Abstract
This pilot randomized controlled trial aimed to determine the feasibility, acceptability, and preliminary efficacy of parental problem solving skills training (PSST) compared to treatment as usual (TAU) on improving parental mental health symptoms, physical health and well-being, and parenting behaviors. Effects of parent PSST on child outcomes (pain, emotional and physical functioning) were also examined. Participants included 61 parents of children aged 10–17 years with chronic pain randomized to PSST (n = 31) or TAU (n = 30). Parents receiving PSST participated in 4–6 individual sessions of training in problem solving skills. Outcomes were assessed at pre-treatment, immediately post-treatment, and at 3-month follow up. Feasibility was determined by therapy session attendance, therapist ratings, and parent treatment acceptability ratings. Feasibility of PSST delivery in this population was demonstrated by high compliance with therapy attendance, excellent retention, high therapist ratings of treatment engagement, and high parent ratings of treatment acceptability. PSST was associated with post-treatment improvements in parental depression (d = −0.68), general mental health (d = 0.64), and pain catastrophizing (d = −0.48), as well as in child depression (d = −0.49), child general anxiety (d = −0.56), and child pain-specific anxiety (d = −0.82). Several effects were maintained at 3-month follow-up. Findings demonstrate that PSST is feasible and acceptable to parents of youth with chronic pain. Treatment outcome analyses show promising but mixed patterns of effects of PSST on parent and child mental health outcomes. Further rigorous trials of PSST are needed to extend these pilot results.
Keywords: problem solving skills training, chronic pain, parents, children and adolescents, randomized controlled trial
Introduction
Chronic pain is as prevalent in childhood as adulthood, with 8% of children reporting severe pain and disability [24]. Pediatric chronic pain is embedded in a broader context of parent and family factors that may directly or indirectly influence the child’s adjustment and coping with pain [36]. For example, higher levels of parental psychological distress [32] and less healthy family functioning [31, 38] are associated with greater child pain-related disability. In addition, parents are themselves affected by caring for a child with chronic pain which may lead to changes in their own psychological and behavioral functioning. Many parents of children with chronic pain report clinically significant role stress, anxiety, and depressive symptoms [14]. Therefore, interventions that alleviate parent distress may also improve health and well-being for children with chronic pain.
Interventions have been developed and evaluated for parents of children with chronic medical conditions [3, 13, 30]. In a Cochrane review on this topic [15], problem-solving skills training (PSST) interventions were effective in reducing distress (i.e., improving parental mental health) in parents of children with chronic conditions (e.g., cancer, asthma). In contrast, there was no evidence for the effectiveness of cognitive-behavioral, family, or multi-systemic therapy in improving parental mental health or behavioral outcomes. PSST is based on the social problem-solving model of D’Zurilla and Nezu [8, 9] and is hypothesized to change interpersonal interactions and behaviors associated with stress. Efficacy of PSST has been evaluated in caregivers of both adult and pediatric medical populations, gaining considerable empirical support, e.g., [41, 43, 44], but had not been applied to chronic pain. Thus, our research team adapted an existing PSST intervention developed for caregivers of children with cancer [43] for caregivers of children with chronic pain [37]. In line with prior studies of PSST, we also sought to evaluate the effects of PSST alone (without other interventions) in order to specifically test the preliminary benefits achieved by PSST.
Although parent interventions have been included in pediatric cognitive-behavioral pain interventions [20], their purpose has been to modify parent behavior that may inadvertently reinforce maladaptive coping (such as teaching parents to reward activity participation) based on social learning theory. Most typically, interventions directed towards parents have been brief (e.g., 1–2 sessions) and do not aim to modify parent distress [20]. Thus, applying interventions only to parents and directed toward reducing parental distress is novel in this population.
In this pilot RCT we aimed to determine feasibility, acceptability, and preliminary efficacy of PSST versus treatment as usual (TAU) for parents of children with chronic pain. We hypothesized that feasibility would be shown by high levels of participation, retention, and high ratings of intervention acceptability. We hypothesized that PSST would impact both parent and child outcomes at post-treatment and 3-month follow-up. Specifically, parents receiving PSST would report improved mental health symptoms, health and well-being, and more adaptive parenting compared to parents receiving TAU; children of parents receiving PSST would report decreased pain and improved physical and emotional functioning compared to children of parents receiving TAU.
Methods
Participants
Participants were 61 parents and their children aged 10–17 years with chronic pain. The clinical trial was registered and the full protocol is available (PSST; Problem Solving Skills Training for Parent Caregivers of Youth with Chronic Pain, ClinicalTrials.gov Identifier NCT01496378). Parent-child dyads were enrolled from May 2012 to October 2014 from two interdisciplinary pediatric pain clinics (Seattle Children’s Hospital and Oregon Health and Science University); follow up data were complete by May 2015. The study was approved by each site’s Institutional Review Board.
We have published one paper concerning adaptation and initial piloting of PSST for parents of youth with chronic pain [37]; however, that paper did not include any of the participants or outcome analyses of the pilot randomized controlled trial results presented here.
Inclusion/Exclusion Criteria
Inclusion criteria for the trial were: 1) parent of a child between the ages of 10 and 17 years with chronic pain, 2) child’s pain of a duration ≥ 3 months and interfering with daily functioning, 3) child received evaluation for chronic pain from one of the two pain clinics, and 4) parents were English-speaking. Exclusion criteria were: 1) child diagnosed with a serious comorbid health condition (e.g., cancer), 2) parent resided with child for < 1 year, and 3) parent had serious or life-threatening mental health issues (e.g., active psychosis, suicidal ideation).
Recruitment
Providers at the two pain centers gave potential participants a flyer about the study and asked if they would be willing to be contacted by study staff to learn more about the study and receive additional screening. Providers then sent potential participant’s contact information to study staff via secure email. Potential participants could also contact study staff directly by calling a phone number provided on the study flyer. Study staff screened for eligibility and held a consent conference with parents and children by telephone. Parents signed the consent forms and returned them to study staff. Children signed assent forms for their study participation.
Trial Design and Randomization
This pilot clinical trial used a balanced (1:1) randomized parallel group design. Assessments were sent to participants’ homes and were returned to study staff via postal mailings. Assessments were completed at pre-randomization (baseline), immediately post-treatment (6–8 weeks), and at 3-month follow-up. A fixed allocation randomization scheme was used. Order of randomization to the two treatment conditions was generated separately for each site with an online program (randomizer.org). A blocked method design was used, with blocks of 4 for each ID number. Using the output provided by the online program, study staff created a password protected electronic document that linked each ID number to a group assignment. Only the research coordinator had the password to the randomization table. Group assignment was concealed by formatting the document to block out group assignment until the time of randomization. Following completion of pre-treatment assessments, the research coordinator revealed participants’ group assignment. Interventionists were informed when participants were allocated to the active treatment group, and they contacted parents directly to schedule the first treatment session. Participants allocated to the TAU group were contacted by the research coordinator and were provided with instructions to continue with their usual care during the treatment phase. Thus, participants were not blinded to their group assignment. All study assessments were self-report measures completed in participants’ homes via mailings; children and parents were instructed to complete measures independently.
Study Flow
Figure 1 shows a CONSORT diagram depicting the flow of study participants through each phase of the study. Referrals were received from the two pain clinics for 151 families of youth with chronic pain. Of those families who were referred to participate, a total of 90 were excluded: 7 did not meet eligibility criteria because the child had a serious comorbid chronic medical condition, 58 declined participation due to lack of time and/or travel burden to the treatment center, 22 were unable to be reached during the recruitment period, and 3 were unable to be reached to complete pre-treatment assessments (passive refusals). The final sample consisted of 61 families. Demographics (child age and sex) did not differ between those families who did and did not choose to participate.
Figure 1.
