In this ongoing research effort, we now reveal that deletion of the PGE2-EP3 receptor improves anatomical and functional outcomes following intracerebral hemorrhage in aged mice, confirming our previous results in young mice, and investigate possible mechanisms of EP3-mediated neurotoxicity.
Keywords: gliosis, iron, neuroinflammation, stroke, VEGF
Abstract
With the population aging at an accelerated rate, the prevalence of stroke and financial burden of stroke-related health care costs are expected to continue to increase. Intracerebral hemorrhage (ICH) is a devastating stroke subtype more commonly affecting the elderly population, who display increased mortality and worse functional outcomes compared with younger patients. This study aimed to investigate the contribution of the prostaglandin E2 (PGE2) E prostanoid (EP) receptor subtype 3 in modulating anatomical outcomes and functional recovery following ICH in 24-mo-old mice. EP3 is the most abundant EP receptor in the brain and we have previously shown that signaling through the PGE2-EP3 axis exacerbates ICH outcomes in young mice. Here, we show that EP3 receptor deletion results in 17.9 ± 6.1% less ICH-induced brain injury (P < 0.05) and improves neurological functional recovery (P < 0.01), as identified by lower neurological deficit scores, decreased resting time, and more gross and fine motor movements. Immunohistological staining was performed to investigate possible mechanisms of EP3-mediated neurotoxicity. Identified mechanisms include reduced blood accumulation and modulation of angiogenic and astroglial responses. Using this aged cohort of mice, we have confirmed and extended our previous results in young mice demonstrating the deleterious role of the PGE2-EP3 signaling axis in modulating brain injury and functional recovery after ICH, further supporting the notion of the EP3 receptor as a putative therapeutic avenue for the treatment of ICH.
NEW & NOTEWORTHY
In this ongoing research effort, we now reveal that deletion of the PGE2-EP3 receptor improves anatomical and functional outcomes following intracerebral hemorrhage in aged mice, confirming our previous results in young mice, and investigate possible mechanisms of EP3-mediated neurotoxicity.
intracerebral hemorrhage (ICH) is a type of stroke associated with high disability and mortality rates, yet there are no effective treatments (21). Accounting for 10–15% of all strokes, the incidence of ICH is lower than ischemic stroke, but ICH carries a substantially greater mortality rate of 35–52% in the first 30 days (8). Age is an important risk factor and outcome predictor for ICH, with the risk doubling every decade (6, 34) and elderly patients having been reported to have worse functional outcomes than their younger counterparts (PMID: 25497513). With about 88% of deaths caused by stroke occurring in people over the age of 65, and the population aging over the next 20 yr, the burden of stroke-related health care costs is expected to increase drastically (10). As such, it is increasingly important to test novel therapeutic strategies for the treatment of ICH in preclinical stroke models using aged animals.
Excitotoxic and neuroinflammatory processes triggered by the presence of foreign blood components in the brain, especially hemoglobin and iron, cause secondary brain injury and cell death, aggravating ICH outcomes (7, 20, 21, 41). These processes are modulated by prostaglandins, of which PGE2 is the most abundant in the body and is often called the proinflammatory prostaglandin (29). PGE2 levels increase after ICH through a two-step enzymatic process involving the substrate arachidonic acid and cyclooxygenase (COX) and PGE2 synthase (PGES) enzymes. The inducible isoforms of these enzymes, COX-2 and mPGES-1, are increased after brain injury and have been reported to have damaging effects in stroke models (11, 20). PGE2 exerts a broad range of physiologic and pathologic effects that can be both neuroprotective and neurotoxic through its ability to bind mainly to four different EP receptors, EP1-4, which are a family of G protein-coupled receptors with different downstream signaling pathways (1, 2, 19, 20, 24). In this study, we focus on the role of the EP3 receptor, the most abundant EP receptor in the body, including the brain (20, 36). The mouse EP3 receptor has three different isoforms: EP3α, EP3β, and EP3γ that are generated by alternative splicing and vary at the carboxy-terminus (27, 29); of note, in the EP3 knockout studied here, all isoforms are deleted. Signaling pathways downstream of these EP3 receptor isoforms include modulation of cyclic adenosine monophosphate (cAMP) levels via Gαs and Gαi resulting in more or less cAMP, respectively, activation of phospholipase C to increase intracellular calcium via Gαq, and activation of the Rho kinase (ROCK) pathway by G12/13. Although in regard to regulation of cAMP levels, inhibition of adenylate cyclase and reduction of cAMP levels, through coupling with Gαi, has been suggested as the prevailing pathway (35). Therefore, activation of the EP3 receptor can result in a wide range of cellular responses depending on the downstream pathway that is activated.
We have previously shown that signaling through the PGE2-EP3 pathway exacerbates outcomes following N-methyl-d-aspartate-induced excitotoxicity or transient focal ischemia (1, 30) and recently have reported similar results after ICH in young mice (20). The present study aimed to investigate and extend our findings demonstrating the role of the PGE2-EP3 signaling axis following ICH by using aged 24-mo-old mice in an analogous experimental approach. In line with our previous findings, we show that genetic deletion of the EP3 receptor results in smaller lesion volumes with less blood accumulation and improved neurobehavioral functional outcomes. We also investigated ferric iron accumulation, gliosis, neutrophil infiltration, blood brain barrier (BBB) integrity, and vasculogenesis/angiogenesis as possible operating mechanisms of EP3-mediated neurotoxicity.
MATERIALS AND METHODS
Mice.
All mice used in this study were bred and maintained in our temperature-controlled (23 ± 2°C) animal facilities on a reverse light cycle (12-h light-dark) so that neurobehavioral testing could be conducted during the awaken phase. Studies were performed using 24-mo-old male C57BL/6 wild-type (WT; 23.7 ± 0.5 mo, n = 14) and EP3 receptor knockout (EP3−/−; 22.7 ± 0.4 mo, n = 14) mice. Mice were allowed free access to food and water before and after surgery. All experimental procedures were conducted by blinded individuals in accordance with our protocols as approved by the Institutional Animal Care and Use Committee at the University of Florida.
ICH model.
