For the first time, we have identified an impairment in microvascular function in patients with cystic fibrosis compared with demographically matched controls. In addition, relationships between microvascular function, exercise capacity, and lung function suggest a possible link between microvascular dysfunction and disease severity in this patient population.
Keywords: endothelium-dependent vasodilation, endothelial function, microvascular function, cystic fibrosis
Abstract
Cystic fibrosis (CF) is a genetic, multisystemic disorder with broad clinical manifestations apart from the well-characterized pulmonary dysfunction. Recent findings have described impairment in conduit vessel function in patients with CF; however, whether microvascular function is affected in this population has yet to be elucidated. Using laser-Doppler imaging, we evaluated microvascular function through postocclusive reactive hyperemia (PORH), local thermal hyperemia (LTH), and iontophoresis with acetylcholine (ACh). PORH [518 ± 174% (CF) and 801 ± 125% (control), P = 0.039], LTH [1,338 ± 436% (CF) and 1,574 ± 620% (control), P = 0.045], and iontophoresis with ACh [416 ± 140% (CF) and 617 ± 143% (control), P = 0.032] were significantly lower in patients with CF than control subjects. In addition, the ratio of PORH to LTH was significantly (P = 0.043) lower in patients with CF (55.3 ± 5.1%) than control subjects (68.8 ± 3.1%). Significant positive correlations between LTH and forced expiratory volume in 1 s (%predicted) (r = 0.441, P = 0.013) and between the PORH-to-LTH ratio and exercise capacity (r = 0.350, P = 0.049) were observed. These data provide evidence of microvascular dysfunction in patients with CF compared with control subjects. In addition, our data demonstrate a complex relationship between microvascular function and classical markers of disease severity (i.e., pulmonary function and exercise capacity) in CF.
NEW & NOTEWORTHY
For the first time, we have identified an impairment in microvascular function in patients with cystic fibrosis compared with demographically matched controls. In addition, relationships between microvascular function, exercise capacity, and lung function suggest a possible link between microvascular dysfunction and disease severity in this patient population.
cystic fibrosis (CF) is an inherited disorder that is predominantly characterized by pulmonary dysfunction; however, broader clinical manifestations, such as gastrointestinal, immune, endocrine, and musculoskeletal dysfunctions, are also present (36). Recently, our group reported conduit vascular endothelial dysfunction in young patients with CF (39). Importantly, this vascular impairment was observed in a population that exhibited preserved spirometric function and exercise capacity, which suggests a systemic consequence prior to the decrease in pulmonary function. Despite these recent observations, there appears to be a lack of understanding of the general functionality of the endothelium in CF.
Impairments in endothelial function are highly related to a reduction in the bioavailability of nitric oxide (NO), a key vasodilator involved in the regulation of vessel tone and endothelium integrity (24). Decreases in NO balance have also been shown to contribute to impaired endothelial microvascular vasodilation (44). With use of laser-Doppler imaging technology, cutaneous blood flux can be quantified (45) to represent an accessible model of microvascular function (17). Postocclusive reactive hyperemia (PORH), local thermal hyperemia (LTH), and iontophoresis with acetylcholine (ACh), in combination with laser technology, have each been used to evaluate microvascular function in humans. While PORH is partly dependent on prostanoids and NO has a less crucial role (21, 58), the plateau phase of LTH is considered to be mediated principally by NO (27, 28, 44), and the response to ACh iontophoresis stimulates the activation of different factors, including NO, cyclooxygenase, prostanoids, and endothelium-derived hyperpolarizing factor-dependent pathways (6, 20, 33). Accordingly, the combination of these three protocols can provide a comprehensive assessment of microvascular function that includes evaluation of the microvascular hyperemic response by different mechanisms.
Thus the purpose of this study was to determine whether patients with CF exhibit a reduced microvascular function compared with control subjects. Using a comprehensive assessment, we hypothesized that microvascular function assessed by many different mechanisms (i.e., PORH, LTH, and iontophoresis) will be lower in patients with CF than control subjects.
MATERIALS AND METHODS
Experimental Design
All participants reported to the Laboratory of Integrative Vascular and Exercise Physiology at 8 AM following an overnight fast and having abstained from moderate-to-vigorous physical activity for 24 h prior to the experimental session. Patients with CF were instructed to adhere to the timing of their daily treatments and come to the laboratory following their morning airway clearance technique and inhaled medicines. It is worth noting that the use of inhaled β-agonists as part of routine treatment of CF does not affect cardiac or peripheral hemodynamics in these patients (52). Microvascular function was assessed; then a pulmonary function test was performed and a blood sample was collected for assessment of standard laboratory values. Subsequently, each participant completed a maximal exercise test to evaluate exercise capacity, an independent predictor of survival in CF (35).
