The pathogenesis of diabetic cardiomyopathy is unclear, but an important clue is that it is strongly linked to hyperglycemia. Here, we provide novel evidence that thioredoxin-interacting protein is responsible for impaired cardiac inotropic reserve by hyperglycemia through regulation of glucose transport in the development of diabetic cardiomyopathy.
Keywords: isoproterenol, α-arrestins, GLUT1
Abstract
Although the precise pathogenesis of diabetic cardiac damage remains unclear, potential mechanisms include increased oxidative stress, autonomic nervous dysfunction, and altered cardiac metabolism. Thioredoxin-interacting protein (Txnip) was initially identified as an inhibitor of the antioxidant thioredoxin but is now recognized as a member of the arrestin superfamily of adaptor proteins that classically regulate G protein-coupled receptor signaling. Here we show that Txnip plays a key role in diabetic cardiomyopathy. High glucose levels induced Txnip expression in rat cardiomyocytes in vitro and in the myocardium of streptozotocin-induced diabetic mice in vivo. While hyperglycemia did not induce cardiac dysfunction at baseline, β-adrenergic challenge revealed a blunted myocardial inotropic response in diabetic animals (24-wk-old male and female C57BL/6;129Sv mice). Interestingly, diabetic mice with cardiomyocyte-specific deletion of Txnip retained a greater cardiac response to β-adrenergic stimulation than wild-type mice. This benefit in Txnip-knockout hearts was not related to the level of thioredoxin activity or oxidative stress. Unlike the β-arrestins, Txnip did not interact with β-adrenergic receptors to desensitize downstream signaling. However, our proteomic and functional analyses demonstrated that Txnip inhibits glucose transport through direct binding to glucose transporter 1 (GLUT1). An ex vivo analysis of perfused hearts further demonstrated that the enhanced functional reserve afforded by deletion of Txnip was associated with myocardial glucose utilization during β-adrenergic stimulation. These data provide novel evidence that hyperglycemia-induced Txnip is responsible for impaired cardiac inotropic reserve by direct regulation of insulin-independent glucose uptake through GLUT1 and plays a role in the development of diabetic cardiomyopathy.
NEW & NOTEWORTHY
The pathogenesis of diabetic cardiomyopathy is unclear, but an important clue is that it is strongly linked to hyperglycemia. Here, we provide novel evidence that thioredoxin-interacting protein is responsible for impaired cardiac inotropic reserve by hyperglycemia through regulation of glucose transport in the development of diabetic cardiomyopathy.
diabetic cardiomyopathy has been defined as “diabetes-associated ventricular dysfunction that occurs independently of coronary artery disease and hypertension” and is supported by extensive clinical data (6). The pathogenesis of diabetic cardiomyopathy is unclear, but an important clue is that it is strongly linked to hyperglycemia (51). Although a specific pathway connecting hyperglycemia to downstream pathophysiological events has remained elusive, potential mechanisms include increased oxidative stress (3, 19), autonomic nervous dysfunction (5), and altered substrate metabolism (3). A predominant change in diabetes is a suppression of cardiac glucose utilization (3), which is tightly regulated by a protein family of glucose transporters (GLUTs). The well-established GLUT isoforms in the heart are GLUT1 and GLUT4, which are known to have distinct regulatory properties. GLUT1 is an insulin-independent glucose transporter responsible for the basal glucose uptake required to sustain energy production, whereas GLUT4 is the rate-limiting transporter regulated by insulin (1). Understanding how cardiomyocytes adapt to changes of extracellular and intracellular glucose concentrations is of critical importance.
Thioredoxin-interacting protein (Txnip) is recognized for important roles in metabolism and redox regulation (39), but its in vivo mechanisms remain unknown. Txnip was initially identified as a protein that stably binds to thioredoxin (42). Thioredoxin is an oxidoreductase that acts as an antioxidant by facilitating the reduction of other proteins via cysteine thiol-disulfide exchange. Txnip binds to the reduced form of thioredoxin, thus suggesting originally that it could inhibit thioredoxin's antioxidant function to modulate redox status and reactive oxygen species (ROS)-mediated signaling (39).
However, Txnip is now known to be part of the arrestin superfamily of proteins (2). β-Arrestins are well known as important regulators of receptor signaling, including β-adrenergic signaling. In mammals, a related class of proteins called α-arrestins includes Txnip and five other α-arrestin proteins (2), which share the arrestin fold and have predicted structural similarities with β-arrestins. Ancestral α-arrestins can coordinate receptor endocytosis, ubiquitination, and downstream signaling, similar to the functions of β-arrestins (28). Nevertheless, how the arrestin fold contributes to the functions of Txnip and the other mammalian α-arrestins remains unknown.
Importantly, Txnip is a highly glucose-responsive gene via a carbohydrate response element in the promoter of Txnip (38) and one of the most dramatically upregulated genes in response to glucose (57). Hyperglycemia-induced Txnip plays a critical role in diabetes etiology (44, 60) and its complications such as nephropathy (56), retinopathy (50, 59), neuropathy (52), and vasculopathy (17, 55). Glucose-induced Txnip contributes to glucotoxicity, as observed in pancreatic β-cells (12), endothelial cells (17, 49), and cardiomyocytes (22, 61). These data suggest that Txnip may serve as a key mechanism in regulating diverse cellular signaling events in diabetic organs. Despite these strong links between Txnip and glucose metabolism, the precise role of myocardial Txnip in the functional adaptation to diabetic stress remains unknown. Therefore, we investigated whether Txnip contributes to the development of hyperglycemia-induced cardiac disorders, using mice with cardiac-selective deletion of Txnip, and discovered a mechanistic link between myocardial glucose metabolism and functional reserve in diabetic cardiomyopathy.
MATERIALS AND METHODS
Diabetic mouse model.
To generate an insulin-deficient diabetic mouse model, 8-wk-old animals were treated with streptozotocin (STZ) according to the low-dose STZ induction protocol (50 mg/kg ip for 5 days) of the Animal Models of Diabetic Complications Consortium. Animals with intraperitoneal injection of 0.01 M Na-citrate (vehicle lacking STZ) served as controls. In each mouse, whole blood was obtained from the tail vein to confirm the increased levels of blood glucose with a glucometer (Bayer). Four weeks after STZ injections, temporally inducible cardiomyocyte-specific Txnip deletion was achieved in αMHC-MerCreMer/Txnipflox/flox mice (C57BL/6;129Sv strain) injected with 0.5 mg of 4-hydroxytamoxifen per day for 2 wk as described previously (68). Age- and sex-matched littermates of αMHC-MerCreMer/Txnipflox/flox mice treated with vehicle lacking 4-hydroxytamoxifen served as controls; we verified that there was no change in Txnip expression in these mice compared with wild-type (WT) mice (68). Mice were maintained in accordance with the Institutional Animal Care and Use Committees of Harvard Medical School. The protocol was approved by the Harvard Medical Area Standing Committee on Animals.
Cell culture.
The embryonic rat cardiac H9c2 (CRL-1446), mouse fibroblast L929 (CCL-1), and human embryonic kidney HEK-293 (CRL-1573) and HEK-293T (CRL-3216) cell lines were obtained from American Type Culture Collection (Manassas, VA). Mouse embryonic fibroblasts (MEFs) were prepared from a systemic Txnip-null mouse and its littermate control WT mouse (14). For glucose uptake and expression analyses, cells were treated with the indicated concentrations of d-glucose and/or d-mannitol (Sigma-Aldrich, St. Louis, MO) in Dulbecco's modified Eagle's medium (DMEM).
Gene and protein expression analyses.
Gene expression of Txnip was analyzed by real-time polymerase chain reaction (PCR) with specific oligonucleotides for the mouse txnip gene (69). Protein expression of Txnip was analyzed by Western blot analysis using the anti-Txnip antibody JY2 (available from MBL International, Woburn, MA) (69). For signaling assays, Western blot analyses were performed with phospho (Thr202/Tyr204)- and total ERK1/2 antibodies (no. 9101 and no. 9102, Cell Signaling Technology, Danvers, MA) and phospho(pSer16)- and total phospholamban antibodies (A010-14 and A010-12, Badrilla, Leeds, UK) in whole heart homogenates. GLUT1 and GLUT4 protein expressions were analyzed in cellular membrane fractions. To extract the cellular membrane fraction, whole hearts were homogenized with a silicon homogenizer at medium speed in buffer containing 50 mM HEPES, 50 mM sodium pyrophosphate, 5 mg/ml NaF, 10 mM EDTA (10 mM), 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1% Triton X, 10 mM Na3VO4, and 2 mM PMSF (pH 7.4). Samples were then centrifuged at 4°C for 15 min at 9,000 rpm. The supernatant was removed without disturbing the pellet and ultracentrifuged for 1 h at 4°C and 47,000 rpm. With a syringe and needle, the protein layer (internatant) was removed and electrophoresed on an SDS-PAGE gel. Immunostaining was carried out with anti-GLUT4 (AB1346, Chemicon) or anti-GLUT1 (AB1340, Chemicon) antibodies. Signals were quantified by densitometry of scanned autoradiographs by Scion Image 4.02 (Scion, Frederick, MD).
In vivo assessments of left ventricular performance.
