Abstract
Fibrin degradation results in the formation of fibrin degradation products (FDPs) of different molecular weights, which include D-dimer. Commercial D-dimer assays recognize multiple forms of FDP with different specificity. As a result, the absence of an international D-dimer standard and the marked discrepancy in the D-dimer values in the same samples measured by assays from different manufacturers have become the primary problems that clinicians face in the D-dimer determination. We consider that an assay with equal specificity to all FDP forms regardless of their molecular weights could help to solve these problems. We aimed to produce mAbs that could equally recognize high-molecular-weight FDP (HMW FDP) and D-dimer. mAbs against D-dimer were produced. The HMW FDP/D-dimer ratios in plasma samples were analyzed following protein separation by gel filtration using the developed fluoroimmunoassay. A sandwich immunoassay with equal specificity to HMW FDP and D-dimer was developed and applied to determine HMW FDP/D-dimer ratios in patients with different diseases. Although the HMW FDP levels prevailed in thrombotic patients, the FDP and D-dimer levels were comparable in septic patients. Meanwhile, the D-dimer levels often exceeded the HMW FDP levels in patients who had undergone surgery. The ‘D-dimer’ levels that were detected by different assays also varied greatly depending on the assay specificities to FDP and D-dimer. Our findings show that the introduction of assays with equal specificities to FDP and D-dimer in clinical practice is a possible way of standardizing D-dimer measurements.
Keywords: antibodies, fibrin degradation products, fibrin fragment D-dimer, specificity, thrombosis
Introduction
Blood coagulation includes a cascade of enzymatic reactions that lead to the conversion of fibrinogen into fibrin. The reverse process is called fibrinolysis and this destroys fibrin clots through the enzymatic cleavage of fibrin into soluble fragments.
Fibrin degradation occurs under the action of plasmin, which cleaves fibrin into many fragments of various molecular weights and in doing so forms the so-called fibrin degradation products (FDPs) [1]. D-dimer is the smallest product of fibrin degradation (MW 180 kDa), it is relatively stable and considered to be a final product of fibrin lysis. It consists of two subunits that are connected by two isopeptide bonds, which are formed under the action of factor XIIIa [2].
Increased D-dimer is a marker of a provoked coagulation process as fibrin formation is followed by fibrin degradation by plasmin. This results in an increase in the FDP concentration in the bloodstream.
Fibrinogen clotting underlies the pathogenesis of many disorders and therefore elevated levels of D-dimer have been found in the blood of patients with deep vein thrombosis [3,4], pulmonary thromboembolism [5], atherosclerosis [6,7], disseminated intravascular coagulation [8,9], sepsis [10,11], cancer [12], and other diseases, as well as after major surgery [13]. In clinical practice, D-dimer analysis is mainly used to exclude deep venous thrombosis, pulmonary thromboembolism, and estimate the risk of VTE recurrence following the discontinuation of anticoagulant therapy [14–18]. Moreover, many articles have been devoted to the prognostic value of elevated D-dimer levels in oncological and cardiovascular diseases. High plasma D-dimer levels have been found to be a marker of poor outcome in patients with colorectal, lung, breast, prostate, and bowel cancers [19–22], and reflect the intensity of the metastatic process [23]. High D-dimer levels may also predict such cardiovascular events as atrial fibrillation [24], ischemic and hemorrhagic outcomes following acute myocardial infarction [25], and permit the exclusion of aortic dissection in patients with chest pain [26].
Despite the long history of using the D-dimer test in clinical practice, there are many problems associated with the quantitative determination of D-dimer in plasma samples. The major problem that clinicians face is the discrepancy in the D-dimer values that are determined by D-dimer assays from different manufacturers. The results of analyte measurements from the same sample can vary by up to 20-fold or more between assays [27,28]. This finding suggests the hypothesis that each assay detects a particular form of the analyte in plasma samples and stresses the importance of obtaining a better understanding regarding which fibrin degradation products can be found in the blood of patients with different diseases and the specificity of the assay that could be used for their precise and reproducible measurement.
As fibrin degradation is a multistage process, a wide range of FDPs with different molecular weights is formed before D-dimer is generated. These intermediate products were found following fibrin digestion by plasmin in vitro[29]. However, only D-dimer and fragment E, as well as their complex (DDE complex), were initially assumed to be present in blood [30]. Nevertheless, two decades ago, Gaffney et al.[31] reported the presence of high-molecular-weight fibrin degradation products in the plasma of patients with disseminated intravascular coagulation (DIC) that were recognized by anti-D-dimer mAbs. The findings led to the conclusion that the fibrin degradation products in the plasma of DIC patients mainly consist of high-molecular-weight cross-linked fragments. Similar results were reported by Pfitzner et al.[32], who demonstrated that the separation of pooled plasma samples of patients with septic DIC by the gel filtration method allows the detection of D-dimer immunoreactivity in fractions eluted prior to a fibrinogen peak. Nowadays, it is common knowledge that a variety of different-sized cross-linked FDPs circulates in the blood and can be detected by D-dimer assays along with D-dimer itself [33]. Therefore, with regard to D-dimer assays, the term ‘D-dimer’ should be widely interpreted as the totality of all cross-linked soluble materials that are derived from fibrin [34].
