Cardiac ischemia causes a loss of sarcolemmal KATP channel density by a dynamin-mediated and CaMKII-dependent internalization mechanism. Ischemic preconditioning protects against the loss of surface KATP channels in a PKC-dependent manner. Maintenance of surface KATP channel expression must be considered as a potential protective mechanism of ischemic preconditioning.
Keywords: KATP channels, endocytic recycling, ischemia, ischemic preconditioning
Abstract
Myocardial ischemia remains the primary cause of morbidity and mortality in the United States. Ischemic preconditioning (IPC) is a powerful form of endogenous protection against myocardial infarction. We studied alterations in KATP channels surface density as a potential mechanism of the protection of IPC. Using cardiac-specific knockout of Kir6.2 subunits, we demonstrated an essential role for sarcolemmal KATP channels in the infarct-limiting effect of IPC in the mouse heart. With biochemical membrane fractionation, we demonstrated that sarcolemmal KATP channel subunits are distributed both to the sarcolemma and intracellular endosomal compartments. Global ischemia causes a loss of sarcolemmal KATP channel subunit distribution and internalization to endosomal compartments. Ischemia-induced internalization of KATP channels was prevented by CaMKII inhibition. KATP channel subcellular redistribution was also observed with immunohistochemistry. Ischemic preconditioning before the index ischemia reduced not only the infarct size but also prevented KATP channel internalization. Furthermore, not only did adenosine mimic IPC by preventing infarct size, but it also prevented ischemia-induced KATP channel internalization via a PKC-mediated pathway. We show that preventing endocytosis with dynasore reduced both KATP channel internalization and strongly mitigated infarct development. Our data demonstrate that plasticity of KATP channel surface expression must be considered as a potentially important mechanism of the protective effects of IPC and adenosine.
NEW & NOTEWORTHY
Cardiac ischemia causes a loss of sarcolemmal KATP channel density by a dynamin-mediated and CaMKII-dependent internalization mechanism. Ischemic preconditioning protects against the loss of surface KATP channels in a PKC-dependent manner. Maintenance of surface KATP channel expression must be considered as a potential protective mechanism of ischemic preconditioning.
after much research and despite improvements in clinical outcomes, acute myocardial infarction secondary to ischemia is still the primary cause of morbidity and mortality in the United States. The major determinant of the long-term prognosis among patients who survive an infarct is the size of the infarct (the amount of myocardium that is destroyed as a result of ischemic injury). It is critical, therefore, to advance our understanding of the molecular, cellular, and physiological mechanisms involved in the development, progression, and treatment of coronary heart disease and to develop therapeutic strategies that will attenuate myocardial cell death and infarct size in patients.
In 1986 it was found that short bursts of ischemia/reperfusion (I/R) before an index ischemic episode significantly limit infarct size development (29). This phenomenon, termed ischemic preconditioning (IPC), is complex in nature and has a multifactorial mechanism. It is generally agreed that receptor signaling by adenosine and other endogenous autacoids are involved and that they act through intracellular signaling pathways that include PKC, PI3 kinase, Akt, and ERK (44), with multiple effectors including cytoskeleton, KATP channel, connexin 43, and mitochondrial permeability transition pore (14).
Some of the earliest observations implicated KATP channels as being important in the infarct-limiting benefits of IPC. For example, in dogs, the benefit of IPC was prevented by the KATP channel blocker glibenclamide, whereas the KATP channel opener RP-49356 (also named aprikalim) could substitute for IPC (12). A large number of studies with pharmacological approaches have now confirmed that KATP channels have a key role in the cardioprotective mechanism(s) of IPC (9). The initial focus in the literature was on sarcolemmal KATP channels, which consist of pore-forming Kir6.x subunits assembled with regulatory SURx subunits (9). Subsequently, KATP channels were also found to be present in mitochondria (17), but their molecular composition is still unresolved. A large number of published studies have concluded that it is the mitochondrial KATP channel that mediates cardioprotection, but these studies must be interpreted with some caution since they often rely on pharmacological approaches and the use of compounds such as diazoxide, which (at the concentration ranges used in these studies) lack the required specificity and selectivity (6). In contrast, genetic studies in mice and human patients have implicated the sarcolemmal KATP channel subunits in cardiovascular events and protection (9). The focus of the current study is the sarcolemmal KATP channel.
The amount of ionic current is not only determined by alterations in the open probability of a channel but also by the channel density. It is becoming increasingly clear that intracellular trafficking events regulate the number of surface ion channels. Indeed, cells can internalize the equivalent of their entire surface area up to 5× per hour and most endocytosed proteins are recycled back to the membrane (25, 28). Not only is endocytic recycling a rapid process (time scale of minutes) but the rates of endocytosis and recycling are tightly controlled by specialized trafficking proteins and signaling pathways (25). Thus endocytic recycling is a potentially powerful mechanism to regulate the surface density and the availability of ion channels (34). Cellular studies have demonstrated that KATP channels can be endocytosed (4, 16, 23) and that they are recycled to the membrane (24). However, in hearts, KATP channel endocytic recycling has not been studied during ischemia and IPC. The aim of this study was to investigate KATP channel subcellular trafficking during ischemia and ischemic preconditioning. We found that KATP channels are internalized during ischemia, that IPC and adenosine prevent this internalization, and that inhibition of KATP channel internalization is cardioprotective.
METHODS
Generation of cardiac-specific Kir6.2 knockout mice.
The mouse model was generated by inGenious Targeting Labs (iTL; Stony Brook, NY). In brief, a 13.27-kb region was subcloned from a positively identified C57BL/B6 BAC clone (RPCI-23: 222N7). The region with a short homology arm extending ∼2.55kb 3′ to exon 1 and a long 7.52 kb homology arm ending 5′ to exon 1. A loxP/FRT-flanked Neo cassette was inserted on the 3′ side of exon 1, and the single loxP site was inserted ∼1.7kb 5′ of the start of exon 1. The target region was 3,198 bp and included exon 1 and ∼1.7 kb of upstream sequence. The targeting construct was linearized with AscI before electroporation into BA1 (C57BL/6 × 129/SvEv) hybrid embryonic stem cells. After G418 selection, surviving clones were expanded for PCR analysis to identify recombinant ES clones. Hybrid-positive ES cells were injected into C57BL/6 blastocysts. The Neo cassette was removed by breeding to FLP deleter mice (Jackson stock No. 005703), followed by backcrossing to a C57BL/6 background for >10 generations to achieve congenicity. Animals were then crossed with αMHC-Mer-Cre-Mer mice (JAX No. 005650)(36) and bred to homozygosity to allow for tamoxifen-inducible Cre expression and cardiac-specific deletion of Kir6.2, which was performed by treatment for five consecutive days with tamoxifen (intraperitoneal injection, 1 mg of a 10 mg/ml solution in corn oil), or with corn-oil as a control, and used after 3 wk.
Langendorff-perfused mouse hearts.
