Molecular mechanisms determining differential sensitivity of smooth muscles to nitric oxide-mediated relaxation have not been defined. This study uses a genetic mouse model to demonstrate that splice variants of myosin phosphatase set vascular smooth muscle sensitivity to NO and cGMP-mediated relaxation and thereby control blood pressure.
Keywords: smooth muscle, cGMP, nitric oxide, blood pressure, mesenteric artery
Abstract
The cGMP activated kinase cGK1α is targeted to its substrates via leucine zipper (LZ)-mediated heterodimerization and thereby mediates vascular smooth muscle (VSM) relaxation. One target is myosin phosphatase (MP), which when activated by cGK1α results in VSM relaxation even in the presence of activating calcium. Variants of MP regulatory subunit Mypt1 are generated by alternative splicing of the 31 nt exon 24 (E24), which, by changing the reading frame, codes for isoforms that contain or lack the COOH-terminal LZ motif (E24+/LZ−; E24−/LZ+). Expression of these isoforms is vessel specific and developmentally regulated, modulates in disease, and is proposed to confer sensitivity to nitric oxide (NO)/cGMP-mediated vasorelaxation. To test this, mice underwent Tamoxifen-inducible and smooth muscle-specific knockout of E24 (E24 cKO) after weaning. Deletion of a single allele of E24 (shift to Mypt1 LZ+) enhanced vasorelaxation of first-order mesenteric arteries (MA1) to diethylamine-NONOate (DEA/NO) and to cGMP in permeabilized and calcium-clamped arteries and lowered blood pressure. There was no further effect of deletion of both E24 alleles, indicating high sensitivity to shift of Mypt1 isoforms. However, a unique property of MA1s from homozygous E24 cKOs was significantly reduced force generation to α-adrenergic activation. Furthermore 2 wk of high-salt (4% NaCl) diet increased MA1 force generation to phenylephrine in control mice, a response that was markedly suppressed in the E24 cKO homozygotes. Thus Mypt1 E24 splice variants tune arterial reactivity and could be worthy targets for lowering vascular resistance in disease states.
NEW & NOTEWORTHY
Molecular mechanisms determining differential sensitivity of smooth muscles to nitric oxide-mediated relaxation have not been defined. This study uses a genetic mouse model to demonstrate that splice variants of myosin phosphatase set vascular smooth muscle sensitivity to NO and cGMP-mediated relaxation and thereby control blood pressure.
myosin phosphatase (MP) is the primary mediator of smooth muscle relaxation and a key target of signaling pathways that regulate vessel tone (4, 8, 11). Nitric oxide (NO) signaling through the second messenger cGMP increases MP activity, thereby decreasing force at any calcium concentration (20, 45), i.e., calcium desensitization of force production. Although the exact mechanism by which NO/cGMP may activate MP has not been determined (see discussion), in vitro and biochemical studies support a model in which the cGMP-dependent protein kinase (cGK1α) protein is targeted to the myosin phosphatase regulatory subunit (Mypt1) via leucine zipper (LZ) motifs within coiled-coil (cc) domains present in the COOH terminus of Mypt1 and NH2 terminus of cGK1 (7, 13, 15, 37, 41).
Isoforms of Mypt1 are generated by alternative splicing of the 31 nt exon 24 (E24). Inclusion of E24 changes the reading frame and introduces a premature stop codon, thereby coding for a Mypt1 variant that lacks the COOH-terminal LZ motif (LZ−). The splicing of E24 and thus generation of Mypt1 LZ+/− isoforms is highly tissue specific, developmentally regulated, and modulated in disease (4, 36). We and others have shown a correlation between the relative expression of the Mypt1 E24−/LZ+ isoform and sensitivity to NO/cGMP-mediated calcium desensitization of force production, comparing phasic vs. tonic smooth muscle (15, 29), large vs. small arteries (30, 35, 49), and animal models of vascular disease in which expression of the Mypt1 E24/LZ isoforms is altered (10, 14, 18, 21, 22, 30, 34, 47, 48). These biochemical and physiological studies support the hypothesis that the regulated expression of the Mypt1 E24/LZ isoforms determines smooth muscle sensitivity to NO/cGMP-mediated relaxation. Parenthetically, it has been appreciated since the discovery of NO as the endothelial-derived relaxing factor that smooth muscle tissues vary in their sensitivity to NO- and cGMP-mediated relaxation (2, 6, 28, 31), yet mechanisms for this differential sensitivity remain poorly described.
The testing of this MP “LZ hypothesis” and determination of the magnitude of the effect of the isoforms in determining vasodilator response to NO/cGMP in vivo require a model in which the expression of the Mypt1 E24/LZ isoforms can be manipulated as independent variables in vivo. To accomplish this, we inserted LoxP sites into the introns flanking mouse Mypt1 E24. Crossing these mice into a line in which Cre is conditionally expressed specifically within smooth muscle (SMMHCCreERT2) (43) leads to the knockout of E24 (E24 cKO), thereby shifting smooth muscle toward the Mypt1 E24−/LZ+ isoform. In our initial studies, we used heterozygous E24 cKO mice to recapitulate changes in Mypt1 E24/LZ isoforms in models of arterial maturation (35) and sepsis (34). We showed that shift toward the Mypt1 E24−/LZ+ isoform lowered blood pressure (BP) and increased sensitivity to cGMP-mediated relaxation of the mesenteric arteries (MAs) from otherwise normal adult mice. The goals of the present study were twofold: 1) to determine the dose-response relationship between expression of Mypt1 E24/LZ +/− variants and arterial function and BP, and 2) to test the hypothesis that forced expression of the Mypt1 E24−/LZ+ isoform (E24 cKO) will have salutary effects on arterial function and BP in a disease model, in this instance, the stress of a high-salt diet.
MATERIALS AND METHODS
Animal model.
