Abstract
Recent reports suggest that aerobic exercise may boost the hypertrophic response to short-term resistance training. This study explored the effects of an acute aerobic exercise bout on the transcriptional response to subsequent resistance exercise. Ten moderately trained men performed ∼45 min cycling on one leg followed by 4 × 7 maximal knee extensions for each leg, 15 min later. Thus, one limb performed aerobic and resistance exercise (AE + RE) while the opposing leg did resistance exercise only (RE). Biopsies were obtained from the vastus lateralis muscle of each leg 3 h after the resistance exercise bout. Using DNA microarray, we analyzed differences [≥1.5-fold, false discovery rate (FDR) ≤10%] in gene expression profiles for the two modes of exercise. There were 176 genes up (127)- or downregulated (49) by AE + RE compared with RE. Among the most significant differentially expressed genes were established markers for muscle growth and oxidative capacity, novel cytokines, transcription factors, and micro-RNAs (miRNAs). The most enriched functional categories were those linked to carbohydrate metabolism and transcriptional regulation. Upstream analysis revealed that vascular endothelial growth factor, cAMP-response element-binding protein, Tet methylcytosine dioxygenase, and mammalian target of rapamycin were regulators highly activated by AE + RE, whereas JnK, NF-κβ, MAPK, and several miRNAs were inhibited. Thus, aerobic exercise alters the skeletal muscle transcriptional signature of resistance exercise to initiate important gene programs promoting both myofiber growth and improved oxidative capacity. These results provide novel insight into human muscle adaptations to diverse exercise modes and offer the very first genomic basis explaining how aerobic exercise may augment, rather than compromise, muscle growth induced by resistance exercise.
Keywords: affymetrix, endurance, gene expression, microarray, strength
it is often put forth that aerobic endurance-type exercise (e.g., running and cycling) interferes with muscle adaptations to strength/resistance exercise executed as part of the same training program (11, 12). Conversely, we have reported that concurrent aerobic exercise may in fact boost skeletal muscle hypertrophic response to resistance exercise, accompanied by molecular signaling responses that could favor increased net protein turnover (25, 27). At the same time, the idea that aerobic exercise alone, particularly cycle training, may produce hypertrophy has been given more attention (17, 32). These reports are supported by studies showing that low-load exercise may produce muscle growth and increased strength (17, 31). However, the mechanisms explaining how aerobic exercise could act synergistically with resistance exercise facilitating muscle growth remain largely unexplored.
Any change in muscle mass arises from an altered balance between protein synthesis and breakdown, favoring either muscle hypertrophy or atrophy (19). However, the precise molecular networks underpinning altered protein balance and changes in muscle mass in response to diverse exercise modes such as aerobic and resistance exercise are not completely understood. Based on acute measurements, we (24) and others (7) reported that concurrent exercise may produce a more “anabolic” molecular and protein synthetic response compared with resistance exercise alone. Indeed, muscle hypertrophy is typically accompanied by mammalian target of rapamycin (mTOR) activation and increased muscle protein synthesis (29), yet these markers appear to have minute predictive value to explain large individual variations in exercise-induced hypertrophy (8, 30). Thus, the reductionistic “single marker” approach from acute studies remains somewhat unsatisfactory to explain mechanisms governing muscle adaptations to chronic exercise.
As newly introduced high-throughput techniques allow for in-depth analysis of the mRNA responses to exercise, the transcriptional regulation of muscle hypertrophy has received increased attention lately. In particular, microarray analysis makes it possible to simultaneously assess a large number of muscle transcripts involved in muscle regenerative processes in response to acute and chronic exercise (1, 35, 38). Studies have shown that young individuals and/or high responders to resistance exercise may show a different signature with regard to myogenic growth transcripts, micro-RNAs (miRNAs), and key transcription factors than old individuals and/or low responders (1, 16). Moreover, while it appears that several hundred genes covary with gains in lean mass, there are apparent distinctions between acute and chronic exercise, as well as between aerobic- and resistance-type exercise (33). To date, no study has examined muscle gene expression profile to gain insight about the molecular regulation responsible for adaptations to combined aerobic and resistance exercise.
In an attempt to reveal the molecular basis of why concurrent aerobic and resistance exercise may produce different phenotypic adaptations (e.g., greater hypertrophy) than resistance exercise alone, we examined subjects who had undertaken 5 wk of resistance exercise with (AE + RE) or without (RE) concurrent aerobic exercise. The specific aim was to analyze the acute transcriptome response to a novel exercise bout of either AE + RE or RE. Gene expression profiles were assessed by means of microarray analysis. Because 5 wk of training using the current AE + RE regimen produced substantially greater muscle hypertrophy than RE (27), we hypothesized that an acute AE + RE bout would elicit a gene signature indicative of greater muscle mass accretion compared with RE.
METHODS
Experimental design.
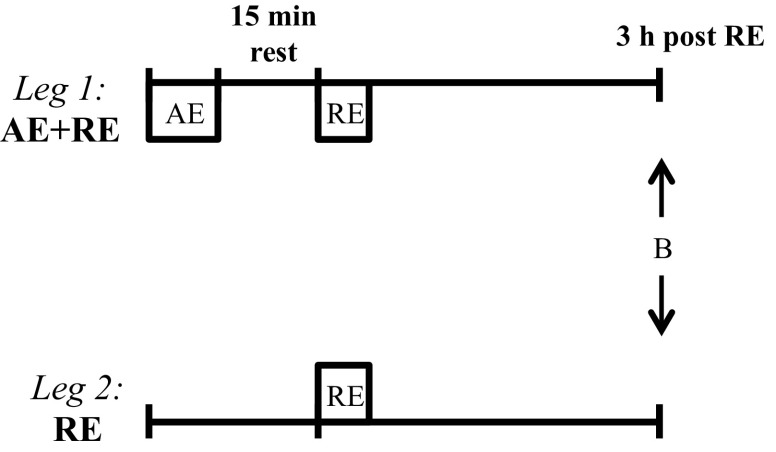
After familiarization with the protocols and exercise equipment, 10 participants performed an acute exercise bout of knee extensor AE + RE for one leg and RE only for the contralateral limb (Fig. 1). The leg assigned to AE + RE was randomized in a counterbalanced manner. A DNA microarray analysis was performed in biopsies obtained from vastus lateralis muscle of both legs 3 h after the resistance exercise bout. Subsequently, and as we have reported previously (27), the subjects completed a 5-wk training program using these identical exercise modes (AE + RE vs. RE).