Left ventricular function was followed by serial echocardiographic measurements and by invasive hemodynamic assessment via catheterization at the time of death (30). Echocardiographic acquisition was performed without anesthesia with a Sonos 4500 (Philips) and a 15-MHz transducer. Hemodynamic parameters were acquired under inhalational anesthesia with isoflurane with a Millar pressure catheter (Millar Instruments, Houston, TX), which was advanced retrograde through the aortic valve into the left ventricle (LV) and positioned to obtain LV pressure. Isoproterenol (1.6 ng/g BW) was given to a subset of animals to generate β-adrenergic stimulation by intravenous bolus injection via the right jugular vein cannulated with PE-10 tubing.
Histopathological examination.
The LVs were paraffin embedded, sectioned at 10 μm, and stained with PicroSirius Red to evaluate collagen deposition and myocyte cross-sectional area as described previously (30). To analyze capillary density in the myocardium, endothelial cells were detected by staining sections with biotinylated GSL-I (100 μg/ml; Vector Laboratories, Burlingame, CA) (29). GSL-I staining was quantified manually by counting the number of vessels per field. Three to five random fields were scanned and quantified for each section.
GLUT1 protein expression was analyzed by confocal microscopy (Olympus Fluoview 1500). After fixation with 4% paraformaldehyde, cells or heart tissues were stained with GLUT1 (Santa Cruz Biotechnology) with anti-rabbit secondary antibody conjugated to Alexa Fluor 594 and were costained with DAPI (Thermo Fisher Scientific). Positive staining of GLUT1 on the cell surface was quantified by image processing software (ImageJ).
Thioredoxin activity and ROS.
Thioredoxin reducing activity was measured with an insulin disulfide reduction assay in whole heart homogenates (47). To evaluate oxidative damages by ROS, tissue levels of lipid peroxide (malondialdehyde) were estimated in whole heart homogenates (68).
Txnip pull-down assay.
The coding sequences of human Txnip and other arrestins were subcloned into pCDH-CMV-MCS-EF1-GFP-T2A-Puro (System Biosciences, Mountain View, CA) with a dual Strep/FLAG (SF) tag (18). Human GLUT1 (SLC2A1) and β1-adrenergic (ADRB1) receptors were subcloned from commercially available cDNA plasmids (Open Biosystems, Huntsville, AL) into the above mentioned vector with a hemagglutinin (HA) tag and cotransfected with Txnip or empty control vector into HEK-293T cells with PureFection transfection reagent (System Biosciences). Cells were lysed in 0.5% Triton X-100, 500 mM NaCl, 50 mM Tris, 1 mM PMSF, and protease inhibitors (Sigma-Aldrich), pH 7.8. Txnip pulldown was performed with magnetic Strep-Tactin beads (IBA, Göttingen, Germany) according to the manufacturer's instructions. Western blot analyses of pulled down proteins were performed with anti-FLAG (Sigma-Aldrich) and anti-HA.11 (16B12, Covance, Princeton, NJ) antibodies.
β-Adrenergic receptor density and internalization assays.
β-Adrenergic receptor density was measured by the methods of Maisel et al. (35) with modifications. Sarcolemmal membrane and light vesicle fractions were prepared from the LV. The total β-adrenergic receptor density was determined as the amount of bound radioligand [3H]CGP-12177 (PerkinElmer, Waltham, MA). Filtered radioactivity was counted in a liquid scintillation counter (Beckman), and data were analyzed with a GraphPad Radioactivity Calculator (La Jolla, CA).
β-Adrenergic receptor internalization was evaluated as described previously (34) with modifications. Briefly, HEK-293 cells were plated in complete DMEM and transiently transfected with β1-adrenergic receptor and β-arrestin 1 (as a positive control), Txnip, or empty vector (as a negative control). Cells were then incubated in 125I-cyanopindolol (PerkinElmer) at 37°C for 5 min. Incubations were stopped by placing the cells on ice and rapidly washing twice with ice-cold PBS. The cells were kept on ice for 10 min in acid wash solution (150 mM NaC1 and 50 mM acetic acid) to remove the surface-bound radioligand. The supernatant containing the acid-released radioactivity was collected, and the cells were treated with 0.5 M NaOH and 0.05% SDS to solubilize the acid-resistant (internalized) radioactivity. Radioactivity was measured, and the percent internalization at each point was calculated from the ratio of the acid-resistant binding to the total binding.
Glucose uptake measurements.
Arrestin-mCherry fusion constructs were overexpressed in HEK-293 cells with a lentiviral expression system (System Biosciences) as described previously (47). After 2–3 days, expression was verified by red epifluorescence. Four hours prior to labeling, medium was changed to 5.5 mM glucose to reduce endogenous Txnip expression. Cells were then incubated with 100 μM 2-deoxyglucose and 125 μM 2-[3H]deoxyglucose (1 μCi/ml, PerkinElmer Life Sciences) for 30 min. Cells were lysed in 0.2 N NaOH and then neutralized with 6 N HCl.
Zero-trans sugar uptake was measured in MEFs and L929 cells as described previously (15). Briefly, cells were placed in serum-free DMEM for 2 h at 37°C and then placed on ice in glucose-free medium to deplete intracellular sugar levels. Cells were treated with increasing concentrations of 3-O-[3H]methylglucose (PerkinElmer). Uptake proceeded for 180 s before cells were lysed. Samples were counted by liquid scintillation spectrometry.
Isolated, perfused heart experiments.
Ex vivo cardiac function was assessed in isolated mouse hearts perfused in the Langendorff mode. The heart was excised and perfused with a constant pressure of 80 mmHg (33). A water-filled balloon was inserted into the LV to record isovolumic ventricular function with a data-acquisition system (PowerLab, AD Instruments, Colorado Springs, CO). The KH buffer perfusate contained the following (in mM): 118 NaCl, 25 NaHCO3, 5.3 KCl, 2 CaCl2, 1.2 MgSO4, 0.5 EDTA, and 10 glucose, equilibrated with 95% O2-5% CO2 (pH 7.4). After an equilibration period, the heart was subjected to isoproterenol (0.05 μM) with KH buffer containing glucose as the sole oxidative energy substrate for 30 min. The heart was then perfused with a solution containing 100 μM iodoacetate (IAA) (Sigma-Aldrich) for another 30 min. Temperature was maintained at 37.5°C throughout the protocol.
Statistical analysis.
All data are presented as means ± SE. Statistical analysis was performed with the unpaired t-test between the groups, one-way ANOVA with the Dunnett's test or two-way ANOVA with a post hoc test of Fisher's least significant difference among the groups. Statistical significance was achieved at a value of P < 0.05.
RESULTS
Myocardial Txnip expression is upregulated by glucose in vitro and with diabetes in vivo.
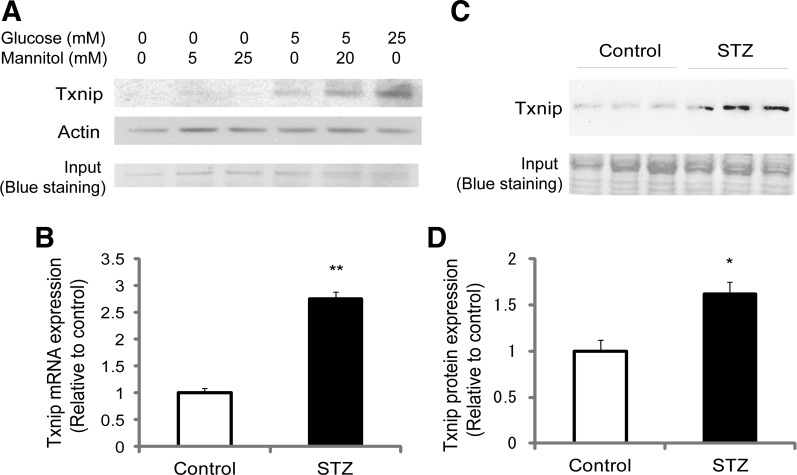
We and others have previously found that Txnip's expression levels are governed by extracellular glucose levels in an insulin-independent fashion (44, 55). Thus we first checked that cardiomyocytes incubated with increasing concentrations of glucose show a concentration-dependent upregulation of Txnip as reported in other cell types (44, 55). In the absence of glucose, H9c2 cardiomyocytes expressed very little endogenous Txnip protein (Fig. 1A). However, incubation with glucose markedly induced expression of Txnip in cardiomyocytes upon stimulation with both 5 mM glucose and 25 mM glucose for 24 h. Identical concentrations of mannitol in cultures did not induce expression of Txnip in a similar fashion, excluding a possible hyperosmolaric effect of glucose on this induction. We next showed that in diabetic WT mice after STZ treatments (blood glucose level 392 ± 65 mg/dl vs. 124 ± 18 mg/dl in control; n = 4, P < 0.01) myocardial gene and protein expressions of Txnip increased in vivo (Fig. 1, B–D). These results led us to hypothesize that hyperglycemia-induced Txnip contributes to the development of diabetic cardiomyopathy.
Fig. 1.
Txnip expression is upregulated by glucose in cardiomyocytes. A: rat cardiac H9c2 cells were treated with the indicated concentrations of glucose and/or mannitol in DMEM after a starvation period. Western blot analysis was performed with the anti-Txnip antibody JY2. Actin and Coomassie blue staining serve as loading controls in the gel. Higher extracellular glucose levels significantly increase intracellular protein expression of Txnip. B–D: gene (B) and protein (C and D) expressions of Txnip were analyzed in cardiac tissues harvested from streptozotocin (STZ)-induced diabetic or nondiabetic wild-type (Control) mice. Gene expression was analyzed by real-time PCR. Hyperglycemia increased mRNA and protein expressions of Txnip in whole heart homogenates. Values are means ± SE. *P < 0.05, **P < 0.01 vs. control. n = 3 or 4 each.