The discrepancy between the results obtained by different D-dimer assays is currently explained by the different specificities of anti-D-dimer antibodies utilized in such assays to various forms of FDP in plasma [31,35]. For example, an immunoassay that better recognizes D-dimer than high-molecular-weight FDP would underestimate the ‘D-dimer’ value in plasma with the high level of high-molecular-weight FDP and low level of D-dimer. Conversely, an assay that better recognizes high-molecular-weight FDP would show a higher ‘D-dimer’ value in the same sample.
The heterogeneity of cross-linked material in plasma and the different specificities of the antibodies used in assays make it difficult to create an appropriate standard that is suitable for all D-dimer assays. It cannot be D-dimer or FDP for the reasons outlined above. Some researchers believe that the standardization of methods for D-dimer detection is principally impossible [33,36]. However, the pooled plasma of patients with elevated D-dimer levels was proposed as a reference material for D-dimer assays [37].
Another approach for solving this problem is the harmonization of the assay results by using a conversion factor [33,36,38] that also requires reference material in the assays. The ISTH Scientific and Standardization Committee proposed the use of a mixture of different FDP as a reference material for the harmonization procedure (http://c.ymcdn.com/sites/www.isth.org/resource/resmgr/yearly_subcommittee_minutes/all_subcommittee,_standing_c.pdf).
In our opinion, this approach cannot really solve the problems of D-dimer assay standardization as the underlying reason for this problem is the different specificities of the antibodies utilized in assays to different FDP. We consider that the only way of achieving real between-assay harmonization is to utilize monoclonal antibodies with equal specificity to D-dimer and to high-molecular-weight FDP in all D-dimer assays. It is only this approach that would permit the detection of the real values of cross-linked fibrin-derived material in blood.
The aim of the current work has been to develop monoclonal antibodies with equal specificities to D-dimer and high-molecular-weight FDP and to evaluate the ratio between them in the plasmas of patients with different diseases.
Methods
Reagents
All of the chemicals were purchased from Sigma-Aldrich (Carlsbad, California, USA), human fibrinogen (purity >90%) was purchased from Calbiochem (San Diego, California, USA), cell culture reagents were obtained from Invitrogen (Waltham, Massachusetts, USA), D-dimer was obtained from HyTest (Turku, Finland), and DELFIA assay buffer and enhancement solution for fluoroimmunoassays was obtained from Perkin Elmer (Salt Lake City, Utah, USA).
Development of monoclonal antibodies
Hybridoma cell lines that produce D-dimer-specific mAbs were obtained following the hybridization of Sp2/0 myeloma cells with spleen cells of Balb/c mice immunized with D-dimer using a standard technique. All D-dimer-specific mAbs were tested through direct ELISA to ensure they have no (or very low) cross-reactivity with fibrinogen.
Sandwich fluoroimmunoassays
The mAb pairs for assay design were selected through a one-step immunoassay. The capture mAbs were adsorbed on plates in phosphate buffer saline (1 μg/100 μl/well; incubation for 30 min). Following three washes with TBST, 75 μl of the detection mAbs in DELFIA assay buffer (200 ng/well) labeled with a stable Eu3+ chelate [39] and 25 μl of the D-dimer-containing sample were added and the mixture was incubated for 1 h. Following six washes with TBST, the enhancement solution (300 μl/well) was added and the mixture was incubated for 3 min under vigorous shaking. The signals were detected using a 1420 Multilabel Counter Victor instrument (Perkin Elmer, USA).
FDP and D-dimer preparations with equal amounts of cross-linked material
FDP was produced from clotted fibrinogen by proteolysis. To briefly explain, fibrinogen was salted in from 100 ml of human citrate plasma through the addition of ammonium sulfate to 25% of saturation. The pellet was collected by centrifugation, washed with 8% ethanol, and dissolved in 40 ml of the 20 mmol/l sodium acetate solution, pH 7.5, containing 0.15 mol/l NaCl and 5 mmol/l CaCl2. 14 NIH U of bovine thrombin was added, quickly stirred, and left for 3 h at 37°C to form a clot. Ten units of streptokinase in 5 ml of the sodium acetate solution were added to the resultant clot and were incubated overnight at 37°C under mild shaking. A liquid fraction of FDP was collected when approximately 30% of the clot had been digested and this fraction was divided into two portions. The reaction in the first portion was stopped immediately by 20 mmol/l PMSF and used as the HMW FDP preparation. Meanwhile, the reaction in the second portion was continued for another 24 h, stopped by PMSF, and used as the D-dimer preparation.
Gel filtration procedure
Gel filtration studies of D-dimer, fibrinogen, FDP, and the plasma samples of patients were performed using the AKTA pure system (GE Healthcare, USA). Hundred to thousand microliters of the samples was loaded onto a Superdex200 16/60 column (GE Healthcare), and the proteins were eluted by using 50 mmol/l Tris-HCl buffer, pH 7.5, containing 0.15 mol/l NaCl at a rate of 2 ml/min. One-milliliter fractions were collected. The D-dimer immunoreactivity in the fractions was measured by different FIAs and quantified as the areas under the corresponding peaks using the OriginPro 8 program.
SDS-PAGE
FDP and D-dimer were analyzed by 3–10% gradient SDS-PAGE according to Laemmli under nonreducing conditions.