All animal handling procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committees of New York University School of Medicine. Male mice (8–12 wk) were injected with heparin and anesthetized with pentobarbital sodium (120 mg/kg ip). Hearts were rapidly excised into ice-cold modified Krebs-Henseleit (KH) buffer containing (in mM) 118.5 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 5 glucose, 0.05 Na-acetate, 0.06 Na-pyruvate, 1 Na-L-lactate, and 1.4 CaCl2. After removal of the lung and surrounding tissues, the aorta was rapidly cannulated with a blunted 21-gauge hypodermic needle and tied to the cannula with surgical silk (size 4). Hearts were then continuously perfused with KH solution, equilibrated with 95% O2-5% CO2 at a constant perfusion pressure of 80 mmHg (100 cm H2O) and a constant temperature of 37°C. Aortic flow was monitored (T206; Transonic Systems, Ithaca, NY), and hearts with a flow rate of <1 ml/min or >6.5 ml/min were excluded. Following an initial observational and stabilization period of 15 min, hearts were pseudo-randomized into one of three experimental groups. The IPC group consisted of three periods of 5-min global ischemia and 5-min reperfusion, before a 30-min index ischemia, achieved by stopping the coronary flow. The heart was submersed in prewarmed KH solution during the ischemic period to maintain the temperature. The ischemia group consisted of a time-matched 30-min extension of the stabilization period before a 30-min index ischemia, whereas the no-ischemia group was normoxically perfused for 1 h following the stabilization period. At the end of the perfusion protocol, hearts were either flash-frozen in liquid nitrogen and kept at −80°C for biochemical analysis or used to measure infarct size. Drugs were added during the stabilization period.
Membrane fractionation.
Flash-frozen hearts were ground to a fine powder in liquid nitrogen using a pestle and mortar. Samples were homogenized on ice with 30 strokes of a glass-glass homogenizer, followed by 30 strokes in a Dounce homogenizer in (in mM) 250 sucrose, 1 EDTA, 10 HEPES, 1 DTT, and pH 7.4 supplemented with protease inhibitor cocktail (Roche Applied Science). Following brief centrifugation (1,000 g for 5 min at 4°C), the pellet was rehomogenized with 25 strokes of a tight-fitting Dounce and cleared by brief centrifugation (1000 g, 5 min, 4°C). The resulting supernatant was combined with that of the previous step. The supernatant was then brought to 10% Optiprep by using a 60% stock solution (Sigma Aldrich), with 1 mM DTT, and protease inhibitors and layered on top of a 20% Optiprep cushion (1 ml). Any remaining homogenate was used to overlay the 10% Optiprep. The tubes were centrifuged at 267,100 g using a TH660 rotor (Thermo Scientific, Waltham, MA) for 1 h at 4°C. The 0/10% and 10/20% interfaces, respectively representing the plasma membrane and endosomal fractions, were collected with a syringe. These fractions were diluted with TE buffer (containing 10 mM Tris, 1 mM EDTA, pH 7.5) to 1.5 ml volume and centrifuged at 370,000 g for 15 min at 4°C. The resulting membrane pellets were solubilized overnight at 4°C in 20 mM HEPES, 0.5% Triton X-100, pH 7.4. Protein concentrations were measured using the Bradford Assay.
Western blotting.
For SDS-PAGE, samples with equal amounts of protein were mixed with sample buffer (ThermoFisher Scientific, Waltham, MA) containing 200 mM DTT and resolved using 10% polyacrylamide gels. Protein samples were not boiled before gel loading. After transfer to polyvinylidene difluoride (PVDF) membranes (Bio-Rad), and blocking (5% milk in TBS/0.05% Tween for 1 h), blots were incubated overnight at 4°C and 1 h at room temperature, respectively, with primary and secondary antibodies (dissolved in blocking buffer) and detected with chemiluminesence (SuperSignal West Dura; Thermo Scientific) and photographic film. Band intensities were quantified using ImageJ (National Institute of Health).
Immunofluorescence microscopy.
With the use of previously described methods (15), mouse heart cryosections with 9 μm thickness were fixed with 4% paraformaldehyde for 10 min and permeabilized with blocking solution (0.1% Triton X-100 and 20% serum in PBS) for 0.5 h at room temperature. Primary antibodies buffered in blocking solution were applied for 1 h. After wash with PBS, secondary antibodies buffered in blocking solution and DAPI were applied for 30 min. After antibodies were mounted, images were taken using Zeiss-700 confocal microscope. Regions of images that include the intercalated disk were converted to line plots (using ImageJ), and peak fluorescence intensities at the intercalated disk were normalized by the baseline fluorescence outside of the intercalated disk.
Antibodies used.
Primary antibodies used were rabbit anti-Kir6.2 (W62b developed by us; 1:10,000) (45) or goat anti-Kir6.2 (N18; Santa Cruz; 1:200), goat anti-SUR2A (M19; Santa Cruz; 1:1,000), rabbit anti-Cx43 (C6219; Sigma; 1:200), mouse anti-GADPH (GAPDH; Millipore; 1:10,000), rabbit anti-GLUT4 (G404 C-terminal; Sigma; 1:10,000), mouse Na+/K+ pump α-subunit (Millipore; 1:1,000), and mouse anti-NCX1 (H-300; Santa Cruz; 1:1,000). Secondary antibodies used were donkey anti-mouse-HRP (sc-2096; Santa Cruz Biotechnology; 1:50,000), donkey anti-goat-HRP (sc-2056; Santa Cruz Biotechnology; 1:5,000), goat anti-rabbit-HRP (sc-2054; Santa Cruz Biotechnology; 1:5,000), donkey anti-goat-dylight549 (705-505-147; Jackson; 1:200), and donkey anti-rabbit-dylight488 (711-485-152; Jackson; 1:200).
Determination of infarct size.
After the heart was removed from the perfusion cannula, it was wrapped in clear Saran wrap to prevent frost burn and placed at −20°C for 1 to 2 h, after which it was cut into 1-mm thick slices. The slices were incubated in a 1% solution of 2,3,5-triphenyl tetrazolium chloride (TTC) in phosphate buffer (pH 7.4) for 15–20 min at a temperature of 37°C. The slices were agitated at least once a minute to evenly stain all sides. Once color development was achieved (TTC stains viable tissue brick red, whereas infarcted tissue is not stained and remained a pale tan/white color), the slices were washed and fixed in 10% formaldehyde for 20 min at room temperature before they were placed at 4°C overnight to improve contrast between the stained and unstained tissue. Slices were placed between two glass slides and imaged using a stereo microscope at 1.0× magnification and a charge-coupled device color camera (OptixCam OCS-3.0). Infarct analysis was performed using a custom MATLAB program developed in-house. Using searching routines and contrast detection algorithms, the program automatically detects infarcted portions of heart in the green channel of a red, green, blue image and all non-infarcted portions of heart in the red channel. This information is user verified and used to calculate the heart size, the infarct size, and the percentage of the infarct as a function of total heart size.
Drugs used.
