Herein we describe new mouse models of cardiomyopathies induced by chronic plus single or multiple ethanol binges [National Institute on Alcohol Abuse and Alcoholism (NIAAA) models], which are characterized by impaired cardiovascular performance, mitochondrial dysfunction, and cardiac steatosis. Accordingly, the chronic plus binge ethanol feeding NIAAA mouse models are good candidates for the future investigations targeting alcoholic cardiomyopathy.
Keywords: alcoholic cardiomyopathy, heart failure, oxidative stress, mitochondrial dysfunction, cardiac steatosis
Abstract
Alcoholic cardiomyopathy in humans develops in response to chronic excessive alcohol consumption; however, good models of alcohol-induced cardiomyopathy in mice are lacking. Herein we describe mouse models of alcoholic cardiomyopathies induced by chronic and binge ethanol (EtOH) feeding and characterize detailed hemodynamic alterations, mitochondrial function, and redox signaling in these models. Mice were fed a liquid diet containing 5% EtOH for 10, 20, and 40 days (d) combined with single or multiple EtOH binges (5 g/kg body wt). Isocalorically pair-fed mice served as controls. Left ventricular (LV) function and morphology were assessed by invasive pressure-volume conductance approach and by echocardiography. Mitochondrial complex (I, II, IV) activities, 3-nitrotyrosine (3-NT) levels, gene expression of markers of oxidative stress (gp91phox, p47phox), mitochondrial biogenesis (PGC1α, peroxisome proliferator-activated receptor α), and fibrosis were examined. Cardiac steatosis and fibrosis were investigated by histological/immunohistochemical methods. Chronic and binge EtOH feeding (already in 10 days EtOH plus single binge group) was characterized by contractile dysfunction (decreased slope of end-systolic pressure-volume relationship and preload recruitable stroke work), impaired relaxation (decreased time constant of LV pressure decay and maximal slope of systolic pressure decrement), and vascular dysfunction (impaired arterial elastance and lower total peripheral resistance). This was accompanied by enhanced myocardial oxidative/nitrative stress (3-NT; gp91phox; p47phox; angiotensin II receptor, type 1a) and deterioration of mitochondrial complex I, II, IV activities and mitochondrial biogenesis, excessive cardiac steatosis, and higher mortality. Collectively, chronic plus binge EtOH feeding in mice leads to alcohol-induced cardiomyopathies (National Institute on Alcohol Abuse and Alcoholism models) characterized by increased myocardial oxidative/nitrative stress, impaired mitochondrial function and biogenesis, and enhanced cardiac steatosis.
NEW & NOTEWORTHY
Herein we describe new mouse models of cardiomyopathies induced by chronic plus single or multiple ethanol binges [National Institute on Alcohol Abuse and Alcoholism (NIAAA) models], which are characterized by impaired cardiovascular performance, mitochondrial dysfunction, and cardiac steatosis. Accordingly, the chronic plus binge ethanol feeding NIAAA mouse models are good candidates for the future investigations targeting alcoholic cardiomyopathy.
alcohol has been consumed in many cultures for centuries, being a most often used psychoactive substance. According to recently published data, chronic alcoholism along with its harmful effects ranks among the top five risk factors for disease and death (26). Whether regular alcohol consumption has beneficial or harmful effects is still a subject of intensive debates. Although low to moderate daily consumption is considered to have favorable consequences on cardiovascular mortality (5, 19), chronic and heavy drinking might lead to cardiac dysfunction or subsequent heart failure (30) and increases the risk of sudden cardiac death (15). Long-term, heavy alcohol consumption leads to the development of alcoholic cardiomyopathy, a specific heart disease associated with characteristic tissue injury and histological and functional alterations (34).
Several contributing factors have been identified in the development of alcoholic cardiomyopathy such as oxidative and nitrative stress, myocardial hypertrophy, acetaldehyde protein adduct formation, apoptosis, increased activation of angiotensin II - angiotensin II receptor signaling, and fibrotic remodeling (34).
During the metabolism of ethanol (EtOH) it is degraded by the alcohol-dehydrogenase (ADH) or cytochrome P-450 2E1 (CYP2E1) enzymes into acetaldehyde (55). Because cardiac tissues have very low levels of ADH and CYP2E1 (1), EtOH may exert direct toxic effects in cardiomyocytes by interfering with the cardiac contractile protein synthesis or by inhibition of the enzymes of the citrate cycle and disturbance of the main mitochondrial biogenesis regulator, the peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) (46). The main product of EtOH metabolism is acetaldehyde, whereas reactive oxygen species (ROS) are formed as by-products. Acetaldehyde is a highly reactive molecule that binds to proteins or other macromolecules forming protein-adducts, which may further aggravate the toxic effects of EtOH (4). Superoxide anion bind to nitric oxide (NO) forming the highly reactive molecule peroxynitrite (32). Peroxynitrite then binds to different structural and contractile proteins and enzymes that contributes to subsequent cellular dysfunction (32). In chronic alcoholism, excessive NO production was found with increased endothelial and inducible NO synthase levels (8), which might further contribute to the oxidative/nitrative stress.
It is noteworthy, that binge drinking is the most common form of alcohol abuse in cases of young adults, according to recently published data in 2013 (38). Binge drinking was reported to exert adverse cardiovascular effects including macro- and microvascular dysfunction (11), increased atherosclerotic plaque development (27), coronary calcification (35), and myocardial injury (52, 54). Although, there are several studies in the literature that investigate the effects of either long-term (16, 17, 21, 43) or acute (12, 18, 25, 40) EtOH consumption, animal models that are mimicking the human drinking patterns are limited. Bertola et al. (3) reported a mouse model of chronic plus binge EtOH feeding, the so-called NIAAA (National Institute on Alcohol Abuse and Alcoholism) model, which was developed to conform with human drinking behavior often seen in chronic alcoholics.
In this study we aimed to develop mouse models of alcoholic cardiomyopathies induced by chronic and binge ethanol (EtOH) feeding based on NIAAA mouse alcohol model, and characterize in detail cardiovascular function and pathological and biochemical alterations in these models.
METHODS
Animals.
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the NIAAA. Forty-two young male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) at the age of 12–14 wk were included in the study and were kept in a specific pathogen-free animal facility under constant temperature (22 ± 2°C), humidity and with 12-h alternating light cycles.
Experimental protocol and treatment groups.
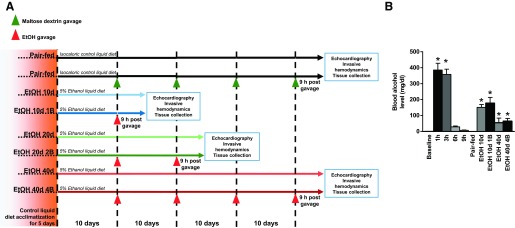
Mice were fed according to the modified NIAAA model described previously (3). In brief, all mice were fed the Lieber-DeCarli liquid diet (Bio-Serv, Frenchtown, NJ) ad libitum for the first 5 days as acclimatization. Then, the feeding protocol was switched to one of the following seven protocols (Fig. 1A): for chronic feeding: 1) free access to ethanol (EtOH) Lieber-DeCarli (Bio-Serv) diet containing 5% (vol/vol) ethanol for 10 days (EtOH 10d, n = 6); 2) for 20 days (EtOH 20d, n = 5); 3); for 40 days (EtOH 40d, n = 4); for chronic feeding combined with EtOH binge gavage early in the morning: 4) 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg body wt EtOH solution (EtOH 10d 1B, n = 8); 5) 5% (vol/vol) EtOH for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg body wt EtOH solution (EtOH 20d 2B, n = 6); 6) 5% (vol/vol) EtOH for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg body wt EtOH solution (EtOH 40d 4B, n = 6); and 7) pair-feeding: control mice were isocalorically pair-fed with control Lieber-DeCarli (Bio-Serv) diet for 10, 20, and 40 days. Half of the control animals with isocalorical maltose-dextrin (9 g/kg body wt) gavage served as controls for EtOH binge groups. Because we found no significant differences in the hemodynamic parameters of the pair-fed groups, we combined the animals into one pair-fed group (n = 7). An additional set of animals (without chronic EtOH feeding) was used to investigate the time course of blood alcohol levels at different time points (baseline, 1 h, 3 h, 6 h, and 9 h; n = 4 at each time point) after 5 g/kg body wt EtOH oral gavage. To exclude acute, direct effects of EtOH, we decided to perform all our in vivo experiments and sampling after 9 h of EtOH gavage, where EtOH levels returned near to the baseline values (Fig. 1B).
Fig. 1.
Protocol outline and blood alcohol levels in the study groups. A: feeding protocol used in our study. Green arrows represent maltose dextrin [9 g/kg body wt (BW) gavage]; red arrows represent ethanol (5 g/kg BW) gavage in the early morning. Animals were euthanized at the end of the feeding period, 9 h after gavage. Groups that did not receive gavage were euthanized at the end of the feeding period. At the end of the feeding period, echocardiography, invasive hemodynamic examination, and tissue collection were performed. Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B). B: time course and results of blood alcohol levels in different study groups. *P < 0.05 vs. baseline (in case of the time course study) or vs. Pair-fed in chronic EtOH-fed groups.