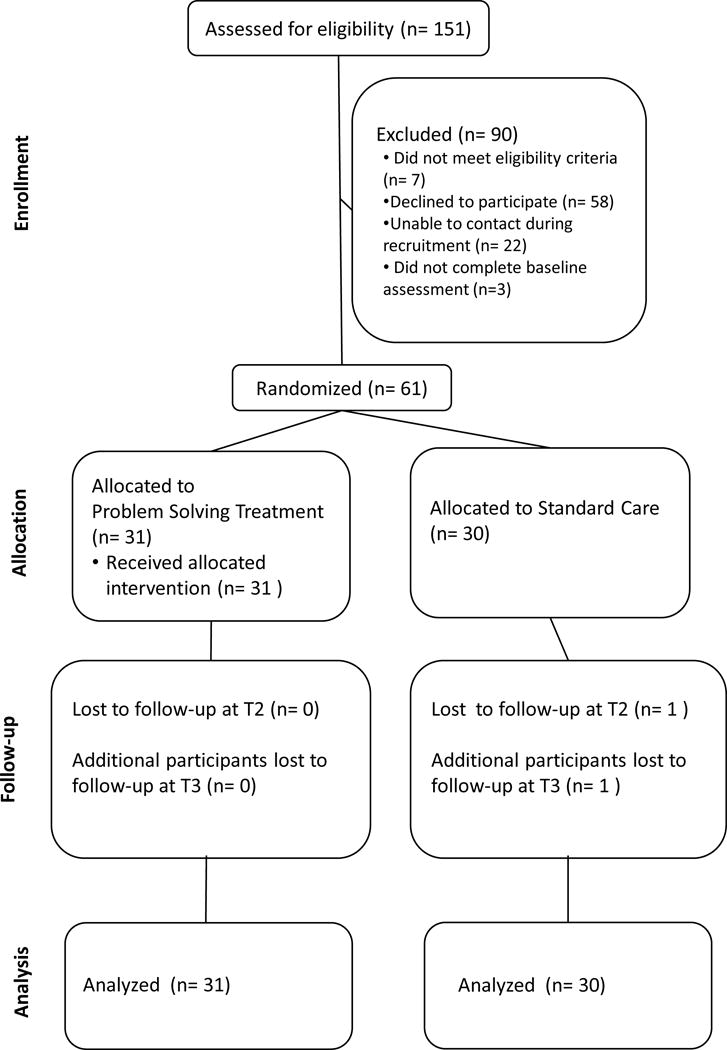
The 61 eligible families were randomly assigned to PSST (n = 31) or TAU (n = 30). All 31 families who were randomized to PSST received the allocated intervention. One participant did not complete the immediate post-treatment assessment (n = 0 PSST, n = 1 TAU). An additional participant did not complete the three-month follow-up assessment (n = 0 PSST, n = 1 TAU) for an overall retention rate of 97%. All enrolled participants were included in final analyses, including 31 participants from the PSST group and 30 participants from the TAU group.
Procedures
All child participants were patients who had received evaluation and treatment in one of the two collaborating interdisciplinary pain clinics. Resulting from this evaluation, participants may have received recommendations for treatment. These usual care recommendations were not altered for the clinical trial. All study-related procedures and interventions were adjunctive to the usual care participants received in their pain clinic. Parents and children were provided with study incentives (giftcards) following each completed assessment.
Participants completed all assessments in their homes and then returned the questionnaires to study staff via postal mailings. After study staff received the completed pre-treatment assessment, participants were randomized to PSST or TAU.
PSST
Parents assigned to the intervention condition received PSST as adapted for parents of children with chronic pain[37] in addition to the usual care their child received in pain clinic. This was a parent only intervention and the children did not participate. Our intervention was adapted from treatment materials developed by Sahler and colleagues [43] for caregivers of children with cancer (“Bright IDEAS”). Drawing from D’Zurilla and colleagues’ conceptualization of effective problem solving ability [8, 9], Sahler et al.’s intervention emphasizes a positive problem-solving orientation characterized by optimism and problem-solving self-efficacy (Bright), as well as the major components of rational problem solving. These include problem definition and formulation (Identify the Problem), generation of alternative solutions (Determine the options), decision-making (Evaluate options), solution implementation (Act), and verification (See if it worked). Content was modified to be relevant to parents of children with chronic pain, including removal of references and examples specific to cancer and the addition of examples specific to chronic pain. Additional modifications included the creation of a list of common challenges faced by caregivers of youth with chronic pain and a booklet illustrating the problem-solving process using a vignette of a family with a child with chronic pain (see [37], for details).
In our initial pilot testing of the intervention [37], we found that parents learned problem solving skills quickly and were rated by therapists as ready to terminate within 4–6 sessions. In addition, we found that it was difficult for parents to schedule 8 sessions in an 8 week intervention period. Thus, we made the decision to shorten treatment to be delivered in 4 to 6 sessions, and telephone sessions were included as a mode of treatment delivery in an effort to increase feasibility of treatment. However, few parents made use of telephone sessions in the pilot RCT (24 out of 167 total sessions; 14%).
Sessions were designed to be delivered individually over one hour, and were conducted in the order prescribed by the treatment manual. The goal of the first session was to orient the caregiver to the intervention and establish rapport. Parents were asked to tell the story of their child’s pain, with follow-up prompts regarding how having a child with pain impacted various aspects of their lives (i.e., family functioning, emotional functioning, finances). They were provided with an overview of the intervention and a copy of the manual, which included the problem-solving vignette. Subsequent sessions focused on developing a positive problem solving orientation and learning how to enact the rational problem-solving skills. Therapists first presented a description and rationale for each skill and then encouraged the caregiver to enact the skill in session, with the aid of manualized worksheets. Training in positive problem solving orientation included education about the importance of a positive outlook when solving problems as well as instruction in cognitive restructuring when a negative problem solving orientation was identified. Parents completed a worksheet in which common problems experienced by families with chronic pain were listed (e.g., financial problems, lack of time for social activities, worry about their child, relationship problems). Therapists could use this worksheet to help parents choose problems to address in sessions. Example problems that parents worked on in sessions included dealing with sibling jealousy over time spent with the child with chronic pain, communication with school personnel about the child’s pain, and negative communications and interactions with their child.
The subsequent steps of problem solving were taught using behavioral rehearsal, role play, and positive reinforcement. Therapists could also provide brief training in abdominal breathing when parents generated a solution that involved the study therapist teaching them how to relax. At the end of each session, parents were provided with a take-home assignment, which was then closely reviewed and discussed in the following session. The full treatment manual can be obtained from the first author.
Treatment as Usual
Parents and children continued with the care that was prescribed by the pain clinic for their child’s pain problem. Care was not altered by participation in this pilot RCT. Clinical recommendations may have included physical therapy, psychological therapy, medication management, and/or complementary and alternative modalities such as acupuncture. Families may also have chosen to not pursue any other treatments. PSST is not a part of usual clinical care and was not offered to parents at either collaborating pain center.
Therapists, Supervision, and Treatment Fidelity
Four therapists were postdoctoral psychology fellows and two were licensed clinical psychologists, all of whom had formal training and experience in treatment of pediatric chronic pain. Therapists underwent a didactic training that was delivered in a group and individual format including review of treatment materials and role-play of treatment sessions with a trained therapist. All sessions were audio-recorded. After each session, therapists completed a fidelity record detailing the problems parents chose to address during the session, progress in acquisition of specific problem-solving skills, and tasks assigned for homework. Cross-site group supervision occurred weekly via conference call or individually with a licensed clinical psychologist (EL) who had experience in PSST to review sessions and compliance to the manual.
Measures
Treatment Acceptability and Satisfaction
At post-treatment and three-month follow-up, parents in the PSST group completed an adapted version of the Treatment Evaluation Inventory-Short Form [28, 29], a 9-item scale designed to assess acceptability and satisfaction with the treatment process and outcomes. Select items were adapted to be specific to pediatric pain (e.g., “I find this treatment to be an acceptable way of dealing with children’s pain”). Items are rated on a 5-point Likert type scale, ranging from 1 (Strongly Disagree) to 5 (Strongly Agree). Items are summed to create a total score ranging from 9–45, with higher scores indicating greater treatment acceptability and satisfaction. “Moderate” treatment satisfaction and acceptability is indicated by a score of 27 or higher [28]. This measure has demonstrated good reliability and validity [29].