Patients
The study was conducted in accordance with the current revision of the Helsinki declaration. Seven patients with deep vein thrombosis, one patient with mesenteric thrombosis, 15 patients with sepsis of different etiologies, nine patients undergoing abdominal surgical operations, and seven patients with pulmonary embolism were enrolled in the study after they provided informed consent. In case of surgical operation, the blood samples were collected 1 day before and 1 day after the operation.
Blood samples
Blood samples from patients and healthy volunteers were taken using vacutainers containing ethylenediaminetetraacetic acid. The plasma samples were prepared using a routine procedure and stored frozen at −70°C.
Results
Preparation of FDP and D-dimer solutions with equal amounts of cross-linked material
To select mAbs with equal specificities to D-dimer and FDP, preparations of D-dimer and FDP with an equal amount of the cross-linked material were produced from a fibrin clot through proteolysis. As one can observe from the gel electrophoresis results (Fig. 1), the FDP preparation mainly contained high-molecular-weight fibrin degradation products and, to a lesser extent, D-dimer. Meanwhile, the D-dimer preparation contained D-dimer and minor (if indeed any) amounts of high-molecular-weight FDP. The disappearance of high-molecular-weight products and intensification of the D-dimer band confirmed that prolonged proteolysis resulted in D-dimer formation from FDP. As the D-dimer preparation was produced from the FDP preparation, the total protein content as well as the total amounts of cross-linked material in both preparations should be equal.
Fig. 1.
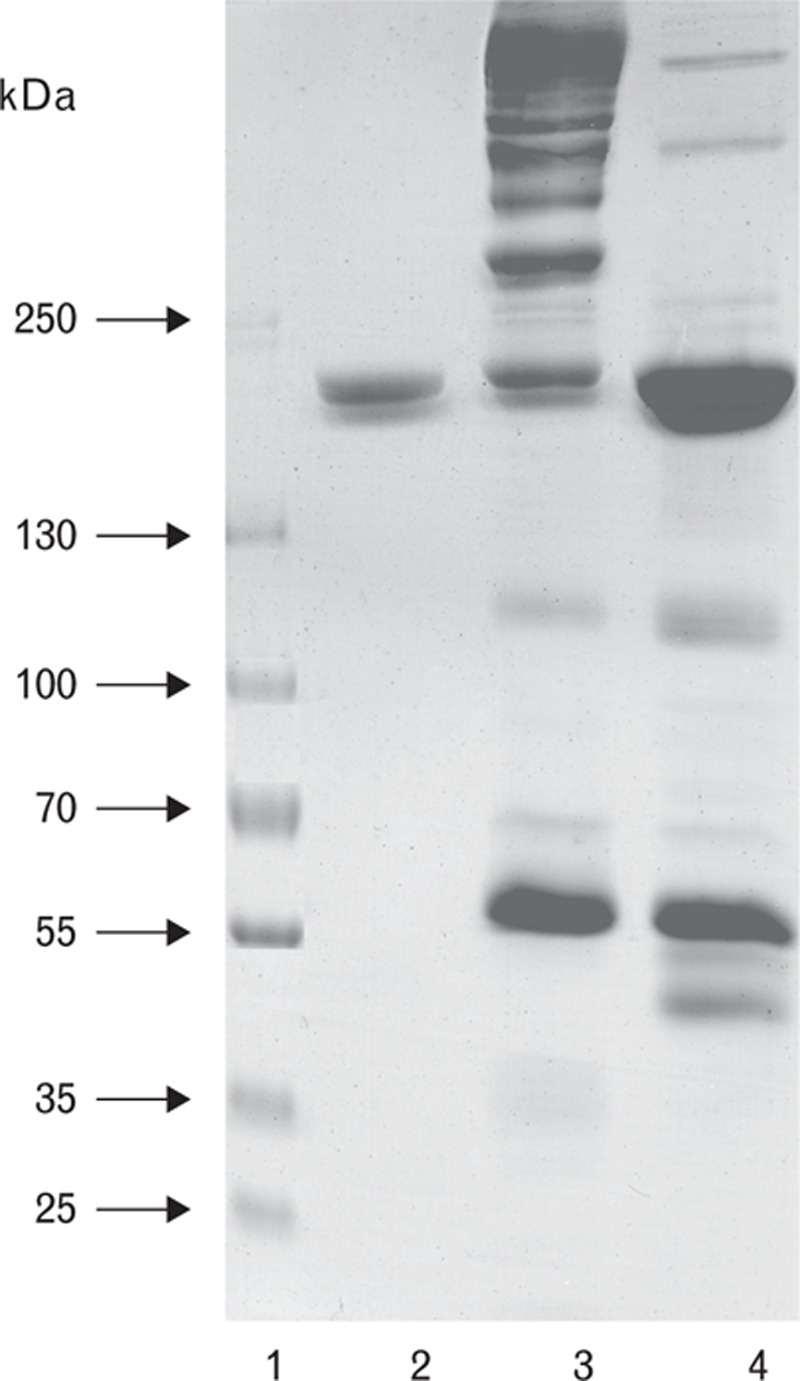
SDS PAGE of FDP and D-dimer prepared sequentially from the same fibrin clot. 1, molecular weight standards; 2, D-dimer standard; 3, high-molecular-weight fibrin degradation product (HMW FDP) solution; 4, D-dimer prepared from HMW FDP solution.
