ANG II-elicited increases in coronary arterial mitochondrial superoxide caused a loss of ferrochelatase, which appeared to promote a depletion of soluble guanylate cyclase and impaired relaxation to nitric oxide by decreasing heme availability. The heme precursor δ-aminolevulinic acid appeared to prevent these processes by promoting cGMP stimulation of protein kinase G.
Keywords: δ-aminolevulinic acid; guanosine 3′,5′-cyclic monophosphate; protoporphyrin IX, nitric oxide
Abstract
Oxidation of the soluble guanylate cyclase (sGC) heme promotes loss of regulation by nitric oxide (NO) and depletion of sGC. We hypothesized that angiotensin II (ANG II) stimulation of mitochondrial superoxide by its type 1 receptor could function as a potential inhibitor of heme biosynthesis by ferrochelatase, and this could decrease vascular responsiveness to NO by depleting sGC. These processes were investigated in a 24-h organoid culture model of bovine coronary arteries (BCA) with 0.1 μM ANG II. Treatment of BCA with ANG II increased mitochondrial superoxide, depleted mitochondrial superoxide dismutase (SOD2), ferrochelatase, and cytochrome oxidase subunit 4, and sGC, associated with impairment of relaxation to NO. These processes were attenuated by organoid culture with 8-bromo-cGMP and/or δ-aminolevulinic acid (a stimulator of sGC by protoporphyrin IX generation and heme biosynthesis). Organoid culture with Mito-TEMPOL, a scavenger of mitochondrial matrix superoxide, also attenuated ANG II-elicited ferrochelatase depletion and loss of relaxation to NO, whereas organoid culture with Tempol, an extramitochondrial scavenger of superoxide, attenuated the loss of relaxation to NO by ANG II, but not ferrochelatase depletion, suggesting cytosolic superoxide could be an initiating factor in the loss of sGC regulation by NO. The depletion of cytochrome oxidase subunit 4 and sGC (but not catalase) suggests that sGC expression may be very sensitive to depletion of heme caused by ANG II disrupting ferrochelatase activity by increasing mitochondrial superoxide. In addition, cGMP-dependent activation of protein kinase G appears to attenuate these ANG II-stimulated processes through both preventing SOD2 depletion and increases in mitochondrial and extramitochondrial superoxide.
NEW & NOTEWORTHY
ANG II-elicited increases in coronary arterial mitochondrial superoxide caused a loss of ferrochelatase, which appeared to promote a depletion of soluble guanylate cyclase and impaired relaxation to nitric oxide by decreasing heme availability. The heme precursor δ-aminolevulinic acid appeared to prevent these processes by promoting cGMP stimulation of protein kinase G.
angiotensin ii (ANG II) is thought to be a prominent factor in many aspects of oxidant-associated vascular dysfunction and the progression of hypertension-associated disease processes (5–7, 16–18, 21, 23, 27, 29). There is substantial evidence for ANG II increasing the activities of NADPH oxidases (Nox oxidases) and mitochondrial superoxide levels through the ANG II type 1 receptor in endothelium and vascular smooth muscle. In addition to decreasing the availability of endothelium-derived nitric oxide (NO), ANG II-elicited increases in Nox oxidase activity have been associated with decreasing the responsiveness of vascular smooth muscle to NO through mechanisms including soluble guanylate cyclase (sGC) depletion (21). The oxidation of sGC-bound heme in vascular smooth muscle from its normal NO-stimulated Fe2+ form to Fe3+ is thought to occur in disease processes such as hypertension, and this results in a loss of sGC stimulation by NO, depletion of its heme, and a subsequent ubiquitination-activated proteolytic degradation of sGC (28). Peroxynitrite generated from the reaction of superoxide with NO has been observed to oxidize the heme of sGC in vascular tissue (28). Thus, it is well established that impairment of sGC activation by endothelium-derived NO is a major factor in many cardiovascular diseases, including hypertension (6, 16, 21), and modulation of its bound Fe2+ heme can be an important contributor to the vascular dysfunction that is observed.
There has been minimal previous consideration of how the biosynthesis of heme influences the function of sGC or is altered in cardiovascular disease processes. It has been proposed that heme biosynthesis is impaired by aging associated with studies observing that subunit 4 of cytochrome c oxidase in the mitochondrial electron transport chain is rapidly depleted when heme biosynthesis by ferrochelatase (FECH) is impaired (2). A recent study examining the in vivo consequences in heterozygous mice deficient in the mitochondrial matrix manganese-containing form of superoxide dismutase (SOD2) in erythrocyte precursor cells detected evidence for decreased activity of FECH (3). Based on evidence for superoxide disrupting iron-sulfur clusters (20) and FECH having an essential iron-sulfur center (4), it was suggested that increased mitochondrial superoxide-elicited impairment of this enzyme's function was responsible for the observed decrease in hematocrit and hemoglobin that were rescued by an in vivo 2,2,6,6-tetramethyl-4-{[5-(triphenylphosphonio)pentyl]oxy}-1-piperidinyloxy bromide (Mito-TEMPOL) therapy targeting the increased mitochondrial superoxide (3). Although the actual actions of superoxide on FECH are not well documented, high levels of NO potentially associated with the formation of reactive NO-derived species have been observed to inactivate FECH (8). Because increased mitochondrial superoxide has been detected in vascular disease models (15) and in the actions of ANG II on vascular smooth muscle (6), we hypothesized that increases in mitochondrial superoxide could impair mitochondrial ferrochelatase and disrupt mitochondrial heme biosynthesis and its ability to maintain the heme needed for sGC regulation.
Initial studies were conducted to investigate if ANG II-induced mitochondrial superoxide causes a loss of FECH, and it was observed that organoid culture of endothelium-denuded bovine coronary arteries for 24 h with 0.1 μM ANG II decreased FECH activity in a manner that was inhibited by organoid culture with Mito-TEMPOL. We then investigated if this exposure to ANG II showed evidence for depletion of heme and sGC. The depletion of subunit 4 of cytochrome oxidase (COx4), but not catalase, was used as an indicator to detect conditions of heme depletion on proteins whose expression is very sensitive to low heme (2). In addition, since we previously reported that 24-h organoid culture of bovine arteries with the heme precursor δ-aminolevulinic acid (ALA) caused an increase in sGC activity by generating protoporphyrin IX (PpIX) (19), as well as finding that ALA attenuated endothelin-1-induced mitochondrial superoxide in bovine pulmonary arteries associated with prevention of pulmonary hypertension (PH) (1), we also investigated if ALA and cGMP mechanisms could protect the function of FECH through lowering mitochondrial superoxide generation.
