In individually perfused intact microvessels, we showed that circulating tumor cells prefer to adhere to the microvessel sites with higher NO concentrations, which are generated by the localized shear stresses and shear stress gradients induced by normal flows. Endothelial nitric oxide synthase inhibition reduces tumor cell adhesion and also decreases microvessel permeability.
Keywords: postcapillary venule in rat mesentery, curved and straight portions, flow-induced nitric oxide production, microvessel permeability to albumin, NG-monomethyl-l-arginine
Abstract
Nitric oxide (NO) at different concentrations may promote or inhibit tumor growth and metastasis under various conditions. To test the hypothesis that tumor cells prefer to adhere to the locations with a higher endothelial NO production in intact microvessels under physiological flows and to further test that inhibiting NO production decreases tumor cell adhesion, we used intravital fluorescence microscopy to measure NO production and tumor cell adhesion in postcapillary venules of rat mesentery under normal and reduced flow conditions, and in the presence of an endothelial nitric oxide synthase (eNOS) inhibitor, NG-monomethyl-l-arginine (l-NMMA). Rats (SD, 250–300 g) were anesthetized. A midline incision (∼2 inch) was made in the abdominal wall, and the mesentery was taken out from the abdominal cavity and spread over a coverslip for the measurement. An individual postcapillary venule (35–50 μm) was first loaded with 4,5-diaminofluorescein diacetate (DAF-2 DA), a fluorescent indictor for NO. Then the DAF-2 intensity was measured for 30 min under a normal or reduced flow velocity, with and without perfusion with MDA-MB-231 breast cancer cells, and in the presence of l-NMMA. We found that tumor cells prefer to adhere to the microvessel locations with a higher NO production such as curved portions. Inhibition of eNOS by l-NMMA attenuated the flow-induced NO production and reduced tumor cell adhesion. We also found that l-NMMA treatment for ∼40 min reduced microvessel permeability to albumin. Our results suggest that inhibition of eNOS is a good approach to preventing tumor cell adhesion to intact microvessels under physiological flows.
NEW & NOTEWORTHY
In individually perfused intact microvessels, we showed that circulating tumor cells prefer to adhere to the microvessel sites with higher NO concentrations, which are generated by the localized shear stresses and shear stress gradients induced by normal flows. Endothelial nitric oxide synthase inhibition reduces tumor cell adhesion and also decreases microvessel permeability.
tumor cell metastasis through blood circulation is a complex process and is one of the great challenges in cancer research since metastatic spread is responsible for ∼90% of cancer-related mortality (25). Tumor cell adhesion to the microvessel wall is one critical step in metastatic spread (19, 64). Many in vivo, ex vivo, and in vitro studies under static and flow conditions have been conducted to understand underlying mechanisms by which tumor cells interact with endothelial cells lining the microvessel wall for the adhesion (10, 11, 20, 24, 38, 60, 62). Specific biochemical factors, e.g., cell adhesion molecules (CAMs), nitric oxide (NO), endothelial NO synthase (eNOS), and chemokines, have been found to play a crucial role in preferred metastasis (17, 18, 20, 21, 47, 49, 50, 54, 59, 71, 77). Mechanical factors have also been postulated to interact with the biochemical factors for tumor metastasis (53, 70). However, how the localized hydrodynamic factors induced by the microcirculation affect tumor cell arrest and adhesion has not been systematically studied, especially for intact microvessels under normal conditions.
NO is the smallest signaling molecule that regulates a variety of important physiological functions (13). eNOS is responsible for most of the vascular NO production (6). In cancer biology, NO can promote or inhibit tumor growth and metastasis, depending on its concentrations (54, 71). Elevated NO and eNOS have been observed in cancer patients in malignancy states (42). Although high concentrations of NO are cytotoxic to the circulating tumor cells (39, 45, 52, 68), low levels of NO promote tumor cell arrest and adhesion (58, 71, 77). NO at some optimal levels can inhibit cancer cell adhesion to cytokines-stimulated endothelial cells (41, 71), decrease tumor cell adhesion to naive and lipopolysaccharide (LPS)-treated postcapillary venules (34), and reduce invasion ability of cancer cells (31).
Previous studies found that malignant breast cancer cells MDA-MB-231 preferred to adhere at the curved portions of microvessels (72, 73) and at the bifurcation of microvasculature (22), where there are localized shear stresses, shear stress gradients, and vorticities (22, 40). Shen et al. (60) also demonstrated that there are more adherent tumor cells in intact postcapillary venules of rat mesentery under normal flows than those under reduced flows. On the other hand, blood flow-induced shear stress has been shown to activate eNOS to produce NO in endothelial cells lining the wall of large and small vessels (4, 7, 23, 51). A recent study on the individually perfused postcapillary venules of rat mesentery found that NO production under a normal flow is ∼1.5-fold of that under a reduced flow after 60 min perfusion (75). Combining these studies led us to the hypothesis that tumor cells prefer to adhere to the locations with higher NO concentration, which is induced by the localized shear stresses or shear stress gradients, in intact microvessels under normal flows. Accordingly, another hypothesis is that inhibition of eNOS activity prevents tumor cell adhesion to the microvessel wall by suppressing endothelial NO production. To test these hypotheses, we used intravital fluorescence microscopy and a membrane permeable fluorescent NO indicator, 4,5-diaminofluorescein diacetate (DAF-2 DA), to measure the endothelial cell (EC) NO production (75, 79) in individually perfused postcapillary venules of rat mesentery under normal and reduced flow conditions, with and without MDA-MB-231 tumor cell perfusion, and in the presence and absence of an eNOS inhibitor, NG-monomethyl-l-arginine (l-NMMA).
Previous studies also reported that enhanced microvessel permeability increases tumor cell adhesion to endothelial cells under static and flow conditions (11, 15, 38, 60). To further investigate whether attenuation of tumor cell adhesion by suppressing flow-induced NO production is due to the decrease in microvessel permeability, we measured microvessel permeability to albumin under the treatment of l-NMMA.
MATERIALS AND METHODS
Animal preparation.
All experiments were performed on female Sprague-Dawley rats (250–300 g, age 3 to 4 mo), supplied by Hilltop Laboratory Animals (Scottsdale, PA). All procedures were approved by the Animal Care and Use Committees at the City College of the City University of New York. The methods used to prepare rat mesenteries, perfusion solutions, and micropipettes for microperfusion experiments have been described in detail (5, 14, 60). Briefly, rats were first anesthetized with pentobarbital sodium given subcutaneously at the initial dosage of 65 mg/kg followed by an additional 3 mg/dose when needed. A midline surgical incision (2 to 3 cm) was made in the abdominal wall. Then the rat was transferred to a tray and kept warm at 37°C on a heating pad and monitored by a thermometer. The mesentery was carefully taken out from the abdominal cavity and spread on a glass coverslip, which formed the base of the observation platform as previously described (60). During the entire experiment, the upper surface of the tissue was continuously superfused by a dripper with mammalian Ringer solution at ∼37°C, which was regulated by a controlled water bath and monitored by a thermometer probe. The microvessels chosen for the study were postcapillary venules of diameter 35–50 μm, which had naturally curved portions, and some of them had Y-branched vessels at their upstream. All vessels had brisk blood flow immediately before cannulation and had no marginating white cells. At the end of experiments, the animals were euthanized with excess anesthetic.
Solutions and reagents.
Mammalian Ringer solution was used for all dissections, perfusate, and superfusate. The solution composition was (in mM) 132 NaCl, 4.6 KCl, 1.2 MgSO4, 2.0 CaCl2, 5.0 NaHCO3, 5.5 glucose, and 20 HEPES. Its pH was balanced to 7.4 by adjusting the ratio of HEPES acid to base. In addition, the perfusate into the microvessel lumen contained BSA (Sigma-Aldrich, St. Louis, MO) at 10 mg/ml (1% BSA-Ringer solution). FITC-labeled BSA (molecular mass ∼67 kDa; A9771), 4,5-diaminofluorescein diacetate (DAF-2 DA), NG-monomethyl-l-arginine (l-NMMA), and sodium nitroprusside (SNP) were purchased from Sigma (Sigma-Aldrich). Texas Red (TR)-BSA was from ThermoFisher Scientific (A23017; Waltham, MA). The stock solutions of DAF-2 DA (10 mM) were prepared with 100% DMSO. The concentrations of DAF-2 DA (5 μM), l-NMMA (1 mM), and SNP (100, 500 μM) were achieved by dilutions of the stock with 1% BSA-Ringer solution (79). All of the solutions described above were made at the time when the experiment was performed and were discarded at the end of the day.
Cell culture.
Human breast carcinoma cells (MDA-MB-231, MCF-7) and mouse brain microvascular endothelial cells (bEnd3) from ATCC (Manassas, VA) were cultured in DMEM/Nutrient Mixture F-12 Ham (DMEM/F-12), 2 mM l-glutamine, and 100 U/ml penicillin and 1 mg/ml streptomycin, all from Sigma-Aldrich, supplemented with 10% FBS (Atlanta Biologicals, Flowery Branch, GA). All the cells were incubated in the humidified atmosphere with 5% CO2 at 37°C.