All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Maryland and adhere to NIH guidelines. Using Zinc Finger Nuclease methodology in the inbred C57Bl/6J mouse line (Sage Laboratories, Boyertown, PA), LoxP sequences were inserted in the introns flanking mouse Mypt1 E24 outside of conserved putative cis-regulatory splicing sequences (Fig. 1) (3, 39) (NCBI reference sequence: NC_000076.6 genomic coordinates 109001-115256). Targeted integration genotyping was achieved by placing restriction enzyme sequences (BsiWI and Hind III) next to each LoxP site. Mice with the E24 floxed allele were then bred to the smooth muscle specific SMMHCCreERT2 mouse (43). Cre was activated in male mice via intraperitoneal injection of Tamoxifen (Sigma; 50 mg/kg in sunflower oil) for 3 consecutive days at 3 wk of age. These mice are described as E24 cKO. Control Cre+ mice without floxed alleles were treated in the same manner. Mice were studied at 8–12 wk of age. A subset of mice were placed on a high-salt (4% NaCl) diet for 2 wk before study. The control mice in these experiments were continued on their normal-salt diet (0.4% NaCl).
Fig. 1.
Cre-Lox-mediated deletion of Mypt1 exon 24 (E24) in vascular smooth muscle. A: sequence of the mouse Mypt1 gene including E24 and flanking introns. The highly conserved and putative splicing regulatory sequence is shaded. Exon sequence is in green, and splice site sequence is in blue. Inserted LoxP sequences are in orange. B: schematic diagram of alternative splicing of Mypt1 E24. Skipping of E24 codes for the COOH-terminal leucine zipper (LZ) motif as shown that mediates heterodimerization with the NH2-terminal LZ motif in cGK1α. Inclusion of the 31 nt E24 changes the reading frame and codes for a unique COOH-terminal sequence, designated LZ−, and a premature termination codon. In the presence of Cre recombinase, intronic LoxP sites undergo recombination, resulting in knockout of E24 (E24 cKO). C: total RNA was purified and reverse transcribed from the mesenteric arterial arcade, aorta, portal vein, and femoral artery of control (Cre+) and E24 cKO mice (heterozygotes: Cre+//F/+; homozygotes: Cre+//F/F). These experiments used the smooth muscle-specific and Tamoxifen-inducible smMHCCreERT2. In all experiments, mice were treated with Tamoxifen at age 3 wk and studied as adults (age 8–12 wk) as described in materials and methods. All mice in all experiments were Cre+ and treated with Tamoxifen, thus only the Mypt1 E24 genotype is shown in the graphs. PCR was performed on cDNAs using a single set of primers flanking E24 to amplify Mypt1 E24+ and E24− splice variants in a single reaction. PCR products were gel separated and quantified with a LiCor imager and graphed as a percentage of Mypt1 E24+. D: protein lysates from mouse mesenteric arterial arcade were subjected to Western blot analysis. Membranes were probed with rabbit polyclonal antibodies specific for the LZ− and LZ+ isoforms of Mypt1 (∼130 kDa) and then stripped and reprobed with an antibody that recognizes all Mypt1 isoforms. The LZ+ antibody also recognizes the LZ motif present in the Mypt family member p85. The ratio of Mypt1 LZ−/LZ+ for mice of the different genotypes is plotted, normalized to the control value (n = 3 each) *†P < 0.05 vs. control; ‡P < 0.05, F/+ vs. F/F
RNA analysis.
Blood vessels were dissected from the mice and stored in RNALater before homogenization and column purification of RNA (RNEasy; Qiagen). Total RNA (100 ng) was reverse transcribed using Superscript III enzyme (1,000 U), and cDNAs were subjected to conventional and quantitative real-time PCR as previously described (35). The Mypt1 E24 alternative exon splice variants were amplified in a single PCR using IR-labeled primers that flank the alternative Mypt1 E24 exon. PCR products (E24+ and E24−) were gel separated, amplicon bands directly quantified with a Li-Cor Odyssey digital imager, and data reported as %Mypt1 E24 inclusion. TaqMan probes (Applied Biosystems) were used to quantify mRNAs by real-time PCR and normalized to cyclophilin A (Ppia), which was invariant. Data are expressed as fold change of transcripts using the 2−ddCt method.
Protein analysis.
MAs and aortas were homogenized using a Next Advance Bullet Blender in a lysis buffer containing 125 mM Tris·HCl (pH 6.8), 20% sucrose, 10% SDS, and 1% proteinase inhibitor cocktail. Protein lysates (10 μg) were loaded into 4–15% Tris-glycine gels (Mini-PROTEAN TGX; Bio-Rad), separated at 80 V for 1.5 h, and transferred to nitrocellulose at 25 V for 2 h. Membranes were blocked with Li-Cor Odyssey blocking buffer and incubated with primary antibodies overnight at 4°C. Rabbit polyclonal antibodies specific for the Mypt1 LZ+ and LZ− isoforms were used at 1:3,000 dilutions as previously described (35). Membranes were incubated with secondary IRDye antibodies (800CW and 680LT) (1:10,000), imaged in the Li-Cor Odyssey digital scanner, and quantified with Image Studio 3.0 software. Membranes were then stripped and reprobed with a rabbit polyclonal antibody that detects all isoforms of Mypt1 (ab24670; Abcam). The LZ− signal in each sample was divided by the LZ+ signal in each sample and then by the Mypt1 signal for internal normalization, which was invariant. The values of the Mypt1 LZ−/LZ+ ratios are reported as fold change vs. control samples.
Vascular function.
First-order MAs (MA1s, 2-mm length; 200–250-μm ID) were dissected free of connective tissue in a HEPES-bicarbonate physiological saline solution containing the following concentrations (in mM): 112 NaCl, 25.7 NaHCO3, 4.9 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 11.5 glucose, and 10 HEPES. The solution was at pH 7.4 and maintained at 37°C for experiments. Arteries were mounted on a four-chamber wire myograph (Model 610M; Danish Myo Technology). Force was continuously recorded and measured at steady state. Starting tension (IC90) was equally applied to all arteries as previously described (35). Arteries were then primed with two separate dose of phenylephrine (PE, 10 μM) and allowed to equilibrate for 20 min in fresh, heated HEPES-bicarbonate buffer. Intact arteries were subjected to dose response to PE (α-adrenergic agonist; 1 nM-100 μM), U46619 (thromboxane mimetic; 1 nM-10 μM), and angiotensin II (1 nM-10 μM). Maximal response of MAs to depolarization was assessed with 100 mM KCl. A subset of arteries were permeabilized with α-toxin (1,000 U/ml) and subjected to calcium clamp in high relaxing solution (pCa9) containing the following (in mM): 60 KMS, 5 EGTA, 0.02 CaCl2, 9.26 MgCl2, 5.2 Na2ATP, 25 creatine phosphate, and 25 BES with pH 7.1 (intracellular pH) by 1 N KOH. Permeabilized arteries were subjected to dose response of calcium. PE-induced calcium sensitization was performed under calcium clamp at a submaximal concentration of calcium (pCa6; 1 μM) with intracellular calcium stores depleted by preincubation of arteries with 10 μM A-23187. Vessels were activated with 10 μM PE with or without preincubation with nitro-l-arginine methyl ester (l-NAME) (100 μM). Vasorelaxation responses to the NO donor diethylamine-NONOate (DEA/NO) were assessed in intact arteries activated with a submaximal concentration of PE (10 μM). Dose responses to 8-bromo-cGMP (8-Br-cGMP) (1 nM-100 μM) were assessed in α-toxin permeabilized, calcium-clamped (pCa6; 1 μM) arteries. Relaxation data are presented as a percentage of maximal force. All chemicals were purchased from Sigma-Aldrich.