Echocardiography.
At the end of the feeding period, depending on the feeding protocol, echocardiography was performed 9 h after the last EtOH or maltose-dextrin gavage. Mice were anesthetized with 1% to 2% isoflurane in 100% oxygen, placed on a temperature controlled heating pad, shaved and prepared for echocardiographic examination. Echocardiography was conducted by using a Vevo-770 Imaging system (FUJIFILM VisualSonics, Toronto, ON, Canada) coupled with RMV-707B (30 MHz) scanhead, as described previously (14). M-mode images were taken on short-axis plane at the midpapillary level. B-mode images, acquired in the long-axis and in the short-axis at the midpapillary level, were used to measure left ventricular (LV) anterior wall (AW) and posterior wall (PW) thickness and LV internal diameter (ID) in end diastole (d) and in end systole (s). Pulse-wave Doppler measurements were performed to assess the ratio of the early (E) to late (A) ventricular filling velocities (E/A ratio). End systole was defined at minimal, whereas end diastole was defined at maximal, LVID. Values presented here were averages of three consecutive cycles. Fractional shortening (FS) was determined as FS = [(LVIDd − LVIDs)/LVIDd] × 100. LV mass was calculated by the following equation: LVmass = 1.04× [(LVAWd + LVIDd + LVPWd)3 − LVIDd3] (6). LV volume was estimated according to the Teichholz formula (9). Ejection fraction (EF) was defined as the ratio of stroke volume and end-diastolic volume. Relative wall thickness (RWT) was calculated as RWT = (LVAWd + LVPWd)/LVIDd (23).
Hemodynamic measurements.
After the echocardiographic examination, invasive hemodynamic measurements were performed by a pressure-conductance catheter system (MPVS-Ultra; Millar Instruments, Houston, TX) and a PVR-1045 1F pressure-volume (P-V) microcatheter (Millar Instruments) to assess detailed LV and vascular performance as described earlier (33). Mice were anesthetized with 1% to 2% isoflurane in 100% oxygen and placed on a temperature controlled heating pad. Hemodynamic parameters were analyzed by the PVAN software (Millar Instruments). Mean arterial pressure (MAP), heart rate, EF, mechanical efficiency (efficiency; the ratio of stroke work and P-V area), maximal slope of systolic pressure increment (dP/dtmax) and decrement (dP/dtmin), time constant of LV pressure decay (Tauw; Weiss method), LV end-diastolic pressure (LVEDP), and arterial elastance (Ea) were analyzed. The slope (Ees) of the LV end-systolic P-V relationships (ESPVR; linear model), the preload recruitable stroke work (PRSW), and the dP/dtmax-end-diastolic volume (EDV) were evaluated as pre- and afterload-independent systolic indexes, and the slope of the end-diastolic P-V relationship (EDPVR) was calculated as LV stiffness parameter. Ventriculo-arterial coupling was determined as the ratio of Ea and Ees. Total peripheral resistance (TPR) was expressed as the ratio of MAP and cardiac output. At the end of the hemodynamic measurements, animals were euthanized and fresh frozen (in liquid nitrogen) LV samples were collected and stored at −80°C for further experimentation. For cryosectioning and histological staining, LV samples were fixed on dry ice in optimal cutting temperature compound (Tissue-Tek; Fisher Scientific, Pittsburgh, PA) and stored at −80°C for long term. For histological purposes, LV samples were fixed in 10% neutral buffered formalin and embedded in paraffin.
RT and real-time PCR analysis.
LV samples were homogenized and total RNA was isolated by using QIAzol reagents (Qiagen, Valencia, CA) according to the manufacturer's protocol. Then, RNA was treated with Rnase-free DNAse I (Ambion; Thermo Fisher Scientific, Waltham, MA) to remove potential genomic DNA contamination. RNA (2 μg) was reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) according to the manufacturer's guidelines with the following cycling conditions: 1.25°C for 10 min, 37°C for 120 min, 85°C for 5 min. Real-time PCR was performed using specific primer sets (Invitrogen, Carlsbad, CA) and SyberSelect PCR Master Mix (Invitrogen) in a ABI 7900HT Realtime PCR Instrument (Applied Biosystems, Foster City, CA) for the following targets (primer sequences provided in Table 1): P0, large, ribosomal protein (RPLP0); atrial natriuretic peptide (ANP); p47phox; gp91phox; PGC1α; peroxisome proliferator-activated receptor α (PPARα); medium-chain acyl-CoA dehydrogenase; estrogen-related receptor α (ERRα); acetyl-CoA oxidase 1; acetyl-CoA carboxylase 1 and 2; collagen 1a1 (Col1a1); fibronectin 1; and angiotensin II receptor, type 1a. Relative quantification was calculated using the comparative CT method. P0, large, ribosomal protein was used as a housekeeping gene to normalize gene expression data.
Table 1.
Primer sequences used in quantitative RT-PCR experiments
| Primer |
||
|---|---|---|
| Target Gene | Forward | Reverse |
| P0, large, ribosomal protein | TCCAGGCTTTGGGCATCA | CTTTATCAGCTGCACATCACTCAGA |
| Atrial natriuretic peptide | TTTCAAGAACCTGCTAGACCACCT | GCAGAGCCCTCAGTTTGCTT |
| p47phox | TTCCATCCCCAAATGCAAAG | TCAGATGCCCTAAAACCGGAG |
| gp91phox (NOX2) | ATCACCAAGGTGGTCACCCA | TGAATAGCCCCTCCGTCCAG |
| Peroxisome proliferator-activated receptor c coactivator 1α | AGACAGGTGCCTTCAGTTCAC | AGCAGCACACTCTATGTCACT |
| Peroxisome proliferator-activated receptor α | TCGGCCTGGCCTTCTAAACATAG | TCTTGCAACAGTGGGTGCAGCG |
| Medium-chain acyl-CoA dehydrogenase | GATCGCAATGGGTGCTTTTGATAGAA | AGCTGATTGGCAATGTCTCCAGCAAA |
| Estrogen-related receptor α | ACTGCCACTGCAGGATGAG | CACAGCCTCAGCATCTTCAA |
| Acetyl-CoA oxidase 1 | CCTGTTGGCCTCAATTACTCC | CCTCGAAGATGAGTTCCGTGG |
| Acetyl-CoA carboxylase 1 | TGGAGAGCCCCACACACA | GACAGACTGATCGCAGAGAAAG |
| Acetyl-CoA carboxylase 2 | CCCAGCCGAGTTTGTCACT | GGCGATGAGCACCTTCTCTA |
| Collagen 1a1 | GACCGATGGATTCCCGTTCG | GGTGCTGTAGGTGAAGCGAC |
| Fibronectin 1 | AGAAGACAGATGAGCTTCCCCA | CGTTGTCCGCCTAAAGCCAT |
| ANG II receptor, type 1a | CTGTCTGGCCGGAGAGGACT | GCACTTGATCTGCTGATGGCTT |
The table shows the primer sequences for the target genes shown.
Mitochondrial complex activity measurement.
Mitochondrial complex I, II, and IV activites were determined from whole heart lysates using microplate colorimetric assay kits (ab109721, ab109908, and ab109911; Abcam, Cambridge, MA) according to the supplied protocol, as described previously (31). Complex activities were expressed as fold change compared with the pair-fed group.
Myocardial 3-nitrotyrosine measurement.
3-Nitrotyrosine levels in LV myocardial samples were determined by a nitrotyrosine enzyme immunoassay (EIA) according to the manufacturer's protocol (BIOXYTECH Nitrotyrosine-EIA; OxisResearch, Portland, OR) as described earlier (14).
Measurement of blood alcohol levels.
Blood was collected from the inferior caval vein at the end of the functional experiments, then serum samples were prepared and stored at −80°C. Serum alcohol levels were assessed by the Ethanol Colorimetric Assay (BioVision, Milpitas, CA) according to the kit instructions.
Cardiac oil red O staining.
For Oil Red O staining, LV samples were fixed on dry ice in optimal cutting temperature compound and 10 μm thin section were cut. Oil Red O (0.5%), dissolved in isopropanol (Sigma-Aldrich, St. Louis, MO), was prepared freshly, filtered through 0.22-μm filters, and diluted 1:5 in distilled water. Sections were then fixed in 10% neutral buffered formalin, rinsed in distilled water, and equlibrated in 60% isopronanol. After that, sections were stained for 10 min with Oil Red O working solution, differentiated in 60% isopropanol and distilled water. Finally, they were counterstained with hematoxylin, washed in tap water, and covered with aqueous mounting medium. Slides were examined under light microscope (400× magnification).
Histology.
Thin LV sections (5 μm) were cut from formalin-fixed, paraffin-embedded tissues. After deparaffination, slides were stained with hematoxylin and eosin to assess myocardial structure. A second set of slides was stained with Picro-Sirius Red according to standard pathological methods (for 1 h). Cardiac fibrosis was investigated under a light microscope (400×), and fibrotic area was quantified by ImageJ software (NIH Public Domain) using color thresholding in 10 random LV area.
