Genetic deletion, or female-specific downregulation, of the soluble epoxide hydrolase gene and potentiation of NO activity attenuate pressure-induced coronary vasoconstrictions, which provides 1) a mechanistically based rationale to further explore the therapeutic potential of soluble epoxide hydrolase inhibitors in improving coronary circulation and 2) mechanistic insight for the lower incidence of ischemic heart diseases in women.
Keywords: sex, soluble epoxide hydrolase, myogenic response, coronary artery
Abstract
Epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid via CYP/epoxygenases, which are catabolized by soluble epoxide hydrolase (sEH) and known to possess cardioprotective properties. To date, the role of sEH in the modulation of pressure-induced myogenic response/constriction in coronary arteries, an important regulatory mechanism in the coronary circulation, and the issue as to whether the disruption of the sEH gene affects the myogenic response sex differentially have never been addressed. To this end, experiments were conducted on male (M) and female (F) wild-type (WT) and sEH-knockout (KO) mice. Pressure-diameter relationships were assessed in isolated and cannulated coronary arteries. All vessels constricted in response to increases in intraluminal pressure from 60 to 120 mmHg. Myogenic vasoconstriction was significantly attenuated, expressed as an upward shift in the pressure-diameter curve of vessels, associated with higher cardiac EETs in M-KO, F-WT, and F-KO mice compared with M-WT controls. Blockade of EETs via exposure of vessels to 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) prevented the attenuated myogenic constriction in sEH-KO mice. In the presence of 14,15-EEZE, pressure-diameter curves of females presented an upward shift from those of males, exhibiting a sex-different phenotype. Additional administration of Nω-nitro-l-arginine methyl ester eliminated the sex difference in myogenic responses, leading to four overlapped pressure-diameter curves. Cardiac sEH was downregulated in F-WT compared with M-WT mice, whereas expression of endothelial nitric oxide synthase and CYP4A (20-HETE synthase) was comparable among all groups. In summary, in combination with NO, the increased EET bioavailability as a function of genetic deletion and/or downregulation of sEH accounts for the female-favorable attenuation of pressure-induced vasoconstriction.
NEW & NOTEWORTHY
Genetic deletion, or female-specific downregulation, of the soluble epoxide hydrolase gene and potentiation of NO activity attenuate pressure-induced coronary vasoconstrictions, which provides 1) a mechanistically based rationale to further explore the therapeutic potential of soluble epoxide hydrolase inhibitors in improving coronary circulation and 2) mechanistic insight for the lower incidence of ischemic heart diseases in women.
the myogenic response is the pressure-sensitive behavior of blood vessels, predominantly in resistance vessels. The myogenic response is characterized as a maintained or reduced vessel diameter (vasoconstriction) in response to increases in intravascular pressure and dilation with reductions in pressure. This pressure-sensitive mechanism contributes to autoregulation, and is one of the primary mechanisms responsible for locally governing the tone of resistance arteries, and therefore peripheral resistance and blood pressure. In the coronary circulation, coronary arteries are specifically exposed to a rhythmic compression generated by myocardial contraction. To this end, the pressure-sensitive mechanism becomes critically important in the regulation of coronary resistance and cardiac flow/perfusion (23, 26, 30, 31, 37). Although this pressure-sensitive behavior originates from the smooth muscle layer of blood vessels that depolarizes in response to increases in pressure, and is therefore named as “myogenic response,” the endothelial cells constitutively produce a variety of vasodilators such as endothelial nitric oxide (NO) that counteracts the pressure-induced vasoconstriction. In some pathological situations, an increase in myogenic constriction of arterioles due to the inadequate synthesis of NO could profoundly affect the regulation of peripheral resistance (17), and, visa versa, the attenuated myogenic responses as a function of increases in NO bioavailability possess beneficial properties to lower blood pressure (14, 16, 18). In this context, actions of NO in the attenuation of coronary arteriolar tone have been extensively studied (6, 23, 26). In the normal heart, coronary arterioles are responsible for the maintenance of the high level of coronary vasomotor tone, whereas increases in endothelial dilator stimuli elicit a significant elevation of coronary flow, as a function of reduced arteriolar tone (8). Particularly, endothelial epoxyeicosatrienoic acids (EETs; cytochrome P-450/epoxygenase-generated metabolites of arachidonic acid) have been demonstrated to act as endothelium-derived hyperpolarizing factors (EDHFs) to dilate coronary arteries (9, 10, 15). In endothelial cells, epoxygenases synthesize EETs that initiate vasodilation (3), and are then metabolized by soluble epoxide hydrolase (sEH; encoded by Ephx2 gene) via hydrolysis of EETs to their corresponding diols [dihydroxyeicosatrienoic acids (DHETs)] that generally lack vasoactive properties (7). As such, compromising EET hydrolysis via inhibition of sEH to increase EET bioavailability has become the therapeutic intervention in the treatment of cardiovascular diseases (20, 25, 27, 35). In this regard, both endothelial nitric oxide synthase (eNOS) and sEH merit consideration as important regulators of blood vessels that dilate/hyperpolarize in response to NO/EETs to modulate pressure-induced myogenic constriction. In this context, any factors that favor bioactivity of endothelial NO/EETs can physiologically improve coronary perfusion by lowering arterial tone. We have demonstrated that genetic disruption of the sEH gene and/or pharmacological inhibition of sEH activity initiates an EET-dependent reduction of coronary resistance to increase coronary perfusion and improve cardiac function (30). Additionally, female-specific downregulation of sEH elicits EET-mediated increases in shear stress-induced vasodilation in skeletal muscle arterioles (29). However, roles of sex in the EET/sEH-dependent regulation of coronary circulation have never been explored. Given that the pressure-sensitive behavior is crucial for the control of coronary resistance, and that the sex-specific regulation of myogenic responses via an EET-dependent mechanism remains unknown, we tested the hypothesis that potentiating endothelial EETs via diminishing their degradation attenuates coronary myogenic constriction, a response that is intrinsically present in a female-favorable manner due to downregulation of sEH.
MATERIALS AND METHODS
Animals.
Twelve- to 15-wk-old male (M) and female (F) Ephx2−/− [sEH-knockout (KO)] and Ephx2+/+ [wild-type (WT)] mice, abbreviated as M-KO, F-KO, M-WT, and F-WT, were used. As described previously (36), cryorecovered heterozygoues (Ephx2+/−, B6.129X-Ephx2tm1Gonz/J) and WT mice were received from the Jackson Laboratory (Bar Harbor, ME), and the homozygous (sEH-KO) mice were developed in the Department of Comparative Medicine, New York Medical College.