On the day of experiment, MDA-MB-231 or MCF-7 cells were collected by brief trypsinization, then counted and suspended in PBS (Sigma-Aldrich). To remove any remaining cell clumps, the cell suspension was filtered through a 40-μm nylon mesh. Then tumor cells were fluorescently labeled using 0.5 μM Cell Tracker Red, excitation/emission = 577/602 nm (Invitrogen, Eugene, OR) in serum-free DMEM medium for 30 min. Concentration of cell suspension was adjusted for the final perfusate ∼4 million/ml in 1% BSA mammalian Ringer. The cell survival rate was ∼98% before perfusion and ∼95% after ∼60 min perfusion at driving pressures of 1–15 cmH2O in perfusing micropipettes. bEnd3 cells were seeded at ∼66,000 per cm2 onto fibronectin-coated coverslip at the bottom of a cultured dish or a flow chamber (μ-Slide I 0.2 Luer ibiTreat; ibidi, Madison, WI) and cultured for ∼3.5 days to form confluent monolayers (11, 12).
Intravital microscopy.
A Nikon Eclipse TE2000-E inverted fluorescent microscope was used to observe the mesentery and cultured cell monolayer. The tissue/monolayer was observed with either transmitted white light from a light pipe suspended above the preparation or with fluorescent light from an illumination system (the monochromator with a xenon lamp FSM150Xe; Bentham Instrument). The monochromator generated the light of wavelength from 200 to 700 nm. The observation of the DAF-2-labeled microvessel wall and adherent tumor cells and the measurement of microvessel permeability to albumin were done by a high-performance digital 12-bit charge-coupled device (CCD) camera (SensiCam QE; Cooke Corporation, Romulus, MI) with a Super Fluor 20× objective lens [numerical aperture (NA) = 0.75; Nikon] and recorded by InCyt Im imaging and analyzing system (Intracellular Imaging, Cincinnati, OH). The excitation/emission wavelengths for observing DAF-2, cell tracker red labeled-tumor cells were 490/520 nm and 577/602 nm, respectively.
Measurement of endothelial NO production in a microvessel.
The method used to measure NO production in the endothelial cells forming the microvessel wall was similar to that previously described (75, 79). Endothelial NO levels were visualized in individually perfused microvessels using DAF-2 DA, a membrane permeable fluorescent indictor for NO, and a fluorescence imaging system. Briefly, in each experiment, a microvessel was first loaded with DAF-2 DA (5 μM) in 1% BSA-Ringer solution for 45 min at a perfusion velocity ∼300 μm/s, then at a velocity of ∼1,000 μm/s, which is the typical mean blood flow velocity in the postcapillary venules of rat mesentery or a reduced flow (∼300 μm/s) for 30 min. In the case without tumor cell (TC) perfusion, the midplane of a vessel was focused, whereas in the case with TC perfusion, a focused plane near the bottom of a vessel was focused and DAF-2 images (0.5-s exposure time for imaging) were taken every 5 min for 30 min. Figure 1 demonstrates the technique used to measure NO production in the microvessel wall without (Fig. 1A) and with (Fig. 1B) TC perfusion. To inhibit the eNOS activity, 1 mM l-NMMA was present in the perfusate during the loading and perfusion with TCs. All DAF-2 images of the vessels were analyzed with the public domain National Institutes of Health ImageJ program by selecting a region of interest (ROI) either focused on the midplane of the vessel without TC perfusion (Fig. 2), or a ROI with an oval shaped area of ∼40 μm for the long axis and ∼25 μm for the short axis from the focal plane of the vessel with TC perfusion (Fig. 3). The intensity of DAF-2 at the end of 45-min DAF-2 DA loading in the respective ROIs was used for the normalization.
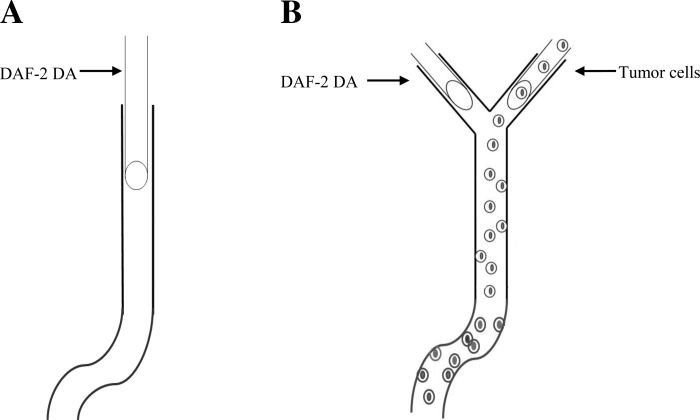
Fig. 1.
Schematic showing the technique used to measure nitric oxide (NO) production in the endothelial cells forming the microvessel wall with and without tumor cell perfusion. A: without tumor cell perfusion. A postcapillary venule of 500∼1000 μm long with straight and curved portions is cannulated with a micropipette and loaded with 4,5-diaminofluorescein diacetate (DAF-2 DA; 5 μM) in 1% BSA-Ringer solution for 45 min at a reduced perfusion rate of ∼300 μm/s; DAF-2 NO images of the microvessel are then taken every 5 min up to 30 min under a perfusion rate of ∼1,000 μm/s (normal flow) or ∼300 μm/s (reduced flow). B: with tumor cell perfusion. A postcapillary venule of 500∼1,000 μm long with straight and curved portions and with a Y-branch at its upstream is found first. One branch is cannulated and loaded with DAF-2 DA (5 μM) in 1% BSA-Ringer solution for 45 min at a reduced perfusion rate of ∼300 μm/s while another branch is occluded by a glass pipette blocker. At the end of 45 min, the branch vessel for DAF-2 DA loading is blocked and another branch is cannulated and perfused with cell tracker red-labeled tumor cells in 1% BSA-Ringer solution at a normal or reduced rate. Every 5 min up to 30 min, DAF-2 NO and tumor cell adhesion images of the vessel are then taken alternately by switching the light to green (excitation 490/emission 520 nm] for DAF-2 NO and to red (excitation 577/emission 602 nm) for tumor cells.
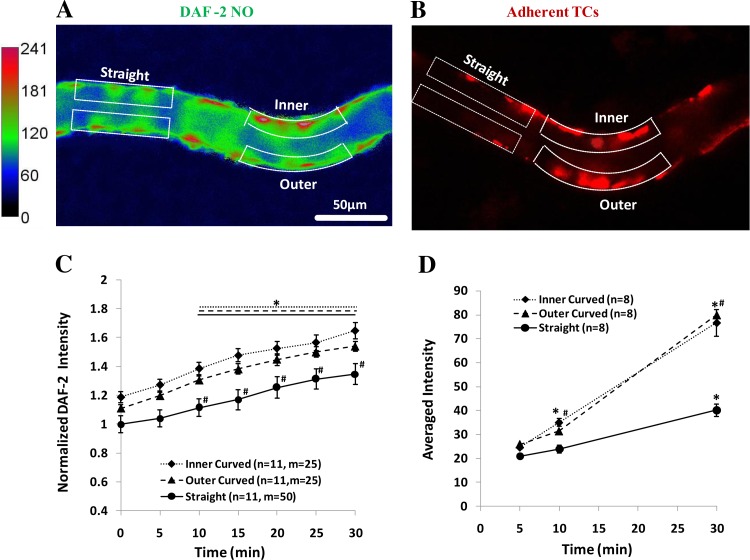
Fig. 2.
Images for endothelial NO production (A) and tumor cell (TC) adhesion (B) along microvessels with straight and curved portions after 30 min perfusion at normal flow. The focus is on the midplane of the vessel. In A, the microvessel is perfused without tumor cells. The intensity scale bar at left is for the DAF-2 NO. The region of interests (ROIs) for the curved portion has 2 locations, the inner and outer curved ROI, which is the white dotted line-enclosed region with the length of 50–100 μm and the width of 10–15 μm along the vessel border. Correspondingly, there are straight portions (2 ROIs for each portion) of the equal size and number in the same vessel. In B, the microvessel is perfused with tumor cells. The red spots are adherent tumor cells labeled with cell tracker red. The ROIs for adherent tumor cells are the same as those defined for the DAF-2 NO. For each vessel, there are 2 to 3 curved portions and 2 to 3 straight portions. C: normalized DAF-2 NO intensity as a function of time during 30 min perfusion under normal flow for the inner and outer curved part and the straight portion in microvessels without tumor cell perfusion. *P < 0.05, compared NO production with that at the end of DAF-2 DA loading (t = 0); #P < 0.05, compared NO production between straight and curved portions at the same time. D: averaged fluorescent intensity for adherent tumor cells as a function of time during 30 min perfusion under normal flow at the inner and outer curved part and the straight portion in the microvessels. *P < 0.05, compared TC adhesion with that at 5 min; #P < 0.05, compared TC adhesion between straight and curved portions at the same time; n, number of vessels; m, number of ROIs. Values are means ± SE.