The third set of slides was used to assess cardiomyocyte cross-sectional area. Thin, paraffin-embedded sections (5 μm) were deparaffinized, washed in 1 × PBS, and stained with wheat germ agglutinin conjugated with Alexa Fluor-594 (W11262, Molecular Probes; Life Technologies, Eugene, OR) for 10 min at RT (5 μg/ml, 10 min). After 1 × PBS was washed, slides were nuclear counterstained with DRAQ5 Fluorescent Probe (4084S, 1:1,000; Cell Signaling Technologies, Danvers, MA) for 5 min at RT. Finally, sections were washed in 1 × PBS and mounted with aqueous medium. Sections were analyzed in a confocal microscope (LSM710; Carl Zeiss AG, Jena, Germany), images were taken (200 × magnification), and cardiomyocyte cross-sectional area (in μm2) was determined by using ImageJ (NIH) software in 10 random fields of each section.
Statistics.
Data are presented as means ± SE. Shappiro-Wilk test was used to confirm normal distribution of our data. One-way ANOVA was performed. Tukey honestly significant difference post hoc testing was carried out to determine intergroup differences. Where data did not show normal distribution, Kruskal-Wallis ANOVA was applied with Dunn's post hoc test. A P value of <0.05 was considered significant.
RESULTS
The effect of EtOH binge and chronic EtOH feeding on blood alcohol levels.
Blood alcohol level increased significantly and reached its peak at 1 h and decreased dramatically by 9 h after oral gavage by 5 g/kg body wt EtOH in control animals (Fig. 1B). Additionally, we observed increased alcohol levels in chronic and binge EtOH feeding groups compared with the pair-fed (Fig. 1B). However, there was no statistical difference between the chronic plus binge EtOH feeding groups (blood was collected 9 h post-binge) in comparison with their corresponding chronic EtOH-fed group (Fig. 1B).
The effect of chronic alcoholism and binge drinking on body weight and heart weight.
We did not observe any differences in the body weight, tibia length, and heart weight-to-tibia length ratio at the time of euthanization (Table 2). However, heart weight-to-body weight ratio was statistically different in EtOH 10d 1B from its corresponding chronic EtOH-fed group (Table 2).
Table 2.
Study group characteristics in alcoholic cardiomyopathy
| Variable | Pair-fed | EtOH 10d | EtOH 10d 1B | EtOH 20d | EtOH 20d 2B | EtOH 40d | EtOH 40d 4B |
|---|---|---|---|---|---|---|---|
| Body weight, g | 26.3 ± 0.4 | 25.0 ± 0.07 | 26.1 ± 0.7 | 26.3 ± 0.08 | 26.6 ± 0.5 | 27.8 ± 0.6 | 26.4 ± 0.6 |
| Heart weight/tibia length, mg/mm | 6.5 ± 0.1 | 6.9 ± 0.02 | 6.4 ± 0.2 | 6.7 ± 0.5 | 6.2 ± 0.5 | 7.3 ± 0.1 | 6.6 ± 0.2 |
| Heart weight/body weight, mg/g | 5.0 ± 0.1 | 5.4 ± 0.2 | 4.6 ± 0.1# | 4.8 ± 0.4 | 4.4 ± 0.3 | 4.9 ± 0.1 | 4.8 ± 0.1 |
| Tibial length, mm | 19.3 ± 0.3 | 18.8 ± 0.3 | 18.9 ± 0.1 | 18.9 ± 0.2 | 18.9 ± 0.3 | 19.0 ± 0.4 | 19.0 ± 0.3 |
| Heart rate, beats/min | 599 ± 10 | 616 ± 7 | 490 ± 13*# | 608 ± 10 | 511 ± 18*# | 623 ± 12 | 504 ± 19*# |
Values are means ± SE. The table shows the basic characteristic of the study groups. Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg body wt EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg body wt EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg body wt EtOH solution (EtOH 40d 4B).
P < 0.05 vs. Pair-fed;
P < 0.05 vs. corresponding chronic EtOH-fed group.
Hemodynamic alterations: cardiac dysfunction in chronic alcoholism and binge drinking.
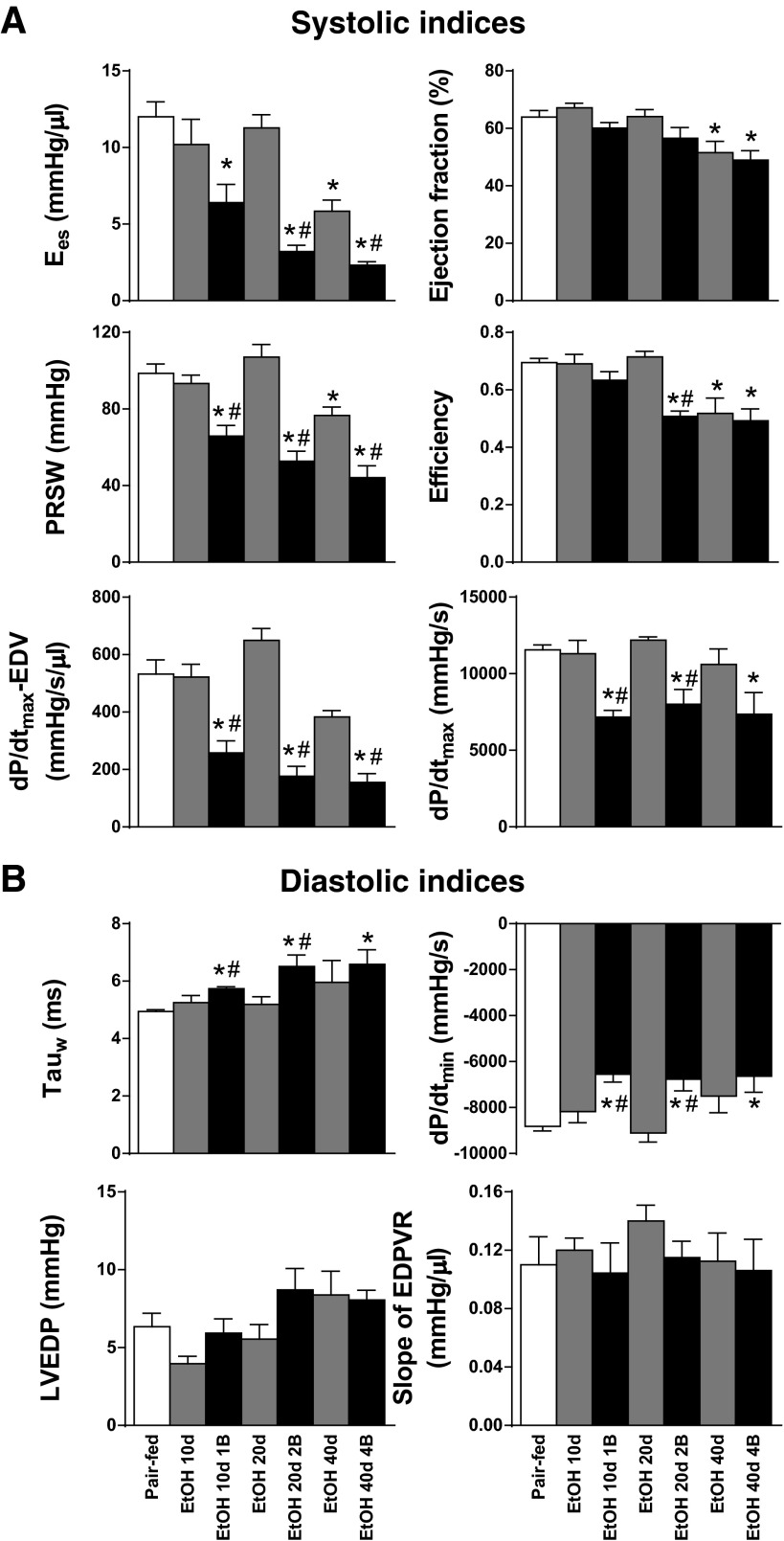
Invasive hemodynamic investigation revealed that the development of alcoholic cardiomyopathy was associated with overt contractile dysfunction (Figs. 2A and 3A) and impaired relaxation (Fig. 3B) of the LV. In comparison with the Pair-fed group, the pre- and afterload independent, sensitive parameters of LV contractility (Ees, PRSW and dP/dtmax-EDV) were already significantly impaired in the EtOH 10d 1B group, which phenomenon worsened by the repetitive EtOH binges and increased duration of alcohol feeding in EtOH 20d 2B and EtOH 40d 4B groups, respectively (Fig. 3A). We observed a similar pattern in the case of the classic systolic parameter dP/dtmax (Fig. 3A). Interestingly, Ees and PRSW were significantly decreased in the EtOH 40d group than in the pair-fed group (Fig. 3A). Additionally, EF showed significant decrease in only EtOH 40d and EtOH 40d 4B groups when compared with the pair-fed group (Fig. 3A). Cardiac efficiency markedly worsened in the chronic EtOH feeding groups EtOH 20d 2B, EtOH 40d, and EtOH 40d 4B (Fig. 3A). Contractile function showed marked deterioration in the chronic feeding plus binge groups when compared with their corresponding chronic feeding groups (Fig. 3A).