mAb development and analysis
Twenty-two mAbs specific to D-dimer and with no (or low) cross-reactivity to fibrinogen were obtained and selected for the studies. All of the selected mAbs were tested in two-site combinations by using FDP and D-dimer preparations that contained equal amounts of cross-linked material. Most assays, such as an assay utilizing the DD162 (capture) and DD186 (detection) mAbs, were able to better recognize FDP than D-dimer (Fig. 2a). However, there were also assays, such as the DD143-DD195 assay, which were able to better recognize D-dimer than FDP (Fig. 2b). Only one assay that utilized the DD189 and DD255 mAbs as the capture and detection antibodies respectively was found to give similar signals with both forms of the antigen (Fig. 2c).
Fig. 2.
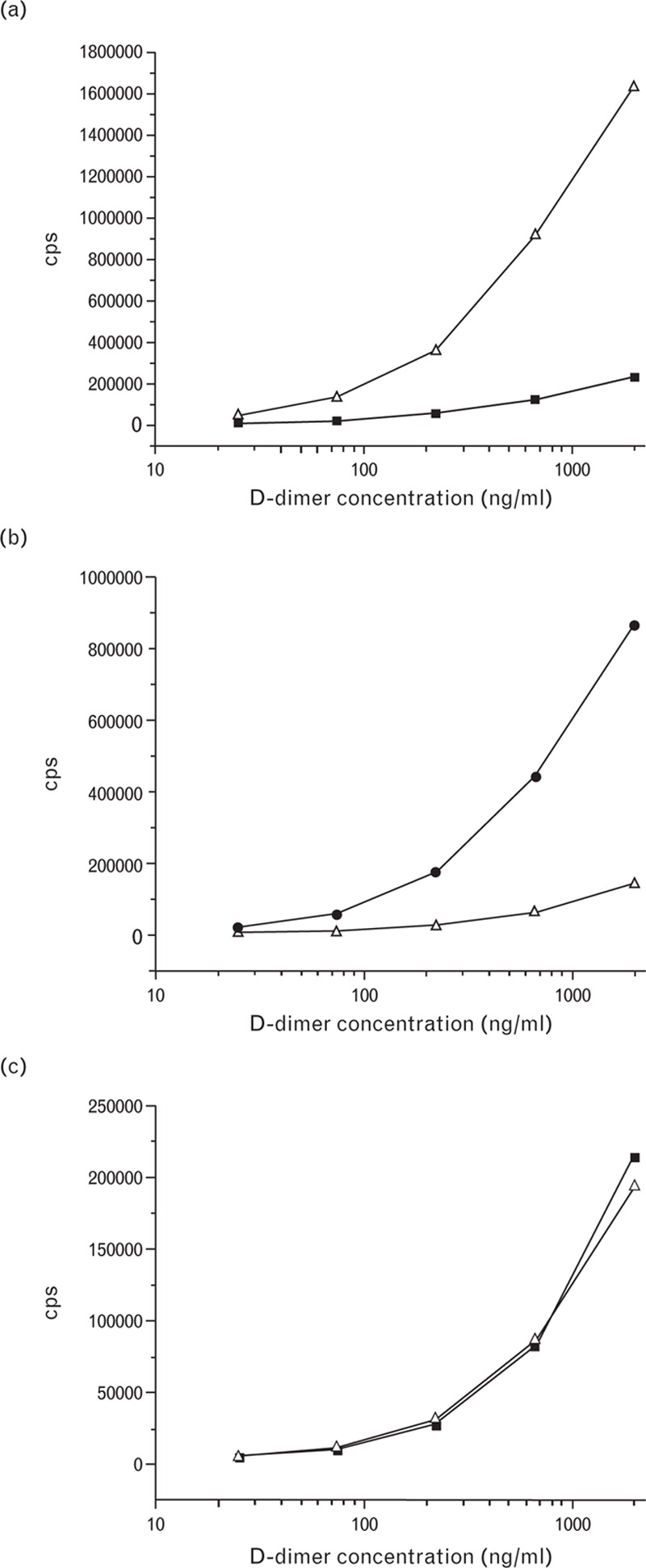
Titration curves of D-dimer and fibrin degradation product (FDP) preparations in the DD162-DD186 (a), DD143-DD195 (b), and DD189-DD255 (c) assays. Empty triangles, FDP; black squares, D-dimer.
The D-dimer and FDP samples were separated through the gel filtration method and the fractions were analyzed by using the DD189-DD255 immunoassay to detect immunoreactive products. In the case of the FDP preparation, the protein profile was very similar to the immunoreactivity profile with the exception of several minor peaks of smaller molecular weights (Fig. 3a). The HMW FDP immunoreactivity peak coincided with the major protein peak, whereas the D-dimer peak was considerably smaller. The comparison of the A280 and immunoreactivity measurement results demonstrates that both protein forms, namely HMW FDP and D-dimer, were almost equally recognized by the DD189-DD255 immunoassay. A gel filtration profile of the D-dimer preparation did not contain HMW products and consisted of lower-molecular-weight products. A major product was D-dimer whereas several minor peaks did not display D-dimer immunoreactivity (Fig. 3b). The major protein peak in the D-dimer preparation did not coincide with the D-dimer peak in the FDP preparation and corresponded to the lower-molecular-weight, which indicates that the D-dimer peak in the FDP preparation was most likely the DDE complex. Therefore, it can be concluded that the gel filtration results are in good agreement with the SDS-PAGE results (Fig. 1).