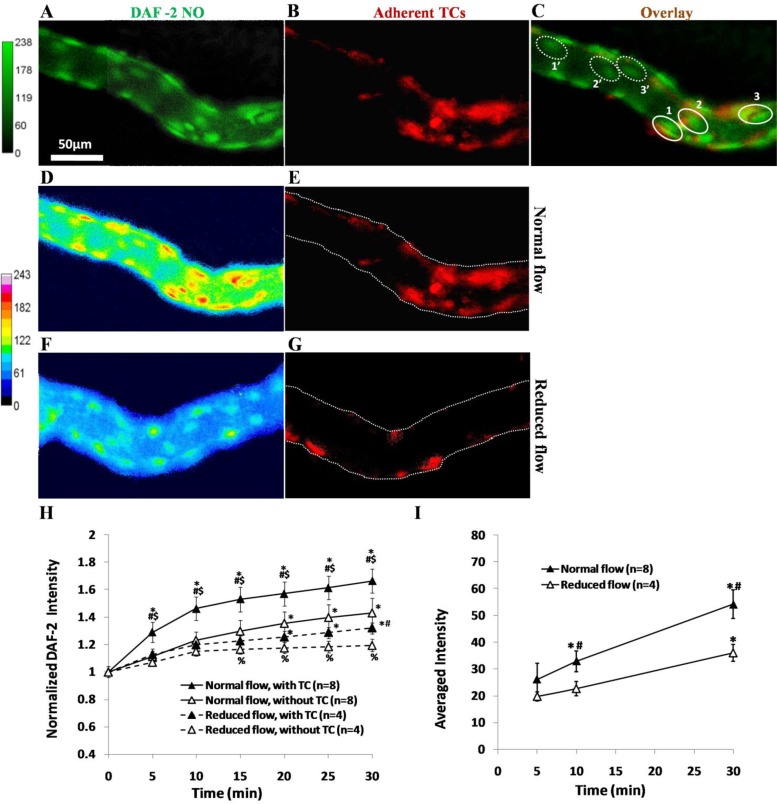
Fig. 3.
A representative experiment showing endothelial NO production (A) and tumor cell (TC) adhesion (B) along the same microvessel after 30 min perfusion under normal flow. The focus is on a focal plane for endothelial cells (ECs) near the bottom of the vessel. C: overlay of A and B. In C, we define 3 typical ROIs, solid line-enclosed oval-shaped regions 1, 2, and 3, for NO production in ECs with adherent TCs, and 3 ROIs, dotted line-enclosed regions 1′, 2′, and 3′ for NO production in ECs without adherent TCs in the same vessel. The ROI is an oval-shaped area with ∼40 μm for the long axis and 25 μm for the short axis. For a vessel with 350–550 μm focused length, there are 12–18 ROIs with adherent TCs and equal amount of ROIs without TCs under normal flow, and a total of 123 ROIs with adherent TCs in 8 vessels. Under the reduced flow, there are only 8–12 ROIs with adherent TCs in each vessel with a total of 38 ROIs with adherent TCs in 4 vessels. Endothelial NO production and TC adhesion along the same microvessel after 30 min perfusion under normal flow (D and E) and under the reduced flow (F and G) are shown. H: comparison of temporal NO production profiles in ECs with adherent TCs (▲) and those without adherent TCs (△) in the same vessels under normal (solid line) and reduced (dashed line) flows. *P < 0.05, compared with that at the end of DAF-2 DA loading (t = 0); #P < 0.05, compared NO production in the ECs with adherent TCs and those without in the same vessel at the same time; $P < 0.05, compared NO production in the ECs with adherent TCs under normal and reduced flows at the same time; %P < 0.05, compared NO production in the ECs without adherent TCs under normal and reduced flows at the same time. I: comparison of temporal TC adhesion profiles under normal and reduced flows. *P < 0.05, compared TC adhesion with that at 5 min; #P < 0.05, compared TC adhesion under normal and reduced flows at the same time; n, number of vessels. Values are means ± SE.
MDA-MB-231 cell adhesion in individually perfused microvessels.
To simultaneously measure tumor cell adhesion and endothelial NO production in a vessel, a microvessel with a Y-branch at the upstream was chosen. Figure 1B shows the technique for the measurement. To control the perfusion velocity in the vessel, the perfusion flow velocity versus the driving pressure from the water manometer connecting to the micropipette was calibrated from the movement of a marker TC (5, 60). Usually, a driving pressure of 12∼15 cmH2O in the micropipette cannulating the side vessel at the Y-branch generated a mean flow velocity of ∼1,000 μm/s in the downstream vessel (Fig. 1B). The reduced velocity of ∼300 μm/s was achieved by decreasing the driving pressure to ∼3–5 cmH2O. We also controlled the rate of TCs out of the micropipette to ∼1 cell/s. The adhesion process was recorded at ∼2 frames/s in a ∼5–20 min interval for 30 min in each experiment. Our imaging system with 20×/NA 0.75 objective lens has the depth of light collection ∼100 μm (5), which can collect all the fluorescent light from the adherent TCs when we focused at the midplane or a focal plane at the bottom of the vessel. Because of the vessel curvature, it is hard to distinguish between one versus two adjacently adherent TCs. Therefore, we used the fluorescent intensity instead of number of TCs for the quantification. In vitro calibration in a prior study showed that the number of TCs is proportional to the fluorescent intensity of TCs (60) by our imaging system.
Measurement of NO production in cultured endothelial cell monolayers and tumor cells in the parallel plate flow chamber.
To investigate how much NO production comes from the TCs in the microvessel under normal flow, we measured NO production in ECs alone and TCs alone using the flow chamber under the similar conditions as in the microvessels. For EC alone, after confluence, bEnd3 monolayer in the flow chamber was loaded with DAF-2 DA for 45 min at a very low flow, then for 30 min at the flow with ∼3 dyn/cm2 wall shear stress, the same as that in the microvessel with ∼1,000 μm/s mean velocity (62). The DAF-2 images of EC monolayers were taken at the end of 30 min. For TC alone, 0.5 million/ml MB-MDA-231 TCs in DAF-2 DA 1% BSA-Ringer was perfused into the flow chamber for 30 min under the flow with ∼3 dyn/cm2 wall shear stress. After the nonadherent TCs were washed away, the DAF-2 images of TCs (green light) and the images of TCs (red light) were taken correspondingly.
Measurement of NO production in cultured endothelial cell monolayers and tumor cells under static conditions.
To test if TC adhesion to ECs increases NO production of ECs, we measured NO production in cultured EC monolayers without and with adding TCs under static conditions. After DAF-2 DA loading for 45 min, the DAF-2 images of EC monolayers were taken. For those without adding TCs, the DAF-2 images were taken again at 30 min after loading. For those with adding TCs, after DAF-2 DA loading, ∼0.2 million TCs (∼0.25 million/cm2) were added and adhered for 30 min. After the nonadherent TCs were washed away, the DAF-2 images of EC monolayer with adherent TCs were taken. We also measured the NO production of TCs alone. The same amount of TCs were added to the fibronectin-coated dish along with DAF-2 DA in 1% BSA-Ringer solution for 45 min, after washing away the floating TCs and waited for another 30 min, the DAF-2 images of TCs and TC images were taken by switching the excitation/emission lights.
To test whether increasing NO production of ECs attracts more TCs, we further measured the TC adhesion to EC monolayers with enhanced NO production by a NO donor, SNP. After 45 min DAF-2 DA loading, the EC monolayer was treated by 100 and 500 μM SNP for 20 min. These concentrations of the NO donor were in the physiological ranges (69). After the SNP was washed away, the DAF-2 images of EC monolayer were taken, then the TCs were added and allowed to adhere for 30 min. After the nonadherent TCs were washed away, the adherent TCs were counted under each condition.
Measurement of apparent microvessel permeability to albumin P.
To test whether the higher NO production location at the microvessel and the preferred location of TC adhesion (curved) are the same locations with the higher microvessel permeability, and to test if reducing NO production by inhibiting eNOS decreases microvessel permeability, we measured microvessel permeability P to albumin in individual microvessels using θ pipette as described (14, 60). Briefly, when FITC/TR-BSA was perfused into the vessel and the vessel was exposed to a 495/561 nm wavelength light for 30–60 s, the images were recorded simultaneously at a rate of 3 images/s by a high-performance digital 12-bit CCD camera (SensiCam QE; Cooke Corporation) with a Super Fluor 20× objective lens (NA = 0.75; Nikon). The P was then determined offline. The total fluorescence intensity (I) in the lumen of a vessel and surrounding tissue was determined by image analysis software (Intracellular Imaging, Cincinnati, OH). The measuring window was 50–150 μm long and 100–200 μm wide for both curved and straight portions and was set at least 100 μm from the cannulation site and from the base of the bifurcation to avoid solute contamination from the cannulation site and from the side arms. Permeability P was calculated by P = (1/ΔI0)(dI/dt)0(r/2), where ΔI0 was the step increase in fluorescence intensity in the measuring window when the perfused dye just filled up the vessel lumen, (dI/dt)0 was the initial rate of increase in fluorescence intensity after the dye filled the lumen and began to accumulate in the tissue, and r was the vessel radius. In general, 3 to 4 curved portions and equal numbers of straight portions were measured for each vessel.
Data analysis.
Data are presented as means ± SE, unless indicated otherwise. Statistical analyses were performed by t-test or two-way ANOVA followed by a Tukey's post hoc analysis using Sigma Plot 11.2 from Systat Software (San Jose, CA). Significance was assumed for probability levels P < 0.05.
RESULTS
Effect of curvature on NO production and tumor cell adhesion in postcapillary venules.