ICH was induced using our previously described model (20, 21). Briefly, mice were anesthetized with isoflurane (4% induction, 1.5–2% maintenance) and immobilized on a stereotactic frame (Stoelting, Wood Dale, IL). After a small incision was made in the skin to expose the skull, a craniotomy was performed at an angle matching that of the stereotactic frame that was set to 40° from the vertical plane. WT and EP3−/− mice were injected with 0.04 units of collagenase type VII-S (Sigma, St. Louis, MO) dissolved in 0.4 μl of sterile water directly into the striatum 3.6 mm ventral to the skull surface using an automated injector (Stoelting) at a rate of 0.2 μl/min at the following coordinates relative to bregma: 0.0 mm anterior, 3.8 mm left. The needle was left in place for 5 min and then slowly removed over a 15-min period to prevent backflow. Rectal temperature was monitored and maintained at 37.0°C ± 0.5°C during surgery. Mice were allowed to fully recover in a humidity- and temperature-controlled chamber postoperatively.
Behavioral assessments.
Functional outcomes were assessed daily following induction of ICH by measuring open field locomotor activity and performing neurological deficit scoring (NDS). All behavioral tests were conducted in the same order and at the same time of day during the dark cycle (awaken phase). With the use of an automated activity monitor with video tracking software sensitive to both gross and fine motor movements (MED Associates, St. Albans, VT), the locomotor activity of four mice were simultaneously recorded while they were individually housed in transparent acrylic cages. The ambulatory distance, stereotypic time, and resting time of each mouse was recorded for a 30-min test period. Ambulatory distance is reported as the total distance travelled by the mouse during the testing period. Stereotypic time is defined as fine motor movements confined within a 4.8 × 4.8 cm space centered at the midline of the mouse. Resting time is defined as the time the animal remained stationary. The first 5 min of data were excluded from all three analyses to control for the initial acclimation to the new environment and anxiety responses. Focal neurological deficits were independently assessed by two blinded experimenters. Six individual parameters (body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling) were scored by the investigators on a four-point scale, where zero represents normal behavior and higher scores signify increasingly more severe deficits, as we have defined (12). For each mouse, each of the six investigators scores were summed for a maximum NDS of 24 and data are reported as an average of the two investigators summed scores for each day.
Histology.
Following 72 h of behavioral testing, mice were euthanized by deeply anesthetizing with isoflurane and then transcardially perfusing with ice-cold PBS (pH = 7.4) followed by chilled 4% paraformaldehyde in PBS (PFA). Brains were collected and kept in PFA on a rocking platform at room temperature for ≤ 24 h after perfusion and then transferred to a 30% sucrose solution for cryoprotection and stored at 4°C until sectioning. Ten sets of 16 sections equally distributed throughout the entire hematoma and anteroposterior brain regions were processed at 30 μm and stored at −80°C for later histological staining. Before staining, slides were thawed at room temperature for 24–48 h to allow firm tissue adherence to the glass. Once stained, all slides were scanned using a Scanscope XT and analyzed using ImageScope Software (Aperio Technologies, Vista, CA).
Cresyl violet staining was used to assess lesion volume, blood accumulation, incidence of intraventricular hemorrhage (IVH), and hemispheric enlargement. To estimate ferric iron content, Perls' iron staining was used. Immunohistochemical staining was performed to identify microgliosis, astrogliosis, neutrophil infiltration, BBB integrity, and vasculogenesis/angiogenesis using the following primary antibodies: ionized calcium-binding adaptor protein 1 (Iba1; 1:1,000; Wako, Richmond, VA), glial fibrillary acidic protein (GFAP; 1:1,000; Dako, Carpinteria, CA), myeloperoxidase (MPO; 1:500; Pierce, Dallas, TX), immunoglobulin G (IgG; 1:300; Vector Laboratories, Burlingame, CA), and vascular endothelial growth factor (VEGF; 1:300; Santa Cruz Biotechnology, Dallas, TX). Iba1 and GFAP slides were not counterstained. MPO and IgG slides were counterstained with Cresyl violet and VEGF slides were counterstained with nuclear fast red. Cresyl violet, Perls' iron, and immunohistochemical staining and quantification (see below) were performed following the same procedures as in our previous study (20).
Quantification procedures.
To reduce any potential bias, an investigator blinded to experimental groups performed the quantification. Furthermore, to eliminate interindividual variability in quantification, a single individual quantified all slides for a particular stain. Lastly, a single experienced investigator reviewed all stains and quantifications to confirm consistency throughout the entire project. For quantifications that analyzed total brain pathology including lesion volume, ipsilateral hemispheric enlargement, blood accumulation, ferric iron content, neutrophil infiltration, BBB integrity, and vasculogenesis/angiogenesis, all sections with a lesion were quantified for each animal. To assess microgliosis and astrogliosis, the same four sections that represented maximal lesion area were analyzed. First, lesion volume: injured brain areas were outlined and these areas were then extracted from the ImageScope software. Lesion volume was calculated using these areas, known distance between sections, and section thickness. Second, percent ipsilateral hemispheric enlargement: the ipsilateral and contralateral hemispheres were each outlined and volumes were calculated in an identical manner as lesion volume. Percent ipsilateral hemispheric enlargement was then calculated as 100 × [(ipsilateral volume-contralateral volume)/contralateral volume]. Third, ICH: incidence of IVH was determined based on the visual presence of red blood cells within the lateral ventricles. Fourth, an ImageScope Positive Pixel Count algorithm was used for quantification of blood accumulation, ferric iron content, and immunohistochemical stains after the appropriate brain regions were outlined (see below). Each of these algorithms were first tuned for each stain such that the appropriate signal and signal intensity were automatically detected (20). Blood accumulation, ferric iron content neutrophil infiltration, and BBB integrity were analyzed by outlining the ipsilateral hemisphere. Cortical microgliosis was analyzed by placing identically sized 1,000 by 1,000 pixel boxes in the ipsilateral and contralateral motor cortex. Data are presented as the relative ipsilateral to contralateral signal. Striatal microgliosis was analyzed by circling the ipsilateral striatum, excluding the lesion area, and contralateral striatum. Data are presented as the ipsilateral signal per area quantified, with normalization for the contralateral signal per area quantified. Astrogliosis was analyzed similar to microgliosis, except no correction for the contralateral signal was performed (20). Vasculogenesis/angiogenesis was analyzed by outlining the ipsilateral and contralateral hemispheres. Since potential baseline differences in VEGF immunoreactivity were noted, as evidenced by the contralateral signal, ipsilateral and contralateral data are presented separately, along with the ipsilateral signal normalized to the contralateral signal. After analyses, all signal data was extracted from the ImageScope software. The angiogenesis/vasculogenesis, iron content, neutrophil infiltration, and BBB integrity results for each mouse were individually corrected for lesion volume to separate the effects of different hematoma volumes on the signal data.