Dynasore hydrate was prepared as a 200-mM stock in DMSO and used at 80 μM by dissolving into KH solution. Chelerythrine chloride was prepared as a 380-mM stock in DMSO and KN-93 as a 1.6-mM stock in DMSO and respectively used at 3 μM and 0.5 μM by dissolving into KH solution. Adenosine (100 μM; Sigma-Aldrich, St. Louis, MO) was directly dissolved in KH solution.
Statistical analysis.
When comparing two groups we used the Student's t-test. For comparison of multiple groups we used one-way ANOVA. If overall significance was achieved, ad hoc pairwise comparison was performed using the Tukey t-test, or the Dunnett's t-test when comparisons were made to a single control. Statistical significance was assumed at P < 0.05.
RESULTS
The infarct-limiting effect of IPC is absent in mouse hearts deficient in Kir6.2 expression.
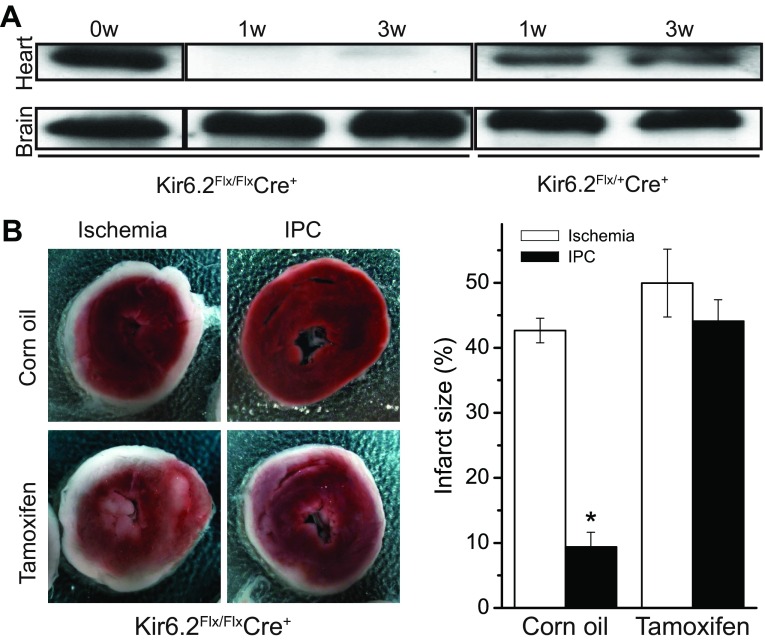
We verified the crucial role of sarcolemmal KATP channels in mediating IPC by using a conditional Kir6.2 knockout mouse model, which was crossed with αMHC-Mer-Cre-Mer mice (cardiac myocyte-specific Cre recombinase expression; JAX No. 005650) (36). Tamoxifen treatment of the heterozygous and homozygous Cre+ offspring, respectively, led to ∼50% and near complete reduction of cardiac Kir6.2 protein expression (Fig. 1A). Brain Kir6.2 expression was unaffected. To assess effects on ischemia and IPC, Kir6.2-Creflx/flx mice were injected with tamoxifen, or with corn oil as a control, and used after 8 wk. Isolated hearts were subjected to 30-min global ischemia, either preceded by an IPC perfusion protocol (3 × 5 min ischemia) or a time-matched normoxic perfusion period. As expected, IPC strongly diminished the infarct size in the control group. In contrast, the infarct-reducing protection afforded by IPC was strongly mitigated in the tamoxifen-treated Kir6.2 knockout mouse hearts (Fig. 1B). These data demonstrate an essential role for Kir6.2-containing sarcolemmal KATP channels in mediating the protective effects of IPC.
Fig. 1.
The protective effect of ischemic preconditioning (IPC) to reduce infarct size is lost in mice with cardiac-specific deletion of the KATP channel subunit, Kir6.2. A: mice were developed for cardiac-specific deletion of Kir6.2 by floxing the single exon that contains the coding region, and then bred with tamoxifen-inducible, cardiac-specific Cre-expressing transgenic mice to produce Kir6.2flx/flx-Cre+ or Kir6.2flx/−-Cre+. Western blotting with an anti-Kir6.2 antibody was performed with heart and brain following 1 and 3 wk after tamoxifen treatment. B: effects of 30 min of global ischemia on infarct size [determined 2,3,5-triphenyl tetrazolium chloride (TTC) staining] of isolated, perfused hearts, with or without preceding ischemic preconditioning (3 × 5 min ischemia) perfusion protocols. The Kir6.2flx/flx-Cre mice were treated with tamoxifen or corn-oil as control. Left: representative TTC-stained cardiac sections (red is viable tissue). Right: summary data of infarct size, expressed as a percentage of heart size. Infarct sizes without (n = 6 each, respectively, for corn oil and tamoxifen treatment) and with (n = 6 for corn oil group and n = 7 for tamoxifen treatment) an IPC perfusion protocol preceding a 30-min index ischemic episode are denoted. *P < 0.05 when comparing the infarct sizes between the ischemia and IPC groups.
Ischemia causes internalization of KATP channels, which is prevented by IPC.
