phospholamban (Pln), and Igf2. We selected genes from functional categories that were likely to be related to changes in gross morphology or physiology of the heart. We picked hypoxia-responsive genes (Hif1α and Ldha, which is a known Hif1α target), calcium signaling genes involved in cardiac development and smooth muscle contraction (Ryr2, Pln), growth factors that could cause hyperplasia (VegfA, Igf2, PdgfB), and structural proteins that could underlie hypertrophic growth (Mybpc3, Myh6, Ttn). We picked 18S rRNA and Gapdh as potential house-keeping genes.
Primers for real-time PCR (Table 1) were designed with Primer Express Software v2.0 (Applied Biosystems) and purchased from Integrated DNA Technologies (Coralville, IA). We designed primers to amplify PCR products of similar size: PCR products were 76 bp or 77 bp for all genes except Ttn, which had an 83-bp product. Standards were made as previously described (57, 58). Standard curves were log-linear over eight orders of magnitude, which allowed quantification of gene expression in attograms of cDNA (i.e., PCR product) for each nanogram of input RNA-1. Given the slightly different lengths of the PCR products, we converted attograms (ag) of cDNA into number of copies of cDNA per nanogram of input per RNA using the following formula: ag PCR product × Avogadro's number/length of PCR product × 1018 ag/g × 650 g/mol (molar mass of dsDNA).
Table 1.
Primer sets for quantitative PCR of indicated genes, product length (base pairs), and annealing temperature
| Gene | Forward Primer | Reverse Primer | Product Length, bp | TM, °C |
|---|---|---|---|---|
| 18Sr | 5′-GTTCAGCCACCCGAGATTGA-3′ | 5′-CCCATCACGAATGGGGTTCA-3′ | 145 | 60 |
| GAPDH | 5′-TGGAAGGTCTCATGACCACA-3′ | 5′-CAGTGGATGCTGGAATGATG-3′ | 123 | 60 |
| Hif1α | 5′-TTGTGTCAAAGCTGGAGCCA-3′ | 5′-TCAGGCGTTTCTTGCATCTG-3′ | 76 | 60 |
| IGF2 | 5′-CCCGTGGGACGAAATAACAG-3′ | 5′-CCCGTGGGACGAAATAACAG-3′ | 76 | 60 |
| LDHA | 5′-AGGCTTTGCACCCTGAGTTG-3′ | 5′-CTGTCCACCACCTGCTTGTG-3′ | 76 | 60 |
| MYBPC3 | 5′-GTGAAGTGTCCGAGGAAGGG-3′ | 5′-TTGAAAGCCTCTTCCCGTGT-3′ | 76 | 60 |
| MYH6 | 5′-CCACCACATGTTCGTGCTG-3′ | 5′-CCATGCCGAAGTCAATGAACT-3′ | 77 | 60 |
| PDGFB | 5′-TGAGTGCAAGACGCGAACAG-3′ | 5′-CACCACAAAGTTGGCATTGG-3′ | 76 | 58.5 |
| PLN | 5′-TCCTCCACTTTGCTGTTGCC-3′ | 5′-TCAATAGTTGAGGCTCTCCGC-3′ | 76 | 60 |
| RYR2 | 5′-CTCAGGCAGTCCGCCTTTC-3′ | 5′-TCCTGGCAAATTCTGCAAGTG-3′ | 77 | 60 |
| TTN-l | 5′-AGGAGACAGGCCCACACCT-3′ | 5′-CTTCCACCCTCAGCTGTTGAG-3′ | 83 | 58.5 |
| vegfA | 5′-CAATCGAGACCCTGGTGGAC-3′ | 5′-GGGTACACAGGATGGCTTGAA-3′ | 77 | 59 |
TM, annealing temperatue.
Total RNA was extracted from heart using a Zymo Research Tissue and Insect RNA MicroPrep kit (Zymo Research, Irvine, CA) and treated with RNase-free DNase. The concentration and purity of total RNA were measured using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). All samples had 260/280 absorbance ratios of 1.8–2.0 and displayed discrete 18S and 28S rRNA bands when analyzed on agarose gels. In the case of rRNA degradation, samples were excluded from further analysis.
We synthesized cDNA by reverse transcribing 1 μg of intact total RNA in a 20 μl reaction using iScript Reverse Transcription Supermix, as per the manufacturer's instructions (Bio-Rad, Hercules, CA). Real-time PCR was performed with SsoFast EvaGreen Supermix, according to the manufacturer's instructions (Bio-Rad, Hercules, CA). Each reaction contained 5 μl of 2× SsoFast EvaGreen Supermix, 0.3 μl of forward primer and 0.3 μl of reverse primer (final primer concentration = 300 nM), 2 μl of heart cDNA (equivalent to 100 ng of input RNA), and water to bring the total volume to 10 μl. Reactions were run on white 384-well plates in a CFX384 real-time PCR Detection System. The thermal profile was 94°C for 30 s to activate the DNA polymerase followed by 40 cycles of two-step PCR (94°C for 1 s and 60°C for 10 s). Negative control reactions (no reverse transcriptase controls and water controls) demonstrated no contamination with genomic DNA or exogenous PCR products. A melt curve at the end of each run verified that a single product was amplified.
Statistical analyses.
For the 2013 study, Pm and fH pre- and post-drug injections were taken as the average over a 5-min period following Pm and fH stabilization, ∼20 min postinjection. Paired t-tests were used to compare control Pm and fH to the hypoxic or drug responses within treatment groups, as previously conducted (26). To compare responses between treatment groups, fractional responses of Pm and fH were arcsine square root transformed, as previously described (17, 26, 65). Transformed fractions were compared using a one-way ANOVA, with incubation condition as the independent variable, and significant effects were followed by SNK post hoc comparisons (65). Egg masses in 2013 and 2014 were analyzed using a one-way ANCOVA with treatment group used as the independent variable and year as the covariate and SNK post hoc comparison. For both the 2013 and 2014 studies, wet embryonic and yolk mass was analyzed using a two-way ANCOVA, with treatment group and year used as the independent variable and egg mass as the covariate. This analysis was selected because of the known correlation between egg mass and hatchling mass (18). Individual tissues wet masses between treatment groups were analyzed using a one-way ANOVA and SNK post hoc comparison, as previously conducted (23, 25, 27). The fraction of wet organ mass to wet embryo mass [organ mass (g) × embryo mass (g)−1] was arcsine square root transformed, and values were compared between treatment groups using a one-way ANOVA and SNK post hoc comparisons. For tests outlined above, statistical significance was determined on the basis of P < 0.05. We used a two-way ANCOVA to analyze mRNA concentrations with treatment group and clutch identity as the independent variables and 18S rRNA or Gapdh as the covariate. A significant main effect of treatment was followed by comparisons of experimental groups to the control group. The Dunn-Sidák method was used to correct for multiple comparisons. The nominal significance level was calculated as α′ = 1 − (1-α)1/k, where k is the number of comparisons for an experiment-wise α = 0.05. Throughout the text, values are given means ± SE (Statistica v12; StatSoft, Tulsa, OK). Sample size is indicated in the figures and tables.
RESULTS
Morphological traits.
Egg mass did not differ between years or incubation treatments (73.4 ± 0.7 g in 2013 and 72.6 ± 0.8 g in 2014). Mass parameters (Table 1) for the N embryos in both years were similar to those previously reported for American alligator embryos (14, 25, 64). Hypoxic incubation significantly reduced (∼30%) embryo mass (P < 0.001) at 90% of incubation compared with N embryos, similar to previous reports (14, 23, 25, 26). Given embryonic mass was unaffected by a year of study, mass parameters were pooled in further analysis.
Organ masses.
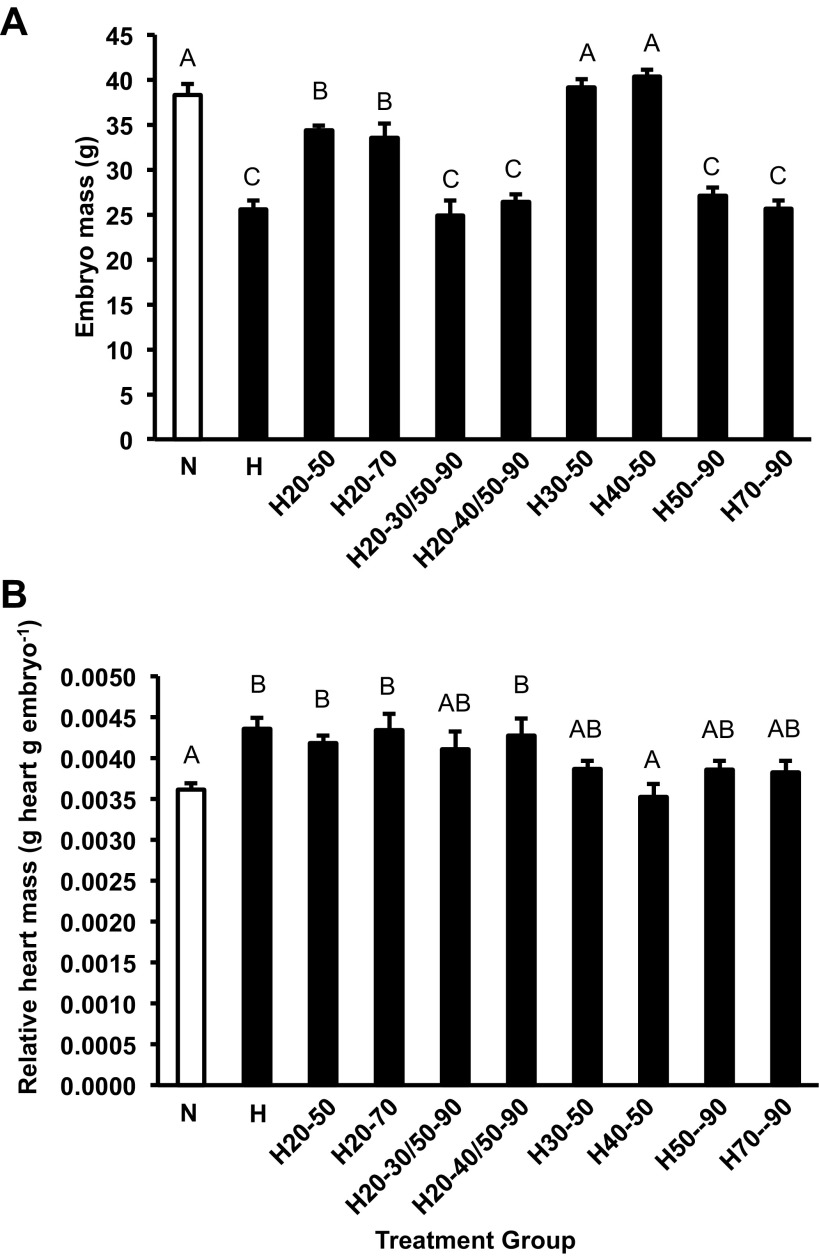
Hypoxia from 70–90% of incubation was a period that strongly affected embryo mass in American alligator embryos. Mean body mass for embryos exposed to hypoxia from 20 to 90% (H), from 50 to 90% (H50-90), and from 70 to 90% (H70-90) of incubation were similar in size to each other, but smaller than the other groups (P < 0.001; Fig. 2A). This was also the case for the H20-30/50-90 and H20-40/50-90 treatments groups (Fig. 2A). Embryos that experienced brief periods of hypoxic exposure (H30-50 and H40-50) were similar in embryonic mass to the N group (Fig. 2A).