Fig. 1.
Schematics of the acute exercise experiment. Muscle biopsies from each leg (B) were obtained 3 h after an acute bout of resistance exercise with [aerobic exercise + resistance exercise (AE + RE)] or without (RE) preceding aerobic exercise.
Subjects.
Ten men (26 ± 5 yr, 183 ± 7 cm, and 77 ± 9 kg) volunteered for the study. Subjects were moderately trained college students performing recreational exercise 2–3 days/wk (e.g., running and team sports). At the time of the study, they had not experienced any lower-limb injury for the last 6 mo or performed structured/intense resistance training in the past year. After being informed about study procedures, risks, and discomforts, subjects gave their written informed consent to participate. The study experiments were approved by the Regional Ethical Review Board in Stockholm.
Exercise experiments.
Exercise equipment, protocols, and their efficacy in stimulating robust muscle adaptations to 5 wk training have been described in detail elsewhere (25, 27). After a standardized warm-up, the aerobic exercise bout was performed in the morning of the experimental day (∼8:00 A.M.). It consisted of 40 min isolated and dynamic knee extensions on a modified one-legged ergometer (model 828E; Monark Exercise, Varberg, Sweden) at ∼70% of maximal workload (previously determined by a maximal incremental test) with a cadence of 60 revolutions/min. Rate of perceived exertion was assessed regularly to confirm strenuous effort. Upon completion of the 40 min, the workload was increased (∼20 W) until subjects failed to maintain target cadence (∼2–4 min). After 15 min recovery, resistance exercise for both legs (one leg at a time) was carried out. Four sets of seven maximal repetitions were performed on a knee extension isoinertial flywheel ergometer [YoYo Technology, Stockholm, Sweden (37)] with 2 min rest between sets. This exercise device uses the inertia of a spinning flywheel (inertia, 0.11 kg/m2) to offer unlimited resistance during coupled concentric and eccentric muscle actions. The range of motion was from 90° knee flexion to almost full extension (i.e., 180°). Power was measured in all repetitions. During all tests, subjects received strong verbal encouragement from research personnel.
Muscle biopsies and diet control.
Three hours after completion of the resistance exercise bout, biopsies were obtained from vastus lateralis muscle of both legs. Under local anesthesia, a 5-mm Bergström needle with suction was employed to obtain muscle tissue samples (2). After excess blood, connective tissue, and/or fat had been removed, the tissue was frozen in liquid nitrogen and stored at −80°C until further analysis. Subjects received a standardized dinner the night before the experiments (pasta, tomato sauce, and juice; 2.21 g carbohydrates/kg body wt, 0.22 g protein/kg body wt, and 0.04 g fat/kg body wt). In addition, standardized breakfast 2 h before the aerobic exercise bout was provided to participants (1.01 g carbohydrates/kg body wt, 0.31 g protein/kg body wt, and 0.24 g fat/kg body wt; Ensure Plus, Abbott Laboratories, Maidenhead, UK). Subjects then remained fasted during the 3-h resting time before biopsy collection.
RNA extraction and microarray analysis.
Total RNA was extracted from ∼20-mg wet muscle samples using a bead beater device (BioSpec Products, Bartlesville, OK) and TRIzol (Invitrogen Life Technologies, Carlsbad, CA). Total RNA was then purified using the PureYield RNA Midiprep System (Promega, Madison, WI). Each of the 20 samples was subjected to analysis on the Affymetrix HuGene-2.1-st platform. Hybridization, washing, staining, and scanning of the arrays were performed according to the manufacturer's instructions (Affymetrix). To control the quality, in addition to the standard quality assessments including scaling factors and chip housekeeper 5′-to-3′ ratios, all individual arrays were examined using hierarchical clustering and Normalized Unscaled Standard Error (NUSE, a variance-based metric to identify outliers before statistical analysis). All chips passed these quality control steps. The probe-set level intensities for the arrays were normalized using the Robust Multi-Array Analysis method (RMA) implemented within the R statistical software environment using the “Oligo” package (Bioconductor project). Raw data and RMA-normalized expression values are publicly available through Gene Expression Omnibus (GEO) accession no. GSE74194. Before differential expression analysis, probe sets without available annotation and probe sets displaying low variance across samples (interquartile range <0.5) were excluded from further analysis. Annotation was carried out in the R environment using “hugene21sttranscriptcluster.db” and the “annotation” and “genefilter” packages from Bioconductor. Differential expression was estimated through pairwise statistical analysis of microarrays (SAM) using 1,000 permutations on the R platform. Significantly up- and downregulated probe sets were analyzed for gene-ontology enrichment using the web-based Database for Annotation, Visualization, and Integrated Discovery (DAVID) version 6.7 (13, 14). Probe sets with a FDR of ≤10% and a fold change of ≥1.5-fold were analyzed for enrichment of “biological functions.” To correct for possible selection bias of genes, only probe sets used in the SAM analysis were used as background. Enriched ontologies with a FDR of ≤10% were categorized as significant. For further pathway analysis, we used the web-based bioinformatics tool Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com; Qiagen). IPA is a knowledge database generated from peer-reviewed scientific publications that enables discovery of biological networks in gene expression data and determines the biological functions most significant to those networks. Affymetrix transcript identifiers were uploaded onto IPA and were reannotated and queried against the verified IPA knowledge database, and probe sets analyzed in the SAM analysis were used as a reference set. Both up- and downregulated probe sets were considered, and interactions annotated as predicted with high confidence or experimentally validated were used in the IPA analysis. Enrichment of the focus genes in networks in IPA is assessed via Fisher's exact test. Furthermore, the software identifies top functions associated with each network via enrichment scores (z-score), highlighting the predicted biological significance of the results.
