Abstract
The consensus view of the ventromedial nucleus of the hypothalamus (VMH) is that it is a key node in the rodent brain network controlling sympathoadrenal counterregulatory responses to hypoglycemia. To identify the location of hypoglycemia-responsive neurons in the VMH, we performed a high spatial resolution Fos analysis in the VMH of rats made hypoglycemic with intraperitoneal injections of insulin. We examined Fos expression in the four constituent parts of VMH throughout its rostrocaudal extent and determined their relationship to blood glucose concentrations. Hypoglycemia significantly decreased Fos expression only in the dorsomedial and central parts of the VMH, but not its anterior or ventrolateral parts. Moreover, the number of Fos-expressing neurons was significantly and positively correlated in the two responsive regions with terminal blood glucose concentrations. We also measured Fos responses in the paraventricular nucleus of the hypothalamus (PVH) and in several levels of the periaqueductal gray (PAG), which receives strong projections from the VMH. We found the expected and highly significant increase in Fos in the neuroendocrine PVH, which was negatively correlated to terminal blood glucose concentrations, but no significant differences were seen in any part of the PAG. Our results show that there are distinct populations of VMH neurons whose Fos expression is suppressed by hypoglycemia, and their numbers correlate with blood glucose. These findings support a clear division of glycemic control functions within the different parts of the VMH.
Keywords: counterregulation, glucosensing neurons, motivated behaviors, periaqueductal gray
endocrine counterregulatory responses (CRRs) are critical homeostatic responses to a fall in blood glucose. They consist of increased secretion of epinephrine (and norepinephrine), glucagon, growth hormone, and glucocorticoid and occur when blood glucose concentrations fall below ∼3.8 mM (12, 33, 41). These hormones act rapidly (epinephrine and glucagon) or more slowly (glucocorticoid and growth hormone) to mobilize glucose stores from liver, muscle, and adipose tissue thereby raising blood glucose back into the euglycemic range.
How fast glucose falls into hypoglycemia determines whether peripheral glucosensors are required for initiating CRRs (4, 14, 48). Many studies implicate the ventromedial nucleus of the hypothalamus (VMH) as a key nucleus for sensing glucose deficits and controlling epinephrine and glucagon CRRs during the rapid-onset hypoglycemia that develops between 20 and 30 min after insulin treatment (13, 16, 27, 28, 34, 49, 53). In particular, three studies have manipulated the local environment of the ventromedial hypothalamus, which includes the VMH, adjacent parts of the lateral hypothalamic area, and the arcuate nucleus (ARH) to implicate a regional control network for epinephrine and glucagon CRRs during systemic hypoglycemia: 1) neurotoxic lesions of this region disrupt CRRs induced by hypoglycemia (7); 2) locally maintained euglycemia blunts these responses to systemic hypoglycemia (5); 3) locally applied 2-deoxyglucose (2-DG) combined with systemic euglycemia produces epinephrine and glucagon CRRs (6).
Electrophysiology has identified distinct glucose-excited (GE) and glucose-inhibited (GI) neurons in the VMH (47), where it is estimated that up to half of its neurons possess some glucosensing capability (38, 46). These neurons then influence hypothalamic and hindbrain integrative networks to control endocrine CRRs (57). However, given the functional complexity of the VMH and the division of function across its constituent parts (23, 35, 57), elaborating a detailed glucoregulatory role for the VMH requires a more precise localization of the participating neurons.
Determining the location of neurons that are functionally related to a stimulus is typically achieved by measuring changes in Fos expression (58); for example, in neuroendocrine parts of the paraventricular nucleus of the hypothalamus following glycemic challenges (PVH) (11, 37, 39). Systemic or cerebral hyperglycemia increases Fos in VMH neurons (11, 15, 16), demonstrating that changes in blood glucose can promote VMH Fos responses. On the other hand, despite many attempts, there are no reports of changes in Fos expression in the VMH after hyperinsulinemic hypoglycemia or 2-DG-induced whole body glucopenia (13, 19, 37, 39, 40, 42, 43).
One explanation for these observations is that hypoglycemia inhibits rather than stimulates Fos expression in the VMH, meaning that changes in Fos expression will be more difficult to detect. To address this question we performed a high-spatial resolution analysis of hypoglycemia-associated Fos expression in the VMH. We identified Fos immunohistochemically in a closely spaced series of brain sections through the rostrocaudal extent of the VMH of euglycemic and hyperinsulinemic hypoglycemic rats. We first quantified and mapped the Fos expression within the four constituent parts of the VMH using the Swanson rat brain atlas as a reference (52). We then determined the relationship between the numbers of Fos neurons in each part of the VMH and terminal blood glucose concentrations. We also performed a similar analysis of Fos expression in the medial parvicellular (neuroendocrine) part (mp) of the PVH, which is robustly activated by glycemic challenges (11, 39, 41, 46). Finally, to determine whether there were accompanying changes in the Fos expression in major downstream targets of VMH projections, we examined the Fos expression in various part of the periaqueductal gray (PAG). This large and functionally diverse midbrain region receives one of the heaviest descending projections from the VMH (10, 36) and is heavily implicated in coordinating functions involving the VMH (25, 55, 56).
Some of the results in this paper have previously been presented in abstract form (22).
METHODS
Animals
Male Wistar rats (275–300 g body wt upon arrival) were housed singly in plastic cages with wood chip bedding. They had unrestricted access to water, chow, and a short piece of polyvinyl chloride tubing (∼10 cm diameter) for environmental enrichment. The vivarium was kept on a 12:12 light cycle, with lights off at 18.00 h. All methods were approved by the University of Southern California Institutional Animal Care and Use Committee.
Experimental Procedures
To allow habituation to the experimental procedures, all animals were exposed to the test environment during the 2 days immediately preceding the test day. These 3 days were similar in all respects except that 0.9% saline was injected on days 1 and 2 and insulin (or vehicle) on day 3. Each day at ∼09.30 h animals were weighed, food was removed, cage bedding was changed, and their cages transferred into an adjacent room that was illuminated by a 60-Watt lamp. Rats were then injected intraperitoneally (ip) with 0.9% saline (1 ml/kg body wt) between 13.30 and 15.00 h. To follow blood glucose concentrations before and after the intraperitoneal injections, blood was obtained several times between 09.30 h and 16.30 h by tail nick following local topical lidocaine application. Blood was analyzed with an AlphaTrak glucose meter with No. 7 test strips (Abbott Laboratories).