Telemetry blood pressure.
Arterial pressure was measured via telemetry in conscious mice (12–16 wk of age) using PA-C10 transmitters (Data Sciences, St. Paul, MN), detected using telemetry-receiving platforms, and analyzed using Dataquest software as previously described (5). The transmitters were implanted into the left carotid artery, and mice were allowed to recover for 1 wk before the transmitters were turned on. Blood pressure was measured continuously over the course of three consecutive days (day and night readings) at 10-min intervals and reported as the average of the mean arterial pressure (MAP) over the 3-day period.
Statistics.
All data are presented as means ± SE. Data were analyzed and graphed using SigmaPlot software (SYSTAT). mRNA and protein data and MAPs were analyzed using a one-way ANOVA. Vascular function data were analyzed via one-way ANOVA and a Bonferroni post hoc test or two-way repeated-measures ANOVA and a Bonferroni post hoc test. EC50 values were calculated via standard curve analysis. Significance was accepted with a P < 0.05.
RESULTS
Efficient deletion of E24 from Mypt1 in vascular smooth muscle.
Treatment of male mice with Tamoxifen (50 mg/kg ip) for 3 days at 3 wk of age resulted in efficient smMHCCreERT2-mediated deletion of E24 from the Mypt1 mRNA measured at 8–12 wk of age (Fig. 1C). In mice with one floxed allele (F/+), there was an approximate 50% relative reduction in Mypt1 transcripts that were E24+, whereas, in the homozygotes (F/F), there was nearly complete deletion of E24. The absolute magnitude of the change was dependent on the basal level. The phasic smooth muscle of the portal vein (PV) has the highest basal level of inclusion and thus the largest absolute drop, whereas the mesenteric and femoral arteries are intermediate, and the tonic smooth muscle of the aorta has the lowest level of inclusion and thus the smallest, yet still significant, reduction. The Mypt1 E24 ratios in the control mice (Cre+ treated with Tamoxifen; Fig. 1C) were not different from untreated wild-type mice (35). Similarly, mice of the genotype Cre+//F/F that were not treated with Tamoxifen had normal Mypt1 E24 ratios (MA: %Mypt1 E24+: 51.9 ± 1.2%), indicating that insertion of LoxP sites or treatment with Tamoxifen in the absence of recombination did not alter the splicing of Mypt1 E24.
Isoform-specific antibodies confirmed that cKO of E24 caused the predicted reduction in the LZ- isoform of Mypt1 in the mesenteric arteries, with no change in the level of total Mypt1 and a corresponding increase in the Mypt1 LZ+ isoform (Fig. 1D). Again a dose-response was observed between the number of Mypt1 E24 floxed alleles and the decrease in the LZ- isoform and ratio of LZ−/LZ+ (P < 0.05).
Conditional KO of Mypt1 E24 lowers systemic blood pressure.
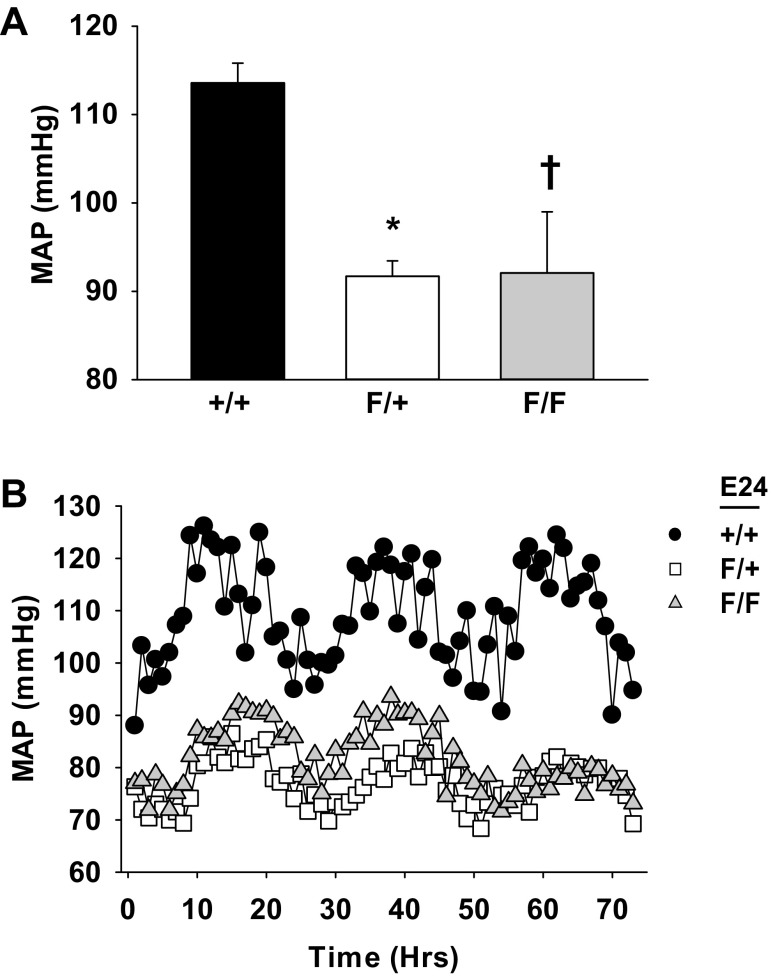
Arterial pressure was measured by telemetry in conscious mice over the course of three consecutive days at age 12 wk. Mice that were heterozygous for deletion of E24 had a ∼15 mmHg decrease in their MAP compared with control mice (Fig. 2A; P < 0.05). There was no further reduction in MAP in mice that were homozygous for deletion of E24. There was no change in the diurnal pattern of BP in these mice (Fig. 2B).