Validation of microarray.
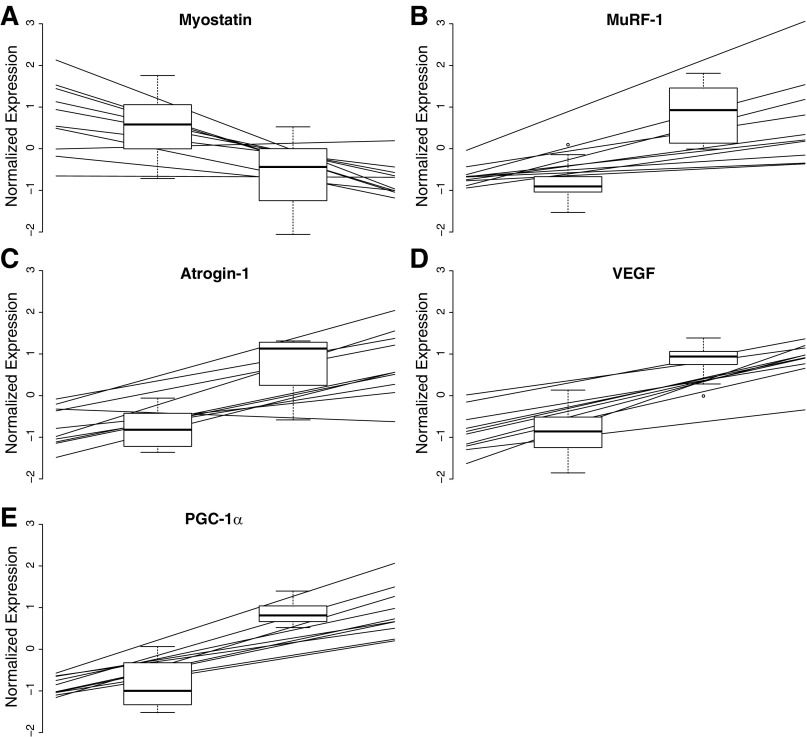
Real-time PCR for five selected mRNA targets was carried out on the same RNA extract as the array, using TaqMan probes and primers as previously reported (27). Target gene expression was reported as a ratio to reference genes (GAPDH and 18S) using the 2−ΔCT formula. Real-time PCR data were then scaled and plotted (Fig. 2) together with the corresponding normalized probe-set expression levels of the array (Probe-set identification: 16974830, 17009093, 16906471, 16683771, and 17080788).
Fig. 2.
Validation of the microarray and previously reported real-time PCR data. Lines represent individual real-time PCR observations, whereas the microarray data are depicted as box plots. These are plotted as median, first and fourth quartile, and range.
RESULTS
Exercise responses and functional measures.
During the acute resistance exercise bout, the leg that had completed aerobic exercise 15 min earlier produced 10% lower peak power than the rested RE leg. The end-point results of the 5-wk training program have been reported previously (27). In brief, after 5 wk training, gains in peak power (∼20%) were similar between AE + RE and RE. Likewise, the increase in peak torque did not differ between AE + RE (10%) and RE (12%). With regard to muscle size, AE + RE increased (P < 0.05) quadriceps muscle volume by 6% compared with 3% for RE. AE + RE, but not RE, increased endurance performance (22%) and citrate synthase activity (18%).
Gene expression analysis.
After annotation and filtering 11,869 probe sets were analyzed using pairwise SAM analysis. Of these probe sets, 49 were down- and 127 upregulated (≥1.5-fold) by AE + RE compared with RE, having a Q-value (corresponding to FDR) of ≤10% [see Supplemental Table 1, Differential Expression Analysis (The online version of this article contains supplemental data.)]. Analysis of “biological functions” significantly enriched among the upregulated genes using DAVID-identified seven ontologies, all of which can be defined as variations of carbohydrate metabolism (Table 1). In contrast, 11 biological functions were identified as enriched among the downregulated genes. Most notably were variations of “response to hormone stimulus,” “MAPKKK cascade,” “regulation of skeletal muscle tissue development,” and “angiogenesis” (Table 1). Some key downregulated genes found in several of these clusters were transcriptional and epigenetic regulators such as histone deacetylase and thioredoxin-interacting protein. The 25 most significantly differentially expressed genes (up- and downregulated by AE + RE compared with RE) are displayed in Table 2. Several of these genes are well-known markers involved in muscle adaptations to exercise [e.g., AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor-γ, coactivator-1α (PGC-1α), muscle-specific RING finger protein 1 (MuRF-1), myostatin]. Some cytokines (IL-18 and IL-311RA) and several miRNAs (mir-133, mir-1, mir-623) appeared among the downregulated genes. As indicated earlier, we compared five exercise-relevant differentially expressed genes [PGC-1α, vascular endothelial growth factor (VEGF), myostatin, MuRF-1, atrogin-1] from the array with qPCR data previously published from the same RNA (27). In both the microarray and the qPCR, the five genes were found to be significantly differentially expressed in the corresponding manner (Fig. 2).
Table 1.