Subjects
Sixteen patients with CF (range 13–43 yr) and 15 age-matched control subjects (range 14–42 yr) volunteered to participate in the study. The majority of the patients were homozygous ΔF508del (n = 14); however, one patient was ΔF508/621+1G->T and another was ΔF508/G551D. Participants were excluded from the study if they 1) had a forced expiratory volume in 1 s (FEV1) <50 %predicted, 2) had a resting O2 saturation <90%, 3) had a clinical diagnosis of pulmonary hypertension or cardiovascular disease, 4) were taking vasoactive medications, 5) had a clinical diagnosis of sleep apnea or any other sleep disorders, 6) were pregnant, or 7) self-reported to be a smoker. All participants and parents were informed of the objectives and possible risks of the investigation before written consent/assent for participation was obtained. The study followed the principles of the Declaration of Helsinki and was approved by the Institutional Review Board at Augusta University.
Pulmonary Function Test
A pulmonary function test was performed by all participants using the EasyOne Pro LAB spirometer (NDD Medical, Andover, MA) to evaluate FEV1, forced vital capacity (FVC), FVC-to-FEV1 ratio, and forced expiratory flow at 25–75% FVC (FEF25–75) according to the American Thoracic Society standards. FEV1 (%predicted) was also determined following spirometric reference standards (40).
Exercise Testing
All participants performed a maximal exercise test using the Godfrey protocol on a cycle ergometer. Briefly, after a 2-min baseline and 2-min unloaded warm-up, participants started an exercise protocol that increased 15–20 W/min (depending on the height of the participant) (14). Expired gases were analyzed breath-by-breath by a metabolic cart (True One 2400, ParvoMedics, Sandy, UT). O2 consumption (V̇o2) was obtained and normalized for total body weight (V̇o2/kg) as previously described (13). Maximal exercise capacity (V̇o2 max) was verified using the American College of Sports Medicine exercise testing criteria (1a). Specifically, a test was considered maximal if the subject met three of the four following criteria: 1) volitional fatigue (>17 on rating of perceived exertion), 2) plateau in V̇o2, 3) ≥85% of predicted maximum heart rate, and 4) respiratory exchange ratio >1.1.
Microvascular Function
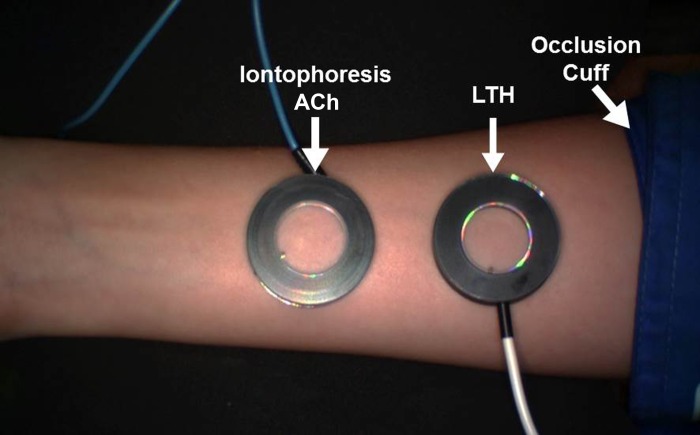
In a supine position, the right arm of each participant was extended laterally at ∼80° of shoulder abduction and the distal forearm was secured in a vacuum-packed pillow (Vac Pac, Baltimore, MD). A forearm cuff was placed immediately distal to the medial epicondyle, and an iontophoresis gel electrode was placed on the ventral part of the wrist. Two 20-mm ring-shaped chambers (Moor Instruments, Wilmington, DE), secured with double-sided adhesive tape, were placed on the ventral surface of the forearm, with care taken to avoid any area with a tattoo or damage to the skin. The experimental setup is illustrated in Fig. 1. A laser-Doppler imager (Moor Instruments) was placed <30 cm above the forearm. After a 20-min acclimation period in a temperature-controlled (22 ± 2°C) room to achieve a hemodynamic steady state, microvascular function was determined using three different protocols: PORH followed by concurrent assessment of LTH and ACh iontophoresis. For all protocols, cutaneous blood flux is expressed in arbitrary perfusion units (PU). Baseline (BL) flux was determined by calculation of a 30-s average prior to initiation of the pertinent protocol. A biological zero (B0), to control for the Brownian movement of macromolecules in cutaneous interstitial space, was determined while the forearm cuff was inflated during the PORH protocol and subtracted from both baseline and peak responses. For each of the protocols, results are presented for 1) the peak response, 2) the relative change in flux expressed as a percentage: relative change (%) = [peak − (BL − B0)] × (BL − B0)−1 × 100, and 3) the time to peak (TTP), which represents the time from the start of the stimulus to the peak flux response.
Fig. 1.
Experimental setup of microvascular function using a laser-Doppler imager with the occlusion cuff for postocclusive reactive hyperemia and the 2 chambers for local thermal hyperemia (LTH) and acetylcholine (ACh) iontophoresis.
Postocclusive Reactive Hyperemia Protocol
Using a forearm cuff inflated to 250 mmHg to provoke reactive hyperemia, we determined microvascular function by recording the flux on the forearm before and after a 5-min occlusion period (44). Cutaneous flux response to this protocol was evaluated in the chamber proximal to the occlusion cuff (Fig. 1). All variables related to this protocol are defined with the subscript PORH.