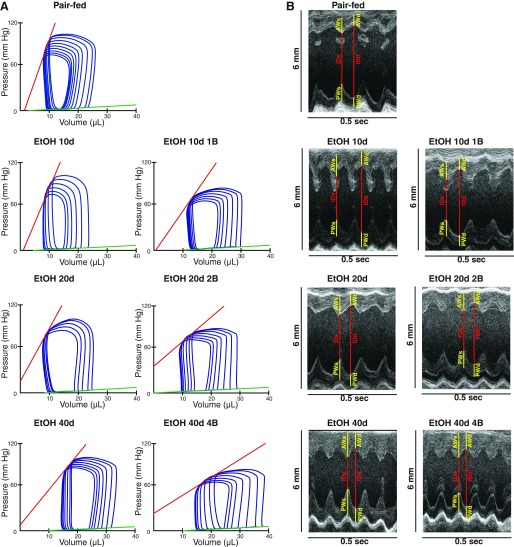
Fig. 2.
Chronic alcohol feeding and binge drinking impairs cardiac performance. A: representative pressure-volume loops in the study groups. B: representative M-mode images taken of the short-axis view at the midpapillary level of the left ventricle in the study groups. Yellow lines represent anterior and posterior wall thickness in end systole (AWs and PWs) and in end diastole (AWd and PWd). Red lines represent internal diameter in end systole (IDs) and in end diastole (IDd). Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B).
Fig. 3.
Chronic alcohol feeding and binge drinking is associated with impaired cardiac function. Systolic indexes were derived from pressure-volume analysis of the study groups. The following indexes are shown: slope of end-systolic pressure-volume relationship (Ees), preload recruitable stroke work (PRSW), maximal slope of systolic pressure increment (dP/dtmax), dP/dtmax-end-diastolic volume (dP/dtmax-EDV), ejection fraction (EF), and efficiency. B: indexes of diastolic function: time constant of left ventricular pressure decay (Tauw, according to Weiss method) and maximal slope of systolic pressure decrement (dP/dtmin). Stiffness parameters: left ventricular end-diastolic pressure (LVEDP) and slope of end-diastolic pressure-volume relationship (EDPVR). Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B). *P < 0.05 vs. Pair-fed; #P < 0.05 vs. corresponding chronic EtOH-fed groups.
Beside the significant worsening of the contractile function, alcoholic cardiomyopathy was associated with significant impairment of LV relaxation (Tauw, dP/dtmin) in binge drinking combined EtOH-fed groups (EtOH 10d 1B, EtOH 20d 2B, EtOH 40d 4B) in comparison with the pair-fed and chronic EtOH-fed groups (Fig. 3B). Despite the impaired global cardiac function observed in our model, LVEDP and the slope of EDPVR did not differ significantly between the study groups (Fig. 3B).
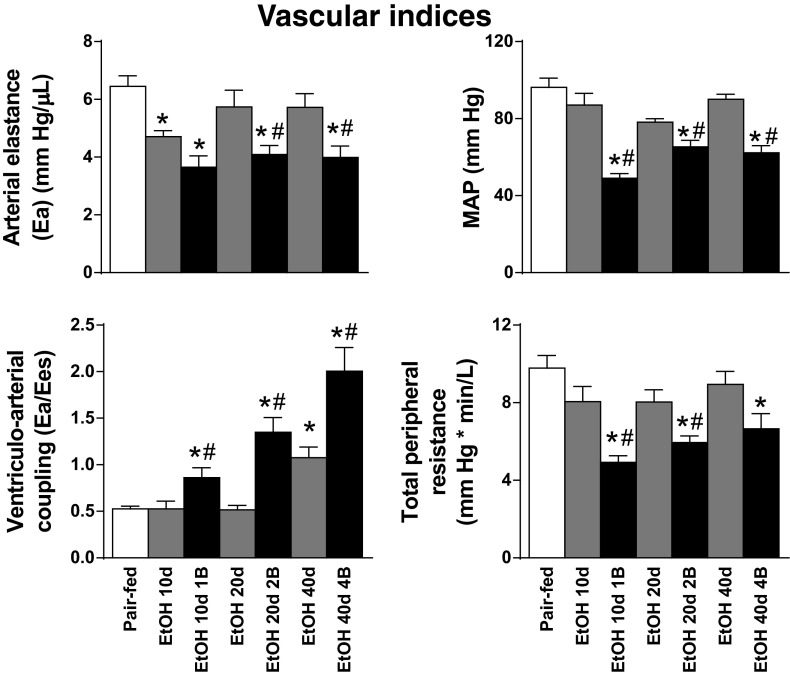
Regarding the vascular indexes, we observed decreased MAP values in the EtOH 10d 1B, EtOH 20d 2B, and EtOH 40d 4B groups (Fig. 4) compared with the Pair-fed animals. In relation to these changes, alcohol intake induced remarkable decrease of TPR in the binge-fed EtOH 10d 1B, EtOH 20d 2B, and EtOH 40d 4b groups (Fig. 4). Although Ea was significantly lower already in the EtOH 10d and in binge-combined EtOH 10d 1B, EtOH 20d 2B, and EtOH 40d 4B groups (Fig. 4), the ventriculo-arterial coupling ratio showed significant increase in EtOH 10d 1B, EtOH 20d 2B, EtOH 40d, and EtOH 40d 4B groups compared with the Pair-fed and chronic EtOH-fed groups, respectively (Fig. 4).
Fig. 4.
Chronic alcohol feeding and binge drinking induces vascular dysfunction. Vascular indexes: arterial elastance (Ea), mean arterial pressure (MAP), ventriculo-arterial coupling and total peripheral resistance. Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B). *P < 0.05 vs. Pair-fed; #P < 0.05 vs. corresponding chronic EtOH-fed groups.
Despite the significant worsening of cardiac function detected by invasive hemodynamic investigation (Fig. 3A), echocardiographic examination did not show any differences in the EF and FS between the study groups (Table 3). However, echocardiography revealed marked alterations in cardiac morphology in alcoholic cardiomyopathy (Fig. 2B). LVAWd and LVAWs values were significantly increased in EtOH 40d and EtOH 40d 4B groups compared with the Pair-fed (Table 3). Additionally, LVAWs was significantly increased in every EtOH-fed groups (Table 3), whereas LVIDd and LVIDs did not differ among the study groups (Table 3). LVPWd and LVPWs showed marked elevation in only the EtOH 10d 1B, EtOH 20d 2B, and EtOH 40d 4B groups (Table 3). According to these, we found significant increase in RWT in EtOH 40d and EtOH 40d 4B groups compared with Pair-fed groups (Table 3). Despite this, the diastolic function marker E/A ratio and the LVmass did not differ among the study groups (Table 3).
Table 3.
Echocardiography parameters in alcoholic cardiomyopathy
| Variable | Pair-fed | EtOH 10d | EtOH 10d 1B | EtOH 20d | EtOH 20d 2B | EtOH 40d | EtOH 40d 4B |
|---|---|---|---|---|---|---|---|
| Ejection fraction, % | 60 ± 3 | 64 ± 3 | 64 ± 1 | 61 ± 2 | 63 ± 3 | 63 ± 3 | 62 ± 2 |
| Fractional shortening, % | 33.8 ± 2.0 | 36.5 ± 3.4 | 33.2 ± 0.7 | 29.9 ± 0.6 | 32.4 ± 1.6 | 30.6 ± 3.5 | 33.9 ± 0.5 |
| LVAWd, mm | 0.80 ± 0.04 | 0.89 ± 0.01 | 0.81 ± 0.03 | 0.81 ± 0.03 | 0.78 ± 0.03 | 0.97 ± 0.03* | 0.93 ± 0.03* |
| LVAWs, mm | 1.02 ± 0.03 | 1.26 ± 0.05* | 1.19 ± 0.03* | 1.20 ± 0.05* | 1.19 ± 0.05* | 1.38 ± 0.04* | 1.34 ± 0.04* |
| LVIDd, mm | 3.78 ± 0.07 | 3.44 ± 0.14 | 3.63 ± 0.12 | 3.72 ± 0.05 | 3.62 ± 0.07 | 3.58 ± 0.09 | 3.49 ± 0.09 |
| LVIDs, mm | 2.64 ± 0.12 | 2.25 ± 0.19 | 2.47 ± 0.09 | 2.61 ± 0.03 | 2.45 ± 0.08 | 2.48 ± 0.12 | 2.39 ± 0.04 |
| LVPWd, mm | 0.75 ± 0.02 | 0.78 ± 0.02 | 0.90 ± 0.04*# | 0.81 ± 0.04 | 0.93 ± 0.04* | 0.81 ± 0.04 | 0.88 ± 0.04* |
| LVPWs, mm | 1.00 ± 0.03 | 1.08 ± 0.03 | 1.18 ± 0.06* | 1.11 ± 0.05 | 1.22 ± 0.09* | 1.10 ± 0.04 | 1.17 ± 0.06* |
| E/A ratio | 1.51 ± 0.07 | 1.54 ± 0.26 | 1.61 ± 0.16 | 1.57 ± 0.29 | 1.37 ± 0.07 | 1.43 ± 0.05 | 1.37 ± 0.08 |
| LVmass, mg | 106.3 ± 4.5 | 100.1 ± 5.5 | 105.7 ± 7.8 | 105.6 ± 6.4 | 105.0 ± 7.0 | 111.3 ± 7.3 | 105.9 ± 5.1 |
| Relative wall thickness (LVAWd + LVPWd/LVIDd) | 0.41 ± 0.02 | 0.46 ± 0.02 | 0.46 ± 0.02 | 0.44 ± 0.01 | 0.46 ± 0.02 | 0.50 ± 0.02* | 0.51 ± 0.02* |
Values are means ± SE. The table shows the results of the following echocardiographic parameters in alcoholic cardiomyopathy: ejection fraction, fractional shortening, left ventricular (LV) anterior wall thickness in diastole (LVAWd), LVAW thickness in systole (LVAWs), LV internal diameter in diastole (LVIDd), LVID in systole (LVIDs), LV posterior wall thickness in diastole (LVPWd), LVPW in systole (LVPWs), ratio of the early (E) to late (A) ventricular filling velocities (E/A ratio), LVmass, and relative wall thickness. Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg body wt EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg body wt EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B)
P < 0.05 vs. Pair-fed
P < 0.05 vs. corresponding chronic EtOH-fed group.