Fig. 2.
Mypt1 E24 cKO lowers systemic blood pressure. Conscious blood pressure was continuously recorded over the course of 3 days 1 wk after the implantation of the telemetry devices as described in materials and methods. Adult male mice were of genotypes as shown; all were Cre+ and treated with Tamoxifen. Mean arterial pressure (MAP) (A) and representative tracings (B) of circadian fluctuations in MAP in a representative mouse from each group over the course of 72 h of recording are shown. All data are expressed as means ± SE; n = 3–5/group. *†P < 0.05 vs. control.
Conditional KO of Mypt1 E24 increases mesenteric arterial sensitivity to NO and cGMP.
To test the role of the Mypt1 LZ in determining arterial function, MA1s from E24 cKO mice were studied ex vivo by wire myography. MA1s from E24 cKO heterozygotes after preconstriction with PE (10 μM) had markedly increased sensitivity of relaxation to the NO donor DEA/NO (Fig. 3A; EC50: CON: 18.2 ± 5.6 μM vs. E24 F/+: 3.3 ± 0.5 nM; P < 0.05). The maximal response was also significantly increased with complete relaxation of E24 cKO MA1s at the highest concentration of DEA/NO. MA1s from E24 cKO homozygotes exhibited the same dose response to DEA/NO as did the heterozygotes (Fig. 3A; EC50: E24 F/F: 2.1 ± 0.5 nM; P < 0.05 vs. control). In MAs that were α-toxin permeabilized and activated with calcium (pCa6; 1 μM), sensitivity of relaxation to the cGMP analog 8-Br-cGMP was markedly increased in MA1s from the Mypt1 E24 cKO heterozygotes (Fig. 3B; EC50: CON: 95.2 ± 11.0 nM; E24 F/+: 3.0 ± 2.5 nM; P < 0.05). The maximal response was also significantly increased with complete relaxation of E24 cKO MA1s at the highest concentration of 8-Br-cGMP. MA1s from E24 cKO homozygotes exhibited the same dose response to 8-Br-cGMP as did the heterozygotes (EC50: E24 F/F: 6.0 ± 1.5 nM; P < 0.05).
Fig. 3.
Mypt1 E24 cKO increases mesenteric arterial relaxation to diethylamine-NONOate (DEA/NO) and 8-Br-cGMP. First-order mesenteric arteries (MA1) were harvested from adult mice with the genotypes as shown (all were Cre+ and treated with Tamoxifen) and mounted on a wire myograph. Force was continuously recorded from intact and α-toxin-permeabilized MA1s. A: MA1s were activated by 10 μM phenylephrine (PE) followed by dose response to the NO donor DEA/NO. B: MA1s were activated with submaximal concentrations of calcium (pCa6; 1 μM) followed by dose response to 8-bromo-cGMP (8-Br-cGMP). Data are presented as percentages of the maximum force generated. All data are expressed as means ± SE; n = 5–6/group. *P < 0.05 control vs. heterozygotes; †P < 0.05 control vs. homozygotes.
Conditional KO of Mypt1 E24 and vasoconstrictor responses.
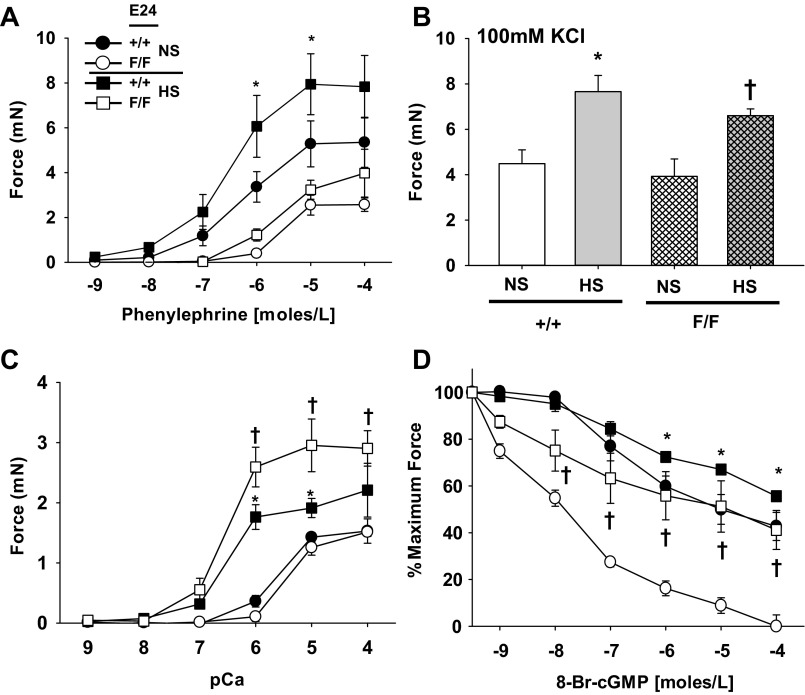
MA1s from E24 cKO heterozygotes had normal force generation to a variety of contractile agonists including the α-adrenergic agonist PE, the thromboxane mimetic U46619, and angiotensin II (Fig. 4, A–C). Similarly, force generation to depolarization induced with 100 mM KCl (Fig. 4D) and to calcium in α-toxin-permeabilized preparations (Fig. 4E) was unchanged. Interestingly, MA1s from E24 cKO homozygotes had markedly reduced force generation that was selective for PE (Fig. 4A), as there was no change in the response to the other contractile agents (Fig. 4, B–E). There was no change in the sensitivity of the MA1 E24 cKO homozygotes to PE (EC50: CON: 0.5 ± 0.1 μM; E24 F/+: 0.6 ± 0.2 μM; E24 F/F: 0.9 ± 0.2 μM). Preincubation with the arginine analog l-NAME (100 μM) to suppress endogenous NO synthesis had no effect on force production to PE in MA1 from wild-type mice (Fig. 5A), increased force to PE in the E24 cKO heterozygotes (Fig. 5B), and increased but did not normalize force to PE in E24 cKO homozygotes (Fig. 5C). This suggests an intrinsic and specific defect in the contractile response to PE in the E24 cKO homozygote mice. This did not appear to be a function of a change in the expression of α1a-adrenergic receptor mRNAs in the MAs, as there was no difference between groups (E24 F/+: 1.1 ± 0.3; E24 F/F: 1.1 ± 0.2; fold change vs. CON; n = 5–6). In MA1s that were α-toxin permeabilized and activated with submaximal calcium (pCa6; 1 μM), there was no difference between the groups in the increment in force with addition of PE (10 μM) and no effect in this assay of suppression of NO synthesis by preincubation with l-NAME (100 μM) (Fig. 4F).