MATERIALS AND METHODS
Materials.
All physiological buffers were prepared using analytical-grade reagent salts purchased from J. T. Baker Chemical. All other chemicals were obtained from Sigma Chemical unless otherwise mentioned. Catalase, SOD2, and ferrochelatase antibodies were purchased from Abcam. Phospho-vasodilator-stimulated phosphoprotein (VASP), total VASP, and voltage-dependent anion channel (VDAC) antibodies were purchased from Cell Signaling (Beverly, MA). β-Actin antibody was purchased from Sigma Chemicals. 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and spermine-NONOate were purchased from Cayman Chemical (Ann Arbor, MI). Mito-TEMPOL was obtained from Abcam. COx4 antibody, MitoSox, and dihydroethidium (DHE) were purchased from Life Technologies. All gases were obtained from Air Gas (Allentown, PA).
Tissue preparations.
Bovine hearts were obtained from a slaughterhouse in ice-cold PBS. Left anterior descending arteries were used for bovine coronary artery (BCA) experiments. BCAs were cleaned of their connective tissue and then cut into rings of 2–3 mm in diameter and width. The endothelium was removed by rubbing the lumen. Organoid-cultured BCA rings were used in experiments for vascular reactivity, superoxide measurements, PpIX fluorescence, FECH activity, and Western blot protein analysis. As indicated in results, organoid cultures were performed (22) with BCA rings in the absence and presence of 0.1 μM ANG II, 100 μM ALA, 50 μM gp91 ds-tat, 100 μM 8-bromo-cGMP, 1 mM Mito-TEMPOL, and 1 mM Tempol with DMEM containing 10% FBS and 1% antibiotics (penicillin, streptomycin, and amphotericin B) for 24 h at 37°C with 5% CO2.
Ferrochelatase activity assay.
As published previously (1), BCA rings were pulverized in liquid nitrogen, incubated on ice for 45 min with 50 μl of buffer (0.25 M Tris·HCl buffer, pH 8.2, containing 1% Triton X-100 and 1.75 mM of palmitic acid), and then sonicated on ice for 10 s. Samples were then centrifuged, and the supernatant was assayed for protein content by the Bradford method. The amount of FECH activity present after 60 min incubation was assayed by the accumulation of zinc PpIX (ZnPpIX) from 67 μM PpIX and 42 μM zinc acetate, using 20 μg protein in a final volume of 30 μl, as previously described (30). At the end FECH reaction was stopped by using dimethyl sulfoxide-methanol (30:70) solution. HPLC measurement of the amount of ZnPpIX formation was used to determine the FECH activity in each sample. At the beginning of the experiment, to generate a standard curve for ZnPpIX, increasing concentrations of ZnPpIX were loaded in the column. We used an Agilent 1100 HPLC system using a normal-phase Phenomenex column (Luna 5μ Silica-2 100A, 250 × 4.6 mm) with acetone-methanol-water-formic acid (560:240:200:2) and 1 ml/min as the mobile phase. ZnPpIX was detected based on the amount of fluorescence observed with excitation and emission wavelengths of 415 and 580 nm, respectively.
Detection of changes in mitochondrial and extramitochondrial superoxide.
HPLC measurement of the superoxide-specific hydroxylated products of MitoSox and dihydroethidium were employed for quantifying changes in mitochondrial matrix and extramitochondrial matrix superoxide, using previously described methods (32). At the beginning of the experiment, a standard curve was generated by injecting increasing concentrations of Mito-2-hydroxyethidium or 2-hydroxyethidium in the column. After 24 h of organoid culture, BCA rings were incubated with either 5 μM MitoSox or DHE for 1 h in the dark (1, 9) under conditions described in results to measure mitochondrial and extramitochondrial superoxide, respectively. They were washed several times with Krebs solution buffered with 10 mM HEPES-NaOH (pH 7.4) and then flash-frozen with liquid nitrogen. Tissues were first weighed and then pulverized in the presence of liquid nitrogen, dissolved in a solution of 100% acetonitrile (HPLC grade). These samples were incubated at −20°C for 1 h. After 1 h samples were centrifuged, and the supernatant was used for HPLC analysis of the superoxide-specific hydroxylated product of MitoSox (Mito-2-hydroxyethidium) or of dihydroethidine (2-hydroxyethidium) using an HPLC system with a Jasco FP-1520 fluorescence detector and a Beckman ultrasphere reverse column (C18) (5μ, 250 × 4.6 mm).
Measurement of PpIX fluorescence.
PpIX fluorescence from BCA rings was measured by a method described previously by our laboratory (19). Increases in PpIX fluorescence were measured using an excitation wavelength of 485 ± 20 and emission of 620 ± 40 nm in 24-h organoid-cultured BCA rings with or without ANG II and ALA. Arterial rings were placed in the bottom of the ∼6-mm-diameter wells of a sterile 96-well microplate with 200 μl of Krebs containing 10 mM HEPES buffer (pH 7.4). PpIX fluorescence was measured from the bottom surface of the plate using a BIOTEK fluorescent microplate reader (model FLx800i), as previously described (19). Data are reported in arbitrary fluorescence units measured after subtraction of the low levels of background fluorescence observed in the absence of BCA rings.
Western blot analysis.
Frozen BCAs were pulverized and then homogenized in lysis buffer containing protease and phosphatase inhibitors, as previously described (25). Bradford method was used for protein quantification assay, and samples were prepared for gel electrophoresis. Proteins were separated using a 10% SDS-polyacrylamide gel under reducing and denaturing conditions. Gels were transferred to polyvinylidene difluoride membranes, and the membranes were blocked with Tris-buffered saline with Tween 20 + 5% milk for 1 h. After this the membranes were exposed to primary and secondary antibodies as per the manufacturer's protocol. Protein bands were visualized with an enhanced chemiluminescence kit (Pierce, Rockford, IL) on X-OMAT autoradiography paper (Kodak, Rochester, NY) in a dark room. Protein levels were measured using densitometry analysis with the UN-SCAN-IT gel software by Silk Scientific (Orem, UT). Molecular mass (kDa) of different proteins are as follows: SOD2, 25; sGCβ1, 70; FECH, 50; catalase, 60; and COx4, 15. For detection of changes in the expression of mitochondrial proteins (SOD2, FECH, and COx4), the mitochondrial protein VDAC was used as a loading control. For detection of changes in the expression of sGCβ1 and catalase, β-actin was used as a loading control. The expression of these loading controls was not altered under the conditions examined in results.