Figure 2A demonstrates endothelial NO production profiles along a typical microvessel with a straight and a curved portion under normal flow for 30 min without TC perfusion. When compared with the straight portion, there was a higher NO production at the curved portion. Figure 2B shows a typical photomicrograph for TC adhesion in a microvessel with straight and curved portions after 30 min perfusion under normal flow in another group of the vessels perfused with TCs. Figure 2C summarized the DAF-2 intensity in 11 vessels with 25 curved portions (25 ROIs for the inner side and 25 ROIs for the outer side) and 25 straight portions (50 ROIs). The averaged DAF-2 intensity in the ROIs of the straight portions at the end of DAF-2 DA loading (t = 0) was used for the normalization in each vessel. For both straight and curved portions, the NO production was significantly increased after 10 min perfusion. There was a significant difference in the NO production between the curved and straight portions 10 min after normal flow, but no significant difference between the inner and outer sides although there was a slightly higher NO production at the inner side of the curved portion. After 30 min, the NO production increased to 1.3-fold in straight portions and to ∼1.6-fold in curved portions. Correspondingly, there were significantly more TCs adhering to the curved portions starting at 10 min but there was no significant difference between the inner and outer sides. After 30 min, the adherent TCs at the curved portions were approximately twofold those at the straight portions (Fig. 2D).
Effect of flow on NO production and tumor cell adhesion in postcapillary venules.
Figure 3, A–C, shows how to quantify the NO production in a microvessel with and without adherent TCs. Figure 3A is the DAF-2 intensity profile (green) in a microvessel, and Fig. 3B is the TC adhesion (red) in the same microvessel; Fig. 3C is the overlay of Fig. 3, A and B. Dotted lines enclosed elliptic areas indicate ECs without adherent TCs, whereas solid lines enclosed areas indicate ECs with adherent TCs. Figure 3, D–G, demonstrates NO production (D, F) and TC adhesion (E, G) in microvessels after 30 min perfusion under normal (D, E) and reduced (F, G) flows, respectively. Figure 3H summarizes NO production in ECs with and without adherent TCs in the same vessels under normal or reduced flows. Under the normal flow, starting at 5 min, there was significant difference in the NO production in ECs with adherent TCs and in ECs without adherent TCs, indicating higher NO production locations are preferred TC adhesion locations in the same vessels. Under the reduced flow, the NO production in ECs with adherent TCs differed with that in ECs without adherent TCs only after 30 min perfusion. NO productions were significantly higher in the microvessels under the normal flow compared with those under the reduced flow for both ECs with and without adherent TCs. Correspondingly, there were more TCs adhering to the microvessels under the normal flow than those under the reduced flow (Fig. 3I).
Effect of inhibiting endothelial NO production on tumor cell adhesion in postcapillary venules under normal flows.
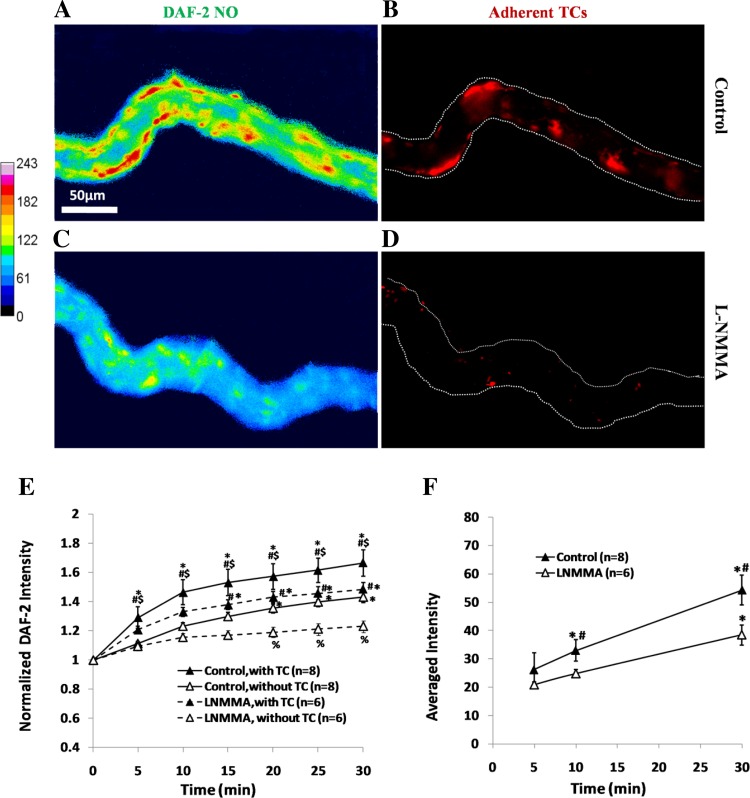
As shown in Figs. 2 and 3, the locations with higher NO production in microvessels are preferred locations for TC adhesion. To test the hypothesis that suppression of EC NO production reduces TC adhesion to the microvessels, we used an eNOS inhibitor l-NMMA to pretreat the microvessels before TC perfusion. Figure 4 shows NO production and TC adhesion profiles in vessels without (Fig. 4, A and B) and with (Fig. 4, C and D) l-NMMA treatment. Figure 4E summarizes temporal NO production in the microvessels with and without l-NMMA treatment and in the ECs with and without adherent TCs in the same vessels. Inhibition of eNOS reduced NO production in the microvessels (Fig. 4, A, C, and E) and indeed decreased TC adhesion to the microvessel wall (Fig. 4, B, D, and F).
Fig. 4.
Endothelial NO production and TC adhesion along the same microvessel after 30 min perfusion under control (A and B) and under NG-monomethyl-l-arginine (l-NMMA) treatment (C and D) under normal flow. The focus is on a focal plane for endothelial cells (ECs) near the bottom of the vessel. With the same definitions for ROIs with and without adherent TCs as in Fig. 3C, there are 8–12 ECs with TC adhesion and the same amount of ROIs without TC adhesion in each vessel with l-NMMA treatment, and a total of 58 ECs with TC adhesion in 6 vessels. E: comparison of temporal NO production profiles in ECs with adherent TCs (▲) and those without adherent TCs (△) in the same vessels under control (solid line) and l-NMMA treatment (dashed line). *P < 0.05, compared with that at the end of DAF-2 DA loading (t = 0); #P < 0.05, compared NO production in the ECs with adherent TCs and those without in the same vessel at the same time; $P < 0.05, compared NO production in the ECs with adherent TCs under control and l-NMMA treatment at the same time; %P < 0.05, compared NO production in the ECs without adherent TCs under control and l-NMMA treatment at the same time. F: comparison of temporal TC adhesion profiles under control and l-NMMA treatment. *P < 0.05, compared TC adhesion with that at 5 min; #P < 0.05, compared TC adhesion under control and l-NMMA treatment at the same time; n, number of vessels. Values are means ± SE.
Enhanced NO production in endothelial cells increases tumor cell adhesion in vitro under static conditions.
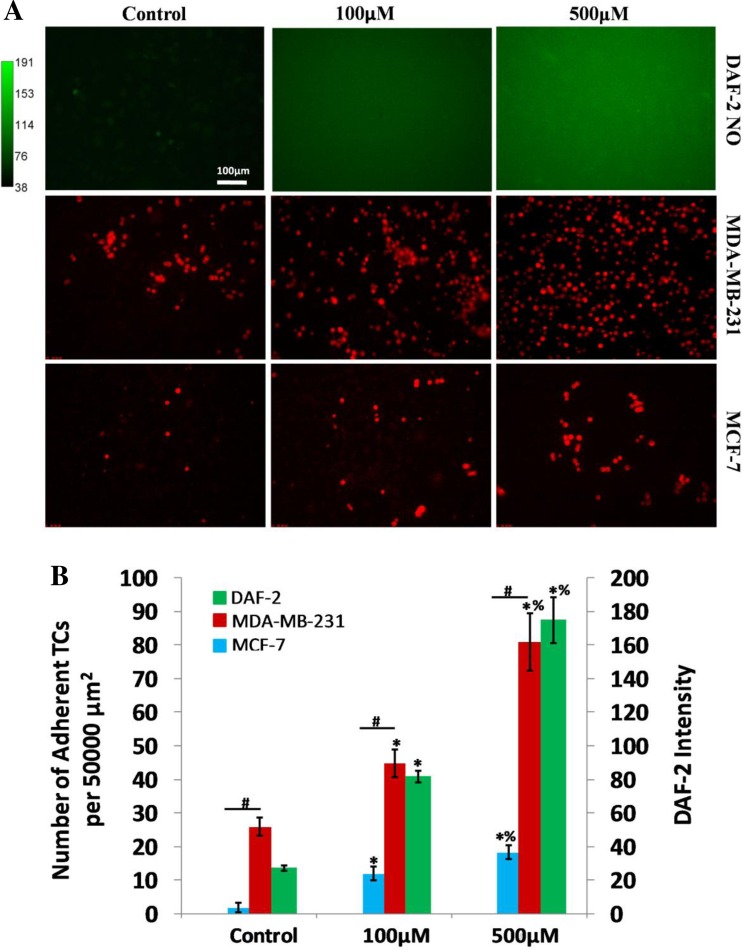
To single out the contribution of the NO effect on TC adhesion from that of the flow, in vitro TC adhesion was performed on bEnd3 (mouse brain microvascular endothelial cells) monolayers under static conditions. The first row in Fig. 5A shows the DAF-2 images of bEnd3 monolayers under control and after 20 min pretreatment with 100 and 500 μM SNP, a NO donor. Our calibration showed that 20-min application of SNP at these two concentrations enhanced the endothelial NO levels comparable with those under normal and reduced flows, and with l-NMMA treatment under normal flows (Figs. 3 and 4). The second row shows the adherent MDA-MB-231 with a high metastatic potential after 30-min adhesion in each case, and the third row shows the corresponding adhesion for a mammary TC MCF-7 with a low metastatic potential (74). Figure 5B indicates that without flow both TCs still prefer to adhere at ECs with higher NO concentration. The adherent amount of the highly metastatic MDA-MB-231 is 13.8-, 6.8-, and 9.5-fold of that of the low metastatic MCF-7, under control, 100, and 500 μM SNP treatment, respectively.