Fig. 4.
Mypt1 E24 cKO reduces MA force generation to the α-adrenergic agonist PE selectively in homozygotes. Force (in mN) was continuously recorded in intact (A–D) and α-toxin-permeabilized (E and F) MA1s from adult mice with the genotypes as shown (all mice were Cre+ and treated with Tamoxifen). Dose response to phenylephrine (A), the thromboxane mimetic U-46619 (B), and angiotensin II (C) is shown. Force generation to 100 mM KCl depolarization (D), calcium (E), submaximal concentration of calcium (pCa6; 1 μM) (F) followed by PE (10 μM) with and without nitro-l-arginine methyl ester (l-NAME) preincubation (100 μM) to suppress synthesis of endogenous NO is shown. All data are expressed as means ± SE; n = 5–6/group. †P < 0.05 control vs. homozygotes; ‡P < 0.05 heterozygotes vs. homozygotes.
Fig. 5.
Suppression of endogenous nitric oxide synthesis does not normalize MA force production to PE in E24 cKO homozygotes. Force (in mN) was continuously recorded in intact MA1s from adult mice with the genotypes as shown (all mice were Cre+ and treated with Tamoxifen). Dose response to PE with and without preincubation with l-NAME (100 μM) in control (A), E24 heterozygote (B), and E24 homozygote (C) mice is shown. All data are expressed as means ± SE; n = 5–6/group. *P < 0.05.
Conditional KO of Mypt1 E24 suppresses increased arterial contractility on a high-salt diet.
To determine how E24 cKO may affect vascular function under conditions of stress, adult mice were fed a high-salt (4% NaCl) diet for 2 wk. High-salt feeding did not change the ratio of Mypt1 E24+/− splice variants (Fig. 6A) or the levels of Mypt1, CPI-17, and MLCK mRNAs (Fig. 6B) in the MAs of control and E24 cKO (F/F) mice. Interestingly, only in the E24 cKO mice on a high-salt diet was smooth muscle myosin heavy chain mRNA significantly increased by approximately fourfold (Fig. 6B).
Fig. 6.
Effect of 2 wk of a high-salt diet (4% NaCl) on mesenteric arterial gene expression. Adult mice of the genotypes as shown (all were Cre+ and treated with Tamoxifen) were fed a normal chow or high-salt (4% NaCl) diet for 2 wk. The mesenteric arterial arcade was isolated (A) and assayed for Mypt1 E24 splice variants as described above (B). Contractile gene mRNAs were assayed by qPCR using TaqMan-based probes and normalized to the invariant cyclophilin A. Data are expressed as fold change. All data are expressed as means ± SE; n = 4–5/group. CPI-17, C-kinase potentiated inhibitory protein 1; MLCK, myosin light chain kinase; smMHC, smooth muscle myosin heavy chain. *†P < 0.05 vs. control.
We next examined the effect of the high-salt diet on MA contractile function. In MA1s from control mice on a high-salt diet for 2 wk, maximum force to the α-adrenergic agonist PE was markedly increased, whereas there was no change in the sensitivity (Fig. 7A; EC50: CON + NS: 0.9 ± 0.4 μM; CON + HS: 1.0 ± 0.5 μM; P > 0.05). As noted above, MA1s from E24 cKO homozygotes had reduced maximal force generation to PE under basal condition and, in contrast to the control mice, had no augmentation in force production after 2 wk of the high-salt diet (Fig. 7A) and no change in the sensitivity (E24 F/F + NS: 0.9 ± 0.2 μM; E24 F/F + HS: 3.0 ± 1.0 μM). The suppressed response of E24 cKO MA1 to PE was again specific. MA1s from both control and E24 cKO mice had increased force generation to KCl depolarization (Fig. 7B) and to calcium (Fig. 7C) after 2 wk of the high-salt diet. In contrast, the high-salt diet reduced vasorelaxant sensitivity of MA1s to 8-Br-cGMP in α-toxin-permeabilized and calcium-activated (pCa6; 1 μM) preparations (Fig. 7D), with the magnitude of the shift greater in the E24 cKO compared with the control mice.
Fig. 7.
Mypt1 E24 cKO specifically suppresses augmentation in MA1 force generation to PE after 2 wk of high-salt diet. MA1s were harvested from adult mice of the genotypes shown (all were Cre+ and treated with Tamoxifen) on normal chow or high-salt diet (4% NaCl) for 2 wk. Force (mN) was continuously recorded from intact (A–C) and α-toxin-permeabilized (D) arteries. A: dose response to the α-adrenergic agonist PE. B: maximal force generated with 100 mM KCl depolarization. C: dose response to calcium. D: force was activated with submaximal concentration of calcium (pCa6; 1 μM) followed by dose response to 8-Br-cGMP. All data are expressed as means ± SE; n = 3–4/group. *P < 0.05 CON: normal chow vs. high salt; †P < 0.05 E24 cKO: normal chow vs. high salt.
DISCUSSION
Mypt1 LZ motif and MP activation.