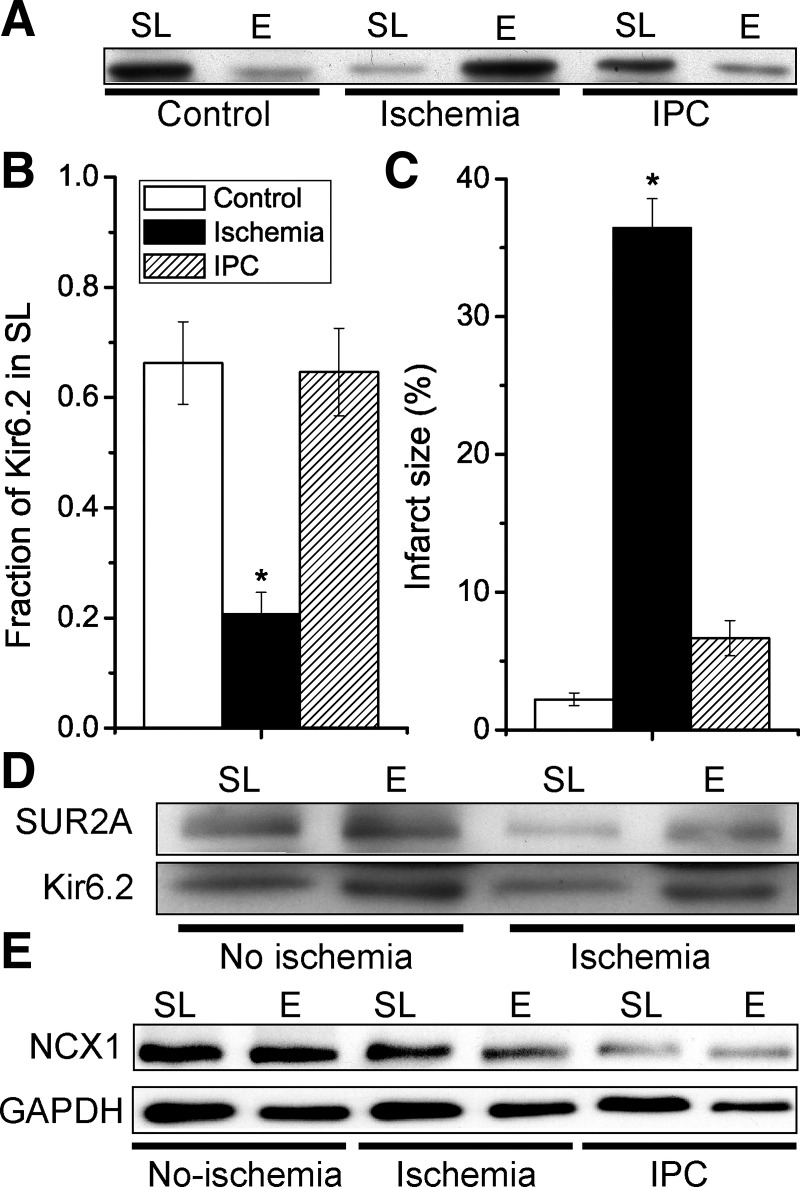
Because KATP channels undergo rapid endocytosis and recycling, we investigated the effects of ischemia on the subcellular distribution of KATP channels in hearts. We used density gradient ultracentrifugation with OptiPrep to separate sarcolemmal (SL; 0–10% fraction) and endosomal (E; 10–20% fraction) membranes from mouse hearts. In normoxic mouse hearts, on average ∼60% of Kir6.2 was present in the sarcolemmal membrane fraction. After 30-min global ischemia, however, the averaged SL expression decreased to ∼20% (Fig. 2, A and B). Ischemia-induced internalization of SUR2A was also observed (Fig. 2D). We next investigated the effects of IPC. Remarkably, not only did IPC limit the infarct development during ischemia but it also prevented the ischemia-induced internalization of Kir6.2 (Fig. 2). Another membrane protein, NCX1, showed only minor SL/E distribution changes (Fig. 2E). Thus ischemia causes a loss of surface KATP channel expression, whereas KATP channel surface expression is maintained by IPC.
Fig. 2.
Ischemic preconditioning not only limits infarct size but also prevents ischemia-induced internalization of the Kir6.2 KATP channel subunit. Isolated mouse hearts were made globally ischemic for 30 min, either with (IPC) a 3 × 5 min IPC protocol or without (ischemia group). A time-matched control normoxic group was also included. A: Western blot with an anti-Kir6.2 antibody of sarcolemmal (SL; 0–10% Optiprep) and endosomal (E; 10–20% Optiprep) membranes for the various groups. B: summary data of sarcolemmal Kir6.2 (as a fraction of the total amount; SL/[SL + E]) for the control (n = 9), ischemia (n = 9), and IPC (n = 7). C: in a different set of hearts, infarct sizes were determined by TTC staining; control (n = 8), ischemia (n = 12), and IPC (n = 7). *P < 0.05 vs. the control group. D: redistribution of Kir6.2 from sarcolemmal to endosomal membrane fractions with ischemia was also observed with SUR2A. E: internalization of Kir6.2 during ischemia is not a general phenomenon. Sarcolemmal and endosomal membranes were prepared from mouse hearts with normoxic perfusion (control), after 30-min global ischemia (ischemia) or with 3 × 5 min ischemia before a 30-min index ischemia (IPC). Immunoblotting was performed with antibodies against Na+/Ca2+ exchanger (NCX)1 and GAPDH. Data are representative of n = 3 experiments.
Adenosine also prevents ischemia-induced KATP channel internalization.
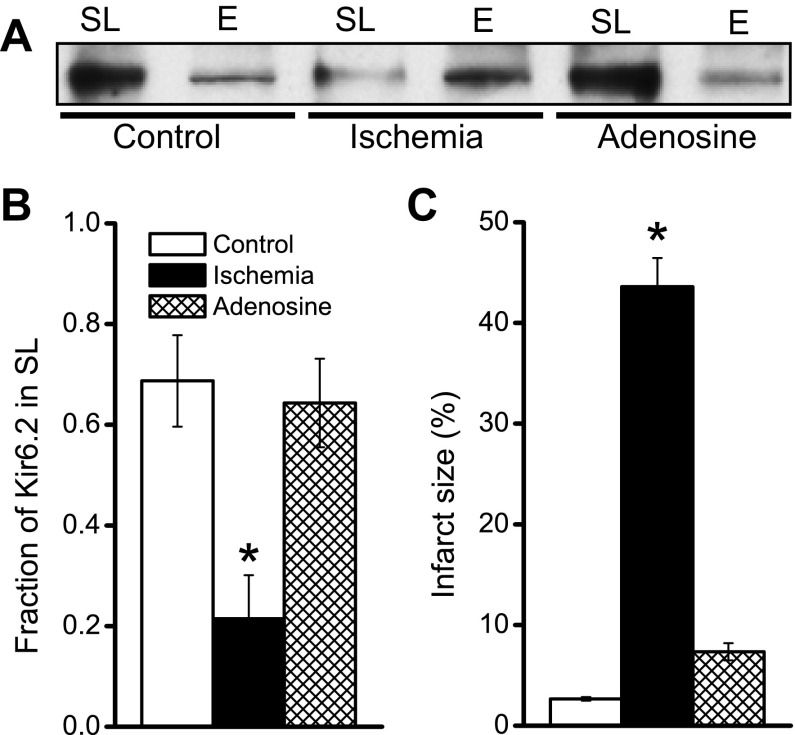
Several endogenous molecules, including adenosine, can mimic the protective effect of ischemic preconditioning (14), a process also referred to as pharmacological preconditioning. We investigated the effects of pharmacological preconditioning with adenosine on the plasticity of KATP channel surface expression. As expected, perfusion of mouse hearts with 100 μM adenosine before a 30-min index ischemia effectively decreased infarct size (Fig. 3C). Interestingly, as we have observed with IPC, adenosine also fully prevented the ischemia-induced surface loss of KATP channel subunits (Fig. 3, A and B). Thus two forms of cardioprotection (IPC and adenosine) share the phenomenon of preventing the surface loss of KATP channels during ischemia.
Fig. 3.
Adenosine perfusion prevents ischemia-induced internalization of the Kir6.2 KATP channel subunit and limits infarct size. Isolated mouse hearts were made globally ischemic for 30 min. A time-matched control normoxic group was also included. The time-matched adenosine group was perfused with 100 μM for 10 min before the 30-min index ischemia. A: Western blot with an anti-Kir6.2 antibody of sarcolemmal (0–10% Optiprep) and endosomal (10–20% Optiprep) membranes for the various groups. B: summary data of sarcolemmal Kir6.2 for the control (n = 9), ischemia (n = 9), and adenosine (n = 3) groups. C: in a different set of hearts, infarct sizes were determined by TTC staining: control (n = 8), ischemia (n = 12), and adenosine (n = 9). *P < 0.05 vs. the control group.