Fig. 3.
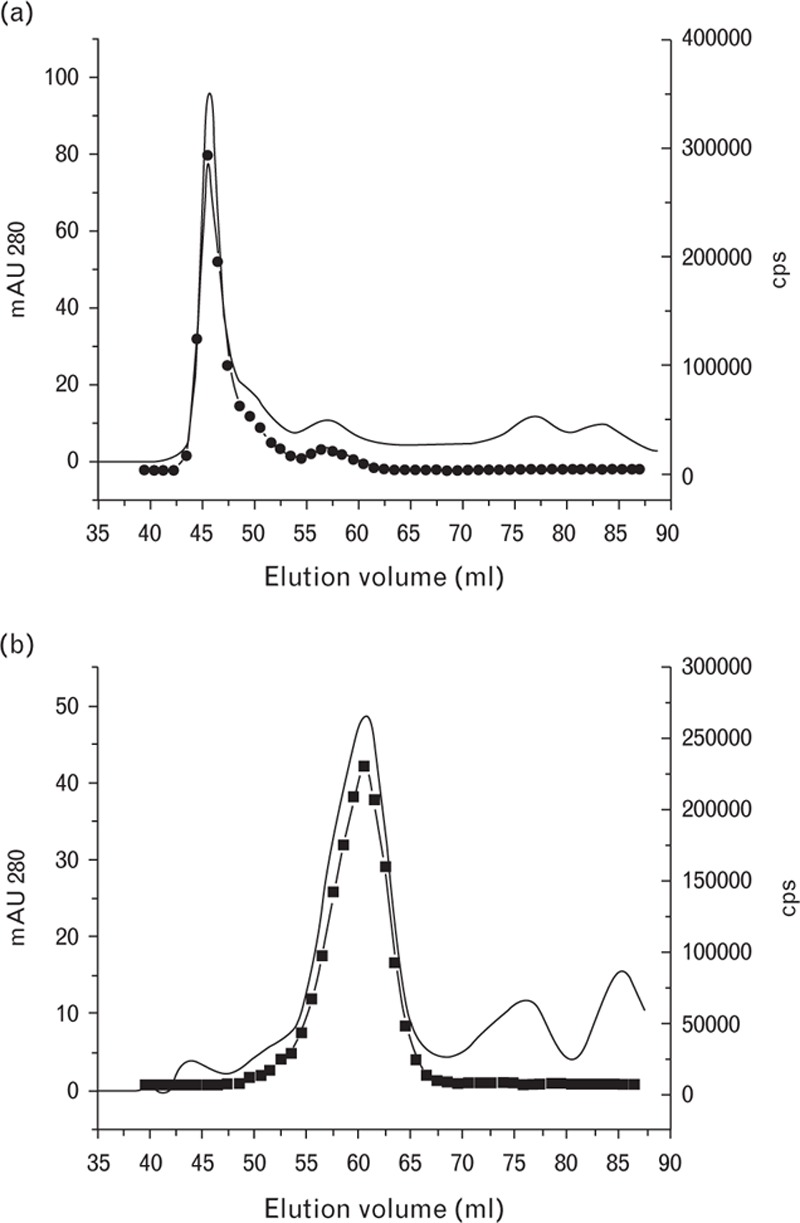
Gel filtration of fibrin degradation product (a) and D-dimer (b) preparations. Solid line, mAU 280; black circles, immunological activity measured by the DD189-DD255 assay.
Analysis of cross-reactivity of the DD189-DD255 assay with fibrinogen
During the preliminary testing of the DD189-DD255 and other assays with fibrinogen, we observed a cross-reactivity of approximately 0.5–5% compared with the signals that were obtained with the same molar amount of D-dimer. To elucidate the question of the cross-reactivity of DD189-DD255 assay to fibrinogen, we separated the fibrinogen sample (which had a purity >90% according to the manufacturer's data) by the gel filtration method, and the resulting profile was analyzed using the DD189-DD255 assay (Fig. 4). The protein profile was represented by two peaks: a major protein peak of approximately 340 kDa that belonged to fibrinogen and a smaller peak of higher-molecular-weight proteins prior to the fibrinogen peak. The D-dimer immunoreactivity was connected with the smaller (high-molecular-weight) peak, whereas the fibrinogen peak did not yield a response with the DD189-DD255 assay. The comparison of the results shown in Figs. 3a and Fig. 4 demonstrates that the immunoreactive peak of the fibrinogen preparation profile coincided with the FDP peak of the FDP preparation profile. This suggests that the fibrinogen preparation contains minor contamination displaying D-dimer immunoreactivity and consisting of HMW FDP. These data prove that the DD189-DD255 assay has no cross-reactivity with fibrinogen and only recognizes protein forms that contain D-dimer-associated immunoreactivity.
Fig. 4.