On day 3 rats were injected with either 0.9% saline (n = 8) or insulin (n = 9; Humulin R, 7.5 U·ml−1·kg−1; Eli Lilly, Indianapolis, IN) between 13.30 and 15.00 h. Blood glucose was measured throughout the day as just described. Preliminary studies showed that 7.5 U/kg insulin was the threshold dose for producing blood glucose concentrations of <3.0 mM glucose. However, we found that some insulin-injected animals remained euglycemic (>5.55 mM) and required another small bolus of insulin (50–90 μl) to induce hypoglycemia. This procedure was effective in most animals. Thirty minutes later blood was sampled to assess the extent of hypoglycemia. Once blood glucose began to fall, animals were returned to their cages, and 60 min later (i.e., 90 min after the initial injection) a final glucose measurement was taken.
Between 15.00 and 16.30 h rats were rapidly anesthetized with isoflurane and perfused. All perfusions were performed as previously described (28). In brief, ∼100 ml of 0.9% saline was followed by 500 ml ice-cold 0.1 M borate-buffered 4% paraformaldehyde. Brains were first postfixed at 4°C overnight in paraformaldehyde solution with 12% sucrose (wt/vol), then frozen in liquid hexanes cooled with dry ice, and stored at −70°C until sectioning.
Histology and Antibodies
Six series of 1:6 frozen sections (30-μm) were cut through hypothalamus and midbrain, collected into polyethylene glycol-based antifreeze (30), and stored at −20°C until processed for immunohistochemistry (IHC). All sections were processed in one of two IHC runs, each of which contained sections from hypoglycemic and euglycemic animals to ensure that staining parameters were identical across groups. Sections were rinsed 6 × 5 min in 0.02 M potassium phosphate-buffered saline (KPBS), blocked for 1 h in 2% normal donkey serum and 0.1% Triton X-100 in KPBS, rinsed in KPBS, and transferred into primary antibodies for NeuN (1:6k mouse anti-NeuN, Millipore no. MAB377) or Fos (1:2k rabbit monoclonal anti-Fos, Cell Signaling Technology no. 2250) in the blocking solution for 72 h at 4°C. Sections were rinsed 6 × 5 min in KPBS and placed into fluorophore-conjugated secondary antibodies in blocking solution (1:1k all: AlexaFluor488 donkey anti-mouse, Life Technologies no. A21202; or CY3 donkey anti-rabbit, Jackson ImmunoResearch no. 711-165-152) for 24 h at 4°C. Sections were rinsed 6 × 5 min, then mounted onto slides, air dried, and then coverslipped with a mounting solution of 1:1 glycerol and 0.1 M sodium PBS (final pH 8.75).
As part of a larger study, all rats received unilateral stereotaxically guided injections of the neuronal tracers Phaseolus vulgaris leucoagglutinin or Fluorogold into the VMH, with cholera toxin B into the PVH 2 wk before the experiment reported here. Some of the tracing results have been presented elsewhere (22). To avoid any possible impact from the tracer injections on Fos expression in the VMH and PVH, only the uninjected side of the brain was used to obtain the Fos results reported here.
Image Analysis
Sections were photographed with a Zeiss Axioimager Z1 epifluorescence microscope through a ×10 plan-apochromat objective lens (numerical aperature 0.45). Images were captured with a Hamamatsu Orca ER monochromatic digital camera controlled with Volocity 6.1 software (Perkin Elmer, Waltham, MA). Signals from the green (NeuN) and red (Fos) fluorophores were captured at 300- and 200-ms exposures, respectively. All photoimages were then imported into Adobe Illustrator (CS6; Adobe System, San Jose, CA).
With the use of the NeuN channel and the Swanson Rat Brain Maps (52) as reference, we used the Adobe Illustrator tools to trace the borders of the PVH and PAG. In the ventromedial hypothalamus, the ARH, the four different parts of the VMH, and the capsular area bounding the dorsal and medial borders of the VMH were all identified at atlas levels (ALs) 26–30 of the Swanson rat brain atlas (52). The anatomical borders were then drawn in a separate layer. These borders were then transferred in perfect register to the Fos channel to enable projection mapping of Fos-immunoreactive (ir) nuclei onto the appropriate ALs of the Adobe Illustrator version of the Swanson atlas (52). The locations of all Fos-ir nuclei from each rat were plotted in a separate layer of a single file.
Quantitation.
Adobe Illustrator tools were used to count the total number of Fos-ir nuclei at the five rostrocaudal levels noted above. Counts were obtained from the ARH, the entire VMH (together with separate counts for each of its 4 different parts), and the capsular area bounding the dorsal and medial borders of the VMH. Fos-ir nuclei were counted from the appropriate photomicrographs of the medial parvicellular part of the PVH (PVHmp) (AL 26), the commissural nucleus (Com), the dorsal (d), dorsolateral (dl), ventrolateral (vl), rostromedial, and medial (m) divisions of the PAG (ALs 34, 38, 41–43). The locations of Fos-ir nuclei in the PVH and PAG were not plotted onto ALs.
Prism 4 (GraphPad Software, La Jolla, CA) was used to determine the statistical significance of differences between the various group means of Fos-ir nuclei in all anatomical areas using two-tailed Welch's t-test at a 0.05 significance level. Regression analyses were made between the number of Fos-ir nuclei in the four parts of the VMH, the PVHmp, and the final (90 min) blood glucose concentration of each animal.
Topography.
Topographical patterns of Fos expression at AL 26–30 (52) were generated for the euglycemic and hypoglycemic groups. This was accomplished by collapsing the Illustrator layers of each animal onto a single map thereby retaining perfect registration between animals. To ensure that the same number of animals per group was used to produce these maps, the five animals whose total number of Fos-ir nuclei in the VMH were closest to the median (based on their rank order) were selected from each group.