CaMKII signaling is involved in ischemia-induced KATP channel internalization.
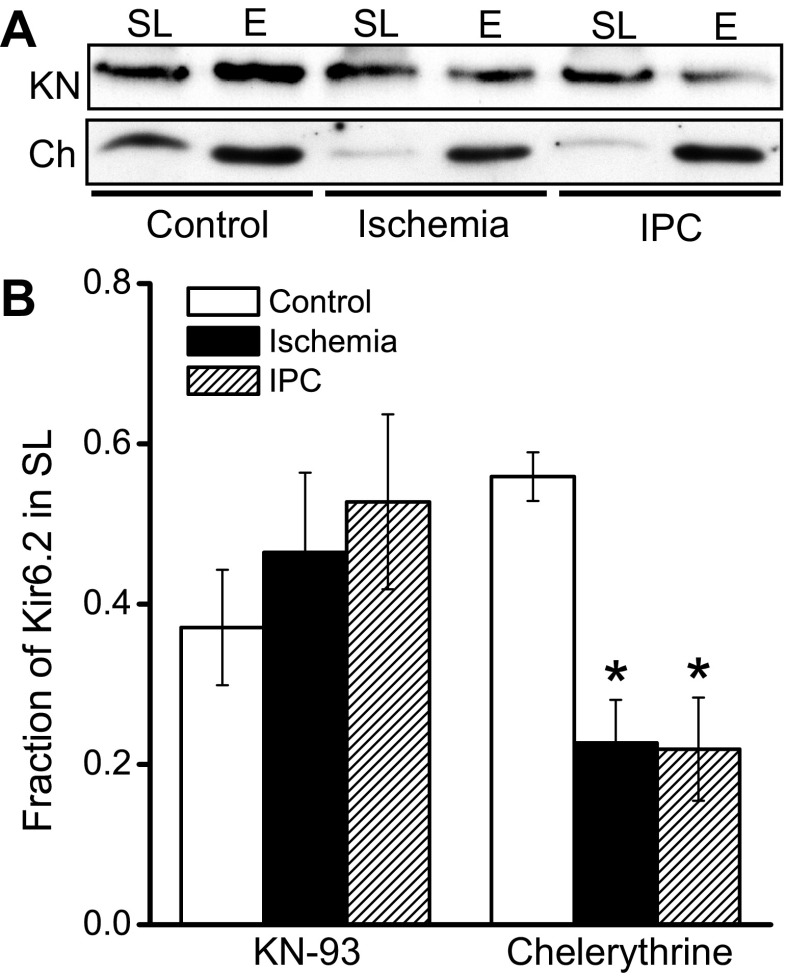
We investigated some of the signaling mechanisms involved in modulating ischemia-induced KATP channel internalization. During ischemia, Ca2+/calmodulin-dependent protein kinase II (CaMKII) is activated (2), partly as a result of an elevated [Ca2+]i. Considerable evidence indicates a deleterious role for CaMKII and a beneficial effect of CaMKII inhibition during ischemia (2). Moreover, CaMKII inhibition enhances the KATP current density (19), whereas CaMKII activation stimulates KATP channel endocytosis in cardiomyocytes (35). We therefore investigated the significance of CaMKII signaling during ischemia. When mouse hearts were perfused with the CaMKII inhibitor KN-93 before the index ischemia, Kir6.2 internalization did not occur (Fig. 4), suggesting that ischemia-induced KATP channel internalization occurs though a CaMKII-mediated mechanism.
Fig. 4.
Roles of CaMKII and PKC signaling on KATP channel trafficking during ischemia and IPC. Mouse hearts were perfused with the CaMKII inhibitor (KN-93, or KN; 0.5 μM) or the PKC inhibitor chelerythrine (3 μM) before subjecting the hearts to ischemia or IPC protocols (or a no-ischemia control). A: sarcolemmal (SL) and endosomal (E) membrane preparations were subjected to immunoblotting with an anti-Kir6.2 antibody. B: fractions of SL Kir6.2 in the presence of KN-93: control (n = 3), ischemia (n = 3), and IPC (n = 3). Fractions of SL Kir6.2 in the presence of chelerythrine are also shown: control (n = 4), ischemia (n = 4), and IPC (n = 4). *P < 0.05 vs. the control group.
PKC signaling during IPC maintains the surface KATP channel density.
Adenosine and opioids mediate their cardioprotective effects through PKC activation. We therefore investigated the hypothesis that PKC activation positively regulates KATP channel surface expression during IPC. The PKC inhibitor chelerythrine did not prevent internalization of Kir6.2 during ischemia (Fig. 4). However, the protective effect of IPC on surface KATP channel expression was eliminated (Fig. 4). Thus we conclude that IPC causes re-expression of surface KATP channels in a PKC-dependent manner.
Plasticity of KATP channel distribution can be directly observed with microscopy.
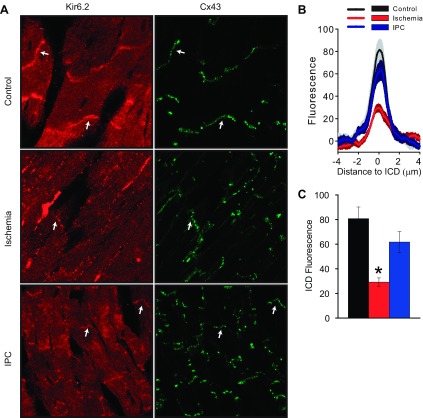
We next sought to investigate ischemia-induce KATP channel internalization using an independent assay by performing immunofluorescence microscopy of mouse hearts. Consistent with our previous report (15), we found KATP channel expression in punctate patterns in cardiomyocytes with enrichment at the intercalated disk (ICD) (Fig. 5A). For quantitative analysis, we focused on the ICD regions identified by connexin 43 (Cx43) expression (arrows). Consistent with the biochemical assays, the Kir6.2 staining at the ICD was significantly reduced by ischemia compared with nonischemic hearts. Moreover, IPC prevented this ischemia-induced redistribution of KATP channels (Fig. 5, B and C). Although previous studies have described distribution changes of Cx43 with chronic ischemia and with ischemic cardiomyopathies (26), during acute ischemia and IPC, we found Cx43 redistribution to be minimal relative to that of KATP channels (data not shown). Thus plasticity of KATP channel expression and subcellular redistribution could be observed with two independent assays.
Fig. 5.
Immunohistochemistry (IHC) of mouse heart cryosections, demonstrating ischemia-induced KATP channel subcellular redistribution and its prevention by IPC. A: representative images obtained with antibodies against Kir6.2 and Connexin 43 (Cx43) in the control, ischemia, and IPC groups. B: relationship between Kir6.2 fluorescence intensity and distance across the intercalated disk (ICD) in the control (black), ischemia (red), and IPC (blue) groups. C: quantification of the peak Kir6.2 fluorescence at the ICD in the control. For each group, data are from 3 hearts and 3 separate sections for each heart (n = 9). *P < 0.05 vs. the control group.
Inhibition of endocytosis is cardioprotective.