The conditional and inducible approach is applied to test the effects of myocardial Txnip in the diabetic mouse model.
To determine whether Txnip contributes to diabetic damages in the heart, cardiomyocyte-specific Txnip deletion was induced by 4-hydroxytamoxifen treatments in STZ-induced diabetic mice. In control animals treated with vehicle only, there were no significant differences in blood glucose levels between Txnip-KO mice (105 ± 5 mg/dl) and their littermate control mice (81 ± 6 mg/dl) at 8 wk after the injections. STZ injections increased blood glucose levels to the same degree (P < 0.01 vs. vehicle) in both Txnip-KO (393 ± 14 mg/dl) and WT (430 ± 12 mg/dl) mice [P = not significant (N.S.) between the genotypes]. Thus the cardiomyocyte-specific Txnip-KO model was able to separate systemic effects of Txnip on blood glucose levels (14).
Of 14 Txnip-KO and 14 WT control mice that were treated with STZ, 4 Txnip-KO (at 8 wk) and 5 WT (between 8 and 16 wk) mice died during the protocol. Gross postmortem analyses showed no significant signs of heart failure such as pulmonary edema, visceral congestion, or cardiomegaly. Of five Txnip-KO and four WT mice that were injected with vehicle lacking STZ, one Txnip-KO mouse and one WT mouse died of natural causes (e.g., fighting) during the protocol. These rates were not statistically different by Fisher's exact test (P = N.S.).
Insulin-deficient diabetes does not induce cardiac hypertrophy, fibrosis, and basal dysfunction throughout 16 wk after STZ injections in mice.
To assess cardiac dysfunction in these diabetic animals, following multiple previous reports (10, 63), echocardiographic parameters were measured at baseline and 8, 12, and 16 wk after STZ injections. There were no differences between the genotypes in left ventricular dimensions or wall thickness (Table 1). Left ventricular mass and fractional shortening were not significantly changed after STZ injections in both genotypes (Fig. 2, A, B, and D). All groups treated with either vehicle or STZ in Txnip-KO and wild-type mice had similar trends in left ventricular wall thickness, dimensions, and fractional shortening throughout the protocol. Invasive hemodynamic analysis at the time of death also showed no significant changes in left ventricular developed pressure (dP/dt) between diabetic and nondiabetic mice at 16 wk after STZ injections in both Txnip-KO and WT mice (Table 1).
Table 1.
Echocardiographic and hemodynamic analyses of cardiac function at baseline and at 16 wk after STZ treatment
| Baseline |
16 wk |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | STZ | KO | KO/STZ | Control | STZ | KO | KO/STZ | |
| Echocardiography, n | 4 | 14 | 5 | 14 | 3 | 9 | 4 | 10 |
| Heart rate, beats/min | 653 ± 31 | 639 ± 9† | 708 ± 12* | 662 ± 12 | 660 ± 1 | 613 ± 28 | 690 ± 17* | 588 ± 23 |
| Anterior wall thickness, mm | 1.0 ± 0.2 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| Posterior wall thickness, mm | 0.9 ± 0.2 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 |
| End-diastolic dimensions, mm | 2.4 ± 0.2 | 2.5 ± 0.2 | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.8 ± 0.4 | 3.0 ± 0.4 | 2.9 ± 0.4 | 3.0 ± 0.4 |
| End-systolic dimensions, mm | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.1 | 1.1 ± 0.3 | 1.3 ± 0.4 |
| Fractional shortening, % | 69 ± 4 | 66 ± 2 | 74 ± 1* | 60 ± 4 | 62 ± 7 | 55 ± 5 | 64 ± 4 | 58 ± 3 |
| Left ventricular mass, g | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 |
| Hemodynamics | ||||||||
| Heart rate, beats/min | N/A | N/A | N/A | N/A | 515 ± 18 | 510 ± 19 | 525 ± 19 | 480 ± 37 |
| End-systolic pressure, mmHg | N/A | N/A | N/A | N/A | 84 ± 8 | 81 ± 5 | 80 ± 10 | 74 ± 5 |
| End-diastolic pressure, mmHg | N/A | N/A | N/A | N/A | 3.5 ± 0.9 | 2.3 ± 0.3 | 3.6 ± 0.8 | 2.7 ± 0.6 |
| dP/dt maximum, mmHg/s | N/A | N/A | N/A | N/A | 6,625 ± 975 | 6,605 ± 933 | 4,890 ± 730 | 5,349 ± 561 |
| dP/dt minimum, mmHg/s | N/A | N/A | N/A | N/A | 6,008 ± 732 | 5,766 ± 683 | 5,322 ± 758 | 5,484 ± 885 |
Values are means ± SE. STZ, streptozotocin; KO, Txnip-knockout mice; N/A, not applicable.
P < 0.05 vs. KO/STZ;
P < 0.01 vs. KO.
Fig. 2.
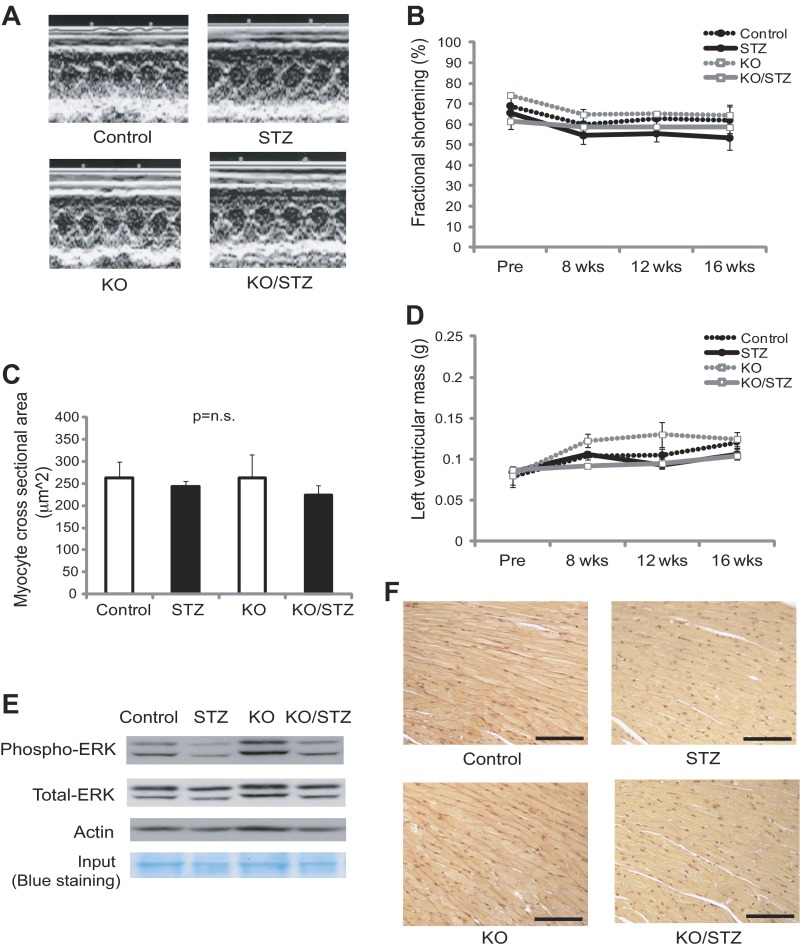
Streptozotocin (STZ)-induced diabetes does not induce cardiac hypertrophy, fibrosis, and dysfunction at 16 wk after STZ injections in cardiomyocyte-specific Txnip-knockout (KO) and wild-type (Control) mice. A, B, and D: echocardiographic analysis was performed without anesthesia at baseline and 8, 12, and 16 wk after STZ injections. Representative M-mode echocardiograms at 16 wk after STZ injections are shown. Left ventricular mass and fractional shortening were comparable among 4 groups of wild-type mice without (Control) or with (STZ) diabetes and Txnip-KO mice without (KO) or with (KO/STZ) diabetes. C: cardiac hypertrophy was assessed by histological myocyte cross-sectional area. E: activation of ERK1/2, one of the signal transduction pathways associated with cardiac hypertrophy, was assessed by Western blot analysis. There was no difference in the ERK1/2 phosphorylation state between wild-type and Txnip-KO hearts after STZ injections. F: diabetes did not induce interstitial collagen depositions in the myocardium from both control and Txnip-KO mice as assessed by PicroSirius Red staining. Scale bars, 50 μm. Values are means ± SE. P = N.S. among 4 groups.
Previous studies have suggested that pathological changes of diabetic cardiomyopathy include compensatory cardiomyocyte hypertrophy and interstitial fibrosis (7). In this study, however, prolonged hyperglycemia did not induce cardiac hypertrophy, as measured by myocyte cross-sectional area (Fig. 2C) or heart weight normalized by tibial length in Txnip-KO (KO/STZ 517 ± 36 g/cm vs. KO 596 ± 24 g/cm, P = N.S.) and WT (STZ 498 ± 38 g/cm vs. control 495 ± 39 g/cm, P = N.S.) mice. The activation of ERK1/2, one of the signal transduction pathways associated with cardiac hypertrophy, was also comparable between WT and Txnip-KO hearts under diabetic conditions (Fig. 2E). Diabetic stress also did not result in interstitial fibrosis in the myocardium from both Txnip-KO and wild-type mice (Fig. 2F).
β-Adrenergic challenge reveals that Txnip-KO hearts manifest greater functional response to β-adrenergic stimulation under diabetic conditions.