Fig. 2.
Alligator embryo wet mass (A) and relative heart mass (B) at 90% of incubation for the control group (open column) and all shifted groups (closed columns). Groups were either maintained in normoxic conditions (N), 10% O2 conditions (H) or shifted as follows; from 21 to 10% O2 at 50% of incubation (H50-90), from 10% to 21% O2 at 50% of incubation (H20-50) from 21 to 10% O2 at 70% of incubation (H70-90), from 10% to 21% O2 at 70% of incubation (H20-70) from 21% to 10% O2 at 30% and back to 21% at 50% of incubation (H30-50), from 10% to 21% O2 at 30% and back to 10% at 50% of incubation (H20-30/50-90), from 21% to 10% O2 at 40% and back to 21% at 50% of incubation (H40-50) incubation and from 10% to 21% O2 at 30% and back to 10% at 50% of incubation (H20-40/50-90). Dissimilar letters indicate statistically distinct values where noted (P < 0.05). Data are presented as means ± SE. Sample sizes are presented in Table 2.
Yolk mass was larger in embryos that experienced hypoxia at any point in incubation compared with N embryos (P < 0.001; Table 2). Yolk mass of the H treatment group was similar to that previously reported for embryonic alligators exposed to 10% O2 until 90% of incubation (14). Data indicated a graded effect on yolk utilization dependent on the period of exposure to hypoxic developmental conditions. However, exposure to hypoxia between 30 and 50% of incubation had no effect on yolk depletion or use relative to the N group (Table 2).
Table 2.
Mass of yolk, heart, liver, lung, kidney and the ratio of heart, liver, lung, and kidney mass to body mass ratio of treatment groups of alligator embryos
| Group | Yolk, g | Heart, g | Liver, g | Liver/Body, g/g | Lung, g | Lung/Body, g/g | Kidney, g | Kidney/Body, g/g |
|---|---|---|---|---|---|---|---|---|
| N (18) | 9.7 ± 0.5A | 0.138 ± 0.004A | 0.774 ± 0.028A | 0.020 ± 0.0004A | 0.406 ± 0.016CD | 0.011 ± 0.001AC | 0.232 ± 0.013A | 0.006 ± 0.0003A |
| H (19) | 19.2 ± 0.5B | 0.111 ± 0.004B | 0.432 ± 0.018B | 0.017 ± 0.0004A | 0.254 ± 0.017A | 0.010 ± 0.0006A | 0.140 ± 0.007B | 0.006 ± 0.0002A |
| H20-50 (18) | 13.0 ± 0.6C | 0.146 ± 0.003A | 0.674 ± 0.016A | 0.019 ± 0.0004A | 0.417 ± 0.017D | 0.012 ± 0.0005AC | 0.221 ± 0.009A | 0.006 ± 0.0002A |
| H20-70 (13) | 13.1 ± 0.5CD | 0.152 ± 0.007A | 0.660 ± 0.031A | 0.019 ± 0.0008A | 0.316 ± 0.023AB | 0.009 ± 0.0004B | 0.232 ± 0.013A | 0.007 ± 0.0003A |
| H20-30/50-90 (7) | 20.2 ± 1.6B | 0.101 ± 0.007B | 0.454 ± 0.037B | 0.018 ± 0.0010A | 0.250 ± 0.021A | 0.010 ± 0.0004A | 0.153 ± 0.013B | 0.006 ± 0.0003A |
| H20-40/50-90 (7) | 18.3 ± 1.3B | 0.112 ± 0.004B | 0.479 ± 0.017B | 0.018 ± 0.0010A | 0.255 ± 0.010A | 0.010 ± 0.0003A | 0.164 ± 0.011B | 0.006 ± 0.0003A |
| H30-50 (7) | 11.1 ± 1.0AD | 0.151 ± 0.004A | 0.729 ± 0.014A | 0.019 ± 0.0010A | 0.367 ± 0.025CB | 0.009 ± 0.0006B | 0.227 ± 0.007A | 0.006 ± 0.0001A |
| H40-50 (7) | 10.6 ± 1.3A | 0.142 ± 0.006A | 0.774 ± 0.031A | 0.019 ± 0.0010A | 0.343 ± 0.026B | 0.009 ± 0.0005B | 0.230 ± 0.008A | 0.006 ± 0.0002A |
| H50-90 (19) | 17.9 ± 0.5B | 0.104 ± 0.004B | 0.410 ± 0.027B | 0.015 ± 0.0007B | 0.277 ± 0.011A | 0.010 ± 0.0003AB | 0.155 ± 0.010B | 0.006 ± 0.0004A |
| H70-90 (13) | 18.6 ± 0.8B | 0.097 ± 0.003B | 0.440 ± 0.032B | 0.017 ± 0.0009AB | 0.323 ± 0.015AB | 0.013 ± 0.0008C | 0.170 ± 0.068B | 0.007 ± 0.0003A |
Data are presented as the means ± SE. Groups consisted of those maintained in normoxic conditions (N), 10% O2 (H) shifted from 21% to 10% O2 at 50% of incubation (H50-90), shifted from 10% to 21% O2 at 50% of incubation (H20-50), shifted from 21% to 10% O2 at 70% of incubation (H70-90) and shifted from 10% to 21% O2 at 70% of incubation (H20-70). N to H at 30% and back to N at 50% of incubation (H30-50), moved from H to N at 30% of incubation and back to H at 50% of incubation (H20-30/H50-90), from N to H at 40% of incubation and back to N at 50% of incubation (H40-50) incubation and moved from H to N at 30% of incubation and back to H at 50% of incubation (H20-40/H50-90). In all cases, differing superscripted letters indicate significant differences (P < 0.05) in values between the treatment groups. Sample size (n) is indicated by numbers in parentheses.
Absolute heart mass of the N treatment group was similar to that previously reported for embryonic alligators at 90% of incubation (14, 25). Absolute heart mass was 18%, 21%, and 26% smaller in H, H50-90, and H70-90 hypoxic treatment groups, respectively, compared with the N group (P < 0.001; Table 2). A decrease in absolute heart mass was also evident in the hypoxic exposure groups H20-30/50-90 (−33%) and H20-40/50-90 (−25%). Overall, those embryos held in 10% O2 from 70 to 90% incubation regardless of the exposure prior to this window had the smallest absolute heart mass (Table 2). However, relative heart mass (tissue mass to body mass) was greater in all alligator embryos that were exposed to 10% O2 from 20 to 40% of incubation compared with the N treatment group, H (increased 21%), H20-50 (increased 15%), H20-40/50-90 (+21%) and H20-70 (increased 19%) treatment groups (P < 0.001; Fig. 2B). Finally, embryos moved from normoxia into hypoxia at 30% (H30-50) or 40% (H40-50) and returned to normoxia at 50% of incubation had similar relative heart mass to that of the N treatment group (Fig. 2B).
All other organ masses were impacted by prolonged incubation in hypoxia (Table 2). Liver mass was reduced in the H (−42%), H50-90 (−43%), H70-90 (−47%), H20-30/50-90 (−45%), and H20-40/50-90 (−42%) groups compared with the N group with no impact on the H30-50 or H40-50 groups (P < 0.001; Table 2). This indicated the window of substantial liver growth spans from 50 to 90% of incubation. When liver mass was corrected relative to embryo mass, only the H50-90 (25% lower) group was significantly different compared with the N group (P < 0.001; Table 2). Similar reductions in lung and kidney mass were recorded for the H, H20-30/50-90, H20-40/50-90, H50-90, and H70-90 treatments groups compared with the N group (Table 2). Reduction in kidney mass was related to overall reduction in embryonic size (Fig. 2A) given that the relative kidney mass was similar across all treatment groups (Table 2). Interestingly, embryonic alligators incubated in hypoxia from 40 to 50% of incubation (H30-50 H40-50, and H20-70) had relative lung mass that was lower than the N group (Table 2).
2013 baseline cardiovascular parameters.