RESULTS
Insulin-Induced Hypoglycemia
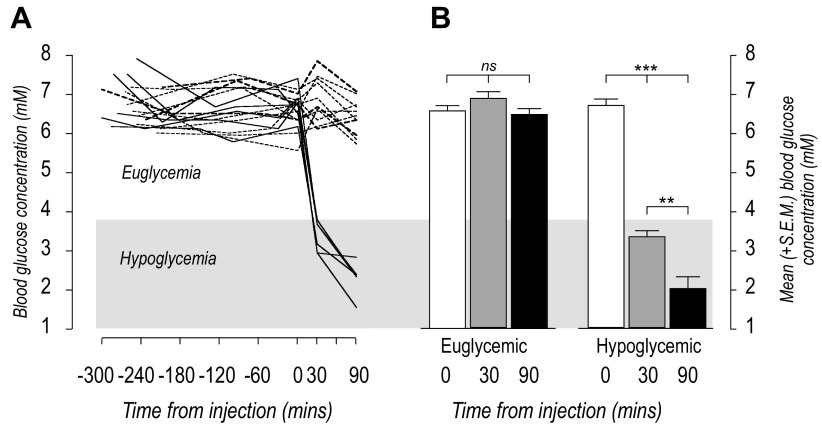
Figure 1A shows that on day 3 all rats had blood glucose concentrations between 5.5 and 8 mM for at least 4 h before receiving saline or insulin injections. There were no significant differences between control and experimental groups at the time of injections (Fig. 1B). After the injections all saline-injected rats remained in the euglycemic range until the terminal sample immediately before perfusion (P = 0.35), while the blood glucose concentrations of 5 insulin-injected rats had already fallen into the hypoglycemic range (<3.8 mM) by 30 min (P < 0.001). These had fallen further at the time of perfusion (Fig. 1B). Therefore, this treatment resulted in a rapid-onset hypoglycemia compared with slow-onset hypoglycemia that takes at least 60 min to develop (14, 48).
Fig. 1.
A: individual blood glucose concentrations up to 300 min before and 90 min after intraperitoneal injections of saline (dashed lines) or insulin (solid lines) at 0 min. Note that three insulin-injected animals maintained euglycemia throughout (heavy dashed lines). These animals were included in the euglycemic group for data analyses. Euglycemic and hypoglycemic ranges are indicated by the light and dark gray shadings respectively. B: mean (+SE) blood glucose concentrations immediately before (0 min, open bars), 30 min (gray bars), and 90 min (black bars) after intraperitoneal injections of saline or insulin. Animals that became hypoglycemic did so within 30 min of intraperitoneal insulin injections (P < 0.001), with blood glucose concentrations declining further by 90 min (P < 0.002 compared with 30 min).
Three rats given insulin maintained blood glucose concentrations of 5.5–8 mM throughout the test period (Fig. 1A, heavy dashed lines). These animals were included with the euglycemic rats for statistical analyses.
Ventromedial Nucleus of the Hypothalamus
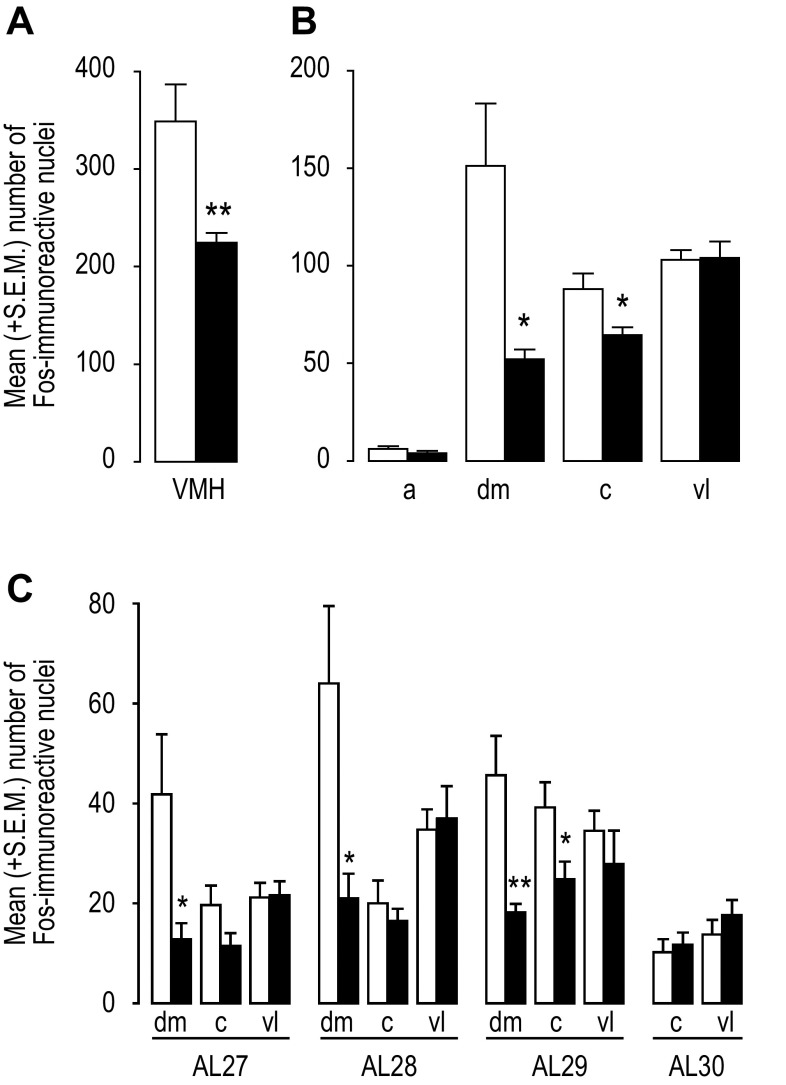
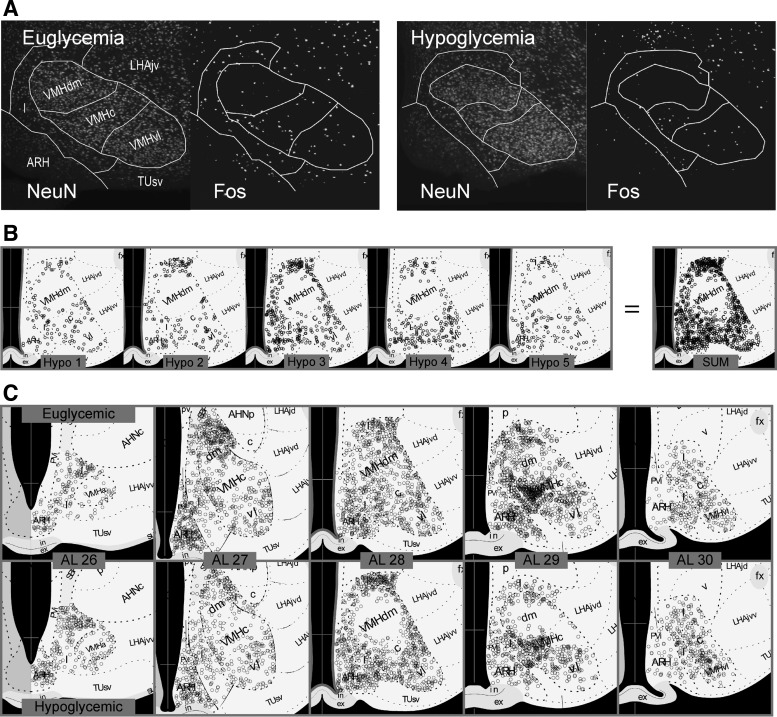
Hypoglycemia was associated with significantly reduced Fos expression in the VMH when the entire nucleus was considered (Fig. 2A; P < 0.01). However, examining constituent parts of the VMH at a higher spatial resolution revealed that these reductions were not distributed evenly across all four VMH parts or at different rostrocaudal levels (Fig. 2, B and C and Fig. 3). Figure 3A illustrates how the locations of Fos-ir nuclei were mapped in the VMH using the NeuN and Fos immunoreactivity in the ventromedial hypothalamus of representative euglycemic and hypoglycemic animals. The anatomical borders of its various regions are drawn as described in the Image Analysis section of the methods.