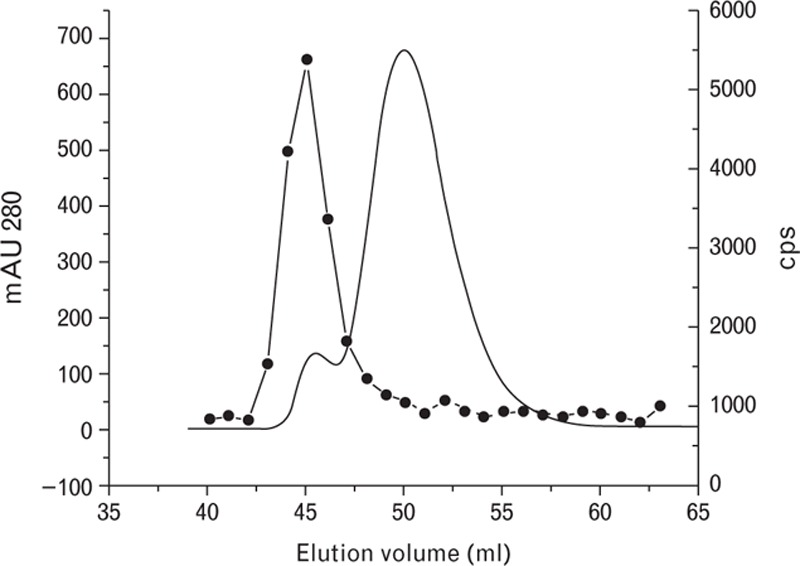
Gel filtration studies of human fibrinogen. Solid line, mAU 280; black circles, immunological activity measured by the DD189-DD255 assay.
Determination of D-dimer in plasma samples from patients
Plasma samples from patients with sepsis, thrombosis, and pulmonary thromboembolism, as well as of patients undergoing surgical operations, were analyzed using the DD189-DD255 assay. The results are summarized in Table 1. The D-dimer levels were significantly elevated in all of the patients with the highest levels being found in the thrombotic patients. The samples with the highest D-dimer levels in each group of patients were selected for further analysis.
Table 1.
Determination of D-dimer levels in plasma samples of patients with different diseases
| Disease | N | DD, μg/ml | Range, μg/ml |
| Sepsis | 15 | 2.5 | 0.52–4.9 |
| Thrombosis | 8 | 3.1 | 0.98–6.9 |
| The surgery (before/after) | 9 | 0.28/2.6 | 0.25–0.32/1.2–3.6 |
| Pulmonary thromboembolism | 7 | 2.7 | 1.3–6.7 |
Analysis of the FDP/D-dimer ratios in plasma samples from patients
To evaluate the ratio of D-dimer to FDP in the blood of patients with elevated D-dimer levels, 18 plasma samples from patients with different diseases (five with thrombosis, six following surgical operations, and seven with sepsis) were separated by gel filtration and the D-dimer immunoreactivity in the fractions was analyzed with the DD189-DD255 assay. In all of the cases, the activity profiles consisted of two distinctly separated peaks representing FDP and D-dimer. The areas under the peaks were calculated to quantify the amounts of FDP and D-dimer. As the DD189-DD255 assay equally recognizes both forms of the protein, the peak area ratios were assumed to be the ratios of FDP to D-dimer. The results showed that the FDP levels exceeded the D-dimer levels in the plasma of patients with thrombotic diseases by up to 3.5 times (see representative profile in Fig. 5a). In septic patients, the FDP and D-dimer levels were comparable with the slight prevalence of the FDP levels. Furthermore, in patients undergoing surgical operations, the FDP and D-dimer levels measured 1 day following surgical operation were also similar to the slight prevalence of D-dimer (Fig. 5b). The ratios of the FDP to D-dimer levels in the blood of patients with different diseases are shown in Fig. 6.
Fig. 5.