Measurement of vascular reactivity in BCA.
Endothelium-rubbed 24-h organoid-cultured BCA rings were mounted on Grass FT-03 or Coulborne Instruments force displacement transducers for recording isometric force development through a Powerlab data acquisition system obtained from ADInstruments (Colorado Springs, CO), as previously described (25). Arterial rings were incubated at 37°C in Krebs-bicarbonate buffer (pH 7.4) containing (in mM) 118 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, and 5.6 glucose under an atmosphere of 21% O2-5% CO2-74% N2 (pH 7.4) for 1 h under resting tension of 5 g. In all studies, arterial rings were depolarized with 123 mM KCl containing Krebs-bicarbonate buffer, and the rings were then reequilibrated with Krebs-bicarbonate buffer for 30 min and subsequently contracted with Krebs-bicarbonate containing 30 mM KCl. Arterial rings were either relaxed to increasing cumulative concentrations of spermine-NONOate (10−9 to 10−5 M) or isoproterenol (10−10 to 10−5 M).
Statistical analysis.
Data values are means ± SE of the number of arterial segments (n) from different animals. Statistical analyses between two groups were performed with paired and unpaired Student's t-test, and a one-way ANOVA with Newman Keuls correction was used for comparison between multiple groups. A value of P < 0.05 was used to establish statistical significance.
RESULTS
Organoid culture of BCA with ANG II decreases ferrochelatase activity through processes prevented by scavenging mitochondrial superoxide and by ALA.
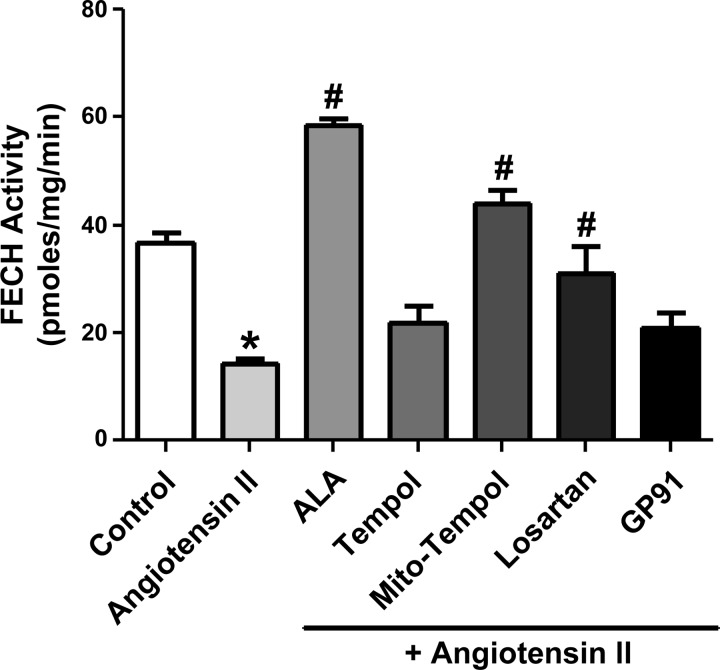
Endothelium-denuded BCA were organoid cultured with 0.1 μM ANG II for 24 h to examine the direct effects of ANG II on coronary arterial smooth muscle in the absence of its effects on endothelium and changes in the influence of endothelium-derived mediators. Initial experiments for developing this study shown in Fig. 1 suggested that decreased FECH activity was readily detected in BCA exposed to the ANG II-organoid culture conditions. As shown in Fig. 1, ANG II decreased FECH activity in a manner that was prevented by organoid culture in the presence of Mito-TEMPOL, a mitochondrial superoxide scavenger, ALA, and the ANG II type 1 receptor antagonist losartan. The Nox oxidase inhibitor gp91 ds-tat (11) or a relatively large 1 mM dose of the extramitochondrial superoxide scavenger Tempol did not have a significant effect in preventing the inhibition of FECH by ANG II.
Fig. 1.
Effects of 24-h organoid culture of bovine coronary arteries (BCA) with angiotensin II (ANG II) on ferrochelatase (FECH) activity in the presence or absence of 1 mM 2,2,6,6-tetramethyl-4-{[5-(triphenylphosphonio)pentyl]oxy}-1-piperidinyloxy bromide (Mito-TEMPOL, a mitochondrial superoxide scavenger), δ-aminolevulinic acid (ALA), losartan, gp91 ds-tat [NADPH oxidase (Nox oxidase) inhibitor], or Tempol (superoxide scavenger). *P < 0.05 vs. control; #P < 0.05 vs. ANG II; n = 4 arterial segments.
ANG II induces increases in generation of mitochondrial superoxide and extramitochondrial superoxide through processes attenuated by ALA.
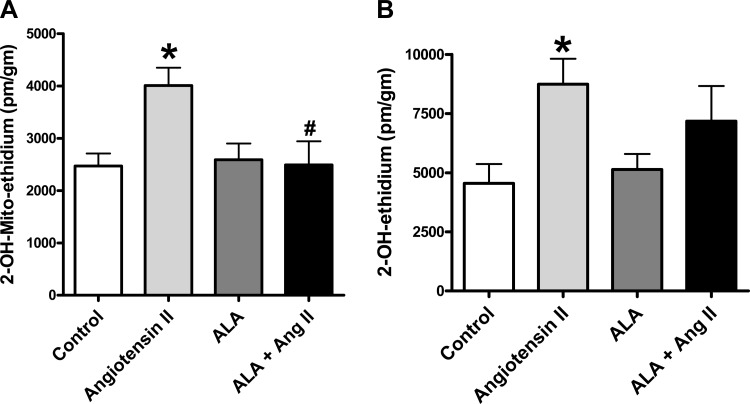
Organoid culture of BCA with ANG II caused increases in mitochondrial superoxide (Fig. 2A) based on the detection of a MitoSox-derived superoxide-specific product by HPLC. Under these conditions, ANG II also increased extramitochondrial superoxide (Fig. 2B) based on the detection of a dihydroethidine-derived superoxide-specific product by HPLC. The increase in mitochondrial superoxide elicited by ANG II was significantly attenuated by ALA (Fig. 2A). The conditions of ALA cotreatment with ANG II did not appear to show a significant increase in extramitochondrial superoxide compared with the control. While this suggests that ALA attenuates the ANG II-induced increase in extramitochondrial superoxide generation, the levels of superoxide detected under these conditions were not significantly different from those observed with ANG II in the absence of ALA (Fig. 2B). Thus, the influence of ALA on the detection of changes in superoxide is consistent with ANG II promoting a loss of FECH activity under conditions where it is increasing mitochondrial superoxide.