Local Thermal Hyperemia Protocol
LTH was determined in the chamber placed proximal to the occlusion cuff on the volar surface of the forearm (Fig. 1). The LTH chamber was filled with 2 ml of water and then heated at >0.1°C/s to 44°C for 25 min (44). This temperature and time have been shown to elicit maximal microvascular dilation (19, 28). We have also expressed the peak reactive hyperemia values as the percentage of the peak hyperemia during local thermal heating (PORH-to-LTH ratio) to represent the hyperemic response in relation to maximal microvascular blood flow (55). All variables related to the heating protocol are defined with the subscript LTH.
ACh Iontophoresis Protocol
ACh iontophoresis was performed by filling the ventral chamber distal to the occlusion cuff with 2 ml of 2% ACh solution with a purity >99% TLC (Sigma-Aldrich, St. Louis, MO). A rectangular-shaped electrode was placed on the wrist, and both the electrode (cathode) and the chamber (anode) were connected to a battery-powered iontophoresis controller (model MIC2, Moor Instruments). After a baseline recording, ACh was delivered using an anodal current of 100 μA for 20 s and repeated seven times at 60-s intervals. All variables related to this protocol are defined with the subscript ACh.
Statistical Analysis
All measurements are expressed as means ± SD. All statistical analyses were performed using SPSS version 23 (SPSS, Chicago, IL), and significance was set at P < 0.05. The Shapiro-Wilk test was used to analyze the normality of the measurement distribution. Independent group t-tests were performed to identify group differences, and Pearson's correlations were utilized to identify relationships between microvascular function and pulmonary function and between microvascular function and exercise capacity. Because of the wide age range of our participants, we have also considered age as a covariate factor for the analysis of correlations based on the close relationship of age with disease severity in CF. Effect sizes (Cohen's d) were calculated for the relative change in flux response to each protocol, with 0.2, 0.5, and 0.8 representing small, medium, and large effects, respectively (31).
RESULTS
Participant Characteristics and Laboratory Values
Demographic characteristics and laboratory values for patients with CF and control subjects are presented in Table 1. No differences in age, sex, height, weight, or body mass index were observed between patients and controls. However, patients with CF exhibited significantly (P < 0.05) lower concentrations of lipids (total cholesterol and high- and low-density lipoproteins) than control subjects. In addition, resting O2 saturation was significantly (P = 0.01) lower in patients than controls, although all values were still within normal limits at 98%.
Table 1.
Participant characteristics and laboratory values
| Variable | Patients with CF (n = 16) | Control Subjects (n = 15) | P Value |
|---|---|---|---|
| Sex, M/F | 7/9 | 7/8 | 0.621 |
| Age, yr | 22 ± 9 | 28 ± 8 | 0.087 |
| Height, cm | 158 ± 11 | 163 ± 25 | 0.626 |
| Weight, kg | 63 ± 14 | 66 ± 12 | 0.729 |
| BMI, kg/m2 | 22 ± 4 | 22 ± 6 | 0.431 |
| Body fat, % | 27.5 ± 7.7 | 29.5 ± 5.6 | 0.517 |
| SBP, mmHg | 108 ± 12 | 115 ± 15 | 0.128 |
| DBP, mmHg | 65 ± 6 | 66 ± 8 | 0.812 |
| Resting O2 saturation, % | 98 ± 1 | 99 ± 1 | 0.010* |
| TC, mmol/l | 3.3 ± 0.5 | 4.4 ± 1.1 | 0.001* |
| HDL, mmol/l | 1.0 ± 0.3 | 1.5 ± 0.3 | 0.001* |
| LDL, mmol/l | 1.8 ± 0.5 | 2.5 ± 0.8 | 0.008* |
| Triglycerides, mmol/l | 1.1 ± 0.4 | 0.9 ± 0.3 | 0.084 |
| TC-to-HDL ratio | 3.3 ± 0.2 | 3.0 ± 0.3 | 0.505 |
| Glucose, mmol/l | 6.6 ± 2.7 | 4.8 ± 0.4 | 0.149 |
| hsCRP, mg/l | 3.12 ± 1.41 | 0.74 ± 0.42 | 0.125 |
| Hemoglobin, mmol/l | 9.5 ± 0.3 | 9.0 ± 0.3 | 0.203 |
| Hematocrit, % | 45.6 ± 3.4 | 43.8 ± 4.2 | 0.253 |
Values are means ± SD.
CF, cystic fibrosis; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL, high-density lipoproteins; LDL, low-density lipoproteins; hsCRP, high-sensitive C-reactive protein.
Statistically significant.
Disease Severity Markers
Spirometric dysfunction and exercise intolerance are common phenotypes in patients with CF (36) and represent classical markers of disease severity. Spirometric function values are presented in Table 2. All spirometric values [FVC, FEV1, FEF25–75, FEV1 (%predicted), and FEV1-to-FVC ratio] were significantly lower (P < 0.05) in patients than controls.
Table 2.