All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conform to the guidelines of the National Institutes of Health and the American Physiological Society for the care and use of laboratory animals.
Measurement of blood pressure.
Mice were anesthetized by inhalation of Isothesia (isoflurane). Mice were placed in an isoflurane induction chamber with the vaporizer, and the flow of oxygen was set at 5% and 500 ml/min, respectively. The anesthetized mice were then transferred to an operation table (37°C) and masked with an isoflurane nose cone. The isoflurane vaporizer and oxygen flow were then adjusted to 2% and 200 ml/min, respectively, and the mice were breathing room air mixed with oxygen (100% O2)-enriched isoflurane vapor. Blood pressure was recorded using a carotid artery catheterization at a controlled heart rate of ∼500 beats/min by adjusting the depth of anesthesia (21).
LC/MS/MS-based measurements for cardiac EETs and DHETs.
Isolated hearts were pulverized in liquid nitrogen. EETs and DHETs were extracted with the Bligh-Dyer method from 30 to 50 mg of heart tissue using ethyl acetate, followed by alkali hydrolysis to release their esterified form quantified with a Q-trap 3200 linear ion trap quadruple LC/MS/MS (AB ScieX; Qtrap 3200) equipped with a Turbo V ion source operated in negative electrospray mode (Applied Biosystems, Foster City, CA), as described previously (30, 36). Protein concentration of samples was determined by the Bradford method (Bio-Rad) and was used to normalize the detected lipids. Data are presented as total EETs and DHETs and expressed as picogram per milligram protein.
Myogenic response.
Mice were killed by inhalation of 100% CO2 after recording blood pressure. The heart was rapidly removed via thoracotomy and placed in a petri dish containing physiological salt solution (PSS). As described previously (19), left anterior descending coronary arteries (∼0.7–1 mm in length with all branches ligated) were isolated and then cannulated in a vessel chamber perfused with PSS (0–4°C). Isolated arteries were equilibrated under 60 mmHg intraluminal pressure for 1 h. During this period of time, all vessels developed spontaneous tone (60–80% of their passive diameter). The intraluminal pressure was then lowered to 20 mmHg and subsequently increased to 120 mmHg in a 20-mmHg step. Each pressure step was maintained for 5–10 min to allow the vessels to reach a stable condition. Changes in diameter of coronary arteries in response to the incremental pressure were recorded. The pressure-diameter relationship in the four groups of mice was assessed in control conditions, and in the presence of 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, 10−5 M; a putative EET antagonist) (11) and additional Nω-nitro-l-arginine methyl ester (l-NAME, 5 × 10−4 M; nitric oxide synthase inhibitor) for 45 min to evaluate effects of endothelial EETs and NO on the responses. At the conclusion of each experiment, the suffusion solution was changed to a Ca2+-free PSS containing 10−3 M EGTA. Vessels were incubated for 10 min, and passive diameter (PD) was recorded at each pressure step.
Western blot analysis.
As described previously (30), isolated hearts were pulverized in liquid N2. Equal amounts of total protein (25 μg) extracted from hearts were loaded on and separated by a 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was probed with specific primary antibodies for sEH and eNOS (Santa Cruz Biotechnology), and CYP4A (Biodesign), followed by appropriate secondary antibodies conjugated with horseradish peroxidase. Specific bands were visualized with a chemiluminescence kit and normalized to GAPDH. The X-ray film was scanned into a computer, and band densitometry was digitalized with UN-SCAN-IT software.
Calculation and statistical analysis.
Changes in diameter in response to intraluminal pressure were normalized to their corresponding PD and expressed as percent PD. The myogenic index was calculated by using the formula: 100 × Δri/ri/ΔP, where ri is the internal radius of vessels, Δri is the change in vessel radius in response to one step of pressure increment, and ΔP is the change in perfusion pressure (19, 36). Data are expressed as means ± SE, and n refers to the number of mice. Statistical analysis was performed using two-way ANOVA followed by the Tukey-Kramer post hoc test. Student's t-test was used where appropriate. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Reduced blood pressure in sEH-KO mice.
Blood pressure and heart rate were summarized in Table 1. Female mice (F-WT and F-KO) had significantly lower systolic and diastolic blood pressure than their male (M-WT and M-KO) counterparts. Compared with M-WT mice, deletion of sEH (M-KO) significantly reduced blood pressure to a level comparable to that of F-WT mice. F-KO mice had the lowest blood pressure among the four groups of mice, revealing a female-specific potential in sEH deficiency-induced reduction in blood pressure.
Table 1.
Hemodynamic parameters and body weights of mice
| M-WT (n = 6) | F-WT (n = 8) | M-KO (n = 6) | F-KO (n = 6) | |
|---|---|---|---|---|
| SBP, mmHg | 114.4 ± 0.5 | 103.9 ± 0.5* | 104.3 ± 0.7* | 99.5 ± 1.0*# |
| DBP, mmHg | 83.0 ± 0.6 | 74.8 ± 0.7* | 72.6 ± 0.7* | 68.0 ± 1.5*# |
| HR, beats/min | 506 ± 7.0 | 514 ± 8.0 | 517 ± 6.0 | 512 ± 9.0 |
| Body wt, g | 22.8 ± 0.9 | 20.9 ± 1.1 | 22.6 ± 0.5 | 21.8 ± 0.4 |
Values are means ± SE; n, no. of mice.
M, male; F, female; WT, wild type; KO, knockout; SBP, systolic blood pressure. DBP, diastolic blood pressure; HR, heart rate.
Significant difference from M-WT mice.
Significant difference from M-KO mice.
Matching pattern of cardiac EET and DHET metabolism in M-KO, F-WT, and F-KO mice.