GO categories related to biological processes induced by AE + RE compared with RE as generated by DAVID
| GO Term | Fold Enrichment | FDR, % |
|---|---|---|
| Upregulated processes | ||
| GO:0010906∼regulation of glucose metabolic process | 15.71 | 2.81 |
| GO:0010675∼regulation of cellular carbohydrate metabolic process | 14.89 | 3.30 |
| GO:0006109∼regulation of carbohydrate metabolic process | 14.89 | 3.30 |
| GO:0019318∼hexose metabolic process | 4.67 | 5.27 |
| GO:0015980∼energy derivation by oxidation of organic compounds | 7.69 | 5.59 |
| GO:0006006∼glucose metabolic process | 5.30 | 7.58 |
| GO:0005996∼monosaccharide metabolic process | 4.12 | 9.47 |
| Downregulated processes | ||
| GO:0007167∼enzyme linked receptor protein signaling pathway | 6.66 | 0.06 |
| GO:0010033∼response to organic substance | 4.08 | 0.32 |
| GO:0009725∼response to hormone stimulus | 5.43 | 0.76 |
| GO:0042127∼regulation of cell proliferation | 3.62 | 0.85 |
| GO:0009719∼response to endogenous stimulus | 4.94 | 1.32 |
| GO:0007169∼transmembrane receptor protein tyrosine kinase signaling pathway | 6.77 | 2.40 |
| GO:0001525∼angiogenesis | 7.61 | 5.43 |
| GO:0009266∼response to temperature stimulus | 12.18 | 5.72 |
| GO:0051789∼response to protein stimulus | 11.63 | 6.48 |
| GO:0000165∼MAPKKK cascade | 6.48 | 9.44 |
| GO:0048641∼regulation of skeletal muscle tissue development | 23.10 | 9.21 |
GO, gene ontology; AE + RE, aerobic and resistance exercise; RE, resistance exercise only; DAVID, Database for Annotation, Visualization, and Integrated Discovery; FDR, false discovery rate.
Table 2.
The 25 top up- and downregulated genes by AE + RE compared with RE out of 176 differentially expressed genes (FDR ≤10%)
| Fold Change | Gene Symbol | Entrez Gene Name |
|---|---|---|
| Top Genes Upregulated by AE + RE Ccompared with RE | ||
| 10.85 | PRKAG2 | Protein kinase, AMP-activated, γ2-noncatalytic subunit |
| 2.96 | TRIM63 | Tripartite motif containing 63, E3 ubiquitin protein ligase |
| 2.87 | KCNQ4 | Potassium channel, voltage-gated KQT-like subfamily Q, member 4 |
| 2.85 | RPS6KA2-IT1 | RPS6KA2 intronic transcript 1 |
| 2.75 | PPARGC1A | Peroxisome proliferator-activated receptor-γ, coactivator-1α |
| 2.61 | CHST15 | Carbohydrate (N-acetylgalactosamine 4-sulfate 6-O)sulfotransferase 15 |
| 2.61 | FAM102A | Family with sequence similarity 102, member A |
| 2.60 | SNAI3 | Snail family zinc finger 3 |
| 2.48 | MAFF | v-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog F |
| 2.39 | ESRRG | Estrogen-related receptor-γ |
| 2.37 | CES3 | Carboxylesterase 3 |
| 2.37 | SLC20A1 | Solute carrier family 20 (phosphate transporter), member 1 |
| 2.36 | NR4A3 | Nuclear receptor subfamily 4, group A, member 3 |
| 2.36 | PFKFB2 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 |
| 2.32 | GPR157 | G protein-coupled receptor 157 |
| 2.32 | SLC25A33 | Solute carrier family 25 (pyrimidine nucleotide carrier), member 33 |
| 2.26 | SLC38A1 | Solute carrier family 38, member 1 |
| 2.26 | SYNJ2 | Synaptojanin 2 |
| 2.25 | SLC16A6 | Solute carrier family 16, member 6 |
| 2.18 | PXDC1 | PX domain containing 1 |
| 2.16 | LOC101928327 | Uncharacterized LOC101928327 |
| 2.14 | CIART | Circadian-associated repressor of transcription |
| 2.11 | ANGPTL2 | Angiopoietin-like 2 |
| 2.08 | MIR3147 | micro-RNA 3147 |
| 2.05 | BHLHE40 | Basic helix-loop-helix family, member e40 |
| Top Genes Downregulated by AE + RE Compared with RE | ||
| 0.36 | OTUD1 | OTU deubiquitinase 1 |
| 0.41 | ATF3 | Activating transcription factor 3 |
| 0.42 | SERPINE1 | Serpin peptidase inhibitor, clade E, member 1 |
| 0.44 | IL18 | Interleukin-18 |
| 0.45 | IL31RA | Interleukin-31 receptor A |
| 0.47 | KCNQ5-IT1 | KCNQ5 intronic transcript 1 |
| 0.47 | GADD45A | Growth arrest and DNA-damage-inducible, α |
| 0.47 | ARRDC4 | Arrestin domain-containing 4 |
| 0.48 | NEXN-AS1 | NEXN antisense RNA 1 |
| 0.48 | CYR61 | Cysteine-rich, angiogenic inducer, 61 |
| 0.51 | BTG2 | BTG family, member 2 |
| 0.51 | IER5 | Immediate early response 5 |
| 0.52 | RUNX1-IT1 | RUNX1 intronic transcript 1 |
| 0.53 | mir-133 | Micro-RNA 133b |
| 0.54 | CTGF | Connective tissue growth factor |
| 0.54 | ENAH | Enabled homolog (Drosophila) |
| 0.54 | CA4 | Carbonic anhydrase IV |
| 0.55 | FOS | FBJ murine osteosarcoma viral oncogene homolog |
| 0.56 | PLAU | Plasminogen activator, urokinase |
| 0.56 | SPNS2 | Spinster homolog 2 (Drosophila) |
| 0.57 | PFKFB3 | 6-Phosphofructo 2-kinase/fructose-2,6-biphosphatase 3 |
| 0.57 | SLC8A3 | Solute carrier family 8 (sodium/calcium exchanger), member 3 |
| 0.57 | TIAM2 | T cell lymphoma invasion and metastasis 2 |
| 0.57 | ARID5A | AT-rich interactive domain 5A (MRF1-like) |
| 0.58 | MSTN | Myostatin |
Upstream analysis.