Baseline Pm and fH at 90% of incubation for N embryos were similar to those previously reported for embryonic alligator embryos incubated at 30°C (14, 23, 26, 50, 64).
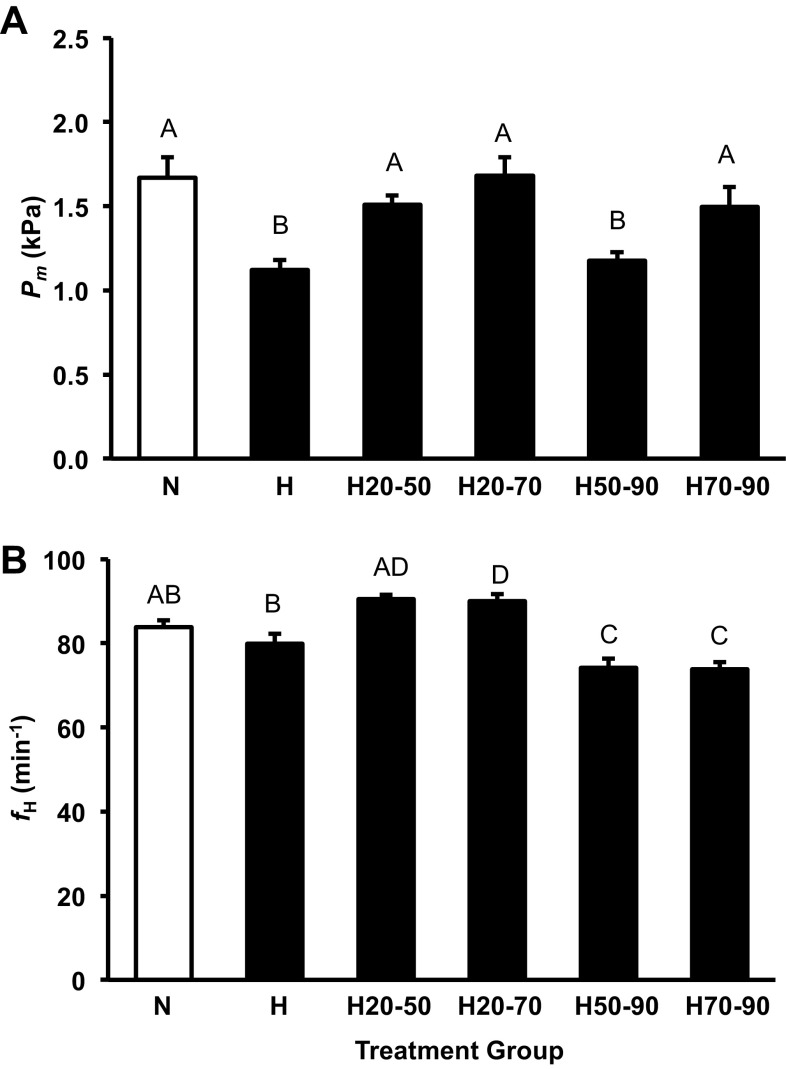
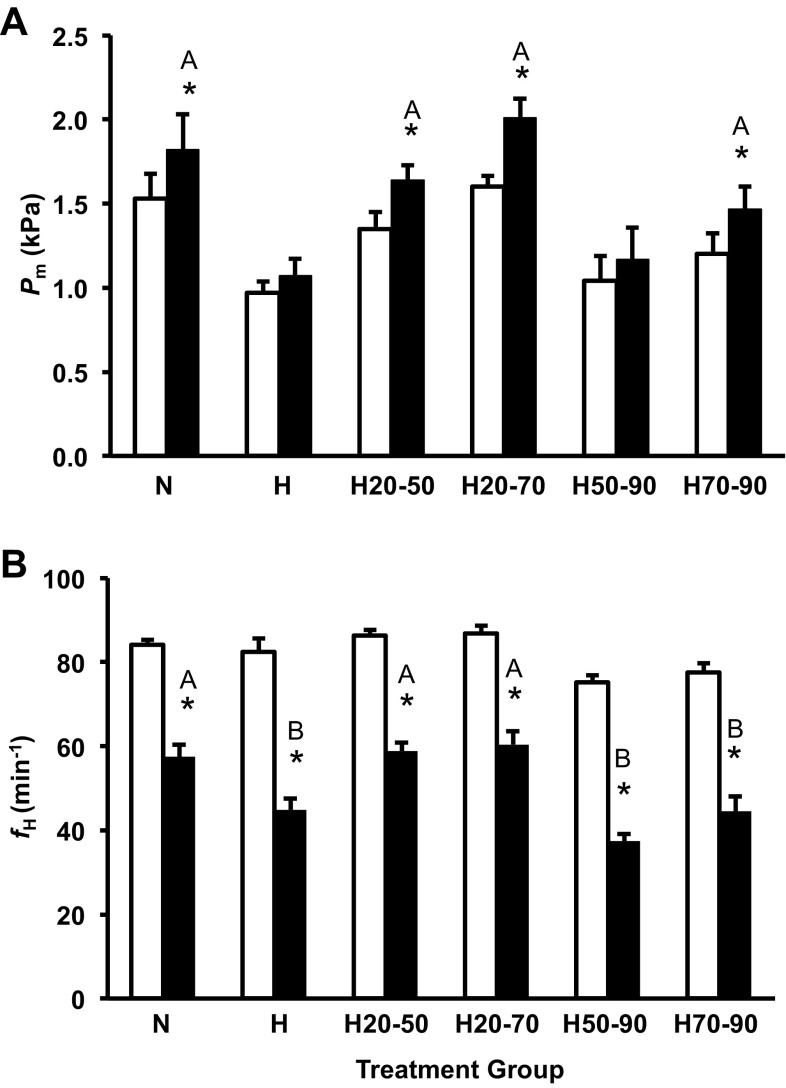
The H treatment group was hypotensive relative to the N group, similar to that previously reported for alligator embryos incubation in these conditions (14, 23, 26, 50). Pm in the H20-50 treatment group was lower than the N group (∼0.5 kPa) and similar to the H group (Fig. 3A). Hypoxia prior to 50% (H20-50) or 70% (H20-70) of incubation had no impact on baseline Pm relative to the N group, which was also the case in the H70-90 group (Fig. 3A). This indicates the window of exposure to hypoxia that results in the embryonic hypotension in the American alligator spans from 50 to 70% of incubation (Fig. 3A).
Fig. 3.
Alligator embryo baseline Pm (A) and fH (B) at 90% of incubation for the control group (open column) and all shifted groups (closed columns). Groups were either maintained in normoxic conditions (N), 10% O2 conditions (H) or shifted as follows: from 21 to 10% O2 at 50% of incubation (H50-90), from 10% to 21% O2 at 50% of incubation (H20-50), from 21 to 10% O2 at 70% of incubation (H70-90), or from 10% to 21% O2 at 70% of incubation (H20-70). Dissimilar letters in the groups indicate statistically distinct values where noted (P < 0.05). Data are presented as means ± SE. Sample sizes N (n = 9), H (n = 5), H50-90; (n = 17), H20-50 (n = 17), H70-90 (n = 10), and H20-70 (n = 10).
fH of the different treatment groups was more complex than the Pm response (Fig. 3B). fH was slightly, but not significantly depressed in the H group compared with the N treatment similar to that previously reported (14, 23, 26, 50). H50-90 and H70-90 groups were bradycardic (−10 beats/min) compared with the N group (P < 0.05; Fig. 3B). H20-50 embryo fH was qualitatively elevated (+6 beats/min), and H20-70 embryos displayed a significantly increased fH (+6 beats/min) compared with the N group (Fig. 3B).
2013 cardiovascular response to cholinergic receptor blockade.
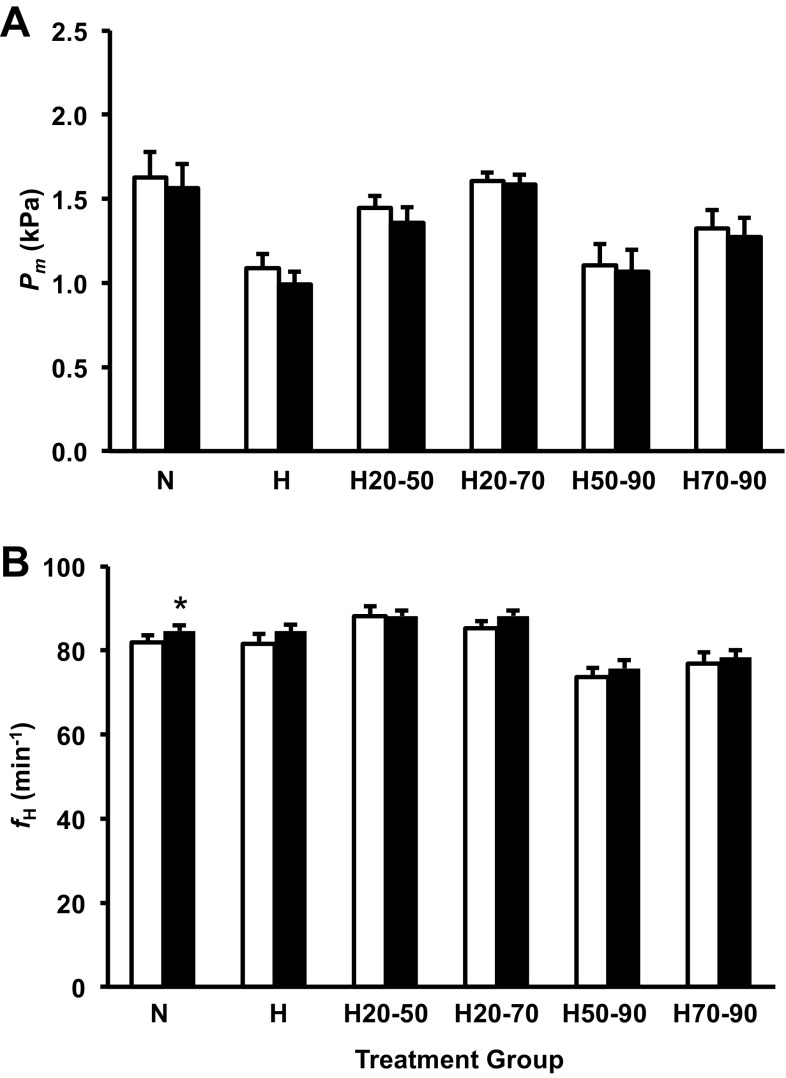
Cholinergic receptor blockade with atropine (3 mg/kg) significantly reduced Pm (∼0.1 kPA) in the H treatment group and increased fH slightly (∼2 beats/min) in the N treatment group (Fig. 4, A and B). Cholinergic blockade had no effect on any of the remaining treatment groups.