In this study, we used smooth muscle-specific inducible deletion of Mypt1 E24 (E24 cKO) to test the role of the COOH-terminal LZ motif of Mypt1 in the control of arterial function and blood pressure. E24 cKO increased sensitivity of MA relaxation to the NO donor DEA/NO and to its second messenger cGMP, ex vivo, and lowered BP in vivo, thereby defining the critical role of the Mypt1 COOH-terminal LZ motif in controlling these functions. Yeast two-hybrid and in vitro biochemical studies demonstrated the binding of the NH2-terminal LZ of PKG1α with the COOH terminus of Mypt1 in vitro (7, 13, 15, 37, 41). However, one study suggested that it was the cc domain just upstream of the LZ motif in Mypt1 that mediated this interaction (7), whereas a second study suggested that the cc and LZ motif mediated heterodimerization (37). These studies were limited by the highly reductionist approaches to the study of this complex enzyme, working mostly with peptide fragments and in simplified systems in vitro. The genetically engineered mouse model described here specifically introduced the LZ motif into the COOH terminus of Mypt1 in vivo. We used the classic assay to test MP function in situ–permeabilization of smooth muscle with force activated by calcium clamp (20, 45) to show that the expression of Mypt1 LZ+ isoform (E24 cKO) increased sensitivity to cGMP by 15–30-fold and increased the maximal response from ∼50% to nearly complete relaxation. This is in agreement with a prior study that, by introducing leucine-to-alanine mutations into the LZ motif of PKG1α, showed that the PKG1α LZ is required for its heterodimerization with Mypt1 and for the regulation of BP and vascular tone (24). In contrast in a recent study of MAs from smooth muscle-specific KO of Mypt1 (Exon 1 flox), contractile responses to agonists such as norepinephrine and to the antagonist NO donor sodium nitroprusside were unchanged (32). The homozygous cKO mice did have the predicted increase in systemic BP (∼20 mmHg) and increased force generation of MAs to KCl depolarization. The difference between the effects of Mypt1 isoform switch (E24 cKO) in the present study vs. Mypt1 KO in the prior study is not certain. It should be noted that neither that study nor the prior study of PKG1α LZ mutation (24) examined effects of the introduced mutation on MP activity, as was done in the present study. The absence of the effect of Mypt1 KO on arterial reactivity could reflect redundancy with the highly related Mypt family member p85. p85 mRNA is expressed at levels similar to or greater than Mypt1 in the MAs (33). p85 and the small subunit of MP (M21) each contain the identical COOH-terminal LZ motif (3); the role of these Mypt family members in control of arterial reactivity has not yet been tested or incorporated into the models for the control of MP function.
The present study defines the critical physiological role of the Mypt1 LZ motif in the function of MP in vivo; however, we cannot distinguish between hypotheses that the LZ- isoform is required for binding of vs. activation by PKG1α. In our prior studies of phasic smooth muscle (chicken gizzard, rat PV) that express nearly exclusively the Mypt1 LZ isoform, we showed by coimmunoprecipitation and functional and biochemical studies that PKG1α does not bind Mypt1 and that cGMP does not increase MP activity, causing dephosphorylation of myosin (15, 29). This leads us to favor the hypothesis that the Mypt1 LZ motif is required for PKG1α binding in vivo. Perhaps the absence of the LZ-containing small M21 subunit in in vitro experiments has obscured the effect of relative affinities on subunit interactions. After binding, the mechanism by which cGMP/PKG1α activate MP is uncertain although, given that it is a serine-threonine kinase, it would presumably involve phosphorylation event(s). Studies of tissue homogenates have suggested that PKG1α phosphorylates Mypt1 at S695 and that this does not activate MP but rather prevents Rho kinase-mediated phosphorylation at the adjacent T696 and the resultant inhibition of MP (disinhibition model) (9, 26, 44). Consistent with this proposed model, in the present study, switch to Mypt1 LZ+ isoform (E24 cKO) caused a much larger increase in sensitivity to DEA/NO when the artery was activated by the α-adrenergic agonist compared with the increase in sensitivity to 8-Br-cGMP when permeabilized arteries were activated by calcium alone, although alternative explanations are also possible, including cGMP-independent effects of NO. In contrast, a recent in vitro biochemical study using expressed constructs in a heterologous system suggested that PKG phosphorylation at S668 of Mypt1 was responsible for activation of MP (46). The very small size of the MAs used in the present study confounds biochemical assays such as coimmunoprecipitation to define protein interactions and assays of protein phosphorylation. However, substantial caution is required in using phospho-epitopes as surrogate indicators of kinase and phosphatase activities. Many phosphorylation sites on Mypt1 have been described, as reviewed above; even for one well accepted as a marker, RhoA-associated protein kinase phosphorylation of Mypt1 at T852, T852A mutation in vivo had no effect on bladder smooth muscle force production (1).
Mypt1 E24 dosage and arterial function.
The increase in sensitivity to NO/cGMP-mediated vasorelaxation and reduction in BP was highly sensitive to Mypt1 E24 variants, as deletion of one or both E24 alleles had the same effect on these parameters. There are several points worth noting here. First, the mature mouse MA1 at baseline has an ∼50:50 mix of the Mypt1 E24+/− isoforms, and in functional assays its response to cGMP-mediated calcium desensitization is intermediate between that of 1) the mature PV, which expresses nearly exclusively the Mypt1 E24+/LZ− isoform and shows little response (15, 29); and 2) the aorta, which expresses nearly exclusively the Mypt1 E24−/LZ+ isoform and completely relaxes (15), similar to that of the MA1 from the Mypt1 E24 cKO mouse. Thus it is possible that PV, or rat MA1 in which the E24+/− ratio is 80:20 (30), would show a more graded dose response to E24 cKO. In any event, these results are consistent with prior studies indicating that NO is an important vasodilator in all arteries, but its role as the endothelium-derived relaxing factor diminishes as vessel size diminishes (25, 38). Of course NO-mediated vasodilation is a function of its effect on calcium sensitivity and calcium flux, the latter not evaluated in the calcium clamp experiments and beyond the scope of this study. We establish the 20% Mypt1 E24+ (LZ−) as the lower threshold beyond which no further increase in response to NO/cGMP-mediated vasodilation and lowering of BP will occur. The difference between the response of arteries expressing E24+ vs. E24− variants of Mypt1 thus represents the NO vasodilator reserve that can be recruited for full vasodilator response to NO when needed under pathological conditions (30, 34, 47, 48). This concept of reserve has been applied to other components of the NO signaling pathway, guanylate cyclase activity (23), for example, and is a generalized phenomenon of receptor signaling.