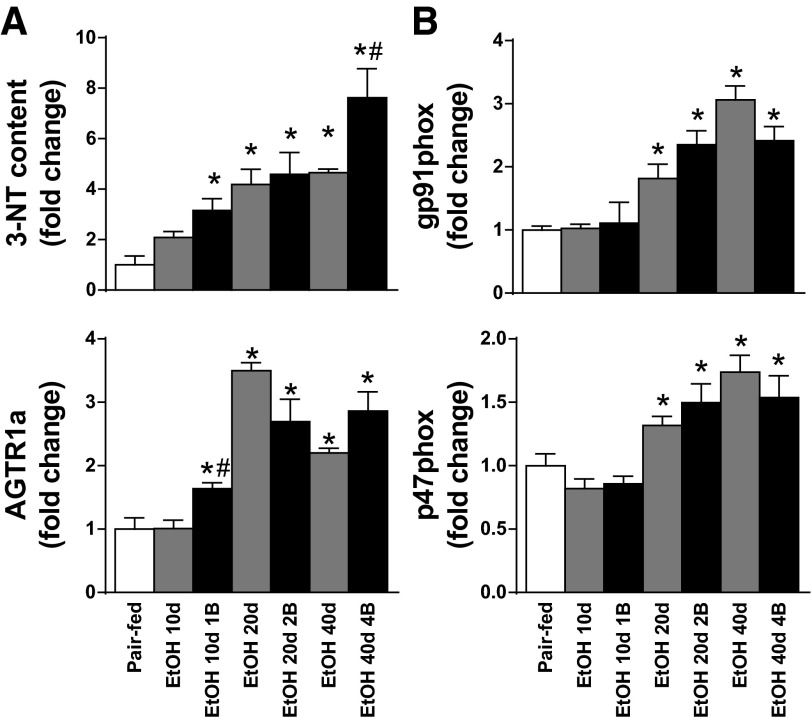
Alcoholic cardiomyopathy in chronic alcoholism combined with binge drinking is associated with excessive oxidative/nitrative stress and mitochondrial dysfunction.
Myocardial 3-nitrotyrosine content was significantly elevated already in the EtOH 10d 1B group and increased with the increment of EtOH feeding duration and the number of binges in our model (Fig. 5A). Furthermore, we found significant elevation of the myocardial gene expression values of gp91phox, p47phox, and angiotensin II receptor, type 1a in hearts of mice on alcohol diets (Fig. 5B).
Fig. 5.
Chronic alcohol intake and binge drinking increases myocardial oxidative/nitrative stress. A: myocardial 3-nitrotyrosine (3-NT) content in the left ventricle. B: gene expression values of gp91phox, p47phox, and angiotensin II receptor, type 1a (AGTR1a) of the LV cardiac tissue are represented. Groups: Pair-fed (isocalorically fed mice with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B). *P < 0.05 vs. Pair-fed; #P < 0.05 vs. corresponding chronic EtOH-fed groups.
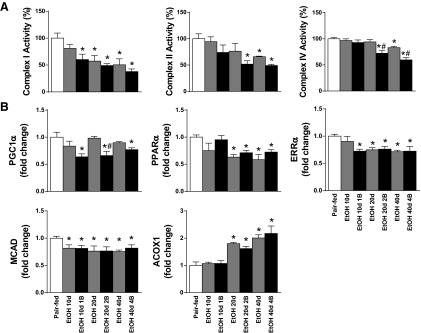
Chronic alcoholism combined with single or multiple binges impairs mitochondrial function, biogenesis, and fatty acid metabolism.
The marked oxidative/nitrative stress resulted in myocardial mitochondrial dysfunction in our model (Fig. 6A). It is noteworthy, that the mitochondrial complex I activity was significantly lower at an early time point in EtOH 10d 1B group and continued to decrease by duration of EtOH feeding and by the number of EtOH binges (Fig. 6A). However, mitochondrial complex II and IV activities were intact at early time points but attenuated in EtOH 20d 2B, EtOH 40d, and EtOH 40d 4B groups compared with Pair-fed and chronic EtOH-fed groups (Fig. 6A).
Fig. 6.
Chronic and binge drinking impairs mitochondrial function and biogenesis. A: mitochondrial complex I, II, and IV activites are shown in the myocardium. B: gene expression values of the following members of mitochondrial biogenesis are shown: PGC1α, peroxisome proliferator-activated receptor alpha (PPARα), estrogen-related receptor α (ERRα), medium-chain acyl-CoA dehydrogenase (MCAD), acetyl-CoA oxidase 1 (ACOX1). Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B). *P < 0.05 vs. Pair-fed; #P < 0.05 vs. corresponding chronic EtOH-fed groups.
Beside the observed dysfunction of the mitochondrial complexes in the alcoholic cardiac tissue, we found significant differences among the regulators of mitochondrial biogenesis and metabolism. EtOH feeding combined with binge drinking was associated with significant decrease of PGC1α mRNA levels in EtOH 10d 1B, EtOH 20d 2B, and EtOH 40d 4B groups (Fig. 6B). PPARα gene expression was remarkably reduced, whereas acetyl-CoA oxidase 1 gene expression level was markedly elevated in chronic EtOH-fed plus multiple binges groups (Fig. 6B). Additionally, ERRα and medium-chain acyl-CoA dehydrogenase mRNA expression significantly decreased in LV samples in alcoholic cardiomyopathies (Fig. 6B).
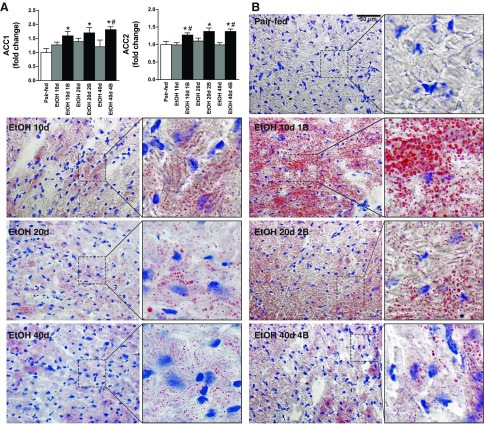
Alcoholic cardiomyopathy is associated with massive lipid accumulation in the cardiomyocytes.
Markedly elevated gene expression values of acetyl-CoA carboxylase 1 and 2 were presented in study groups treated with single or multiple EtOH binges (EtOH 10d 1B, EtOH 20d 2B, EtOH 40d 4B) (Fig. 7A). In parallel with these, chronic alcoholism combined with single binge (EtOH 10d 1B) induced massive myocardial steatosis compared with Pair-fed mice detected by Oil Red O staining (Fig. 7B). Cardiac steatosis was an early phenomenon, which was largely amplified by binges (EtOH 10d, EtOH 10d 1B, EtOH 20d 2B), and had tendency for attenuation by the increased duration of EtOH feeding (Fig. 7B).
Fig. 7.
Chronic alcoholism and binge drinking leads to myocardial fat accumulation. A: gene expression values of acetyl-CoA carboxylase 1 and 2 (ACC1, ACC2) of the left ventricle. B: representative images of Oil Red O stained left ventricle sections with lipid droplets in the cardiomyocytes. Magnification: 400×. Scale bar: 50 μm. Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B). *P < 0.05 vs. Pair-fed; #P < 0.05 vs. corresponding chronic EtOH-fed groups.