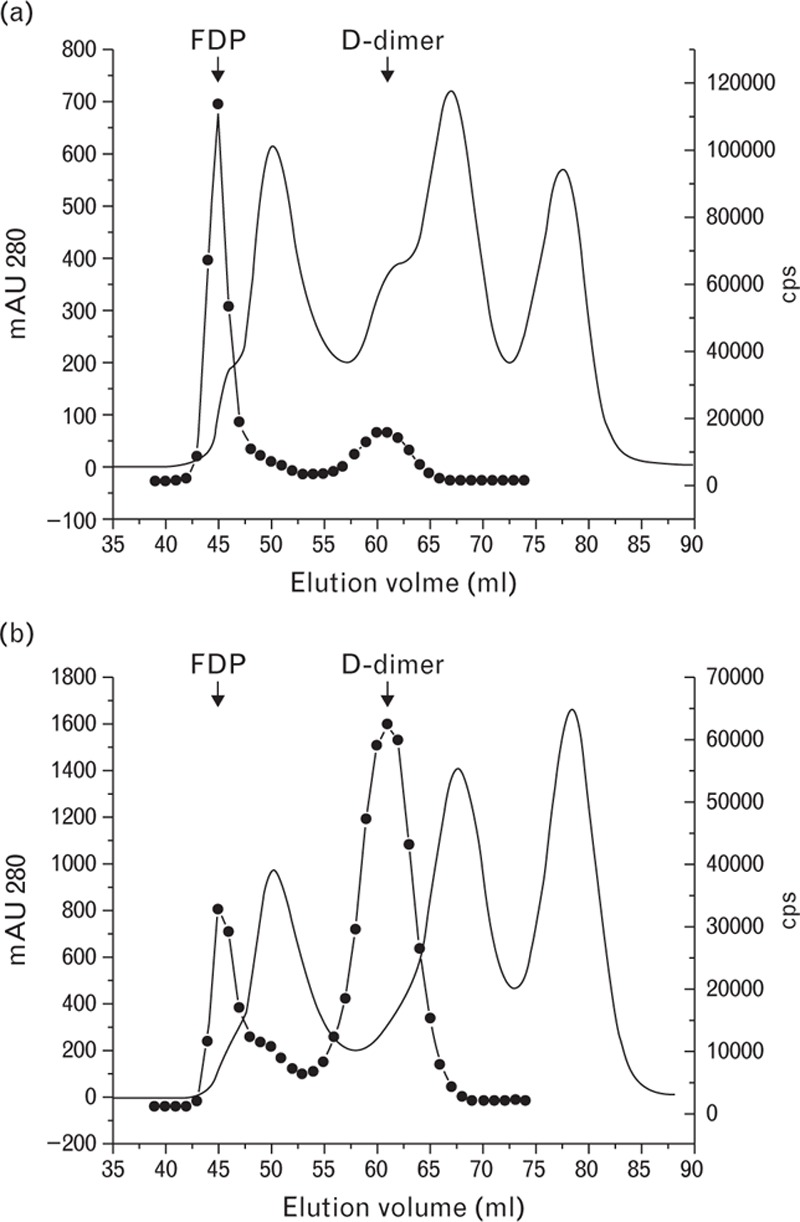
Gel filtration study of plasma samples from patients with thrombosis (a) and one day after surgical operation (b). Solid line, mAU 280; black circles, immunological activity measured by the DD189-DD255 assay.
Fig. 6.
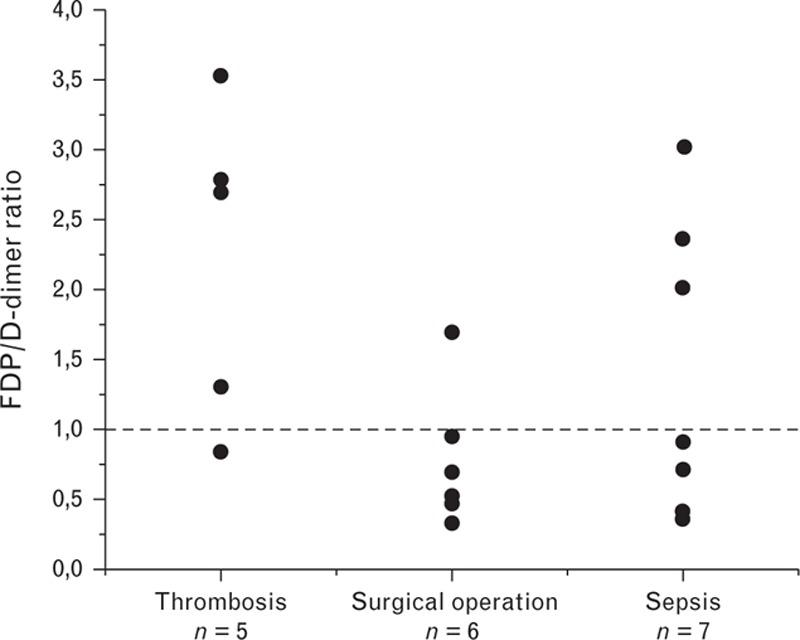
Ratios of the fibrin degradation product to D-dimer levels in the blood of patients with different diseases measured by the DD189-DD255 assay.
FDP and D-dimer measurements by the assays with different specificity to different fibrin degradation products
We used two assays – one of which is much better at recognizing FDP than D-dimer (DD162-DD186 assay), whereas the other is more specific to D-dimer (DD143-DD195 assay) – to analyze the gel filtration profile of a plasma sample from a septic patient. As shown in Fig. 7, the plasma profile obtained with the DD162-DD186 assay only detected the FDP peak and did not detect the D-dimer peak. Meanwhile, the DD143-DD195 assay detected the D-dimer peak better than the FDP peak. These results show that different cross-linked products in patient plasma samples may either be overestimated or underestimated depending on the specificities of the antibodies utilized in the assay.
Fig. 7.
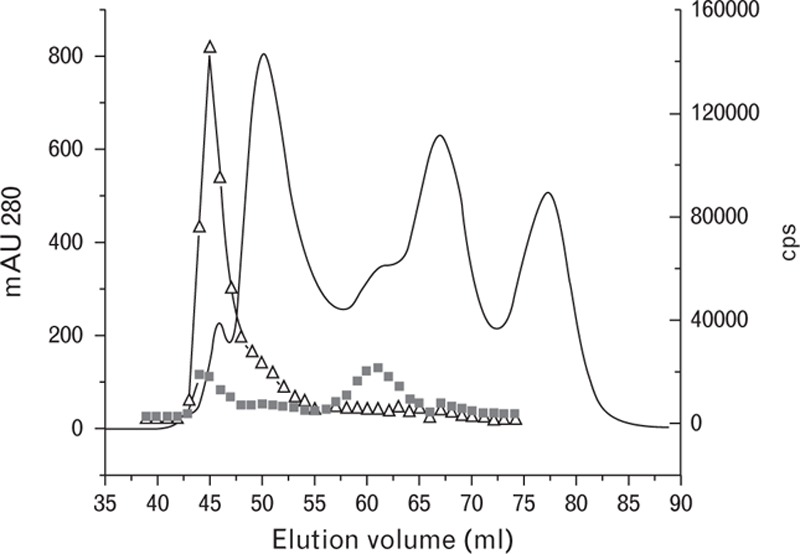
Gel filtration and immunochemical studies of the plasma sample from a septic patient. Solid line, mAU 280; empty triangles, immunological activity measured by the DD162-DD186 assay; black squares, immunological activity measured by the DD143-DD195 assay.