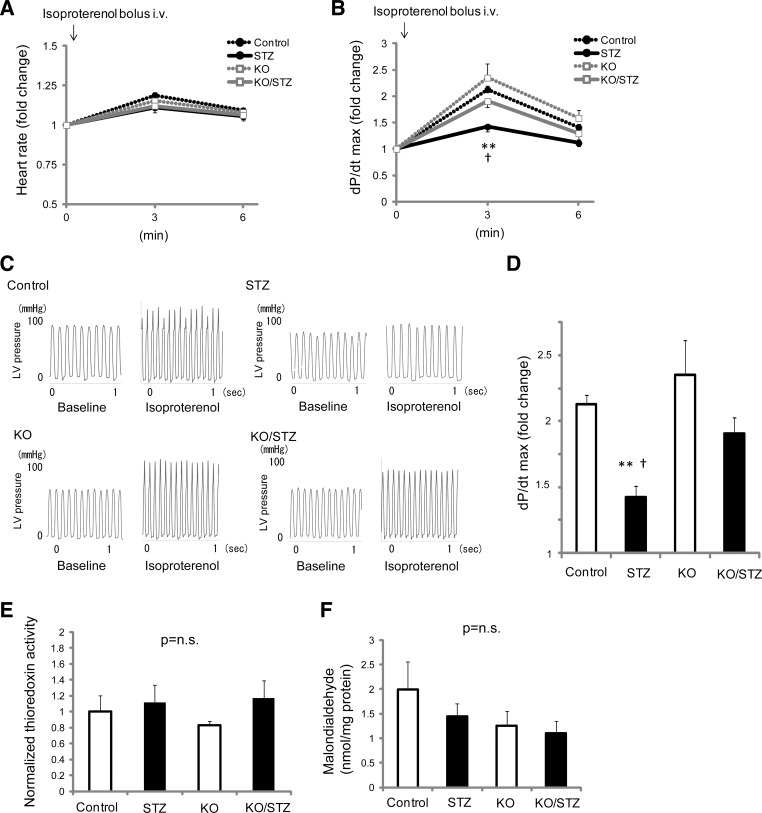
Resting echocardiographic and hemodynamic analyses showed no significant differences in basal LV functional parameters between diabetic and nondiabetic conditions even in WT mice. Since these techniques may not be sensitive enough to reveal early changes caused by diabetic stress in the myocardium, we next performed an in vivo stress test to evaluate contractile provocation and inotropic reserve. β-Adrenergic challenge by isoproterenol increased heart rate at 3 min after bolus intravenous injection through the jugular vein in all groups: WT nondiabetic control (119 ± 9%, n = 4), WT STZ-induced diabetic (111 ± 5%, n = 9), Txnip-KO nondiabetic (115 ± 8%, n = 5), and Txnip-KO STZ-induced diabetic (112 ± 6%, n = 4) mice (Fig. 3A). Cardiac inotropic response, as measured by dP/dt max, was also increased by β-adrenergic stimulation in all groups (Fig. 3, B and C). However, in diabetic mice, the inotropic response was significantly lower than that of control WT mice during β-adrenergic stimulation. Interestingly, in diabetic Txnip-KO hearts, the functional response to β-adrenergic stimulation was preserved compared with diabetic WT hearts (Fig. 3, B and D). Thus we identified that, in contrast to baseline conditions, stress induced by β-adrenergic challenge uncovered masked mechanical dysfunction in the STZ-induced diabetic mouse model. These results demonstrated that Txnip promotes hyperglycemia-induced impairment of myocardial functional reserve in vivo.
Fig. 3.
Stress in vivo hemodynamic analysis uncovers the evidence of masked mechanical dysfunction by hyperglycemia and that Txnip-KO hearts have a greater inotropic reserve under diabetic conditions. Hemodynamic parameters were acquired under anesthesia with a catheter positioned in the left ventricle (LV). Isoproterenol (1.6 ng/g BW) was given to animals by intravenous bolus injection via the right jugular vein. A: β-adrenergic challenge minimally increased heart rate at 3 min after the injection in wild-type nondiabetic (Control), streptozotocin-induced diabetic (STZ), Txnip-KO nondiabetic (KO), and Txnip-KO diabetic (KO/STZ) mice. B: the maximal rate of rise of LV pressure (dP/dt max) was increased by isoproterenol injection. C and D: representative traces of LV pressure and comparisons of dP/dt max among 4 groups at 3 min, respectively. Data are expressed as means ± SE. n = 4–9. **P < 0.01 between Control and STZ; †P < 0.05 between STZ and KO/STZ. E and F: despite these mechanical functional differences, neither thioredoxin activities (E) nor tissue levels of malondialdehyde (F), both indicators of oxidative stress, were different in whole heart homogenates among the groups. P = N.S. n = 4 or 5 each.
The diabetic heart is sometimes characterized by a severe decrease in capillary density. Since capillary density can influence cardiac functional reserve, we evaluated vessel density in the myocardium by lectin staining. In our model, hyperglycemia did not change the number of vessels per field of the myocardium. We found no statistical difference in vessel density between WT (7.3 ± 0.2 ×103 vessels/mm2) and Txnip-KO (7.0 ± 0.2 ×103 vessels/mm2) hearts under STZ-induced diabetic conditions (P = N.S.).
A better functional reserve in Txnip-KO hearts is not associated with level of thioredoxin activity or oxidative stress.
Thioredoxin, a binding partner of Txnip, is a potent antioxidant in cardiomyocytes (67). To determine whether alterations of myocardial ROS levels are associated with better cardiac functional reserve in Txnip-KO hearts, we measured cellular thioredoxin activities and ROS levels in whole heart homogenates from Txnip-KO and WT mice. No significant differences in myocardial activities of thioredoxin were seen at baseline (Txnip-KO, 83 ± 5% of WT) or after STZ injections between Txnip-KO (116 ± 22% of WT baseline) and WT (111 ± 22% of WT baseline) mice (Fig. 3E). Levels of cellular lipid peroxide, an indicator of oxidative stress estimated as malondialdehyde, were also comparable between the myocardium from Txnip-KO mice and WT mice at baseline (WT 1.9 ± 0.5 nmol/mg, KO 1.2 ± 0.2 nmol/mg; P = N.S.) or after STZ treatments (WT 1.4 ± 0.2 nmol/mg, KO 1.1 ± 0.1 nmol/mg; P = N.S.) (Fig. 3F). Thus the functional benefits of Txnip-KO hearts under hyperglycemia were not attributed to decreased levels of ROS in the myocardium.
Enhanced sensitivity of Txnip-deletion myocardium to β-adrenergic stimulation is not associated with sarcolemmal expressions of β-adrenergic receptors.
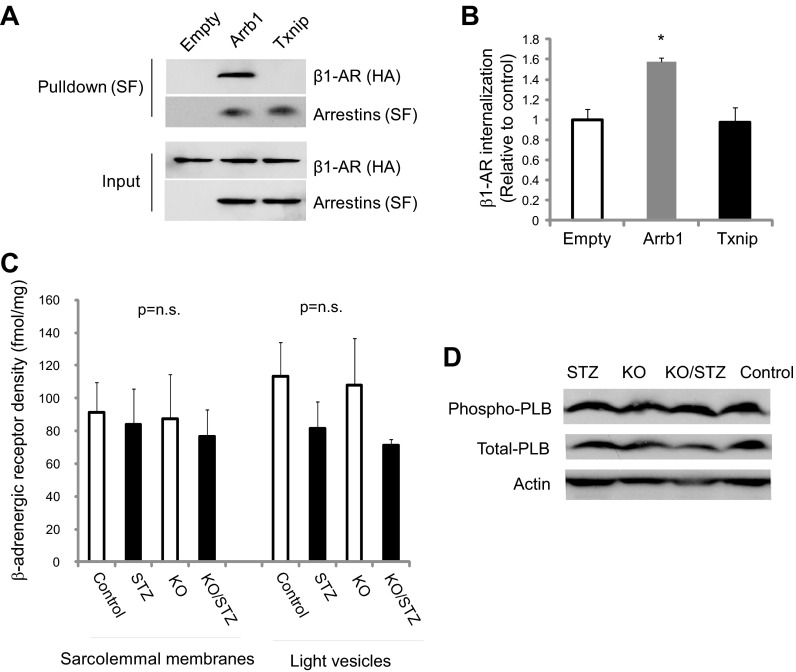
Members of the arrestin superfamily participate in agonist-mediated desensitization of G protein-coupled receptors. β-Arrestins bind to and desensitize β-adrenergic receptors through clathrin-mediated endocytosis (45, 48). Because Txnip is a member of the arrestin superfamily (48), we sought to determine whether Txnip, like β-arrestin 1, plays a role in desensitization of β1-adrenergic receptor and causes specific dampening of cellular responses to isoproterenol. Txnip and β-arrestin 1 were overexpressed in HEK-293T cells, and the interaction was assayed by coimmunoprecipitation (Fig. 4A). Unlike β-arrestin 1, no interaction between β1-adrenergic receptor and Txnip was detected. While overexpression of β-arrestin 1 resulted in a 57 ± 4% increase in β1-adrenergic receptor internalization compared with empty vector control, overexpression of Txnip showed no significant effects on internalization or endocytosis of the β1-adrenergic receptor (Fig. 4B).
Fig. 4.