Fig. 4.
Mean arterial pressure measured through the CAM artery (Pm; A) and frequency of heart beats (fH; B) for the control group and all shifted groups precholinergic (open column) and postcholinergic (solid columns) blockade in alligator embryos at 90% of incubation. Groups were either maintained in normoxic conditions (N), 10% O2 conditions (H), or shifted as follows: from 21 to 10% O2 at 50% of incubation (H50-90), from 10% to 21% O2 at 50% of incubation (H20-50) from 21 to 10% O2 at 70% of incubation (H70-90), or from 10% to 21% O2 at 70% of incubation (H20-70). Asterisks indicate a significant response to cholinergic blockade (P < 0.05). Dissimilar letters in the groups indicate statistically distinct differences in the response intensity between compared groups. Data are presented as means ± SE. Sample sizes N (n = 9), H (5), H50-90 (n = 15), H20-50 (n = 15), H70-90 (n = 9), and H20-70 (n = 9).
2013 cardiovascular response to adrenergic receptor blockade.
β-adrenergic receptor blockade with propranolol (3 mg/kg) significantly increased Pm with the exception of H and H50-90 treatment groups (Fig. 5A). The intensity of the hypertensive response to β-adrenergic blockade for the N, H20-50, H70-90, and H20-70 was similar, ranging from 0.26 to 0.40 kPa (Fig. 5A). The β-adrenergic receptor blockade resulted in a significant reduction in fH, ranging from 26 to 37% in all groups (Fig. 5B); however, the intensity of the blockade response differed in the treatment groups (P < 0.001; Fig. 5B). Embryos incubated in hypoxia from 70 to 90% of incubation (H, H50-90, and H70-90) had a greater bradycardic response to β-adrenergic receptor blockade of ∼36% compared with those embryos incubated in normoxia (N, H20-50, and H20-70) in which fH decreased ∼26% (Fig. 5B).
Fig. 5.
Pm (A) and fH (B) for the control group, and all shifted groups pre- (open column) and post-β-adrenergic (solid columns) blockade in alligator embryos at 90% of incubation. Groups were either maintained in normoxic conditions (N), 10% O2 conditions (H), or shifted as follows: from 21 to 10% O2 at 50% of incubation (H50-90), from 10% to 21% O2 at 50% of incubation (H20-50), from 21 to 10% O2 at 70% of incubation (H70-90), or from 10% to 21% O2 at 70% of incubation (H20-70). Asterisks indicate a significant response to β-adrenergic blockade (P < 0.05). Dissimilar letters in the groups indicate statistically distinct differences in the response intensity between compared groups. Data are presented as means ± SE. Sample sizes N (n = 9), H (n = 5), H50-90 (n = 12), H20-50 (n = 12), H70-90 (n = 8), and H20-70 (n = 9).
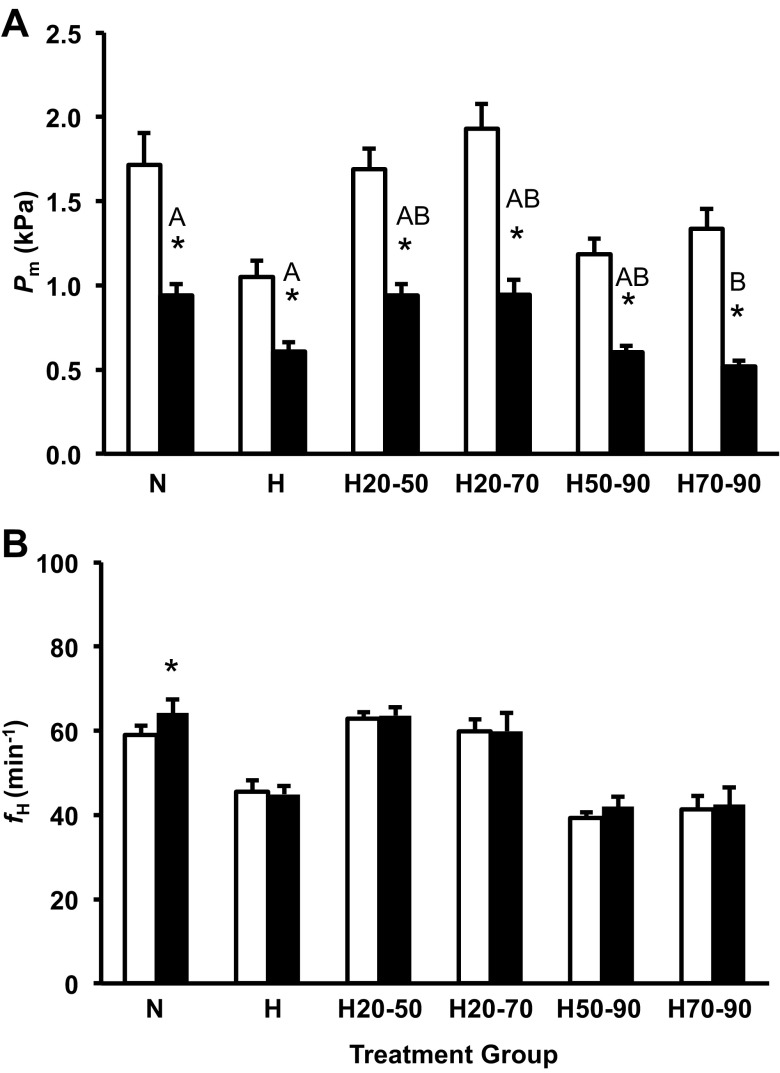
Blockade of the α-adrenergic receptors with phentolamine (3 mg/kg) significantly reduced Pm in all embryos, and the intensity of the response ranging from 42% to 60% of preinjection values was similar between all groups except the H70-90 treatment group (Fig. 6A). Following α-adrenergic receptor blockade, fH was unaffected in most treatment groups, with the exception of N embryos that showed a slight but significant increase in fH (P < 0.001; Fig. 6B).
Fig. 6.
Pm (A) and fH (B) for the control group and all shifted groups pre- (open column) and post-α-adrenergic (solid columns) blockade in alligator embryos at 90% of incubation. Groups were either maintained in normoxic conditions (N), 10% O2 conditions (H), or shifted as follows: from 21 to 10% O2 at 50% of incubation (H50-90), from 10% to 21% O2 at 50% of incubation (H20-50), from 21 to 10% O2 at 70% of incubation (H70-90), or from 10% to 21% O2 at 70% of incubation (H20-70). Asterisks indicate a significant response to ɑ-adrenergic blockade (P < 0.05). Dissimilar letters in the groups indicate statistically distinct differences in the response intensity between the compared groups. Data are presented as means ± SE. Sample sizes N (n = 9), H (5), H50-90 (n = 10), H20-50 (n = 12), H70-90 (n = 7), and H20-70 (n = 7).
2013 effects of developmental hypoxia on cardiac gene expression.
Gene expression studies were conducted on a subset of the cardiac tissues: the hypoxic group (H) was excluded from our analyses because those hearts were processed at a different time and in a different location (at UNT) from hearts in the other groups (at University of North Dakota). After excluding the H group, expression of 18S rRNA in hearts did not vary among treatment groups or between clutches. We could, therefore, use 18S rRNA expression as a covariate (i.e., a housekeeping gene) to control for sample-to-sample variation in RNA extraction or cDNA synthesis efficiency.
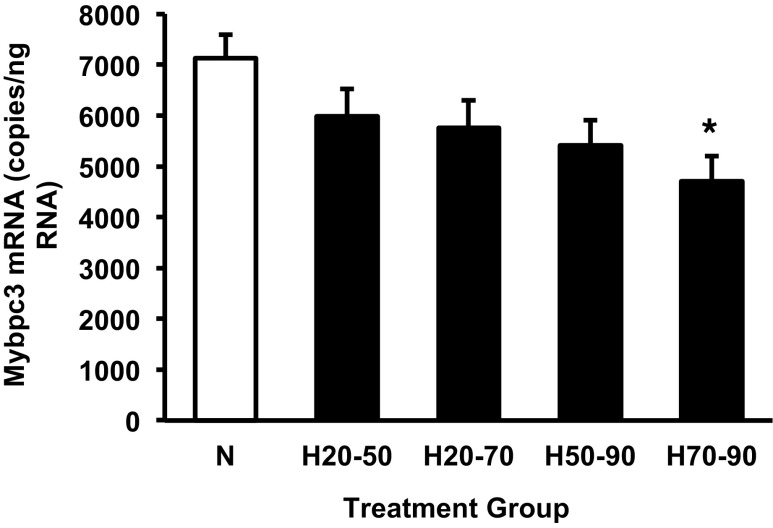
Cardiac expression of Mybpc3 in hearts was significantly affected by hypoxic treatment (P < 0.05) and covaried with 18S rRNA levels (P < 0.05), but was not affected by clutch identity. Although Mybpc3 expression tended to be lower in all hypoxic groups, expression was only significantly lower in the H70-90 group after correction for multiple comparisons (Fig. 7). Expression of Hif1α, Ldha, Ryr2, Pln, VegfA, PdgfB, Myh6, Igf2, and Ttn was unaffected by hypoxic incubation. However, clutch identity did have a significant effect on Ldha expression (P < 0.01). Igf2 expression was significantly correlated with 18S rRNA levels (P < 0.005).