Fig. 2.
Effects of organoid culture of BCA with ANG II in the presence or absence of ALA on mitochondrial superoxide (A) and extramitochondrial (cytosolic) superoxide (B) detected by HPLC measurements of the superoxide-specific products of MitoSox and dihydroethidium. *P < 0.05 vs. control; #P < 0.05 vs. ANG II; n = 4–6.
Effects of ANG II and ALA during 24-h organoid culture of BCA on the expression of mitochondrial matrix SOD2.
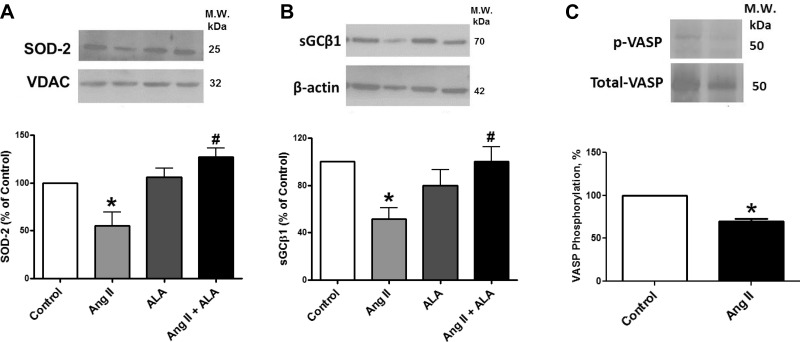
Treatment of BCAs with ANG II decreased SOD2 (Fig. 3A) expression under conditions where mitochondrial superoxide was elevated. Treatment of BCA with ALA under organoid culture conditions did not significantly alter the expression of SOD2. However, cotreatment with ANG II together with ALA prevented depletion of SOD2 by ANG II.
Fig. 3.
Effects of organoid culture of BCA with ANG II in the presence or absence of ALA for 24 h on the expression of superoxide dismutase (SOD)-2 (A), soluble guanylate cyclase (sGC, B), and vasodilator-stimulated phosphoprotein (VASP, C) phosphorylation detected by Western analysis. *P < 0.05 vs. control; #P < 0.05 vs. ANG II; n = 4–6. The mitochondrial protein voltage-dependent anion channel (VDAC) was the loading control for SOD2.
Effects of ANG II and ALA during 24 h organoid culture of BCA on the expression of sGCβ1 and the influence of ANG II on PKG activation.
Treatment of BCAs with ANG II decreased sGCβ1 (Fig. 3B) expression under conditions where both mitochondrial and extramitochondrial superoxide were elevated. Treatment of BCA with ALA under organoid culture conditions did not significantly alter the expression of sGCβ1. However, cotreatment with ANG II together with ALA prevented depletion of sGCβ1 by ANG II. The treatment of BCA with ANG II significantly decreased endogenous protein kinase G (PKG) activity as documented by a decrease in Ser239 phosphorylation of VASP (Fig. 3C). These observations suggest that ANG II is promoting a depletion of sGC associated with a decrease in PKG activity detected through the decrease in its phosphorylation of VASP.
Effects of organoid culture of BCA with ANG II and ALA on FECH expression and PpIX fluorescence.
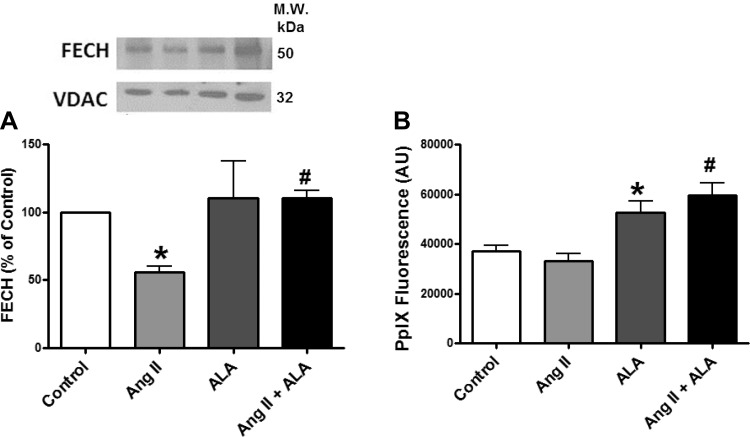
The expression of FECH was measured in BCA to determine if changes in the activity of FECH shown in Fig. 1 were associated with changes in the expression of this protein. Treatment of BCAs with ANG II decreased FECH expression (Fig. 4A) under conditions where mitochondrial superoxide was elevated. Measurements of changes in the surface fluorescence of PpIX (19) were examined to detect if ANG II influences BCA levels of PpIX in the absence and presence of promoting PpIX accumulation with ALA. While ANG II did not significantly alter the levels of PpIX, ALA increased PpIX levels in 24-h organoid-cultured BCAs both in the absence or presence of ANG II (Fig. 4B). While the increase in PpIX fluorescence elicited by ALA together with ANG II appeared slightly greater than ALA alone, this difference was not statistically significant.
Fig. 4.
Effects of organoid culture of BCA with ANG II in the presence or absence of ALA on FECH expression detected by Western analysis (A) and protoporphyrin IX (PpIX) levels detected by its surface fluorescence (B). *P < 0.05 vs. control; #P < 0.05 vs. ANG II; n = 4–6. The mitochondrial protein VDAC was the loading control for FECH.
