Abstract
We have previously reported that methylene blue (MB) can counteract hydrogen sulfide (H2S) intoxication-induced circulatory failure. Because of the multifarious effects of high concentrations of H2S on cardiac function, as well as the numerous properties of MB, the nature of this interaction, if any, remains uncertain. The aim of this study was to clarify 1) the effects of MB on H2S-induced cardiac toxicity and 2) whether L-type Ca2+ channels, one of the targets of H2S, could transduce some of the counteracting effects of MB. In sedated rats, H2S infused at a rate that would be lethal within 5 min (24 μM·kg−1·min−1), produced a rapid fall in left ventricle ejection fraction, determined by echocardiography, leading to a pulseless electrical activity. Blood concentrations of gaseous H2S reached 7.09 ± 3.53 μM when cardiac contractility started to decrease. Two to three injections of MB (4 mg/kg) transiently restored cardiac contractility, blood pressure, and V̇o2, allowing the animals to stay alive until the end of H2S infusion. MB also delayed PEA by several minutes following H2S-induced coma and shock in unsedated rats. Applying a solution containing lethal levels of H2S (100 μM) on isolated mouse cardiomyocytes significantly reduced cell contractility, intracellular calcium concentration ([Ca2+]i) transient amplitudes, and L-type Ca2+ currents (ICa) within 3 min of exposure. MB (20 mg/l) restored the cardiomyocyte function, ([Ca2+]i) transient, and ICa. The present results offer a new approach for counteracting H2S toxicity and potentially other conditions associated with acute inhibition of L-type Ca2+ channels.
Keywords: calcium channels, cardiac contractility, sulfide toxicity
hydrogen sulfide (h2s) remains a significant hazard in the gas and oil industry (4, 26, 33, 69), and more recently, it has been used as a method of suicide (68, 85) in Japan (34) and in the United States (6). The severity of H2S acute toxicity lies with the development of a rapid cardiac asystole (79), in the form of a pulseless electrical activity (PEA) and a depression in breathing (42). These symptoms occur in anesthetized rats as soon as the blood concentration of dissolved H2S reaches no more than 2–5 μM (28, 79, 80). Studies performed on isolated heart or isolated cardiac cells (28, 81, 101) have shown that H2S depresses cardiomyocytes contractions, when in contact with a solution containing 100 μM of dissolved H2S, a concentration, which is in the high range of what can be found in the blood during lethal H2S intoxication (39, 42). Similar results have been confirmed using left ventricular dP/dt max as a marker of cardiac contractility (28, 79, 80).
In all of these studies, it is difficult to ascertain a unique mechanism of action to account for H2S toxicity on the heart, due to the multiple potential impacts and interactions of dissolved/free H2S with molecules involved in cardiomyocyte contraction. These modalities of interaction include 1) a depression of L-type Ca2+ channel activity (81, 102) or activation of K+ATP channels (14, 28); 2) a possible reconfiguration of proteins containing cysteine residues (102); 3) a direct impediment of actin-myosin interaction in a condition wherein ATP regeneration is depressed (19); 4) an accumulation of reactive O2 species during impediment of the mitochondrial electron chain (7), affecting, in turn, the activity of various critical ion channels (106); and 5) an interaction of H2S with NO pathway, resulting in an increase in cyclic guanosine monophosphate (cGMP) production (1, 3, 18), as well as a depression in cAMP pathway (99), both potentially leading to cardiac depression.
We have recently established that cobalt or ferric-compounds (35, 74, 75, 84, 88) are very effective in scavenging free/dissolved H2S in vivo (36). However, these compounds lose their effectiveness after the end of exposure (15), since the pool of free sulfide, accessible to trapping, disappears extremely rapidly from the blood and the tissue (36, 42, 89, 98). Intriguingly, we have also found in an anesthetized rat model that methylene blue (MB; 4 to 8 mg/kg), currently used for the treatment of methemoglobinemia and postsurgical vasoplegic shock (17), can counteract the circulatory failure produced by sulfide (79). In unsedated animals, MB also decreased the mortality of H2S-induced coma, if administered within 2 min after the onset of the coma (78). This occurs despite the fact that MB appears to have no direct interaction with the pools of dissolved or combined H2S (79). MB is an old molecule that possesses various properties and biological functions as summarized in these recent reviews (29, 54) and as developed in the discussion. With respect to the relevance of these properties to the cardiovascular system, MB at a low dose inhibits the soluble guanylate cyclase (32), leading to a decrease in cGMP (29, 97), the second messenger used by NO to transduce its cellular effects (53, 59, 96), which could, in turn, increase cardiac contractility. This anti-NO effect (96), which is well described on vessels, is the basis for treating refractory postoperative vasoplegic shock in humans (31, 43, 45, 47). Also, LeucoMB, the reduced form of MB that is produced in the blood and cells, is a strong reducing agent (29), which could counteract the modification of proteins, including calcium channels (81), which result from the creation of new disulfide bonds in the presence of H2S (66). LeucoMB could additionally oppose the consequences of the effects of reactive O2 species (106), created by the decrease in the rate of the electron chain activity.
Our present study was designed to clarify and confirm whether cardiac contractility, determined by echocardiography, is affected during severe H2S intoxication in both sedated and unanesthetized rats, whether MB specifically counteracts sulfide intoxication-induced cardiac depression; and whether these in vivo observations translate into measurable effects on cell contractility, intracellular calcium concentrations ([Ca2+]i), and [Ca2+]i transient and L-type Ca2+ currents (ICa) in isolated mouse cardiomyocytes.
METHODS
In Vivo Experiments
Sedated rat model.
ANIMAL PREPARATION.
Six adult male Sprague-Dawley rats weighing 448 ± 107 g were studied. The protocol was approved by the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee.
Anesthesia was induced by isoflurane 3.5% in O2 for a few minutes, followed by an intraperitoneal injection of urethane (1.2 g/kg). The rats were tracheostomized and mechanically ventilated, to maintain PaO2 around 80 mmHg, as previously described (79, 80). A catheter (PE-50 tubing) was inserted into the right femoral artery for continuous monitoring of systemic arterial blood pressure and arterial blood sampling. Venous catheters were inserted into the right femoral and right jugular veins for H2S infusion and antidote administration, respectively. Adequate ventilation was monitored by periodic arterial blood gas measurements using i-STAT1 blood gas analyzer (Abaxis, Union City, CA). The electrocardiogram was recorded by a bioelectric amplifier (Differential AC amplifier, model 1700, A-M Systems, Sequim, WA).
Expiratory flow was measured using a pneumotachograph (1100 Series, Hans Rudolph, Shawnee, KS) (42), and minute ventilation was determined (40). Mixed expired O2, CO2, and H2S fractions were measured continuously using O2 (Oxystar-100; CWE Ardmore, PA), CO2 (model 17630; VacuMed, Ventura, CA), and H2S (Interscan RM series; range: 0–200 ppm; Interscan, Simi Valley, CA) analyzers. On the basis of the determination of minute ventilation and mixed expired O2, oxygen uptake (V̇o2) was computed (40). Alveolar H2S was computed, and the concentration of dissolved H2S in the arterial blood was estimated, as previously described (39).
EXPERIMENTAL PROTOCOLS.
H2S solution was prepared using sodium hydrosulfide hydrate (NaHS; Sigma, St. Louis, MO). The molecular weight of the “hydrated” form of H2S was 74.08 g/mol, as the crystals used for preparing the solution contained 75% H2S-25% H2O. H2S was diluted in saline at a concentration of 0.8 mg/ml (10.8 mM) and prepared in airtight syringes immediately prior to each experiment. The level of exposure to H2S was determined on the basis of the relationship we have previously established between the blood concentrations, rate of exogenously administered H2S, and the corresponding clinical symptoms, i.e., H2S-induced apnea (37) and cardiac toxicity (80) using continuous infusion of H2S solution (38). Since a solution of NaHS contains H2S and HS−, the terms H2S or NaHS solution will be interchangeably used in this paper.
The rats were exposed to the H2S solution at a rate of 24 μmol·kg−1·min−1, a level we previously found to decrease blood pressure very rapidly and to be lethal within 5 min (79, 80). The changes in hemodynamics in response to infusion of H2S have been described in detail in our previous publications (79, 80). MB (4 mg/kg) in saline (1 ml) or only saline (1 ml) was injected as soon as cardiac contractility was found to decrease by at least 20%. A second injection of MB was performed 1 min later. One rat received a third injection of MB during the last minute of infusion. Infusion was stopped after 4.5 min in all instances, which corresponded to the time at which PEA would occur in all untreated intoxicated animals (see results).
Unsedated rat model.
ANIMAL PREPARATION.
We have previously found that intraperitoneal administration of 20 mg/kg H2S (5 mg/ml) can lead to a rapid coma in about one-third of rats receiving this injection, while a second injection performed 10 min later in the remaining rats, produced a coma in two-thirds of them. A third injection typically produces a coma in all animals. The coma develops in less than 2 min, with no difference in the clinical picture or outcome between the number of injections (79, 80). The protocol used in the present study was modified from these protocols by reducing the time between injections to 5 min (instead of 10 min) to create a more severe form of intoxication. Our objective was to describe the temporal profile of the changes in cardiac contractility without the interference of the anesthesia.
EXPERIMENTAL PROTOCOLS.
A total of 17 rats weighing 431 ± 132 g were used for this part of the study. This specific protocol was also approved by the Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee.
Four hours before intraperitoneal injection of H2S, animals were sedated with isoflurane (5%, then 2%) using a small “face mask”. Body temperature was maintained around 37°C using a heating pad. A catheter was placed in the dorsal vein of the tail and filled with heparinized saline (25 units/ml in saline). This procedure lasted no more than 10 min, during which baseline cardiac function was established by echocardiography (within 5 min following the onset of anesthesia). Every animal recovered within 5 min following the cessation of isoflurane inhalation and spent the following 3 to 4 h in their cage. They all displayed strictly normal behavior before starting the protocol.
Rats received an intraperitoneal injection of 20 mg/kg H2S solution (5 mg/ml ip) every 5 min (instead of 10 min) until coma was produced, which always occurred within 1 or 2 min after the injection (see Refs. 79 and 80 for more details). Clinical examination was performed every 30 s for 30 min following H2S administration, or until death of the animal, as previously described (79, 80). This examination comprised the determination of 1) response to hand clapping; 2) the flexion reflex, a spinal cord reflex that produces a flexion of the limb in response to a pinch of the toes; 3) the righting reflex, determined by the presence or absence of immediate turn over when the animal was put in a lateral position. The breathing pattern was also monitored by determination of breathing frequency and observations of a gasping pattern of breathing. Coma was defined by the disappearance of response to external stimuli with a loss of the righting reflex. As soon as the animal developed a coma, a continuous determination of the cardiac function by echography was performed.