Fig. 7.
Cardiac expression of Mybpc3 mRNA for the control normoxic group and experimental groups exposed to hypoxia for the indicated periods of development. Groups were either maintained in normoxic conditions (N) or shifted to hypoxic conditions as follows: exposed to 10% O2 between 50% and 90% of incubation (H50-90), exposed to 10% O2 between 20% and 50% of incubation (H20-50), exposed to 10% O2 between 70% and 90% of incubation (H70-90), or exposed to 10% O2 from 20% to 70% of incubation (H20-70). *Significant difference from the normoxic control group at a P < 0.013 (probability adjusted using Dunn-Sidák correction for multiple comparisons, four experimental groups compared with controls). Data are presented as means ± SE. Sample sizes N (n = 10), H20-50 (n = 10), H20-70 (n = 11), H50-90 (n = 12), and H70-90 (n = 12).
We also tested for correlations between gene expression and cardiac mass. We used treatment and clutch as independent variables in ANCOVA and included embryo mass as a covariate to control for the overall correlation between heart size and body size. We then included expression values for each gene as a covariate. Statistically controlling for treatment, clutch, and embryo mass, Pdgf was the only gene to covary with cardiac mass at 90% of development (P < 0.01). There was a positive correlation between Pdgf expression and cardiac mass.
2013 effects of developmental hypoxia on gene expression in limbs.
Limb tissues were all processed together at the same time and in the same location (at UNT). Therefore, we were able to analyze gene expression in limbs for all experimental groups. In contrast to hearts, expression of 18S rRNA in limbs was significantly affected by hypoxic treatment (P < 0.005) and by clutch identity (P < 0.05). Thus, we could not use 18S rRNA as a covariate to control for sample-to-sample variation in RNA extraction or cDNA synthesis efficiency. We examined Gapdh as another potential housekeeping gene. Expression of Gapdh in limbs did not vary among treatment groups or between clutches. Therefore, Gapdh was used as the covariate for expression analyses of other genes in limbs.
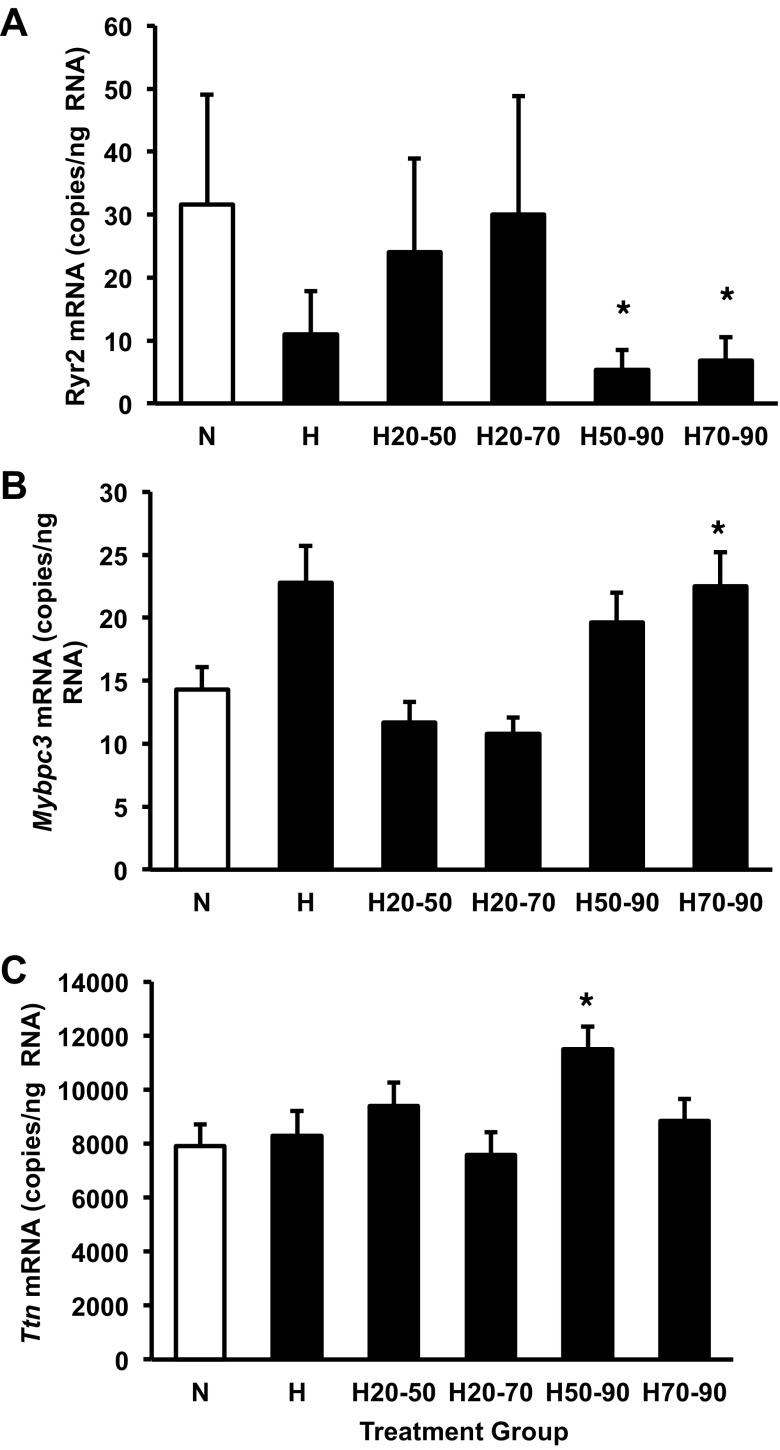
Expression of Ryr2 was significantly affected by hypoxic treatments (P < 0.005) and by clutch identity (P < 0.0001). Hypoxic exposure during the latter part of embryogenesis decreased Ryr2 expression: H50-90 and H70-90 groups had significantly lower levels of Ryr2 in limbs than the normoxic group (Fig. 8A). Mybpc3 in limbs was significantly affected by hypoxic treatment (P < 0.0001), by clutch identity (P < 0.001), and covaried with Gapdh expression (P < 0.0001). The H70-90 group was the only group that differed significantly from the normoxic control group after correction for multiple comparisons (Fig. 8B). Expression of Ttn in limbs was significantly affected by hypoxic treatment (P < 0.01) and covaried with Gapdh expression (P < 0.0001). The H50-90 group was the only group that differed significantly from the normoxic control group after correction for multiple comparisons (Fig. 8C).
Fig. 8.
Expression of Ryr2 (A), Mybpc3 (B), and Ttn (C) mRNA in limbs from the control normoxic group and experimental groups exposed to hypoxia for the indicated periods of development. Groups were either maintained in normoxic conditions (N) or shifted to hypoxic conditions as follows: exposed to 10% O2 between 50% and 90% of incubation (H50-90), exposed to 10% O2 between 20% and 50% of incubation (H20-50), exposed to 10% O2 between 70% and 90% of incubation (H70-90), or exposed to 10% O2 from 20% to 70% of incubation (H20-70). *Significant difference from the normoxic control group at a P < 0.01 (probability adjusted using Dunn-Sidák correction for multiple comparisons, 5 experimental groups compared with controls). Data are presented as means ± SE. Sample sizes N (n = 10), H (n = 7), H20-50 (n = 10), H20-70 (n = 11), H50-90 (n = 12), and H70-90 (n = 12).
Expression of Hif1α, Ldha, Pln, VegfA, Igf2, PdgfB, and Myh6 were not affected by hypoxia treatments. However, Ldha expression was affected by clutch (P < 0.05) and was correlated with Gapdh expression (P < 0.0001). Clutch identity affected Igf2 expression (P < 0.05) and PdgfB expression (P < 0.05). Myh6 expression was affected by clutch (P < 0.05) and was correlated with Gapdh expression (P < 0.0001). Additionally, Gapdh expression was a significant covariate for Hif1α (P < 0.0001), Pln (P <0.0001), VegfA expression (P < 0.001).
DISCUSSION
The environment has the potential to markedly alter the phenotype of developing animals (14, 23, 24, 25, 26, 27, 50, 67, 68). Brief changes in the developmental environment for short periods can impact the pattern of organogenesis, which may have both short- and long-term effects on the organism (10, 22, 67). The potential for irreversible changes in organ phenotype is greatest if a stressor is applied early in ontogeny. During embryogenesis in egg laying reptiles, which naturally experience fluctuations in abiotic conditions, such as O2 availability, plasticity could conceivably produce an animal with greater tolerance to low O2 environments later in life. These data support our hypotheses that developmental hypoxia alters cardiac morphology, gene expression, and cardiovascular regulation in reptiles, including embryonic alligators. Recently, Wearing et al. (70) found that snapping turtles raised for 3 yr in normoxia following hypoxic incubation were smaller with reduced fH and increased V̇o2 following feeding (70), relative to their normoxic counterparts. It is possible that alligators incubated in hypoxic conditions could show similar responses.