Statistics.
All behavioral statistical analyses were performed using JMP (SAS, Cary, NC). All remaining data sets were analyzed using Prism 6 (GraphPad, San Diego, CA). Mortality and IVH were analyzed using a χ2-test. Functional recovery was analyzed using a linear mixed model accounting for baseline neurobehavioral function. All data sets were first checked for differences in variances between groups and normality. If the data did not follow a normal distribution, then a Mann-Whitney U-test was used. If the data were normally distributed but the variances in each group were not equal, an unpaired two-tailed Student's t-test with Welch's correction was used. The same statistical test was used for normally distributed data with equal variances among groups, except no Welch's correction was applied. Data are expressed as mean ± SE with P < 0.05 considered statistically significant.
RESULTS
Experiments were repeated on two separate occasions, with equal numbers of WT and EP3−/− mice on each day, and the same results were obtained. Consistent striatal hemorrhages were seen in the WT and EP3−/− mice.
EP3 receptor deletion reduces ICH-related mortality.
No significant differences in body weight were observed before ICH or at any time point post-ICH between the genotypes. Following ICH, EP3−/− mice visually appeared healthier and tended to have reduced mortality compared with WT controls, with mortality rates of 0.0% (0 out of 14) and 21.4% (3 out of 14), respectively (P = 0.0668).
EP3 receptor deletion reduces ICH-induced brain damage.
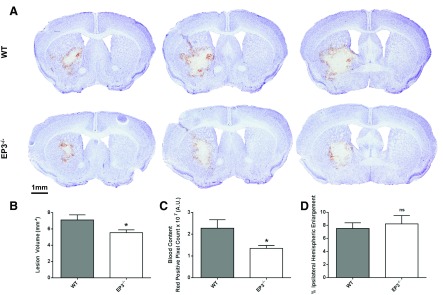
Compared with WT controls, EP3−/− mice displayed significantly smaller lesions and reduced blood accumulation (Fig. 1A). Quantification of lesion volume showed that EP3−/− mice had 17.9 ± 6.1% smaller lesions (7.1 ± 0.6 vs. 5.5 ± 0.4 mm3, P = 0.0336; Fig. 1B). Analysis of red/brown-positive pixel count demonstrated that EP3−/− mice had 36.0 ± 7.1% less blood accumulation (2.27 ± 0.39 × 107 vs. 1.35 ± 0.13 × 107 AU, P = 0.0277; Fig. 1C). No significant differences in percent ipsilateral hemispheric enlargement were seen between the groups (WT: 7.5 ± 0.9% vs. EP3−/−: 8.2 ± 1.3%, P = 0.6715; Fig. 1D). The overall rate of IVH was 44%, with no significant difference observed between the genotypes (WT: 36.4% vs. EP3−/−: 53.9%, P = 0.4954).
Fig. 1.
E prostanoid receptor subtype 3 (EP3) receptor deletion reduces intracerebral hemorrhage (ICH)-induced brain damage. Cresyl violet staining of coronal brain sections collected at 72 h post-ICH was performed to evaluate brain injury in wild-type (WT) and EP3−/− mice. A: representative images showing characteristic hematoma profiles for WT (top) and EP3−/− (bottom) mice. Left and right correspond to anterior to posterior and center is at the needle insertion site. B: lesion volume quantification demonstrated that EP3−/− mice had significantly less brain injury following ICH. C: red/brown-positive pixel count analysis showed that EP3−/− mice had significantly less blood accumulation within the injured brain areas. AU, arbitrary units. D: no significant difference in percent ipsilateral hemispheric enlargement was seen between WT and EP3−/− mice. All comparisons include n = 11 WT and n = 13 EP3−/− mice; ns, not significant; *P < 0.05.
EP3 receptor deletion improves functional outcomes after ICH.