Disease severity values in patients with CF and control subjects
| Variable | Patients with CF | Control Subjects | P Value |
|---|---|---|---|
| Pulmonary function | |||
| FVC, liters | 3.61 ± 1.24 | 4.54 ± 1.10 | 0.023* |
| FEV1, liters | 2.78 ± 1.01 | 3.74 ± 0.81 | 0.006* |
| FEF25–75, l/s | 2.56 ± 1.30 | 3.77 ± 0.99 | 0.007* |
| FEV1, %predicted | 75 ± 16 | 97 ± 9 | 0.001* |
| FEV1/FVC, % | 72 ± 8 | 91 ± 6 | 0.044* |
| Exercise capacity | |||
| V̇o2peak | |||
| l/min | 1.6 ± 0.5 | 2.3 ± 0.8 | 0.017* |
| ml·kg−1·min−1 | 28.4 ± 4.8 | 34.5 ± 7.0 | 0.012* |
| %predicted | 73 ± 12 | 98 ± 14 | 0.001* |
| Heart rate peak, beats/min | 160 ± 6 | 184 ± 4 | 0.003* |
| Work peak, W | 139 ± 14 | 205 ± 13 | 0.001* |
Values are means ± SD.
CF, cystic fibrosis; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25–75, forced expiratory flow at 25-75% FVC; V̇o2peak, peak O2 consumption.
Statistically significant.
Exercise testing values are also presented in Table 2. Given that only 30 of the 31 participants achieved a true maximal exercise test following the criteria defined by the American College of Sports Medicine (1a), values are expressed as V̇o2 peak, rather than V̇o2 max. V̇o2 peak (liters), V̇o2 peak/kg, V̇o2 peak (%predicted), heart rate peak, and work rate peak were significantly (P < 0.05) lower in patients with CF than control subjects.
Microvascular Function
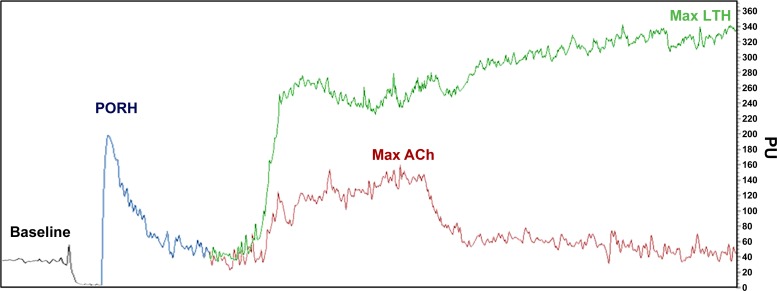
A representative trace of the following microvascular function protocols is illustrated in Fig. 2.
Fig. 2.
Typical cutaneous vascular flux assessment using a laser-Doppler imager with baseline and responses for postocclusive reactive hyperemia (PORH), local thermal hyperemia (LTH), and iontophoresis with ACh. PU, perfusion units.
Postocclusive reactive hyperemia.
Data obtained from the PORH protocol are presented in Table 3. The BL (P = 0.748) and peak (P = 0.187) flux responses were similar between patients and controls. Likewise, B0 was similar (P = 0.355) between patients with CF (15 ± 3 PU) and control subjects (17 ± 5 PU). The relative changePORH was significantly (P = 0.039, Cohen's d = 0.73) reduced in patients with CF compared with control subjects.
Table 3.
Microvascular function in patients with CF and control subjects
| Variable | Patients with CF | Control Subjects | P Value |
|---|---|---|---|
| Postocclusive reactive hyperemia | |||
| Baseline, PU | 42 ± 8 | 40 ± 5 | 0.748 |
| PeakPORH, PU | 167 ± 43 | 207 ± 22 | 0.187 |
| Relative changePORH, % | 518 ± 174 | 801 ± 125 | 0.039* |
| TTPPORH, s | 16 ± 6 | 15 ± 2 | 0.296 |
| Local thermal hyperemia | |||
| Baseline, PU | 36 ± 8 | 35 ± 6 | 0.676 |
| PeakLTH, PU | 302 ± 44 | 301 ± 51 | 0.275 |
| Relative changeLTH, % | 1,338 ± 436 | 1,574 ± 620 | 0.045* |
| TTPLTH, s | 781 ± 197 | 806 ± 182 | 0.113 |
| Iontophoresis with ACh | |||
| Baseline, PU | 40 ± 11 | 34 ± 11 | 0.083 |
| PeakACh, PU | 129 ± 54 | 122 ± 41 | 0.094 |
| Relative changeACh, % | 416 ± 140 | 617 ± 143 | 0.032* |
| TTPACh, s | 16 ± 6 | 15 ± 2 | 0.494 |
Values are means ± SD.
CF, cystic fibrosis; PORH, postocclusive reactive hyperemia; LTH, local thermal hyperemia; ACh, acetylcholine; TTP, time to peak; PU, perfusion units.
Statistically significant.
Local thermal hyperemia.