During the first 24 h following H2S intoxication, each surviving animal was watched carefully for signs of distress or discomfort, including signs of prostration, inability to walk, eat or drink, paralysis, or visual deficit. The observations of the intoxicated animals were performed every hour in the first 8 h after the coma and then observed at 16 and 24 h after the coma. All the surviving rats were euthanized 36 h after the coma.
Evaluation of cardiac function.
In both studies, the cardiac function was evaluated by transthoracic echocardiography. The left hemithorax was shaved during the initial period of sedation by isoflurane. Two-dimensional (2D) and M-mode studies were performed with a Philips-ATL HDI 5000 ultrasound system, using a 12-MHz linear array transducer usually designed for musculoskeletal and superficial vascular studies. Three subcutaneous electrodes were placed to obtain simultaneous ECG recording in the anesthetized model. Left longitudinal parasternal views were obtained, and the settings were optimized to obtain the best image quality, adjusting the focus between 1 and 2 cm from the surface of the transducer. M-mode was used because of its excellent time resolution to determine the changes in left ventricular dimension or contractility. Images were obtained at mid left ventricle (LV), to allow a clear visualization of LV anterior and posterior wall endocardial borders, avoiding papillary muscles and mitral chordae. Simultaneous 2D and M-mode imaging of the left ventricle was obtained throughout the experimentation. Ten-second clips were recorded every 15–30 s and stored on magnetic optical disks for subsequent analysis with the Synapse Cardiovascular software (Fujifilm).
Data analysis.
In the sedated animals, all signals—blood pressure, respiratory flow, expiratory fraction of O2, CO2, and H2S—were digitized at 400 Hz using an analog-to-digital data acquisition system (PowerLab 16/35, AD Instruments, Colorado Springs, CO) and were visualized on line. All data were stored for further analysis by LabChart7 (AD Instruments, Colorado Springs, CO). Blood pressure, heart rate, and V̇o2 were averaged over 15 s and time aligned with the echography measurements.
An experienced reader analyzed the images and video obtained from the echography studies in a blinded manner. LV end-diastolic (Dd) and end-systolic (Sd) diameters were measured from M-mode views, guided by 2D images to position the sampling line at mid ventricle, through the largest diameter, just above the posterior papillary muscle tip. For each evaluation, five to seven consecutive cycles were measured and averaged. Fractional shortening (FS) was computed as (Dd − Sd)/Dd, the ejection fraction (EF) was calculated on the basis of the Teichholz formula to estimation of the end-diastolic volume (Dv) and end-systolic volume (Sv) from the diameters (82).
Statistical analysis.
All results are presented in the text as means ± SD unless indicated otherwise. The changes in echocardiographic data vs. time were analyzed using ANOVA with repeated measures. If significant, differences between specific time points were compared using a Mann-Whitney U-test. For the anesthetized model, variables of interest were compared at baseline and during the last 30 s of infusion (one-way ANOVA). In the unsedated rats, ejection fraction data were averaged every minute, including the values of animals in PEA, for comparison against baseline. The occurrence of death was also compared between the saline and MB groups, using a χ2-test. All statistical analyses were conducted using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
In Vitro Experiments
Studies on isolated contracting cardiomyocytes.
ISOLATION OF ADULT MOUSE LEFT VENTRICULAR (LV) MYOCYTES.
Cardiac myocytes were isolated from the septum and LV-free wall of C57BL/6 mice, according to the protocol of Zhou et al. (105) and as modified by us (77, 86). Isolated myocytes were plated on laminin-coated coverslips and used on the same day.
MYOCYTE SHORTENING MEASUREMENTS.
Myocytes adherent to coverslips were bathed in 0.7 ml of air- and temperature-equilibrated (37°C), HEPES-buffered (20 mM, pH 7.4) medium 199 (1.8 mM [Ca2+]o), and paced to contract (2 Hz). Myocyte images were captured by a charge-coupled device video camera, and myocyte motion was analyzed offline with edge detection algorithm, as previously described (76, 77, 86, 91, 93, 94).
[Ca2+]i TRANSIENT MEASUREMENTS.
Fura-2-loaded (0.67 μM fura-2 AM, 15 min, 37°C) myocytes were field-stimulated to contract (2 Hz, 37°C) in medium 199 containing 1.8 mM [Ca2+]o. [Ca2+]i transient measurements, daily calibration of fura-2 fluorescent signals, and [Ca2+]i transient analyses were performed as previously described (76, 77, 86, 91–94).
L-TYPE Ca2+ CURRENT (ICA) MEASUREMENTS.
Whole cell patch-clamp recordings were performed at 30°C, as previously described (77, 86, 93). The pipette diameter was 4–6 μm, and the pipette resistance was 0.8–1.4 MΩ when filled with standard pipette solution containing (in mM): 110 CsCl, 20 TEA·Cl, 10 HEPES, 5 MgATP, and 10 EGTA at pH 7.2. Extracellular bathing solution contained (in mM): 137 N-methyl-d-glucamine, 5.4 CsCl, 2 CaCl2, 1.3 MgSO4, 20 HEPES, 4 4-aminopyridine, and 15 glucose; at pH 7.4. Our solutions were designed to be Na+- and K+-free. To ensure steady-state Ca2+ loading in the sarcoplasmic reticulum, six conditioning pulses (from −70 to 0 mV, 100 ms, 2 Hz) were delivered to the myocyte before the arrival of each test pulse (from −90 to +50 mV, 10 mV increments, 60 ms). Leak-subtracted inward currents were used in analysis for ICa amplitudes and inactivation and deactivation kinetics. Inward currents obtained under these conditions were blocked by 1 μM verapamil (data not shown). ICa was normalized to membrane capacitance (Cm) before comparisons. All ICa amplitudes and τinact values, when given in results, were measured at −10 mV.
IN VITRO STUDY PROTOCOL.
At time zero, myocytes were exposed to either H2S (100 μM; prepared from NaHS, saline, MB (20 μg/ml) or H2S + MB. Contraction and [Ca2+]i transients were measured at 0 and 10 min in myocytes on different glass coverslips at each time point. This enabled data collection from 3 to 5 myocytes on each coverslip at each time point. ICa was measured at 0 and 3 min in myocytes on different glass coverslips at each time point. In a separate series of experiments, we sought to eliminate the possibility that MB may neutralize H2S in solution and also to evaluate whether MB is effective after myocytes were exposed to H2S. Myocytes were exposed to H2S or saline at time 0, MB or saline was added at 3 min, and contractions were measured at 10 min, while ICa was measured at 7 min.
The reproducibility of our approach over time was determined by measuring the contractility of myocytes in different glass coverslips exposed to saline at 0, 3, and 10 min and ICa at 0, 3, and 7 min.
DATA AND STATISTICAL ANALYSES.
All results are expressed as means ± SE. For analysis of the magnitude of contraction, [Ca2+]i transient, ICa amplitudes, and τinact, one-way ANOVA was used. A commercially available software package (JMP version 10, SAS Institute, Cary, NC) was used. In all analyses, P < 0.05 was taken to be statistically significant.
Kinetics of H2S in the dish in the presence of Media 199.
H2S solution (100 μM) was mixed in the medium (Media 199) used for the study of the cardiomyocytes in three different dishes. H2S concentrations were determined after reaction with N, N-dimethyl-p-phenylenediamine (NNDP; Sigma) and iron chloride (FeCl3) in acidic conditions (73, 88). Briefly, the reagents comprised a 20 mM solution of NNDP, in 7.2 N HCl (Sigma), and 30 mM FeCl3 (Sigma) in 1.2 N HCl. Each reagent was added to H2S at 0, 5, and 10 min. Absorbance of the solutions between 550–700 nm was measured by spectrophotometer (DU730, Beckman Coulter) (90). Tests were performed in triplicate.
In vitro interaction between H2S and MB.
A 500-ml Erlenmeyer flask was equipped with a rubber stopper with sealed catheter ports for injection of solutions. A solution of H2S was injected into the flask (2 μmol) containing 20 ml of Media 199 or Media 199 + 20 mg/l of MB to produce a final concentration 100 μM. The solutions were incubated for 10 min, corresponding to the maximal time of sulfide exposure during the study of the cardiomyocytes. The clamps were released at 10 min and the head-space of the flask was ventilated at a rate of 2 l/min using room air, while the solution was constantly agitated using a magnetic stir bar. The gas flow rate was precisely determined by a pneumotachograph (1100 Series, Hans Rudolph, Shawnee, KS), and the concentration of H2S leaving the flask was continuously measured by the H2S analyzers (Interscan range: 0–1 and 200 ppm) until the concentration of gaseous H2S retuned to zero. H2S and flow rate signals were recorded, and the total quantity of gaseous H2S that diffused from the media was computed by integration of the flow rate of gaseous H2S measured from the output outlet of the flask. The same procedure was repeated by flushing the head-space of the flask with nitrogen instead of room air. Reactions were performed in triplicate.
RESULTS
Effects of H2S and MB on Cardiac Contractility in Urethane-Anesthetized Rats
Baseline left ventricular ejection fraction: effects of anesthesia.
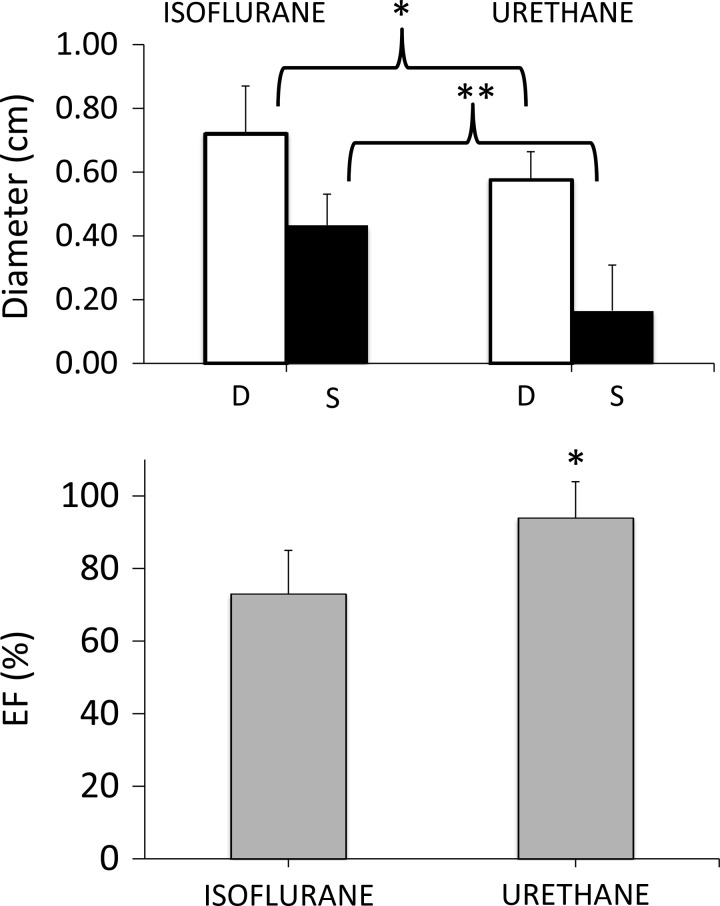
Baseline left ventricular ejection fraction (LVEF) and the fractional shortening (FS) averaged 94 ± 9% and 73 ± 21%, respectively, in the six urethane-anesthetized rats. These figures differ from the baseline values found during isoflurane anesthesia used in the protocol of H2S-induced coma (Fig. 1). EF and FS were significantly lower in the latter (74 ± 12% and 39 ± 11%, respectively, P < 0.01), with higher end-systolic and end-diastolic diameters than in urethane-sedated rats. Of note, echocardiographic measurements in the unsedated model could only be performed when the animals were in coma, so no baseline comparison could be done between sedated and nonsedated animals.