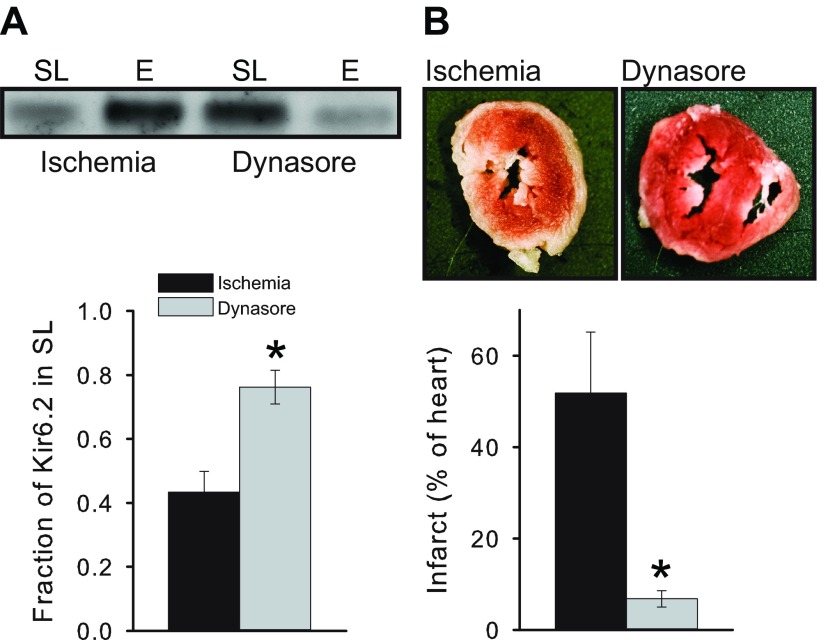
Many membrane proteins, including KATP channels (9), are internalized by clathrin- and dynamin-dependent endocytosis. To examine the role of endocytosis during ischemia, we used the dynamin inhibitor, dynasore (22). Consistent with a role for dynamin-dependent endocytosis during ischemia, we found that perfusing mouse hearts with 80 μM dynasore largely prevented ischemia-induced KATP channel internalization, either when measuring KATP channel subcellular distribution biochemically (Fig. 6A) or with microscopy (Fig. 7). Consistent with the hypothesis that ischemia-induced endocytosis is detrimental, we found dynasore to be powerfully cardioprotective in that it mimicked IPC by preventing infarct formation (Fig. 6B).
Fig. 6.
Inhibiting endocytosis prevents ischemia-induced KATP channel redistribution and protects the heart from ischemic damage. A, top: Western blot with an anti-Kir6.2 antibody of sarcolemmal and endosomal membranes for ischemia and dynasore groups. A, bottom: summary data of sarcolemmal Kir6.2 for ischemia (n = 3) and dynasore (n = 3) groups. B, top: representative TTC-stained heart sections obtained after ischemia in the absence and presence of dynasore. B, bottom: summary data of infarct sizes for the ischemia (n = 4) and dynasore (n = 4) groups. *P < 0.05.
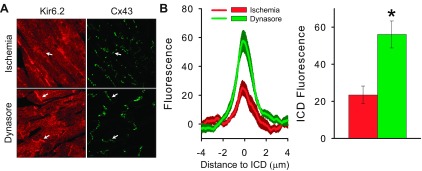
Fig. 7.
Confocal microscopy of mouse heart cryosections confirms the ischemia-induced KATP channel subcellular redistribution and its prevention by IPC. A: representative IHC images of mouse heart cryosections obtained with antibodies against Kir6.2 and Cx43 after 30-min global ischemia in the absence (top) or presence (bottom) of dynasore. B: summary data of the Kir6.2 fluorescence intensity for ischemia (n = 9; red) and dynasore (n = 9; green) groups. *P < 0.05 vs. the ischemia group.
DISCUSSION
Using cardiac-specific KATP channel knockout animals, we demonstrated an essential role for Kir6.2-containing sarcolemmal KATP channels in the infarct-limiting effect of IPC in the mouse heart. A major finding of the current study is that ischemia causes a loss of surface KATP channels and that IPC restores this defect. This plasticity of surface KATP channel expression comes about from an internalization process during ischemia that depends on dynamin and CaMKII signaling (possibly as a result of intracellular Ca2+ accumulation). The KATP channel surface density is maintained by IPC and by adenosine, and this appears to occur as a result of a PKC-mediated re-expression of surface KATP channels. Preventing endocytosis with dynasore mimics the cardioprotective infarct size-limiting effects of IPC. On balance, our data suggest that maintenance of the surface KATP channel density represent a novel cardioprotective mechanism.
Sarcolemmal KATP channels are necessary for the protective effects of IPC.
Sarcolemmal KATP channels contribute to cardioprotection and cardiovascular disease, as demonstrated by a multitude of animal studies using knockout mouse models of KATP channel subunits (13, 38, 39, 43) and by the association of genetic variation in KATP channel genes and human cardiovascular disease (3, 7, 30, 32, 33) and risk of myocardial infarction (27). There has been much controversy regarding the relative roles of mitochondrial and sarcolemmal KATP channels in mediating the protective effects of IPC (9, 10). We used a novel mouse model in which cardiac myocytes are specifically deficient of the KATP channel subunit, Kir6.2, which is thought to be a component of the sarcolemmal, but not the mitochondrial, KATP channel (9). Interestingly, the infarct size in the hearts of these animals was not significantly larger during ischemia, which suggests that KATP channel opening during ischemia might not directly contribute to cardioprotection. This finding is consistent with the observation made in several animal models that KATP channel blockers by themselves generally have little effect on infarct size development (9). In striking contrast, we found that the protective effect of IPC on infarct development was completely absent in the hearts of mice that lack the sarcolemmal KATP channel subunit, Kir6.2. This observation is consistent with a multitude of studies demonstrating that structurally diverse KATP channel openers (including aprikalim, bimakalim, pinacidil, nicorandil, and diazoxide) are cardioprotective by reducing the size of the developing infarct when they are applied before index ischemia (instead of IPC) and that the protective effects of IPC can be prevented by KATP channel blockers such as glibenclamide, 5-hydroxydecanoate, and HMR-1098 (9). Our data are also consistent with previous studies demonstrating an essential role for Kir6.2 in the genesis of diazoxide-induced preconditioning in mouse hearts (38, 43). Overall, our finding underscores the fact that the sarcolemmal KATP channel has a crucial role in mediating the beneficial effects of IPC.
Loss of surface KATP channels during ischemia.
Sarcolemmal KATP channel opening can be protective by conserving ATP consumption due to the negative inotropic effect caused by action potential shortening (the “energy sparing hypothesis”), by preventing Ca2+-induced mitochondrial dysfunction, and through other mechanisms (9, 37). Even though sarcolemmal KATP channels are strongly linked to the cardioprotective effects of IPC (13, 19, 39, 43), the underlying molecular mechanisms have not been resolved. Our studies demonstrate that KATP channels are internalized during ischemia, which would mitigate any protective roles of these channels.