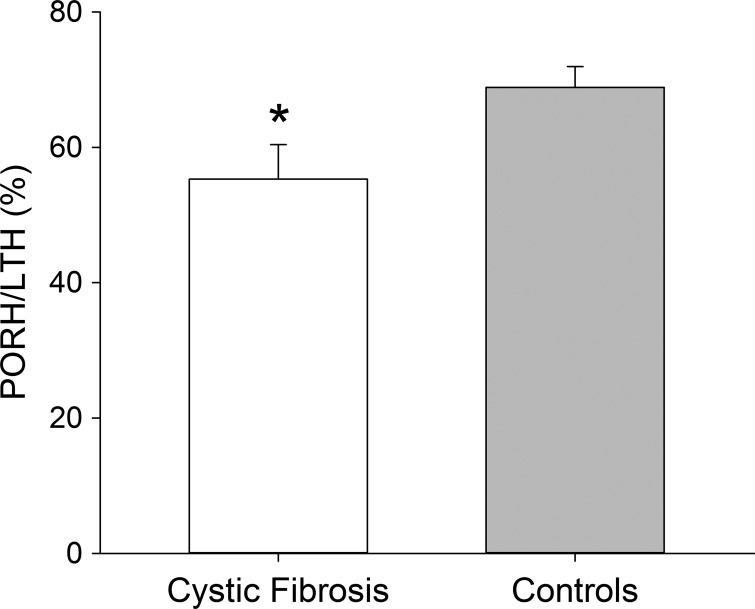
Data from the LTH protocol are presented in Table 3. BL and B0 values during the LTH protocol were similar (P > 0.05) in both groups. However, relative changeLTH was significantly (P = 0.045, Cohen's d = 0.87) lower in patients than controls. In addition, the PORH-to-LTH ratio (Fig. 3) was significantly (P = 0.043) lower in patients with CF (55.3 ± 5.1%) than control subjects (68.8 ± 3.1%).
Fig. 3.
Postocclusive reactive hyperemia (PORH) response normalized to maximal hyperemia during local thermal hyperemia (LTH). Values are means ± SD. *Significantly different from controls.
ACh iontophoresis.
Variables obtained from the cutaneous iontophoresis protocol with ACh are shown in Table 3. Relative changeACh was significantly (P = 0.032, Cohen's d = 0.69) lower in patients with CF than control subjects. All other parameters were similar (P > 0.05) between groups.
Relationships Between Microvascular Function and Markers of Disease Severity
Significant correlations between microvascular function and markers of disease severity (lung function and exercise capacity) were identified. Specifically, a significant correlation (r = 0.441, P = 0.013) between relative changeLTH and FEV1 (%predicted) was identified. Additionally, the PORH-to-LTH ratio was significantly correlated with V̇o2 peak (r = 0.350, P = 0.049). Furthermore, TTPLTH was positively associated with FVC (r = 0.395, P = 0.028), FEV1 (r = 0.405, P = 0.024), FEF25–75 (r = 0.373, P = 0.039), and FEV1 (%predicted) (r = 0.384, P = 0.033). After adjustment by age, a significant correlation between TTPLTH and FEV1 (%predicted) was also observed (r = 0.475, P = 0.046).
DISCUSSION
CF is associated with many different systemic consequences. For the first time, the present study documents that patients with CF exhibit cutaneous microvascular dysfunction compared with demographically matched control subjects. Our data obtained from comprehensive assessment of microvascular function (PORH, LTH, and iontophoresis with ACh) suggest that patients with CF exhibit some evidence of microvascular dysfunction compared with control subjects. Perhaps more clinically relevant, our data support a positive relationship between microvascular function, exercise capacity, and lung function, such that the disease severity may be utilized to predict microvascular function in CF.
Microvascular Function in Patients with CF
The microvascular network, consisting of arterioles, capillaries, and venules, is responsible for the distribution of blood within tissues. Oxidative stress, a common phenotype in CF, reduces the bioavailability of NO in the arterioles (8, 30), leading to subsequent development of microvascular dysfunction (48, 56).
Postocclusive reactive hyperemia.
The PORH protocol represents a sensitive assessment of microvascular function anatomically as well as functionally (15). Although no group differences were observed in the peak response or in the TTP response within the PORH protocol, findings from the present study have identified a significantly reduced relative change in reactive hyperemia in patients with CF compared with control subjects. Expression of the values as a relative change, as reported by others (55), eliminates possible spatial variations and allows for a more accurate comparison not only between groups, but also between sites on the same individual. In addition, our results may suggest that the structure of the blood microvessels is preserved (41, 51), despite impairment of the mechanisms that contribute to the reactive hyperemia response. The exact mechanism is unknown; however, prostanoids are key players in the PORH response (21), and many factors, including metabolic and endothelial vasodilators, sensory nerves, and NO, have also been shown to be involved (23, 50). In fact, abnormally elevated release of prostanoids has been observed in patients with CF (10). Considering the role of these components as vasoconstrictor factors (22), findings from the present study in patients during the PORH protocol are supported.
Local thermal hyperemia.