Fig. 1.
Means ± SD values of end-diastolic, end-systolic diameter, and left ventricular ejection fraction (EF) in animals anesthetized with urethane (current protocol) vs. isoflurane (determined during the placement of the tail catheters in 17 rats). This figure shows a more hyperdynamic heart in urethane-sedated animals compared with exposure to isoflurane. As a result, baseline EFs were always above 90% in our protocol involving sedated rats (see discussion for more details). *P < 0.05, **P < 0.01.
Untreated intoxication in urethane-anesthetized rats.
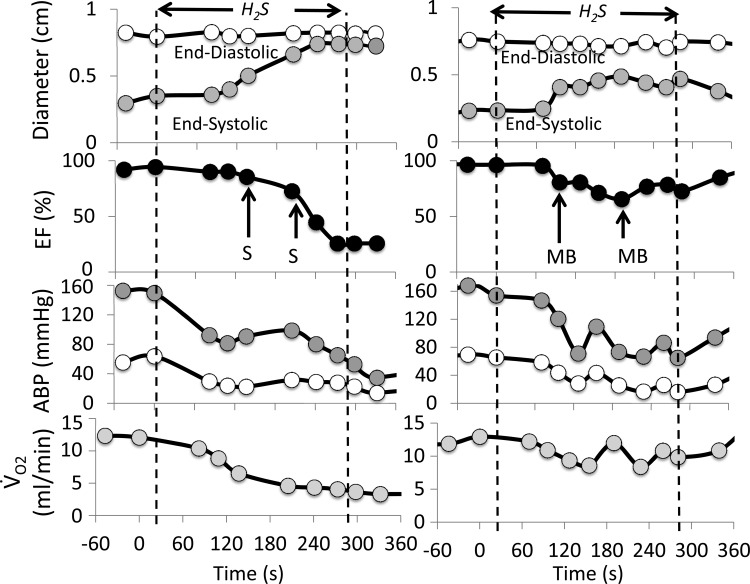
A total of 603 cardiac cycles were analyzed for this part of the study in the six animals. Infusion of H2S (24 μmol·kg−1·min−1) produced a stereotypical response. An example is shown in Fig. 2, while the averaged data obtained in the three untreated animals are displayed on Fig. 3. Within 30 s, a transient hyperdynamic myocardial response with sinus tachycardia and increased LV contractility was observed in half of the animals that did not reach significance. However, 1.8 ± 1.2 min into infusion, arterial blood pressure (BP), heart rate (HR), and V̇o2 started to decrease (Figs. 2 and 3), dropping from 96 ± 20 to 26 ± 5 mmHg, 368 ± 46 to 116 ± 11 beats/min, and 13 ± 2 to 3 ± 1 ml/min, respectively, from baseline to the end of sulfide infusion. Our main original finding was that the ejection fraction also dropped very rapidly from 97.3 ± 2.6% to 73.8 ± 9.5% (−15 ± 5%, P < 0.05) at 2 min, reaching 28 ± 2.5% at 4.5 min (P < 0.05) when H2S exposure was stopped. LV end-diastolic diameter increased from 0.68 ± 0.10 cm (baseline) to 0.94 ± 0.34 cm (P < 0.05) along with an almost fourfold increase in LV end-systolic diameter, which rose from 0.29 ± 0.14 cm (baseline) up to 0.83 ± 0.27 cm (Fig. 4, P < 0.01). Asynchrony was also observed in all instances, with a delayed contraction of the LV posterior wall compared with the anteroseptal wall, likely related to a left bundle branch block. Frequent PVC were observed with a profound bradycardia. Various examples of TM (time-motion) mode and 2D echocardiography pictures are shown on Fig. 4. During the last minute of exposure, spontaneous formation of intraventricular contrast consisting in an increased 2D echodensity with slow twirling motion, also referred to as “sludge” or “smoke” was observed, reflecting prethrombotic state created by the stagnation of blood in a motionless LV cavity. Despite the cessation of infusion (Fig. 4), a state of pulseless electrical activity (PEA) led to asystole in all untreated animals (Fig. 4): in spite of a persistent electrical sinus electrical activity, LV contractions were virtually abolished (Supplemental Video S1 and S2). The LV walls remained flat, and the cavity filled with prethrombotic material.
Fig. 2.
Examples of the effects of continuous infusion of hydrogen sulfide (H2S; 12 μmol/min for 4.5 min) on end-systolic and end-diastolic left ventricular diameter, left ventricular ejection fraction (EF), arterial blood pressure (ABP), and oxygen uptake (V̇o2) in two urethane-anesthetized rats. Left: response of an untreated animal (saline, S), wherein H2S infusion provoked a rapid reduction in cardiac contractility along with a drop in blood pressure and V̇o2 leading to complete electromechanical dissociation (PEA). Right: time course of the same variables in an animal treated with methylene blue (MB) injections (4 mg/kg, vertical arrows). Note that MB allowed the maintenance of cardiac contractility and hemodynamics until the end of infusion.
Fig. 3.
Means ± SD values of LVEF, heart rate (HR), arterial blood pressure (ABP), and oxygen uptake (V̇o2) before (open bars) and at the end of the 4.5-min period of sulfide infusion (black bars) in control rats and animals treated with MB. Note that the animals receiving MB had a significant higher EF, ABP, and V̇o2 at the end of infusion (*P < 0.05). Although MB increased HR toward baseline levels, the difference in heart rate between treated and nontreated animals did not reach significance.
Fig. 4.
Various examples of the effects of H2S and MB on cardiac function (end-systolic and end-diastolic diameters) determined by echocardiography (TM in A) and in 2D (B and C) in the same rat. Note the decrease in contractility and heart rate produced by H2S (A). B: baseline recordings. C: development of a PEA at the end of sulfide infusion in the same rat. D: illustration of the average change in end-systolic and end-diastolic diameters in the three untreated rats exposed to H2S for 4.5 min. *P < 0.05.
The estimated concentration of gaseous H2S in the arterial blood, based on the determination on alveolar H2S, was null in baseline condition, reaching 7.09 ± 3.53 μM, 1–2 min into infusion, when cardiac contractility started to decrease, reaching 33.26 ± 6.43 μM by the end of the 4.5 min of infusion.
Effects of MB
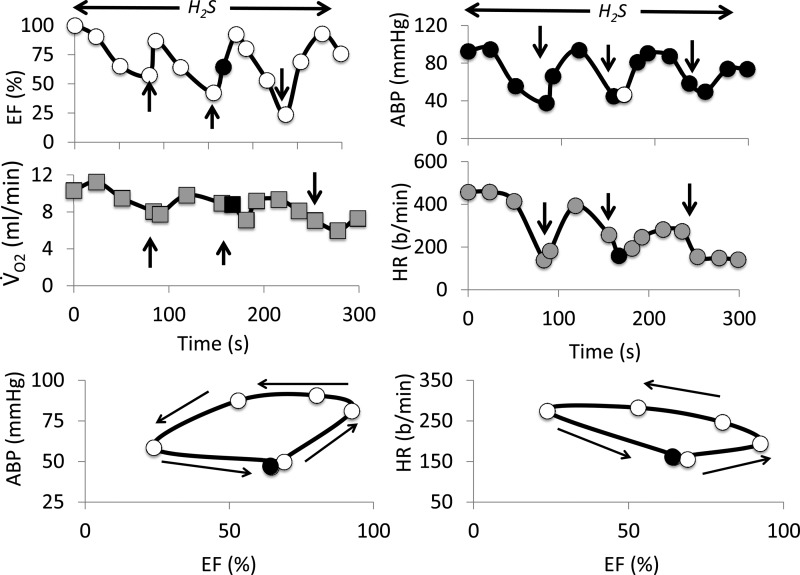
The three treated animals received MB as soon as EF dropped by about 20% (−17 ± 10%). A second injection of MB was administered 1 min later (Fig. 2). In one rat, an additional injection of MB was performed during the last minute of the sulfide infusion (Fig. 5). The response to H2S was strikingly affected by MB administration: EF increased immediately after MB bolus infusions (Figs. 2 and 5). Indeed, as illustrated in Fig. 5, the response to MB consisted in an immediate increase in LVEF (within 15 s), followed by an increase in BP and HR. The EF response was, however, of short duration, while BP and HR continued to rise as EF returned toward the preinjection values, resulting in a hysteresis of the EF-BP or EF-HR relationships (Fig. 5). By the end of the H2S perfusion, EF averaged 67 ± 20% in the MB-treated animals (2.5-fold increase, compared with untreated animals, P < 0.05), BP and V̇o2 were also significantly higher than in untreated animals (Fig. 3). None of animals treated with MB presented an episode of PEA, and EF returned to baseline less than 2 min after the cessation of perfusion.
Fig. 5.
Time course of the effects of three injections of MB on LVEF, V̇o2, arterial blood pressure (ABP), and heart rate (HR) in one rat receiving a continuous infusion of H2S (12 μmol/min). The two lower panels show the x-y relationship between EF and ABP and EF and heart rate. This figure exemplifies the rapid change in EF following MB injection (vertical arrows), while blood pressure and HR increased with delay and remained elevated for a longer period, responsible for a hysteresis of the RF-BP and EF-HR relationship. Because blood pressure relies on cardiac output, which in the rat depends on HR, as well as systolic volume and the changes in peripheral resistance, these x-y relationships illustrate that MB exerts its effects on hemodynamics not only via an increase in contractility, but also through its effects on HR and possibly on peripheral vascular resistance (see discussion for further details).
Effects of H2S Toxicity and MB on Cardiac Contractility in Spontaneously Breathing Unsedated Rats
All animals fell into coma within 1–2 min following the last intraperitoneal injection, after an average of two injections of H2S in each group. A reduction in cardiac contractility was observed as soon as the first echocardiography recording could be performed, i.e., 1 to 2 min after onset of coma. Three out of seventeen rats exhibited an immediate episode of either ventricular fibrillation or PEA, which occurred within less than 2 min after sulfide injection. Since no antidote or saline solution could be injected before cardiac arrest, these animals were excluded from the study. The fourteen remaining rats received MB (n = 6) or saline (n = 8). A total of 763 cardiac cycles were analyzed. In all animals, the depression in cardiac contractility took about 3 min to develop. Fig. 6 illustrates the change in EF, averaged every minute up to 10 min, in the animals receiving H2S and treated with saline (n = 8). The limit of 10 min was chosen as no more animals in the MB-treated group would die after this period (see below).