Several important points are shown in Table 2. 1) M-KO mice exhibited significant increases in cardiac EETs, as a function of reduced EET hydrolysis to DHETs. This led to a greater ratio of EETs/DHETs compared with M-WT controls, providing functional evidence for the genetic disruption of the Ephx2 gene. 2) F-WT mice had a significantly higher level of EETs and lower DHETs to form a greater ratio of EETs/DHETs than M-WT mice, revealing a sex difference in EET metabolism. 3) This sex difference in cardiac EET/DHET parameters was essentially eliminated by sEH deletion, as evidenced by the comparable level of EETs and DHETs, as well as the ratio of EETs/DHETs, in the myocardium of M-KO and F-KO mice. 4) Unlike M-KO mice, F-KO mice failed to initiate statistically significant changes in the total level of EETs, DHETs, or the EET-to-DHET ratio compared with F-WT mice, thus indicating a sex-different response to sEH deficiency. 5) Comparable profiles of cardiac metabolism of EETs/DHETs among M-KO, F-WT, and F-KO mice imply the presence of redundant or overlapping regulatory mechanism(s) between female sex and sEH deficiency.
Table 2.
Myocardial cytochrome P-450 metabolites of mice
| M-WT (n = 9) | F-WT (n = 5) | M-KO (n = 5) | F-KO (n = 5) | |
|---|---|---|---|---|
| Total EETs | 0.845 ± 0.19 | 1.167 ± 0.19* | 1.23 ± 0.27* | 1.518 ± 0.15* |
| Total DHETs | 0.177 ± 0.02 | 0.071 ± 0.01* | 0.083 ± 0.03* | 0.081 ± 0.02* |
| EETs/DHETs | 6.30 ± 2.17 | 18.88 ± 3.57* | 20.84 ± 5.59* | 24.57 ± 6.53* |
Values are means ± SE; n, no. of mice.
EETs, epoxyeicosatrienoic acids; DHET, dihydroxyeicosatrienoic acids.
Significant difference from M-WT mice.
Attenuated myogenic constriction in sEH-KO and F-WT mice.
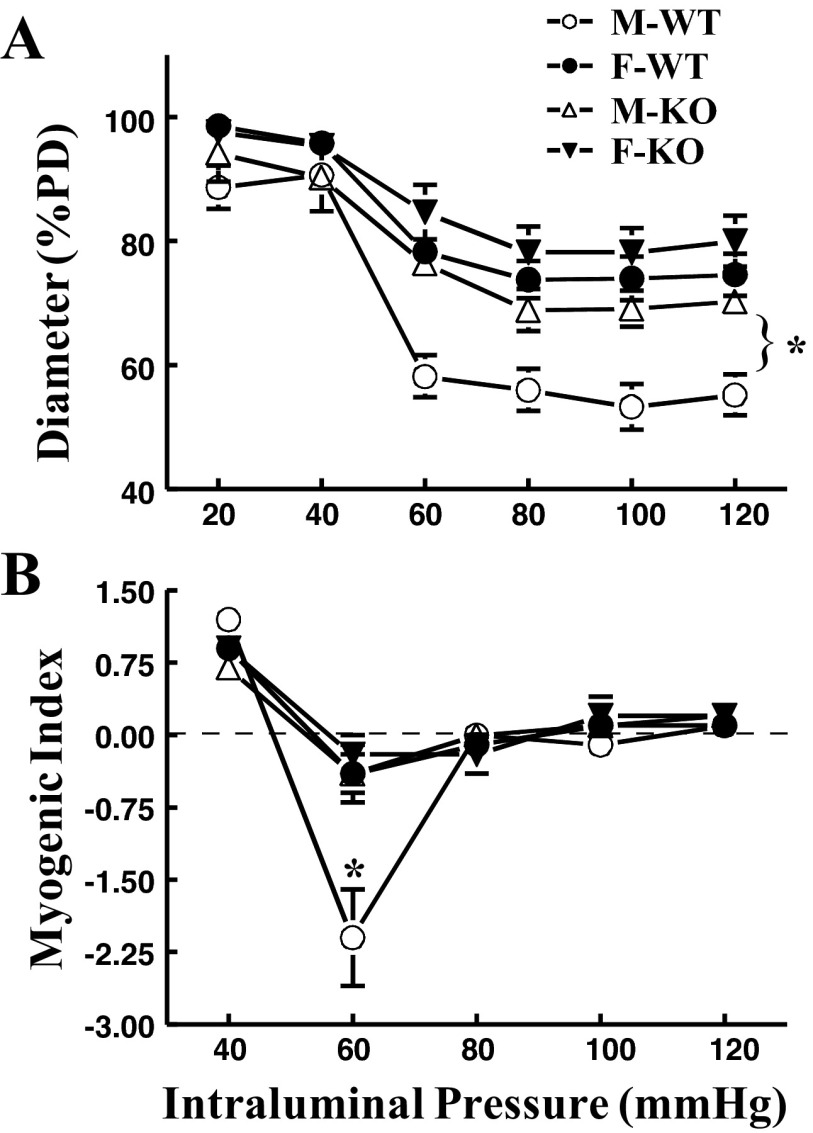
The pressure-PD curves of coronary arteries were comparable in all four groups of mice (data not shown). The average PD at 60 mmHg was 143.6 ± 7.1, 137.9 ± 10.4, 149.0 ± 10.9, and 139.0 ± 11.8 μm in M-WT, M-KO, F-WT, and F-KO, respectively. Figure 1A shows that there was a minimal myogenic constriction at pressures of 20 and 40 mmHg (expressed as %PD). Upon an increase in pressure to 60 mmHg and further increases to 120 mmHg, coronary arteries exhibited myogenic constriction. The constriction was significantly reduced (expressed as bigger diameter) in vessels of both sexes of sEH-KO (M-KO and F-KO) mice than that of M-WT mice, suggesting that sEH deficiency attenuates pressure-induced constriction. Interestingly, vessels of F-WT mice also displayed a similar attenuation in myogenic constriction to those of sEH-KO (M-KO and F-KO) mice, as evidenced by a comparable upward shift of pressure-diameter curves in the three groups of mice. Coincidently, myocardial EET metabolism profiles of sEH-KO mice were identical to that of F-WT mice (Table 2). These results point to a characteristic of female-specific imitation of sEH deficiency to attenuate pressure-induced constriction. The myogenic index, defined as a percentage change in diameter in response to per unit of pressure increment (20 mmHg), was used to assess the dynamic reaction of vascular smooth muscle (VSM) to changes in pressure. Paralleled with the results that show the strongest pressure-induced myogenic constriction (Fig. 1A), coronary arteries of M-WT mice also exhibited significantly deeper slope of myogenic index as the pressure increased from 40 to 60 mmHg (Fig. 1B). Thus, compared with M-WT controls, not only was the basal myogenic tone at each pressure step reduced, but also the dynamic reaction of VSM was significantly alleviated, as a function of either female sex or sEH deficiency.