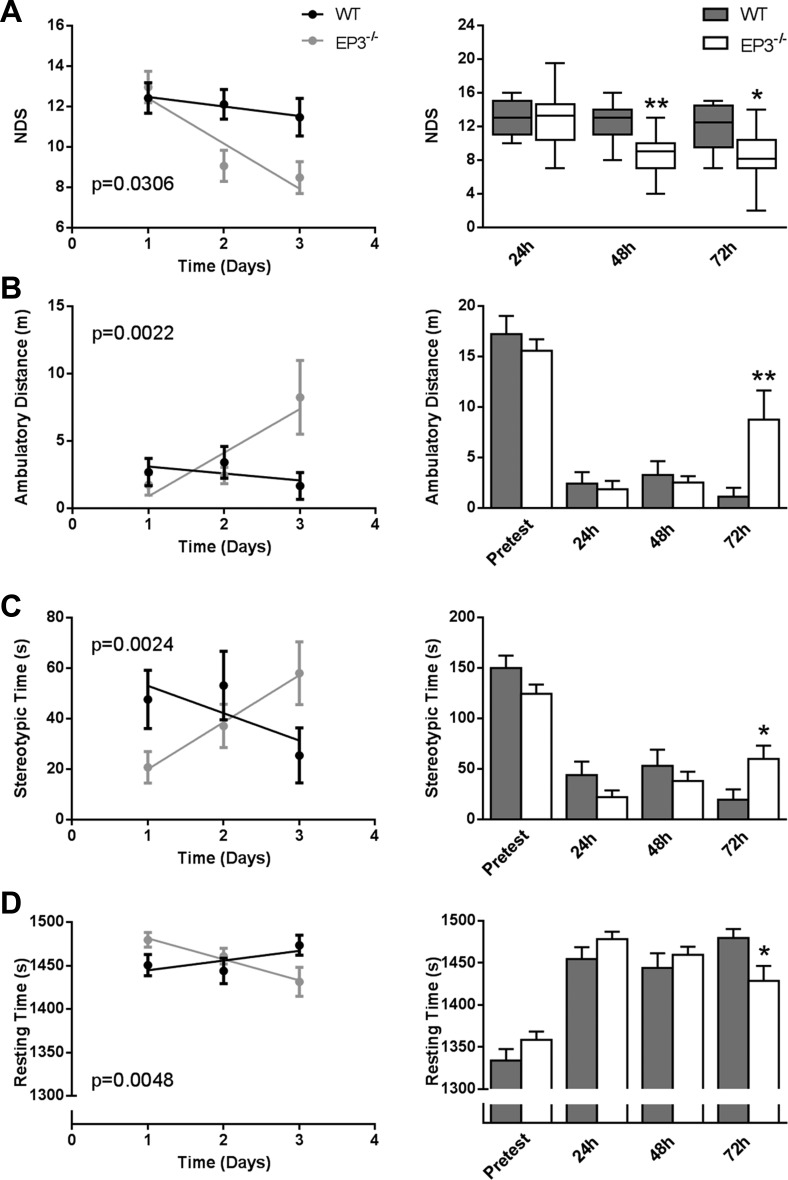
In agreement with less brain damage, EP3−/− mice also displayed significantly less focal neurological deficits at the 48 h (P = 0.0086; Fig. 2A) and 72 h (P = 0.0139; Fig. 2A) time points. Deletion of the EP3 receptor did not affect baseline open field locomotor activity, as measured by ambulatory distance, stereotypic time, or resting time. EP3−/− mice showed significantly improved functional outcomes at the 72-h endpoint with regard to ambulatory distance (P = 0.0035; Fig. 2B), stereotypic time (P = 0.0149; Fig. 2C), and resting time (P = 0.0233; Fig. 2D). Additionally, EP3−/− mice demonstrated improved recovery for NDS (P = 0.0306; Fig. 2A), ambulatory distance (P = 0.0022; Fig. 2B), stereotypic time (P = 0.0024; Fig. 2C), and resting time (P = 0.0048; Fig. 2D).
Fig. 2.
EP3 receptor deletion improves functional outcomes after ICH. At 24, 48, and 72 h following ICH, investigators blinded to genotype performed neurobehavioral testing to evaluate functional outcomes of WT and EP3−/− mice using 2 separate tests: neurological deficit scoring (NDS) and open field locomotor activity, which consists of stereotypic time, ambulatory distance, and resting time. A: EP3−/− mice displayed a significantly improved rate of recovery in focal neurological deficits (left). EP3−/− mice had significantly better neurological function as identified by lower NDS scores at the 48- and 72-h endpoints (right). B-D, left: EP3−/− mice demonstrated a significantly improved rate of recovery in ambulatory distance (B), stereotypic time (C), and resting time (D). WT and EP3−/− mice had similar locomotor activity at baseline and 24 and 48 h. However, EP3−/− mice perform better on measures of stereotypic time (B), ambulatory distance (C), and resting time (D) at 72 h post-ICH. All comparisons include n = 11 WT and n = 14 EP3−/− mice; *P < 0.05; **P < 0.01.
Effect of EP3 receptor deletion on brain ferric iron content following ICH.
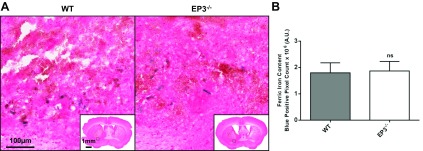
Perls' iron stain was performed to assess brain ferric iron content. For all mice in the study, ferric iron was found in perihematomal regions (Fig. 3A). No Perls' iron staining was seen in the contralateral hemisphere for any of the mice within the study. No difference in ferric iron content was found between the genotypes (WT: 1.8 ± 0.4 × 106 AU vs. EP3−/−: 1.9 ± 0.4 × 106 AU, P = 0.8481; Fig. 3B). For each animal, after individually correcting iron content for lesion volume, we still saw no difference between groups (WT: 2.8 ± 0.8 × 104 AU vs. EP3−/−: 3.3 ± 0.8 × 104 AU, P = 0.6349).
Fig. 3.
Effect of EP3 receptor deletion on brain ferric iron content following ICH. Perls' iron staining of coronal brain sections collected at 72 h post-ICH was performed to evaluate total brain ferric iron content in WT and EP3−/− mice. A: representative high magnification images showing ferric iron accumulation (blue) in perihematomal regions of WT (left) and EP3−/− (right) mice. Square selections in the inserts denote the location of magnified regions. No Perls' iron staining was observed in the contralateral hemisphere of WT or EP3−/− mice. B: blue-positive pixel count analysis showed no significant difference in total brain ferric iron content between the WT and EP3−/− groups. The comparison includes n = 11 WT and n = 13 EP3−/− mice; ns, not significant.
Effect of EP3 receptor deletion on gliosis after ICH.
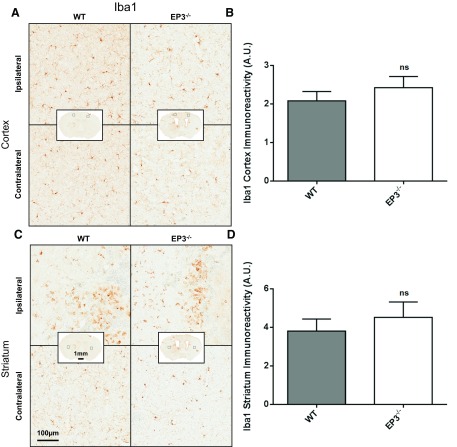
Immunohistochemical staining for Iba1 and GFAP was conducted to assess cortical and striatal microgliosis and astrogliosis, respectively. Cortical microgliosis (WT: 2.1 ± 0.2 AU vs. EP3−/−: 2.4 ± 0.3, P = 0.2934; Fig. 4, A and B) and striatal (WT: 3.8 ± 0.6 AU vs. EP3−/−: 4.5 ± 0.8 AU, P = 0.5114; Fig. 4, C and D) microgliosis were not significantly different between WT and EP3−/− mice. Additionally, no significant difference was observed between WT and EP3−/− mice in the contralateral striatum (P = 0.9252) and cortex (P = 0.7427). Significantly greater ipsilateral microglial activation was seen in both the cortex (WT: P = 0.0214, EP3−/−: P = 0.0126) and striatum (WT: P = 0.0004, EP3−/−: P = 0.0024) compared with the contralateral for both groups.