Ingenuity's Upstream Regulator Analysis in IPA is a tool that predicts upstream regulators from gene expression data based on the literature and compiled in the Ingenuity Knowledge Base. A Fisher's Exact Test P value was calculated to assess the significance of enrichment of the gene expression data for the genes downstream of an upstream regulator. In addition, directionality and magnitude of the differentially expressed genes was calculated to a z-score of directional consistency. A high positive z-score indicates activation for that pathway, whereas a negative z-score is associated with an inhibition by AE + RE compared with RE. The top-ranking regulatory molecules based solely on overlap (i.e., P value of Fisher's Exact test) were v-myc avian myelocytomatosis viral oncogene homolog and TNF-α (−log10 P value >10), followed by hepatocyte growth factor, IL-1β, and forkhead box O3. However, z-scores of all of these pathways were in the range of −0.6 to 0.2, illustrating that, while many genes in these pathways are differentially expressed, the directionality of the altered gene expression is inconsistent with an activation or inhibition of the pathway.
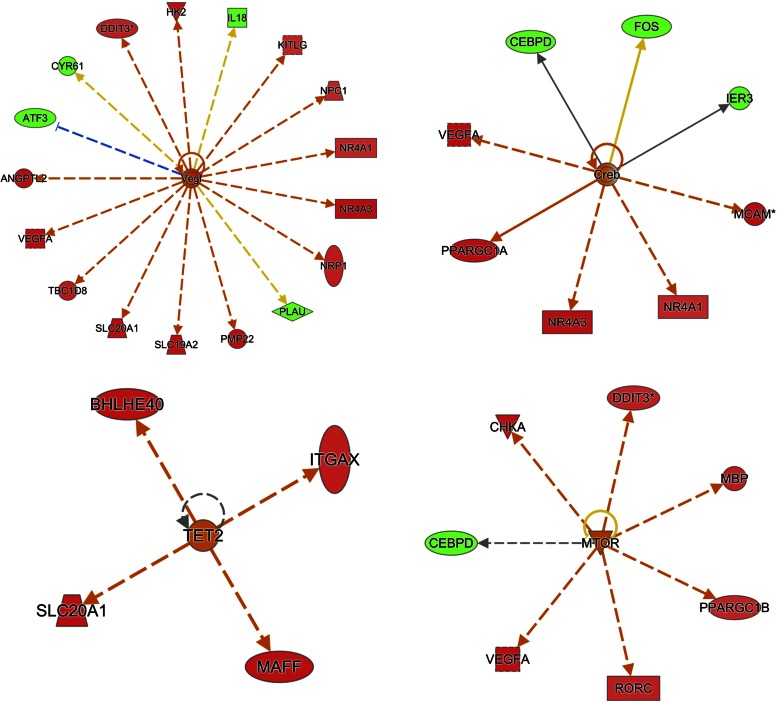
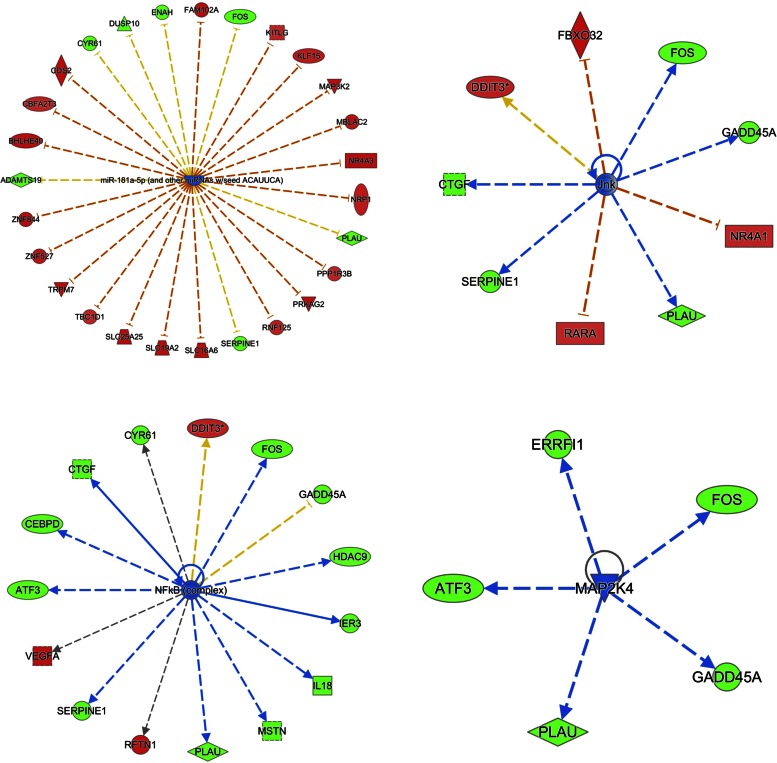
When considering the regulatory molecules with a gene expression pattern consistent with what is expected (i.e., z-score >2), the number one pathway predicted to be activated was VEGF (P value 3−7, z-score 2.7) and miR-181a, which was predicted to be inhibited (P value 1.7−7, z-score −2.6; see Figs. 3 and 4 and Table 3). The pathways predicted to be activated second to VEGF were cAMP-response element-binding protein [cAMP-response element-binding protein (CREB), P value 5.6−4, z-score 2.2], Tet methylcytosine dioxygenase (TET2, P value 6.9−4, z-score 2.0), and mTOR (P value 5.3−3, z-score 2.4). The second and third most inhibited regulators were JnK (P value 4.5−5, z-score −2.4) and NF-κβ followed by MAP2K4 and MAP3K8 (P value 7.8−5, z-score −2.5). See Figs. 3 and 4, Table 3, and Supplemental Table 2 (Upstream Analysis).
Fig. 3.
Upstream regulator analysis by ingenuity pathway analysis (IPA). Data illustrate the top-ranking pathways predicted as “activated” in upstream regulator analysis. Genes in red were greater expressed in AE + RE compared with RE, whereas genes in green were lower expressed. An orange line indicates predicted upregulation (by AE + RE), whereas a blue line indicates predicted downregulation. A yellow line indicates expression being contradictory to the prediction. Gray line indicates that direction of change is not predicted. Solid or broken edges indicate direct or indirect relationships, respectively.