Treatment Feasibility
Feasibility of delivering PSST to parents with chronic pain was assessed by documenting the number of sessions completed by parents as well as therapist ratings of parent motivation to learn, receptivity to learning, understanding of the PSST process, and rapport. At the end of each session, therapists completed these ratings on 0–10 Likert scales, which were averaged across sessions.
Pre-treatment Measures
Demographics
Parents completed an information form to assess their relationship to the child (i.e., biological mother, father), family composition, marital status, race, and education. Parents also provided information regarding their child’s age, sex, and race.
Psychological distress
The Brief Symptom Inventory 18 (BSI 18) [11] was used to screen for parent psychological distress and psychiatric disorders at pre-treatment. Parents rate their level of distress over the past week using a 5-point scale ranging from 0 (not at all) to 4 (extremely). Items are summed to create a Global Severity Index. The BSI 18 has demonstrated strong validity and reliability [11]. In the present study, Cronbach’s α was 0.84 for the global severity index score.
Selection of Outcome Measures
A goal of our pilot trial was to determine optimal measurement tools for parent behavior and mental health outcomes to inform a future larger definitive trial. To achieve this goal, where possible we administered two types of measures within each parent outcome domain: 1) general measures that have been used in previous trials of PSST with other caregiver populations, and 2) measures specifically developed for use with parents of children with chronic pain. Consistent with prior trials of PSST [41–43, 48], our primary outcome used for estimating sample size was a general measure of parent depressive symptoms.
Parent Mental Health Outcomes
General parent mental health
The primary outcome was parent depression as measured by the Beck Depression Inventory-II (BDI-II) [4]. The BDI-II is a 21-item questionnaire that assesses the presence and severity of depressive symptoms over the preceding two weeks. Items are rated on a 4 point scale, ranging from 0 to 3. Items are summed to create a total score, with higher scores indicating greater depressive symptom severity. The psychometric properties of the BDI-II have been extensively evaluated, with consistently strong support for its validity and reliability (e.g., [2, 12]). In the present study, Cronbach’s α ranged from 0.85–0.94.
The Profile of Mood States-Standard (POMS) [34] was used as a secondary outcome to assess transitory mood. The POMS is a 65-item questionnaire that assesses transitory mood states (i.e., exhausted, relaxed). Items are rated on a 5 point Likert type scale ranging from 0 to 4, with higher scores indicating more intense mood during the past week. Items load onto six subscales: Tension-Anxiety, Anger-Hostility, Fatigue-Inertia, Depression-Dejection, Vigor-Activity, and Confusion-Bewilderment. Subscale scores are summed to create a Total Mood Disturbance score. In the present study, we report the effect of treatment on the Total Mood Disturbance score. The POMS has strong psychometric properties [35, 40]. Cronbach’s α ranged from 0.91–0.92 in the present study.
Pain-specific parent mental health
Secondary pain-specific mental health outcomes included subscales from the Bath Adolescent Pain-Parental Impact Questionnaire (BAPQ-PIQ) [27] and the Pain Catastrophizing Scale for Parents (PCS-P) [21]. The BAPQ-PIQ is a 61-item questionnaire designed to assess the impact of caring for a child with chronic pain on parents’ functioning. Items are rated on a 5 point frequency response scale ranging from 0 (Never) to 4 (Always). Higher scores are indicative of more impaired functioning for all subscales. To reflect pain-specific mental health symptoms, we used scores on the Depression and Anxiety subscales. The BAPQ-PIQ has demonstrated good reliability and validity among parents of youth with chronic pain [27]. In the present study, Cronbach’s α ranged from 0.88–0.89.
The PCS-P is a 13-item questionnaire designed to assess catastrophic thoughts and feelings about the child’s pain [21]. Items are rated on a 5 point frequency response scale ranging from 0 to 4, and load onto three subscales: Rumination, Magnification, and Helplessness. Subscale scores are summed to create a Parent Pain Catastrophizing Total score, with higher scores indicative of greater catastrophizing. In the present study, we report the effect of treatment on the Parent Pain Catastrophizing Total score. The PCS-P has demonstrated good reliability and validity among parents of youth with chronic pain [21]. In the present study, Cronbach’s α ranged from 0.90–0.91.
General Parent Health and Well-being
General parent health and well-being was assessed with the Short Form Health Survey 12 (SF-12) [49], which is a brief health survey measure to assess functional health and well-being. Items ask about limitations and problems with emotions, health, and functional activities over the prior 4 weeks. The 12 items are combined to calculate physical and mental health summary scores. The SF-12 is a well-established health status measure that has demonstrated adequate content validity, discriminant validity, and test-retest reliability [49].
Exploratory Parenting Outcomes
General parenting stress
The Parenting Stress Index-Short Form (PSI-SF) [1] is a 36-item questionnaire that assesses general parenting stress. Items are rated on a 5 point scale, ranging from 1 to 5, with higher scores indicative of greater difficulties related to parenting. Items load onto 3 subscales: Parental Distress, Parent-Child Dysfunctional Interaction, and Difficult Child. Subscale scores are summed to calculate a Total Parenting Stress score. Raw scores were transformed to percentile scores for analyses. In the present study, we report on the Total Parenting Stress score. The PSI has demonstrated adequate reliability and validity [5, 23]. Cronbach’s α ranged from 0.93–0.95 in the present study.
Pain-specific parent behaviors
Our primary measure of parent behavioral responses to pain was the Parent Behavior subscale of the BAPQ-PIQ [27]. This subscale contains 11 items about parent behaviors directed at encouraging or discouraging child activity. Items are rated on a 5 point frequency response scale ranging from 0 to 4. Higher scores are interpreted as more problematic parent behaviors. The BAPQ-PIQ has demonstrated good reliability and validity among parents of youth with chronic pain [27]. In the present study, Cronbach’s α was 0.88.
A secondary measure of parent behavioral responses to pain was the Helping for Health Inventory (HHI) adapted for chronic pain [18, 22]. The HHI assesses miscarried helping, a maladaptive interactional process characterized by parents’ attempts to help their child that are met with resistance. Two versions (parent and child) are available that provide perceptions from each viewpoint. Example items include “The more my parents try to involve themselves in my pain, the more I resist their involvement,” and “I find that the more I try to help my child with his/her pain, the more he/she resists my involvement.” Items are rated on a 5 point scale, with higher scores indicative of greater miscarried helping. Items are summed to create an HHI Total score. In the present study, we report parent and child-reported HHI Total scores. The HHI has demonstrated good reliability and validity in pediatric populations, including in outpatient samples of youth with chronic pain [18]. In the present study, Cronbach’s α was 0.83 (parent) and 0.88 for child report.
Child Physical and Mental Health Outcomes
Pain intensity
Pain intensity was assessed using a questionnaire previously validated with youth with chronic pain [39]. Children reported on their average pain intensity over the past month on an 11-point numerical rating scale (NRS), ranging from 0 (No pain) to 10 (Worst pain). The NRS is recommended for assessment of pain intensity in children and adolescents with chronic pain [47].
Emotional functioning and functional impairment
Youth completed the Bath Adolescent Pain Questionnaire (BAPQ) [16], a 61-item questionnaire that assesses the multidimensional impact of chronic pain on children’s functioning. Outcomes representing child emotional functioning outcomes were drawn from three subscales (Depression, General anxiety, Pain-specific anxiety). Child functional impairment was assessed by two subscales (Social functioning, Physical functioning). Items are rated on a 5 point scale, with higher scores indicative of more impaired functioning. The BAPQ was developed for use in clinical populations of youth with chronic pain to evaluate treatment efficacy and has demonstrated good validity and test-retest reliability in outpatient pain samples [16]. In the present study, Cronbach’s α = 0.85–0.88.
Adverse Events
Participants provided open-ended responses concerning any adverse events occurring during the study at post-treatment and follow up.