Fig. 4.
Effect of EP3 receptor deletion on microgliosis after ICH. At 72 h following ICH, immunohistochemical staining for Iba1 was used to evaluate cortical and striatal microglial activation and morphological changes in WT and EP3−/− mice. A and C: representative high magnification images of coronal brain sections showing the ipsilateral and contralateral cortex (A) and striatum (C) for WT (left) and EP3−/− (right) mice. Square selections in the insets denote the location of magnified regions. B and D: brown-positive pixel count analysis showed no difference in cortical (B) or striatal (D) microgliosis between WT and EP3−/− mice. All comparisons include n = 11 WT and n = 11 EP3−/− mice; ns, not significant.
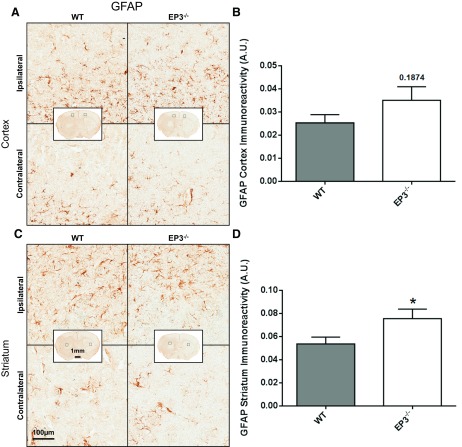
When compared with WT controls, EP3−/− mice had significantly increased striatal astrogliosis and tended to have increased cortical astrogliosis. Quantification showed 35.1 ± 21.8% more ipsilateral striatal astrogliosis for EP3−/− mice (0.054 ± 0.006 vs. 0.075 ± 0.008 AU, P = 0.0498; Fig. 5, C and D). No significant difference was found in cortical astrogliosis between the groups, although EP3−/− mice tended to have more GFAP immunoreactivity (0.025 ± 0.004 vs. 0.035 ± 0.006 AU, P = 0.1874; Fig. 5, A and B). No astrogliosis differences were seen between the genotypes in the contralateral striatum (P = 0.5696) or cortex (P = 0.5623). Significantly greater ipsilateral astroglial activation was seen in both the cortex (WT: P = 0.0475, EP3−/−: P = 0.0001) and striatum (WT: P = 0.0081, EP3−/−: P = 0.0003) compared with the contralateral for both groups.
Fig. 5.
Effect of EP3 receptor deletion on astrogliosis after ICH. At 72 h following ICH, immunohistochemical staining for glial fibrillary acidic protein (GFAP) was used to evaluate cortical and striatal astrocyte activation and morphological changes in WT and EP3−/− mice. A and C: representative high magnification images of coronal brain sections showing the ipsilateral and contralateral cortex (A) and striatum (C) for WT (left) and EP3−/− (right) mice. Square selections in the insets denote the location of magnified regions. B and D: brown-positive pixel count analysis showed no significant difference in cortical astrogliosis (B) between WT and EP3−/− groups, although EP3−/− mice tended to have increased cortical GFAP immunoreactivity. EP3−/− mice have significantly increased striatal astrogliosis (D). All comparisons include n = 11 WT and n = 13 EP3−/− mice; ns, not significant; *P < 0.05.
Effect of EP3 receptor deletion on peripheral neutrophil infiltration following ICH.
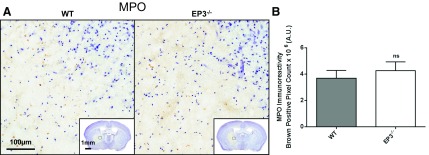
Immunohistochemical staining for MPO was performed to identify peripheral neutrophil infiltration after ICH. Neutrophils were found in the internal regions of the hematoma (Fig. 6A), with no cells found outside the hematoma or in the contralateral hemisphere. No difference in MPO staining was seen between genotypes (WT: 2.4 ± 0.3 × 107 AU vs. EP3−/−: 2.4 ± 0.4 × 107 AU, P = 0.9799). After correcting for lesion volume, we still found no difference between WT and EP3−/− mice (WT: 3.7 ± 0.6 × 106 AU vs. EP3−/−: 4.3 ± 0.7 × 106 AU, P = 0.5356; Fig. 6B).
Fig. 6.
Effect of EP3 receptor deletion on peripheral neutrophil infiltration following ICH. At 72 h after ICH, immunohistochemical staining for myeloperoxidase (MPO) was used to evaluate peripheral neutrophil infiltration in WT and EP3−/− mice. A: representative high magnification images of coronal brain sections showing MPO-positive cells (brown) diffusely localized within injured brain areas. Square selections in the insets denote the location of magnified regions. No neutrophils were seen outside of the injured brain areas for any of the mice in the study. B: brown-positive pixel count analysis showed no difference in neutrophil infiltration between WT and EP3−/− mice. The comparison includes n = 11 WT and n = 13 EP3−/− mice; ns; not significant.
Effect of EP3 receptor deletion on BBB integrity following ICH.
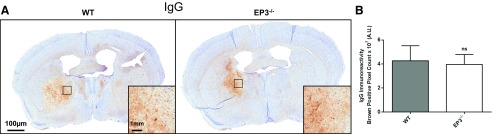
Immunohistochemical staining for IgG was performed as a marker for BBB integrity following ICH. In all animals, IgG staining was predominantly constrained to the hematoma and surrounding regions (Fig. 7A). No difference in IgG staining in the ipsilateral hemisphere was seen between genotypes (WT: 4.3 ± 1.3 × 107 vs. EP3−/−: 4.0 ± 0.8 × 107 AU, P = 0.6034; Fig. 7B). After correcting for lesion volume, we still found no difference between WT and EP3−/− mice (WT: 6.5 ± 1.5 × 106 AU vs. EP3−/−: 7.8 ± 1.9 × 106 AU, P = 0.8891).
Fig. 7.