Fig. 2.
A: mean (+SE) number of Fos expressing nuclei in the entire ventromedial nucleus of the hypothalamus (VMH) summed across its rostrocaudal extent in euglycemic and hypoglycemic animals. B: mean (+SE) number of Fos expressing nuclei in the anterior (a), dorsomedial (dm), central (c), and ventrolateral (vl) parts of the VMH summed across its rostrocaudal extent in euglycemic and hypoglycemic animals. C: mean (+SE) number of Fos expressing nuclei in the anterior (a), dorsomedial (dm), central (c), and ventrolateral (vl) parts of the VMH at atlas levels (AL; 52) 27 to 30. Euglycemic animals are shown by open bars and hypoglycemic animals by closed bars in all panels.*P < 0.05; **P < 0.01.
Fig. 3.
A: two channel epifluoresence photomicrographs of NeuN and Fos-immunoreactive cells in the VMH and immediately adjacent regions from a representative euglycemic and hypoglycemic animal. The anatomical borders derived from the NeuN-stained sections are show by the solid white lines. B: locations of Fos-immunoreactive cells in the VMH and immediately adjacent regions from five different hypoglycemic animals (Hypo 1, etc.) mapped onto atlas level (AL) 28 of the Swanson rat brain atlas (52). The far right (SUM) shows the locations of locations of Fos-immunoreactive cells from all five animals collapsed onto a single map. See text for the methods for how this was achieved. C: locations of Fos-immunoreactive cells in the VMH and the immediately adjacent regions onto the collapsed maps from five euglycemic and five hypoglycemic animals across the rostrocaudal extent of the VMH (AL26-30, 52). AHNc, central part, anterior hypothalamic nucleus; AHNp, posterior part, anterior hypothalamic nucleus; ARH, arcuate nucleus; ex, external layer of the median eminence; fx, fornix; I, internuclear area, hypothalamic periventricular region; in, internal layer of the median eminence; LHA, lateral hypothalamic area; LHAjd, juxtadorsomedial region of the LHA; LHAjvd, dorsal zone of the juxtaventromedial region of the LHA; LHAjvv, ventral zone of the juxtaventromedial region of the LHA; PVi, intermediate part of the periventricular nucleius; SBP, subparaventricular zone; TUsv, subventromedial part of the tuberal nucleus; VMHc, central part of the ventromedial nucleus; VMHdm, dorsomedial part of the ventromedial nucleus; VMHvl, ventrolateral part of the ventromedial nucleus.
Figure 2C shows that the significantly reduced Fos expression in the VMHdm (P < 0.05) was evident at all of the ALs containing the VMHdm (AL 27, P < 0.05; AL 28, P < 0.05; AL 29, P < 0.01). Significantly reduced Fos expression in the VMHc (P < 0.05) was only apparent in the more caudal AL 29 (Fig. 2C; P < 0.05). There was no effect of hypoglycemia on the Fos in the VMHvl or the already negligible numbers of Fos-ir nuclei in the VMHa (Fig. 2B).
Figure 3A shows two channel epifluoresence photomicrographs of NeuN and Fos-ir cells in the VMH and adjacent region from a representative euglycemic and hypoglycemic animal. To examine the detailed topographic distribution of Fos-ir nuclei in the various parts of the VMH of euglycemic and hypoglycemic animals, the location of all the labeled neurons within the VMH of 5 representative animals were collapsed onto single ALs as described in the Image Analysis (methods) section (Fig. 3B). Using this method, Fig. 3C shows that the effects of hypoglycemia on the number of Fos-ir nuclei were most pronounced in the dorsomedial part of the ventromedial nucleus (VMHdm) at ALs 28 and 29. Reductions in the VMHc were less apparent than in the VMHdm but were most evident at AL 29. The lack of effect of hypoglycemia on Fos expression in the VMHvl was apparent throughout its rostrocaudal extent.
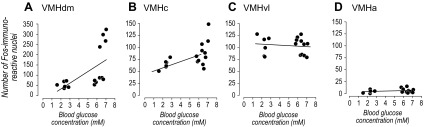
Significant correlations between the terminal blood glucose concentrations and Fos expression occurred differentially within the VMH (Fig. 4). Significant positive correlations were seen in the VMHdm (Fig. 4A; P = 0.011, r2 = 0.36) and the VMHc (Fig. 4B; P = 0.017, r2 = 0.324), but not in the VMHvl (Fig. 4C; P = 0.634, r2 = 0.015) or VMHa (Fig. 4D; P = 0.398, r2 = 0.048).
Fig. 4.
Scatter plots of the terminal blood glucose concentrations and the number of Fos-immunoreactive nuclei of individual rats in the dorsomedial (A), central (B), ventrolateral (C), and ventromedial (D) parts of the ventromedial nucleus of the hypothalamus (VMH). Significant correlations were only found in the dorsomedial (A) and central (B) parts of the VMH. See text for the regression analyses and levels of statistical significance.