Fig. 4.
Upstream regulator analysis by IPA. Data illustrate the top-ranking pathways predicted as “inhibited” in upstream regulator analysis. Genes in red were greater expressed in AE + RE compared with RE, whereas genes in green were lower expressed. An orange line indicates predicted upregulation (by AE + RE), whereas a blue line indicates predicted downregulation. A yellow line indicates expression being contradictory to the prediction. Gray line indicates that direction of change is not predicted. Solid or broken edges indicate direct or indirect relationships, respectively.
Table 3.
The top-ranked activated/inhibited central regulators in the IPA analysis
| Upstream Regulator | Predicted Activation State | Z-Score | P Value of Overlap |
|---|---|---|---|
| miR-181a-5p (and other miRNAs with seed ACAUUCA) | Inhibited | −2.57 | 0.000000167 |
| Vegf | Activated | 2.67 | 0.000000303 |
| Jnk | Inhibited | −2.35 | 0.0000447 |
| NF-κB (complex) | Inhibited | −2.52 | 0.0000775 |
| MAP2K4 | Inhibited | −2.19 | 0.000101 |
| MAP3K8 | Inhibited | −2.00 | 0.000107 |
| miR-141-3p (and other miRNAs with seed AACACUG) | Inhibited | −2.81 | 0.000341 |
| miR-291a-3p (and other miRNAs with seed AAGUGCU) | Inhibited | −3.25 | 0.000544 |
| Creb | Activated | 2.18 | 0.000561 |
| miR-17-5p (and other miRNAs with seed AAAGUGC) | Inhibited | −2.82 | 0.000633 |
| TET2 | Activated | 2.00 | 0.000685 |
| MTOR | Activated | 2.41 | 0.00525 |
DISCUSSION
This study provides the first comprehensive gene expression analysis comparing concurrent aerobic and resistance exercise (AE + RE) with resistance exercise alone (RE). The main finding was that an exhaustive aerobic exercise insult, performed before resistance exercise, augmented important gene signatures that regulate contrasting functions such as oxidative metabolism on one hand and myofiber growth and tissue regeneration on the other. Thus, we have characterized a molecular signature of concurrent exercise that could aid in explaining how aerobic exercise may boost, rather than compromise, muscle hypertrophy to chronic resistance training.
While we and others have reported on single molecular markers in response to concurrent aerobic and resistance exercise (7, 24, 27), neither study provided an in-depth transcriptional map of this exercise mode. We hypothesized that there must be substantial differences in the transcriptome response of an exercise regime (AE + RE) producing twice as robust hypertrophy as RE alone (27). Indeed, our results showed that 176 genes were differently expressed ≥1.5-fold when aerobic exercise preceded a bout of resistance exercise. This would suggest that these genes, and their associated targets and regulators, play important roles in eliciting phenotype differentiation depending on exercise stimulus imposed over 5 wk.
In an effort to explore the rather substantial difference in expression across exercise modes, we performed analysis for enrichment of gene ontologies related to biological functions. Perhaps not surprising, the most activated biological functions induced by AE + RE were processes involved in carbohydrate metabolism. Indeed, it has been shown that aerobic exercise promotes gene programs involved in glucose homeostasis and oxidative metabolism (28). Similarly, the gene showing the most significant difference in expression across exercise modes was AMPK, a well-known regulator of carbohydrate metabolism. Furthermore, PGC-1α was highly upregulated by AE + RE, confirming that this gene and its exercise-responsive splice variants are upregulated by both aerobic and resistance exercise, and more so by combined exercise (26, 42). The fact that AMPK and PGC-1α were prominent genes in this unbiased expression analysis further underlines their role in promoting an oxidative phenotype (20). It also fits with the impressive increase in endurance performance and CS activity reported by us following AE + RE training (27).
Among functions being downregulated by AE + RE compared with RE, it is worth noting that we observed genes involved in regulation of skeletal muscle tissue development. Genes clustered in this group have a negative effect on muscle hypertrophy, and one of the most downregulated genes was myostatin, an established negative regulator of hypertrophy (36). In addition, we found key genes involved in muscle protein breakdown to be upregulated, e.g., MuRF-1 and atrogin-1. This adds further credibility to the recent notion that these ubiquitin ligase proteins may be critical in the healthy skeletal muscle remodeling process, in fact facilitating contraction-induced muscle growth (15). Overall, the response of key genes and associated biological functions activated by AE + RE concord with the greater muscle mass accretion following the concurrent exercise regime.
To identify transcriptional regulators potentially explaining the observed gene expression differences between AE + RE and RE, we used IPA upstream regulator analysis. The analysis revealed that factors centralized around the key regulators VEGF, CREB, TET2, and mTOR were highly activated by AE + RE, whereas JnK, NF-κβ, MAPK, and several miRNAs were inhibited regulators. While mTOR is a growth inducer, its role in regulating muscle mass is not entirely unequivocal. Thus, recent research suggests high responders to resistance exercise display an inhibited mTOR activation signature (33) and that the muscle anabolic response to exercise is not solely dependent on mTOR activation (34). Nevertheless, mTOR aligns with muscle hypertrophy (29) and is deemed crucial for the hypertrophic response and for protection against atrophy (3). Because our first-hand physiological data would be hard to challenge (27), the demonstration of greater activation of mTOR following AE + RE than RE would be indicative of a greater protein synthetic response. Ultimately, this aids in explaining the more robust hypertrophic response to concurrent execise.