Effect of EP3 receptor deletion on blood brain barrier (BBB) integrity following ICH. At 72 h after ICH, immunohistochemical staining for IgG was used to evaluate BBB integrity in WT and EP3−/− mice. A: representative images of coronal brain sections. Square selections denote location of magnified regions. B: brown-positive pixel count analysis showed no difference in BBB integrity between WT and EP3−/− mice. The comparison includes n = 5 WT and n = 9 EP3−/− mice; ns, not significant.
Effect of EP3 receptor deletion on VEGF expression following ICH.
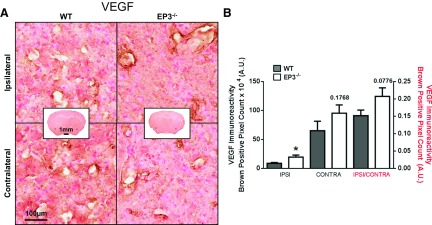
It has been reported in tumor models that baseline VEGF expression was lower in EP3−/− mice (3, 40), so we investigated potential baseline differences and changes after ICH in the brain. Diffuse VEGF expression was noted in blood vessels, and in some cases cortical neurons, of both the ipsilateral and contralateral hemispheres of WT and EP3−/− mice (Fig. 8A). Compared with the WT group, a trend toward increased VEGF expression was observed in the EP3−/− mice at baseline as shown by the differences in contralateral signal (WT: 65.2 ± 16.1 × 104 AU vs. EP3−/−: 95.2 ± 14.3 × 104 AU, P = 0.1027; Fig. 8B). The ipsilateral signal followed similar trends (WT: 64.8 ± 14.9 × 104 AU vs. EP3−/−: 100.5 ± 16.7 × 104 AU, P = 0.1289), and there was no difference in VEGF signal between the ipsilateral and contralateral hemispheres in the WT or EP3−/− mice (WT: P = 0.9875, EP3−/−: P = 0.8115). After the ipsilateral VEGF immunoreactivity signal was corrected for lesion volume, the EP3−/− group had significantly greater VEGF expression (WT: 8.7 ± 1.3 × 104 AU vs. EP3−/−: 19.0 ± 3.5 × 104 AU, P = 0.0115).
Fig. 8.
Effect of EP3 receptor deletion on VEGF expression following ICH. At 72 h after ICH, immunohistochemical staining for VEGF was used to evaluate angiogenesis/vasculogenesis in WT and EP3−/− mice. A: representative high magnification images of coronal brain sections showing the ipsilateral perihematomal region and contralateral equivalent for WT (left) and EP3−/− (right) mice. Square selections in the insets denote the location of magnified regions. B: brown-positive pixel count analysis showed significantly more ipsilateral VEGF expression in EP3−/− mice after individual signal normalization for lesion volume. A trend toward increased baseline VEGF expression, as observed by contralateral VEGF immunoreactivity, was seen in the EP3−/− mice. Ipsilateral (ipsi) and contralateral (contra) signal corresponds to left y-axis. Ipsilateral signal normalized by contralateral signal (ipsi/contra) corresponds to the right y-axis. All comparisons include n = 11 WT and n = 12 EP3−/− mice, ns, not significant; *P < 0.05.
DISCUSSION
In the current study, we examined the role of the EP3 receptor in modulating anatomical and functional outcomes following ICH in an aged cohort of mice. We reveal that genetic deletion of the EP3 receptor reduces ICH-induced brain injury as evident by smaller lesions with less blood accumulation. Notably, these improvements in anatomical outcomes are accompanied by significantly less focal neurological deficits and better recovery of motor skills and ambulatory ability. EP3−/− mice display increased striatal astrogliosis, tend to have more cortical astrogliosis, and have increased VEGF immunoreactivity. No differences in ferric iron content, striatal and cortical microgliosis, peripheral neutrophil infiltration, and BBB integrity were observed between the groups.
These findings are in line with those from our previous study investigating the role of the EP3 receptor after ICH in younger mice (20). Specifically, we show that genetic deletion of the EP3 receptor results in less ICH-induced brain injury in both young (2–4 mo) and aged (22–26 mo) mice. In comparing the previous and current studies, it appears that aged EP3−/− mice have less relative reduction in lesion volume compared with the young mice, although this suggestion should be taken with caution since the studies were performed at different times. Additionally, in contrast to the younger cohort, we now observe a greater relative functional recovery of aged EP3−/− mice compared with their WT counterparts at 48 and 72 h post-ICH. Also importantly, in the aged group, EP3−/− mice have reduced mortality and visually appeared healthier at all time points post-ICH compared with WT controls. It is unclear why there appears to be less relative reduction in lesion volume but improved functional recovery and less mortality in aged EP3−/− mice; additional experiments performed at the same time would be necessary to confirm and address these findings. In the young group, we observed differences in ferric iron content, striatal microgliosis, and peripheral neutrophil infiltration (20), although in the present study with aged mice, we do not see such differences. Actually, we observe opposite trends in astrocyte activation responses, suggesting the importance of age on EP3-mediated responses to brain injury. Nevertheless, these results confirm a deleterious role for the PGE2-EP3 signaling axis after ICH and further suggest the EP3 receptor as a putative therapeutic target. Ongoing work with delayed administration of selective EP3 antagonists will clarify the therapeutic potential of targeting the EP3 receptor after ICH.
The EP3 receptor has been reported to be the most abundantly expressed PGE2 receptor subtype in the brain, where it has been shown to be present on astrocytes and microglia (9, 15, 18, 25, 31, 33, 36, 38). Therefore, in combination with the upregulation of PGE2 synthesis under pathological conditions (14, 42), the PGE2-EP3 signaling axis likely plays an important modulatory role on neuroinflammatory processes after brain injury. While the deleterious effects of signaling through the EP3 receptor are evident following ICH, the specific mechanism and effector molecules downstream of the EP3 receptor are not yet fully understood. Moreover, it is not clear why different patterns of microglial, astroglial, and neutrophil accumulation, all cellular responses to brain injury, are seen in the young vs. the aged cohort of mice. It is likely that EP3 expression levels vary with age, and given the three isoforms of EP3, which each have different binding affinities, G-protein coupling, and downstream effector molecules, there are multiple and complex interactions possible. Additional work is needed to fully understand the complicated mechanisms and pathways downstream of the EP3 receptor responsible for the neurotoxicity after ICH.