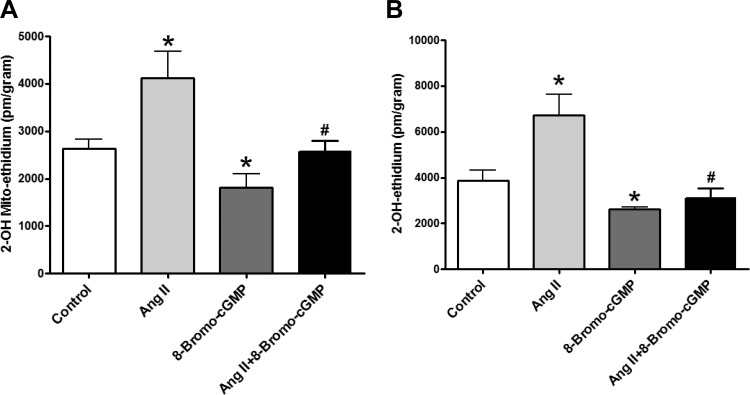
8-Bromo-cGMP cotreatment reverses ANG II-induced increases in generation of mitochondrial superoxide and extramitochondrial superoxide.
The PKG activator 8-bromo-cGMP was used to examine if cGMP/PKG signaling functions to attenuate increases in mitochondrial superoxide and potential consequences of its actions on FECH that were observed with ALA. These experiments were designed to help define processes potentially regulated by cGMP as a result of PpIX generated from ALA stimulating sGC (19). ANG II-elicited increases in mitochondrial superoxide (Fig. 5A) and extramitochondrial (cytosolic) (Fig. 5B) superoxide observed in organoid-cultured BCA were significantly attenuated by the PKG activator 8-bromo-cGMP, suggesting that cGMP may have a major role in superoxide regulation and the actions of ALA.
Fig. 5.
Effects of organoid culture of BCA with ANG II in the presence or absence of 8-bromo-cGMP on mitochondrial superoxide (A) and extramitochondrial (cytosolic) superoxide (B). *P < 0.05 vs. control; #P < 0.05 vs. ANG II; n = 5–9.
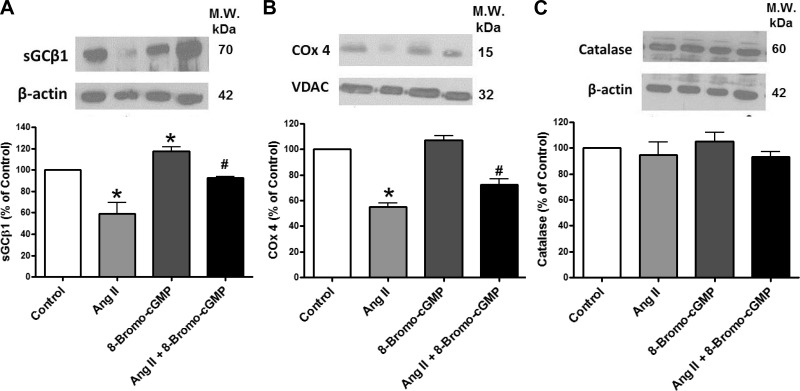
Effects of ANG II and 8-bromo-cGMP during 24-h organoid culture of BCA on expression of the heme-containing protein subunits sGCβ1, COx4, and catalase.
Organoid culture of BCA with ANG II also depleted sGCβ1 (Fig. 6A) and COx4 (Fig. 6B), but not catalase (Fig. 6C), suggesting that ANG II depletes heme and that sGC expression may be very sensitive to the depletion of heme caused by ANG II. These effects of ANG II were significantly attenuated by coorganoid culture with the PKG activator 8-bromo-cGMP.
Fig. 6.
Effects of organoid culture of BCA with ANG II in the presence and absence of 8-bromo-cGMP on expression of the heme-containing proteins sGC (A), subunit 4 of cytochrome oxidase (COx4, B), and catalase (C). *P < 0.05 vs. control; #P < 0.05 vs. ANG II; n = 4. The mitochondrial protein VDAC was the loading control for COx4.
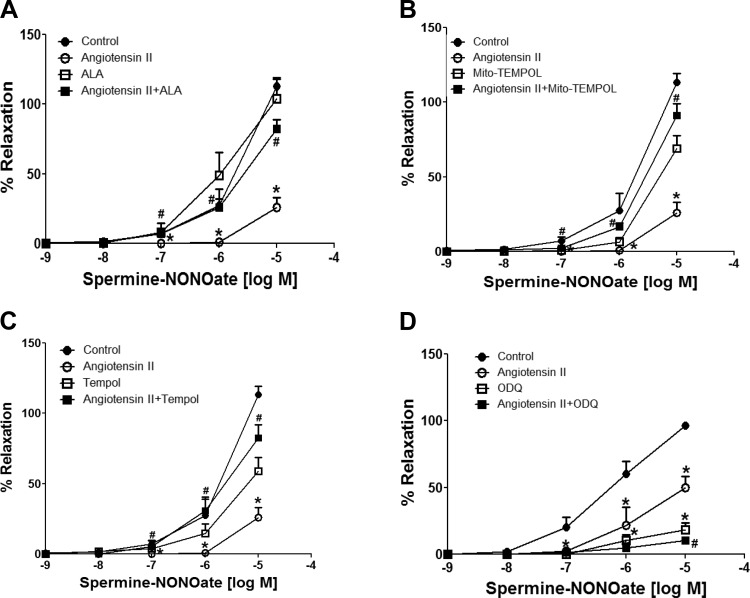
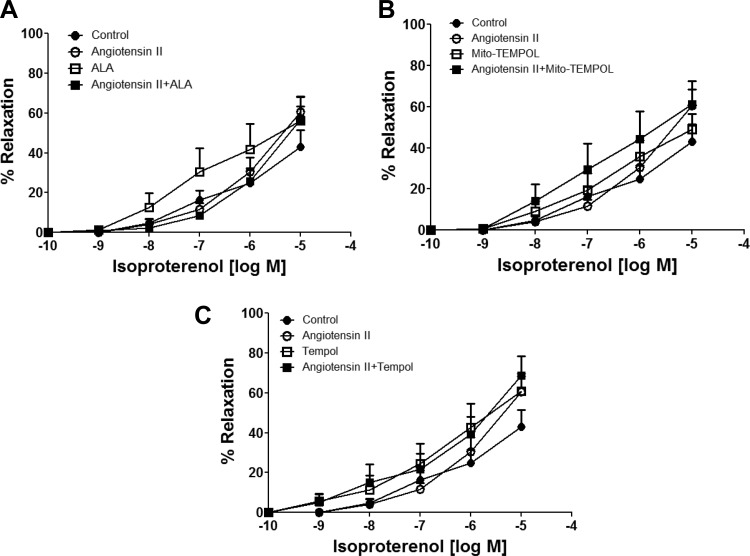
Effects of ANG II during 24-h organoid culture of BCA on relaxation to a NO donor and isoproterenol.