Process Measure
Problem-solving
Parents completed the Social Problem-Solving Skills Inventory-Revised (SPSI-R) [10], a 52-item questionnaire that assesses a five dimensional model of social problem solving including two types of problem orientation (positive and negative) and three problem-solving styles (rational problem solving, impulsivity-carelessness, and avoidance). A dysfunctional problem solving score was constructed from negative problem solving, avoidance and impulsivity-carelessness scores, and a constructive problem solving score was calculated from positive problem orientation and rational problem solving scores. The SPSI-R total score is the weighted average of the five subscale scores, with higher scores indicating better problem solving skills. The SPSI-R has strong psychometric properties [10]. In the present study, Cronbach’s α ranged from 0.82–0.87. We report the effect of treatment on dysfunctional problem solving, constructive problem solving, and total problem solving.
Sample Size and Power Calculations
Data available from previous caregiver studies of PSST allowed us to conduct sample size calculations on parent depressive symptoms at post-treatment under the assumptions that the primary purpose of this pilot study is to examine feasibility and produce estimates of effect sizes to power a future larger scale trial. Therefore we sought to calculate estimates of effect sizes with confidence intervals less precise than for a definitive large-scale trial. Based on previous studies of PSST in caregivers [41–43, 48] we calculated a standardized mean difference (SMD) across these trials for change in caregiver depressive symptoms to be −0.83 with 95% CI of −1.51 to −0.14. This effect size would be considered large per Cohen [7]. Using this estimate, the probability of detecting a true difference at a two-sided .05 significance level is 81% with a total sample size of 50. Based on estimates of sample attrition, we sought to enroll 61 parents to achieve a sample size of 50, which would allow us to test the primary study hypotheses with sufficient power and to create effect size estimates for a future larger scale trial.
Data Analysis Plan
Data analyses were conducted using IBM SPSS v21 [46]. Measures were scored and missing items addressed per the scoring manual for each measure. When scoring manuals did not include specific instructions to address missing items, mean imputation was used to replace missing items when at least 80% of items were completed. Overall missingness was very low. Descriptive statistics were used to summarize the demographic characteristics of the sample. For categorical variables frequency statistics are reported, and for continuous variables we report means and standard deviations. Table 1 shows demographic characteristics of the sample. Table 2 shows means and standard deviations for process and outcome variables at pre-treatment, post-treatment and three-month follow-up. Table 3 provides all coefficient estimates from the multilevel modeling analyses testing the group × time treatment effect for each outcome from baseline to post-treatment and baseline to 3-month follow-up.
Table 1.
Parent and child demographic characteristics at baseline (pre-randomization)
| Parent Demographic Characteristics | Total (n=61) |
PSST (n=31) |
Treatment as Usual (n=30) |
|---|---|---|---|
| Gender (% female) | 98.4% | 96.8% | 100% |
| Race | |||
| White | 93.4% | 93.5% | 93.3% |
| Black or African American | 1.6% | — | 3.3% |
| Asian | 1.6% | 3.2% | — |
| Other | 3.3% | 3.2% | 3.3% |
| Marital Status | |||
| Married | 73.8% | 67.7% | 80% |
| Not Married | 26.2% | 32.3% | 20% |
| Education | |||
| High School or less | 9.8% | 9.7% | 10.0% |
| Vocational School/Some College | 24.6% | 19.4% | 30.0% |
| College | 44.3% | 48.4% | 40.0% |
| Graduate/Professional School | 21.3% | 22.6% | 20.0% |
| Household Annual Income | |||
| 10,000–29,999 | 9.8% | 6.5% | 13.3% |
| 30,000–49,999 | 18.0% | 25.8% | 10.0% |
| 50,000–69,999 | 9.8% | 6.5% | 13.3% |
| 70,000–100,000 | 24.6% | 25.8% | 23.3% |
| >100,000 | 29.5% | 22.6% | 36.7% |
| Missing | 8.2% | 12.9% | 3.3% |
| Employment Status | |||
| Full time | 32.8% | 35.5% | 30.0% |
| Part time | 23.0% | 22.6% | 23.3% |
| Not working | 42.6% | 38.7% | 46.7% |
| Missing | 1.6% | 3.2% | — |
| Brief Symptom Inventory (M, SD) | |||
| Global Severity Index | 52.02(8.78) | 50.71(8.96) | 53.41(8.51) |
|
| |||
| Child Demographic Characteristics | Total (n=61) |
PSST (n=31) |
Treatment as Usual (n=30) |
|
| |||
| Gender (% female) | 80.3% | 80.6% | 80.0% |
| Age (M, SD) | 14.34(1.91) | 14.61(2.01) | 14.07(1.80) |
| Race | |||
| White | 90.2% | 93.5% | 86.7% |
| Black or African American | 1.6% | — | 3.3% |
| Asian | 1.6% | — | 3.3% |
| Other | 6.6% | 6.5% | 6.7% |
| Primary Pain Location | |||
| Headache | 29.5% | 29.0% | 30% |
| Stomach | 29.5% | 29.0% | 30% |
| Musculoskeletal | 39.3% | 38.7% | 39.9% |
| Missing | 1.6% | 3.2% | — |
| Pain Frequency | |||
| None | 1.6% | 3.2% | — |
| 1 time per week | 4.9% | 6.5% | 3.3% |
| 2–3 times per week | 13.1% | 6.5% | 20.0% |
| 3–6 times per week | 6.6% | 6.5% | 6.7% |
| Daily | 73.8% | 77.4% | 70.0% |
Table 2.
Unadjusted Descriptive Statistics on Primary and Secondary Treatment Outcomes by Treatment Condition
| Measure | PSST (n = 31) Mean (SD) |
Treatment as Usual (n =30) Mean (SD) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| T1 | T2 | T3 | T1 | T2 | T3 | ||||
| General Parent Mental Health | |||||||||
| Depression (BDI-II) | 13.45(7.89) | 7.87(5.82) | 7.21(8.26) | 10.00(6.85) | 9.33(8.51) | 7.16(8.61) | |||
| Mood Disturbance, Total (POMS) | 32.81(34.39) | 29.35(33.60) | 18.00(26.70) | 27.70(28.10) | 26.87(36.87) | 21.43(31.95) | |||
| Pain-Specific Parent Mental Health | |||||||||
| Depression (BAPQ-PIQ) | 14.03(5.55) | 10.58(5.41) | 10.29(5.70) | 10.97(4.99) | 9.83(4.62) | 10.77(6.88) | |||
| Anxiety (BAPQ-PIQ) | 9.23(4.03) | 7.48(4.44) | 5.74(4.14) | 8.47(4.14) | 6.40(4.64) | 8.17(5.09) | |||
| Pain Catastrophizing, Total (PCS-P) | 39.23(10.17) | 32.65(8.67) | 31.77(9.01) | 37.17(9.17) | 34.79(8.44) | 34.93(8.70) | |||
| General Parent Health and Well-Being | |||||||||
| Physical Health (SF-12) | 44.77(12.69) | 46.69(11.73) | 49.60(10.60) | 47.03(12.37) | 48.34(13.73) | 46.71(13.74) | |||
| Mental Health (SF-12) | 42.12(11.47) | 46.41(10.34) | 46.33(10.12) | 45.02(9.65) | 41.57(12.67) | 46.30(11.02) | |||
| Exploratory Parenting Outcomes | |||||||||
| Parenting Stress, Total (PSI) | 83.16(24.34) | 78.29(23.70) | 83.23(27.97) | 78.59(22.58) | 82.43(19.71) | 77.33(19.46) | |||
| Parent Behavior (BAPQ-PIQ) | 26.10(4.17) | 21.93(5.02) | 18.32(5.98) | 22.73(5.56) | 21.15(7.33) | 21.98(5.90) | |||
| Miscarried Helping, Child Report (HHI) | 40.29(10.53) | 36.97(10.53) | 39.71(9.27) | 35.60(8.72) | 38.27(9.59) | 34.70(11.25) | |||
| Miscarried Helping, Parent Report (HHI) | 37.03(9.49) | 33.42(8.69) | 34.23(9.60) | 34.67(10.16) | 34.03(9.57) | 33.00(9.03) | |||
| Child Physical and Mental Health | |||||||||
| Pain Intensity (NRS) | 6.45(1.06) | 5.58(2.03) | 5.42(2.05) | 6.37(1.35) | 5.70(2.05) | 5.30(2.12) | |||
| Depression (BAPQ) | 13.97(5.58) | 12.03(5.13) | 11.53(5.37) | 10.47(5.39) | 11.20(5.37) | 8.71(5.60) | |||
| General Anxiety (BAPQ) | 13.87(6.25) | 11.42(5.33) | 12.61(6.05) | 12.27(5.88) | 13.00(6.03) | 11.21(5.55) | |||
| Pain-Specific Anxiety (BAPQ) | 14.87(5.96) | 11.03(5.04) | 10.71(5.63) | 12.33(6.27) | 13.03(5.96) | 10.23(6.47) | |||
| Social Functioning (BAPQ) | 14.83(5.75) | 14.81(6.34) | 15.52(7.64) | 15.14(7.79) | 15.07(6.62) | 13.61(6.70) | |||
| Physical Functioning (BAPQ) | 8.90(3.97) | 9.52(6.47) | 7.84(5.50) | 9.60(5.42) | 8.10(4.28) | 8.75(4.64) | |||
| Parent Problem Solving | |||||||||
| Dysfunctional Problem Solving (SPSI-R) | 22.84(10.29) | 22.33(10.86) | 21.58(9.95) | 25.91(16.19) | 24.41(13.38) | 28.46(16.46) | |||
| Constructive Problem Solving (SPSI-R) | 57.77(17.24) | 65.19(14.52) | 65.68(16.37) | 51.93(17.01) | 53.04(18.81) | 55.85(13.98) | |||
| Problem Solving, Total (SPSI-R) | 14.16(1.94) | 14.73(2.00) | 14.92(2.04) | 13.49(2.56) | 13.75(2.39) | 13.47(2.37) | |||
Table 3.