Cerebral angiogenesis and vasculogenesis are fundamental processes to brain development and repair following injury through formation of new blood vessels from preexisting ones or de novo production, respectively (23). Angiogenesis/vasculogenesis are stimulated by increased expression of VEGF. Genetic deletion of the EP3 receptor has been shown to reduce VEGF expression in tumor development, highlighting the importance of the PGE2-EP3 signaling axis in modulating angiogenesis/vasculogenesis under pathologic conditions (3). VEGF and one its receptors, VEGFR-1, have been shown to be upregulated following injection of a topical EP3 agonist in a sponge implantation tumor model (3). Two separate pathways have been elucidated that link the EP3 receptor to VEGF expression. In humans, increased VEGF/VEGFR-1 expression occurs through EP3 coupling to Gαi, which sequentially activates phosphatidylinositol 3-kinase and extracellular signal-regulated kinases 1/2 (40). Additionally, EP3 has been previously associated with induction of RhoA through G12 and G13, and this signaling axis has been shown to mediate neurite retraction and vascular contraction, as well as in neurotoxic responses following glutamate-induced excitotoxicity (4, 14, 16, 17, 32). Activated RhoA can then activate mDia1, which has been implicated in the regulation of Src kinase activity in vitro (26). Last, Src kinase has been reported to modulate outcomes following both ICH and ischemic stroke through regulation of VEGF expression (5, 28).
Here, we observe that EP3−/− mice tend to have more VEGF immunoreactivity at baseline, as evident by the contralateral hemisphere signal. These results seemingly contradict previous findings in peripheral tumors demonstrating that EP3−/− mice have reduced VEGF/VEGFR-1 expression. However, its effects in the brain and in other pathologies beyond tumors may vary from those previously studied. For example, VEGF responses following ICH may be global, with increases in the ipsilateral and contralateral hemispheres, and thus the contralateral signal is not indicative of baseline VEGF expression differences. Furthermore, we do not observe differences in VEGF immunoreactivity when comparing the ipsilateral and contralateral hemispheres, suggesting that ICH did not affect VEGF expression. This finding also seemingly contradicts other studies, which have mostly suggested that VEGF expression increases with time post-ICH in the perihematomal region, although decreased expression has also been reported (22, 37, 39, 43). Several possible explanations exist to explain these apparent discrepancies: 1) quantification and other experimental methods were different between the studies, 2) VEGF responses following ICH are age dependent, 3) a global VEGF response after ICH inhibited detection of ipsilateral to contralateral differences, 4) animals were investigated at different time points post-ICH, and 5) a combination of the aforementioned possibilities. In the current study, the diffuse staining and large lesions produced by this model led us to quantify ipsilateral and contralateral hemispheres. We should note that we did see sites of VEGF immunoreactive vessels in the perihematomal regions, which were seemingly more evident in the EP3−/− mice most likely due to their smaller lesions and relative preservation of the striatum. Therefore, results were corrected for lesion volume in an attempt to separate the effects of lesion size on VEGF immunoreactivity such that the EP3-dependent effect on VEGF expression could be evaluated. In both cases, increased VEGF immunoreactivity is noted in the ipsilateral hemisphere and thus we propose that EP3 has a direct negative modulatory role on VEGF expression in the brain after ICH. The increased VEGF expression in EP3-deficient mice could be a possible mechanism for improved ICH outcomes, although more work is needed to establish this correlation.
We provide additional evidence suggesting an injurious role for the PGE2-EP3 signaling axis following ICH in an aged cohort of mice, where EP3 receptor deletion improved anatomical outcomes and functional recovery. Specifically, EP3−/− mice have reduced mortality, smaller lesion volumes with less blood accumulation, less focal neurological deficits, more recovery of gross and fine motor skills, improved ambulatory ability, and generally appear healthier after ICH. The EP3 receptor continues to represent a new putative therapeutic target for the treatment of ICH.
GRANTS
This work was supported by National Institutes of Neurological Disorders and Stroke Grants R01-NS-046400 (to S. Doré) and F31-NS-086441 (to J. L. Leclerc).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.L.L. and S.D. conception and design of research; J.L.L., A.S.L., and M.A.D. performed experiments; J.L.L., A.S.L., M.A.D., and S.D. analyzed data; J.L.L., A.S.L., M.A.D., and S.D. interpreted results of experiments; J.L.L., A.S.L., and M.A.D. prepared figures; J.L.L. and A.S.L. drafted manuscript; J.L.L., A.S.L., M.A.D., and S.D. edited and revised manuscript; J.L.L., A.S.L., M.A.D., and S.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all members of the Doré laboratory for helpful discussions and technical assistance, with a special acknowledgment of the many talented undergraduate students who contributed to the brain sectioning, immunohistochemical staining, and blinded quantification and behavioral procedures.