Fig. 6.
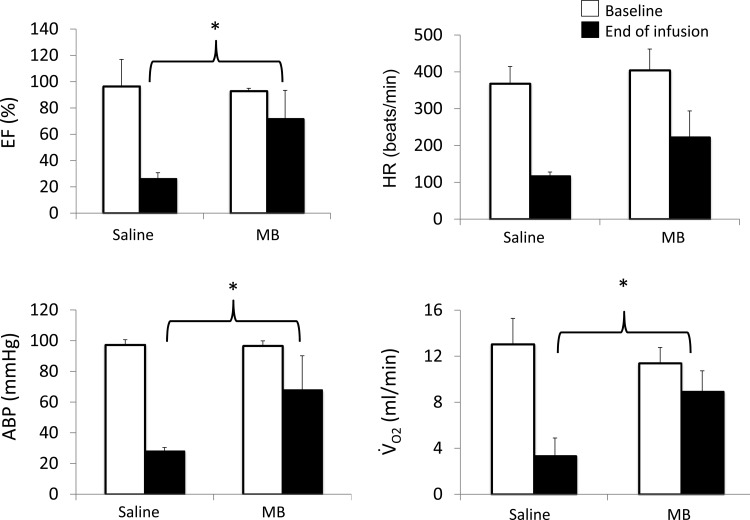
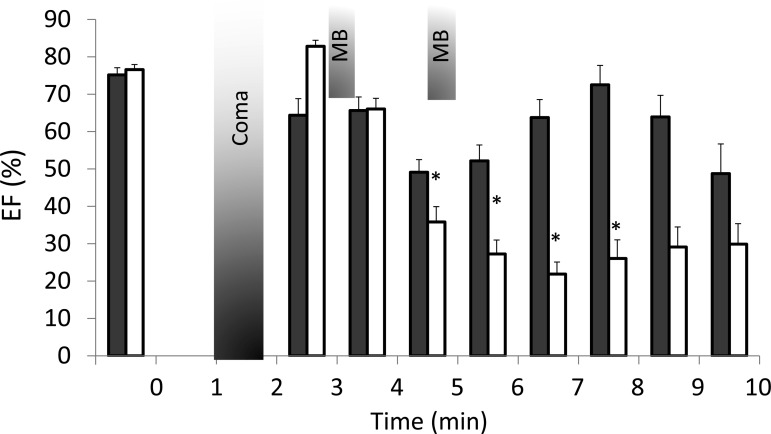
Time course of the changes in LVEF (means ± SE) in all the animals receiving an intraperitoneal injection of H2S. In all animals, EF decreased within 2 min following coma (gray bar), leading to asystole in five out of the eight nontreated animals (white bars) within less than 8 min. All of the rats that received MB (black bars), but one, increased and maintained their LVEF up to 8 min (*Significantly different from baseline, P < 0.05). None of MB rats died during this period, while three of treated rats died between 8 and 10 min.
Five out of these eight untreated rats exhibited a progressive and continuous drop in cardiac contractility resulting in a complete cessation of cardiac contractions, 3.9 to 6.8 min after the last intraperitoneal injection, i.e., all within 4 min after the onset of coma. Frequent episodes of premature ventricular contractions and ventricular tachycardia occurred while bradycardia was consistently observed, in addition to bundle branch blocks. Of note, cardiac depression was always associated with a continuous gasping breathing pattern, which was still present beyond the phase of cardiac arrest. One out of three remaining rats resumed normal breathing but displayed a persistent low ejection fraction, around 40%. In the two other rats the ejection fraction and breathing recovered at 7.1 and 7.4 min, respectively, followed by the awakening of the animal.
The six treated animals received a bolus injection of MB at 3 and 5 min after H2S administration. These animals had a different pattern of response compared with the control intoxicated rats. All of the treated rats displayed a transient increase in EF after the first MB injection (Fig. 7), followed by second decrease and a progressive recovery after the second injection (see Supplemental Videos S3 and S4). As a result, in contrast to the untreated animals, no treated animals died during the first 8 min following H2S injection (χ2-test, P < 0.05), while the EF was maintained (Fig. 6). Indeed, in all MB-treated animals but one, EF was above 67% at 8 min. Three animals eventually died, but one of them developed ventricular fibrillation and not PEA after 8 min. The three other rats survived with a normalization of end-systolic volume and EF.
Fig. 7.
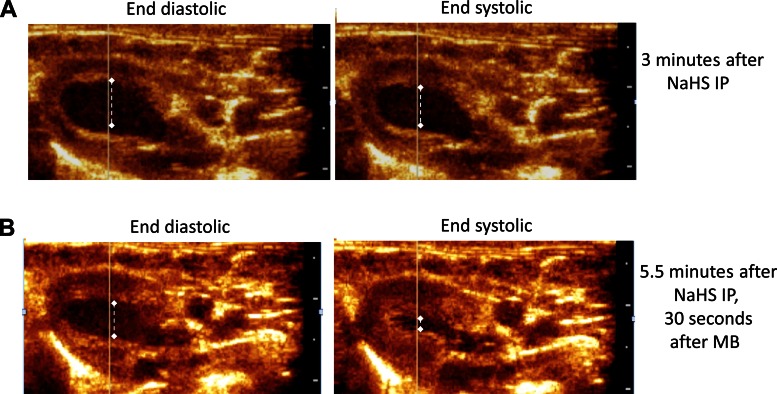
Example of a response produced by of MB (4 mg/kg) during H2S-induced coma. End-systolic and end-diastolic diameters were determined by echocardiography are shown. Note the decrease in contractility and heart rate produced by H2S (A), 3 min after the intraperitoneal injection of H2S, which was improved by an acute injection of MB (4 mg/kg) (B).
Effects of H2S Toxicity and MB on Isolated Cardiomyocytes
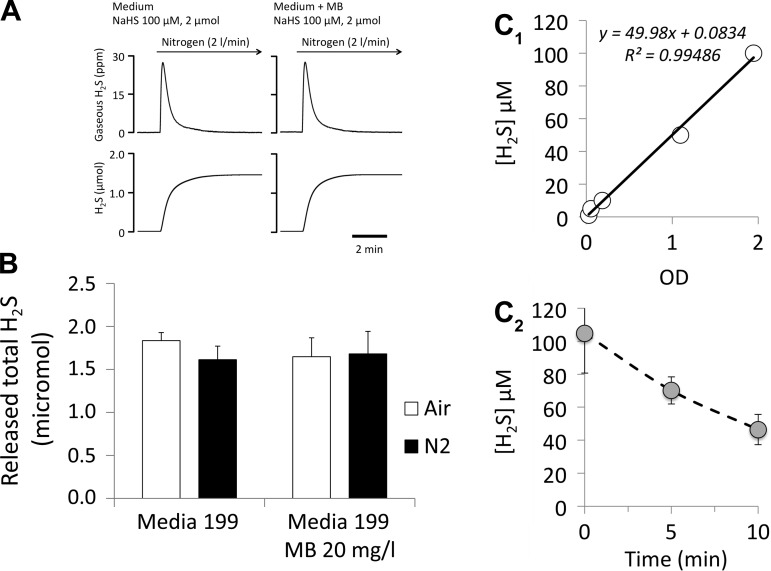
The incubation of H2S (100 μM) with MB (20 μg/ml) for 10 min did not alter the concentration of H2S dissolved in the media. Figure 8A illustrates the kinetics of H2S evaporation, with and without MB, in the media used for the cardiomyocyte experiments (Fig. 8A) after 10 min of incubation. Note that evaporation of H2S was determined while the head-space of the flask was ventilated at a rate of 2 l/min, and the solution was constantly agitated using a magnetic stir bar. Results were identical whether air or N2 was used (Fig. 8B). These data confirm the absence of direct interaction between MB and H2S in vitro.
Fig. 8.
A: example of the time course of H2S diffusion from a solution of H2S with and without MB in the media 199. H2S was recovered in air or nitrogen after incubating with MB for 10 min. B: quantity of H2S (average of three tests in each condition) recovered from diffusion, following 10 min of incubation of H2S with MB at a concentration of 20 mg/l for 10 min in air and N2. The initial quantity of sulfide in solution was 2 μmol (100 μM H2S dissolved in the media). Note that, in contrast to measurements of H2S in dishes (shown in C), H2S evaporation in A and B was determined, while the head-space of the flask was ventilated at a rate of 2 l/min, and the solution was constantly agitated using a magnetic stir bar. Results were identical whether air or N2 was used. These data confirm the absence of direct interaction between MB and H2S in vitro. C1: relationship between H2S concentration in the solution and the absorbance (OD) at 450 nm determined by spectrophotometry (see text). C2: mean ± SD concentrations of H2S (triplicate) in solution vs. time present in a dish, determined by the spectrophotometry method. Initial solution concentration was 100 μM (see methods for more details). H2S concentrations in our cell culture dish dropped by half within 10 min. In contrast to the measurement performed in the flask, the solution in the dish was not agitated and the interface gas-solution was not flushed with a gas.
In addition, we found that H2S concentration in cell culture dish dropped by half within 10 min (Fig. 8C). In contrast to the measurement in the flask, this determination was made while solution was neither agitated nor “ventilated” in an attempt to reproduce the experimental conditions of the studies performed on cardiac cells.
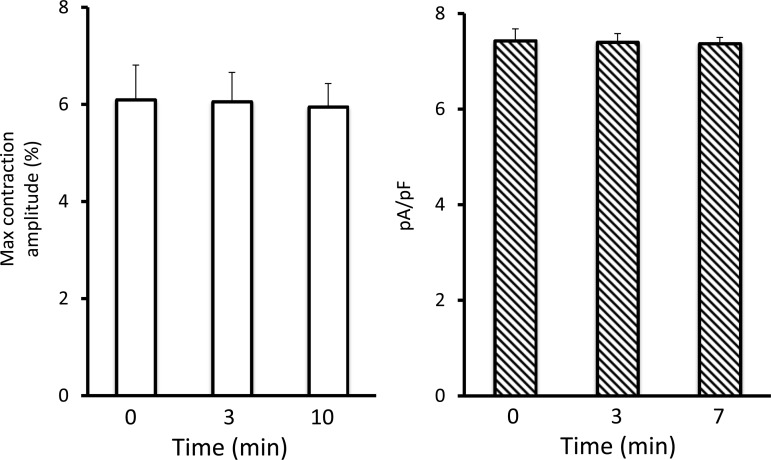
Figure 9 illustrates the reproducibility and stability over time of both myocyte contractility and ICa in cardiomyocytes from different glass coverslips exposed to saline following the modalities of study used in the actual protocol. The latter relies on data collection from three to five myocytes, randomly chosen in a blind manner, on each coverslip at each time point.