Linear Growth Models Testing Treatment Effects for Parent and Child Treatment Outcomes
| Group × Wave Treatment Effect from Baseline, Post-treatment |
Group × Wave Treatment Effect from Baseline, 3 Month Follow-up |
|||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Measure | β | SE | p | d [95% CI] | β | SE | p | d [95% CI] |
| General Parent Mental Health | ||||||||
| Depression (BDI-II) | −4.91 | 2.59 | 0.06 | −0.68 [−1.39, 0.03] | −3.39 | 2.59 | 0.19 | −0.41 [−1.02, 0.21] |
| Mood Disturbance, Total (POMS) | −0.93 | 3.42 | 0.79 | −0.09 [−0.73, 0.55] | −2.52 | 3.42 | 0.46 | −0.28 [−1.04, 0.48] |
| Pain-Specific Parent Mental Health | ||||||||
| Depression (BAPQ-PIQ) | −2.32 | 1.90 | 0.23 | −0.46 [−1.22, 0.29] | −3.54 | 1.90 | 0.07 | −0.57 [−1.17, 0.04] |
| Anxiety (BAPQ-PIQ) | 0.32 | 1.59 | 0.84 | 0.07 [−0.62, −1.40] | −3.18 | 1.59 | 0.047 | −0.67 [−1.33, −0.01] |
| Pain Catastrophizing, Total (PCS-P) | −4.09 | 1.91 | 0.03 | −0.48 [−0.92, −0.04] | −4.68 | 1.92 | 0.02 | −0.52 [−0.95, −0.10] |
| General Parent Health and Well-being | ||||||||
| Physical Health (SF-12) | −0.73 | 2.49 | 0.77 | −0.06 [−0.45, 0.33] | 4.48 | 2.50 | 0.08 | 0.37 [−0.04, 0.78] |
| Mental Health (SF-12) | 7.52 | 2.93 | 0.01 | 0.64 [0.15, 1.14] | 2.68 | 2.93 | 0.36 | 0.26 [−0.30, 0.81] |
| Exploratory Parenting Outcomes | ||||||||
| Parenting Stress, Total (PSI) | −12.54 | 11.44 | 0.28 | −0.42 [−1.18, 0.34] | −3.46 | 11.44 | 0.76 | −0.11 [−0.85, 0.63] |
| Parent Behavior (BAPQ-PIQ) | −2.58 | 1.82 | 0.16 | −0.42 [−0.99, 0.16] | −7.02 | 1.82 | 0.0001 | −1.14 [−1.72, −0.55] |
| Miscarried Helping, Child report (HHI) | −5.99 | 3.00 | 0.045 | −0.60 [−1.19, 0] | 0.32 | 3.00 | 0.92 | 0.03 [−0.53, 0.60] |
| Miscarried Helping, Parent report (HHI) | −2.98 | 2.92 | 0.31 | −0.33 [−0.97, 0.31] | −0.97 | 2.98 | 0.75 | −0.10 [−0.74, 0.53] |
| Child Physical and Mental Health | ||||||||
| Pain Intensity (NRS) | −0.20 | 0.53 | 0.70 | −0.10 [−0.62, 0.42] | 0.03 | 0.53 | 0.95 | 0.02 [−0.49, 0.53] |
| Depression (BAPQ) | −2.56 | 1.35 | 0.06 | −0.49 [−1.00, 0.02] | −0.59 | 1.37 | 0.67 | −0.11 [−0.59, 0.38] |
| General Anxiety (BAPQ) | −3.18 | 1.57 | 0.045 | −0.56 [−1.10, −0.01] | −0.13 | 1.59 | 0.93 | −0.02 [−0.56, 0.52] |
| Pain-Specific Anxiety (BAPQ) | −4.54 | 1.67 | 0.008 | −0.82 [−1.41, −0.22] | −2.00 | 1.71 | 0.25 | −0.33 [−0.90, 0.23] |
| Social Functioning (BAPQ) | −0.24 | 2.06 | 0.91 | 0.33 [−0.20, 0.86] | 1.99 | 2.08 | 0.34 | −0.02 [−0.50, 0.46] |
| Physical Functioning (BAPQ) | 2.11 | 1.72 | 0.22 | −0.46 [−0.95, 0.02] | −0.15 | 1.74 | 0.93 | −0.12 [−0.65, 0.42] |
| Parent Problem Solving | ||||||||
| Dysfunctional Problem Solving (SPSI-R) | 1.23 | 2.85 | 0.67 | 0.10 [−0.37, 0.57] | −3.41 | 2.87 | 0.24 | −0.25 [−0.66, 0.50] |
| Constructive Problem Solving (SPSI-R) | 6.09 | 3.33 | 0.07 | 0.34 [−0.03, 0.72] | 3.50 | 3.35 | 0.30 | 0.22 [−0.20, 0.64] |
| Problem Solving, Total (SPSI-R) | 0.25 | 0.39 | 0.52 | 0.11 −0.23, 0.45] | 0.67 | 0.39 | 0.09 | 0.29 [−0.05, 0.62] |
Independent samples t-tests with Bonferroni correction and chi-square analyses were conducted to confirm that randomization produced equal groups. We also used independent samples t-tests to examine group differences on treatment expectancies at pre-treatment. For parents in the PSST group, we examined treatment engagement and treatment satisfaction/acceptability using descriptive statistics.
Multilevel modeling (MLM) procedures were used to test primary hypotheses for continuous outcomes. MLM accounts for repeated measures within subjects, accommodates missing observations, and includes all available observations in analyses. Procedures for linear growth model specifications were based on Shek and Ma [45]. Assessment wave (baseline, post-treatment, follow-up) was treated as a categorical variable and baseline values were specified as the reference point so that results were interpreted as change from baseline to immediate post-treatment and baseline to three-month follow-up. A full conditional model tested the effects of wave, treatment group, and a group × wave interaction. The group × wave interaction represents the change from baseline to post-treatment and the change from baseline to three-month follow-up for the PSST group relative to the TAU group. Separate linear growth model analyses were conducted for each outcome variable. In Table 3, we report the beta and effect size for the group × wave interaction for each outcome variable at post-treatment and at three-month follow-up.