Txnip does not bind to and promote internalization of β1-adrenergic receptor (AR). A: Txnip-Strep/FLAG (SF)-tag, β arrestin-1 (Arrb1)-SF-tag (positive control), or empty vector (Empty)-SF-tag (negative control) were coexpressed with β1-adrenergic receptors (ADRB1)-HA-tag in HEK-293T cells. Complexes were pulled down with Strep-Tactin resin, subjected to SDS-PAGE, and immunoblotted with anti-HA or anti-SF antibody. B: β-adrenergic receptor internalization was evaluated in HEK-293 cells by eluting 125I-cyanopindolol selectively internalized from the cell surface into intracellular vesicles. *P < 0.05 vs. empty vector. C: β-adrenergic receptors were extracted from homogenized mouse cardiac tissues from streptozotocin-induced diabetic (STZ) and nondiabetic wild-type (Control) and Txnip-KO (KO) mice. Membrane and internalized β-adrenergic receptors (vesicles from endocytosis) were labeled with radioactive dihydroalprenolol. Liquid scintillation counter was used to detect radiolabeled β-adrenergic receptors. Values are means ± SE. n = 3. D: expression level and phosphorylation state of phospholamban (PLB), a key mediator of the cardiac β-adrenergic pathway, were assessed by Western blot analysis; no differences were found among the groups.
STZ-induced diabetes has been shown to downregulate the expression level of β1-adrenergic receptors in the heart (37). Thus densities of β-adrenergic receptors in sarcolemmal and light vesicle fractions from cardiac tissues were measured based on [3H]CGP-12177 binding studies (Fig. 4C). The calculated β-adrenergic receptor density was 91 ± 18 fmol/mg protein in the sarcolemmal fraction of WT hearts. We observed no statistical difference in β-adrenergic receptor density between WT and Txnip-KO samples within nondiabetic and STZ-induced diabetic conditions.
One of the most critical mediators of the cardiac β-adrenergic pathway is phospholamban, a key protein that regulates sarcoplasmic reticular Ca2+ uptake in cardiomyocytes. Recent reports indicate that depressed cardiac performance in diabetes is associated with reduced sarcoplasmic reticular Ca2+ uptake by an impaired regulation of phospholamban (64). We found no difference in the expression level and the phosphorylation state of phospholamban between Txnip-KO and WT hearts (Fig. 4D).
Taken together, these results indicate that the preserved inotropic response to isoproterenol in Txnip-KO hearts was due to neither higher levels of β1-adrenergic receptor density nor altered regulation of phospholamban under diabetic conditions.
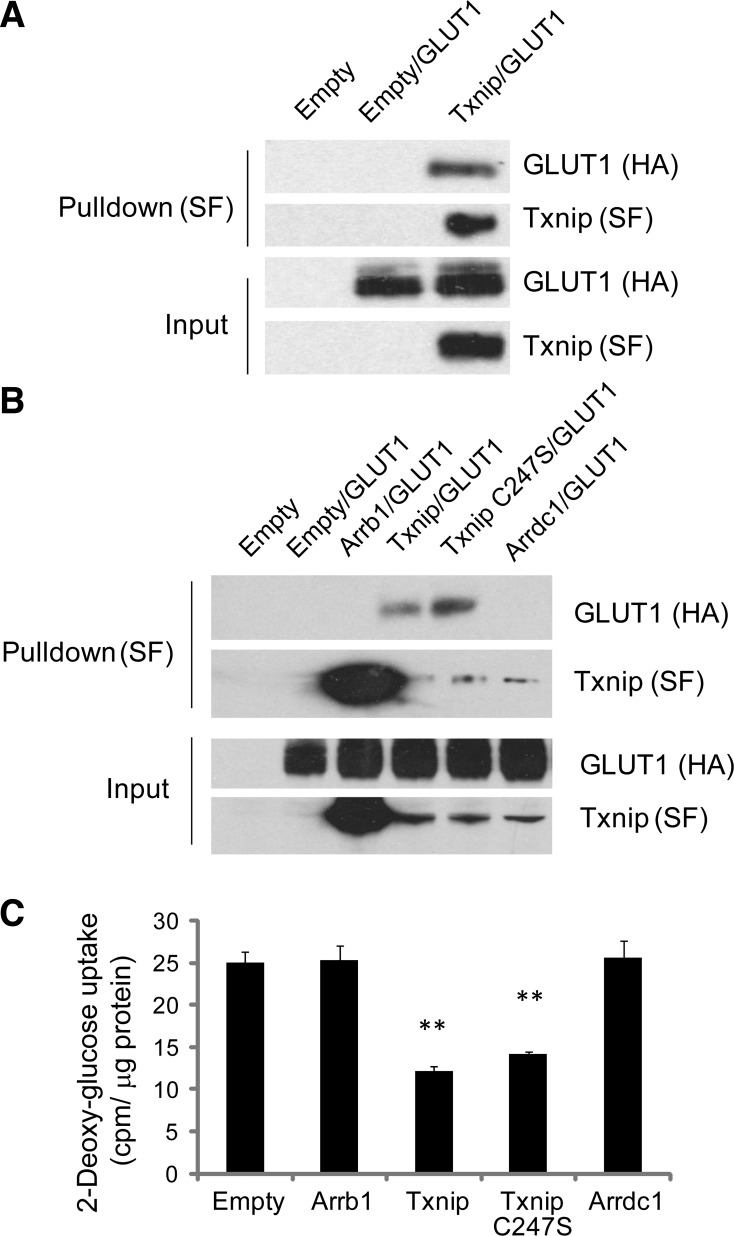
Txnip binds to GLUT1 and inhibits GLUT1-mediated cellular glucose uptake.
The rate of glucose transport in cardiomyocytes is GLUT1 dependent under insulin-deficient conditions. We conducted an unbiased proteomic-based interaction screen to explore signaling downstream of Txnip (26) and identified GLUT1 as a Txnip-interacting protein. We confirmed this interaction with standard coimmunoprecipitation experiments (Fig. 5A). Since a single cysteine-to-serine mutation (C247S) abolishes the ability of Txnip to bind to thioredoxin (47), we used the Txnip C247S mutant and found that thioredoxin binding was not required for Txnip to interact with GLUT1 (Fig. 5B). Overexpression of Txnip decreased glucose uptake to 48 ± 2% of control levels (P < 0.01 vs. mCherry only) in the absence of insulin stimulation (Fig. 5C). Overexpression of Txnip C247S mutant also decreased glucose uptake to 56 ± 1% of control levels (P < 0.001 vs. mCherry only), while overexpression of other related arrestins, β-arrestin 1 (Arrb1) and arrestin domain containing 1 (Arrdc1), had no effects on interaction with GLUT1 as well as on cellular glucose uptake. These data confirm that Txnip's binding to GLUT1, unlike Arrb1 and Arrdc1, regulates glucose uptake.
Fig. 5.
Txnip binds to GLUT1 and inhibits cellular glucose uptake. A and B: Txnip-Strep/FLAG (SF)-tag, Txnip C247S mutant-SF-tag, β-arrestin 1 (Arrb1)-SF-tag, arrestin domain containing 1 (Arrdc1)-SF-tag, or empty vector (Empty)-SF-tag (negative control) was coexpressed with GLUT1-HA-tag in HEK-293T cells. Complexes were pulled down with Strep-Tactin resin, subjected to SDS-PAGE, and immunoblotted with anti-HA or anti-SF antibody. C: 2-[3H]deoxyglucose uptake was measured in HEK-293 cells after lentiviral overexpression of arrestin-mCherry fusions. Uptake was normalized to total protein content. 2-Deoxyglucose uptake was significantly inhibited by both Txnip and Txnip C247S mutant but not by other related arrestins. Values are means ± SE. n = 8. **P < 0.01 vs. empty vector.