In a previous study we reported that ischemia increased the SL KATP channel subunit abundance in rat hearts harvested from dead rats (∼20 min after euthanasia) (1). We have now repeated the study with mouse hearts, by carefully controlling all experimental variables, including the temperature, optimization of the membrane preparation protocols, having time-matched controls, the duration of ischemia, and the presence of IPC. A main finding of the present study is that both Kir6.2 and SUR2A are internalized during ischemia (i.e., they translocate from SL to E membranes). Species differences are an unlikely explanation for the different result since we also observed Kir6.2 internalization with Langendorff-perfused rat hearts (not shown). The ischemia-induced internalization of KATP channels is akin to the internalization of the Na+/K+ pump α-subunit that occurs in cellular models of simulated ischemia (31). The KATP channel internalization that we observed during ischemia is unlikely to reflect massive endocytosis (MEND), described in fibroblasts and in isolated cardiac myocytes subjected to anoxia (21). Hallmarks of MEND are that it is cargo-indiscriminate, it is dependent on palmitoylation, and it occurs without the involvement of classical endocytic proteins (18). In contrast, we show that internalization during ischemia discriminates between various cargos and can be blocked by inhibitors of endocytosis. The fact that dynasore blocks the process suggests that ischemia-induced internalization occurs through classical endocytosis described in other cells, namely cargo recognition mediated by binding of Kir6.2 to the μ2 subunit of the AP-2 adaptor complex (23) and an internalization pathway mediated by clathrin and dynamin (23, 35).
The role of CaMKII.
We showed that CaMKII inhibition with KN-93 prevents ischemia-induced KATP channel internalization. A potential role for this Ca2+-dependent signaling pathway in KATP channel trafficking was previously suggested by the finding that CaMKII inhibition increases the KATP channel density in membrane patches from cardiomyocytes (19). In a subsequent study, it was shown that CaMKII activation reduces KATP channel surface density in isolated cardiac myocytes by stimulating endocytosis (35). Moreover, a Kir6.2 tyrosine-based internalization motif (330YSKF333) was necessary for CaMKII-induced endocytosis. Our finding provides further credence to the concept that internalization is mediated in part by CaMKII activation. A decreased number of sarcolemmal KATP channels during ischemia due to internalization would be expected to reduce the protective role of KATP channels. Rescuing KATP channel internalization with CaMKII inhibition (19) is therefore a potential mechanism for preventing cardiac damage.
Is trafficking inhibition the key to prevent ischemic damage?
Our data show that a specific inhibitor of dynamin-dependent endocytosis (dynasore) mimics IPC by being powerfully protective against infarct size development. The interpretation of this result is not simple, since all forms of dynamin-dependent endocytosis are being inhibited by the drug. Given the positive regulation of endocytosis by CaMKII (19, 35), it is tempting to speculate that CaMKII inhibitors and internalization inhibitors (such as dynasore) share a common mechanism of action. The literature is not consistent in this regard, however, since it has been suggested that internalization of G protein-coupled receptor signaling complexes may serve as a mediator of IPC (40). It should be noted that endocytosis of GPCRs can occur via multiple mechanisms, including caveolae, clathrin-coated vesicles, or uncoated vesicles (5), and the potential deleterious or protective roles of specific endocytic pathways remain to be elucidated. Because dynasore improves the ischemic outcome, our data suggest that dynamin-dependent processes are deleterious to the ischemic heart. Further challenges remain, including the identities of the specific proteins involved (likely to be multifactorial) and their relative roles. The KATP channel appears to be one of these candidates, but it is unlikely to be the only relevant protein involved. Regardless, our data raise the tantalizing question whether trafficking inhibition is a key to prevent ischemic damage and whether more targeted pharmacological or molecular approaches might be useful to mediate cardioprotection.
KATP channel surface density is maintained by IPC and adenosine.
A major finding of this study is that IPC not only limits infarct development, it also maintains the KATP channel surface density by affecting subcellular distribution patterns. We verified this finding with two independent methods (biochemically and with immunohistochemistry). Given the key role of KATP channels in mediating the protective effect of IPC on infarct size (9), also demonstrated with the conditional Kir6.2 knockout animals, we postulate that the beneficial role of IPC is caused (at least in part) by preserving the surface density of sarcolemmal KATP channels. The IPC protocol by itself, in the absence of index ischemia, did not cause a significant redistribution of KATP channel subunits (not shown), suggesting that IPC sets in motion a cascade of events that operate during a subsequent ischemic period. Indeed, we found that adenosine, an endogenous autacoid that mimics the infarct-limiting effects of IPC (8), similarly protects against ischemia-induced KATP channel internalization. Our data are also in full support of the known cross-reaction between adenosine signaling and KATP channels. For example, the protective effect of adenosine is prevented by KATP channel blockers (20, 41) and is absent in KATP channel-deficient Kir6.2−/− mice (42). Moreover, our data are also in full support of the notion that the protective effects of IPC and adenosine occur through PKC activation (11). Indeed, PKC inhibition did not prevent ischemia-induced KATP channel internalization, but disabled the protective effect of IPC to maintain the surface KATP channel density. Thus, taken together, our data show that IPC, adenosine, and intracellular signaling through PKC converge on the preservation of the sarcolemmal KATP channel density and that this response may be an important element in the protection afforded by these processes.
Conclusions
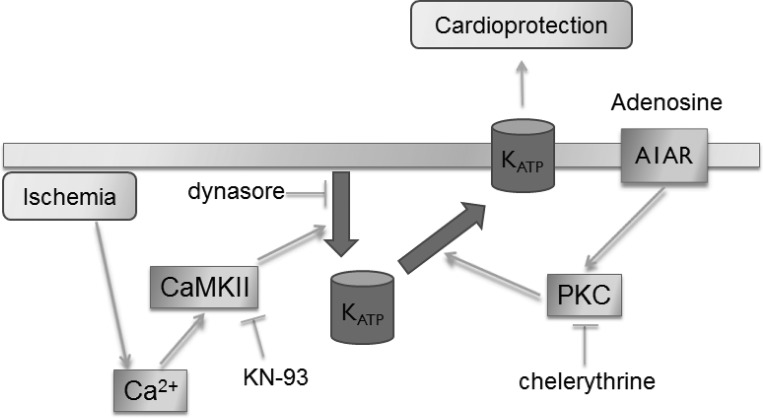
Our studies show that cardiac ischemia causes a loss of the sarcolemmal KATP channel density by internalization through a pathway mediated by dynamin-dependent endocytosis and CaMKII-mediated signaling. Importantly, IPC counteracts this loss and restores the density of the sarcolemmal KATP channels. Adenosine mimics IPC in this regard, and PKC signaling mediates the maintenance of surface KATP density. Inhibition of endocytosis mimics IPC by protecting against infarct development. We propose that maintenance of the KATP channel surface density must be considered as a protective mechanism of ischemic preconditioning (Fig. 8).
Fig. 8.