Paraventricular Nucleus of the Hypothalamus
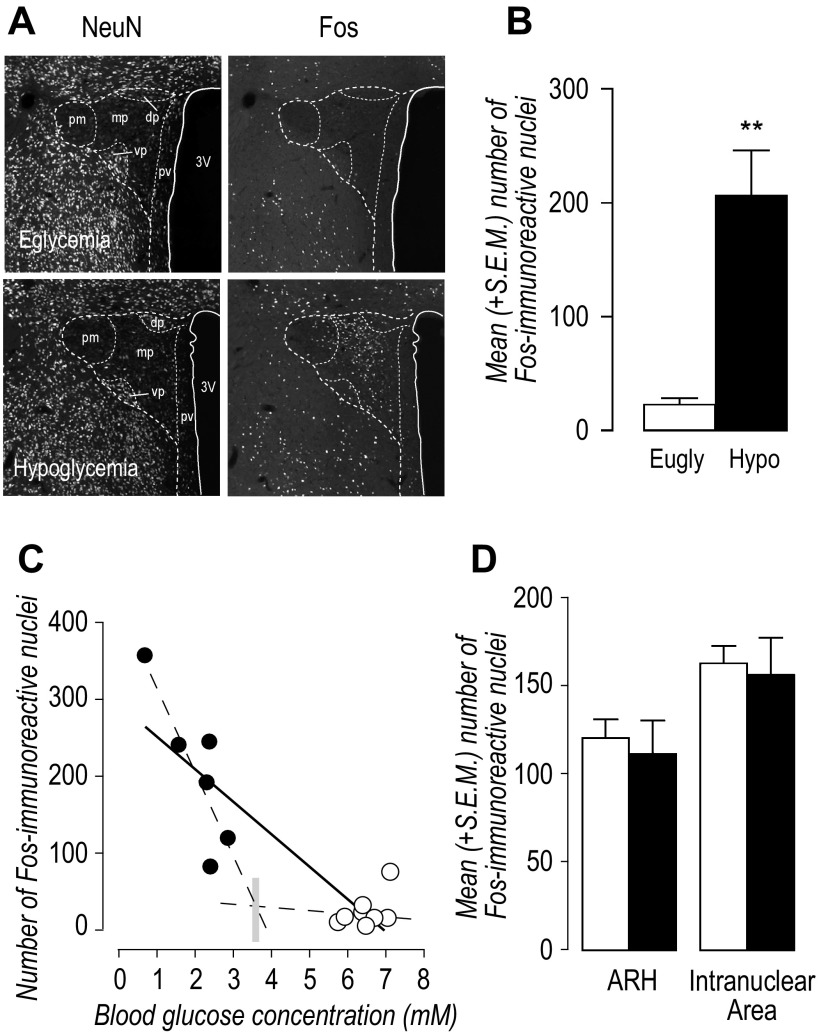
Hypoglycemic rats exhibited robust Fos expression in the PVHmp (AL 26) compared with euglycemic controls (Fig. 5, A and B; P < 0.01). Regression analysis of all animals showed a significant negative correlation between the number of Fos-ir nuclei in the PVHmp and terminal blood glucose concentrations (Fig. 5C; P < 0.0001, r2 = 0.803). When animals were divided into euglycemic and hypoglycemic animals, only hypoglycemic animals showed a significant negative correlation between the number of Fos-ir nuclei in the PVHmp and terminal blood glucose concentrations (Fig. 5C. euglycemic animals P = 0.185, r2 = 0.187; hypoglycemic animals P = 0.03, r2 = 0.742).
Fig. 5.
A: two channel epifluoresence photomicrographs of NeuN and Fos-immunoreactive nuclei in the paraventricular nucleus of the hypothalamus (PVH) of a euglycemic and a hypoglycemic animal at approximately level 26 of the Swanson rat brain atlas (52). B: mean (+SE) number of Fos-immunoeactive nuclei in the medial parvicellular part of the PVH (PVHmp) of euglycemic (open bar) and hypoglycemic (solid bar) animals. **P < 0.01. C: scatter plots of the terminal blood glucose concentrations and the number of Fos-immunoeactive nuclei of individual rats in the PVHmp. There was a significant correlation in all animals (solid line), and in hypoglycemic (solid circles) but not euglycemic (open circles) animals (the two dashed lines). See text for the regression analyses and levels of statistical significance. The vertical gray bar is at the intersection of the regression lines for hypoglycemic and euglycemic animals and approximates a glycemic threshold for Fos activation. D: mean (+SE) number of Fos-immunoreactive nuclei in the arcuate nucleus (ARH) and the internuclear area of the hypothalamic periventricular region (I) of euglycemic (open bars) and hypoglycemic (solid bars) animals. No statistically significant differences were apparent.
Arcuate Nucleus and the Internuclear Area
Neither the ARH nor the internuclear area that borders the VMH had significantly different numbers of Fos-ir nuclei between groups (Fig. 5D).
Periaqueductal Gray
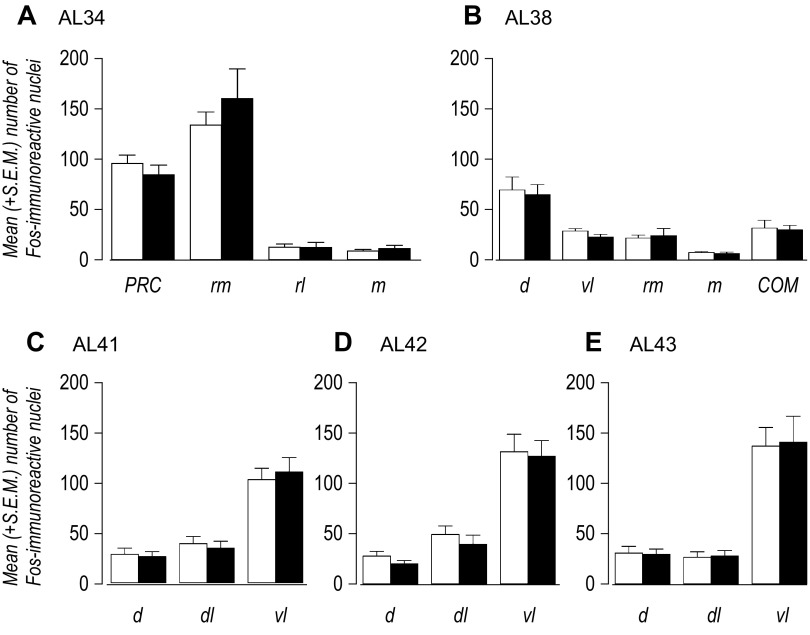
We measured the number of Fos-ir nuclei in those parts of the dorsal PAG that receive dense projections from the VMHdm and VMHc (10). These were from sections at the level of the posterior commissure (AL 34), the level of the commissure of the superior colliculus (AL 38), and from ALs 41–43. Figure 6 shows that there were no significant differences between euglycemic and hypoglycemic animals at any of the PAG divisions that were sampled.