One potentially important difference between heterozygous and homozygous E24 cKO was a specific reduction in the force generated to the α-agonist PE. This was not due to the increased sensitivity to NO/cGMP because it was only partially normalized by the arginine antagonist l-NAME, nor does it reflect a generalized effect because force generated to other agonists (thromboxane analog, angiotensin II), KCl depolarization, and calcium were all normal. In this study, we were not able to identify a specific mechanism for this effect, but it does not appear to be due to an inability of PE to activate MP under these conditions. PE caused a similar degree of calcium sensitization in permeabilized preparations in control and E24 cKO, albeit the magnitude of this effect was rather small in the mouse MA1s. One possible explanation is that the switch to the Mypt1 LZ+ isoform causes a basal locked-in activation of MP that is not reversed by inhibition of NO synthesis with l-NAME, similar to what has been proposed in thromboxane activation of force in rat cerebral arteries (27). Interestingly, in a prior study using a similar smooth muscle-specific cKO strategy, it was shown that cKO of Gαq-Gα11 specifically abolished constrictor response to PE and angiotensin II, whereas cKO of Gα12-Gα13 had more potent effects on ET-1 and thromboxane analog constriction, demonstrating specificity in the coupling of the G protein-coupled receptors (GPCRs) to the contractile machinery (43). Thus it is possible that E24 cKO had a specific effect on the PE signaling pathway downstream of the GPCR, perhaps via cross talk through regulator of G protein signaling 2, which is activated by NO/cGK1α and suppresses α-agonist activation of force (19, 40, 42). However, there are many points of cross talk between these constrictor and dilator signaling pathways, and further studies are required.
Mypt1 E24 cKO: Effect of high-salt diet.
Here we used 2 wk of a high-salt diet as the model to determine how E24 cKO affects arterial function under stressed conditions. MAs from control mice under high salt had increased force generation to all modes of activation, including KCl depolarization, calcium, and the α-adrenergic agonist PE, without a change in sensitivity. This suggests an increase in the force-generating capacity of the myofilaments, without a change in the signaling pathways that activate them. MAs from E24 cKO homozygotes under high salt also had increased force generation to KCl depolarization and calcium, indicating that the ability to augment force in this condition was not affected by E24 cKO. However, force generation to PE in E24 cKO was basally suppressed and did not augment under salt loading in the E24 cKO homozygotes, suggesting that E24 cKO causes a sustained suppression of α-agonist-mediated vasoconstriction. As discussed above, the mechanism for this specific effect on contractility requires further study.
These mice did not have elevations in BP after the 2 wk of salt loading, consistent with prior studies that showed that additional stressors such as angiotensin II are required to raise BP in this Bl6 strain of mice (5). These results have interesting parallels with prior studies of Dahl salt-sensitive rats in which it was shown that high-salt diets (7% NaCl) augmented vasoconstrictor response to norepinephrine and nerve stimulation in the perfused mesenteric preparation. This enhanced response was present in the prehypertensive phase, at 5 days and 3 wk of high-salt feeding, but had normalized by 5–6 wk, at which time hypertension was evident (16, 17). In the present study, the sensitivity of the MAs to cGMP studied ex vivo under calcium clamp was reduced in mice on the high-salt diet for 2 wk. This effect was greater in the E24 cKO compared with the wild-type mice, such that the difference in response to cGMP in mice on a high-salt diet was still significant but significantly narrowed between E24 cKO and WT mice. We do not have an explanation for this change in sensitivity, but it did not appear to be due to a change in Mypt1 E24 isoform expression. PKG1α has many targets in smooth muscle that regulate calcium sensitivity and calcium flux (12), and further studies are required to address this question.
Conclusion.
In conclusion, Mypt1 E24 splice variant isoforms tune arterial reactivity under basal and pathological conditions and thereby control blood pressure. Sensitivity to NO/cGMP-mediated vasorelaxation is highly sensitive to increasing expression of the Mypt1 E24−/LZ+ isoform. Targeting of E24 may thus be a worthy goal for the treatment of the many human conditions in which systemic vascular resistance and blood pressure are increased.
GRANTS
This study was funded by NIH R01-HL066171, 7R37-HL023081, T32-HL072751, and T32-AR007592.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.R. and S.A.F. conception and design of research; J.J.R., D.K., and L.A. performed experiments; J.J.R., D.K., L.A., and S.A.F. analyzed data; J.J.R. and S.A.F. interpreted results of experiments; J.J.R. and D.K. prepared Figs.; J.J.R. and S.A.F. drafted manuscript; J.J.R. and S.A.F. edited and revised manuscript; J.J.R., L.A., and J.J.R., D.K., L.A., and S.A.F. approved final version of manuscript.