Fig. 5.
Effects of a NO donor, sodium nitroprusside (SNP), on EC NO production and TC adhesion in cultured bEnd3 monolayers. A: first row shows DAF-2 NO images in bEnd3 monolayers under control and after 20 min treatment of 100 and 500 μM SNP, respectively; second row and third rows show adherent MDA-MB-231 and MCF-7 cells after 30 min adhesion on EC monolayers under control and after 100 and 500 μM SNP treatment, respectively. B: comparison of NO production in EC monolayers under control and after 20 min treatment of 100 and 500 μM SNP and corresponding TC adhesion. For each condition, 3 culture dishes were measured, and 4 regions of ∼750 × 560 μm were imaged in each dish. For NO production, the DAF-2 NO intensity was normalized by that under control condition. *P < 0.05, compared with control for TC adhesion and NO production, respectively; #P < 0.05, compared adhesion of MDA-MB-231 cells with that of MCF-7 cells; %P < 0.05, compared effect of 500 μM SNP with that of 100 μM SNP; n = 3. Values are means ± SE.
Tumor cell adhesion to endothelial monolayers does not increase NO production in endothelial cells under static conditions.
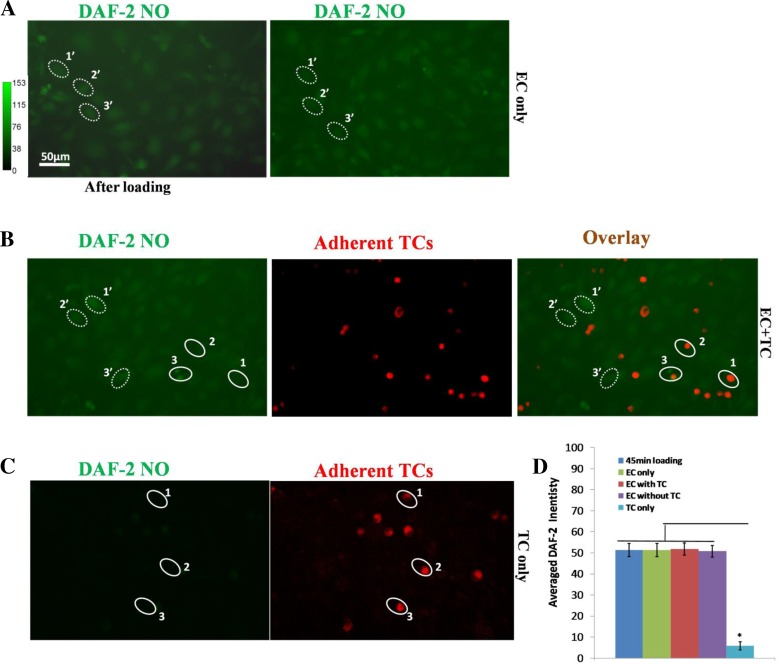
To test whether TC adhesion alone contributes to the NO production in ECs, we measured NO production in endothelial monolayer alone (Fig. 6A), endothelial monolayer with TC adhesion for 30 min (Fig. 6B), and TC alone (Fig. 6C). Figure 6D compares the NO production in EC only right after DAF-2 DA loading (blue bar), 30 min after loading (green bar), in EC monolayers added with TCs (red bar for ECs with adherent TCs and purple bar for those without adherent TCs), and in TCs alone (cyan bar). We can see from Fig. 6D that there was no significant difference in NO production in ECs under all the conditions, with and without adherent TCs. TC adhesion does not increase NO production in ECs. In addition, the NO production in TCs alone was ∼8% of that in ECs.
Fig. 6.
DAF-2 NO images under static conditions in bEnd3 monolayer with ECs only (A), in bEnd3 monolayer with adherent TCs (B), and in TCs only (C). Left image in A is that after 45-min DAF-2 DA loading, and right image is that for 30 min later. Left image in B is for DAF-2 NO 30 min after 45-min DAF-2 DA loading in bEnd3 monolayer with adherent TCs, middle image is for adherent TCs, and right image is the overlay of the left and middle images. Left image in C is for DAF-2 NO 30 min after 45-min DAF-2 DA loading in TCs, and the right one is for TCs. The dotted line-enclosed oval-shaped regions are ROIs (∼40 μm for the long axis and 25 μm for the short axis) for NO production quantification in ECs without adherent TCs and the same-sized solid line-enclosed ROIs for those in ECs with adherent TCs or that in TCs. Four regions of ∼750 × 560 μm were taken from each experiment. Twenty to thirty ROIs for each type were chosen on each region. D: comparison of NO production in ECs and TCs under static conditions. The averaged DAF-2 intensity is for the ROIs over 3 experiments for each case. *P < 0.05, compared NO production in TCs with that in ECs. Values are means ± SE.
NO production from tumor cells can be neglected under normal flows.
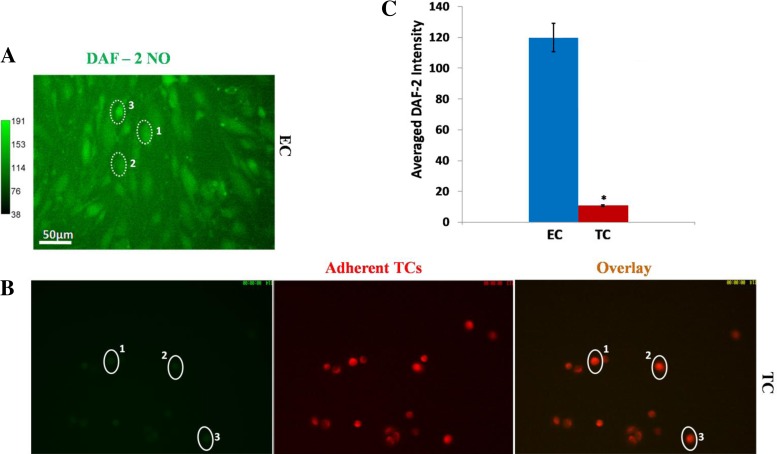
To test whether NO production in TCs under flow is negligible compared with that in ECs, we measured NO production in ECs alone and TCs alone in the flow chambers after 30 min under ∼3 dyn/cm2 wall shear stress, which is the same as that in an intact microvessel under the normal flow of ∼1,000 μm/s mean velocity. Figure 7A demonstrates the DAF-2 image for EC alone and Fig. 7B for TCs alone. Figure 7C shows that the NO production in TCs is only ∼10% that in ECs under normal flow.
Fig. 7.
DAF-2 NO images in ECs of bEnd3 monolayer (A) and TCs (B) under normal flow (or equivalent 3 dyn/cm2 wall shear stress) for 30 min. In B, the left image shows the DAF-2 NO image of TCs, the middle one shows the TCs, and the right one is the overlay of the left and middle images. Three typical ROIs, dotted- or solid line-enclosed oval-shaped regions (∼40 μm for the long axis and 25 μm for the short axis) 1, 2, 3 in A and B, are for NO production quantification in ECs and TCs. Three experiments were done for each cell type. Four regions of ∼750 × 560 μm were taken from each experiment. Seventy to eighty ROIs were chosen on each region. B: comparison of NO production in ECs and TCs under normal flow. The averaged DAF-NO intensity is for the ROIs over 3 experiments for each cell type. *P < 0.05, compared NO production in TCs with that in ECs. Values are means ± SE.
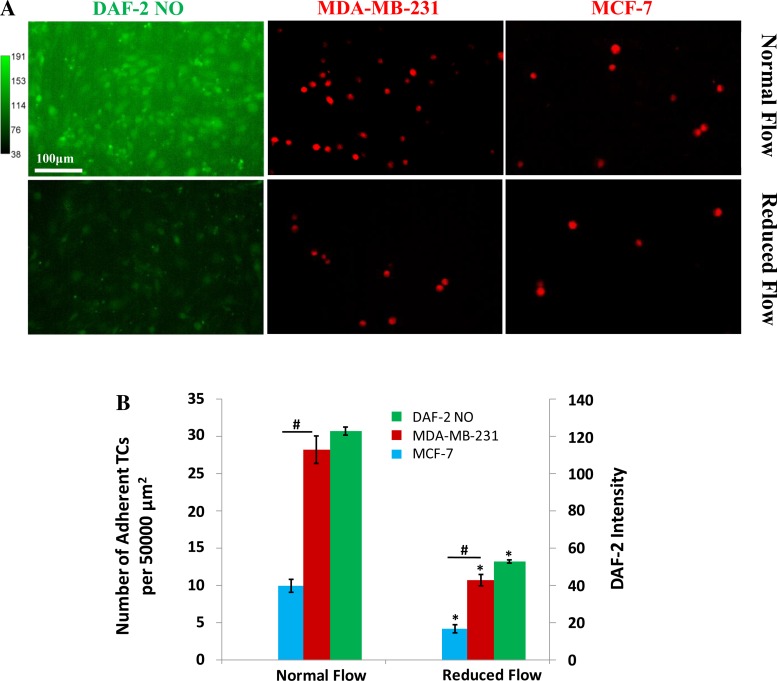
Comparison of adhesion of MDA-MB-231 and MCF-7 under normal and reduced flows.