Fig. 1.
A: normalized diameter [percentage of passive diameter (%PD)] of coronary arteries of male (M) and female (F) wild-type (WT) and soluble epoxide hydrolase (sEH)-knockout (KO) mice in response to stepwise increases in intravascular pressure. B: myogenic index of coronary arteries in the four groups of mice (n = 6–8 in each group). *Significant difference from other groups of mice.
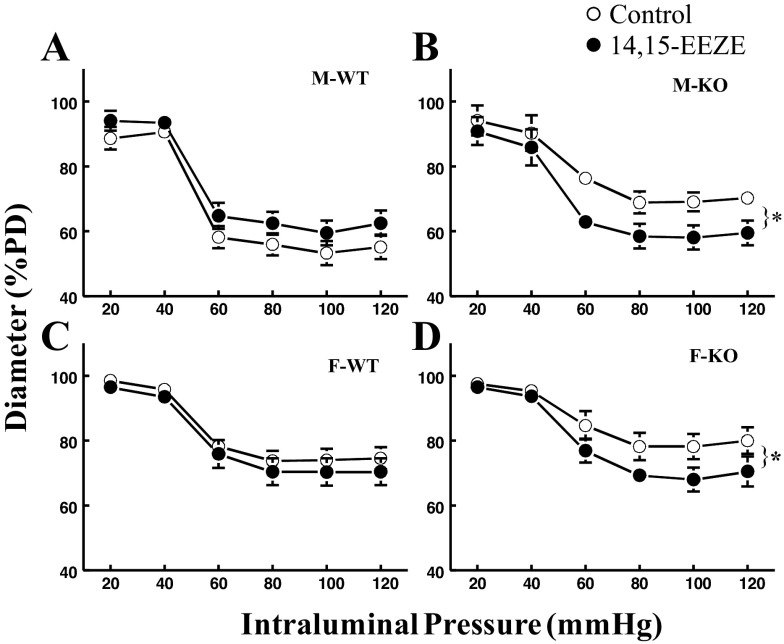
EET-dependent attenuation of myogenic constriction in sEH-KO mice.
Specific roles of EETs in the attenuation of myogenic responses were evaluated and summarized in Fig. 2. Exposure of vessels to 14,15-EEZE shifted the pressure-diameter curve downward significantly (indicative of enhanced myogenic constriction) in vessels of sEH-KO mice (Fig. 2, B and D). This suggests that the attenuated myogenic constriction in sEH-KO mice is EET dependent in nature. Of note, although F-WT mice originally exhibited a similar attenuated myogenic constriction (Fig. 1A) and similar levels of EETs (Table 2) to those of sEH-KO mice, 14,15-EEZE elicited a minimal increase in myogenic constriction in F-WT mice (Fig. 2C), suggesting an EET-independent mediator(s) that may redundantly participate in the modulation of pressure-induced coronary vasoconstriction in F-WT mice. By contrast, in M-WT mice that originally displayed greater myogenic constriction (Fig. 1A), treatment of the vessels with 14,15-EEZE did not elicit any increases in constriction, but instead exhibited a reduced tendency in the response, resulting in an upward-shifted pressure-diameter curve (Fig. 2A). This finding suggests that EETs do not primarily contribute to the modulation of the myogenic response in normal males.
Fig. 2.
Normalized diameter of coronary arteries of M-WT (A), M-KO (B), F-WT (C), and F-KO (D) mice as a function of changes in intravascular pressure in the control condition and in the presence of 10−5 M 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE; n = 8 in each group). *Significant difference between two curves.
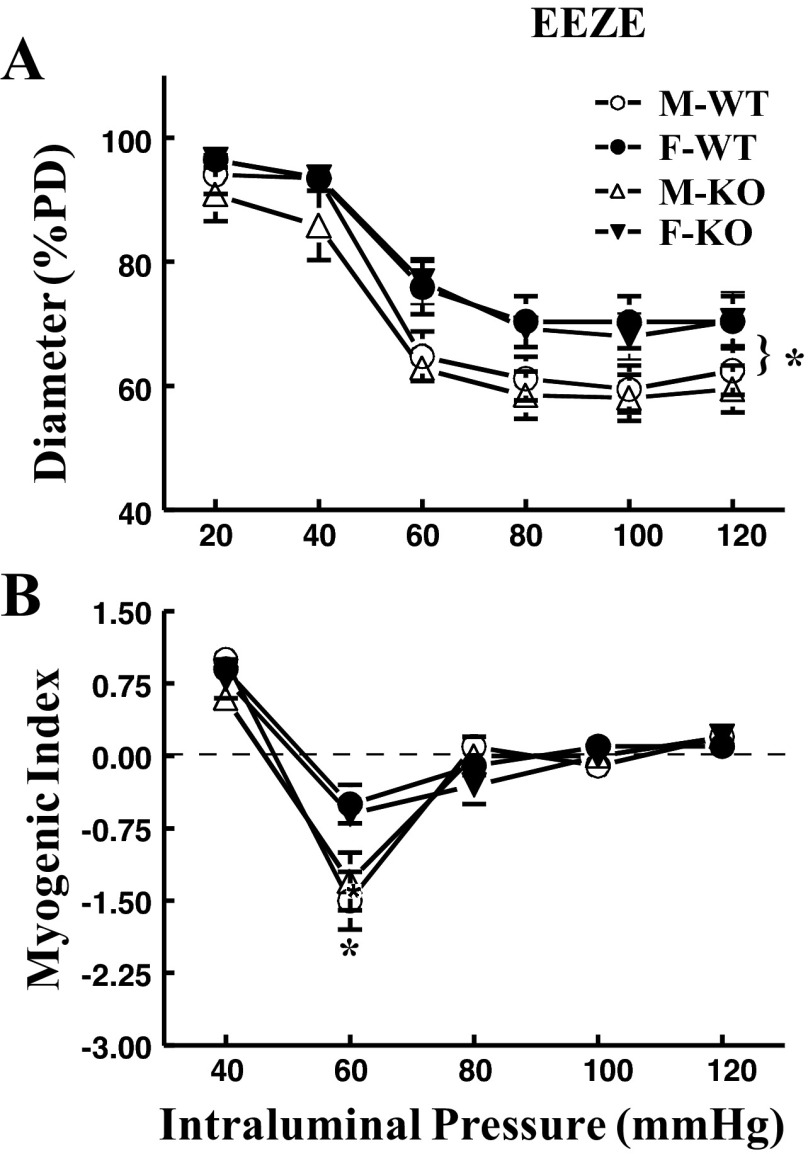
NO-attenuated myogenic constriction was discerned in female vessels treated with an EET inhibitor.