We identified discrete windows where embryonic and cardiac growth were affected by hypoxic exposure. Our prior work in the common snapping turtle demonstrated that hypoxic exposure over an 11-day period of embryonic development (total incubation 55 days) resulted in an embryo with an enlarged heart (27, 67). This relative increase in heart mass could be due to a suppression of somatic growth, a response of the cardiac tissue to hypoxia directly, or to a secondary response due to endocrine or hemodynamic changes in the embryonic system (25, 27). In the present study, we hypothesized that incubation in 10% O2 increases relative cardiac size due to cardiac enlargement and not suppression of somatic growth. Our results supported this hypothesis, as cardiac enlargement results after hypoxic exposure between 20 and 40% of incubation, whereas embryonic growth is suppressed when hypoxia occurs from 70% to 90% of incubation.
Relative cardiac enlargement was evident in embryos from both seasons when they were subjected to chronic 10% O2 prior to 50% of incubation (Fig. 2B). Cardiac mass in ectotherms is an index of stroke volume (38, 39, 72), and therefore, incubation in 10% O2 may have increased stroke volume in embryos from this study. Eme et al. (25) reported that stroke volume increases ∼100% in H embryos at 90% of incubation compared with control embryos from normoxic conditions. Critical windows for environmental effects on cardiac mass have been identified in numerous species, including embryonic fish, common snapping turtles, chickens, and mice, illustrating this may be a common feature of vertebrate ontogeny (10, 22, 45, 51, 55, 61, 67, 72). Clearly, hypoxic conditions during embryogenesis can produce an enlarged heart; however, the mechanisms by which hypoxia elicits this phenotype have yet to be determined.
We measured expression of genes that are known to respond to hypoxia or that have been shown to play a role in cardiac physiology or growth in other species. This included Hif1α and one of its direct transcriptional targets, Ldha. We also examined calcium-signaling genes (Ryr2 and Pln) that are involved in cardiac development and smooth muscle contraction. Finally, we measured growth factors (VegfA, Igf2, PdgfB) and structural genes (Mybpc3, Mhc6, Ttn). Unexpectedly, we found that hypoxia only influenced expression of one of these genes (Mybpc3) in the heart at 90% of incubation. It should be noted that gene expression in this study was based on samples taken at 90% of incubation, when gene expression may have normalized after an initial increase at the onset of hypoxic incubation. A time series would likely detect transient gene expression changes responsible for growth differences; however, with limited egg availability we could not sample for tissues and collect physiological measurements at multiple stages of development.
Changes in mRNA translation as well as post-translational modifications following hypoxic incubation could account for the cardiac enlargement without marked changes in mRNA expression. Nonetheless, hypoxia-induced differences in cardiac Mybpc3 mRNA expression persisted to 90% of incubation (Fig. 7). It is unclear whether lower expression of Mybpc3 is directly related to physiological and morphological changes in the alligator heart, but mutation of this gene in mice and humans results in cardiac enlargement (42). In mammals, the cellular mechanism underlying the increase in heart size depends upon whether individuals are heterozygotes or homozygotes for Mybpc3 mutations. Heterozygotes for Mybpc3 develop hypertrophic cardiomyopathy as a result of myocyte hypertrophy (increased cell size), whereas homozygotes develop dilated cardiomyopathy with cardiomyocyte hyperplasia (increased cell number). Our findings suggest that changes in Mybpc3 expression might provide a mechanistic link between hypoxia and altered cardiovascular phenotype in alligator embryos. Further characterization of Mybpc3 expression throughout embryogenesis is clearly warranted. It will also be critical to examine whether this molecular phenotype is associated with hyperplastic or hypertrophic cardiac growth.
Previously, we proposed that a potential contributor to the hypoxic heart enlargement could be the direct effect of low O2 on gene expression, a mechanically mediated response to changes in cardiac preload and afterload, or a secondary response to hypoxia-induced hormonal release (25, 27). The window of susceptibility identified for cardiac enlargement spanned from 20 to 40% of incubation, a time period of 14 days in embryos incubated at 30°C (Fig. 2B). Eggs moved to normoxic conditions at 30% or 40% of incubation, then returned to hypoxia at 50% of incubation, retained the enlarged cardiac phenotype similar to H embryos (Fig. 2B), thereby identifying this 7–14-day period of susceptibility. This relatively brief period of exposure to hypoxia coincides with the developmental stages 13 through 19, a period of cardiac chamber formation and major outflow vessel development (18, 28, 35, 47). Metabolic rate over this period is quite low, less than ∼0.04 ml O2·g−1·min−1 in embryonic alligators incubated in both N and H conditions (Eme J and Crossley II DA, unpublished data). Therefore, during stages 13 through 19, 10% ambient O2 should be sufficient to meet metabolic demands, and H conditions are unlikely to alter the metabolic demand of the cardiovascular system during this critical window (69). Although possible changes in blood viscosity due to increased hematocrit at the time of hypoxic exposure cannot be ruled out, hematocrit at 70% and 90% of incubation is similar, 32 to 34%, in both normoxic and hypoxic incubated alligator embryos (65; Crossley II DA, unpublished data). Finally, relative cardiac enlargement is absent until 80% of incubation in H embryonic alligators, the same period during which embryo mass is most affected by hypoxic incubation (14, 25). Collectively, findings from this study suggest the relative cardiac enlargement evident at 90% of embryonic alligator development could be due to earlier changes in gene expression induced by hypoxia. For example, research in embryonic mice found that hypoxic exposure applied at ∼50% of embryonic development for 4 h results in elevated expression of the Hif1α (45). While a similar study has yet to be completed in alligator embryos, the Kenchegowda et al. (45) finding lends support to the proposed method of action of hypoxia in this species. As mentioned above, an examination of cardiac gene expression across the entirety of embryonic development would be illuminating. At a minimum, experiments during the sensitive period identified in this study may resolve key questions about the molecular and cellular mechanisms underlying the enlarged heart in alligator embryos.
Normoxia during the latter half of incubation allowed recovery of normal embryonic mass (Fig. 2A). Conversely, hypoxic conditions over a short period late in embryogenesis (from 70 to 90% of incubation) caused a significant decrease in embryonic mass (Fig. 2A). This effect of hypoxia on somatic growth was most strongly associated with decreased expression of Ryr2 mRNA in whole limbs (Fig. 8A). This effect is consistent with the observation that ryanodine receptor operated calcium stores are involved in muscle cell proliferation during tail regeneration in Xenopus laevis tadpoles (68). The negative impact of hypoxia on embryonic mass has been documented in studies of Florida red-bellied turtle (Pseudemys nelson), common snapping turtles, American alligators, and several strains of domestic chickens (14, 15, 22, 23, 25–27, 41, 43–45, 63, 67, 69). In both the common snapping turtle and embryonic chicken, hypoxia-induced reduction in overall embryonic mass is evident at 70% of incubation (27, 41). Common snapping turtles have been suggested to lack a critical window for overall embryonic growth given that overall reduction in embryonic mass is dependent on total time exposed to hypoxic conditions (67). Alligator embryos show a hypoxia-sensitive period for embryo mass from 70 to 90% of incubation (Fig. 2A). The reason for this difference is unclear, as embryonic chickens have been suggested to have a critical window for hypoxia-induced reductions in mass from 30 to 60% of incubation (22, 62). In addition, snapping turtle embryos exhibit a linear pattern of embryonic growth over the latter half of incubation, and on the basis of metabolic rate, the cost of development is also linear (6). During alligator development, embryonic growth is exponential, with the maximal growth rate and maximal metabolic rate occurring between 70% and 90% of incubation at 30°C (18, 69). This maximal growth window corresponds to the critical window for embryonic growth identified in this study (Fig. 2A). Movement to hypoxia during this period results in suppression of normal embryonic alligator growth (Fig. 2A).
The cardiovascular regulatory phenotype is also altered during discrete windows. Hypoxia between 50 and 70% of incubation causes a relatively hypotensive and bradycardic phenotype when hypoxic conditions are experienced from 70 to 90% of incubation. The H group in this study was hypotensive relative to the N group at 90% of incubation (Fig. 3A), as previously reported for this species (14, 23, 25). Hypoxic incubation increases CAM vascularization of a number of embryonic species, including American alligator (12) and domestic chicken (20, 21). Whole body vascular resistance in embryonic domestic chickens also decreases in response to hypoxic incubation (2). Hypoxic incubation increases muscular capillary density and decreases diffusion distances in Canada geese (Branta canadensis); however, the impact on embryonic Pm in this species was not investigated (63). If similar changes in vascular structure occur in hypoxia-incubated alligator embryos, then relative hypotension could be attributed to increases in vascular density, as previously suggested (14). Interestingly, hypoxic exposure of embryonic alligators beginning at 50% of incubation, but not 70%, produced a hypotensive phenotype, indicating that 50% to 70% of incubation is a critical window for establishing normal Pm regulation in alligators (Fig. 3A). This window of development coincides with a doubling of extra-embryonic blood volume in the domestic chicken, while CAM mass remains constant, indicating the vascular cross-sectional area of the CAM is increasing (59). If this phenomenon also occurs in embryonic alligators, it may indicate the capacity to increase CAM vascular density or tissue capillary is limited during the 50 to 70% of incubation window. This limitation to vascular structure may also account for the reduction in overall body size evident in the H70-90 treatment group (Figs. 2A and 4A). In this scenario, embryonic mass is suppressed by hypoxia (Fig. 2A), while the animal no longer possesses the plasticity to increase CAM or body capillary density. Alternatively, embryonic alligators after 70% of incubation may exhibit an increase in cardiac contractility or blood volume to offset any increases in vascular cross-sectional area. However, expression of one gene involved in cardiac muscle contraction, Mybpc3, was depressed in embryos exposed to hypoxia late in incubation, possibly accounting for the relative hypotension. Further investigations of CAM vasculature plasticity, blood volume, and whole body vasculature of embryonic American alligators would help determine the basis for the Pm critical window identified.