Fig. 6.
The mean (+SE) number of Fos-immunoeactive nuclei in different parts of the periaqueductal gray (PAG) at atlas levels (AL; 52) 34 (A), 38 (B), 41 (C), 42 (D), and 43 (E) of euglycemic (open bars) and hypoglycemic (solid bars) animals. No statistically significant differences were apparent between groups at any anatomical level. COM, commissural nucleus, PAG; d, dorsal division PAG; dl, dorsolateral division PAG; m, medial division PAG; PAG, periaqueductal gray; PRC, precommissural nucleus, PAG; rl, rostrolateral division PAG; rm, rostromedial division PAG; vl, ventrolateral division PAG.
DISCUSSION
Our findings are the first to demonstrate hypoglycemia-associated changes in Fos expression in the VMH. We show that the suppression of VMH Fos expression by rapid-onset hypoglycemia is region-specific: effects are restricted to the VMHdm and VMHc, with no change in the VMHvl or the VMHa, which is the smallest part of the VMH. Although many previous studies have examined the effects of manipulating glycemia on Fos expression in various parts of the brain, none have reported changes in the VMH with hypoglycemia, despite its well-described position in the neural networks controlling endocrine counterregulatory responses (13, 15, 19, 37, 39, 40, 43). One reason for this discrepancy has been the tendency to assume that hypoglycemia will activate VMH neurons and therefore increase Fos expression (e.g., 38). But as noted by Evans et al. (19) there is evidence that VMH neurons are inhibited by hypoglycemia, perhaps in response to the reported increases in GABA release within the ventromedial hypothalamus (3, 59). These changes should therefore correlate with reduced Fos expression. However, the relatively few Fos-ir cells seen in control animals has tended to mask any attempts to detect such a change.
Consistent with many previous reports, we found that the number of Fos-ir cells in the VMH of control animals was quite low (9, 15, 50). This basal expression most likely results from the neural activity associated with the handling and control habituation procedures we performed in the days immediately before the insulin injections, as well as the manipulations on the test day. The lack of Fos expression in the PVHmp of control animals confirms the nonstressful nature of these procedures. But even with the relatively low levels of Fos-ir cells we see in the VMH of control animals, we showed that rapid-onset hypoglycemia suppresses Fos expression in the VMHdm and to a lesser extent the VMHc. These regions have long been associated with metabolic control, primarily because of their high expression of the receptors for two key metabolic hormones, ghrelin and leptin (17, 60). In contrast, we found that the VMHvl, which is a major component of the neural networks that control conspecific interactions (25, 35) showed no suppression of Fos expression after rapid-onset hypoglycemia.
How is Fos expression suppressed in the VMHdm and VMHa during hypoglycemia? Three possible mechanisms can account for these observations: suppression is driven by proximal VMH glucosensing mechanisms, by more distal glucosensing mechanisms that are mediated by afferents to the ventromedial hypothalamus, or by a combination of both.
The electrophysiological characterization of glucose-excited (GE) and glucose-inhibited (GI) neurons within the VMH and nearby locations in the ventromedial hypothalamus (47) has had a major impact on how we view the position of the VMH in metabolic control. During hypoglycemia VMH GE neurons respond to a fall in local glucose concentration by opening the ATP-sensitive potassium channel Kir6.2, which then hyperpolarizes the neuron. The importance of this channel for generating endocrine CRRs is shown by the fact that its closure with local delivery of glibenclamide significantly blunts epinephrine and glucagon CRRs to systemic hypoglycemia (18). Therefore, one interpretation of our results is that it is the VMH GE neurons themselves in which Fos expression is suppressed. On the other hand, the fact that we did not see increased Fos expression in any part of the VMH suggests that GI neurons [whose activity is increased when ambient glucose concentrations fall (47)] do not express Fos following rapid-onset hypoglycemia. Because there are no effective or definitive neuroanatomical markers for glucosensing neurons, the idea that Fos expression is reduced in glucosensing neurons following hypoglycemia must remain conjecture.
Afferent pathways from the hindbrain to the hypothalamus convey critical information for some cellular responses to hypoglycemia. We have shown in two studies that specific lesions of ascending norepinephrine/epinephrine-containing pathways to the hypothalamus render pERK responses in the PVH and ARH insensitive to rapid-onset hypoglycemia (30, 31). Therefore, some neuronal responses in the hypothalamus are driven by hindbrain glucosensing mechanisms that are mediated by afferent inputs rather than by the direct actions of proximal glucosensing mechanisms. In this way, the reduced Fos responses we see in parts of the VMH following rapid-onset hypoglycemia may also rely on afferent information from more distal sites. These inputs include cholecystokinin-containing projections from the parabrachial nucleus (20, 23) and ascending catecholaminergic-containing pathways to the ventromedial hypothalamus. We have already shown that lesioning the catecholaminergic pathway impedes sympathoadrenal responses to hypoglycemia (24). Although this dependency was tied to slow- rather than rapid-onset hypoglycemia, these results show the requirement of ascending catecholaminergic pathways for VMH neuronal responses to some types of hypoglycemia.
An alternative interpretation of our findings is that insulin rather than hypoglycemia suppresses Fos in hypoglycemic animals. Although insulin receptors are expressed in the VMH (54) and can reduce the firing rate of VMH neurons when stimulated (32), three sets of observations strongly suggest that insulin is not itself directly responsible for our findings. First, hyperinsulinemic-euglycemic clamps do not alter Fos in the PVH or those parts of the hindbrain where hypoglycemia increases Fos expression (1). Second, direct injections of insulin into the lateral ventricle have no effect on Fos expression in the PVH or VMH (42). Finally, the inability of insulin to alter some intracellular signaling pathways directly is shown by the loss of Fos and pERK responses to insulin-induced hypoglycemia in the PVH and ARH following DSAP lesions (which eliminate catecholaminergic inputs to the medial hypothalamus) but not control lesions (29, 30, 44). In all cases, intact and lesioned animals both experienced the same insulin treatments.