MAPK- and NF-κβ-associated signaling can be both adaptive and maladaptive in skeletal muscle depending on the conditions (18). However, given that exercise seems potent to increase MAPK and NF-κβ in the nondiseased state (18, 41), it strikes that MAPK/JnK and NF-κβ were identified as inhibited regulators in the current study. In support of this notion, among the top genes being downregulated by AE + RE, we found genes involved in myokine (e.g., IL-18, IL-31RA) and MAPK/ERK signaling (activating transcription factor 3). Thus, while activation of these genes in response to acute exercise appears to benefit muscle adaptations, chronic activation could very well exacerbate protein breakdown and hence muscle wasting (18). In light of this, the AE + RE-induced downregulation of these genes was perhaps due to a volume-related attenuation of MAPK signaling (22).
A less studied factor in human skeletal muscle remodeling is the transcriptional regulator CREB, which was significantly activated as an upstream regulator in the current analysis. It has been reported that high-intensity exercise activates CREB in mice, and the activation of this transcriptional complex induces anabolic changes driving muscle hypertrophy during subsequent recovery (4). Furthermore, overexpressing cells with CREB-regulated coregulators induced hypertrophy in transgenic mouse models and cultured myotubes (4). Our findings spur future studies to examine weather CREB and its associated targets play a key role in exercise-induced adaptations of human skeletal muscle.
miRNAs are accepted posttranscriptional regulators of protein translation that appear to block translation of transcribed genes in human skeletal muscle (9). Several miRNA routes were inhibited by AE + RE in the upstream regulator analysis. In further support of this notion, some of the most established “myomirs” (miR-133 and miR-206/miR1) were significantly less expressed after AE + RE compared with RE. Hence, it is tempting to speculate that the blunted miRNA expression in AE + RE could reverse inhibitory effects on target gene transcription. This in turn may allow for more efficient protein translation from available mRNA transcripts, resulting in more robust muscle protein accretion following the combined exercise paradigm. Interestingly, miRNAs also seem to regulate TET2 signaling (6), which was heavily activated by AE + RE in the current study. To the authors' knowledge, we are the first to depict the TET2 pathway as a potential regulator of adaptations to combined training. TET2 is a master epigenetic regulator of smooth muscle plasticity (21), yet its role in human skeletal muscle is relatively unknown. The TET2 transcript was fairly abundant and detectable in all samples, yet it was discarded from further analysis due to low variability across samples (SD ∼0.2). Post hoc analysis of TET2 did not indicate it being differentially expressed. Therefore, a potential role for TET2 in the context of skeletal muscle response to exercise does not seem to involve transcriptional regulation of TET2 itself. However, given that TET2 may be under heavy miRNA regulation (6), recalling we discovered several suppressed miRNAs in our gene list, future studies should explore the potential interaction between miRNA, TET2, and muscle adaptive responses to exercise in more detail.
The ontology analysis surprisingly revealed that AE + RE, while improving endurance capacity, downregulated the biological function angiogenesis. This was due to the fact that several known angiogenic factors were downregulated by combined exercise. In contrast, the proangiogenic growth factor VEGF (10) showed substantially greater expression after AE + RE than RE, and this was confirmed through qPCR. This highlights the risk of drawing conclusions about complex biological processes based on measurements of single factors. In further support of a suppressed angiogenic response, we have reported that 5 wk of AE + RE (25) did not induce capillarization (23). Thus, albeit the training period may have been too short for vascular growth to occur, it cannot be excluded that our finding indicates an interference effect in vascular remodeling induced by adding resistance exercise to aerobic exercise.
It is obvious that, to assess the effects of adding aerobic exercise before resistance exercise, the resistance exercise protocol needs to be matched, and hence total work performed is substantially greater in the AE + RE leg. Although this is an essential part of the design for this and other comparable concurrent exercise studies, it does not rule out the possibility that differences in workloads account for the different gene expression profiles across legs. Because the primary goal of this study was to comprehensively explore gene expression profiles of the two exercise modes, we did not assess protein-related changes of any specific target, nor did we specifically profile transcripts in the polysome pool (5). However, we appreciate that further validation work could have revealed even more significant information. The specific one-legged model was selected because it allows for a unique interindividual design with repeated-measure analysis of samples originating from the same individual and hence the same genetic variance, training history, and nutrient status. We acknowledge that the acute nature of the imposed exercise challenges and subsequent biopsy sampling time point could produce large noise and nonspecific transcriptional responses (39). This was reflected in our dataset where members of several key regulatory pathways were differentially expressed in a discordant manner, i.e., both up- and downregulation. While this highlights the complexity in interpreting acute exercise data, it also emphasizes a major strength of the current study, i.e., first-hand data of muscle adaptations to the exercise modes under study (40). In the absence of such knowledge, it becomes virtually impossible to interpret the acute molecular response to exercise.
Perspectives and Significance
We provide novel data revealing that the skeletal muscle, subjected to concurrent exercise, shows striking transcriptional responses when initiating gene programs generally thought to be antagonistic with each other. In addition to providing unbiased support for factors with established roles in regulating muscle growth and an oxidative phenotype in response to concurrent exercise (e.g., mTOR, myostatin, AMPK, PGC-1α), our in-depth transcriptional map also identified several new candidate genes and putative networks that markedly differed in expression between AE + RE and RE. Thus, central regulators such as CREB, TET2, MAPK, NF-κβ, and several miRNAs appeared as novel factors that could be part in regulating vast differences in types and magnitudes of adaptations across combined exercise paradigms. The current findings also highlight the difficulty in assessing single markers as a proxy of physiological adaptations if no end-point measures of chronic adaptations are at hand. Collectively, our findings should stimulate further exhaustive investigations of the transcriptional and molecular basis of exercise-induced muscle adaptations using different exercise combinations in various populations.