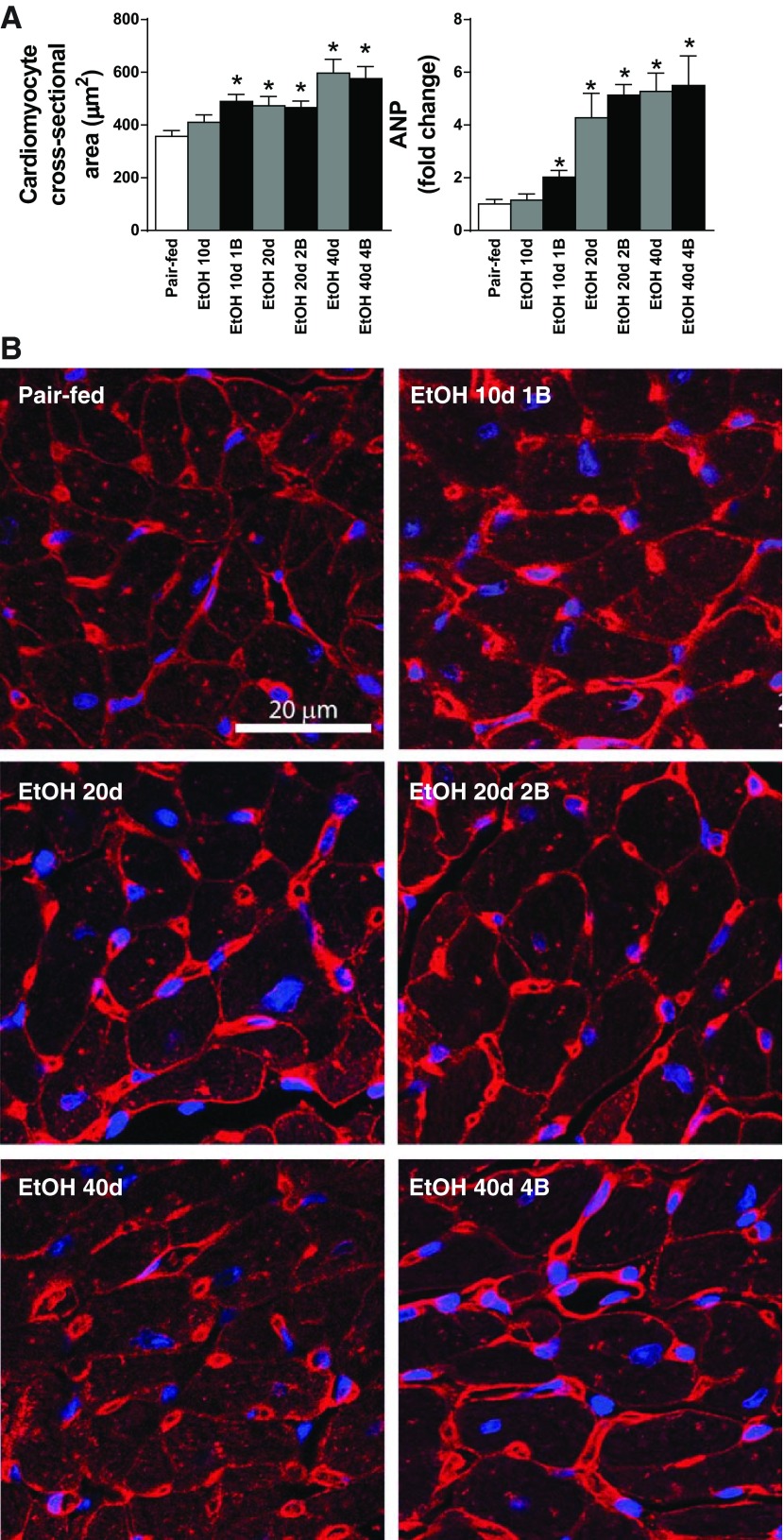
Chronic alcoholism and binge drinking induces mild cardiomyocyte hypertrophy.
Histological analysis of Wheat germ agglutinin-stained sections and measurement of cardiomyocyte cross-sectional area revealed mild cardiomyocyte hypertrophy in alcoholic cardiomyopathy (Fig. 8, A and B). Cardiomyocyte cross-sectional area was significantly elevated in our study groups, except for short-term EtOH feeding (EtOH 10d) in comparison with the Pair-feeding animals (Fig. 8A). Consistently with this, the gene expression of the hypertrophy marker ANP was increased (Fig. 8A).
Fig. 8.
Chronic alcoholism and binge drinking induces mild cardiomyocyte hypertrophy. A: cardiomyocyte cross-sectional area and left ventricular gene expression values of atrial natriuretic peptide (ANP) in our study groups. B: representative images of wheat germ agglutinin (WGA; red color)-stained left ventricular sections. Nuclear counterstaining of DRAQ5 is shown as blue. Magnification: 200×. Scale bar: 20 μm. Groups: Pair-fed (isocalorically fed with Lieber-DeCarli control liquid diet), ethanol (EtOH) Lieber-DeCarli liquid diet containing 5% (vol/vol) EtOH for 10 days (EtOH 10d); for 20 days (EtOH 20d); for 40 days (EtOH 40d); liquid diet containing 5% (vol/vol) EtOH for 10 days and binge feeding on day 11 with 5 g/kg BW EtOH solution (EtOH 10d 1B); for 20 days combined with binge feeding on days 11 and 21 with 5 g/kg BW EtOH solution (EtOH 20d 2B); for 40 days combined with binge feeding on days 11, 21, 31, and 41 with 5 g/kg BW EtOH solution (EtOH 40d 4B). *P < 0.05 vs. Pair-fed.
Chronic alcoholism and binge drinking was not associated with increased myocardial fibrosis.
Picro-Sirius Red staining and fibrotic area measurements did not show any difference among the study groups. mRNA levels of fibronectin 1 and Col1a1 did not differ in our models from the Pair-fed group (data not shown). Chronic alcohol feeding significantly affected the survival rate. Although the mortality was 0% in EtOH 10d and EtOH 10d 1B, it increased up to 25% in the long-term EtOH-fed groups (data not shown).
DISCUSSION
Excessive alcohol consumption leads to the development of nonischemic dilated cardiomyopathy, called alcoholic cardiomyopathy (44, 49). Alcoholic cardiomyopathy is characterized by LV dilation and hypertrophy (13). The pathophysiology of the disease includes the presence of cardiac oxidative/nitrative stress, mitochondrial dysfunction and disturbance of mitochondrial biogenesis, myocardial hypertrophy, LV dysfunction, and remodeling (34). Alcoholism-associated mortality and morbidity is closely related to the deleterious effects of the combination of chronic with binge drinking in humans. However, there are limited studies investigating the effect of binge drinking on the myocardium. Therefore, new animal models that mimic the drinking pattern of the human are warranted (34). Bertola et al. (3) reported the mouse model of chronic plus binge ethanol feeding, which closely mimics the human conditions of chronic long-term alcoholism with occasional binge drinking.
Herein, we report that modified NIAAA chronic and binge alcohol feeding mouse models are associated with 1) severe cardiac dysfunction, 2) excessive oxidative-nitrative stress, 3) deterioration of the mitochondrial complex activity and biogenesis, and 4) cardiac steatosis.
Ethanol is metabolized into acetaldehyde by ADH or CYP2E1, and acetaldehyde is transported to the mitochondria for further degradation through aldehyde-dehydrogenase to acetate and finally to acetyl-CoA (55). During the metabolism of ethanol ROS are produced as by-products, which results in pronounced oxidative stress (43, 45, 51). ROS are highly reactive molecules that bind to different proteins and enzymes, thereby reducing their function and leading to cellular malfunctions (32). Superoxide is able to react with NO to form the reactive nitrogen species peroxynitrite, which in turn damages different cellular targets, including contractile proteins, enzymes, and the mitochondria by oxidation and nitration (32). As a results of the oxidative/nitrative damage, high amount of intramitochondrial ROS is produced (47); thus a vicious cycle of ROS production develops. Consistent with the above investigations, we have found significantly increased myocardial oxidative and nitrative stress. Our experiments showed that the degree of oxidative/nitrative stress positively correlated with the time being on alcohol feeding diet and by the number of EtOH binges. In alcoholism, mitochondrial injury occurs due to the overproduction of ROS, which causes mitochondrial DNA damage (24) and decreases the activity of mitochondrial complexes (20). Additionally, EtOH is metabolized in the heart through the fatty acid (FA) ethyl ester synthase into FA ethyl ester (2) that are potentially toxic products. They interact with several intracellular pathways, including mitochondrial oxidative phopshorylation or ATP production, thus contributing to the deleterious effects of EtOH. We observed decreased mitochondrial complex activity (complex I, II, and IV) in our models. It is noteworthy that the complex I activity was decreased already in the 10 d + 1 binge model, whereas complex II and IV activities were attenuated by increasing the number of binges and the duration of EtOH feeding. PGC1α, the master regulator of mitochondrial biogenesis, is directly damaged by EtOH due to its toxic effects (46). PGC1α is involved in a wide range of mitochondrial pathways through the regulation of different factors including PPARs and ERRα playing a major role in the uptake of energy substrates, ATP production, or in the FA oxidation (48). Additionally, EtOH directly deteriorates the citrate cycle (29). In summary, EtOH consumption leads to the imbalance of energy substrates along with blunted mitochondrial biogenesis. In line with the above findings, we observed significant decrease of PGC1α gene expression in all EtOH-fed groups combined with EtOH binges. Furthermore, EtOH consumption was associated with reduced mRNA levels of different regulators of mitochondrial metabolism, suggesting a complex impairement of mitochondrial biogenesis pathways, energy production, and FA metabolism.