Consistent with previous findings (11, 37, 39), we found that hypoglycemia robustly increased Fos expression in the PVHmp neurons. Moreover, we now find a robust and significant negative correlation between the number of Fos neurons in the PVHmp and the terminal glucose concentrations in hypoglycemic animals, a relationship that contrasts to the positive correlation we found in the Fos-responsive parts of the VMH. This same regression analysis produces an estimated activation threshold for Fos (∼3.5 mM) that is similar to the one we reported for ACTH [3.25–3.30 mM; (24)]. This finding supports three points. First, there is a close link between hypoglycemia and the mechanisms in CRH neurons that control Fos expression and ACTH release. Second, there is an activation threshold for Fos. Finally, once glucose levels fall below this threshold, the degree of Fos activation, like ACTH release, is directly proportional to the stimulus intensity of hypoglycemia, i.e., the deeper the hypoglycemia, the greater the Fos expression. Unfortunately we do not have an exact determination of when each animal crossed this threshold, but we estimate from Fig. 1A that it was at least 45 min before the termination of the experiment, which is sufficient time to increase Fos expression. Data distributions in the VMH do not allow us to determine whether there is a glycemic threshold in the ability of blood glucose to inhibit Fos expression in the VMHdm and VMHc.
In addition to examining the PVH and VMH, we also measured the effects of insulin-induced hypoglycemia on Fos expression in the PAG. The PAG receives the densest descending projection from the VMH (10). The PAG and the VMHdm together form two nodes in a network that controls behavioral responses to predators (25). These fight-or-flight responses comprise a host of physiological changes, including increases in blood glucose, that collectively prepare the animal for significant physical activity (45). As a major downstream effector of VMH actions, together with its connections with hindbrain preautonomic neurons (29), the PAG is well placed to mediate VMH control on adrenomedullary and pancreatic CRRs. However, the fact that we found no hypoglycemia-associated effect on Fos expression in those parts of the PAG that receive significant projections from the VMH indicates that if this pathway is important for activating CRRs, then it must do so in a way that does not alter Fos expression.
The ARH has long been recognized a key nucleus for metabolic control (2, 57). However, as with the PAG, we found no significant increases in Fos expression in the ARH after insulin-induced hypoglycemia. Although several previous studies have reported hypoglycemia-associated Fos increases in the ARH (8, 19, 37, 50), each used longer time courses (3–5 h) than ours (1.5 h). This means that direct comparisons are difficult. However, Soloman et al. (50) used a shorter time course (2h) after intraperitoneal insulin injections in nonfasted animals and reported a significant increase in the small numbers of Fos-labeled neurons in the ARH. Therefore, one reason for our negative result may be the shorter interval after onset of hypoglycemia compared with previous studies.
Perspectives and Significance
The most responsive part of the VMH to hypoglycemia is the VMHdm, which is also the part most closely associated with metabolic function as a consequence of the high concentrations of leptin and ghrelin receptors (17, 60). On the other hand, the least responsive part (the VMHvl) is the one that is most closely related to predator aggression and sexual conspecific interactions (25, 35). These findings support a clear division of glycemic control functions within the different parts of the VMH. To place these results in a wider context, it is useful to consider how glycemia-sensitive neurons in the VMH might interface with the behavioral control processes for which this nucleus is best known. The VMH is located in the hypothalamic part of the brain stem behavioral control column (51). In this context the VMH has evolved to adapt and coordinate behavior in a range of complex environmental situations, particularly the presence of aggressive or sexually attractive conspecifics, or responses to predators (25, 35). Importantly, the expression of each of these behaviors is shaped by interactions between the availability of food sources and internal metabolic status. Therefore, the ability of some VMHdm and VMHc neurons to sense changing glycemia may be one way that nutrient status is used to alter the expression of these key behaviors. But this interaction may work in the other direction. By dynamically sensing changes in glycemia as these behaviors are expressed may allow the VMH to help maintain glycemia in the face of the energy demands placed on the animal. These mechanisms may provide important integrative links between the neural networks that control defensive and sexual behaviors and those that control glycemia.
GRANTS
This work was supported by NS029728 from the National Institute of Neurological Disorders and Stroke/National Institutes of Health (to A. G. Watts).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.N.F. and A.G.W. conception and design of research; N.N.F. performed experiments; N.N.F., S.A., and A.G.W. analyzed data; N.N.F., S.A., and A.G.W. interpreted results of experiments; N.N.F., S.A., and A.G.W. prepared figures; N.N.F. and A.G.W. drafted manuscript; N.N.F. and A.G.W. edited and revised manuscript; N.N.F., S.A., and A.G.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Amanda Sampson for technical assistance.
Current address of N. Foster and S. Azam: Stevens Institute of Neuroimaging and Informatics, Keck School of Medicine, University of Southern California, Los Angeles, CA 90032.