Fig. 9.
Left: time course of the contractility of cardiomyocytes (n = 7) (contraction amplitudes, % of resting cell length) studied at time 0, 3 min, and 10 min after exposure to saline. Right: time course of the change in ICa determined in 5, 3, and 4 cardiomyocytes at time points 0, 3, and 7 min, respectively, after exposure to saline. Data are shown as means ± SE. Note the stability of the preparation over the period of study.
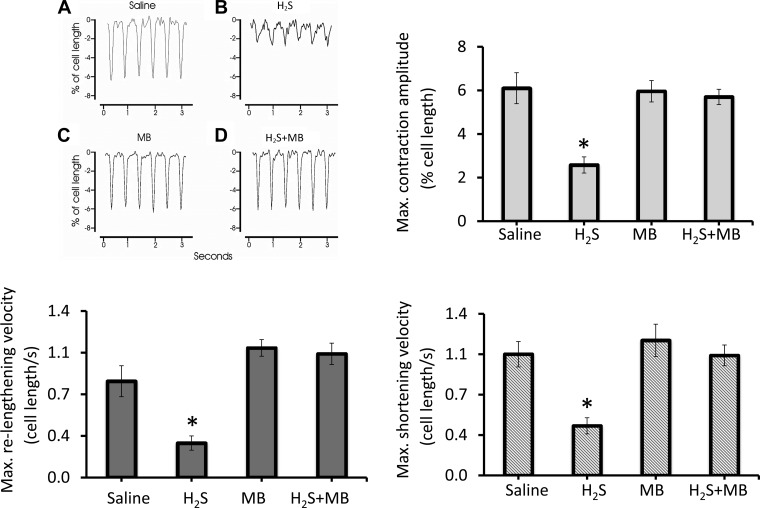
The contraction amplitude of single myocytes exposed for 10 min to H2S (n = 11) was found to be severely depressed compared with saline control (n = 7) (Fig. 10). MB (n = 10) alone had no effects on myocyte contractility but restored contraction amplitude in H2S-treated myocytes (n = 9) to normal (*P < 0.0009; H2S vs. other three groups). In addition to the decrease in contraction amplitudes, H2S significantly decreased the maximum shortening and relengthening velocities. MB restored all these alterations (Fig. 10).
Fig. 10.
MB rescued contractile dysfunction of cardiomyocytes exposed to H2S. Freshly isolated myocytes from mouse LV and septum were plated on laminin-coated coverslips, bathed in medium 199 ([Ca2+]o 1.8 mM) and paced (2 Hz) to contract (37°C) (see methods). Saline (A), H2S (B; 100 μM), MB (C; 20 μg/ml), or H2S + MB (D) were added at time 0, and contractions were measured at 10 min. Representative contraction traces of myocytes exposed to saline, H2S, MB, and H2S + MB are shown. Contraction amplitudes (% of resting cell length), maximum shortening, and relengthening velocities (cell lengths/s) are also shown. Seven myocytes were exposed to saline, 11 to H2S, 10 to MB, and 9 to H2S + MB. *Significantly different from saline, P < 0.05.
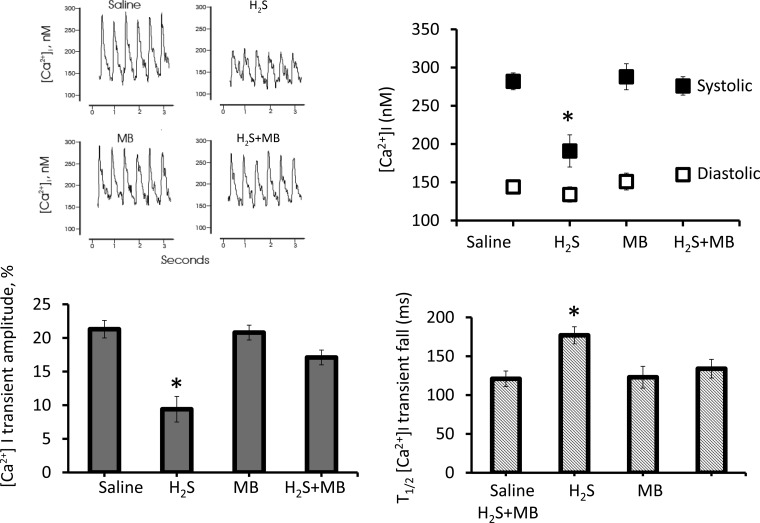
[Ca2+]i transient amplitudes were also severely depressed (P < 0.035) after 10 min of H2S exposure, compared with saline control (Fig. 11). MB prevented the decrease in [Ca2+]i transient amplitudes in H2S-treated myocytes. Systolic [Ca2+]i in H2S (n = 10) was significantly (P < 0.03) lower than saline (n = 10) or H2S+MB (n = 9) myocytes. Diastolic [Ca2+]i were not different among the groups. Importantly, the t1/2 of [Ca2+]i transient decline, a measure of sarcoplasmic reticulum (SR) Ca2+ uptake activity (104), was significantly prolonged in H2S compared with saline or H2S+MB myocytes (P < 0.03), indicating depressed SR Ca2+ uptake activity. MB alone (n = 8) did not have any effect.
Fig. 11.
Methylene blue restored [Ca2+]i dynamics in cardiomyocytes exposed to H2S toward normal. Fura-2-loaded (0.67 μM, 15 min, 37°C) myocytes in Medium 199 (1.8 mM [Ca2+]o, 37°C) were paced to contract (2 Hz). saline, H2S (100 μM), MB (20 μg/ml), or H2S + MB were added at time zero, and [Ca2+]i dynamics were measured at 10 min. Representative [Ca2+]i traces of myocytes exposed to saline, H2S, MB, and H2S + MB are shown. Summary of systolic and diastolic [Ca2+]i (nM), [Ca2+]i transient amplitudes (% increase of fura-2 signal), and half-time (t1/2) of [Ca2+]i transient decline (an estimate of sarcoplasmic reticulum Ca2+ uptake activity; ms) are also shown. Ten myocytes were exposed to saline, 10 to H2S, 8 to MB, and 9 to H2S + MB. Significantly different from saline, *P < 0.05.
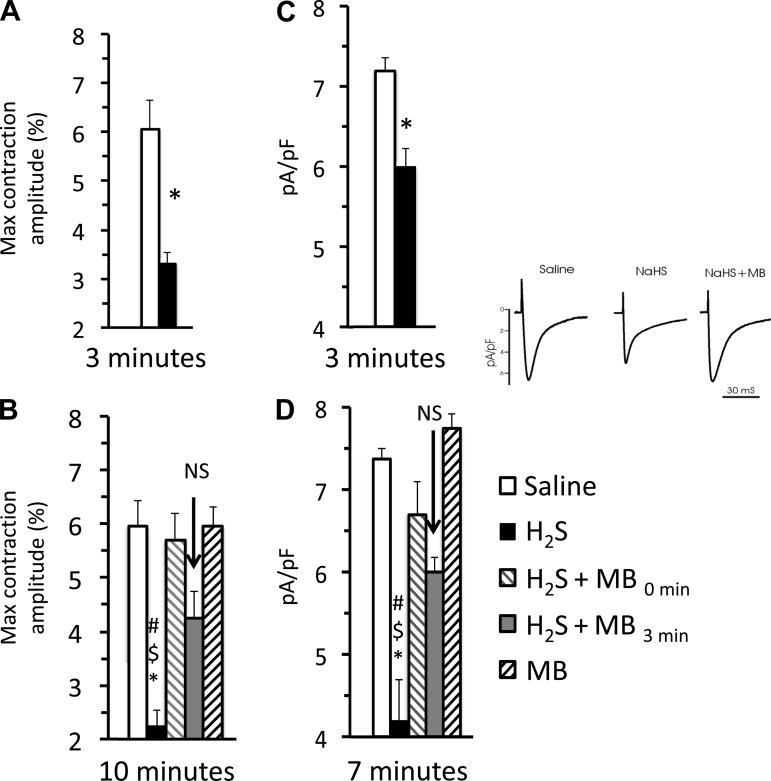
Finally, a pattern similar to that of myocyte shortening was observed with ICa (Fig. 12), which was significantly depressed by H2S (n = 6) compared with saline-treated myocytes (n = 11, P = 0.0009) or those receiving MB (P = 0.01). ICa was rescued by MB, added 3 min after H2S exposure (n = 8, P = 0.0089), while H2S+MB was not different from saline (n = 6, P = 0.1542) as illustrated on Fig. 12. Of note, as shown in Fig. 12, the reduced myocyte shortening was already well established 3 min after H2S exposure (n = 9), compared with saline (n = 7). In addition, adding MB (n = 8) 3 min after exposure to sulfide exposure was still efficacious in rescuing the depressed contractility compared with saline (n = 7). All these data were obtained in a group of myocytes different from those shown in Fig. 10.
Fig. 12.
A, left: effect of H2S on cardiac contractility determined 3 min after the onset of exposure. Contractility was already significantly depressed. B: at time 0, myocytes were exposed to saline, H2S (100 μM), H2S, and MB or MB alone (20 μg/ml), and then maximum contraction amplitude was determined at 10 min. In addition at 3 min, MB (20 μg/ml, gray bar) was added to the cells that were exposed to H2S solution at time zero, and the contractility was measured at 10 min (7 min after MB rescue). The number of myocytes analyzed was 7 for the saline exposure, 9 for H2S, 8 for H2S -MB, 10 for MB alone, and 9 H2S followed by MB at the 3rd min of exposure. Note that methylene blue was effective even when given after H2S toxicity was established. C, right: effect of H2S ICa 3 min after exposure to H2S (n = 5); ICa was already significantly depressed at 3 min. D: MB ameliorates the inhibition of L-type Ca2+ channel current (ICa) in adult myocytes exposed to H2S. ICa was measured in isolated adult cardiomyocytes under patch-clamp (holding potential −90 mV), in solutions that were Na+- and K+-free (see methods). To ensure steady-state sarcoplasmic reticulum Ca2+ load, 6 conditioning pulses (from −70 to 0 mV, 100 ms, 2 Hz) were delivered before the arrival of each test pulse (from −90 to +50 mV, 60 ms). The legend is the same as on the left panel except for the last measurements of ICa (−10 mV) that were made at 7 instead of 10 min. Six cells were exposed to H2S-saline, 6 cells were exposed to H2S + MB, two cells were exposed to MB alone, and 8 cells were exposed to H2S followed by MB 3 min later were studied. Significant different from saline, #P < 0.01. Significant different from H2S + MB, $P < 0.01. Significantly different from H2S followed by MB at the 3rd min of exposure, *P < 0.05. Inset: representative ICa traces (at −10 mV) from myocytes exposed to saline, H2S (100 μM) and H2S + MB (20 μg/ml) for 3 min.