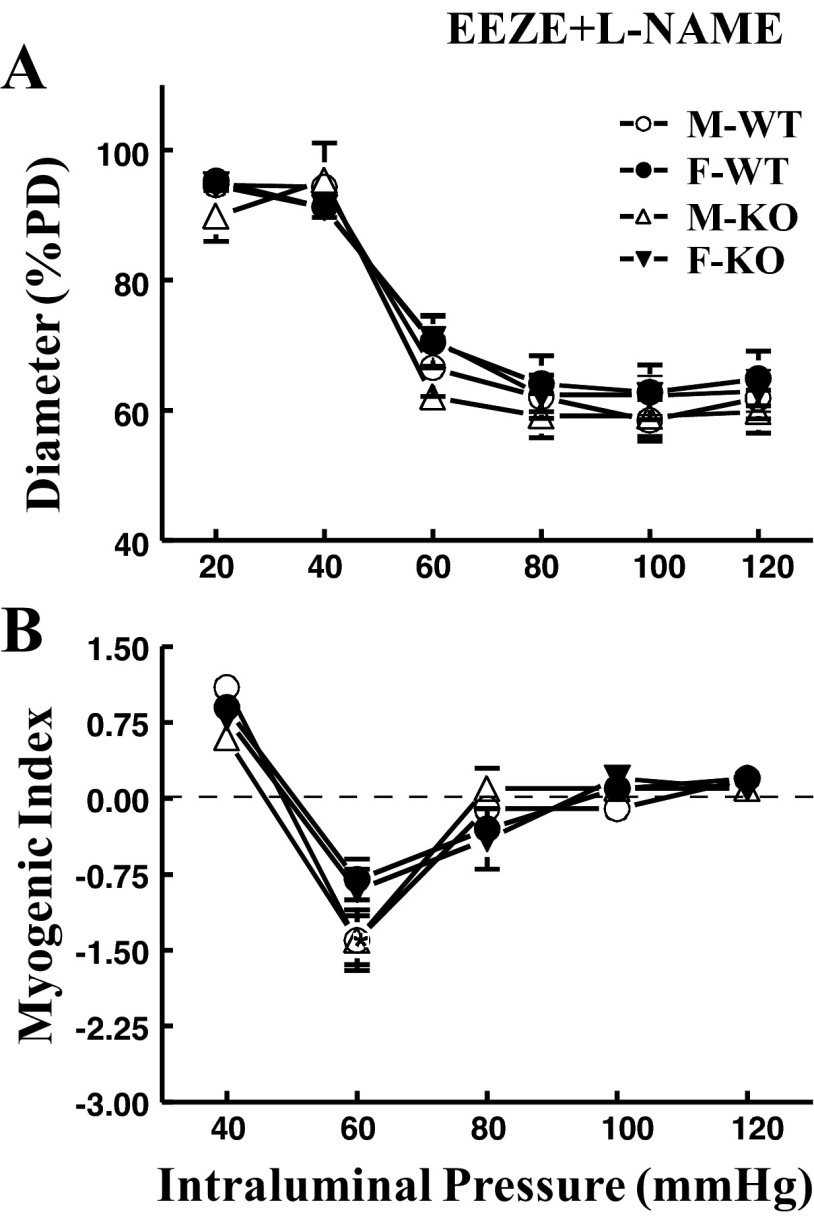
As a consequence of eliminating EET activity, data summarized from Fig. 2 are depicted in Fig. 3 and illustrate a female-specific EET-independent attenuation of myogenic response. Compared with control conditions (Fig. 1), blockade of EET actions with 14,15-EEZE switched the original phenotype of myogenic response to that manifested in a pure sex-different manner. Specifically, arteries obtained from female (F-WT and F-KO) mice exhibited significantly attenuated myogenic constriction (Fig. 3A) and myogenic index (Fig. 3B) compared with their male (M-WT and M-KO) counterparts. Addition of l-NAME enhanced myogenic constriction in vessels of females (F-WT and F-KO), but did not significantly affect the response in those of males (M-WT and M-KO), leading to an overlap of the four pressure-diameter curves (Fig. 4A), as well as a comparable myogenic index among all groups of mice (Fig. 4B). This indicates that, in the absence of EET actions, the female-favorable attenuation of pressure-induced constriction is subsequently mediated by endothelial NO.
Fig. 3.
Summarized data of myogenic response (A) and myogenic index (B) in coronary arteries of M-WT, F-WT, M-KO, and F-KO mice in the presence of 14,15-EEZE (n = 8 in each group). *Significant difference in pressure-diameter curves and myogenic indexes between male and female mice.
Fig. 4.
Summarized data of myogenic response (A) and myogenic index (B) in coronary arteries of M-WT, F-WT, M-KO, and F-KO mice in the presence of 14,15-EEZE and 5 × 10−4 M Nω-nitro-l-arginine methyl ester (l-NAME) (n = 6–8 in each group).
Taken collectively, these data reveal that the female-specific modulation of pressure-induced myogenic constriction is mediated via both EET (Figs. 2 and 3)- and NO (Fig. 4)-dependent signaling.
Downregulation of sEH expression in F-WT mice.