Differences in fH were also evident in alligator embryos introduced to hypoxic conditions at 50% or 70% of incubation (H50-90 and H70-90, respectively). These groups were bradycardic compared with N embryos (Fig. 3B). In this study, the H treatment group's fH was not significantly lower than the N group, which differs from previous reports (14, 23, 26) but similar to late stage embryonic alligators at 95% of incubation (Fig. 3B; Ref. 23). Clearly, the factors that establish control of fH require further investigation.
The effects of vagal blockade were minimal in all groups, as previously reported (17, 23). This suggests cholinergic tone of cardiovascular function is minimal in embryonic alligators and nonresponsive to hypoxic incubation (Fig. 4, A and B).
Embryonic alligator Pm and fH responses to α- and β-receptor blockade revealed possible changes in adrenergic regulation in the different treatment groups (Fig. 5 and Fig. 6). Pm response to β-adrenergic blockade was absent in the H and H50-90 treatment groups, indicating sustained exposure to hypoxia from 50% to 90% of incubation eliminates β-receptor mediated dilation (Fig. 5A). This finding is similar to that previously reported for hypoxia-incubated embryonic alligators and could be attributed to vascular desensitization from chronic elevation of plasma catecholamines (23). Prior analysis of hypoxia-susceptible periods in embryonic snapping turtles indicated that β-adrenergic receptor tone on the vasculature is constant irrespective of the period when hypoxic incubation commences (67). However, this is not the case in the American alligator, which demonstrate greater plasticity in cardiovascular control elements compared with the snapping turtle. While sympathetic nervous system contribution was not addressed in this study, prior work has demonstrated the sympathetic regulation is nonfunctional in embryonic alligators (14, 23). Therefore, increased catecholamine release in response to hypoxia starting midway through incubation could account for the proposed desensitization. However, differences in reductions in fH following adrenergic blockade could have secondarily impacted Pm. Further investigations are needed to isolate the basis of the loss of responsiveness of Pm to β-adrenergic blockade.
In contrast to the suppressed vascular β adrenergic tone, the fH response to β adrenergic blockade intensified in all treatment groups incubated in hypoxia from 70 to 90% of development (Fig. 5B). Increased β tone on fH has previously been reported in alligator embryos studied between 80 and 90% of incubation (23). Cardiac β-adrenoceptor sensitivity is increased in chicken embryos subjected to chronic hypoxic incubation, possibly accounting for our findings in the alligators (48). Importantly, all treatment groups that included hypoxia during a period spanning 70–90% of incubation (i.e., H, H50-90, and H70-90) had similar intrinsic fH, with complete cholinergic and adrenergic receptor blockade, which was lower than all other treatment groups (Fig. 6B). Thus, the difference in β-adrenergic tone may be based on modifications of cardiac pacemaker tissue in the embryonic alligator heart.
Perspectives and Significance
The potency of the developmental environment in dictating the neonatal phenotype has been recognized clinically and is known to influence human physiology. On the basis of our findings, this concept can be extended to the American alligator, which experiences changes in abiotic factors, such as O2, during ontogeny. We propose that ambient O2 concentrations dictate the level of gene expression during critical windows of development, programing cardiac morphological and physiological phenotypes in embryonic alligators. To build a more comprehensive understanding of hypoxia's effects on organ development and gene expression, future studies will address the plasticity in developmental timing, termed heterokairy, of cardiovascular sensitivity to hypoxia (56, 64). For example, when measured at 90% of incubation, snapping turtle embryos exposed to hypoxia between 50 and 70% showed increased heart mass and reduced whole embryo mass (67). However, in Tate et al. (67) and this study, we only sampled embryonic tissue for gene expression analysis at a single time point (90%) toward the end of embryonic development. Future studies should sample organ masses and quantify gene expression at multiple time points in development to determine whether heterokairy can be induced by hypoxia. Heterokairy may be present in developing reptiles in response to hypoxia and warrants further study in the embryonic alligators given the findings presented here. We also suggest that the cardiorespiratory phenotype of juvenile animals may also be affected by early exposure to hypoxia, and experiments measuring juvenile cardiac performance will be a rewarding area of future research. Evidence from snapping turtles incubated in hypoxic environments suggests that the cardiovascular physiology is maintained into juvenile life stages (70), supporting the hypothesis that hypoxic incubation may have lasting effects on juvenile and adult alligators.
GRANTS
This work was supported by National Science Foundation Career Award IBN IOS-0845741 to D. A. Crossley II and NSF award IBN IOS-0923300 to T. Rhen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.B.T., J.E., and D.A.C.I. conception and design of research; K.B.T., T.R., Z.F.K., J.C., and D.A.C.I. performed experiments; K.B.T. and D.A.C.I. interpreted results of experiments; K.B.T. and D.A.C.I. prepared figures; K.B.T. and D.A.C.I. drafted manuscript; K.B.T., T.R., J.E., Z.F.K., R.M.E., and D.A.C.I. edited and revised manuscript; K.B.T., T.R., J.E., Z.F.K., J.C., R.M.E., and D.A.C.I. approved final version of manuscript; D.A.C.I. analyzed data.
ACKNOWLEDGMENTS
The authors sincerely thank the Louisiana Department of Wildlife and Fisheries and Rockefeller Wildlife Refuge for access to alligator eggs.
REFERENCES
- 1.Ackerman RA. Physiological and ecological aspects of gas exchange by sea turtle eggs. Am Zool 20: 575–583, 1980. [Google Scholar]
- 2.Adair TH, Guyton AC, Montani JP, Lindsay JL, Stanek KA. Whole body structural vascular adaptation to prolonged hypoxia in chick embryos. Am J Physiol Heart Circ Physiol 252: H1228–H1234, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJP. In utero programming of cardiovascular disease. Theriogenol 53: 555–574, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Miraźon L, Sultan McNamara J, Metcalf NB, Monaghan P, Spencer HG, S E. Developmental plasticity and human health. Nature 430: 419–421, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, Mould A, Cahill MM, Tonks ID, Grimmond SM. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res 86: e29–e35, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Birchard GF, Reiber CL. Growth, metabolism, and chorioallantoic vascular density of developing snapping turtles (Chelydra serpentina): Influence of temperature. Physiol Zool 68: 799–811, 1995. [Google Scholar]
- 7.Bjarnegård M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fässler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development 131: 1847–1857, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Burggren WW, Christoffels VM, Crossley DA, Enok S, Farrell AP, Hedrick MS, Jensen B, Moorman AFM, Mueller CA, Skovgaard N, Taylor EW, Wang T. Comparative cardiovascular physiology: future trends, opportunities and challenges. Acta Physiol (Oxf) 210: 257–276, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Burggren WW, Mueller CA. Developmental critical windows and sensitive periods as 3-D constructs in time and space. Physiol Biochem Zool 88: 91–102, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Burggren WW, Reyna KS. Developmental trajectories, critical windows and phenotypic alteration during cardio-respiratory development. Respir Physiol Neurobiol 178: 13–21, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Chen CL, Wang CC, Cheng IJ. Effects of biotic and abiotic factors on the oxygen content of green sea turtle nests during embryogenesis. J Comp Physiol B 180: 1045–1055, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Corona TB, Warburton SJ. Regional hypoxia elicits regional changes in chorioallantoic membrane vascular density in alligator but not chicken embryos. Comp Biochem Physiol A 125: 57–61, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Crossley IIDA, Altimiras J. Ontogeny of autonomic control of cardiovascular function in the domestic chicken Gallus gallus. Am J Physiol Regul Integr Comp Physiol 279: R1091–R1098, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Crossley IIDA, Altimiras J. Cardiovascular development in embryos of the American alligator (Alligator mississippiensis): effects of chronic and acute hypoxia. J Exp Biol 208: 31–39, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Crossley DA II, Altimiras J. Effect of selection for commercially productive traits on the plasticity of cardiovascular regulation in chicken breeds during embryonic development. Poult Sci 91: 2628–2636, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Crossley DA II, Burggren WW, Altimiras J. Cardiovascular regulation during hypoxia in embryos of the domestic chicken Gallus gallus. Am J Physiol Regul Integr Comp Physiol 284: R219–R226, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Crossley DA II, Hicks JW, Altimiras J. Ontogeny of baroreflex control in the American alligator Alligator mississippiensis. J Exp Biol 206: 2895–2902, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Deeming DC, Ferguson MWJ. Effects of incubation temperature on growth and development of embryos of Alligator mississippiensis. J Comp Physiol B 159: 183–193, 1989. [Google Scholar]
- 19.DeWitt TJ, Scheiner SM (Eds.). Phenotypic Plasticity: Functional and Conceptual Approaches. Oxford, UK: Oxford University, p. 98–111, 2004. [Google Scholar]