In Fig. 8, we compared the adhesion of highly metastatic MDA-MB-231 cells and that of low metastatic MCF-7 cells. The first column in Fig. 8A shows the DAF-2 images of bEnd3 monolayer under normal (top) and reduced (bottom) flows. The second and third columns show the adhesion of MDA-MB-231 and MCF-7 cells, under normal and reduced flows, respectively. Figure 8B compares the endothelial NO production (green bar) and the adhesion of MDA-MB-231 (red bar) and MCF-7 (blue bar) cells under normal and reduced flows. The adhesion of highly metastatic MDA-MB-231 is 2.8-fold and 2.5-fold that of low metastatic MCF-7 under normal and reduced flows, respectively. However, the adhesion of both TCs increases under normal flows with higher endothelial NO production.
Fig. 8.
Comparison of adhesion of MDA-MB-231 cells with that of MCF-7 cells under normal and reduced flows. A: first column shows DAF-2 NO images in bEnd3 monolayers under normal and reduced flows, respectively; second and third columns show adherent MDA-MB-231 and MCF-7 cells after 30 min adhesion on EC monolayers under normal and reduced flows, respectively. B: comparison of NO production in EC monolayers under normal and reduced flows and corresponding TC adhesion. For each condition, 3 flow chambers were measured and 4 regions of ∼750 × 560 μm were imaged in each chamber. *P < 0.05, compared with control for TC adhesion and NO production, respectively; #P < 0.05, compared adhesion of MDA-MB-231 cells with that of MCF-7 cells; n = 3. Values are means ± SE.
Inhibition of eNOS reduces microvessel permeability to albumin (P) in postcapillary venules.
To investigate the underlying mechanism by which TCs prefer to adhere to the curved portion of a microvessel with higher NO concentration, we measured the microvascular permeability P to albumin at the curved and straight portions of the microvessels. We found that although the whole microvessels have the normal P around 0.92 ± 0.05 × 10−6 cm/s (n = 7) (5), the P of the curved portions is 1.06 ± 0.06 × 10−6 cm/s, which is ∼1.4-fold that of the straight portions, 0.75 ± 0.02 ×10−6 cm/s (P < 0.001). These results are consistent with our previous observations that TCs prefer to adhere to the microvessels with higher permeability (5, 15, 60).
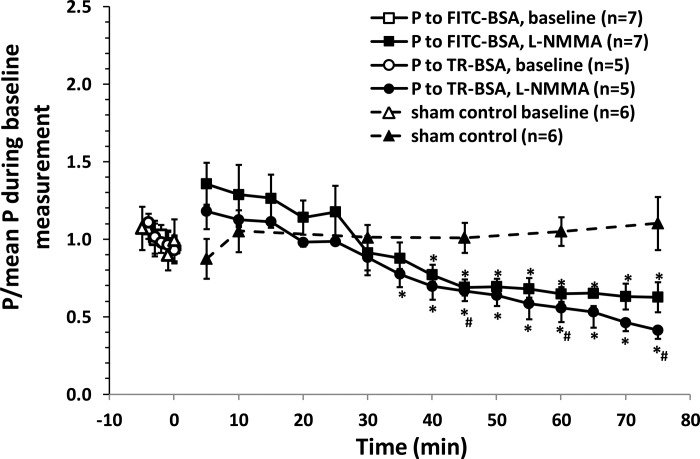
Figure 4 shows that inhibition of eNOS by l-NMMA reduces NO production as well as TC adhesion, and the above results indicate that the microvessel location with the higher NO production has higher permeability. This led to the hypothesis that reducing NO production in the microvessel should reduce its permeability under basal conditions. We then measured P to albumin under l-NMMA treatment. Figure 9 demonstrates that at the onset of l-NMMA treatment, P to FITC-BSA had an insignificant increase compared with its baseline, but it started to gradually decrease after 30-min treatment (significant decrease from 40 min), to ∼65% of its baseline after 60-min treatment. Rumbaut et al. (56) reported that leakage responses to another eNOS inhibitor, NG-nitro-l-arginine methyl ester (l-NAME), differed with fluorescent dye used to label albumin. They found that superfusion with l-NAME increased microvessel leakage to FITC-BSA but not to Texas Red (TR), Oregon Green, and DTAF-labeled BSA. We then used TR labeled albumin to determine the microvessel permeability under control and l-NMMA treatment. The baseline P to TR-BSA, 0.96 ± 0.05 × 10−6 cm/s, was similar to P to FITC-BSA, 0.92 ± 0.05 × 10−6 cm/s (P = 0.39). At the onset of l-NMMA treatment, P to TR-BSA had an insignificant increase from its baseline, but it had a significant decrease after only 35 min treatment, to ∼56% of its baseline after 60 min treatment. There was no significant difference in P to TR-BSA and P to FITC-BSA under the treatment of l-NMMA (P > 0.1) at all times. The difference from what was reported (56) may be due to two possible reasons. One is that we perfused l-NMMA into the microvessel, whereas Rumbaut et al. (56) superfused l-NAME onto the microvessel. Another is that the fluorescent dye may interact with blood-borne constituents when systemically injected into the circulation. The interaction-induced substances may affect microvessel permeability.
Fig. 9.
Effect of l-NMMA treatment on microvessel permeability to albumin. *P < 0.05, compared with baseline; #P < 0.05, compared with sham control at the same time. Values are means ± SE. TR, Texas Red.
DISCUSSION
Elucidating where and why circulating TCs prefer to adhere in the microvasculature, especially in intact microvessels under normal physiological flows, is important in developing antimetastatic strategies at a early stage. The current study clearly showed that circulating breast cancer cells MDA-MB-231 prefer to adhere to the microvessel sites with higher NO concentrations (e.g., curved portions), which are generated by the localized shear stresses and shear stress gradients induced by normal blood flows (40). It also showed that inhibition of eNOS reduces flow-induced NO production in the ECs forming the microvessel wall and attenuates TC adhesion.
Many previous studies have shown that NO is a double-edged sword for tumor metastasis (54, 71). Cytokine-activated ECs can produce NO to lyse TCs (39). In response to shear stress, the EC-generated NO in the pulmonary microvessels can kill the arrested B16 melanoma cells (52). Trapped melanoma cells in the mouse liver induce NO release, which is cytotoxic to TCs (68). Under no flow conditions, Kong et al. (34) found that 30-min pretreatment of diethylenetriamine (DETA)/NO (1 mM), a NO donor, attenuates melanoma cell (RPMI 1846) adhesion in naive and LPS-activated isolated rat postcapillary venules, whereas l-arginine, an NO precursor, fails to decrease TC adhesion to naive venules but abolishes LPS-stimulated TC adhesion. A recent study by Lu et al. (41) demonstrated that another NO donor, S-nitrosocaptopril (CAP-NO), inhibits adhesion of colorectal cancer cells HT-26 to cytokines-stimulated human umbilical vein endothelial cells (HUVECs) via downregulating expression of CAMs (VCAM-1). Caterina et al. (8) also showed that NO donors decrease cytokine-induced activation of human saphenous vein endothelial cells by inhibiting the expression of CAMs including VCAM-1, ICAM-1, and E-selectin. Differently, Yudoh et al. (77) reported that exogenous NO, DETA/NO (20 nM), significantly enhances fibrosarcoma cell HT1080 adhesion to HUVECs and increases HUVEC monolayer permeability. DETA/NO pretreatment does not induce expression of CAMs on TCs, but it greatly enhances expression of CAMs (ICAM-1, ELAM-1) on ECs. Our current study also showed that a NO donor, SNP, significantly enhanced TC adhesion to ECs. Lahiri and Martin (37) investigated NO effect on metastasis of several human breast cancer cells including MDA-MB-231 and found that SNP decreases motility and increases adhesion of these breast cancer cells. These previous studies demonstrated the multiple roles of NO in tumor metastasis. At different concentration levels and under different conditions, NO can kill TCs directly, NO can attenuate TC adhesion to ECs that are stimulated by cytokines, and NO can also enhance TC adhesion to ECs. Our current study directly demonstrated, for the first time in intact microvessels under known flow conditions, that normal physiological flow-induced localized mechanical factors can activate eNOS of ECs forming the microvessel wall to generate NO, which enhances TC adhesion in these locations, such as curved parts of microvessels.