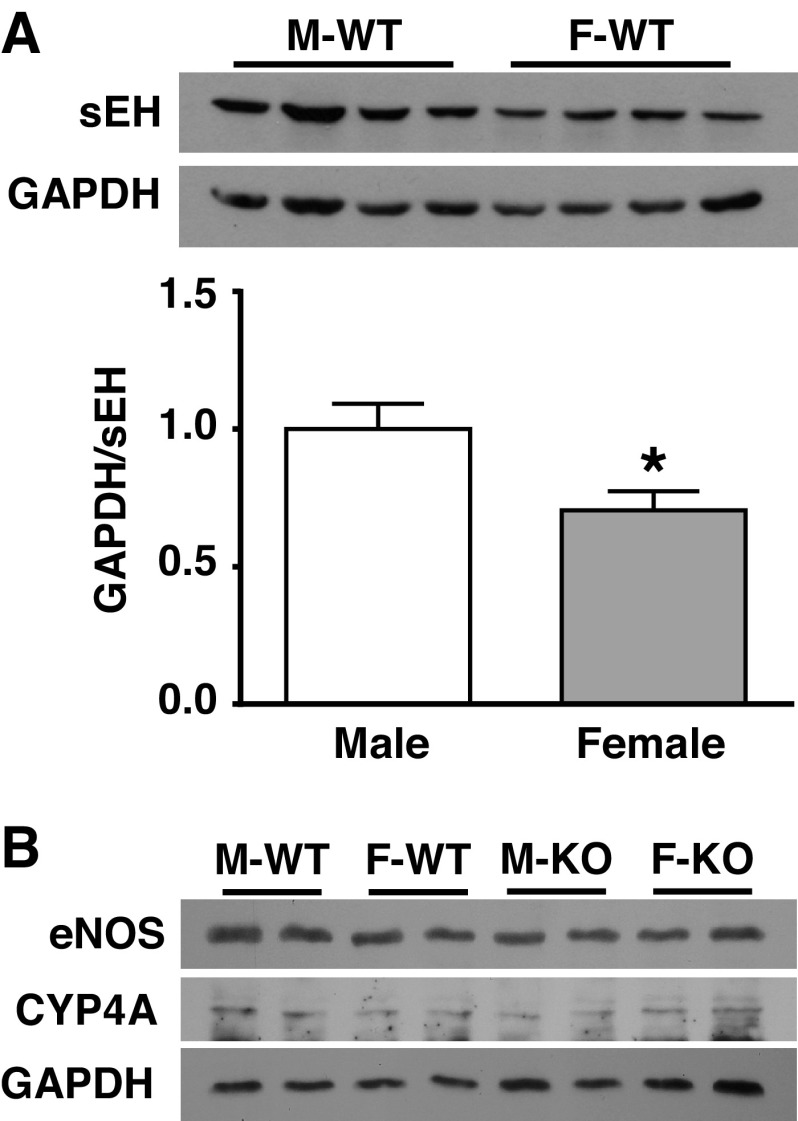
Figure 5 shows the original and summarized data from Western blot analysis, indicating that protein expression of sEH in myocardium was significantly suppressed in F-WT compared with M-WT mice (Fig. 5A) and was undetectable in sEH-KO mice (data not shown) (36). Cardiac expression of eNOS and CYP4A was comparable among the four groups of mice (Fig. 5B). We did not use eNOS phosphorylation as an indicator of eNOS activity because it reflects a stimulated, but not basal release, of NO. Thus, the molecular evidence provided explanations for the reduced DHETs and greater EETs and EETs-to-DHETs ratio in myocardium of F-WT mice.
Fig. 5.
Original and summarized data (n = 3 blots) of protein expression for sEH (A), endothelial nitric oxide synthase (eNOS), and cytochrome P-450 (CYP4A) (B) in the heart of M-WT, M-KO, F-WT, and F-KO mice. *Significant difference from M-WT mice.
DISCUSSION
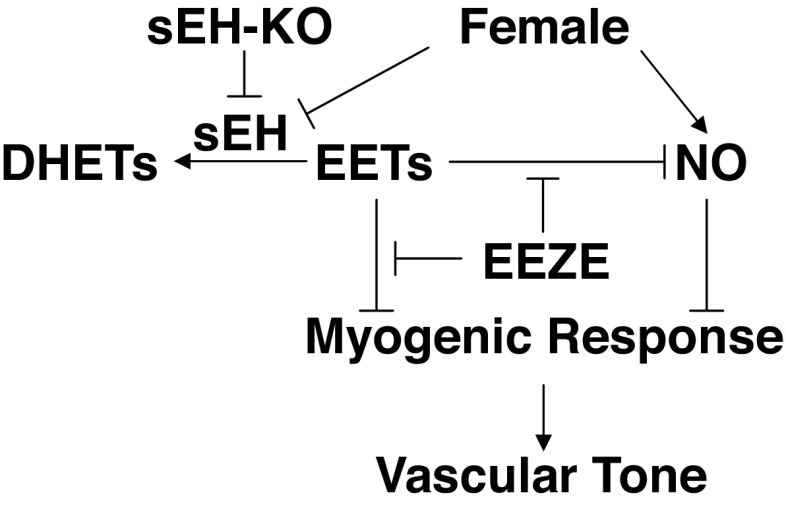
The present study provides direct evidence that was summarized in Fig. 6 to indicate that 1) deletion of the sEH gene evokes an EET-dependent attenuation of pressure-induced constriction in coronary arteries; 2) the female sex exhibits an inherent downregulation in sEH protein expression. This regulatory mechanism duplicates the outcome of Ephx2 gene deletion, leading to less prominent changes in the myogenic response of F-KO mice, a response that is functionally more observable in M-KO mice. As a result, there is an identical phenotype of myogenic response and similar profile of cardiac EET metabolism in M-KO, F-KO, and F-WT mice; and 3) female-specific modulation of myogenic response via an NO-dependent pathway is sequentially discerned and takes responsibility when activities of EETs are inhibited, revealing an interaction between the two endothelial signaling pathways. Collectively, the female-specific regulation of the pressure-sensitive mechanism in coronary arteries involves potentiating endothelial EET and NO bioavailabilities, which promotes vasodilation to counteract pressure-induced constriction. As a result, the balance between the two actions was tipped in favor of dilation, leading to attenuated arteriolar resistance in the coronary circulation.
Fig. 6.
Schematic illustration of reciprocal activation of epoxyeicosatrienoic acids (EETs) and NO in the modulation of myogenic response in coronary arteries. Genetic disruption of sEH gene, or female-specific downregulation of sEH, leads to great increases in EET bioavailability that counteract myogenic constriction and, meanwhile, inhibits NO activity. The female-specific potentiation of NO to modulate myogenic constriction functions predominantly via its disinhibition from EETs by EEZE. Taken together, EETs and NO participate sequentially in the female-favorable attenuation of pressure-induced constriction to reduce coronary resistance. ↓, Stimulation; ⊥, inhibition.