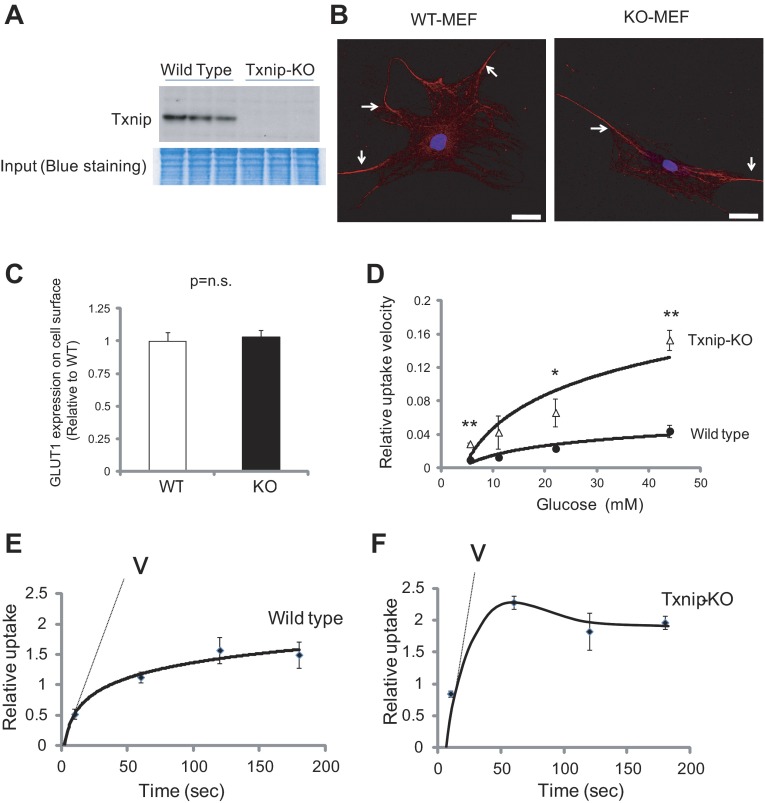
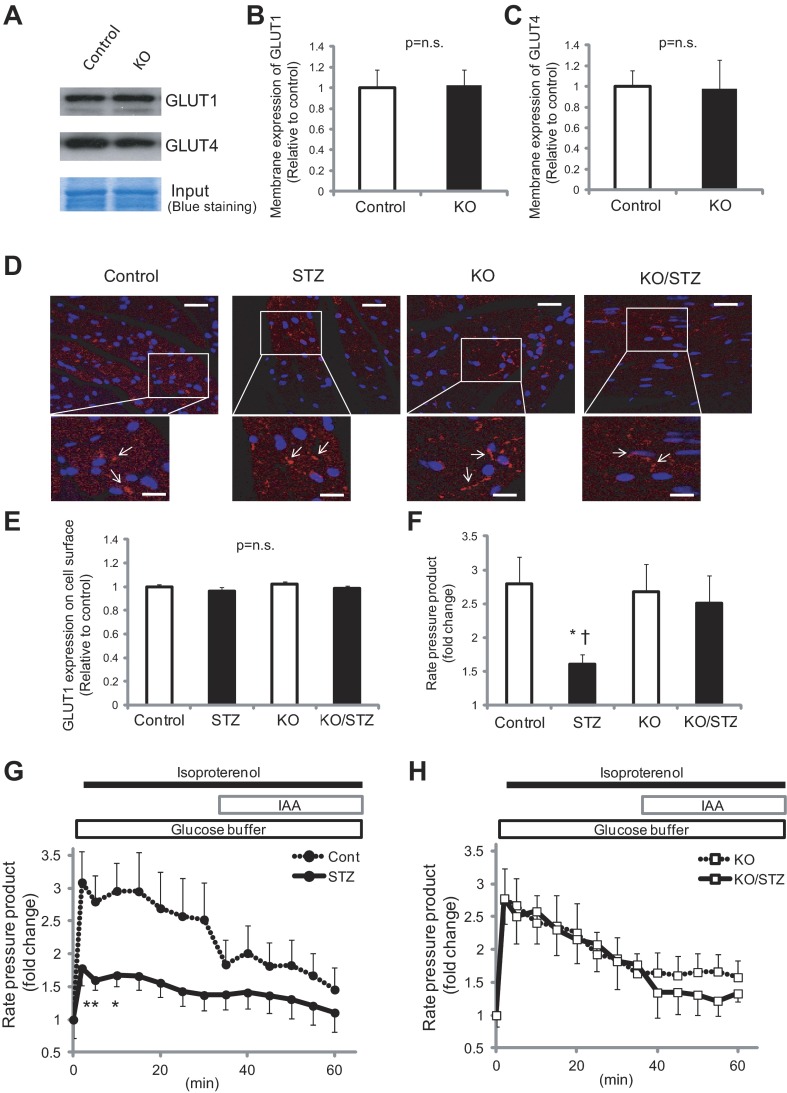
We next sought to identify the mechanism of Txnip function on glucose uptake via GLUT1 using Txnip-KO and WT MEFs. Absence of Txnip protein expression was confirmed in Txnip-KO MEFs compared with WT MEFs (Fig. 6A). It has been suggested by in vitro studies that Txnip regulates GLUT1 through endocytosis (66). In our knockout models, however, the GLUT1 levels on the cell surface were comparable between Txnip-KO and WT MEFs (Fig. 6, B and C). Both our in vivo data and those of others also indicate that modulation of glucose uptake by Txnip does not involve increased expression of GLUT1 and GLUT4 in the isolated plasma membrane fraction from mouse hearts in the fed state (Fig. 7, A–C) (4, 68). In addition, confocal microscopic analyses revealed that GLUT1 levels on the cell surface were comparable in in vivo tissues between Txnip-KO and WT myocardium within nondiabetic and STZ-induced diabetic conditions (Fig. 7, D and E).
Fig. 6.
Deletion of Txnip enhances glucose uptake. A: absence of Txnip protein expression was confirmed in systemic Txnip-KO mouse embryonic fibroblasts (MEFs) compared with wild-type MEFs. B: GLUT1 protein expression in MEFs from wild-type mice (WT-MEF) and Txnip-null mice (KO-MEF). After fixation, cells were stained with GLUT1 with secondary antibody conjugated to Alexa Fluor 594 and were costained with DAPI. Images were acquired by a confocal microscope. Scale bars, 40 μm. Arrows indicate positive staining of GLUT1 in MEF. C: positive staining of GLUT1 on the cell surface was quantified by ImageJ. D: these MEFs were treated with 3-O-[3H]methylglcuose (3-OMG), and concentration dependence of zero-trans 3-OMG uptake was analyzed in WT-MEFs and in KO-MEFs. Vmax and Km values for 3-OMG transporter were obtained by nonlinear regression. Txnip-KO MEFs had increased Vmax for exchange 3-OMG uptake without significant changes of Km compared with wild-type cells. E and F: time course of 5.5 mM 3-OMG uptake in wild-type (E) and Txnip-KO MEFs (F) is shown. V indicates the velocity of the reaction. Values are means ± SE. n = 3. *P < 0.05, **P < 0.01 vs. wild-type MEFs.
Fig. 7.
A–E: GLUT1 levels on the cell surface were comparable between Txnip-KO and wild-type myocardium in nondiabetic and streptozotocin (STZ)-induced diabetic conditions in the fed state. A: GLUT1 and GLUT4 protein expressions on the cellular membrane fraction were analyzed by Western blot. B and C: signals were quantified by densitometry of scanned autoradiographs. D: heart sections were stained with GLUT1 and secondary antibody conjugated to Alexa Fluor 594. Slides were then costained with DAPI and analyzed with confocal microscopy. Scale bars, 40 μm (lower magnification) and 10 μm (higher magnification). E: GLUT1 expression on the cell surface was quantified with ImageJ. P = N.S. among groups. F–H: functional reserve afforded by Txnip-KO hearts with diabetes is related to glucose utilization during β-adrenergic stress. Cardiac phenotype was analyzed by isolated, perfused mouse hearts with the perfusion buffer containing glucose as the sole oxidative energy substrate. Thus mechanical function was primarily supported by glucose. Changes in rate-pressure product (RPP) in response to isoproterenol (0.05 μM) were recorded in wild-type (G) or Txnip-KO (H) mice. Glycolytic inhibition with iodoacetate (IAA; 100 μM) caused a decline in mechanical function in both genotypes. The acute increase in RPP at 5 min (F) was significantly less in STZ-induced diabetic wild-type hearts (STZ) than in nondiabetic wild-type hearts (Control) or in Txnip-KO hearts without (KO) and with (KO/STZ) diabetes. Values are means ± SE. n = 3–9. *P < 0.05 vs. control (by 2-way ANOVA and unpaired t-test); †P < 0.05 vs. KO/STZ (by unpaired t-test).
Nevertheless, a sugar uptake assay using 5.5 mM 3-O-methylglucose (3-OMG), a nonmetabolizable transport substrate, showed a 3.0-fold increase in uptake velocity in Txnip-KO MEFs (Fig. 6F) compared with wild-type MEFs (Fig. 6E). Vmax and Km values for 3-OMG transporter were obtained by nonlinear regression analysis of the concentration dependence of sugar uptake assuming that uptake is described by the Michaelis-Menten equation (Fig. 6D). While Txnip deletion increased Vmax for exchange 3-OMG uptake by 3.5-fold, it had no significant effect on Km for exhange 3-OMG uptake (13 ± 3.6 mM in WT vs. 14 ± 2.3 mM in Txnip-KO; P = N.S.). The reciprocal effect on 3-OMG transporter was confirmed by overexpression of Txnip in the L929 cell line, which expresses GLUT1 as the exclusive glucose transporter (31). Txnip overexpression decreased Vmax for exchange 3-OMG uptake by 38 ± 7% (n = 3, P < 0.05), while it had no significant effect on Km for exchange 3-OMG uptake in L929 cells. These results suggest that deletion of Txnip enhances glucose uptake by increasing GLUT1 catalytic turnover more than changing transporter expression or redistribution from a microsomal storage pool to the cell surface (recruitment). This is supported by in vivo studies from another group, who reported that increased glucose utilization in muscle tissues from Txnip-KO mice is not associated with the abundance of glucose transporters on the plasma membrane (4).
Thus these pieces of evidence identify a new mechanism by which Txnip-KO cells have higher capacity of glucose utilization through increased GLUT1 catalytic efficiency.
Enhanced glucose utilization by deletion of Txnip may contribute to a greater functional reserve in diabetic cardiomyopathy.
The myocardium is generally dependent on glucose metabolism during conditions that stress intracellular calcium homeostasis including β-adrenergic stimulation (40). To determine whether functional reserve afforded by deletion of Txnip was related to increased glucose utilization during isoproterenol exposure, we examined cardiac phenotype by an ex vivo approach with isolated, perfused mouse hearts. In this system, mechanical function was primarily supported by glucose uptake and glycolysis, since the perfusion buffer contained glucose as the sole oxidative energy substrate. Before administration of isoproterenol, there were no significant differences in rate-pressure product (RPP), developed pressure, end-diastolic pressure, or heart rate between WT and Txnip-KO hearts under both nondiabetic and diabetic conditions. Fig. 7, G and H show the changes in mechanical function produced by continuous infusion of isoproterenol. During administration of isoproterenol to WT nondiabetic hearts, RPP rose up to a 3.1 ± 0.5-fold change from baseline within the first 15 min and then mildly decreased to a 2.5 ± 0.6-fold change for the next 15 min (Fig. 7G). Addition of IAA (100 μM), an inhibitor of glycolysis, produced a further decrease in RPP to 1.5 ± 0.3-fold change from baseline. The increase in RPP within the first 15 min was significantly less in STZ-induced diabetic WT hearts compared with nondiabetic WT hearts (Fig. 7, F and G), suggesting an impairment of myocardial glucose utilization in generating left ventricular mechanical force during β-adrenergic stimulation in the diabetic heart. It should be noted that IAA failed to completely block the mechanical function of nondiabetic hearts to the level of diabetic hearts, which may indicate that other mechanisms besides glycolysis are also involved.