- 20.Dusseau JW, Hutchins PM. Hypoxia-induced angiogenesis in chick chorioallantoic membranes: a role for adenosine. Respir Physiol 71: 33–44, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Dusseau JW, Hutchins PM. Microvascular responses to chronic hypoxia by the chick chorioallantoic membrane: a morphometric analysis. Microvasc Res 37: 138–147, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Dzialowski EM, Von Plettenberg D, Elmonoufy NA, Burggren WW. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A 131: 713–724, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Eme J, Altimiras J, Hicks JW, Crossley DA II. Hypoxic alligator embryos: Chronic hypoxia, catecholamine levels and autonomic responses of in ovo alligators. Comp Biochem Physiol A 160: 412–420, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Eme J, Crossley DA II. Chronic hypercapnic incubation increases relative organ growth and reduces blood pressure of embryonic American alligator (Alligator mississippiensis). Comp Biochem Physiol A 182: 53–57, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Eme J, Crossley DA II, Hicks JW. Role of the left aortic arch and blood flows in embryonic American alligator (Alligator mississippiensis). J Comp Physiol B 181: 391–401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eme J, Hicks JW, Crossley DA II. Chronic hypoxic incubation blunts a cardiovascular reflex loop in embryonic American alligator (Alligator mississippiensis). J Comp Physiol B 181: 981–990, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Eme J, Rhen T, Tate KB, Gruchalla K, Kohl ZF, Slay CE, Crossley DA II. Plasticity of cardiovascular function in snapping turtle embryos (Chelydra serpentina): chronic hypoxia alters autonomic regulation and gene expression. Am J Physiol Regul Integr Comp Physiol 304: R966–R979, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson MWJ. Reproductive biology and embryology of the crocodilians. In: Biology of the Reptilia: Development A, edited by Gans C, Billett F, and Maderson PFA. New York: Wiley-Interscience Publication, 1985, p. 329–491. [Google Scholar]
- 29.Ferguson MWJ, Joanen T. Temperature of egg incubation determines sex in Alligator mississippiensis. Nature 296: 850–853, 1982. [DOI] [PubMed] [Google Scholar]
- 30.Garland T, Kelly SA. Phenotypic plasticity and experimental evolution. J Exp Biol 209: 2344–2361, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science 305: 1733–1736, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Grabowski CT. A quantitative study of the lethal and teratogenic effects of hypoxia on the three-day chick embryo. Am J Anat 109: 25–36, 1961. [DOI] [PubMed] [Google Scholar]
- 33.Grabowski CT. The etiology of hypoxia-induced malformations in the chick embryo. J Exp Zool 157: 307–326, 1964. [DOI] [PubMed] [Google Scholar]
- 34.Grabowski CT, Paar JA. The teratogenic effects of graded doses of hypoxia on the chick embryo. Am J Anat 103: 313–347, 1958. [DOI] [PubMed] [Google Scholar]
- 35.Greil A. Beitrage zur vergleichenden Anatomie und entwicklungsgeschichte des Herzens und des truncus arteriosus der wirbelthiere. Morphologisches Jahrbuch 31: 1903. [Google Scholar]
- 36.Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdez EA, Giussani DA, Blanco CE, Hanson MA, Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292: R2234–R2240, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Herrera E, Salinas C, Blanco C, Villena M, Giussani D. High-altitude hypoxia and blood pressure dysregulation in adult chickens. J Dev Orig Health Dis 4: 69–76, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Hillman SS. Cardiovascular correlates of maximal oxygen consumption rates in anuran amphibians. J Comp Physiol 109: 199–207, 1976. [Google Scholar]
- 39.Hillman SS, Hedrick MS. A meta-analysis of in vivo vertebrate cardiac performance: implications for cardiovascular support in the evolution of endothermy. J Exp Biol 218: 1143–1150, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Hodin J. Plasticity and constraints in development and evolution. J Exp Zool 288: 1–20, 2000. [PubMed] [Google Scholar]
- 41.Iversen NK, Wang T, Baatrup E, Crossley DA II. The role of nitric oxide in the cardiovascular response to chronic and acute hypoxia in White Leghorn chicken (Gallus domesticus). Acta Physiol (Oxf) 211: 346–357, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Jiang J, Burgon PG, Wakimoto H, Onoue K, Gorham JM, O'Meara CC, Fomovsky G, McConnell BK, Lee RT, Seidman JG, Seidman CE. Cardiac myosin binding protein C regulates postnatal myocytes cytokinesis. Proc Natl Acad Sci USA 112: 9046–9051, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonker SS, Giraud GD, Espinoza HM, Davis EN, Crossley DA II. Effects of chronic hypoxia on cardiac function measured by pressure-volume catheter in fetal chickens. Am J Physiol Regul Integr Comp Physiol 308: R680–R689, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kam YC. Physiological effects of hypoxia on metabolism and growth of turtle embryos. Respir Physiol 92: 127–138, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Kenchegowda D, Liu H, Thompson K, Luo L, Martin SS, Fisher SA. Vulnerability of the developing heart to oxygen deprivation as a cause of congenital heart defects. J Am Heart Assoc 3: e000841, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kushlan JA, Mazzotti FJ. Population biology of the American crocodile. J Herpetol 23: 7–21, 1989. [Google Scholar]
- 47.Kutsche LM, Van Mierop L. Development of the pulmonary vein in the American alligator (Alligator mississippiensis). Anat Rec 222: 170–176, 1988. [DOI] [PubMed] [Google Scholar]
- 48.Lindgren I, Altimiras J. Chronic prenatal hypoxia sensitizes β-adrenoceptors in the embryonic heart but causes postnatal desensitization. Am J Physiol Regul Integr Comp Physiol 297: R258–R264, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Lutz PL, Dunbar-Cooper A. The nest environment of the American crocodile (Crocodylus acutus). Copeia 1: 153–161, 1984. [Google Scholar]
- 50.Marks C, Kaut K, Moore F, Bagatto B. Ontogenetic oxygen changes alter zebrafish size, behavior, and blood glucose. Physiol Biochem Zool 85: 635–644, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Marks C, Eme J, Elsey RM, Crossley IIDA. Chronic hypoxic incubation blunts thermally dependent cholinergic tone on the cardiovascular system in embryonic American alligator (Alligator mississippiensis). J Comp Physiol B 183: 947–957, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Mazzotti F, Kushlan J, Dunbar-Cooper A. Desiccation and cryptic nest flooding as probable causes of egg mortality in the American crocodile, Crocodylus acutus. Everglades National Park, Florida. Florida Scientist 51: 65–72, 1988. [Google Scholar]
- 53.Miller K, Packard GC. The influence of substrate water potential during incubation on the metabolism of embryonic snapping turtles (Chelydra serpentina). Physiol Zool 65: 172–187, 1992. [Google Scholar]
- 54.Miller NA. Po2 in loggerhead sea turtle (Caretta caretta) nests measured using fiber-optic oxygen sensors. Copeia 4: 882–888, 2008. [Google Scholar]
- 55.Moore FBG, Hosey M, Bagatto B. Cardiovascular system in larval zebrafish responds to developmental hypoxia in a family specific manner. Front Zool 3: 1–8, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller CA, Eme J, Burggren WW, Roghair R, Rundle SD. Challenges and opportunities in developmental integrative physiology. Comp Biochem Physiol A 184: 113–124, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Packard GC, Paukstis GL, Boardman TJ, Gutzke WHN. Daily and seasonal variation in hydric conditions and temperature inside nests of common snapping turtles (Chelydra serpentina). Can J Zool 63: 2422–2429, 1985. [Google Scholar]
- 58.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol 295: R583–R595, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhen T, Metzger K, Schroeder A, Woodward R. Expression of putative sex-determining genes during the thermosensitive period of gonad development in the snapping turtle, Chelydra serpentina. Sex Dev 1: 255–270, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Romanoff AL. Biochemistry of the Avian Embryo. New York: John Wiley and Sons, 1967. [Google Scholar]
- 61.Rossitto JJ, Burggren W. Critical cardiac and renal developmental windows for Atenolol exposure in embryos of the chicken Gallus gallus. FASEB J 26: 684–616., 2012. [Google Scholar]
- 62.Ruijtenbeek K, Le Noble FAC, Janssen GMJ, Kessels CGA, Fazzi GE, Blanco CE, De Mey JGR. Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation 102: 2892–2897, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Snyder GK, Byers RL, Kayar SR. Effects of hypoxia on tissue capillarity in geese. Respir Physiol 58: 151–160, 1984. [DOI] [PubMed] [Google Scholar]
- 64.Spicer JI, Burggren WW. Development of physiological regulatory systems: altering the timing of crucial events. J Zool 106: 91–99, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Stock MK, Metcalfe J. Modulation of growth and metabolism of the chick embryo by a brief (72-hr) change in oxygen availability. J Exp Zool Suppl 1: 351–356, 1987. [PubMed] [Google Scholar]
- 66.Tate KB, Eme J, Swart J, Conlon JM, Crossley DA II. Effects of dehydration on cardiovascular development in the embryonic American alligator (Alligator mississipiensis). Comp Biochem Physiol 162: A252–A258, 2012. [DOI] [PubMed] [Google Scholar]
- 67.Tate KB, Kohl ZF, Eme J, Rhen T, Crossley DA II. Critical windows of cardiovascular susceptibility to developmental hypoxia in Common snapping turtle (Chelydra serpentina) embryos. Physiol Biochem Zool 88: 103–115, 2015. [DOI] [PubMed] [Google Scholar]
- 68.Tu MK, Borodiesky LN. Spontaneous calcium transients manifest in the regenerating muscle and are necessary for skeletal muscle replenishment. Cell Calcium 56: 34–41, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warburton SJ, Hastings D, Wang T. Responses to chronic hypoxia in embryonic alligators. J Exp Zool 273: 44–50, 1995. [DOI] [PubMed] [Google Scholar]
- 70.Wearing OH, Eme J, Rhen Crossley DA. Phenotypic plasticity in the common snapping turtle (Chelydra serpentina): long-term physiological effects of chronic hypoxia during embryonic development. Am J Physiol Regul Integr Comp Physiol 310: R176–R184, 2016. [DOI] [PubMed] [Google Scholar]
- 71.Withers P, Hillman S. Allometric and ecological relationships of ventricle and liver mass in anuran amphibians. Funct Ecol 15: 60–69, 2001. [Google Scholar]
- 72.Zhang H, Burggren W. Hypoxic level and duration differentially affect embryonic organ system development of the chicken (Gallus gallus). Poult Sci 91: 3191–3201, 2012. [DOI] [PubMed] [Google Scholar]