FAs are key energetic source of the myocardium that are transported to the mitochondria for β-oxidation or stored as TG. Alcoholic cardiomyopathy has been described to be associated with the formation of lipid droplets and fat accumulation in the myocardium (16, 44). Hu et al. (16) showed enhanced myocardial lipid accumulation and increased long chain FA uptake during chronic alcohol consumption using a model of 10%, 14%, or 18% EtOH in drinking water for 12 wk, which is somewhat different from the human drinking pattern/condition. In contrast, we found cardiomyocyte lipid accumulation (evidenced by Oil Red O histology) already at an earlier time point (EtOH 10d) in our study, which was markedly enhanced by alcohol binge and tended to be attenuated with increased EtOH feeding period. In parallel, we observed significant upregulation of myocardial ACC 1 and 2, suggesting upregulation of the TG synthesis pathway. Interestingly, despite the application of multiple binges and long-term alcohol feeding, cardiac steatosis was reduced in the 20 and 40 days models indicated by Oil Red O results. The most likely explanation for the observed phenomenon is an adaptive process, which would be interesting to explore in the future studies. It has been reported that excessive amount of adipocytes and steatosis in the myocardium is associated with different cardiovascular diseases (57).
Pathological remodeling of the heart in alcoholic cardiomyopathy is a well-known phenomenon. As part of a complex cardiac pathology myocardial hypertrophy, apoptosis and fibrosis have been described in alcoholic cardiomyopathy (7, 43, 56). However, there are controversies regarding the extent and type of myocardial remodeling (37, 53). These features are mostly dependent on the frequency and the amount of alcohol consumption. In our model, mild cardiomyocyte hypertrophy was presented as indicated by the increased wall thicknesses and RWT on echocardiographic examination and by the enlarged cardiomyocyte cross-sectional area in all EtOH-fed group, except the shortest EtOH 10d group. The presence of mild cardiomyocyte hypertrophy was also supported by the elevated gene expression value of the hypertrophy marker ANP. However, we did not observe fibrotic remodeling in our models.
The deterioration of the cardiac performance is intensively investigated in alcoholic cardiomyopathy by invasive, noninvasive, and in vitro methods (4, 10, 12, 16, 22, 45, 54). However, there are still controversies regarding the positive or negative effects of ethanol consumption. Heavy alcohol drinking is associated with diminished cardiac performance and heart failure (50). In addition to the effects on the heart, alcohol dose-dependently affects the vascular system and leads to either vasoconstriction or vasodilation (36). Conclusively, a complex approach is needed to investigate the effects of ethanol consumption on the cardiovascular performance. Therefore, we performed invasive hemodynamic examination and P-V analysis combined with echocardiography to characterize our models. P-V analysis provides the opportunity to measure pre- and afterload and heart rate independent systolic, contractility parameters, specific diastolic, cardiac stiffness, and vascular markers (33).
We observed marked attenuation of systolic indexes dP/dtmax, Ees, PRSW, and dP/dtmax-EDV indicating LV contractile dysfunction in all groups of chronic ethanol consumption combined with EtOH binges. Interestingly, 40d EtOH feeding by itself showed diminished contractility. Additionally, EF was significantly lower in only the 40d EtOH groups (with or without multiple binges) due to the rightward shift of P-V loops. Beside the prominent systolic dysfunction, we observed the significant worsening of diastolic LV relaxation (as shown by increased Tauw and decreased dP/dtmin) in the EtOH feeding groups combined with single or multiple EtOH binges. In contrast with this, LV stiffness (according to LVEDP and the slope of EDPVR) was not increased in any of the groups investigated. Our functional data are in accordance with the in vitro data suggesting that the marked myocardial oxidative/nitrative stress combined with mitochondrial dysfunction and impaired biogenesis leads to an energetic crisis resulting in the abovementioned impairement of cardiac contractility and diastolic relaxation. However, our model was not associated with cardiac fibrotic remodeling, which is usually an underlying mechanism of cardiac stiffening in many diseases (28, 39).
In addition to the diminished cardiac performance by P-V approach, we found deleterious effect of EtOH consumption on the vascular function as indicated by the lower MAP, TPR, and Ea parameters in single or multiple binges groups. It is noteworthy, that the systolic EF, FS, and the diastolic E/A ratio markers were not different measured with echocardiography among the groups, which is probably a consequence of the observed vascular changes and vasodilation.
Importantly, according to our data, the limitation of cardiac ultrasound examination and the vascular effects of alcohol should be taken into account when performing echocardiographic analysis to study the cardiac effects of ethanol consumption. Echocardiographic measurements are dependent on loading conditions and often not reliable in diseases with significant vascular alterations. Therefore, we performed invasive hemodynamic examination coupled with P-V analysis (33). The indexes derived from P-V analysis are pre- and afterload independent.
We also used P-V analysis to determine different mechanoenergetic parameters, such as efficiency and ventriculo-arterial coupling. We used the ratio of Ees and Ea to determine ventriculo-arterial coupling ratio (42). Ventriculo-arterial coupling ratio increased significantly in long-term alcohol feeding (EtOH 40d) and in single or multiple binge groups (EtOH 10, 20, and 40 with binges) indicating an inappropriate matching between the LV and arterial system. The mechanical efficiency is related to Ees, and the P-V area thus linearly correlates with the total oxygen consumption of the myocardium (41). Long-term alcohol feeding was associated with diminished cardiac efficiency, suggesting the reduction of metabolic efficiency (most probably due to the observed mitochondrial disturbances and oxidative/nitrative stress) in our model. To our knowledge, this is the first study reporting a detailed hemodynamic characterization of mouse alcoholic cardiomyopathy models due to a chronic EtOH feeding plus single or multiple EtOH binges.
Collectively, we demonstrate that chronic and binge drinking is associated with enhanced myocardial oxidative/nitrative stress, deteriorated mitochondrial function and biogenesis, cardiomyocyte hypertrophy, and myocardial steatosis, leading to impaired cardiovascular performance and mechanoenergetics.
GRANTS
The recent work was supported by the Intramural Research Program of NIAAA/NIH (to P. Pacher). C. Matyas was supported by the scholarship of the Hungarian-American Enterprise Scholarship Fund/Council on International Educational Exchange. Z. V. Varga was supported by the Rosztoczy Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.M. and P.P. conception and design of research; C.M., Z.V.V., P.M., J.P., T.L., K.E., B.T.N., and M.N. performed experiments; C.M., Z.V.V., P.M., J.P., K.E., and B.T.N. analyzed data; C.M., Z.V.V., P.M., J.P., G.H., and P.P. interpreted results of experiments; C.M. and P.P. prepared figures; C.M., G.H., B.G., and P.P. drafted manuscript; C.M., G.H., B.G., and P.P. edited and revised manuscript; C.M., Z.V.V., P.M., J.P., T.L., K.E., B.T.N., M.N., G.H., B.G., and P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. George Kunos, the Scientific Director of NIAAA, for support.