Proposed mechanism by which ischemia and ischemic preconditioning affect KATP channel subcellular trafficking. Our data indicate that KATP channels are internalized during ischemia by dynamin-dependent endocytosis and that this process depends on CaMKII signaling. IPC and adenosine may stimulate recycling of the internalized KATP channels back to the sarcolemma, and PKC signaling appears to be involved at this step. The larger surface density of KATP channels may promote cardioprotection against further ischemic episodes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-126905, HL-126905-S1, HL-093563, HL-085820, and HL-085820-S1 (to W. A. Coetzee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.-Q.Y., M.N.F., J.H., M.J.R., and W.A.C. conception and design of research; H.-Q.Y., M.N.F., K.J., J.H., and W.A.C. performed experiments; H.-Q.Y., M.N.F., K.J., J.H., and W.A.C. analyzed data; H.-Q.Y., M.N.F., K.J., J.H., M.J.R., and W.A.C. interpreted results of experiments; H.-Q.Y., J.H., and W.A.C. prepared figures; H.-Q.Y. and W.A.C. drafted manuscript; H.-Q.Y., M.N.F., K.J., J.H., M.J.R., and W.A.C. edited and revised manuscript; H.-Q.Y., M.N.F., K.J., J.H., M.J.R., and W.A.C. approved final version of manuscript.
REFERENCES
- 1.Bao L, Hadjiolova K, Coetzee WA, Rindler MJ. Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits. Am J Physiol Heart Circ Physiol 300: H262–H270, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell JR, Vila-Petroff M, Delbridge LM. CaMKII-dependent responses to ischemia and reperfusion challenges in the heart. Front Pharmacol 5: 96, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O′Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet 36: 382–387, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruederle CE, Gay J, Shyng SL. A role of the sulfonylurea receptor 1 in endocytic trafficking of ATP-sensitive potassium channels. Traffic 12: 1242–1256, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol 66: 61–79, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Coetzee WA. Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol Ther 140: 167–175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper PE, Reutter H, Woelfle J, Engels H, Grange DK, van Haaften G, van Bon BW, Hoischen A, Nichols CG. Cantu syndrome resulting from activating mutation in the KCNJ8 gene. Hum Mutat 35: 809–813, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Foster MN, Coetzee WA. KATP channels in the cardiovascular system. Physiol Rev 96: 177–252, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garlid KD, Halestrap AP. The mitochondrial KATP channel—fact or fiction? J Mol Cell Cardiol 52: 578–583, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto M, Cohen MV, Downey JM. The role of protein kinase C in ischemic preconditioning. Ann N Y Acad Sci 793: 177–190, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res 70: 223–233, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol 284: H2106–H2113, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116: 674–699, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Hong M, Bao L, Kefaloyianni E, Agullo-Pascual E, Chkourko H, Foster M, Taskin E, Zhandre M, Reid DA, Rothenberg E, Delmar M, Coetzee WA. Heterogeneity of ATP-sensitive K+ channels in cardiac myocytes: enrichment at the intercalated disk. J Biol Chem 287: 41258–41267, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron 38: 417–432, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352: 244–247, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Lariccia V, Fine M, Magi S, Lin MJ, Yaradanakul A, Llaguno MC, Hilgemann DW. Massive calcium-activated endocytosis without involvement of classical endocytic proteins. J Gen Physiol 137: 111–132, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Marionneau C, Koval O, Zingman L, Mohler PJ, Nerbonne JM, Anderson ME. Calmodulin kinase II inhibition enhances ischemic preconditioning by augmenting ATP-sensitive K+ current. Channels (Austin) 1: 387–394, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Liang BT. Protein kinase C-dependent activation of KATP channel enhances adenosine-induced cardioprotection. Biochem J 336: 337–343, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin MJ, Fine M, Lu JY, Hofmann SL, Frazier G, Hilgemann DW. Massive palmitoylation-dependent endocytosis during reoxygenation of anoxic cardiac muscle. eLife 2: e01295, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Mankouri J, Taneja TK, Smith AJ, Ponnambalam S, Sivaprasadarao A. Kir6.2 mutations causing neonatal diabetes prevent endocytosis of ATP-sensitive potassium channels. EMBO J 25: 4142–4151, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control KATP channel surface density. J Biol Chem 285: 5963–5973, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Michela P, Velia V, Aldo P, Ada P. Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol 768: 71–76, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Minoretti P, Falcone C, Aldeghi A, Olivieri V, Mori F, Emanuele E, Calcagnino M, Geroldi D. A novel Val734Ile variant in the ABCC9 gene associated with myocardial infarction. Clin Chim Acta 370: 124–128, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev 77: 759–803, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Olson TM, Terzic A. Human KATP channelopathies: diseases of metabolic homeostasis. Pflügers Arch 460: 295–306, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierre SV, Belliard A, Sottejeau Y. Modulation of Na+-K+-ATPase cell surface abundance through structural determinants on the α1-subunit. Am J Physiol Cell Physiol 300: C42–C48, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes S, Park S, Johnson BD, Terzic A, Olson TM. KATP channel Kir6.2 E23K variant overrepresented in human heart failure is associated with impaired exercise stress response. Hum Genet 126: 779–789, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes S, Terzic A, Mahoney DW, Redfield MM, Rodeheffer RJ, Olson TM. KATP channel polymorphism is associated with left ventricular size in hypertensive individuals: a large-scale community-based study. Hum Genet 123: 665–667, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson GA. Endocytic control of ion channel density as a target for cardiovascular disease. J Clin Invest 119: 2531–2534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sierra A, Zhu Z, Sapay N, Sharotri V, Kline CF, Luczak ED, Subbotina E, Sivaprasadarao A, Snyder PM, Mohler PJ, Anderson ME, Vivaudou M, Zingman LV, Hodgson-Zingman DM. Regulation of cardiac ATP-sensitive potassium channel surface expression by calcium/calmodulin-dependent protein kinase II. J Biol Chem 288: 1568–1581, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20–25, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Storey NM, Stratton RC, Rainbow RD, Standen NB, Lodwick D. Kir6.2 limits Ca2+ overload and mitochondrial oscillations of ventricular myocytes in response to metabolic stress. Am J Physiol Heart Circ Physiol 305: H1508–H1518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation 107: 682–685, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest 109: 509–516, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res 94: 1133–1141, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Vanwinkle DM, Chien GL, Wolff RA, Soifer BE, Kuzume K, Davis RF. Cardioprotection provided by adenosine receptor activation is abolished by blockade of the KATP channel. Am J Physiol Heart Circ Physiol 266: H829–H839, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther 324: 234–243, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wojtovich AP, Urciuoli WR, Chatterjee S, Fisher AB, Nehrke K, Brookes PS. Kir6.2 is not the mitochondrial KATP channel but is required for cardioprotection by ischemic preconditioning. Am J Physiol Heart Circ Physiol 304: H1439–H1445, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther 24: 225–234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. KATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol 37: 857–869, 2004. [DOI] [PubMed] [Google Scholar]