Female-specific downregulation of sEH accounts for the sex difference in response to sEH deficiency.
In male mice, deletion of the sEH gene elevated cardiac EET levels (Table 2), and significantly reduced coronary vasoconstriction in response to increases in intravascular pressure (Fig. 1), which coincided with lower blood pressure (Table 1). In contrast, F-WT mice that had originally displayed an attenuated myogenic constriction associated with higher myocardial EETs did not exhibit further changes in response to deletion of the sEH gene (Fig. 1), alluding to a female-specific regulation of EET metabolism. Notably, the attenuated myogenic response in F-WT mice was not significantly changed by sEH deficiency, but their blood pressure was further reduced in response to sEH deletion. This could be attributed to the fact that the regulation of blood pressure is a multifactorial and complex process that can be governed by varying female-favorable mechanisms in addition to the EET-mediated local regulation of vascular tone via the myogenic-dependent mechanism.
To single out the nature of the sex-different responsiveness to sEH deficiency, we tested the hypothesis that the minimal changes in pressure-sensitive behavior in response to Ephx2 deletion in females attribute to their inherently compromised sEH activity. We indeed indicated a sex difference in blood pressure (Table 1) and in vascular behaviors such as myogenic constriction and myogenic index phenotypes (Fig. 1), as well as distinct sex differences in the cardiac EET metabolism (Table 2) between M-WT and F-WT mice. However, knockout of the Ephx2 gene in males (M-KO) attenuated myogenic vasoconstriction to the same level as those observed in both F-WT and F-KO mice. This supports our hypothesis that the female sex possesses a regulatory mechanism that duplicates the outcome of Ephx2 gene deletion. As a result, female sex (e.g., F-WT mice), sEH disruption (M-KO mice), or the redundant presence of both (F-KO mice) generates an identical EEZE-sensitive response (Fig. 2). A downregulation of sEH expression in the coronary vasculature and myocardium of F-WT mice (Fig. 5) provides molecular evidence clarifying a differential expression of sEH that contributes to the sex-different regulation of coronary myogenic responses. Deletion of the Ephx2 gene or inhibition of sEH activity initiates cardiovascular protective actions that have been widely evidenced in a variety of pathological conditions, such as cardiac ischemia (25), hypertension-induced renal injury (24), and cardiac damage induced by ischemia-reperfusion injury (1, 4, 5), and demonstrated to improve cardiac perfusion under physiological conditions (30). During these processes, an EET-dependent modulation of pressure-sensitive behavior was indicated for the first time that elucidates, at least in part, the mechanistic insights for EET-exerted cardioprotective properties in terms of an improvement in myocardial perfusion through a significant reduction in coronary resistance (26). Moreover, consistent with our previous findings showing a female-specific downregulation of sEH in arteries of skeletal muscle and mesentery (29), as well as in the pulmonary circulation (22) to potentiate the bioactivity of EETs, the present study indicates a female-favorable attenuation of myogenic constriction, as a function of suppression of sEH expression in the coronary circulation revealing, from a physiological point of view, the female-specific regulation that possesses universally based characteristics.
Endothelial NO participates in the female-favorable attenuation of pressure-induced vasoconstriction.
Intriguingly, when actions of EETs were eliminated by 14,15-EEZE, the myogenic response manifested solely in a sex-different manner, characterized as the upward shift of both pressure-diameter curves and myogenic index in female (F-WT and F-KO) compared with those of their male littermates (Fig. 3). In this context, possible roles of CYP-ω-hydroxylation (20-HETE synthase) and eNOS in the mediation of sex-different responses emerged, since both enzymes contribute significantly to the control and regulation of myogenic responses in a sex-specific manner. 20-HETE acts as an intracellular second messenger that plays an integral role in the signal transduction processes underlying the development of pressure-dependent myogenic constriction via depolarization of VSM (13). Vascular expression of CYP4A (20-HETE synthase) and production of 20-HETE are altered in different models of hypertension (12, 32, 33), a condition that is characterized by enhanced myogenic constriction and endothelial dysfunction (17, 19). However, in physiological conditions such as in the present study, changes in neither CYP4A expression (Fig. 5) nor 20-HETE production (data not shown) were detected, and inhibition of 20-HETE synthesis did not alter myogenic constriction in healthy coronary arteries of mice (19, 36). These findings argue against the idea that an altered 20-HETE action is responsible for the sex-differential response in pressure-sensitive behaviors, but suggest rather a possible involvement of NO. Indeed, l-NAME treatment prevented the attenuated myogenic constriction in female mice and eliminated the sex difference, as evidenced by the overlap of four pressure-diameter curves (Fig. 4). As such, it is endothelial NO that in combination with EETs participates in the female-favorable modulation of myogenic responses. Notably, the expression of eNOS was originally assessed in the control condition (Fig. 5); therefore, the comparable expression of eNOS between males and females failed to explain the sex difference in the NO-mediated myogenic constriction that was secondary to an acute inhibition of vascular EETs (Figs. 3 and 4). In this regard, the NO-mediated attenuation of myogenic constriction could be attributed to either a nongenomic-based activation of eNOS to increase basal release of NO as a function of female hormones/estrogens (14), or dysinhibition of EET-inhibitory effects on eNOS.
Interactions between EETs and NO.
The sex-different regulation of myogenic responses via NO mediation emerged once the action of EETs had been abolished (Figs. 3 and 4), suggesting the presence of a negative feedback between endothelial EETs and NO. In physiological conditions, NO is the primary mediator contributing to the control of vascular tone, due partially to its inhibitory property on CYP/EETs (12). EETs then act as back-up dilator mediators in pathological circumstances associated with manifestations of “endothelial dysfunction” in which the bioavailability of NO is impaired (2, 28). On the other hand, when EETs' bioavailability dramatically increases to overcome actions of NO, their contribution to the regulation of vascular function becomes discernable, especially in resistance vessels that exhibit more predominant responses to EETs/EDHF than large vessels (34). As indicated, EETs did not seem to participate significantly in the control of coronary myogenic response in M-WT mice (Fig. 2A) but functioned predominantly in vessels that were deficient or had a reduced expression of sEH (Fig. 2, B–D). As such, in the present study, female-favorable NO-mediated attenuation of myogenic constriction became discernable only in the presence of EEZE, which disinhibits the inhibitory effect of EETs on NO activity. In regard to the nonstatistically significant change in EEZE-treated F-WT vessels (Fig. 2C), we interpret the result to mean that, in addition to EETs, other EET-independent vasodilator mediator(s) might redundantly participate in modulating myogenic constriction. Indeed, in the presence of EEZE, the female-specific attenuation of myogenic response was eliminated by additional l-NAME, indicating that NO takes over immediately and becomes responsible for modulating the myogenic response when EET actions are inhibited.