REFERENCES
- 1.Chen CP, Chen X, Qiao YN, Wang P, He WQ, Zhang CH, Zhao W, Gao YQ, Chen C, Tao T, Sun J, Wang Y, Gao N, Kamm KE, Stull JT, Zhu MS. In vivo roles for myosin phosphatase targeting subunit-1 phosphorylation sites T694 and T852 in bladder smooth muscle contraction. J Physiol 593: 681–700, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond J. Lack of correlation between cyclic GMP elevation and relaxation of nonvascular smooth muscle by nitroglycerin, nitroprusside, hydroxylamine and sodium azide. J Pharmacol Exp Ther 225: 422–426, 1983. [PubMed] [Google Scholar]
- 3.Dippold RP, Fisher SA. A bioinformatic and computational study of myosin phosphatase subunit diversity. Am J Physiol Regul Integr Comp Physiol 307: R256–R270, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dippold RP, Fisher SA. Myosin phosphatase isoforms as determinants of smooth muscle contractile function and calcium sensitivity of force production. Microcirculation 21: 239–248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol 297: R1660–R1669, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feletou M, Hoeffner U, Vanhoutte PM. Endothelium-dependent relaxing factors do not affect the smooth muscle of portal vein. Blood Vessels 26: 21–32, 1989. [PubMed] [Google Scholar]
- 7.Given AM, Ogut O, Brozovich FV. MYPT1 mutants demonstrate the importance of aa 888–928 for the interaction with PKGIα. Am J Physiol Cell Physiol 292: C432–C439, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Grassie ME, Moffat LD, Walsh MP, MacDonald JA. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys 510: 147–159, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Grassie ME, Sutherland C, Ulke-Lemée A, Chappellaz M, Kiss E, Walsh MP, MacDonald JA. Cross-talk between Rho-associated kinase and cyclic nucleotide-dependent kinase signaling pathways in the regulation of smooth muscle myosin light chain phosphatase. J Biol Chem 287: 36356–36369, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han YS, Brozovich FV. Altered reactivity of tertiary mesenteric arteries following acute myocardial ischemia. J Vasc Res 50: 100–108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartshorne DJ, Ito M, Erdodi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem 279: 37211–37214, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Huang QQ, Fisher SA, Brozovich FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem 279: 597–603, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Karim SM, Rhee AY, Given AM, Faulx MD, Hoit BD, Brozovich FV. Vascular reactivity in heart failure: role of myosin light chain phosphatase. Circ Res 95: 612–618, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem 276: 37250–37257, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kong JQ, Taylor DA, Fleming WW. Mesenteric vascular responses of young spontaneously hypertensive rats. J Pharmacol Exp Ther 258: 13–17, 1991. [PubMed] [Google Scholar]
- 17.Kong JQ, Taylor DA, Fleming WW. Sustained hypertension in Dahl rats. Negative correlation of agonist response to blood pressure. Hypertension 25: 139–145, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Konik EA, Han YS, Brozovich FV. The role of pulmonary vascular contractile protein expression in pulmonary arterial hypertension. J Mol Cell Cardiol 65: 147–155, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Krzyzanowski MC, Brueggemann C, Ezak MJ, Wood JF, Michaels KL, Jackson CA, Juang BT, Collins KD, Yu MC, L'Etoile ND, Ferkey DM. The C elegans cGMP-dependent protein kinase EGL-4 regulates nociceptive behavioral sensitivity. PLoS Genet 9: 11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca 2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem 272: 5063–5068, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Zhang H, Gokina N, Mandala M, Sato O, Ikebe M, Osol G, Fisher SA. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat l-NAME model of hypertension of pregnancy. Am J Physiol Cell Physiol 294: C564–C571, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Ma H, He Q, Dou D, Zheng X, Ying L, Wu Y, Raj JU, Gao Y. Increased degradation of Mypt1 contributes to the development of tolerance to nitric oxide in porcine pulmonary artery. Am J Physiol Lung Cell Mol Physiol 299: L117–L123, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest 116: 1731–1737, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci USA 105: 6702–6707, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagao T, Illiano S, Vanhoutte PM. Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-l-arginine in rats. Am J Physiol Heart Circ Physiol 263: H1090–H1094, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res 101: 712–722, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Neppl RL, Lubomirov LT, Momotani K, Pfitzer G, Eto M, Somlyo AV. Thromboxane A2-induced bi-directional regulation of cerebral arterial tone. J Biol Chem 284: 6348–6360, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pannen BH, Bauer M. Differential regulation of hepatic arterial and portal venous vascular resistance by nitric oxide and carbon monoxide in rats. Life Sci 62: 2025–2033, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Payne MC, Zhang HY, Prosdocimo T, Joyce KM, Koga Y, Ikebe M, Fisher SA. Myosin phosphatase isoform switching in vascular smooth muscle development. J Mol Cell Cardiol 40: 274–282, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Payne MC, Zhang HY, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. Am J Physiol Heart Circ Physiol 286: H1801–H1810, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Pfitzer G, Merkel L, Ruegg JC, Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflügers Arch 407: 87–91, 1986. [DOI] [PubMed] [Google Scholar]
- 32.Qiao YN, He WQ, Chen CP, Zhang CH, Zhao W, Wang P, Zhang L, Wu YZ, Yang X, Peng YJ, Gao JM, Kamm KE, Stull JT, Zhu MS. Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J Biol Chem 289: 22512–22523, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reho JJ, Shetty A, Dippold RP, Mahurkar A, Fisher SA. Unique gene program of rat small resistance mesenteric arteries as revealed by deep RNA sequencing. Physiol Rep 3: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reho JJ, Zheng X, Asico LD, Fisher SA. Redox signaling and splicing dependent change in myosin phosphatase underlie early versus late changes in NO vasodilator reserve in a mouse LPS model of sepsis. Am J Physiol Heart Circ Physiol 308: H1039–H1050, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reho JJ, Zheng X, Benjamin JE, Fisher SA. Neural programming of mesenteric and renal arteries. Am J Physiol Heart Circ Physiol 307: H563–H573, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reho JJ, Zheng X, Fisher SA. Smooth muscle contractile diversity in the control of regional circulations. Am J Physiol Heart Circ Physiol 306: H163–H172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma AK, Zhou GP, Kupferman J, Surks HK, Christensen EN, Chou JJ, Mendelsohn ME, Rigby AC. Probing the interaction between the coiled coil leucine zipper of cGMP-dependent protein kinase Iα and the C terminus of the myosin binding subunit of the myosin light chain phosphatase. J Biol Chem 283: 32860–32869, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Shukla S, Fisher SA. Tra2beta as a novel mediator of vascular smooth muscle diversification. Circ Res 103: 485–492, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X, Kaltenbronn KM, Steinberg TH, Blumer KJ. RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol Pharmacol 67: 631–639, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 286: 1583–1587, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, Mendelsohn ME. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med 9: 1506–1512, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind S, Offermanns S. G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TAJ. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of serine 695 in response to cyclic nucleotides. J Biol Chem 279: 34496–34504, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Somlyo AV, Somlyo AP. c-GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphatase. Biochem Biophys Res Commun 220: 658–663, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Yuen SL, Ogut O, Brozovich FV. Differential phosphorylation of LZ+/LZ- MYPT1 isoforms regulates MLC phosphatase activity. Arch Biochem Biophys 562: 37–42, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res 100: 730–737, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Pakeerappa P, Lee HJ, Fisher SA. Induction of PDE5 and de-sensitization to endogenous NO signaling in a systemic resistance artery under altered blood flow. J Mol Cell Cardiol 47: 57–65, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng X, Reho JJ, Wirth B, Fisher SA. TRA2β controls Mypt1 exon 24 splicing in the developmental maturation of mouse mesenteric artery smooth muscle. Am J Physiol Cell Physiol 308: C289–C296, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]