After the local increase in skin temperature, the LTH response is characterized by a biphasic hyperemic response (44) and represents the maximal vasodilator capacity of the microvessels. The initial peak vasodilation is predominantly mediated by an axon reflex and substance P (44, 54), followed by a prolonged plateau phase that is mediated primarily by release of NO from the microvascular endothelium (27, 28, 44). In the present study we have identified a similar initial peak between patients with CF and control subjects (data not shown) followed by a significantly attenuated plateau phase in patients with CF (Table 3). In addition, a 20% reduction in the PORH-to-LTH ratio was observed in patients with CF compared with control subjects (Fig. 3). It is important to note that oxidative stress inactivates NO, attenuating the vasodilatory process (32) and, hence, playing an important role in the reaction of the microvasculature during LTH (7). Considering the strong presence of inflammation and oxidative stress in patients with CF (42), it is reasonable to suspect that these damaging mediators contribute to attenuation of microvascular vasodilation to LTH as previously reported in other populations (12, 26). However, future studies are needed to determine the mechanistic contribution of the lower LTH cutaneous response in CF.
ACh iontophoresis.
Cutaneous iontophoresis with ACh has previously been used to investigate microvascular function (47, 49). The infusion of ACh across the skin and into the intradermal space using an electrical charge promotes the activation of NO, although the larger response seems to be mediated by prostanoids (6, 20, 33). Findings from the present study demonstrate that patients with CF exhibit a significantly reduced cutaneous vasodilation to ACh compared with control subjects (Table 3). It is important to note that patients and controls in the present study exhibit similar (P = 0.287) skin resistance (120.937 ± 24.084 and 135.500 ± 18.357 Ω, respectively), which can impact the delivery of ACh with iontophoresis. Accordingly, it is unlikely that differences in skin resistance are contributing to the differences between groups. Bearing in mind the same considerations of elevated prostanoids in patients with CF previously described (10) and the contribution of these mediators in an impaired vascular response (21), it is not surprising that patients with CF also exhibit a lower microvascular response to the ACh iontophoresis. However, TTP values were similar between both groups, confirming that the microvascular integrity and structure are intact and unlikely affecting the ACh response (41, 51). In addition, a previously described reliance of the iontophoresis response to ACh on endothelial function was assessed through brachial artery flow-mediated dilatation, which is also impaired in CF (11). Therefore, findings from the present study confirm that patients with CF exhibit evidence of microvascular dysfunction.
Microvascular Function and Disease Severity: Clinical Relevance
A novel aspect of the present study is the comprehensive evaluation of microvascular function and the relationships between prognostic markers of disease severity, such as pulmonary function and exercise capacity, in patients with CF.
Pulmonary function, specifically FEV1 (%predicted), represents the most traditional assessment of disease severity in CF (1) and is commonly used as a predictor of survival in this patient population (43). Perhaps it is not surprising that the present study has identified positive associations between indexes of microvascular function (relative changeLTH and TTPLTH) and FEV1 (%predicted). These data indicate that patients with a greater lung function may have a more robust response to the LTH protocol. A possible link between LTH and pulmonary function may rely on the role of systemic oxidative stress in the action of free radicals and NO bioavailability (2, 37, 46). In fact, excessive oxidative stress status has been observed in patients with obstructed airways and impaired lung function, including CF (5, 16). Although the present study cannot determine a cause-effect relationship, future studies are needed to elucidate the causal mechanisms.
Patients with CF also exhibit a progressive, longitudinal decline in exercise capacity, which represents an important predictor of mortality in this population (34, 35, 38). It is well known that regular aerobic exercise improves the quality of life of patients with CF through increasing expectoration of the sputum and ameliorating the decline in lung function (29, 35). Exercise also enhances NO bioavailability (3, 4, 9). In the present study a positive correlation between the PORH-to-LTH ratio and exercise capacity suggests that a greater exercise capacity positively impacts microvascular function in CF. Accordingly, it is reasonable to believe that the microvascular dysfunction identified in patients with CF can impact the transport and delivery of O2 to the exercising muscles. However, future studies are needed to determine if improvement in microvascular function in CF contributes to improvements in exercise capacity.
Not all outcomes of microvascular function assessed by each protocol were related to the same markers of disease severity. It is important to remember, however, that the cutaneous response to each protocol is mediated, in part, by different molecular mechanisms, which may likely explain the different relationships between the microvascular function and the disease severity markers in the present study. Nevertheless, future studies are warranted to determine the contribution of microvascular dysfunction to both pulmonary function and exercise intolerance in CF.
Experimental Considerations
The present study included a relatively small sample size; therefore, the possibility of a type II error should be considered. However, we used Cohen's d statistics to identify large effect sizes in all the primary microvascular outcomes between groups. Therefore, we believe that these analyses corroborate statistical differences in microvascular function between patients with CF and control subjects.
In the present study, PORH always preceded the ACh and LTH protocols. It is plausible that the microvascular function responses to ACh iontophoresis and LTH were impacted by PORH preconditioning contributing to microvascular hyperreactivity (18, 57); however, the same protocol was conducted in both groups. In addition, unpublished data from our lab demonstrate a similar peak response to ACh iontophoresis whether it is conducted before (158 ± 44 PU) or after (158 ± 59 PU) a PORH protocol, ruling out possible interferences among responses that may affect the present results.
In the present study, endothelium-independent microvascular dilation through iontophoresis with sodium nitroprusside was not included. Data from our lab support a similar (P = 0.207) conduit vessel endothelium-independent vasodilatory response to sublingual nitroglycerine between patients with CF and control subjects (23.1 ± 3.2% and 21.4 ± 5.8% respectively). It is plausible, however, that the endothelium-independent dilation is different in conduit and microvessels; therefore, further investigation is needed.