DISCUSSION
This study extends our previous results on the effects of H2S toxicity on the heart (78, 79) and demonstrates that the phenothiazinium chromophore methylene blue is capable of reversing the acute cardiac depression produced by H2S toxicity in vivo by partially restoring cardiac contractility. Furthermore, we show that MB is capable of reversing H2S-induced L-type Ca2+ channel inhibition, thereby improving cardiomyocyte contractility in vitro. These results not only form the rational basis for the potential utilization of MB as part of the treatment of H2S intoxication, but they also raise intriguing questions about the potential interest of this family of compounds in other conditions, wherein ICa could be affected.
On the Use of Concentration of High Micromolar H2S in Solution to Study Isolated Cardiomyocytes
Before discussing our present findings, it is essential to clarify the frame of reference that we have been using to elaborate on the toxic effects of H2S and MB on the heart, in keeping with the most relevant literature on this topic (28, 81, 99, 101). Solutions containing H2S (prepared from NaHS) have been used in a large number of in vitro studies to try to clarify the effects of “endogenous” H2S [see for general review and discussion (21, 27, 46, 60, 61)]. This was also true for the studies looking at the interaction between H2S and cardiomyocytes (28, 81, 99, 101), where the primary objective was to identify the possible physiological functions of “endogenous” H2S on cardiac function. H2S in PBS solution is present, at a pH of about 7, under the form of HS− (2/3) and gaseous H2S (1/3) (2, 13, 23). The latter is responsible for H2S partial pressure that controls the diffusion of exogenous H2S from the solution into the cells. In other words, the amount of H2S capable of diffusing to the cells in vitro or in an isolated heart is directly related to the concentration of H2S in solution. The main difference with an in vivo study, wherein H2S would be infused (or inhaled), is that the majority of H2S diffusing into the blood is immediately oxidized or combined with the metalloproteins (hemoglobin). Only the remaining pool of “free”/soluble H2S/HS−, i.e., left after oxidation and trapping, can diffuse into the cells. This pool can be estimated in vivo by directly measuring the amount of H2S able to diffuse via an electrode or by evaporation from a blood sample (60). This free pool of sulfide is also directly measured by its rate of diffusion through the lung (42, 83, 98). Importantly, these measurements reveal that sulfide is virtually absent from the blood in baseline conditions and represents a very small proportion of H2S infused or inhaled (39, 42, 80).
Typically, whenever solutions of H2S at concentrations of 100 μM or above were used on an isolated heart or isolated cells, the effect was a measurable depression in cardiac contractility (28, 81, 99, 101). This depression is an obvious pathological and toxic change in the function at both the level of individual cells and the whole heart. The rationale for using these levels of H2S to predict physiological effects relies on initial reports wherein H2S was found to be in the high micromolar range in the blood, under conditions assumed to be endogenous H2S production (for discussion, see Ref. 27). Research by Furne et al. (27) and Levitt et al. (46) followed by work form Olson's group (95) has shown that these figures, i.e., high micromolar concentrations of H2S, are unrealistically high. Such concentrations do not represent a pool of free H2S (61) in the blood, which was found to be null in baseline conditions (27, 46). We have more recently tried to determine the pool of free H2S in vivo during H2S infusion, and while we could find no evidence for the presence of free H2S in baseline conditions at least in the micromolar range, it was clear that a concentration of H2S of only a few micromoles in the blood is associated with life-threatening symptoms (39, 42, 80). To be more specific, while in vitro, the activity of the mitochondrial cytochrome-c oxidase is abolished by a solution of H2S at concentrations of soluble/free H2S/HS− ranging from 10 to 30 μM (19, 44, 100), in vivo, a depression in respiratory medullary neurons (leading to a fatal apnea within minutes) and a severe depression in cardiac contractility (leading to a terminal asystole within seconds) can be produced in rodents and in large mammals by infusing or inhaling H2S at levels yielding blood concentrations of free H2S/HS− between 2 and 10 μM (39, 42, 80). In the present study, we found that EFs started to decrease for an average level of gaseous sulfide of 7.09 ± 3.53 μM. This corresponds to a total level of dissolved H2S of about 20 μM, assuming that one-third of total soluble H2S is present in gaseous form (2, 13, 23). Other pools of sulfide can be found in the blood, as well as in the tissues, including the heart, but they represent a “fossilized” compartment of sulfide, which requires extremely low pH or strong reducing agents to be released, conditions never met in vivo (for review, see Refs. 21, 27, 46, 60, 61). Finally, extensive necrosis of the cortical and subcortical neurons is observed in about 30% of animals surviving H2S-induced coma (78), which can develop after exposure to blood concentrations of free H2S in low micromolar range for few minutes only. Of note, we found that as long as a solution of 100 μM H2S, present in a dish, is not agitated and no gas is flowing on the solution/air interface, the concentrations of H2S still remained around 50 μM after 10 min.
In summary, we will be considering, throughout this discussion section, that the literature available of the effects of H2S on cardiomyocytes using concentrations of 50 μM and above is reflecting the effects of H2S toxicity. Incidentally, we are not aware of a single example, wherein such an exposure would not produce a significant depression in cardiac contractility and function.
H2S-Induced Cardiac Depression In Vivo
We have previously established that H2S infusion produces a marked decrease in cardiac output as soon as the concentration of dissolved H2S in the blood increases from zero (or inconsequential levels) to about 3–5 μM in the blood (80). We have also found that in urethane-anesthetized animals, H2S infusion at a rate of infusion of 10 μmol/min would lead to such concentrations of dissolved sulfide in the blood and produce a decrease in cardiac output by more than half within 2 min, with little vasodilatory effects (79). As mentioned in the above paragraph, we postulated that the vasodilatory effects reported in vitro at concentration of 50 μM and higher (see Ref. 5 for review) might be a late sign of H2S toxicity. We have also previously found that, in the rat and the sheep, H2S can lead to a complete electromechanical dissociation or PEA within minutes (79). The present study demonstrates and confirms that the decrease in cardiac output produced by H2S includes a rapid and profound depression in cardiac contractility. Various abnormal heart rhythms were also observed, including frequent premature ventricular contractions, bundle branch blocks, bradycardia and rare episodes of ventricular tachycardia or very rarely ventricular fibrillation. Severe signs of cardiac toxicity are always present during H2S-induced coma and were responsible for the immediate outcome of acute sulfide poisoning.
Of note, baseline EFs in our 400-g urethane-anesthetized rats were higher (>90%) than the initial measurements made under isoflurane anesthesia (72 ± 12%). The choice of urethane was dictated by our previous works on H2S intoxication, which were all performed using this anesthetic agent (15, 38, 78, 80), and its moderate depressive effect on cardiac function (25). Urethane also appears to preserve many of the physiological circulatory and respiratory reflexes (49–51). EFs above 90% have also been repeatedly found in 400–500-g rats anesthetized by a mixture of ketamine and xylazine (20) or by barbiturate (65).
Effects of MB on H2S-Induced Cardiac Depression In Vivo
In urethane anesthetized and mechanically ventilated rats, wherein the amount of H2S administered was precisely controlled, acute administration of methylene blue allowed ejection fraction to almost immediately increase. The rapidity of the effects of MB was striking (Fig. 5). The effect of MB was transient, but sufficient (after a second or a third injection) to allow the animals to survive an exposure that would otherwise be lethal. MB also corrected bundle branch block, while heart rate was stimulated contributing to improved blood pressure. Incidentally, the hysteresis of the EF-BP relationship, reflecting the return of EF toward pre-MB injection values faster than that of blood pressure, could be explained by the activation of the arterial baroreflex secondary to the burst increase in blood pressure or could be the result of an acute increase in afterload (Fig. 5). Indeed, the relationship displayed in Fig. 5 shows that MB exerts its effects on hemodynamics not only through changes in contractility but also via an increase in HR and possibly a rise in peripheral resistance explaining the prolonged increase in blood pressure after MB.
The nonsedated animal model produced a more severe intoxication than in the protocol that we previously used (78), wherein injections were repeated every 10 min, instead of 5 min. In addition, the echocardiography procedure, which requires one to apply the probe on the chest wall with some pressure, while the gasping rats were maintained in a (nonphysiological) supine position may have also contributed to the higher mortality of this model. Yet, we could establish that the H2S-induced coma was associated with a very rapid and primary reduction in the ejection fraction and that MB did counteract the effects of H2S. We observed in most animals a progressive recovery of EF delaying death by several minutes. In this model, the outcome of the H2S-induced coma appears to be dictated by the severity of the decrease in cardiac contractility, and the rare occurrence of episodes of ventricular fibrillation, as the animals continued to produce gasps even after complete asystole.
Effects of H2S on Isolated Contracting Cardiomyocytes
Both Zhang et al. (102) and Sun et al. (81) have reported that H2S alters the contractility of an isolated heart preparation or of isolated cardiac cells by inhibiting the activity of L-type Ca2+ channels. In all these studies, exogenous sulfide was shown to produce a measurable effect at a concentration of 100 μM and above. We also found that H2S at a concentration of 100 μM, led to a profound inhibition of cardiac cell contractility. This depression in contractility produced by H2S paralleled the changes in [Ca2+]i transient, including a severe reduction in SR Ca2+ reuptake. The depressed contractility and [Ca2+]i transient amplitudes produced by H2S were at least partly due to the inhibition of ICa. As ICa provides the trigger Ca2+ for SR Ca2+ release and also provides the necessary Ca2+ for steady-state SR Ca2+ loading, an impediment of ICa can account for producing both the observed in vivo and in vitro effects of H2S and explain the mechanical and conduction failure produced by H2S. In addition, the decreased SR Ca2+ uptake by sulfide could lead to a depletion in SR Ca2+ content, resulting in depressed [Ca2+]i transient amplitudes. We did not address the potential effects of H2S on SR Ca2+ leak in this study.
Zhang et al. (102) proposed that ICa inhibition by H2S could result from sulfuration (also referred as sulfhydration) of the free cysteine residues of proteins constituting L-type Ca2+ channels (57, 58). Indeed, the abundance of free cysteine residues on L-type Ca2+ (16, 30, 106) or RyR2 channels (24) may, in the presence of a high partial pressure of H2S, have yielded to such a phenomenon (57, 64), changing protein three-dimensional configuration and affecting, in turn, the function of these channels, akin to S-nitrosylation of proteins by NO. This hypothesis is supported by the observation that DTT, a very potent reducing agent, has “protective effects” against H2S-induced ICa inhibition, an effect that could have resulted from the cleavage of new disulfide bonds (102). This mechanism must, however, be reconciled with our observation that the reversibility of the effects of H2S on the heart, whether spontaneous (38, 42) or following MB, is extremely rapid (within tens of seconds).
Alternative mechanisms implicating L-type Ca2+ channel can be considered: it is indeed possible that the production of free radicals, due to the reduction of the rate of electron chain activity, a consequence of a partial inhibition of the cytochrome-c oxidase activity, could have affected the cysteine residues independently of the presence of H2S. The resulting fast changes in the oxido-reducing environment could rapidly switch off L-type Ca2+ channel activity (106).