Spermine-NONOate was used in this study as an NO donor, under conditions where NO-mediated relaxation functions primarily through a Fe2+ heme-dependent stimulation of sGC (13). As shown in Fig. 7, the 24-h organoid culture of ANG II significantly reduced relaxation to NONOate at doses of 10−7, 10−6, and 10−5 M. ALA (Fig. 7A), Mito-TEMPOL (Fig. 7B), and Tempol (Fig. 7C) cotreatment significantly reversed the effects of ANG II. Acute treatment with 10 μM ODQ markedly inhibited NONOate-induced relaxation (Fig. 7D) at doses of 10−7, 10−6, and 10−5 M in control organoid-cultured BCA rings. While acute treatment with 10 μM ODQ inhibited NONOate-induced relaxation at 10−5 M dose in BCA rings organoid cultured with ANG II, ANG II did alter the minor relaxation to NONOate observed in the presence of ODQ. These data suggest that the increased mitochondrial and extramitochondrial superoxide elicited by ANG II contributes to the observed loss of NO-stimulated vasodilation. Isoproterenol, the β-adrenergic receptor agonist of cAMP-mediated relaxation, was used as a sGC-independent relaxing agent. As shown in Fig. 8, relaxation of BCA to isoproterenol was not altered by ANG II or by any other cotreatments in organoid-treated BCAs. None of these treatments had a significant effect on the force generated by 30 mM KCl used to examine relaxation responses to NONOate or isoproterenol under any of the conditions examined in these studies (data not shown). Thus, the actions of ANG II appeared to selectively attenuate cGMP-associated relaxation to a NO donor, without altering cAMP-associated relaxation to isoproterenol.
Fig. 7.
Effects of 24-h organoid-cultured BCA rings with or without ANG II in the absence or presence of ALA (A), 1 mM Mito-TEMPOL (B), 1 mM Tempol (C), and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, D) on relaxation to increasing cumulative doses of spermine-NONOate. BCA rings from each group were precontracted with 30 mM KCl, and data are reported as %relaxation of the force generated by this contractile agent. Responses from the same animals reported in each panel of data to spermine-NONOate measured in the absence of ANG II, ALA, Mito-TEMPOL, Tempol, or ODQ in the tissue bath were used for the controls. *P < 0.05 vs. control; #P < 0.05 vs. ANG II; n = 5–7.
Fig. 8.
Effects of 24-h organoid-cultured BCA rings with or without ANG II in the absence or presence of ALA (A), Mito-TEMPOL (B), and Tempol (C) on relaxation to increasing cumulative doses of isoproterenol. BCA rings from each group were precontracted with 30 mM KCl, and data are reported as %relaxation of the force generated by this contractile agent. Responses from the same animals reported in each panel of data to isoproterenol measured in the absence of ANG II, ALA, Mito-TEMPOL, or Tempol in the tissue bath were used for the controls. n = 5–7.
DISCUSSION
The data in this study provide evidence that organ culture of endothelium-denuded bovine coronary arteries with ANG II increases mitochondrial and extramitochondrial superoxide generation in a manner that appears to be associated with a depletion of sGC. Under these conditions, ANG II appeared to selectively attenuate cGMP-associated relaxation to a NO donor, without altering cAMP-associated relaxation to isoproterenol. Because this loss of relaxation to a NO donor resulting from organoid-cultured BCAs with ANG II was attenuated by organoid culture in the presence of Tempol or Mito-TEMPOL (Fig. 7), both mitochondrial and extramitochondrial superoxide appear to be contributing to the observed loss of relaxation to NO. Organoid culture of BCA with ANG II also caused a loss of SOD2 expression, ferrochelatase expression and activity, and sGC expression, and decreased PKG phosphorylation of VASP, under conditions that appeared to be associated with a depletion of heme based on the observed depletion of COx4. These data suggest that sGC expression and its ability to participate in promoting relaxation by NO appear to be very sensitive to actions of superoxide, potentially through superoxide influencing the availability of heme and interactions of NO and heme with sGC.
Organoid culture with ALA appeared to reverse many of the effects of ANG II, including restoring sGC-mediated relaxation to NO. ALA is likely to be functioning under the organoid culture conditions employed through promoting the biosynthesis of PpIX and/or heme because increases in PpIX were detected by its fluorescence (Fig. 4B), and the decreased level of COx4 suggesting heme depletion was restored. Because it is known that activators of sGC, which function through binding its PpIX/heme site, prevent sGC degradation once its heme is oxidized (28), this could also be a factor in preventing sGC degradation. However, the observed protection of sGC depletion by the 8-bromo-cGMP activator of PKG suggests additional processes may be involved in controlling the loss of sGC expression caused by ANG II. Because organoid culture of arteries with 8-bromo-cGMP attenuated ANG II-elicited increases in mitochondrial and extramitochondrial (cytosolic) superoxide in a manner similar to ALA, PKG activation as a result of ALA-generated PpIX stimulating cGMP production by sGC (19) appears to be a major factor in the actions of ALA.
Oxidation of the sGC heme and sGC depletion have been observed to be associated with the formation of reactive NO-derived oxidant species (28). However, processes through which superoxide could promote the oxidation of the heme of sGC in an intracellular environment with low levels of NO, such as the organoid culture conditions with endothelium-denuded BCA used in the present study, are not well defined. While the data in Fig. 7 are consistent with the presence of Tempol during organoid culture with ANG II attenuating the loss of sGC-dependent relaxation to the NO donor studied, the high dose of Tempol used leaves open the possibility that it may be functioning through actions that go beyond its intended use as an extramitochondrial scavenger of superoxide. In addition, other processes could prevent the depletion of sGC associated with heme oxidation. The Fe2+ heme form of sGC appears to bind PpIX (13, 31) and other activators of sGC that bind its heme site (28), suggesting heme may be released from sGC even in the absence of substantial heme oxidation. Thus, the availability of PpIX, heme, and perhaps other porphyrins or activators that bind the heme site of sGC could be major factors in controlling or maintaining both the expression of sGC by preventing its degradation by proteolysis (26) and the ability of sGC to maintain its stimulation by mediators such as NO. In addition, any process increasing cGMP or promoting PKG activation could function to attenuate the actions of pathophysiological mediators that are associated with increasing superoxide, disrupting heme biosynthesis, and/or promoting sGC heme oxidation or depletion.