Conclusion
This is the first study, to our knowledge, to complete a comprehensive evaluation of microvascular function using PORH, LTH, and ACh iontophoresis in patients with CF. Findings from the present study indicate that patients with CF exhibit some evidence of microvascular function compared with demographically matched control subjects. In addition, a positive relationship between microvascular function and both exercise capacity and lung function was observed, suggesting that disease severity may contribute, at least in part, to microvascular dysfunction in this population. Future studies are necessary to elucidate whether an enhancement in NO bioavailability, and hence an improvement in microvascular function, contributes to improvements in pulmonary function and exercise capacity (or vice versa) in patients with CF.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R21 DK-100783 (R. A. Harris) and the Vertex Pharmaceuticals Investigator-Initiated Studies Program (R. A. Harris).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.R.-M., J.T., N.S., R.C., and R.A.H. performed the experiments; P.R.-M., J.T., N.S., R.C., and R.A.H. analyzed the data; P.R.-M., J.T., N.S., R.C., K.T.M., C.F., and R.A.H. interpreted the results of the experiments; P.R.-M. and R.A.H. prepared the figures; P.R.-M. and R.A.H. drafted the manuscript; P.R.-M., J.T., N.S., R.C., K.T.M., C.F., and R.A.H. edited and revised the manuscript; P.R.-M., J.T., N.S., R.C., K.T.M., C.F., and R.A.H. approved the final version of the manuscript; R.A.H. developed the concept and designed the research.
ACKNOWLEDGMENTS
The authors thank the patients and volunteers for their commitment and participation in the study. We also acknowledge the entire Augusta University CF care team for their commitment to CF research.
REFERENCES
- 1.Abraham EH, Sterling KM, Kim RJ, Salikhova AY, Huffman HB, Crockett MA, Johnston N, Parker HW, Boyle WE Jr, Hartov A, Demidenko E, Efird J, Kahn J, Grubman SA, Jefferson DM, Robson SC, Thakar JH, Lorico A, Rappa G, Sartorelli AC, Okunieff P. Erythrocyte membrane ATP binding cassette (ABC) proteins: MRP1 and CFTR as well as CD39 (ecto-apyrase) involved in RBC ATP transport and elevated blood plasma ATP of cystic fibrosis. Blood Cells Mol Dis 27: 165–180, 2001. [DOI] [PubMed] [Google Scholar]
- 1a.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription (6th ed). Philadelphia, PA: Lippincott Williams and Wilkins, 2005. [Google Scholar]
- 2.Antus B. Oxidative stress markers in sputum. Oxid Med Cell Longev 2016: 2930434, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med 45: 279–296, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Braga VA, Couto GK, Lazzarin MC, Rossoni LV, Medeiros A. Aerobic exercise training prevents the onset of endothelial dysfunction via increased nitric oxide bioavailability and reduced reactive oxygen species in an experimental model of menopause. PLos One 10: e0125388, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RK, Wyatt H, Price JF, Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J 9: 334–339, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol 112: 2019–2026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunt VE, Minson CT. Cutaneous thermal hyperemia: more than skin deep. J Appl Physiol 111: 5–7, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol 129: 184–192, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol 33: 1379–1385, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Clayton A, Knox AJ. COX-2: a link between airway inflammation and disordered chloride secretion in cystic fibrosis? Thorax 61: 552–553, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cracowski JL, Roustit M. Current methods to assess human cutaneous blood flow. An updated focus on laser based-techniques. Microcirculation. In press. [DOI] [PubMed] [Google Scholar]
- 12.Dupont JJ, Farquhar WB, Townsend RR, Edwards DG. Ascorbic acid or l-arginine improves cutaneous microvascular function in chronic kidney disease. J Appl Physiol 111: 1561–1567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fielding J, Brantley L, Seigler N, McKie KT, Davison GW, Harris RA. Oxygen uptake kinetics and exercise capacity in children with cystic fibrosis. Pediatr Pulmonol 50: 647–654, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey S. Exercise tests in assessing children with lung or heart disease. Thorax 25: 258, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris LW, Wayland M, Lan M, Ryan M, Giger T, Lockstone H, Wuethrich I, Mimmack M, Wang L, Kotter M, Craddock R, Bahn S. The cerebral microvasculature in schizophrenia: a laser capture microdissection study. PLos One 3: e3964, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hector A, Griese M, Hartl D. Oxidative stress in cystic fibrosis lung disease: an early event, but worth targeting? Eur Respir J 44: 17–19, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol 105: 370–372, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Keahey TM, Indrisano J, White MV, Kaliner MA. Analyses of microvascular permeability in response to mediators of immediate hypersensitivity. J Allergy Clin Immunol 87: 586–594, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg DL Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Kellogg DL Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Larkin SW, Williams TJ. Evidence for sensory nerve involvement in cutaneous reactive hyperemia in humans. Circ Res 73: 147–154, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Lobato NS, Filgueira FP, Akamine EH, Tostes RC, Carvalho MH, Fortes ZB. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz J Med Biol Res 45: 392–400, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 60: 1455–1469, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 111: 20–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol 109: 1239–1246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Moorcroft AJ, Dodd ME, Webb AK. Exercise testing and prognosis in adult cystic fibrosis. Thorax 52: 291–293, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol 496: 531–542, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullineaux DR, Bartlett RM, Bennett S. Research design and statistics in biomechanics and motor control. J Sports Sci 19: 739–760, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol 25: 1551–1557, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Newton DJ, Davies J, Belch JJ, Khan F. Role of endothelium-derived hyperpolarising factor in acetylcholine-mediated vasodilatation in skin. Int Angiol 32: 312–318, 2013. [PubMed] [Google Scholar]
- 34.Nguyen S, Leroy S, Cracowski C, Perez T, Valette M, Neviere R, Aguilaniu B, Wallaert B. [Prognostic value of clinical exercise testing in adult patients with cystic fibrosis]. Rev Mal Respir 27: 219–225, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med 327: 1785–1788, 1992. [DOI] [PubMed] [Google Scholar]
- 36.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 373: 1891–1904, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Browne RW, Trevisan M, Iacoviello L, Cassano PA, Schunemann HJ. Oxidative stress and pulmonary function in the general population. Am J Epidemiol 162: 1137–1145, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Pastre J, Prevotat A, Tardif C, Langlois C, Duhamel A, Wallaert B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm Med 14: 74, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poore S, Berry B, Eidson D, McKie KT, Harris RA. Evidence of vascular endothelial dysfunction in young patients with cystic fibrosis. Chest 143: 939–945, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40: 1324–1343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasool AH, Abdul Rahman AS, Abd Ghaffar NA, Nik Mahmood NM, Wong AR. Abnormal microvascular reactivity with hypercholesterolaemia in pregnancy. Malays J Med Sci 17: 14–19, 2010. [PMC free article] [PubMed] [Google Scholar]
- 42.Reverri EJ, Morrissey BM, Cross CE, Steinberg FM. Inflammation, oxidative stress, and cardiovascular disease risk factors in adults with cystic fibrosis. Free Radic Biol Med 76: 261–277, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 126: 412–419, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Roustit M, Cracowski JL. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci 34: 373–384, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Roustit M, Cracowski JL. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 19: 47–64, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Schunemann HJ, Muti P, Freudenheim JL, Armstrong D, Browne R, Klocke RA, Trevisan M. Oxidative stress and lung function. Am J Epidemiol 146: 939–948, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Strain WD, Chaturvedi N, Bulpitt CJ, Rajkumar C, Shore AC. Albumin excretion rate and cardiovascular risk: could the association be explained by early microvascular dysfunction? Diabetes 54: 1816–1822, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Szewczyk A, Jarmuszkiewicz W, Koziel A, Sobieraj I, Nobik W, Lukasiak A, Skup A, Bednarczyk P, Drabarek B, Dymkowska D, Wrzosek A, Zablocki K. Mitochondrial mechanisms of endothelial dysfunction. Pharmacol Rep 67: 704–710, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Harasawa Y, Takeshita A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 90: 2285–2290, 1994. [DOI] [PubMed] [Google Scholar]
- 50.Tee GB, Rasool AH, Halim AS, Rahman AR. Dependence of human forearm skin postocclusive reactive hyperemia on occlusion time. J Pharmacol Toxicol Methods 50: 73–78, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Tur E, Politi Y, Rubinstein A. Cutaneous blood flow abnormalities in hypertriglyceridemia. J Invest Dermatol 103: 597–600, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Van Iterson EH, Karpen SR, Baker SE, Wheatley CM, Morgan WJ, Snyder EM. Impaired cardiac and peripheral hemodynamic responses to inhaled β2-agonist in cystic fibrosis. Respir Res 16: 103, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wesley NO, Maibach HI. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol 4: 843–860, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Wilkins BW, Martin EA, Roberts SK, Joyner MJ. Preserved reflex cutaneous vasodilation in cystic fibrosis does not include an enhanced nitric oxide-dependent mechanism. J Appl Physiol 102: 2301–2306, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol 95: 504–510, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Wood LG, Fitzgerald DA, Gibson PG, Cooper DM, Collins CE, Garg ML. Oxidative stress in cystic fibrosis: dietary and metabolic factors. J Am Coll Nutr 20: 157–165, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Woodward DF, Nieves AL, Williams LS, Gary RK Jr, Wasserman MA, Gleason JG. Interactive effects of peptidoleukotrienes and histamine on microvascular permeability and their involvement in experimental cutaneous and conjunctival immediate hypersensitivity. Eur J Pharmacol 164: 323–333, 1989. [DOI] [PubMed] [Google Scholar]
- 58.Zhao JL, Pergola PE, Roman LJ, Kellogg DL Jr. Bioactive nitric oxide concentration does not increase during reactive hyperemia in human skin. J Appl Physiol 96: 628–632, 2004. [DOI] [PubMed] [Google Scholar]