Interestingly, RPP in diabetic Txnip-KO hearts remained at the same level of inotropic response to isoproterenol compared with nondiabetic hearts (Fig. 7, F and H). These results demonstrate that glucose utilization improved by deletion of Txnip, at least in part, contributes to preserved myocardial functional reserve during β-adrenergic stimulation under diabetic conditions.
DISCUSSION
The underlying basis of diabetic cardiomyopathy remains elusive, since it is a complex pathological manifestation involving abnormalities in multiple cellular and extracellular compartments, including the coronary microvasculature, extracellular space, autonomic nervous system, and cardiomyocytes (9). These changes are not well understood at the molecular level, nor is it clear how they cooperate to produce cardiac dysfunction.
In this study, we showed that hyperglycemia increases Txnip levels in cardiomyocytes. To determine a role of hyperglycemia-induced Txnip in diabetic cardiomyopathy, we characterized mice with cardiac-selective deletion of Txnip with type 1 diabetes. In animal models of type 1 diabetes, either STZ-induced or genetic rodents, systolic and diastolic cardiac dysfunction have been documented (23, 43, 62). Thus we expected that prolonged hyperglycemia would induce cardiac dysfunction; however, there were no differences in basal cardiac function between baseline and 8–16 wk after STZ injections in both WT and Txnip-deletion mice. This discrepancy between our and previous reports might be due to the age and strain of animals, the timing of onset of functional decline, and/or the severity of hyperglycemia (62). Accordingly, we performed stress tests and found that WT diabetic animals displayed a loss of inotropic reserve during β-adrenergic challenge, uncovering the evidence of early subtle mechanical dysfunction induced by contractile provocation under diabetic conditions. Inotropic reserve is a reflection of the contractile response to the stimulation of the myocardial adrenergic signaling pathways, which provides a measure of the integrity of the myocardial contractile apparatus. Higher inotropic reserve is a marker of the overall health of the LV and is an independent predictor of survival (16). Interestingly, Txnip-KO hearts showed a higher inotropic reserve than WT hearts.
Since Txnip has pleiotropic cellular functions (36), we specifically tested three hypotheses for how Txnip controls cardiac inotropic reserve in the diabetic heart. First, Txnip binds to and inhibits thioredoxin, the key component of a major redox system that detoxifies ROS in the cell. Hyperglycemia causes disorders of the oxidative-antioxidative balance in the cell, leading to increased free radical formation (19). Disturbed redox-sensitive signaling contributes to the development of diabetic cardiomyopathy (3). Hence, the increased Txnip expression resulting from high glucose is expected to reduce thioredoxin activity and increase concentrations of ROS, and thereby Txnip may contribute to oxidative stress. However, in our mouse model hyperglycemia did not lead to significant changes in the levels of thioredoxin activity and ROS in the myocardium. Knockout of Txnip did not alter thioredoxin function and levels of oxidative stress, which is consistent with our earlier findings in vivo and those of others (58, 68).
Second, autonomic neuropathy, which may underlie abnormalities of cardiac performance, is recognized as a complication of chronic diabetes mellitus. An isoproterenol infusion study has shown decreased β-adrenoceptor responsiveness in type 1 insulin-dependent diabetic patients (5). Likewise, a reduction in myocardial β-adrenergic receptor density has been shown in diabetic animal models (41, 54). Therefore, we tested whether interacting with β-adrenergic receptors, a defining characteristic of the β-arrestins, is a property of Txnip. The data indicate that Txnip interacts with neither β1- nor β2-adrenergic receptors (21, 46) to cause desensitization of the receptor signaling. Nevertheless, we found better contractile reserve by inotropic stimulation in Txnip-KO hearts.
A third critical cellular function of Txnip is serving as a “glucostat” (38, 44). The hallmark of diabetic insult is a glucose transport system that has been either fully or partially compromised. In both type 1 and type 2 diabetes, glucose uptake and glycolysis are impaired, causing the heart to adapt by using fatty acid for ATP generation (3). Chronically, this maladaptation is believed to lead to the development of diabetic cardiomyopathy (3). In type 1 diabetic animals, because of the dramatic decrease in the rate of glucose utilization and glycolysis, the primary metabolic process that provides ATP as an energy source is fatty acid oxidation (11, 65). Compared with glucose, oxidation of fatty acid consumes more oxygen, which increases oxygen demand, making the heart vulnerable during workload such as β-adrenergic challenge. In this way, the cardiac adaptation in Txnip KO hearts appears to be driven by enhanced glucose metabolism by the interaction between Txnip and GLUT1. Increased glucose utilization can confer cardioprotection, as overexpression of GLUT1 increases myocardial glucose utilization and attenuates cardiac dysfunction after aortic constriction in mice (27, 33). Although we found no changes in the expression level of GLUT1 on the cell surface, recent evidence suggests that GLUT1 can be quickly activated without an increase in either GLUT1 expression or total GLUT1 membrane concentration (20). This regulation is sometimes described as “unmasking” of GLUT1 already present in the membrane for activation (20). Because some thiol-reactive compounds maximally activate GLUT1 in L929 fibroblasts (32, 53), Txnip may activate GLUT1 with a thiol-mediated reaction.
While myocardial changes through diabetic stress may mask subtle cardiac mechanical dysfunction, diabetic hearts display a loss of inotropic reserve in response to β-adrenergic stress. Multiple mechanisms should be involved, leading to reduced contractile reserve in diabetic cardiomyopathy, including impaired insulin signaling (8), lipid accumulation (13), adipokines (25), and advanced glycation end products (24). Our results provide novel evidence that Txnip also contributes to cardiac dysfunction in the diabetic heart during contractile provocation through the interplay with GLUT1. Although further studies are necessary to determine the precise role of myocardial Txnip in metabolic disorders, these data suggest that Txnip may be crucial in the pathogenesis of diabetic cardiomyopathy.
GRANTS
This work was supported by awards from the American Heart Association (13GRNT16870007 to J. Yoshioka) and the German Research Foundation (LE 2728/1-1 to S. Lee) and National Institutes of Health Grants 5F32 DK-098052 (to R. B. Myers), 1F32 HL-106979 (to G. M. Fomovsky), and 1R01 HL-130861 (to J. Yoshioka).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.B.M., G.M.F., S.L., M.T., B.F.W., and J.Y. performed experiments; R.B.M., G.M.F., S.L., M.T., B.F.W., and J.Y. analyzed data; R.B.M., G.M.F., S.L., M.T., B.F.W., P.P., and J.Y. interpreted results of experiments; R.B.M., G.M.F., S.L., M.T., B.F.W., and J.Y. prepared figures; R.B.M., G.M.F., S.L., B.F.W., P.P., and J.Y. edited and revised manuscript; G.M.F., P.P., and J.Y. conception and design of research; J.Y. drafted manuscript; J.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Joseph Gannon for his technical assistance.