REFERENCES
- 1.Ahmad M, Ahmad AS, Zhuang H, Maruyama T, Narumiya S, Doré S. Stimulation of prostaglandin E2-EP3 receptors exacerbates stroke and excitotoxic injury. J Neuroimmunol 184: 172–179, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad M, Saleem S, Zhuang H, Ahmad AS, Echeverria V, Sapirstein A, Doré S. 1-HydroxyPGE1 reduces infarction volume in mouse transient cerebral ischemia. Eur J Neurosci 23: 35–42, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto Y, Murata T, Yoshimura H, Narumiya S, Majima M. Host prostaglandin E2-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med 197: 221–232, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki J, Katoh H, Yasui H, Yamaguchi Y, Nakamura K, Hasegawa H, Ichikawa A, Negishi M. Signal transduction pathway regulating prostaglandin EP3 receptor-induced neurite retraction: requirement for two different tyrosine kinases. Biochem J 340: 365–369, 1999. [PMC free article] [PubMed] [Google Scholar]
- 5.Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke 38: 1621–1625, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 34: 2060–2065, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42: 1781–1786, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS, Vespa P, Zuccarello M; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 38: 2001–2023, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Caggiano AO, Kraig RP. Prostaglandin E receptor subtypes in cultured rat microglia and their role in reducing lipopolysaccharide-induced interleukin-1beta production. J Neurochem 72: 565–575, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet 371: 1612–1623, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Doré S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol 54: 155–162, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Glushakov AV, Robbins SW, Bracy CL, Narumiya S, Doré S. Prostaglandin F2alpha FP receptor antagonist improves outcomes after experimental traumatic brain injury. J Neuroinflammation 10: 132, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero W, Nguyen C, Mir O, Savitz S. Elderly patients with intracerebral hemorrhage have worse outcomes compared with younger patients despite similar severity and complication rates. In: American Academy of Neurology, 2013. [Google Scholar]
- 14.Ikeda-Matsuo Y, Hirayama Y, Ota A, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 and cyclooxygenase-2 are both required for ischaemic excitotoxicity. Br J Pharmacol 159: 1174–1186, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda-Matsuo Y, Tanji H, Narumiya S, Sasaki Y. Inhibition of prostaglandin E2 EP3 receptors improves stroke injury via anti-inflammatory and anti-apoptotic mechanisms. J Neuroimmunol 238: 34–43, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Jeon BT, Jeong EA, Park SY, Son H, Shin HJ, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. The Rho-kinase (ROCK) inhibitor Y-27632 protects against excitotoxicity-induced neuronal death in vivo and in vitro. Neurotox Res 23: 238–248, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Katoh H, Negishi M, Ichikawa A. Prostaglandin E receptor EP3 subtype induces neurite retraction via small GTPase Rho. J Biol Chem 271: 29780–29784, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kitanaka J, Hashimoto H, Gotoh M, Kondo K, Sakata K, Hirasawa Y, Sawada M, Suzumura A, Marunouchi T, Matsuda T, Baba A. Expression pattern of messenger RNAs for prostanoid receptors in glial cell cultures. Brain Res 707: 282–287, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Leclerc JL, Ahmad AS, Singh N, Soshnik-Schierling L, Greene E, Dang A, Doré S. Intracerebral hemorrhage outcomes following selective blockade or stimulation of the PGE2 EP1 receptor. BMC Neurosci 16: 48, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclerc JL, Lampert AS, Diller MA, Doré S. Genetic deletion of the prostaglandin E2 E prostanoid receptor subtype 3 improves anatomical and functional outcomes after intracerebral hemorrhage. Eur J Neurosci 41: 1381–1391, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclerc JL, Lampert AS, Diller MA, Immergluck JB, Doré S. Prostaglandin E2 EP2 receptor deletion attenuates intracerebral hemorrhage-induced brain injury and improves functional recovery. ASN Neuro 7: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei C, Zhang S, Cao T, Tao W, Liu M, Wu B. HMGB1 may act via RAGE to promote angiogenesis in the later phase after intracerebral hemorrhage. Neuroscience 295: 39–47, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Zechariah A, Qu Y, Hermann DM. Effects of vascular endothelial growth factor in ischemic stroke. J Neurosci Res 90: 1873–1882, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Milatovic D, Montine TJ, Aschner M. Prostanoid signaling: dual role for prostaglandin E2 in neurotoxicity. Neurotoxicology 32: 312–319, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol 421: 543–569, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev 28: 65–76, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Negishi M, Sugimoto Y, Irie A, Narumiya S, Ichikawa A. Two isoforms of prostaglandin E receptor EP3 subtype. Different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J Biol Chem 268: 9517–9521, 1993. [PubMed] [Google Scholar]
- 28.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med 7: 222–227, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Reader J, Holt D, Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev 30: 449–463, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saleem S, Kim YT, Maruyama T, Narumiya S, Doré S. Reduced acute brain injury in PGE2 EP3 receptor-deficient mice after cerebral ischemia. J Neuroimmunol 208: 87–93, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Wang Q, Johansson JU, Liang X, Woodling NS, Priyam P, Loui TM, Merchant M, Breyer RM, Montine TJ, Andreasson K. Inflammatory prostaglandin E2 signaling in a mouse model of Alzheimer disease. Ann Neurol 72: 788–798, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shum WW, Le GY, Jones RL, Gurney AM, Sasaki Y. Involvement of Rho-kinase in contraction of guinea-pig aorta induced by prostanoid EP3 receptor agonists. Br J Pharmacol 139: 1449–1461, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slawik H, Volk B, Fiebich B, Hull M. Microglial expression of prostaglandin EP3 receptor in excitotoxic lesions in the rat striatum. Neurochem Int 45: 653–660, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Sturgeon JD, Folsom AR, Longstreth WT Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 38: 2718–2725, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 282: 11613–11617, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto Y, Shigemoto R, Namba T, Negishi M, Mizuno N, Narumiya S, Ichikawa A. Distribution of the messenger RNA for the prostaglandin E receptor subtype EP3 in the mouse nervous system. Neuroscience 62: 919–928, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Wei ZZ, Gu X, Zhang JY, Zhang Y, Li J, Wei L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol 2015. [DOI] [PubMed] [Google Scholar]
- 38.Takemiya T, Matsumura K, Sugiura H, Maehara M, Yasuda S, Uematsu S, Akira S, Yamagata K. Endothelial microsomal prostaglandin E synthase-1 exacerbates neuronal loss induced by kainate. J Neurosci Res 88: 381–390, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Tang T, Liu XJ, Zhang ZQ, Zhou HJ, Luo JK, Huang JF, Yang QD, Li XQ. Cerebral angiogenesis after collagenase-induced intracerebral hemorrhage in rats. Brain Res 1175: 134–142, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi T, Fujino H, Israel DD, Regan JW, Murayama T. Human EP3(I) prostanoid receptor induces VEGF and VEGF receptor-1 mRNA expression. Biochem Biophys Res Commun 377: 1173–1178, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 27: 894–908, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Wu T, Wu H, Wang J, Wang J. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation 8: 22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou HJ, Tang T, Cui HJ, Yang AL, Luo JK, Lin Y, Yang QD, Li XQ. Thrombin-triggered angiogenesis in rat brains following experimental intracerebral hemorrhage. J Neurosurg 117: 920–928, 2012. [DOI] [PubMed] [Google Scholar]