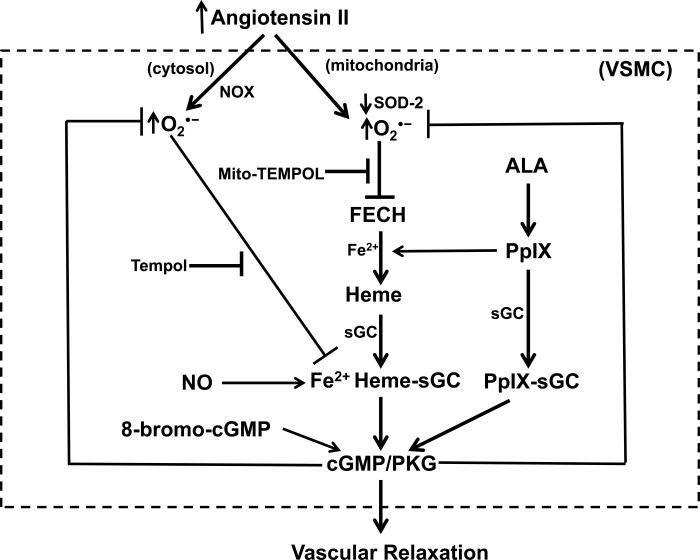
The inhibition and depletion of ferrochelatase caused by ANG II-elicited increases in mitochondrial superoxide in the present study are potentially major factors in impairing the availability of heme and its potential influence in maintaining the expression of sGC. The Fe-S cluster of FECH seems to be essential for maintaining its stability and activity (4). It was recently demonstrated that mice with a conditional knockout of mitochondrial matrix SOD2 in hematopoietic stem cells showed evidence of decreased FECH activity, which could originate from superoxide disrupting its Fe-S cluster (20). These animals also showed decreased hemoglobin or hematocrit that was reversed by treatment of the mice with Mito-TEMPOL, suggesting mitochondrial superoxide may disrupt heme biosynthesis and influence other processes potentially related to iron metabolism and oxidant stress (18). Previous studies inhibiting ferrochelatase with N-methyl-PpIX detected a selective depletion of COx4, but not catalase, suggesting that what is thought to be a small pool of heme may be needed to maintain the expression of some heme-containing proteins. Because Mito-TEMPOL, but not Tempol or the Nox oxidase inhibitor gp91 ds-tat, prevented the decrease in FECH activity in coronary artery organoid cultured with ANG II (Fig. 1), it appears that mitochondrial superoxide is a key factor in inhibiting FECH activity, and that other extramitochondrial sources of superoxide do not seem to be influencing FECH activity under the conditions examined. The pattern of protective effects of ALA and/or 8-bromo-cGMP suggests that PKG activation promoted by these agents is functioning to prevent the depletion of SOD2 and increased mitochondrial matrix superoxide-mediated inhibition of FECH, and the associated depletion of heme needed for maintaining the expression of COx4 and sGC through hypothesized relationships illustrated in the model shown in Fig. 9. Further studies are needed to define the properties of key aspects of this model, such as how cGMP signaling alters the expression of SOD2 and attenuates increased production of mitochondrial superoxide and to examine how these systems function in vivo under pathophysiological conditions.
Fig. 9.
Model showing some of the hypothesized actions of ANG II on vascular smooth muscle cells (VSMC) in the coronary arteries studied that are potentially contributing to the observed depletion of sGC and loss of relaxation to NO, and the potential origins of detected protective effects of ALA, Mito-TEMPOL, and Tempol. ANG II decreases mitochondrial SOD2 and increases mitochondrial superoxide (detected by MitoSox), which should both inactivate and promote depletion of FECH. The loss of FECH is expected to impair heme biosynthesis (as supported by detection of the depletion of cytochrome c oxidase subunit 4), and this is hypothesized to contribute to an acceleration of the depletion of sGC, since its heme is oxidized by processes associated with increased extramitochondrial superoxide derived from systems such as NOX or other oxidant mechanisms. Based on the actions of the protein kinase G (PKG) activator 8-bromo-cGMP, PKG functions to lower superoxide and prevent these actions of ANG II. ALA may function by generating PpIX, a stimulator of cGMP production by sGC, and perhaps by promoting increased generation of heme.
The results of this study provide documentation that promoting PpIX accumulation, preventing the inactivation of ferrochelatase by mitochondrial superoxide, and preserving heme biosynthesis are potentially targets for developing new beneficial therapeutic approaches, especially for maintaining processes such as NO regulation of sGC and aspects of cGMP-mediated regulation (28) such as vasodilation, inhibition of processes involved in inflammatory signaling, and vascular remodeling. For example, mitochondrial superoxide scavengers, which function in a manner similar to Mito-TEMPOL, have the potential for being effective in attenuating the disruption of heme biosynthesis, and Mito-TEMPO has been reported to attenuate vascular dysfunction in the ANG II infusion model (6, 17). ALA has already been demonstrated to prevent hypoxia-induced pulmonary hypertension in mice (1, 24). Although ALA was not protective in a mouse renal ischemia-reperfusion injury model, when used in combination with ferrous iron, it was protective as a result of generating heme in amounts that appeared to both induce and support the beneficial actions of heme oxygenase (8). The use of ALA for fluorescence detection of cancer cells based on their accumulation of PpIX, and for cancer cell phototherapy targeting PpIX, have been extensively studied in humans (11). The actions of ANG II and other pathophysiological mediators that promote increased mitochondrial superoxide and/or reactive NO-derived species in amounts that cause disruption of the function of FECH may allow ALA to promote a selective enhancement of PpIX accumulation in cells with elevated mitochondrial oxidant stress. However, the accumulation of PpIX may then promote processes that reverse these oxidant conditions through promoting a cGMP-mediated inhibition of increased mitochondrial and extramitochondrial generation of superoxide.