GRANTS
This study was supported by grants from the Swedish National Centre for Research in Sports (P. A. Tesch), the European Space Agency (P. A. Tesch, T. Gustafsson), the Marianne & Marcus Wallenberg Foundation (T. Gustafsson), the Swedish Medical Research Council (T. Gustafsson), and the Swedish Society for Medical Research (E. Rullman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.R.L., R.F.-G., P.A.T., and T.G. conception and design of research; T.R.L. and R.F.-G. performed experiments; T.R.L., R.F.-G., P.A.T., E.R., and T.G. interpreted results of experiments; T.R.L. and E.R. prepared figures; T.R.L. and T.G. drafted manuscript; T.R.L., R.F.-G., P.A.T., E.R., and T.G. edited and revised manuscript; T.R.L., R.F.-G., P.A.T., E.R., and T.G. approved final version of manuscript; E.R. analyzed data.
Supplementary Material
ACKNOWLEDGMENTS
We thank BEA, the core facility for Bioinformatics and Expression Analysis service facility at Novum, Karolinska Institutet in Huddinge, for helpful contributions.
REFERENCES
- 1.Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102: 2232–2239, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bergström J. Muscle electrolytes in man. Scand J Lab Med Invest 14: 1–110, 1962. [Google Scholar]
- 3.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bruno NE, Kelly KA, Hawkins R, Bramah-Lawani M, Amelio AL, Nwachukwu JC, Nettles KW, Conkright MD. Creb coactivators direct anabolic responses and enhance performance of skeletal muscle. EMBO J 33: 1027–1043, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YW, Nader GA, Baar KR, Fedele MJ, Hoffman EP, Esser KA. Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. J Physiol 545: 27–41, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng J, Guo S, Chen S, Mastriano SJ, Liu C, D'Alessio AC, Hysolli E, Guo Y, Yao H, Megyola CM, Li D, Liu J, Pan W, Roden CA, Zhou XL, Heydari K, Chen J, Park IH, Ding Y, Zhang Y, Lu J. An extensive network of TET2-targeting MicroRNAs regulates malignant hematopoiesis. Cell Rep 5: 471–481, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donges CE, Burd NA, Duffield R, Smith GC, West DW, Short MJ, Mackenzie R, Plank LD, Shepherd PR, Phillips SM, Edge JA. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. J Appl Physiol 112: 1992–2001, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Gonzalo R, Lundberg TR, Tesch PA. Acute molecular responses in untrained and trained muscle subjected to aerobic and resistance exercise training versus resistance training alone. Acta Physiol 209: 283–294, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher IJ, Scheele C, Keller P, Nielsen AR, Remenyi J, Fischer CP, Roder K, Babraj J, Wahlestedt C, Hutvagner G, Pedersen BK, Timmons JA. Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in type 2 diabetes. Genome Med 2: 9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson T. Vascular remodelling in human skeletal muscle. Biochem Soc Trans 39: 1628–1632, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Hawley JA. Molecular responses to strength and endurance training: are they incompatible? Appl Physiol Nutr Metab 34: 355–361, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol 45: 255–263, 1980. [DOI] [PubMed] [Google Scholar]
- 13.da Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC. Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age. Aging Cell 13: 92–101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol 103: 1488–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Ex Sport Sci Rev 42: 53–61, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-κB signaling in skeletal muscle. J Appl Physiol 103: 388–395, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol 106: 2026–2039, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, Pehmoller C, Sanz N, Sakakibara I, Saint-Amand E, Rimbaud S, Maire P, Marette A, Ventura-Clapier R, Ferry A, Wojtaszewski JF, Foretz M, Viollet B. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J 28: 3211–3224, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128: 2047–2057, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long YC, Widegren U, Zierath JR. Exercise-induced mitogen-activated protein kinase signalling in skeletal muscle. Proc Nutr Soc 63: 227–232, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Lundberg T. The Effects of Aerobic Exercise on Human Skeletal Muscle Adaptations to Resistance Exercise (PhD thesis) Sundsvall, Sweden: Mid Sweden University, 2014, p. 1–73. [Google Scholar]
- 24.Lundberg TR, Fernandez-Gonzalo R, Gustafsson T, Tesch PA. Aerobic exercise alters skeletal muscle molecular responses to resistance exercise. Med Sci Sports Exerc 44: 1680–1688, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg TR, Fernandez-Gonzalo R, Gustafsson T, Tesch PA. Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J Appl Physiol 114: 81–89, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg TR, Fernandez-Gonzalo R, Norrbom J, Fischer H, Tesch PA, Gustafsson T. Truncated splice variant PGC-1alpha4 is not associated with exercise-induced human muscle hypertrophy. Acta Physiol 212: 142–151, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg TR, Fernandez-Gonzalo R, Tesch PA. Exercise-induced AMPK activation does not interfere with muscle hypertrophy in response to resistance training in men. J Appl Physiol 116: 611–620, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19: 1498–1500, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol 107: 1655–1662, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PloS one 9: e89431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 113: 71–77, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki H, Loenneke JP, Thiebaud RS, Abe T. Cycle training induces muscle hypertrophy and strength gain: strategies and mechanisms. Acta Physiol Hung 102: 1–22, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA, Atherton PJ. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet 9: e1003389, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philp A, Schenk S, Perez-Schindler J, Hamilton DL, Breen L, Laverone E, Jeromson S, Phillips SM, Baar K. Rapamycin does not prevent increases in myofibrillar or mitochondrial protein synthesis following endurance exercise. J Physiol 593: 4275–4284, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112: 1625–1636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350: 2682–2688, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Tesch PA, Ekberg A, Lindquist DM, Trieschmann JT. Muscle hypertrophy following 5-week resistance training using a non-gravity-dependent exercise system. Acta Physiol Scand 180: 89–98, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol 110: 846–853, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmons JA, Sundberg CJ. Oligonucleotide microarray expression profiling: human skeletal muscle phenotype and aerobic exercise training. IUBMB Life 58: 15–24, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, Russell AP, Cameron-Smith D. Resistance exercise increases NF-κB activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 302: R667–R673, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Ydfors M, Fischer H, Mascher H, Blomstrand E, Norrbom J, Gustafsson T. The truncated splice variants, NT-PGC-1alpha and PGC-1alpha4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep 1: e00140, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.