REFERENCES
- 1.Awtry EH, Philippides GJ. Alcoholic and cocaine-associated cardiomyopathies. Prog Cardiovasc Dis 52: 289–299, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Beckemeier ME, Bora PS. Fatty acid ethyl esters: potentially toxic products of myocardial ethanol metabolism. J Mol Cell Cardiol 30: 2487–2494, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8: 627–637, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RA, Jefferson L, Sudan N, Lloyd TC, Ren J. Acetaldehyde depresses myocardial contraction and cardiac myocyte shortening in spontaneously hypertensive rats: role of intracellular Ca2+. Cell Mol Biol (Noisy-le-grand) 45: 453–465, 1999. [PubMed] [Google Scholar]
- 5.Bryson CL, Mukamal KJ, Mittleman MA, Fried LP, Hirsch CH, Kitzman DW, Siscovick DS. The association of alcohol consumption and incident heart failure: the Cardiovascular Health Study. J Am Coll Cardiol 48: 305–311, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57: 450–458, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation 119: 1941–1949, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.El-Mas MM, Fan M, Abdel-Rahman AA. Upregulation of cardiac NOS due to endotoxemia and vagal overactivity contributes to the hypotensive effect of chronic ethanol in female rats. Eur J Pharmacol 650: 317–323, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folland ED, Parisi AF, Moynihan PF, Jones DR, Feldman CL, Tow DE. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation 60: 760–766, 1979. [DOI] [PubMed] [Google Scholar]
- 10.Gardner JD, Mouton AJ. Alcohol effects on cardiac function. Compr Physiol 5: 791–802, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Goslawski M, Piano MR, Bian JT, Church EC, Szczurek M, Phillips SA. Binge drinking impairs vascular function in young adults. J Am Coll Cardiol 62: 201–207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo R, Ren J. Alcohol dehydrogenase accentuates ethanol-induced myocardial dysfunction and mitochondrial damage in mice: role of mitochondrial death pathway. PLoS One 5: e8757, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzzo-Merello G, Cobo-Marcos M, Gallego-Delgado M, Garcia-Pavia P. Alcoholic cardiomyopathy. World J Cardiol 6: 771–781, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao E, Mukhopadhyay P, Cao Z, Erdelyi K, Holovac E, Liaudet L, Lee WS, Hasko G, Mechoulam R, Pacher P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol Med 21: 38–45, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hookana E, Junttila MJ, Puurunen VP, Tikkanen JT, Kaikkonen KS, Kortelainen ML, Myerburg RJ, Huikuri HV. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm 8: 1570–1575, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Hu C, Ge F, Hyodo E, Arai K, Iwata S, Lobdell Ht Walewski JL, Zhou S, Clugston RD, Jiang H, Zizola CP, Bharadwaj KG, Blaner WS, Homma S, Schulze PC, Goldberg IJ, Berk PD. Chronic ethanol consumption increases cardiomyocyte fatty acid uptake and decreases ventricular contractile function in C57BL/6J mice. J Mol Cell Cardiol 59: 30–40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing L, Jin CM, Li SS, Zhang FM, Yuan L, Li WM, Sang Y, Li S, Zhou LJ. Chronic alcohol intake-induced oxidative stress and apoptosis: role of CYP2E1 and calpain-1 in alcoholic cardiomyopathy. Mol Cell Biochem 359: 283–292, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Kandadi MR, Hu N, Ren J. ULK1 plays a critical role in AMPK-mediated myocardial autophagy and contractile dysfunction following acute alcohol challenge. Curr Pharm Des 19: 4874–4887, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Ellison RC. Alcohol and coronary heart disease: the evidence for a protective effect. Clin Chim Acta 246: 59–76, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Karadayian AG, Bustamante J, Czerniczyniec A, Lombardi P, Cutrera RA, Lores-Arnaiz S. Alcohol hangover induces mitochondrial dysfunction and free radical production in mouse cerebellum. Neuroscience 304: 47–59, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Kim SD, Beck J, Bieniarz T, Schumacher A, Piano MR. A rodent model of alcoholic heart muscle disease and its evaluation by echocardiography. Alcohol Clin Exp Res 25: 457–463, 2001. [PubMed] [Google Scholar]
- 22.Lang CH, Korzick DH. Chronic alcohol consumption disrupts myocardial protein balance and function in aged, but not adult, female F344 rats. Am J Physiol Regul Integr Comp Physiol 306: R23–R33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Eur J Echocardiogr 7: 79–108, 2006.16458610 [Google Scholar]
- 24.Laurent D, Mathew JE, Mitry M, Taft M, Force A, Edwards JG. Chronic ethanol consumption increases myocardial mitochondrial DNA mutations: a potential contribution by mitochondrial topoisomerases. Alcohol Alcohol 49: 381–389, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Korkmaz S, Loganathan S, Weymann A, Radovits T, Barnucz E, Hirschberg K, Hegedus P, Zhou Y, Tao L, Pali S, Veres G, Karck M, Szabo G. Acute ethanol exposure increases the susceptibility of the donor hearts to ischemia/reperfusion injury after transplantation in rats. PLoS One 7: e49237, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D Pope CA 3rd Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Redmond EM, Morrow D, Cullen JP. Differential effects of daily-moderate versus weekend-binge alcohol consumption on atherosclerotic plaque development in mice. Atherosclerosis 219: 448–454, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matyas C, Nemeth BT, Olah A, Hidi L, Birtalan E, Kellermayer D, Ruppert M, Korkmaz-Icoz S, Kokeny G, Horvath EM, Szabo G, Merkely B, Radovits T. The soluble guanylate cyclase activator cinaciguat prevents cardiac dysfunction in a rat model of type-1 diabetes mellitus. Cardiovasc Diabetol 14: 145, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihailovic D, Nikolic J, Bjelakovic BB, Stankovic BN, Bjelakovic G. Morphometric and biochemical characteristics of short-term effects of ethanol on rat cardiac muscle. Exp Toxicol Pathol 51: 545–547, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Movva R, Figueredo VM. Alcohol and the heart: to abstain or not to abstain? Int J Cardiol 172: 628, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay P, Rajesh M, Cao Z, Horvath B, Park O, Wang H, Erdelyi K, Holovac E, Wang Y, Liaudet L, Hamdaoui N, Lafdil F, Hasko G, Szabo C, Boulares AH, Gao B, Pacher P. Poly (ADP-ribose) polymerase-1 is a key mediator of liver inflammation and fibrosis. Hepatology 59: 1998–2009, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piano MR, Phillips SA. Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol 14: 291–308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol 161: 423–433, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Puddey IB, Zilkens RR, Croft KD, Beilin LJ. Alcohol and endothelial function: a brief review. Clin Exp Pharmacol Physiol 28: 1020–1024, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Regan TJ, Khan MI, Ettinger PO, Haider B, Lyons MM, Oldewurtel HA. Myocardial function and lipid metabolism in the chronic alcoholic animal. J Clin Invest 54: 740–752, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.SAMHSA. 2013 National Survey on Drug Use and Health (NSDUH). Table 6.89B-Binge Alcohol Use in the Past Month among Persons Aged 18 to 22, by College Enrollment Status and Demographic Characteristics: Percentages, 2012 and 2013. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabsPDFWHTML2013/Web/HTML/NSDUH-DetTabsSect6peTabs55to107-2013.htm#tab6.89b. [Google Scholar]
- 39.Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart Fail Rev 19: 173–185, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Shi J, Larson DF, Yang B, Hunter K, Gorman M, Montes S, Beischel J, Watson RR. Differential effects of acute ethanol treatment on cardiac contractile function in young adult and senescent mice. Alcohol 24: 197–204, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Suga H. Cardiac energetics: from Emax to pressure-volume area. Clin Exp Pharmacol Physiol 30: 580–585, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Heart Circ Physiol 245: H773–H780, 1983. [DOI] [PubMed] [Google Scholar]
- 43.Tan Y, Li X, Prabhu SD, Brittian KR, Chen Q, Yin X, McClain CJ, Zhou Z, Cai L. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol 59: 1477–1486, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsiplenkova VG, Vikhert AM, Cherpachenko NM. Ultrastructural and histochemical observations in human and experimental alcoholic cardiomyopathy. J Am Coll Cardiol 8: 22A–32A, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Umoh NA, Walker RK, Al-Rubaiee M, Jeffress MA, Haddad GE. Acute alcohol modulates cardiac function as PI3K/Akt regulates oxidative stress. Alcohol Clin Exp Res 38: 1847–1864, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varga ZV, Ferdinandy P, Liaudet L, Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol 309: H1453–H1467, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venditti P, Di Stefano L, Di Meo S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 13: 71–82, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 79: 208–217, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Vikhert AM, Tsiplenkova VG, Cherpachenko NM. Alcoholic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 8: 3A–11A, 1986. [DOI] [PubMed] [Google Scholar]
- 50.Walker RK, Cousins VM, Umoh NA, Jeffress MA, Taghipour D, Al-Rubaiee M, Haddad GE. The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp Res 37: 1253–1260, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Zhao J, Yang W, Bi Y, Chi J, Tian J, Li W. High-dose alcohol induces reactive oxygen species-mediated apoptosis via PKC-beta/p66Shc in mouse primary cardiomyocytes. Biochem Biophys Res Commun 456: 656–661, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Waszkiewicz N, Szulc A, Zwierz K. Binge drinking-induced subtle myocardial injury. Alcohol Clin Exp Res 37: 1261–1263, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Yuan F, Lei Y, Wang Q, Esberg LB, Huang Z, Scott GI, Li X, Ren J. Moderate ethanol administration accentuates cardiomyocyte contractile dysfunction and mitochondrial injury in high fat diet-induced obesity. Toxicol Lett 233: 267–277, 2015. [DOI] [PubMed] [Google Scholar]
- 54.Zagrosek A, Messroghli D, Schulz O, Dietz R, Schulz-Menger J. Effect of binge drinking on the heart as assessed by cardiac magnetic resonance imaging. JAMA 304: 1328–1330, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health 29: 245–254, 2006. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang B, Turdi S, Li Q, Lopez FL, Eason AR, Anversa P, Ren J. Cardiac overexpression of insulin-like growth factor 1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction but not hypertrophy: roles of Akt, mTOR, GSK3beta, and PTEN. Free Radic Biol Med 49: 1238–1253, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, Pu W, Liu Q, He L, Huang X, Tian X, Zhang L, Nie Y, Hu S, Lui K, Zhou B. Endocardium Contributes to Cardiac Fat. Circ Res 118: 254–265, 2016. [DOI] [PubMed] [Google Scholar]