The most important new aspect of this study appears to be evidence for increased mitochondrial generation of superoxide caused by ANG II (and perhaps other agents) functioning to impair heme biosynthesis by inhibiting ferrochelatase in a manner that results in a loss of the beneficial vascular regulatory effects of heme-dependent expression and/or function of sGC (e.g., stimulation by NO), and perhaps other systems contributing to vascular dysfunction such as mitochondrial functions influenced by a heme-dependent depletion of cytochrome oxidase. These processes appear to be prevented by cGMP stimulation of PKG signaling attenuating both mitochondrial and extramitochondrial increases in superoxide. The data in this study also suggest that ALA could be beneficial in overcoming oxidative inactivation of ferrochelatase and restoring the consequences this has on heme-dependent proteins in a manner that normalizes processes such as vasodilatation.
GRANTS
This study was support by National Heart, Lung, and Blood Institute Grant R01-HL-115124.
DISCLOSURES
Michael S. Wolin, Ph.D. is the inventor on a patent held by New York Medical College based on using protoporphyrin IX accumulation for smooth muscle relaxation.
AUTHOR CONTRIBUTIONS
D.P., D.S., and M.S.W. conception and design of research; D.P., R.A., M.R.K., J.J.A., A.L., and D.S. performed experiments; D.P., R.A., M.R.K., J.J.A., A.L., D.S., and M.S.W. analyzed data; D.P., R.A., J.J.A., A.L., S.A.G., D.S., and M.S.W. interpreted results of experiments; D.P., R.A., and M.S.W. prepared figures; D.P. and M.S.W. drafted manuscript; D.P., R.A., M.R.K., J.J.A., A.L., S.A.G., D.S., and M.S.W. edited and revised manuscript; D.P., R.A., M.R.K., J.J.A., A.L., S.A.G., D.S., and M.S.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Portions of this study were presented at the 2015 Experimental Biology Meeting in Boston, MA (24).
REFERENCES
- 1.Alhawaj R, Patel D, Kelly MR, Sun D, Wolin MS. Heme biosynthesis modulation via delta-aminolevulinic acid administration attenuates chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L719–L728, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atamna H, Liu J, Ames BN. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: relevance to aging. J Biol Chem 276: 48410–48416, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Case AJ, Madsen JM, Motto DG, Meyerholz DK, Domann FE. Manganese superoxide dismutase depletion in murine hematopoietic stem cells perturbs iron homeostasis, globin switching, and epigenetic control in erythrocyte precursor cells. Free Radic Biol Med 56: 17–27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crooks DR, Ghosh MC, Haller RG, Tong WH, Rouault TA. Posttranslational stability of the heme biosynthetic enzyme ferrochelatase is dependent on iron availability and intact iron-sulfur cluster assembly machinery. Blood 115: 860–869, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa T, Kohno H, Tokunaga R, Taketani S. Nitric oxide-mediated inactivation of mammalian ferrochelatase in vivo and in vitro: possible involvement of the iron-sulphur cluster of the enzyme. Biochem J 310: 533–538, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Q, Wolin MS. Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol 295: H978–H989, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupte SA, Kaminski PM, George S, Kouznestova L, Olson SC, Mathew R, Hintze TH, Wolin MS. Peroxide generation by p47phox-Src activation of Nox2 has a key role in protein kinase C-induced arterial smooth muscle contraction. Am J Physiol Heart Circ Physiol 296: H1048–H1057, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman MA, Webber J, Fromm D, Kessel D. Hemodynamic effects of 5-aminolevulinic acid in humans. J Photochem Photobiol B 43: 61–65, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Hou J, Cai S, Kitajima Y, Fujino M, Ito H, Takahashi K, Abe F, Tanaka T, Ding Q, Li XK. 5-Aminolevulinic acid combined with ferrous iron induces carbon monoxide generation in mouse kidneys and protects from renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 305: F1149–F1157, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Iesaki T, Gupte SA, Kaminski PM, Wolin MS. Inhibition of guanylate cyclase stimulation by NO and bovine arterial relaxation to peroxynitrite and H2O2. Am J Physiol Heart Circ Physiol 277: H978–H985, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Ignarro LJ, Wood KS, Wolin MS. Regulation of purified soluble guanylate cyclase by porphyrins and metalloporphyrins: a unifying concept. Adv Cyclic Nucleotide Protein Phosphorylation Res 17: 267–274, 1984. [PubMed] [Google Scholar]
- 15.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res 102: 401–414, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95: 588–593, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Revelles S, Avendano MS, Garcia-Redondo AB, Alvarez Y, Aguado A, Perez-Giron JV, Garcia-Redondo L, Esteban V, Redondo JM, Alonso MJ, Briones AM, Salaices M. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal 18: 51–65, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Mingone CJ, Gupte SA, Chow JL, Ahmad M, Abraham NG, Wolin MS. Protoporphyrin IX generation from delta-aminolevulinic acid elicits pulmonary artery relaxation and soluble guanylate cyclase activation. Am J Physiol Lung Cell Mol Physiol 291: L337–L344, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Missirlis F, Hu J, Kirby K, Hilliker AJ, Rouault TA, Phillips JP. Compartment-specific protection of iron-sulfur proteins by superoxide dismutase. J Biol Chem 278: 47365–47369, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: e58–e65, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Neo BH, Kandhi S, Wolin MS. Roles for soluble guanylate cyclase and a thiol oxidation-elicited subunit dimerization of protein kinase G in pulmonary artery relaxation to hydrogen peroxide. Am J Physiol Heart Circ Physiol 299: H1235–H1241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res 71: 247–258, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Patel D, Alhawaj R, Wolin MS. Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide. Am J Physiol Regul Integr Comp Physiol 307: R426–R433, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α. Am J Physiol Lung Cell Mol Physiol 306: L383–L391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel D, Alhawaj R, Kelly M, Sun D, Wolin M. Role of angiotensin II-associated mitochondrial superoxide in inhibiting ferrochelatase activity and disrupting heme biosynthesis regulation of coronary artery soluble guanylate cyclase expression (Abstract). FASEB J 29: 623.8, 2015.25384422 [Google Scholar]
- 27.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, HSAK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Van Hillegersberg R, Van den Berg JW, Kort WJ, Terpstra OT, Wilson JH. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology 10: 647–651, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Wolin MS, Wood KS, Ignarro LJ. Guanylate cyclase from bovine lung. A kinetic analysis of the regulation of the purified soluble enzyme by protoporphyrin IX, heme, and nitrosyl-heme. J Biol Chem 257: 13312–13320, 1982. [PubMed] [Google Scholar]
- 32.Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med 46: 329–338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]