We calculated effect size estimates for the pre-post treatment design using recommendations put forth by Feingold [19]. Effect sizes (reported as Cohen’s d) were calculated for the group × wave interactions. Guidelines to interpret the effect size estimates are as follows: d = 0.20 indicates a small effect, d = 0.50 indicates a medium effect, and d = 0.80 indicates a large effect (Cohen [6]). Because this is a pilot RCT we provide 95% CI effect size estimates on all outcomes (see Table 3). For this pilot trial, a significance level of .05 was used for all analyses.
Results
Descriptive Statistics
Participants included 61 parents and their children with chronic pain. Children were between the ages of 10 and 17 years (M = 14.3, SD = 1.9) and parents were between the ages of 32 and 67 years (M = 45.7, SD = 6.8). Parents and children were primarily female (98.4% and 80.3%, respectively), Caucasian (93.4%, 90.2%, respectively), and middle class as indicated by annual household income between $50,000 and $100,000 (38%). The majority of parents had completed a college education or higher (65.6%). Demographic characteristics for parents and children in each treatment group are presented in Table 1. Children had pain on average for two years and reported various chronic pain conditions including musculoskeletal pain (41%), abdominal pain (29.5%), and headache (29.5%). Most children (70%) reported experiencing daily pain.
Tests of Group Equivalence
Independent samples t-tests and chi-square tests indicated that participants in the two groups did not differ on any demographic characteristics (see Table 1) or on the BSI Global Severity Index score (p’s > 0.05). The two groups were also similar on all outcome variables at pre-treatment (p’s > 0.05). Non-participants were similar to participating children on age and sex (p’s > 0.05). Only two participants dropped out of the study, and there were no differences from those who completed the study.
Treatment Expectancies
Independent samples t-test indicated there was no difference in treatment expectancies between groups, p > .05.
Treatment Satisfaction and Acceptability
Parents in the PSST group reported high satisfaction and acceptability for the intervention immediately post-treatment and at three-month follow-up. Mean ratings on the TEI were over 27 (post-treatment M = 33.9, SD = 7.3, follow up M = 34.5, SD = 6.1) indicating that treatment was rated as acceptable by parents. Treatment satisfaction and acceptability ratings were not completed by parents in the TAU group.
Treatment Feasibility
Parents in the PSST group completed an average of 5.23 treatment sessions (SD = 0.72, range = 4–6). Therapists rated parents as highly motivated to learn (M = 8.0, SD = 1.5, range = 6–10), receptive to learning (M = 8.1, SD = 1.6, range = 3–10), and with good understanding of the PSST process (M = 7.9, SD = 1.5, range = 4–10). Therapists rated their rapport with parents as generally strong (M = 8.3, SD = 1.0, range = 5–10).
Parent Mental Health Outcomes
General parent mental health
As shown in Table 3, the PSST group demonstrated greater reductions in depression (BDI-II) compared to the TAU group at post-treatment that approached significance (b = −4.91, p = 0.06, d = −0.68, 95% CI [−1.39, 0.03]) which was a medium effect. However, this difference was not maintained at 3-month follow-up. On the POMS total mood disturbance scale there were no effects of treatment on transitory mood through post-treatment or follow-up.
Pain-specific parent mental health
On the BAPQ-PIQ depression scale and the BAPQ-PIQ anxiety scale, there were no between-groups differences through post-treatment. However, through follow-up, the PSST group had greater reductions in both depression and anxiety symptoms compared to the TAU group and this approached significance (b = −3.54, p = 0.07, d = −0.57, 95% CI [−1.17, 0.04]; b = −3.18, p = 0.05, d = −0.67, 95% CI [−1.33, −0.01], respectively), which were medium effects.
The PSST group had significantly greater reductions in pain catastrophizing (PCS-P) compared to the TAU group through post-treatment and follow-up (b = −4.09, p = 0.03, d = −0.48, 95% CI [−0.92, −0.04]; b = −4.68, p = 0.02, d =−0.52, 95% CI [−0.95, −0.10], respectively), which were medium effects.
General Parent Health and Well-being Outcomes
On the SF-12 physical health scale, there were no treatment effects through post-treatment. However, through follow-up, the PSST group had greater improvement in physical health relative to the TAU group that approached significance (b = 4.48, p = 0.08, d = 0.37, 95% CI [−0.04, 0.78]), which was a small effect. On the SF-12 mental health scale, the PSST group had significantly greater improvement in mental health symptoms compared to the TAU group through post-treatment (b = 7.52, p = 0.01, d = 0.64, 95% CI [0.15, 1.14]), which was a medium effect. This effect was not maintained through follow-up.
Exploratory Parenting Outcomes
General parenting stress
On the PSI Total Parenting Stress scale, there were no between-groups differences through post-treatment or follow-up.
Pain-specific parent behaviors
On the BAPQ-PIQ parent behavior scale, there was no between-groups difference through post-treatment. However, through follow-up, the PSST group had a significantly greater decrease in problematic parent behaviors compared to the TAU group (b = −7.02, p < 0.001, d = −1.14, 95% CI [−1.72, −0.55]), which was a large effect.
On the HHI, the PSST group had a significantly greater decrease in child-report of miscarried helping through post-treatment compared to the TAU group (b = −5.99, p = 0.05, d = −0.60, 95% CI [−1.19, 0]), which was a medium effect. This was not maintained through follow-up. On the parent-report HHI, there were no between-group differences through post-treatment or follow-up.
Child Physical and Mental Health Outcomes
In examining downstream effects of PSST intervention on child outcomes, there were no between-groups differences through post-treatment or follow-up on children’s usual pain intensity scores or on child functional impairment (social functioning and physical functioning) through post-treatment and follow-up.
However, PSST was associated with improvements in child mental health outcomes. On the BAPQ depression scale and the BAPQ general anxiety scale, children whose parents received PSST had greater decreases in symptoms compared to children whose parents received TAU through post-treatment and this approached significance (b = −2.56, p = 0.06, d = −0.49, 95% CI [−1.00, 0.02]; b = −3.18, p = 0.05, d = −0.56, 95% CI [−1.10, −0.01], respectfully), which were medium effects. There were not between-groups differences on either scale through follow-up. Similarly, children in the PSST group had a significantly greater decrease in pain-specific anxiety compared to the TAU group through post-treatment (b = −4.54, p = 0.008, d = −0.82, 95% CI [−1.41, −0.22]), which was a large effect. There was no between-groups difference on BAPQ pain-specific anxiety scores through follow-up.
Adverse Events
There were no study-related adverse events reported by participants during the course of the trial in either treatment group. When we collected adverse events at each assessment period, several participants reported major life events and stressors (e.g., hip replacement surgery). However, these were described by parents as being unrelated to study procedures.
Process Measure
The SPSI-R was used as a process measure. On dysfunctional problem solving, there were no between-groups differences through post-treatment or follow-up. However, PSST was associated with improvements in constructive problem solving and total problem solving scores. Parents in the PSST group had a greater increase in constructive problem solving compared to the TAU group through post-treatment that approached significance (b = 6.09, p = 0.07, d = 0.34, 95% CI [−0.03, 0.72]), which was a small to medium effect. This was not maintained through follow-up. For total problem solving scores, there was no between-groups difference through post-treatment. However, at follow-up, the PSST group had a greater improvement in total problem solving relative to the TAU group that approached significance (b = 0.67, p = 0.09, d = 0.29, 95% CI [−0.05, 0.62]), a small effect.
Discussion
This is the first pilot RCT of problem solving skills training in parent caregivers of youth with chronic pain. Although our participation rate for entering the trial was only moderate, the families who agreed to participate stayed in the trial (97%) and were compliant with 4–6 individual sessions of PSST. Parents reported satisfaction with treatment, and were willing to complete outcome assessments. Taken together, these results indicate feasibility in delivering PSST to parents of children with chronic pain.