We conclude that endothelial EETs and NO coparticipate in the modulation of coronary myogenic constriction in a female-susceptible manner. Our studies provide a mechanistically based rationale for the clinical development of therapeutic interventions that target sEH to improve cardiovascular function. In particular, the female-specific downregulation of sEH increases tissue/vascular EETs, leading to reduced coronary resistance and increased coronary blood flow. This noteworthy finding highlights a clinically relevant interpretation for the significantly lower incidence of ischemia heart diseases in female populations.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-070653, HL-129797, and HL-34300.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.F., J.Q., S.K., Y.L., H.J., D.S., and A.H. performed experiments; G.F., J.Q., S.K., Y.L., H.J., D.S., and A.H. analyzed data; G.F., J.Q., S.K., Y.L., H.J., D.S., and A.H. interpreted results of experiments; G.F., J.Q., S.K., D.S., and A.H. prepared figures; G.F. and A.H. drafted manuscript; G.F., J.Q., S.K., Y.L., H.J., M.L., D.S., and A.H. approved final version of manuscript; M.L., D.S., and A.H. conception and design of research; M.L., D.S., and A.H. edited and revised manuscript.
REFERENCES
- 1.Batchu SN, Lee SB, Samokhvalov V, Chaudhary KR, El-Sikhry H, Weldon SM, Seubert JM. Novel soluble epoxide hydrolase inhibitor protects mitochondrial function following stress. Can J Physiol Pharmacol 90: 811–823, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94: 3341–3347, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch 459: 881–895, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhary KR, Abukhashim M, Hwang SH, Hammock BD, Seubert JM. Inhibition of soluble epoxide hydrolase by trans-4- [4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is protective against ischemia-reperfusion injury. J Cardiovasc Pharmacol 55: 67–73, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhary KR, Batchu SN, Seubert JM. Cytochrome P450 enzymes and the heart. IUBMB Life 61: 954–960, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Chilian WM, Kuo L, DeFily DV, Jones CJ, Davis MJ. Endothelial regulation of coronary microvascular tone under physiological and pathophysiological conditions. Eur Heart J 14, Suppl I: 55–59, 1993. [PubMed] [Google Scholar]
- 7.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem 276: 14867–14874, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Feliciano L, Henning RJ. Coronary artery blood flow: physiologic and pathophysiologic regulation. Clin Cardiol 22: 775–786, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisslthaler B, Hinsch N, Chataigneau T, Popp R, Kiss L, Busse R, Fleming I. Nifedipine increases cytochrome P4502C expression and endothelium-derived hyperpolarizing factor-mediated responses in coronary arteries. Hypertension 36: 270–275, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Harder DR, Campbell WB, Roman RJ. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 32: 79–92, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Harder DR, Lange AR, Gebremedhin D, Birks EK, Roman RJ. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J Vasc Res 34: 237–243, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 11: 9–38, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res 96: 376–383, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang A, Sun D, Kaley G, Koller A. Estrogen maintains nitric oxide synthesis in arterioles of female hypertensive rats. Hypertension 29: 1351–1356, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Huang A, Sun D, Koller A. Endothelial dysfunction augments myogenic arteriolar constriction in hypertension. Hypertension 22: 913–921, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Huang A, Sun D, Koller A, Kaley G. Gender difference in myogenic tone of rat arterioles is due to estrogen-induced, enhanced release of NO. Am J Physiol Heart Circ Physiol 272: H1804–H1809, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Huang A, Sun D, Yan C, Falck JR, Kaley G. Contribution of 20-HETE to augmented myogenic constriction in coronary arteries of endothelial NO synthase knockout mice. Hypertension 46: 607–613, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8: 794–805, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandhi S, Froogh G, Qin J, Luo M, Wolin MS, Huang A, Sun D. EETs elicit direct increases in pulmonary arterial pressure in mice. Am J Hypertens In press. [DOI] [PMC free article] [PubMed]
- 22.Kandhi S, Qin J, Froogh G, Jiang H, Luo M, Wolin MS, Huang A, Sun D. EET-dependent potentiation of pulmonary arterial pressure: sex different regulation of soluble epoxide hydrolase. Am J Physiol Lung Cell Mol Physiol 309: L1478–L1486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo L, Hein TW. Vasomotor regulation of coronary microcirculation by oxidative stress: role of arginase. Front Immunol 4: 237, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J 24: 3770–3781, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Dahl M, Grande P, Tybjaerg-Hansen A, Nordestgaard BG. Genetically reduced soluble epoxide hydrolase activity and risk of stroke and other cardiovascular disease. Stroke 41: 27–33, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol 104: 211–227, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA 102: 16747–16752, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol 279: H459–H465, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Qin J, Kandhi S, Froogh G, Jiang H, Meng L, Sun D, Huang A. Sexually dimorphic phenotype of arteriolar responsiveness to shear stress in soluble epoxide hydrolase-knockout mice. Am J Physiol Heart Circ Physiol 309: H1860–H1866, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin J, Sun D, Jiang H, Kandhi S, Froogh G, Hwang SH, Hammock BD, Wolin MS, Thompson CI, Hintze TH, Huang A. Inhibition of soluble epoxide hydrolase increases coronary perfusion in mice. Physiol Rep 3: e12427, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Dube S, Canty JM Jr. Regulation of coronary diameter by myogenic mechanisms in arterial microvessels greater than 100 microns in diameter. Am J Physiol Heart Circ Physiol 268: H788–H793, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Roman RJ, Maier KG, Sun CW, Harder DR, Alonso-Galicia M. Renal and cardiovascular actions of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol 27: 855–865, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem 275: 40504–40510, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Cuevas AJ, Gotlinger K, Hwang SH, Hammock BD, Schwartzman ML, Huang A. Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol 306: H1146–H1153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, Huang A, Kaley G. Mechanical compression elicits NO-dependent increases in coronary flow. Am J Physiol Heart Circ Physiol 287: H2454–H2460, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]