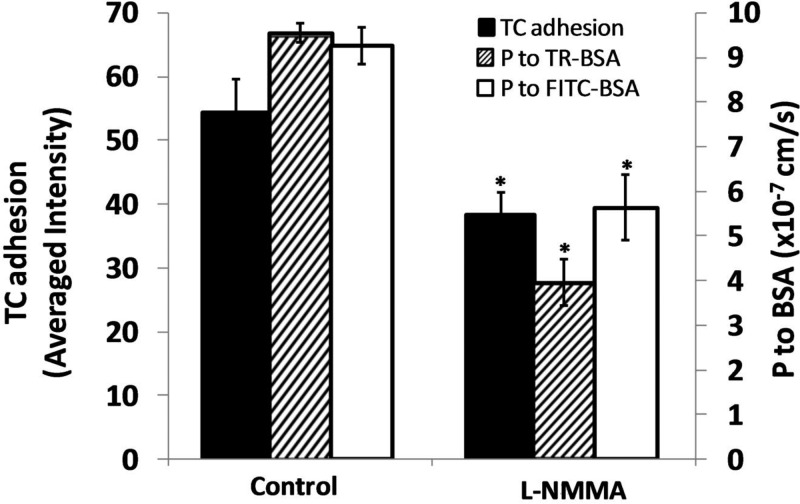
Although the cause for the TC adhesion by the flow-induced endogenous NO has not been clarified yet, we postulated that both enhanced microvessel permeability and CAMs play a role in this process. Previous studies have shown that activation of eNOS by cytokines or inflammatory agents enhances NO release that increases microvessel permeability (9, 16, 26, 29, 48, 80, 81) and may or may not enhance leukocyte adhesion (27). Yuan et al. (76) reported that increasing flow velocity in isolated coronary venules increases vessel permeability to albumin, whereas an eNOS inhibitor, l-NMMA, reduces baseline permeability and abolishes the flow-induced permeability changes. On the contrary, others reported that inhibition of eNOS under resting conditions transiently increases microvessel permeability for 10–30 min (29, 35) and can enhance leukocyte adhesion to postcapillary venules (36). Because we found that curved portions of microvessels have higher NO concentration due to localized shear stresses or shear stress gradients (40) under normal flows, we postulated that microvessel permeability to albumin (P) should be higher at the curved portions compared with that at the straight portions. The averaged P at the curved portions is indeed ∼40% higher than that at the straight portions. TCs also adhered more to the curved portion. This is consistent with the previous observation that TCs preferred to adhere to the microvessel walls or EC monolayers with higher permeability under both static and flow conditions (5, 11, 15, 38, 60). Inhibition of eNOS by l-NMMA initially increases P, similar to what is reported in Kubes and Granger (35) and He et al. (29); however, after 30 min l-NMMA treatment, P starts to decrease. That the longer time treatment with l-NMMA decreases P is also consistent with the observation by Yuan et al. (76). Decreased P by eNOS inhibition also reduces TC adhesion to the microvessel wall by ∼30% after 60–75 min l-NMMA treatment. Figure 10 summarizes the effect of eNOS inhibition on microvessel permeability and tumor cell adhesion in intact postcapillary venules.
Fig. 10.
Summary of the effects of l-NMMA on microvessel permeability to albumin and MDA-MB-231 cell adhesion. *P < 0.05, compared with the control. The permeability shown was at 75 min post-l-NMMA treatment. TC adhesion was at 30 min after 45-min pretreatment of l-NMMA. TR, Texas Red.
Shear stress affects physical and biochemical characteristics of ECs including microvessel (or EC monolayer) permeability and CAMs (2, 7, 30, 32, 44, 46, 66), as well as CAMs at TCs (61, 78). The upregulated CAMs at the TCs and ECs under proper levels of flows (shear stress) increase the opportunity of TC adhesion to ECs. Previous studies found that more than 90% of TCs adhered to EC junctions (11, 12, 15); the increased paracellular permeability allows more CAMs at the ECM expose to TCs for their adhesion (11). The mechanisms by which flow-induced NO under normal physiological flows increases microvessel permeability are not obvious. One possible explanation is that NO, induced by the venular endothelial cells in response to flow, relaxes endothelium and/or pericytes and alters endothelial junctions, resulting in increased microvessel permeability (43, 55). Another explanation is that NO can regulate VEGF expression in ECs (33, 67). VEGF has been known to increase microvessel permeability (3, 14, 15, 60). The cause for decreasing microvessel permeability to below its baseline value after longer time eNOS inhibition is also unclear. One possibility is that reduced production of NO generates lower levels of c-GMP, which can locally elevate intracellular cAMP levels by inhibiting phosphodiesterase 3 (65). cAMP has been known to decrease microvessel permeability (1, 15, 28, 63) and reduce TC adhesion to the microvessel wall (15).
In the current study, we perfused TCs into individual microvessels in the absence of whole blood and found that TC prefer to adhere to the curved portions of the vessels. The same observation was reported by Liu et al. (40) for thrombosis in curved microvessels with whole blood. Guo et al. (22) also reported that TCs systemically injected into the blood circulation preferred to adhere to the bifurcations of microvasculature in rat mesentery, especially at the intersections of the capillary/postcapillary venule and the postcapillary venule with relatively lower velocity. Similar to the curved portions, there are localized shear stresses at the bifurcations, which induce higher NO generation and attract more TCs. But the flow rate is not too high to wash away TCs.
Rumbaut et al. (57) reported that hydraulic conductivity of rat mesenteric venules decreased by 50% by superfusion of l-NAME for 2 to 15 min in the absence of blood-borne constituents. In contrast, He et al. (29) found that perfusion of l-NAME into the microvessel lumen transiently increased its hydraulic conductivity by two- to fivefold, depending on l-NAME concentrations. One possible explanation is that superfusion of l-NAME has a different effect on endothelial NO levels from that by perfusion l-NAME directly into the vessel lumen. However, in the presence of blood-borne constituents, superfusion of l-NAME for 15 min increased hydraulic conductivity by 78%. In Kubes et al. (36), superfusion of l-NAME for 30 min increased leukocyte adhesion to cat mesenteric microvessels. Our results showed that at the onset of l-NMMA treatment, P to albumin increased from its baseline (Fig. 9), which is consistent with their observations.
To further investigate the mechanistic causes, an oxidant or a peroxynitrite scavenger can be used in the future study to see if it can be as effective as l-NMMA in reducing tumor cell adhesion. In addition, chronic NO productions occur in many proinflammatory states, many of which promote the likelihood of metastasis. Therefore, in the clinical application to prevent metastasis by eNOS inhibition, long-term treatment is required. Furthermore, males do get breast cancer so study in male rats is informative.
In summary, our study showed that circulating tumor cells prefer to adhere to the microvessel sites with higher NO concentrations, which are generated by the localized shear stresses and shear stress gradients induced by normal flows. eNOS inhibition reduces tumor cell adhesion and also decreases microvessel permeability.
GRANTS
This work was supported by the National Institutes of Health (National Cancer Institute) grant SC1CA153325-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.Z., M.Z., and B.M.F. conception and design of research; L.Z. and M.Z. performed experiments; L.Z. and B.M.F. analyzed data; L.Z. and B.M.F. interpreted results of experiments; L.Z. prepared figures; L.Z. and B.M.F. drafted manuscript; L.Z. and B.M.F. approved final version of manuscript; B.M.F. edited and revised manuscript.