We also examined preliminary efficacy of PSST compared to usual care on parent and child outcomes. Our hypothesized effects on parent outcomes were partially supported. PSST was associated with improvements on our primary outcome of parent depression at post-treatment and in some other areas of general and pain-specific parent mental health, well being, and behavior. Although children were not involved in PSST treatment, several downstream effects of PSST on child outcomes were found. Specifically, children whose parents received PSST had improved emotional functioning (depression, general anxiety, and pain-specific anxiety) post-treatment compared to children whose parents received usual care. However, there were no effects found on children’s pain or daily functioning. Effect sizes ranged from small to large across outcomes, and confidence intervals for effect sizes were wide. Most changes were not maintained at follow-up.
In a recent Cochrane review of published trials of PSST in children with chronic medical conditions [15], treatment was also associated with improved parent mental health and behavior with similar small effect sizes; however there were no treatment effects observed on child outcomes. In contrast, our study found small improvements in child mental health outcomes. Although results from this pilot trial should be interpreted cautiously, our findings are consistent with Palermo and Chambers’ [36] conceptual model of parent and family influences on chronic pain in children. This model highlights the interrelationship between parent and child functioning, and suggests that downstream effects on child outcomes can be achieved by directly targeting parent distress. Existing behavioral interventions for this population focus primarily on cognitive-behavioral skills training for children and do not address parent mental health [20]. Treatment approaches such as PSST that directly target parent distress may contribute to positive outcomes for children with chronic pain and their families. Future research is needed to determine whether added benefit can be obtained from combining parent PSST with other effective child pain-focused CBT interventions where children are learning pain management skills concurrently. It is possible that synergy between the two interventions would produce more powerful sustained effects on relevant child and parent outcomes. Future trials should also document health services and cost of treatment to understand whether PSST is associated with improvements in health service use and cost reduction.
We examined parent problem solving abilities as a process measure in this pilot trial, and found that PSST produced only small effects. The process measure of problem solving abilities (SPSI-R) used does not have clinical cut-points, and so we are not able to determine whether parents had clinically significant impairments in problem solving skills at baseline that could have improved with treatment. In particular, because our sample had a high portion of college-educated parents the problem-solving skills may have been better than average at the start of the trial. Interestingly, despite the availability of a validated and standardized measure of problem solving abilities, this domain is not routinely assessed in clinical trials of PSST. For example, in a recent meta-analysis only 4 of 12 prior RCTs of PSST for parent caregivers of children with chronic medical conditions reported on change in parents’ problem solving abilities [15]. We encourage future research in this area to include assessment of parent problem solving abilities to further understanding of the mechanisms underlying this intervention. It will also be important to examine the effects of PSST in more socioeconomically diverse samples of parents.
There may also be other treatment mechanisms to consider in future trials of PSST. In particular, the support received from the therapist, normalization of the stress experienced by parents of children with chronic pain (e.g., via the “common problems” worksheet), or other non-specific therapeutic effects may contribute to positive outcomes. The PSST intervention might also serve to improve parent-child interaction patterns and increase psychological flexibility, which may be important for change in the context of chronic pain [33]. Future trials of PSST should include measurement of other key process variables.
In contrast to previous trials of PSST for parents of children with other chronic illnesses, we delivered treatment in just 4–6 sessions rather than 6–8 sessions. Although our initial adaptation of the intervention led us to determine that a shorter intervention would be more feasible to deliver [37], parents of youth with chronic pain differ in important ways from populations evaluated in these previous trials (e.g., children with newly diagnosed cancer; Sahler et al., 2005). In our sample, the children had chronic pain for an average of 2 years and most parents (56.6%) had clinically elevated symptoms of depression. Given the chronicity of problems faced by families of children with chronic pain, it is possible that these caregivers may actually require more rather than less treatment compared to other pediatric populations. Although additional or booster treatment sessions could result in larger effects on parent and child outcomes, the burden and demand of additional sessions may also have a negative impact on feasibility of treatment delivery. Further research is needed to determine the optimal dose of PSST treatment for parents of children with chronic pain.
A goal of our pilot RCT was to test a range of outcome measures in order to help define appropriate outcomes for a future large definitive trial. Prior studies of PSST have used various domains of measurement including health-related quality of life, parent mental health, child medical symptoms, parent behaviors, family functioning, and parenting skills. It is challenging to make direct comparisons between studies due to the variability in specific measures used and lack of consensus about outcome measures for PSST trials [17]. We included both general measures and pain-specific measures in order to determine change in particular areas of mental health and well-being that may be most relevant to our patient population. Indeed, some pain-specific variables (e.g., pain catastrophizing) demonstrated changes in parents receiving PSST compared to TAU. However, overall there was a lack of consistent pattern in findings observed in our pilot RCT. In future trials of PSST, investigators will need to select appropriate outcome domains and balance the issue of defining independent outcomes within each measurement domain. Based on our pilot RCT, in future definitive trials, we recommend inclusion of the outcome domains of parent mental health (with general parent depressive symptoms as a primary outcome), child mental health, child symptoms (e.g., pain), and behavior (e.g., parent behavior, child pain-related functioning).
The study sample was mostly comprised of mothers and thus our experience delivering PSST to fathers is limited. Although both parents were invited to be involved in PSST sessions, most often, mothers chose to attend treatment alone. Qualitative research with parents of youth with chronic pain involving mothers has emphasized the negative and burdensome experience of parenting a child with chronic pain [26]. However, fathers of youth with chronic pain [25] may have a different experience. In a larger trial, it is possible that participating adults may include a larger number of fathers. However, acquiring participation from fathers is a problem in the field and may need to be addressed with increased flexibility in timing and mode of treatment delivery (e.g., offering internet-based treatment).
Our findings should be interpreted in light of several study limitations. This was a pilot RCT and was only powered to detect medium to large effects. Thus we were underpowered to detect small effects. Fitting with our proof of concept pilot RCT design, we included a usual care rather than an attention control comparison arm, which limited our ability to determine the source of the treatment effect. Future studies are needed in larger, more definitive trials with attention control groups. The sample is small and may not be representative of the broader population of parents of children with chronic pain. Because we conducted only short-term follow up, durability of treatment effects are unknown. Children in our trial were not receiving a consistent form of pain treatment and therefore it is difficult to understand any potential synergy between PSST and other child-focused treatment interventions. This remains an important area for future research to understand whether parent treatment with PSST might enhance child or family-focused treatment.
Clinical implications of our findings highlight the importance of including parents in treatment of childhood chronic pain. Applying interventions to reduce parent distress and to support parent coping skills is feasible and parents desire this form of treatment. At this point, a definitive test of the efficacy of PSST on parent mental health and child pain outcomes is needed. Long term effects should be measured as maintenance of treatment gains is critically important in pediatric chronic pain management where children have symptoms for many years.
Acknowledgments
The authors thank the parents and youth who participated in the study. We are also grateful for the contributions of the late Dr. Robert Butler who advised on the treatment protocol and to Drs. Andrew Riley and Bonnie Essner who served as study therapists. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD065180 (PI: Palermo). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest. None of the authors have any conflicts of interest.