Finally, other channels and pathways of interaction between H2S and Ca2+ transient in the heart could have been involved. For instance, the studies of Geng et al. (28) and Chen et al. (14) suggest that KATP channels, as well as PI3K/Akt and sarcoplasmic reticulum phospholamban pathways, are affected by H2S, at concentrations ranging from 100 to 800 μM. In addition, Yong et al. (99) found that H2S could negatively regulate cAMP pathway during activation of β-adrenergic receptor. These findings were not confirmed by the study of Sun et al. (81), who found no effect of H2S on the levels of cAMP in cardiomyocytes, in baseline conditions though; direct measurements of KATP current did not confirm the alteration of these channels by H2S. It should be pointed out that a direct measurement of KATP current (81) could make it difficult to demonstrate a dysfunction of this channel if it were produced by a decrease in cellular ATP. With respect to the cGMP pathway, Coletta et al. (18) have shown that exposure of endothelial cells to H2S increases intracellular cGMP. Whether this effect is also present in the heart remains to be clarified.
In summary, it is clear is that H2S in a toxic range decreases L-type Ca2+ channel activity and is responsible for a reduction in calcium transients, an effect opposed by MB. The contribution of other mechanisms controlling cardiac contractions remain to be clarified.
Mechanism of Action of Methylene Blue on Isolated Contracting Cardiomyocytes Intoxicated with H2S
MB is a cationic triheterocyclic redox compound with a central aromatic thiazine ring system, which confers on this agent a high lipophilicity and the ability to concentrate in the heart after systemic injection, where it accumulates (17, 29, 54). Although this study did not explore the fundamental mechanisms of interaction between MB and H2S on cardiac contractility, MB could have counteracted H2S-induced cardiac failure through two different types of pathways, which are not mutually exclusive: a pathway involving the L-type Ca2+ channels, as demonstrated in the present study, and a nondependent L-type Ca2+ channel pathway, whose modus operandi can, at this point, only be speculated and, thus, will be briefly discussed.
The interaction of MB and H2S at the level of L-type Ca2+ channels could rely on various properties of MB: leucomethylene blue (LMB) (17, 29, 54), readily produced by the reduction of MB in blood and cells, forms with MB a reversible oxidation-reduction system or electron donor-acceptor couple (29, 96) (see also Refs. 62 and 71 for a recent review). As already mentioned above, LMB has strong reducing properties, as evidenced from the very effective treatment of methemoglobinemia by MB (17). MB could have rapidly allowed L-type Ca2+ channels to recover their original configuration by cleaving disulfide bonds created by the presence of H2S/HS−, akin to the effects of DTT (102). Alternatively, if bursts of reactive oxygen species, produced as the mitochondrial electron chain activity is impeded by H2S (9, 10), have altered major components of [Ca2+]i regulation, including L-type Ca2+ channels and RyR2, LMB could well have restored the redox-mediated alteration of the activity of these ion channels. Of note, the potent reducing properties of LMB at low doses have also been suggested to protect selective regions of the brain, wherein memory is encoded and processed (71), and these combined effects of MB on mitochondrial function and as an antioxidant (62) have been proposed to account for the preservation and restoration of memory loss or cognitive deficit in various models of brain dysfunction-induced amnesia (71). Recent studies also suggested a reduction by MB of postanoxic brain injury (52, 55, 56, 96, 97) through a similar mechanism.
It is also possible that MB may have exerted protective effects on ICa without directly interacting with L-type Ca2+ channels. MB, at low doses, exerts a potent anti-nitric oxide effect (32), as it inhibits the soluble guanylyl cyclase (sGC), leading to a decrease in cGMP (29, 97), i.e., the second messenger used by NO to transduce its cellular effects (53, 59, 96). This antiguanylyl cyclase property could result in an increase Ca2+ transient, counteracting the effects of H2S. The alteration in L-type Ca2+ channels that we observed could, therefore, have resulted from the positive interaction between NO and H2S-induced toxicity (1, 3, 18).
Finally, LMB/MB couple has been suggested to support the transfer of protons through the mitochondrial membrane against a concentration gradient, essential for the production of ATP (48, 72, 103). In addition, reduced MB has been shown to increase the cytochrome-c oxidase activity (11). These effects, which in our view remain very speculative, could have clearly antagonized the mechanisms of hydrogen sulfide toxicity (7, 8, 19, 41). Such an effect may also account for the remarkable protection of MB against the toxicity of sodium azide (12, 70), another poison of the mitochondrial activity.
Perspectives and Significance
The strategy to be used by first responders to treat sulfide poisoning must be developed in keeping with the metabolism and targets of H2S. The present study is offering a possible framework to understand and treat H2S-induced acute cardiac failure. MB counteracts the depressive effects of H2S on cardiac contractions, partly via a restoration of L-type Ca2+ channel activity. Although these effects are transient in vivo, injections of MB can be repeated and appears to remain effective. Since H2S spontaneously disappears very rapidly from the body, maintaining circulation homeostasis for several minutes could have a dramatic impact on both survival and long-term outcome.
The effects of MB on other cardiac dysfunctions related to a depression in L-type Ca2+ channel activity remain to be explored. Understanding the precise mechanism of H2S induced L-type Ca2+ channel inhibition and the reversal of this effect by MB has other clinically relevant consequences, as LMB/MB may well be able to restore cardiomyocyte contractility and L-type Ca2+ channel activity in conditions other than sulfide intoxication. Indeed, L-type Ca2+ channel activity can be inhibited in the context of any oxidative stress (106), triggered by an anoxic or ischemic insult or during sepsis. Of note, the potential interest of MB has already been investigated in postischemic conditions (52, 55, 56, 96, 97) or in septic shock (22, 63, 67), showing intriguing protective effects of MB, at least in preclinical studies.
GRANTS
This work has been supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number 1R21NS090017-01 and National Heart, Lung, and Blood Institute RO1-HL74854 and RO1-HL123093 and the National Center for Research Resources and the National Center for Advancing Translational Science, National Institute of Health, through Grant UL1TR000127.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.-H., J.Y.C., and P.H. conception and design of research; A.J.-H., X.-Q.Z., T.S., J.S., J.W., N.T., and P.H. performed experiments; A.J.-H., X.-Q.Z., T.S., J.S., J.W., N.T., J.Y.C., and P.H. analyzed data; A.J.-H., X.-Q.Z., T.S., J.S., M.D.R., J.W., J.Y.C., and P.H. interpreted results of experiments; A.J.-H., X.-Q.Z., T.S., J.S., J.W., J.Y.C., and P.H. prepared figures; A.J.-H., J.Y.C., and P.H. drafted manuscript; A.J.-H., T.S., M.D.R., N.T., J.Y.C., and P.H. edited and revised manuscript; A.J.-H., X.-Q.Z., T.S., J.S., M.D.R., J.W., N.T., J.Y.C., and P.H. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625–634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almgren T, Dyrssen D, Elgquist B, Johannsson O. Dissociation of hydrogen sulfide in seawater and comparison of pH scales. Marine Chem 4: 289–297 1976. [Google Scholar]
- 3.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med 17: 879–888, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp RO Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol 13: 25–97, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life 57: 603–606, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bott E, Dodd M. Suicide by hydrogen sulfide inhalation. Am J Forensic Med Pathol 34: 23–25, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Bouillaud F, Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid Redox Signal 15: 379–391, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Buckler KJ. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflügers Arch 463: 743–754, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med 25: 17–26, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222–230, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav 77: 175–181, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Callaway NL, Riha PD, Wrubel KM, McCollum D, Gonzalez-Lima F. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosci Lett 332: 83–86, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Carroll JJ, Mather AE. The solubility of hydrogen sulfide in water from 0 to 90°C and pressures to 1 MPa. Geochim Cosmochim Acta 53: 1163–1170, 1989. [Google Scholar]
- 14.Chen Y, Zhao J, Du J, Xu G, Tang C, Geng B. Hydrogen sulfide regulates cardiac sarcoplasmic reticulum Ca2+ uptake via K(ATP) channel and PI3K/Akt pathway. Life Sci 91: 271–278, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Chenuel B, Sonobe T, Haouzi P. Effects of infusion of human methemoglobin solution following hydrogen sulfide poisoning. Clin Toxicol (Phila) 53: 93–101, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhary G, Dudley SC Jr. Heart failure, oxidative stress, and ion channel modulation. Congest Heart Fail 8: 148–155, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Clifton J, 2nd, Leikin JB. Methylene blue. Am J Ther 10: 289–291, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 109: 9161–9166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40: 533–539, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Darbandi Azar A, Tavakoli F, Moladoust H, Zare A, Sadeghpour A. Echocardiographic evaluation of cardiac function in ischemic rats: value of m-mode echocardiography. Res Cardiovasc Med 3: e22941, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deleon ER, Stoy GF, Olson KR. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem 421: 203–207, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Donati A, Conti G, Loggi S, Munch C, Coltrinari R, Pelaia P, Pietropaoli P, Preiser JC. Does methylene blue administration to septic shock patients affect vascular permeability and blood volume? Crit Care Med 30: 2271–2277, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Douabul AA, Riley JP. The solubility of gases in distilled water and seawater. V. Hydrogen sulphide. Deep Sea Res 26A: 259–268, 1979. 26A: 259–268 1979. [Google Scholar]
- 24.Dulhunty A, Haarmann C, Green D, Hart J. How many cysteine residues regulate ryanodine receptor channel activity? Antioxid Redox Signal 2: 27–34, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Field KJ, White WJ, Lang CM. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim 27: 258–269, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Fuller DC, Suruda AJ. Occupationally related hydrogen sulfide deaths in the United States from 1984 to 1994. J Occup Environ Med 42: 939–942, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1485, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313: 362–368, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Ginimuge PR, Jyothi SD. Methylene blue: revisited. J Anaesthesiol Clin Pharmacol 26: 517–520, 2010. [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez DR, Treuer A, Sun QA, Stamler JS, Hare JM. S-Nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol 54: 188–195, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayling M, Deakin CD. Methylene blue during cardiopulmonary bypass to treat refractory hypotension in septic endocarditis. J Thorac Cardiovasc Surg 125: 426–427, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Gruetter CA, Kadowitz PJ, Ignarro LJ. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol 59: 150–156, 1981. [DOI] [PubMed] [Google Scholar]