REFERENCES
- 1.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol Heart Circ Physiol 274: H1885–H1894, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol Heart Circ Physiol 271: H2520–H2528, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol 285: C499–C508, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cai B, Fan J, Zeng M, Zhang L, Fu BM. Adhesion of malignant mammary tumor cells MDA-MB-231 to microvessel wall increases microvascular permeability via degradation of endothelial surface glycocalyx. J Appl Physiol (1985) 113: 1141–1153, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke JP, Stamler J, Andon N, Davies PF, McKinley G, Loscalzo J. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol Heart Circ Physiol 259: H804–H812, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duran WN, Breslin JW, Sanchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res 87: 254–261, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earley S, Plopper GE. Disruption of focal adhesion kinase slows transendothelial migration of AU-565 breast cancer cells. Biochem Biophys Res Commun 350: 405–412, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Cai B, Zeng M, Hao Y, Giancotti FG, Fu BM. Integrin beta4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF. Ann Biomed Eng 39: 2223–2241, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan J, Fu BM. Quantification of malignant breast cancer cell MDA-MB-231 transmigration across brain and lung microvascular endothelium. Ann Biomed Eng. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu BM, Shen S. Acute VEGF effect on solute permeability of mammalian microvessels in vivo. Microvasc Res 68: 51–62, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Fu BM, Yang J, Cai B, Fan J, Zhang L, Zeng M. Reinforcing endothelial junctions prevents microvessel permeability increase and tumor cell adhesion in microvessels in vivo. Sci Rep 5: 15697, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98: 2604–2609, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer 6: 521–534, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Fukumura D, Yuan F, Endo M, Jain RK. Role of nitric oxide in tumor microcirculation. Blood flow, vascular permeability, and leukocyte-endothelial interactions. Am J Pathol 150: 713–725, 1997. [PMC free article] [PubMed] [Google Scholar]
- 19.Gassmann P, Haier J. The tumor cell-host organ interface in the early onset of metastatic organ colonisation. Clin Exp Metastasis 25: 171–181, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia 7: 522–527, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glinskii OV, Turk JR, Pienta KJ, Huxley VH, Glinsky VV. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumour cells. J Physiol London 554: 89–99, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo P, Cai B, Lei M, Liu Y, Fu BM. Differential arrest and adhesion of tumor cells and microbeads in the microvasculature. Biomech Model Mechanobiol 13: 537–550, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo X, Kassab GS. Role of shear stress on nitrite and NOS protein content in different size conduit arteries of swine. Acta Physiol (Oxf) 197: 99–106, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad O, Chotard-Ghodsnia R, Verdier C, Duperray A. Tumor cell/endothelial cell tight contact upregulates endothelial adhesion molecule expression mediated by NFkappaB: differential role of the shear stress. Exp Cell Res 316: 615–626, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Hatakeyama T, Pappas PJ, Hobson RW, Boric MP 2nd, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol 574: 275–281, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res 87: 281–290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He P, Zeng M, Curry FE. Dominant role of cAMP in regulation of microvessel permeability. Am J Physiol Heart Circ Physiol 278: H1124–H1133, 2000. [DOI] [PubMed] [Google Scholar]
- 29.He P, Zeng M, Curry FE. Effect of nitric oxide synthase inhibitors on basal microvessel permeability and endothelial cell [Ca2+]i. Am J Physiol Heart Circ Physiol 273: H747–H755, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Jo H, Dull RO, Hollis TM, Tarbell JM. Endothelial albumin permeability is shear dependent, time dependent, and reversible. Am J Physiol Heart Circ Physiol 260: H1992–H1996, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Kielbik M, Szulc I, Brzezinska M, Bednarska K, Przygodzka P, Sulowska Z, Nowak M, Klink M. Nitric oxide donors reduce the invasion ability of ovarian cancer cells in vitro. Anticancer Drugs 25: 1141–1151, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Kim MB, Sarelius IH. Role of shear forces and adhesion molecule distribution on P-selectin-mediated leukocyte rolling in postcapillary venules. Am J Physiol Heart Circ Physiol 287: H2705–H2711, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol 50: 49–59, 2003. [PubMed] [Google Scholar]
- 34.Kong L, Dunn GD, Keefer LK, Korthuis RJ. Nitric oxide reduces tumor cell adhesion to isolated rat postcapillary venules. Clin Exp Metastasis 14: 335–343, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol Heart Circ Physiol 262: H611–H615, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 88: 4651–4655, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahiri M, Martin JH. Nitric oxide decreases motility and increases adhesion in human breast cancer cells. Oncol Rep 21: 275–281, 2009. [PubMed] [Google Scholar]
- 38.Lee TH, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278: 5277–5284, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Li LM, Kilbourn RG, Adams J, Fidler IJ. Role of nitric oxide in lysis of tumor cells by cytokine-activated endothelial cells. Cancer Res 51: 2531–2535, 1991. [PubMed] [Google Scholar]
- 40.Liu Q, Mirc D, Fu BM. Mechanical mechanisms of thrombosis in intact bent microvessels of rat mesentery. J Biomech 41: 2726–2734, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, Yu T, Liang H, Wang J, Xie J, Shao J, Gao Y, Yu S, Chen S, Wang L, Jia L. Nitric oxide inhibits hetero-adhesion of cancer cells to endothelial cells: restraining circulating tumor cells from initiating metastatic cascade. Sci Rep 4: 4344, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masri FA, Comhair SA, Koeck T, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, Erzurum SC, Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med 172: 597–605, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer DJ Jr, Huxley VH. Capillary hydraulic conductivity is elevated by cGMP-dependent vasodilators. Circ Res 70: 382–391, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Morigi M, Zoja C, Figliuzzi M, Foppolo M, Micheletti G, Bontempelli M, Saronni M, Remuzzi G, Remuzzi A. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood 85: 1696–1703, 1995. [PubMed] [Google Scholar]
- 45.Mortensen K, Christensen IJ, Nielsen HJ, Hansen U, Larsson LI. High expression of endothelial cell nitric oxide synthase in peritumoral microvessels predicts increased disease-free survival in colorectal cancer. Cancer Lett 216: 109–114, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Nagel T, Resnick N, Atkinson WJ, Dewey CF Jr, Gimbrone MA Jr. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest 94: 885–891, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9: 274–284, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Noel AA, Fallek SR, Hobson RW 2nd, Duran WN. Inhibition of nitric oxide synthase attenuates primed microvascular permeability in the in vivo microcirculation. J Vasc Surg 22: 661–669, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133: 66–77, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phadke PA, Vaidya KS, Nash KT, Hurst DR, Welch DR. BRMS1 suppresses breast cancer experimental metastasis to multiple organs by inhibiting several steps of the metastatic process. Am J Pathol 172: 809–817, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pohl U, Herlan K, Huang A, Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol Heart Circ Physiol 261: H2016–H2023, 1991. [DOI] [PubMed] [Google Scholar]
- 52.Qiu H, Orr FW, Jensen D, Wang HH, McIntosh AR, Hasinoff BB, Nance DM, Pylypas S, Qi K, Song C, Muschel RJ, Al-Mehdi AB. Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am J Pathol 162: 403–412, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reymond N, d′Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13: 858–870, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD, Wink DA, Isenberg JS. The biphasic nature of nitric oxide responses in tumor biology. Antioxid Redox Signal 8: 1329–1337, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986. [DOI] [PubMed] [Google Scholar]
- 56.Rumbaut RE, Harris NR, Sial AJ, Huxley VH, Granger DN. Leakage responses to l-NAME differ with the fluorescent dye used to label albumin. Am J Physiol Heart Circ Physiol 276: H333–H339, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Rumbaut RE, Wang J, Huxley VH. Differential effects of l-NAME on rat venular hydraulic conductivity. Am J Physiol Heart Circ Physiol 279: H2017–H2023, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Scher RL. Role of nitric oxide in the development of distant metastasis from squamous cell carcinoma. Laryngoscope 117: 199–209, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Sethi N, Kang Y. Unravelling the complexity of metastasis–molecular understanding and targeted therapies. Nat Rev Cancer 11: 735–748, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen S, Fan J, Cai B, Lv YG, Zeng M, Hao YY, Giancotti FG, Fu BMM. Vascular endothelial growth factor enhances cancer cell adhesion to microvascular endothelium in vivo. Exp Physiol 95: 369–379, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shirure VS, Liu T, Delgadillo LF, Cuckler CM, Tees DF, Benencia F, Goetz DJ, Burdick MM. CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions. Am J Physiol Cell Physiol 308: C68–C78, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol 288: C831–C839, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spindler V, Peter D, Harms GS, Asan E, Waschke J. Ultrastructural analysis reveals cAMP-dependent enhancement of microvascular endothelial barrier functions via Rac1-mediated reorganization of intercellular junctions. Am J Pathol 178: 2424–2436, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12: 895–904, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res 101: 811–818, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 87: 320–330, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Buren G 2nd, Camp ER, Yang AD, Gray MJ, Fan F, Somcio R, Ellis LM. The role of nitric oxide in mediating tumour blood flow. Expert Opin Ther Targets 10: 689–701, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res 60: 5862–5869, 2000. [PubMed] [Google Scholar]
- 69.William M, Vien J, Hamilton E, Garcia A, Bundgaard H, Clarke RJ, Rasmussen HH. The nitric oxide donor sodium nitroprusside stimulates the Na+-K+ pump in isolated rabbit cardiac myocytes. J Physiol 565: 815–825, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wirtz DKK, Peter Searson C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11: 512–522, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res 12: 311–320, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Yan WW, Cai B, Liu Y, Fu BM. Effects of wall shear stress and its gradient on tumor cell adhesion in curved microvessels. Biomech Model Mechanobiol 11: 641–653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan WW, Liu Y, Fu BM. Effects of curvature and cell-cell interaction on cell adhesion in microvessels. Biomech Model Mechanobiol 9: 629–640, 2010. [DOI] [PubMed] [Google Scholar]
- 74.Yang H, Wang B, Wang T, Xu L, He C, Wen H, Yan J, Su H, Zhu X. Toll-like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasis. PLoS One 9: e109980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yen WY, Cai B, Yang JL, Zhang L, Zeng M, Tarbell JM, Fu BMM. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. Plos One 10: e0117133, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol Heart Circ Physiol 263: H641–H646, 1992. [DOI] [PubMed] [Google Scholar]
- 77.Yudoh K, Matsui H, Tsuji H. Nitric oxide induced by tumor cells activates tumor cell adhesion to endothelial cells and permeability of the endothelium in vitro. Clin Exp Metastasis 15: 557–567, 1997. [DOI] [PubMed] [Google Scholar]
- 78.Zhao F, Li L, Guan L, Yang H, Wu C, Liu Y. Roles for GP IIb/IIIa and alphavbeta3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett 344: 62–73, 2014. [DOI] [PubMed] [Google Scholar]
- 79.Zhou X, He P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am J Physiol Heart Circ Physiol 301: H108–H114, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou X, He P. Temporal and spatial correlation of platelet-activating factor-induced increases in endothelial [Ca2+]i, nitric oxide, and gap formation in intact venules. Am J Physiol Heart Circ Physiol 301: H1788–H1797, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 288: H2869–H2877, 2005. [DOI] [PubMed] [Google Scholar]