References
- 1.Abidin RR. Parenting Stress Index. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 2.Arnau RC, Meagher MW, Norris MP, Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20(2):112–9. doi: 10.1037//0278-6133.20.2.112. [DOI] [PubMed] [Google Scholar]
- 3.Barlow JH, Ellard DR. The psychosocial well-being of children with chronic disease, their parents and siblings: an overview of the research evidence base. Child Care Health Dev. 2006;32(1):19–31. doi: 10.1111/j.1365-2214.2006.00591.x. [DOI] [PubMed] [Google Scholar]
- 4.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 5.Bigras M, LaFreniere PJ, Dumas JE. Discriminant Validity of the Parent and Child Scales of the Parenting Stress Index. Early Education and Development. 1996;7(2):167–178. [Google Scholar]
- 6.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 7.Cohen J. Statistical power analysis for the behavioral sciences (rev ed) Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1987. p. 474. [Google Scholar]
- 8.D’Zurilla TJ, Nezu AM. Problem solving therapy: A positive approach to clinical intervention. 3rd. New York: Springer Publishing Company, LLC; 2007. [Google Scholar]
- 9.D’Zurilla TJ, Nezu AM. Problem solving therapy: A social competence approach to clinical intervention. 2nd. New York: Springer Publishing; 1999. [Google Scholar]
- 10.D’Zurilla TJ, Nezu AM, Maydeu-Olivares A. Manual for the Social Problem-Solving Inventory-Revised. North Tonawanda, NY: Multi-Health Systems; 2002. [Google Scholar]
- 11.Derogatis LR. The Brief Symptom Inventory-18 (BSI-18): Administration, Scoring and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 12.Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10(2):89–89. [Google Scholar]
- 13.Drotar D. Relating parent and family functioning to the psychological adjustment of children with chronic health conditions: what have we learned? What do we need to know? J Pediatr Psychol. 1997;22(2):149–65. doi: 10.1093/jpepsy/22.2.149. [DOI] [PubMed] [Google Scholar]
- 14.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: Patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108(3):221–9. doi: 10.1016/j.pain.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Eccleston C, Fisher E, Law E, Bartlett J, Palermo TM. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2015;4:CD009660. doi: 10.1002/14651858.CD009660.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eccleston C, Jordan A, McCracken LM, Sleed M, Connell H, Clinch J. The Bath Adolescent Pain Questionnaire (BAPQ): development and preliminary psychometric evaluation of an instrument to assess the impact of chronic pain on adolescents. Pain. 2005;118(1–2):263–70. doi: 10.1016/j.pain.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Eccleston C, Palermo TM, Williams AC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2014;5:CD003968. doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fales JL, Essner BS, Harris MA, Palermo TM. When helping hurts: miscarried helping in families of youth with chronic pain. J Pediatr Psychol. 2014;39(4):427–37. doi: 10.1093/jpepsy/jsu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feingold A. Confidence interval estimation for standardized effect sizes in multilevel and latent growth modeling. J Consult Clin Psychol. 2015;83(1):157–68. doi: 10.1037/a0037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher E, Heathcote L, Palermo TM, de CW AC, Lau J, Eccleston C. Systematic review and meta-analysis of psychological therapies for children with chronic pain. J Pediatr Psychol. 2014;39(8):763–82. doi: 10.1093/jpepsy/jsu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain. 2006;123(3):254–63. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 22.Harris MA, Antal H, Oelbaum R, Buckloh LM, White NH, Wysocki T. Good intentions gone awry: assessing parental “miscarried helping” in diabetes. Families, Systems & Health. 2008;26(4):393–403. [Google Scholar]
- 23.Haskett ME, Ahern LS, Ward CS, Allaire JC. Factor structure and validity of the parenting stress index-short form. J Clin Child Adolesc Psychol. 2006;35(2):302–12. doi: 10.1207/s15374424jccp3502_14. [DOI] [PubMed] [Google Scholar]
- 24.Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9(3):226–36. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Jordan A, Crabtree A, Eccleston C. ‘You have to be a jack of all trades’: Fathers parenting their adolescent with chronic pain. J Health Psychol. 2015 doi: 10.1177/1359105315580461. [DOI] [PubMed] [Google Scholar]
- 26.Jordan A, Eccleston C, Crombez G. Parental functioning in the context of adolescent chronic pain: a review of previously used measures. J Pediatr Psychol. 2008;33(6):640–59. doi: 10.1093/jpepsy/jsm139. [DOI] [PubMed] [Google Scholar]
- 27.Jordan A, Eccleston C, McCracken LM, Connell H, Clinch J. The Bath Adolescent Pain–Parental Impact Questionnaire (BAP-PIQ): development and preliminary psychometric evaluation of an instrument to assess the impact of parenting an adolescent with chronic pain. Pain. 2008;137(3):478–87. doi: 10.1016/j.pain.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Kazdin AE. Acceptability of alternative treatments for deviant child behavior. J Appl Behav Anal. 1980;13(2):259–73. doi: 10.1901/jaba.1980.13-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley ML, Heffer R, Gresham F, Elliot S. Development of a modified treatment evaluation inventory. Journal of Psychopathology and Behavioral Assessment. 1989;11(3):235–247. [Google Scholar]
- 30.Klassen A, Raina P, Reineking S, Dix D, Pritchard S, O’Donnell M. Developing a literature base to understand the caregiving experience of parents of children with cancer: a systematic review of factors related to parental health and well-being. Support Care Cancer. 2007;15(7):807–18. doi: 10.1007/s00520-007-0243-x. [DOI] [PubMed] [Google Scholar]
- 31.Lewandowski AS, Palermo TM, Stinson J, Handley S, Chambers CT. Systematic review of family functioning in families of children and adolescents with chronic pain. J Pain. 2010;11(11):1027–38. doi: 10.1016/j.jpain.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logan DE, Scharff L. Relationships between family and parent characteristics and functional abilities in children with recurrent pain syndromes: an investigation of moderating effects on the pathway from pain to disability. J Pediatr Psychol. 2005;30(8):698–707. doi: 10.1093/jpepsy/jsj060. [DOI] [PubMed] [Google Scholar]
- 33.McCracken LM, Morley S. The psychological flexibility model: a basis for integration and progress in psychological approaches to chronic pain management. J Pain. 2014;15(3):221–34. doi: 10.1016/j.jpain.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 34.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: EdITS; 1992. [Google Scholar]
- 35.Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55(1):79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain. 2005;119(1–3):1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Palermo TM, Law EF, Essner B, Jessen-Fiddick T, Eccleston C. Adaptation of Problem-Solving Skills Training (PSST) for Parent Caregivers of Youth with Chronic Pain. Clin Pract Pediatr Psychol. 2014;2(3):212–223. doi: 10.1037/cpp0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palermo TM, Putnam J, Armstrong G, Daily S. Adolescent autonomy and family functioning are associated with headache-related disability. Clin J Pain. 2007;23(5):458–65. doi: 10.1097/AJP.0b013e31805f70e2. [DOI] [PubMed] [Google Scholar]
- 39.Palermo TM, Witherspoon D, Valenzuela D, Drotar D. Development and validation of the Child Activity Limitations Interview: a measure of pain-related functional impairment in school-age children and adolescents. Pain. 2004;109(3):461–70. doi: 10.1016/j.pain.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Reddon J, Marceau R, Holden R. A confirmatory evaluation of the profile of mood states: Convergent and discriminant item validity. Journal of Psychopathology and Behavioral Assessment. 1985;7(3):243–259. [Google Scholar]
- 41.Rivera PA, Elliott TR, Berry JW, Grant JS. Problem-solving training for family caregivers of persons with traumatic brain injuries: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89(5):931–41. doi: 10.1016/j.apmr.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahler OJ, Fairclough DL, Phipps S, et al. Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: report of a multisite randomized trial. J Consult Clin Psychol. 2005;73(2):272–83. doi: 10.1037/0022-006X.73.2.272. [DOI] [PubMed] [Google Scholar]
- 43.Sahler OJ, Varni JW, Fairclough DL, et al. Problem-solving skills training for mothers of children with newly diagnosed cancer: a randomized trial. J Dev Behav Pediatr. 2002;23(2):77–86. doi: 10.1097/00004703-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Seid M, Varni JW, Gidwani P, Gelhard LR, Slymen DJ. Problem-solving skills training for vulnerable families of children with persistent asthma: report of a randomized trial on health-related quality of life outcomes. Journal of Pediatric Psychology. 2010;35(10):1133–43. doi: 10.1093/jpepsy/jsp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shek DT, Ma CM. Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures and illustrations. Scientific World Journal. 2011;11:42–76. doi: 10.1100/tsw.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SPSS. SPSS for Windows, v.21.0. SPSS Inc.; Chicago, IL: 2012. [Google Scholar]
- 47.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143(3):223–7. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Wade SL, Carey J, Wolfe CR. An online family intervention to reduce parental distress following pediatric brain injury. J Consult Clin Psychol. 2006;74(3):445–54. doi: 10.1037/0022-006X.74.3.445. [DOI] [PubMed] [Google Scholar]
- 49.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]