REFERENCES
- 1.Ao Y, Wu S, Go VL, Toy N, Yang H. Maintaining euglycemia prevents insulin-induced Fos expression in brain autonomic regulatory circuits. Pancreas 31: 142–147, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Arble DM, Sandoval DA. CNS control of glucose metabolism: response to environmental challenges. Front Neurosci Feb 26;7:20. doi: 10.3389/fnins.2013.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beverly JL, De Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am J Physiol Regul Integr Comp Physiol 280: R563–R569, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bohland M, Matveyenko AV, Saberi M, Khan AM, Watts AG, Donovan CM. Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes 63: 2866–2875, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99: 361–365, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44: 180–184, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 93: 1677–1682, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JRS, Wilson S, Williams G. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels. Diabetes 50: 105–112, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Canteras NS, Chiavegatto S, Ribeiro do Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull 44: 297–305, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348: 41–79, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Carrasco M, Portilla F, Larsen PJ, Vallo JJ. Insulin and glucose administration stimulates Fos expression in neurones of the paraventricular nucleus that project to autonomic preganglionic structures. J Neuroendocrinol 13: 339–346, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Cryer PE. Hierarchy of physiological responses to hypoglycemia: relevance to clinical hypoglycemia in type I (insulin dependent) diabetes mellitus. Horm Metab Res 29: 92–96, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Diggs-Andrews KA, Zhang X, Song Z, Daphna-Iken D, Routh VH, Fisher SJ. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes 59: 2271–2280, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donovan CM, Watts AG. Peripheral and central glucose sensing in hypoglycemic detection. Physiology (Bethesda) 229: 314–324, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn-Meynell AA, Govek E, Levin BE. Intracarotid glucose selectively increases Fos-like immunoreactivity in paraventricular, ventromedial and dorsomedial nuclei neurons. Brain Res 748: 100–106, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51: 2056–2065, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CS. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998. [PubMed] [Google Scholar]
- 18.Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, Jacob RJ, Sherwin RS. Hypothalamic ATP-sensitive K+ channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 53: 2542–2551, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Evans SB, Wilkinson CW, Bentson K, Gronbeck P, Zavosh A, Figlewicz DP. PVN activation is suppressed by repeated hypoglycemia but not antecedent corticosterone in the rat. Am J Physiol Regul Integr Comp Physiol 281: R1426–R1436, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Flak JN, Patterson CM, Garfield AS, D'Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JM, Germani M, Jones JC, Rajala M, Satin L, Rhodes CJ, Olson DP, Kennedy RT, Heisler LK, Myers MG Jr. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nature Neurosci 17: 1744–50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster NN, Watts AG. How does the ventromedial nucleus of the hypothalamus control the endocrine counterregulatory response to hypoglycemia? 2013 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2013. [Google Scholar]
- 22.Foster NN, Azam S, Watts AG. Hypoglycemia reduces c-Fos expression in the ventromedial hypothalamic nucleus. 2014 Neuroscience Meeting Planner. Washington DC: Society for Neuroscience, 2014. [Google Scholar]
- 23.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG Jr, Heisler LK. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab 20: 1030–7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorton LM, Khan AM, Bohland M, Sanchez-Watts G, Donovan CM, Watts AG. A role for the forebrain in mediating time-of-day differences in glucocorticoid counterregulatory responses to hypoglycemia in rats. Endocrinology 148: 6026–39, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci 13: 651–658. 2012. [DOI] [PubMed] [Google Scholar]
- 26.Jokiaho AJ, Donovan CM, Watts AG. The rate of fall of blood glucose determines the necessity of forebrain-projecting catecholaminergic neurons for male rat sympathoadrenal responses. Diabetes 63: 2854–2865, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: a virally mediated transsynaptic tracing study. J Neurosci 26: 3423–33, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan AM, Kaminski KL, Sanchez-Watts G, Ponzio TA, Kuzmiski JB, Bains JS, Watts AG. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. J Neurosci 31: 18479–18491, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan AM, Walker EM, Dominguez N, Watts AG. Neural input is critical for arcuate hypothalamic neurons to mount intracellular signaling responses to systemic insulin and deoxyglucose challenges in male rats: implications for communication within feeding and metabolic control networks. Endocrinology 155: 405–416, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LAW, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Bruning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci 14: 911–918, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin BE, Sullivan AC. Glucose, insulin and sympathoadrenal activation. J Auton Nerv Syst 20: 233–242, 1987. [DOI] [PubMed] [Google Scholar]
- 34.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol Regul Integr Comp Physiol 276: R1223–R1231, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470: 221–226, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J Comp Neurol 521: 3167–3190, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Niimi M, Sato M, Tamaki M, Wada Y, Takahara J, Kawanishi K. Induction of Fos protein in the rat hypothalamus elicited by insulin-induced hypoglycemia. Neurosci Res 23: 361–364, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Ono T, Nishino H, Fukuda M, Sasaki K, Muramato KI, Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res 232: 494–499, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endo Rev 22: 502–548, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Paranjape SA, Briski KP. Recurrent insulin-induced hypoglycemia causes site-specific patterns of habituation or amplification of CNS neuronal genomic activation. Neuroscience 130: 957–970, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Patel DG. Role of parasympathetic nervous system in glucagon response to insulin-induced hypoglycemia in normal and diabetic rats. Metabolism 3 3: 1123–1127, 1984. [DOI] [PubMed] [Google Scholar]
- 42.Porter JP, Bokil HS. Effect of intracerebroventricular and intravenous insulin on Fos-immunoreactivity in the rat brain. Neurosci Lett 224: 161–164, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-d-glucose induce Fos-like immunoreactivity in rat brain. Brain Res 641: 111–120, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 144: 1357–1367, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Romero LM, Butler LK. Endocrinology of stress. Int J Comp Psyc 20: 89–95, 2007. [Google Scholar]
- 46.Routh VH. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF). Diabetes Metab Res Rev 19: 348–356, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Routh VH. Glucose sensing neurons in the ventromedial hypothalamus. Sensors (Basel) 10: 9002–9025, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saberi M, Bohland M, Donovan CM. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes 57: 1380–1386, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Silver IA, Erecinska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol 79: 1733–1745. 1998. [DOI] [PubMed] [Google Scholar]
- 50.Solomon A, De Fanti BA, Martínez JA. Peripheral ghrelin participates in the glucostatic signaling mediated by the ventromedial and lateral hypothalamus neurons. Peptides 27: 1607–1615, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res 886: 113–164, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW. Brain Maps: Structure of the Rat Brain (3rd Ed.). Academic Press; (Open access available at http://larrywswanson.com/?page_id=164) 2004. [Google Scholar]
- 53.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 2007 5: 383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unger J, McNeill TH, Moxley IIIRT, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat brain. Neuroscience 31: 143–157, 1989. [DOI] [PubMed] [Google Scholar]
- 55.Veening JG, Coolen LM, Gerrits PO. Neural mechanisms of female sexual behavior in the rat; comparison with male ejaculatory control. Pharmacol Biochem Behav 121: 16–30, 2014. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Chen IZ, Lin D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85: 1344–1358, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol 31: 32–43, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watts AG, Khan AM, Sanchez-Watts G, Salter D, Neuner CM. Activation in neural networks controlling ingestive behaviors: what does it mean, and how do we map and measure it? Physiol Behav 89: 501–510, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Zhu W, Czyzyk D, Paranjape SA, Zhou L, Horblitt A, Szabó G, Seashore MR, Sherwin RS, Chan O. Glucose prevents the fall in ventromedial hypothalamic GABA that is required for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab 298: E971–E977, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494: 528–548, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]