REFERENCES
- 1.Abel ED. Glucose transport in the heart. Front Biosci 9: 201–215, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez CE. On the origins of arrestin and rhodopsin. BMC Evol Biol 8: 222, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 291: H1489–H1506, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Andres AM, Ratliff EP, Sachithanantham S, Hui ST. Diminished AMPK signaling response to fasting in thioredoxin-interacting protein knockout mice. FEBS Lett 585: 1223–1230, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlin I, Grimaldi A, Bosquet F, Puech AJ. Decreased beta-adrenergic sensitivity in insulin-dependent diabetic subjects. J Clin Endocrinol Metab 63: 262–265, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 115: 3213–3223, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11: 31–39, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudina S, Bugger H, Sena S, O'Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119: 1272–1283, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57: 660–671, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 54: 1829–1837, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Chatham JC, Forder JR. A 13C-NMR study of glucose oxidation in the intact functioning rat heart following diabetes-induced cardiomyopathy. J Mol Cell Cardiol 25: 1203–1213, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes 57: 938–944, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144: 3483–3490, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Chutkow WA, Birkenfeld AL, Brown JD, Lee HY, Frederick DW, Yoshioka J, Patwari P, Kursawe R, Cushman SW, Plutzky J, Shulman GI, Samuel VT, Lee RT. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes 59: 1424–1434, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cura AJ, Carruthers A. Acute modulation of sugar transport in brain capillary endothelial cell cultures during activation of the metabolic stress pathway. J Biol Chem 285: 15430–15439, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois-Rande JL, Merlet P, Roudot F, Benvenuti C, Adnot S, Hittinger L, Duval AM, Syrota A, Castaigne A, Loisance D. Beta-adrenergic contractile reserve as a predictor of clinical outcome in patients with idiopathic dilated cardiomyopathy. Am Heart J 124: 679–685, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Dunn LL, Simpson PJ, Prosser HC, Lecce L, Yuen GS, Buckle A, Sieveking DP, Vanags LZ, Lim PR, Chow RW, Lam YT, Clayton Z, Bao S, Davies MJ, Stadler N, Celermajer DS, Stocker R, Bursill CA, Cooke JP, Ng MK. A critical role for thioredoxin-interacting protein in diabetes-related impairment of angiogenesis. Diabetes 63: 675–687, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gloeckner CJ, Boldt K, Schumacher A, Roepman R, Ueffing M. A novel tandem affinity purification strategy for the efficient isolation and characterisation of native protein complexes. Proteomics 7: 4228–4234, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53, Suppl 1: S110–S118, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Gunnink SM, Kerk SA, Kuiper BD, Alabi OD, Kuipers DP, Praamsma RC, Wrobel KE, Louters LL. Alkaline pH activates the transport activity of GLUT1 in L929 fibroblast cells. Biochimie 99: 189–194, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han SO, Kommaddi RP, Shenoy SK. Distinct roles for beta-arrestin2 and arrestin-domain-containing proteins in beta2 adrenergic receptor trafficking. EMBO Rep 14: 164–171, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, Ma Q. Redox regulation by nuclear factor erythroid 2-related factor 2: gatekeeping for the basal and diabetes-induced expression of thioredoxin-interacting protein. Mol Pharmacol 82: 887–897, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joffe II, Travers KE, Perreault-Micale CL, Hampton T, Katz SE, Morgan JP, Douglas PS. Abnormal cardiac function in the streptozotocin-induced non-insulin-dependent diabetic rat: noninvasive assessment with Doppler echocardiography and contribution of the nitric oxide pathway. J Am Coll Cardiol 34: 2111–2119, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Joshi D, Gupta R, Dubey A, Shiwalkar A, Pathak P, Gupta RC, Chauthaiwale V, Dutt C. TRC4186, a novel AGE-breaker, improves diabetic cardiomyopathy and nephropathy in Ob-ZSF1 model of type 2 diabetes. J Cardiovasc Pharmacol 54: 72–81, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Lamounier-Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, Ehrhart-Bornstein M, Bornstein SR, Morano I. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res 105: 326–334, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Min Kim S, Dotimas J, Li L, Feener EP, Baldus S, Myers RB, Chutkow WA, Patwari P, Yoshioka J, Lee RT. Thioredoxin-interacting protein regulates protein disulfide isomerases and endoplasmic reticulum stress. EMBO Mol Med 6: 732–743, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation 106: 2125–2131, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135: 714–725, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290: H232–H239, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lindsey ML, Yoshioka J, MacGillivray C, Muangman S, Gannon J, Verghese A, Aikawa M, Libby P, Krane SM, Lee RT. Effect of a cleavage-resistant collagen mutation on left ventricular remodeling. Circ Res 93: 238–245, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Liong E, Kong SK, Au KK, Li JY, Xu GY, Lee YL, Kwok TT, Choy YM, Lee CY, Fung KP. Inhibition of glucose uptake and suppression of glucose transporter 1 mRNA expression in L929 cells by tumour necrosis factor-alpha. Life Sci 65: PL215–PL220, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Louters LL, Scripture JP, Kuipers DP, Gunnink SM, Kuiper BD, Alabi OD. Hydroxylamine acutely activates glucose uptake in L929 fibroblast cells. Biochimie 95: 787–792, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation 116: 901–909, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Mahan LC, Motulsky HJ, Insel PA. Do agonists promote rapid internalization of beta-adrenergic receptors? Proc Natl Acad Sci USA 82: 6566–6570, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maisel AS, Motulsky HJ, Insel PA. Externalization of beta-adrenergic receptors promoted by myocardial ischemia. Science 230: 183–186, 1985. [DOI] [PubMed] [Google Scholar]
- 36.Masutani H, Yoshihara E, Masaki S, Chen Z, Yodoi J. Thioredoxin binding protein (TBP)-2/Txnip and alpha-arrestin proteins in cancer and diabetes mellitus. J Clin Biochem Nutr 50: 23–34, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda N, Hattori Y, Gando S, Akaishi Y, Kemmotsu O, Kanno M. Diabetes-induced down-regulation of beta1-adrenoceptor mRNA expression in rat heart. Biochem Pharmacol 58: 881–885, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 146: 2397–2405, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Muoio DM. TXNIP links redox circuitry to glucose control. Cell Metab 5: 412–414, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K, Kusuoka H, Ambrosio G, Becker LC. Glycolysis is necessary to preserve myocardial Ca2+ homeostasis during beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol 264: H670–H678, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Nishio Y, Kashiwagi A, Kida Y, Kodama M, Abe N, Saeki Y, Shigeta Y. Deficiency of cardiac beta-adrenergic receptor in streptozocin-induced diabetic rats. Diabetes 37: 1181–1187, 1988. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem 274: 21645–21650, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes 51: 514–521, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze PC, Mazzini MJ, Jensen CB, Krook A, Bjornholm M, Tornqvist H, Zierath JR, Ridderstrale M, Altshuler D, Lee RT, Vaag A, Groop LC, Mootha VK. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4: e158, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parruti G, Peracchia F, Sallese M, Ambrosini G, Masini M, Rotilio D, De Blasi A. Molecular analysis of human beta-arrestin-1: cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. J Biol Chem 268: 9753–9761, 1993. [PubMed] [Google Scholar]
- 46.Patwari P, Emilsson V, Schadt EE, Chutkow WA, Lee S, Marsili A, Zhang Y, Dobrin R, Cohen DE, Larsen PR, Zavacki AM, Fong LG, Young SG, Lee RT. The arrestin domain-containing 3 protein regulates body mass and energy expenditure. Cell Metab 14: 671–683, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem 281: 21884–21891, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patwari P, Lee RT. An expanded family of arrestins regulate metabolism. Trends Endocrinol Metab 23: 216–222, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol 221: 262–272, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis 1: e65, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 98: 596–605, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Price SA, Gardiner NJ, Duran-Jimenez B, Zeef LA, Obrosova IG, Tomlinson DR. Thioredoxin interacting protein is increased in sensory neurons in experimental diabetes. Brain Res 1116: 206–214, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Salie MJ, Oram DS, Kuipers DP, Scripture JP, Chenge J, MacDonald GJ, Louters LL. Nitroxyl (HNO) acutely activates the glucose uptake activity of GLUT1. Biochimie 94: 864–869, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savarese JJ, Berkowitz BA. Beta-adrenergic receptor decrease in diabetic rat hearts. Life Sci 25: 2075–2078, 1979. [DOI] [PubMed] [Google Scholar]
- 55.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem 279: 30369–30374, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Shah A, Xia L, Masson EA, Gui C, Momen A, Shikatani EA, Husain M, Quaggin S, John R, Fantus IG. Thioredoxin-interacting protein deficiency protects against diabetic nephropathy. J Am Soc Nephrol 26: 2963–2977, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology 143: 3695–3698, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Sheth SS, Castellani LW, Chari S, Wagg C, Thipphavong CK, Bodnar JS, Tontonoz P, Attie AD, Lopaschuk GD, Lusis AJ. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res 46: 123–134, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Singh LP. Thioredoxin interacting protein (TXNIP) and pathogenesis of diabetic retinopathy. J Clin Exp Ophthalmol 2013: 4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soriano-Tarraga C, Jimenez-Conde J, Giralt-Steinhauer E, Mola-Caminal M, Vivanco-Hidalgo RM, Ois A, Rodriguez-Campello A, Cuadrado-Godia E, Sayols-Baixeras S, Elosua R, Roquer J. Epigenome-wide association study identifies TXNIP gene associated with type 2 diabetes mellitus and sustained hyperglycemia. Hum Mol Genet 25: 609–619, 2016. [DOI] [PubMed] [Google Scholar]
- 61.Su H, Ji L, Xing W, Zhang W, Zhou H, Qian X, Wang X, Gao F, Sun X, Zhang H. Acute hyperglycaemia enhances oxidative stress and aggravates myocardial ischaemia/reperfusion injury: role of thioredoxin-interacting protein. J Cell Mol Med 17: 181–191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suarez J, Belke DD, Gloss B, Dieterle T, McDonough PM, Kim YK, Brunton LL, Dillmann WH. In vivo adenoviral transfer of sorcin reverses cardiac contractile abnormalities of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 286: H68–H75, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki H, Kayama Y, Sakamoto M, Iuchi H, Shimizu I, Yoshino T, Katoh D, Nagoshi T, Tojo K, Minamino T, Yoshimura M, Utsunomiya K. Arachidonate 12/15-lipoxygenase-induced inflammation and oxidative stress are involved in the development of diabetic cardiomyopathy. Diabetes 64: 618–630, 2015. [DOI] [PubMed] [Google Scholar]
- 64.Vasanji Z, Dhalla NS, Netticadan T. Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 261: 245–249, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Wall SR, Lopaschuk GD. Glucose oxidation rates in fatty acid-perfused isolated working hearts from diabetic rats. Biochim Biophys Acta 1006: 97–103, 1989. [DOI] [PubMed] [Google Scholar]
- 66.Wu N, Zheng B, Shaywitz A, Dagon Y, Tower C, Bellinger G, Shen CH, Wen J, Asara J, McGraw TE, Kahn BB, Cantley LC. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 49: 1167–1175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest 112: 1395–1406, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshioka J, Imahashi K, Gabel SA, Chutkow WA, Burds AA, Gannon J, Schulze PC, MacGillivray C, London RE, Murphy E, Lee RT. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res 101: 1328–1338, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Yoshioka J, Schulze PC, Cupesi M, Sylvan JD, MacGillivray C, Gannon J, Huang H, Lee RT. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation 109: 2581–2586, 2004. [DOI] [PubMed] [Google Scholar]