- 33.Guidotti TL. Hydrogen sulphide. Occup Med (Lond) 46: 367–371, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Hagihara A, Abe T, Omagari M, Motoi M, Nabeshima Y. The impact of newspaper reporting of hydrogen sulfide suicide on imitative suicide attempts in Japan. Soc Psychiatry Psychiatr Epidemiol 49: 221–229, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Haouzi P, Bell H, Van de Louw A. Hypoxia-induced arterial chemoreceptor stimulation and hydrogen sulfide: too much or too little? Respir Physiol Neurobiol 179: 97–102, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Haouzi P, Chenuel B, Sonobe T. High-dose hydroxocobalamin administered after H2S exposure counteracts sulfide-poisoning-induced cardiac depression in sheep. Clin Toxicol (Phila) 53: 28–36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haouzi P, Klingerman CM. Fate of intracellular H2S/HS(-) and metallo-proteins. Respir Physiol Neurobiol 188: 229–230, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In vivo interactions between cobalt or ferric compounds and the pools of sulphide in the blood during and after H2S poisoning. Toxicol Sci 141: 493–504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In vivo interactions between cobalt or ferric compounds and the pools of sulphide in the blood during and after H2S poisoning. Toxicol Sci 141: 493–504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haouzi P, Van de Louw A. Persistent reduced oxygen requirement following blood transfusion during recovery from hemorrhagic shock. Respir Physiol Neurobiol 215: 39–46, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, Lillie LE. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol 103: 482–490, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am J Physiol Regul Integr Comp Physiol 305: R630–R638, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kofidis T, Struber M, Wilhelmi M, Anssar M, Simon A, Harringer W, Haverich A. Reversal of severe vasoplegia with single-dose methylene blue after heart transplantation. J Thorac Cardiovasc Surg 122: 823–824, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta 1725: 201–212, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Levin RL, Degrange MA, Bruno GF, Del Mazo CD, Taborda DJ, Griotti JJ, Boullon FJ. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg 77: 496–499, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal 15: 373–378, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Leyh RG, Kofidis T, Struber M, Fischer S, Knobloch K, Wachsmann B, Hagl C, Simon AR, Haverich A. Methylene blue: the drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg 125: 1426–1431, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Lindahl PE, Oberg KE. The effect of rotenone on respiration and its point of attack. Exp Cell Res 23: 228–237, 1961. [DOI] [PubMed] [Google Scholar]
- 49.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia 42: 109–114, 1986. [DOI] [PubMed] [Google Scholar]
- 50.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: Cardiovascular system. Experientia 42: 292–297, 1986. [DOI] [PubMed] [Google Scholar]
- 51.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia 42: 531–537, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Martijn C, Wiklund L. Effect of methylene blue on the genomic response to reperfusion injury induced by cardiac arrest and cardiopulmonary resuscitation in porcine brain. BMC Med Genomics 3: 27, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther 232: 708–716, 1985. [PubMed] [Google Scholar]
- 54.Mayer B, Brunner F, Schmidt K. Novel actions of methylene blue. Eur Heart J 14 Suppl I: 22–26, 1993. [PubMed] [Google Scholar]
- 55.Miclescu A, Basu S, Wiklund L. Cardio-cerebral and metabolic effects of methylene blue in hypertonic sodium lactate during experimental cardiopulmonary resuscitation. Resuscitation 75: 88–97, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Miclescu A, Basu S, Wiklund L. Methylene blue added to a hypertonic-hyperoncotic solution increases short-term survival in experimental cardiac arrest. Crit Care Med 34: 2806–2813, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olgart C, Wiklund NP, Gustafsson LE. Blockade of nitric oxide-evoked smooth muscle contractions by an inhibitor of guanylyl cyclase. Neuroreport 8: 3355–3358, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal 17: 32–44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson KR. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am J Physiol Regul Integr Comp Physiol 301: R297–R312, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of methylene blue in the nervous system. Med Res Rev 31: 93–117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paciullo CA, McMahon Horner D, Hatton KW, Flynn JD. Methylene blue for the treatment of septic shock. Pharmacotherapy 30: 702–715, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Pawlush DG, Moore RL, Musch TI, Davidson WR Jr. Echocardiographic evaluation of size, function, and mass of normal and hypertrophied rat ventricles. J Appl Physiol 74: 2598–2605, 1993. [DOI] [PubMed] [Google Scholar]
- 66.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA 107: 10,719–10,724, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Preiser JC, Lejeune P, Roman A, Carlier E, De Backer D, Leeman M, Kahn RJ, Vincent JL. Methylene blue administration in septic shock: a clinical trial. Crit Care Med 23: 259–264, 1995. [DOI] [PubMed] [Google Scholar]
- 68.Reedy SJ, Schwartz MD, Morgan BW. Suicide fads: frequency and characteristics of hydrogen sulfide suicides in the United States. West J Emerg Med 12: 300–304, 2011. [PMC free article] [PubMed] [Google Scholar]
- 69.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32: 109–134, 1992. [DOI] [PubMed] [Google Scholar]
- 70.Riha PD, Rojas JC, Gonzalez-Lima F. Beneficial network effects of methylene blue in an amnestic model. Neuroimage 54: 2623–2634, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol 96: 32–45, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scott A, Hunter FE Jr. Support of thyroxine-induced swelling of liver mitochondria by generation of high-energy intermediates at any one of three sites in electron transport. J Biol Chem 241: 1060–1066, 1966. [PubMed] [Google Scholar]
- 73.Siegel LM. A direct microdetermination for sulfide. Anal Biochem 11: 126–132, 1965. [DOI] [PubMed] [Google Scholar]
- 74.Smith RP. Cobalt salts: effects in cyanide and sulfide poisoning and on methemoglobinemia. Toxicol Appl Pharmacol 15: 505–516, 1969. [DOI] [PubMed] [Google Scholar]
- 75.Smith RP, Gosselin RE. Current concepts about the treatment of selected poisonings: nitrite, cyanide, sulfide, barium, and quinidine. Annu Rev Pharmacol Toxicol 16: 189–199, 1976. [DOI] [PubMed] [Google Scholar]
- 76.Song J, Gao E, Wang J, Zhang XQ, Chan TO, Koch WJ, Shang X, Joseph JI, Peterson BZ, Feldman AM, Cheung JY. Constitutive overexpression of phospholemman S68E mutant results in arrhythmias, early mortality and heart failure: Potenial involvement of Na+/Ca2+ exchanger. Am J Physiol Heart Circ Physiol 302: H770–H781, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song J, Zhang XQ, Wang J, Cheskis E, Chan TO, Feldman AM, Tucker AL, Cheung JY. Regulation of cardiac myocyte contractility by phospholemman:Na+/Ca2+ exchange vs. Na+-K+-ATPase. Am J Physiol Heart Circ Physiol 295: H1615–H1625, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sonobe T, Chenuel B, Cooper TK, Haouzi P. Immediate and long-term outcome of acute H2S intoxication induced coma in unanesthetized rats: effects of methylene blue. PLoS One 10: e0131340, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonobe T, Haouzi P. H2S induced coma and cardiogenic shock in the rat: Effects of phenothiazinium chromophores. Clin Toxicol (Phila) 53: 525–539, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sonobe T, Haouzi P. Sulfide intoxication-induced circulatory failure is mediated by a depression in cardiac contractility. Cardiovasc Toxicol 16: 67–78, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37: 7–11, 1976. [DOI] [PubMed] [Google Scholar]
- 83.Toombs CF, Insko MA, Wintner EA, Deckwerth TL, Usansky H, Jamil K, Goldstein B, Cooreman M, Szabo C. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br J Clin Pharmacol 69: 626–636, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Truong DH, Mihajlovic A, Gunness P, Hindmarsh W, O'Brien PJ. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B12a). Toxicology 242: 16–22, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Truscott A. Suicide fad threatens neighbours, rescuers. CMAJ 179: 312–313, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tucker AL, Song J, Zhang XQ, Wang J, Ahlers BA, Carl LL, Mounsey JP, Moorman JR, Rothblum LI, Cheung JY. Altered contractility and [Ca2+]i homeostasis in phospholemman-deficient murine myocytes: Role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol 291: H2199–H2209, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van de Louw A, Haouzi P. Ferric iron and cobalt (III) compounds to safely decrease hydrogen sulfide in the body? Antioxid Redox Signal 19: 510–516, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Van de Louw A, Haouzi P. Inhibitory effects of hyperoxia and methemoglobinemia on H2S induced ventilatory stimulation in the rat. Respir Physiol Neurobiol 181: 326–334, 2012. [DOI] [PubMed] [Google Scholar]
- 90.Van de Louw A, Haouzi P. Oxygen deficit and H2S in hemorrhagic shock in rats. Crit Care 16: R178, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, Chan TO, Zhang XQ, Gao E, Song J, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger transgene: Altered myocyte contractility, [Ca2+]i transients, SR Ca2+ contents and action potential duration. Am J Physiol Heart Circ Physiol 297: H590–H601, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J, Gao E, Chan TO, Zhang XQ, Song J, Shang X, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger does not aggravate myocardial dysfunction induced by transverse aortic constriction. J Card Fail 19: 60–70, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J, Gao E, Rabinowitz J, Song J, Zhang XQ, Koch WJ, Tucker AL, Chan TO, Feldman AM, Cheung JY. Regulation of in vivo cardiac contractility by phospholemman: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol 300: H859–H868, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Gao E, Song J, Zhang XQ, Li J, Koch WJ, Tucker AL, Philipson KD, Chan TO, Feldman AM, Cheung JY. Phospholemman and β-adrenergic stimulation in the heart. Am J Physiol Heart Circ Physiol 298: H807–H815, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol 294: R1930–R1937, 2008. [DOI] [PubMed] [Google Scholar]
- 96.Wiklund L, Basu S, Miclescu A, Wiklund P, Ronquist G, Sharma HS. Neuro- and cardioprotective effects of blockade of nitric oxide action by administration of methylene blue. Ann NY Acad Sci 1122: 231–244, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Wiklund L, Zoerner F, Semenas E, Miclescu A, Basu S, Sharma HS. Improved neuroprotective effect of methylene blue with hypothermia after porcine cardiac arrest. Acta Anaesthesiol Scand 57: 1073–1082, 2013. [DOI] [PubMed] [Google Scholar]
- 98.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol 160: 941–957, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yong QC, Pan TT, Hu LF, Bian JS. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J Mol Cell Cardiol 44: 701–710, 2008. [DOI] [PubMed] [Google Scholar]
- 100.Yong R, Searcy DG. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comp Biochem Physiol B Biochem Mol Biol 129: 129–137, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Zhang H, Guo C, Wu D, Zhang A, Gu T, Wang L, Wang C. Hydrogen sulfide inhibits the development of atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLos One 7: e41147, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang R, Sun Y, Tsai H, Tang C, Jin H, Du J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLos One 7: e37073, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, Rojas JC, Gonzalez-Lima F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox Res 9: 47–57, 2006. [DOI] [PubMed] [Google Scholar]
- 104.Zhang XQ, Ng YC, Moore RL, Musch TI, Cheung JY. In situ SR function in postinfarction myocytes. J Appl Physiol 87: 2143–2150, 1999. [DOI] [PubMed] [Google Scholar]
- 105.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000. [DOI] [PubMed] [Google Scholar]
- 106.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.