To assess how differences in mAb specificity can influence the results of D-dimer determination in plasma samples, we compared the results of the D-dimer measurements obtained by using the DD189-DD255 assay (equal specificities to FDP and D-dimer) and the DD162-DD186 assay (more specific to FDP) (Table 2). Both assays were calibrated using a commercial D-dimer. In patients with deep vein thrombosis and a prevalence of FDP, the DD162-DD186 assay yielded extremely high values of ‘D-dimer’ because of the very high sensitivity of the DD162-DD186 assay to FDP and its low sensitivity to D-dimer used in both assays as a calibrator. In a patient following surgical operation and in a septic patient with the prevalence of D-dimer in the plasma, the ‘D-dimer’ levels were markedly lower than those in thrombotic patients, as measured by the DD162-DD186 assay. Meanwhile, significantly lower levels were obtained with the DD189-DD255 assay.
Table 2.
Comparison of D-dimer concentrations in the blood of representative patients with different diseases measured by D-dimer assays utilizing antibodies with different specificities
| Patient diagnosis | FDP/DD ratio measured by the DD189-DD255 assay | DD measured by the DD162-DD186 assay, μg/ml | DD measured by the DD189-DD255 assay, μg/ml |
| Deep vein thrombosis | 2.7 | 420 | 4.8 |
| Deep vein thrombosis | 3.5 | >1000 | 6 |
| Surgical operation | 0.32 | 65 | 3.3 |
| Sepsis | 0.36 | 145 | 4.6 |
FDP, fibrin degradation product.
Discussion
The D-dimer measurements in the plasma of patients have been widely used in clinical practice for several decades. Although these assays are traditionally known as ‘D-dimer’ assays, one should keep in mind that such assays detect – together with D-dimer – a wide variety of cross-linked fibrin degradation products with different molecular weights. The monoclonal antibodies that are utilized in different assays have different specificities to various cross-linked fibrin degradation products that are present in the plasma of patients. Consequently, the results of D-dimer measurements in the same blood sample by different assays can vary significantly. This situation is aggravated by the fact that the ratio of FDP forms in the blood may be inconsistent. Therefore, the uniqueness of individual FDP spectra for different patients and the different specificities of the antibodies utilized in assays make the standardization or even harmonization of the existing D-dimer assays almost impossible.
We hypothesize that the only way of reaching agreement between different assays is through a proper selection of antibodies. To the best of our understanding, to ensure the precise measurements of D-dimer and other cross-linked fibrin degradation products that contain D-dimer immunoreactivity, the antibodies utilized in D-dimer assays should have equal specificities to different fibrin degradation products, regardless of their weights (degradation level). In this study, we describe D-dimer-specific monoclonal antibodies with equal reactivities to D-dimer and high-molecular-weight FDP. The comparison of the assay that utilizes such antibodies with other assays that display different specificities demonstrates how this approach can improve the accuracy of D-dimer measurements.
The traditional technique of mice immunization and subsequent hybridoma development made it possible to produce a number of monoclonal antibodies that are specific to D-dimer and FDP. However, we applied a novel approach to select mAbs that equally recognize FDP and D-dimer. We produced D-dimer and FDP preparations with equal amounts of cross-linked groups. For this purpose, D-dimer was prepared from FDP through additional lysis as cross-linked regions are mostly stable upon site-specific digestion and their number does not change during the further degradation of FDP to form D-dimer. In our case, the FDP solution was divided into two equal parts and one part was later used as the FDP preparation, whereas the other part was subjected to further proteolysis to obtain the D-dimer preparation. In both cases, the volumes were unchanged; therefore, the concentrations of cross-linked material displaying D-dimer immunoreactivity were the same. Gel filtration and SDS-PAGE analysis showed that despite both preparations not being 100% pure, the major components of the preparations were FDP and D-dimer, respectively. By using such preparations, we were able to select the DD189-DD255 antibody combination out of several hundred tested antibody combinations, which provided similar results with both antigens.
The antibodies utilized in a D-dimer assay should not exhibit cross-reactivity to fibrinogen. As fibrinogen is an abundant protein in human blood, even minor assay cross-reactivity with fibrinogen can result in incorrect D-dimer measurements. Although the preliminary results of the test with fibrinogen for many antibody pairs selected for the study were slightly positive, further analysis revealed that this finding was obtained due to the contamination of the commercial fibrinogen preparation by high-molecular-weight FDP. The DD189-DD255 assay had no cross-reactivity with fibrinogen.
As the DD189-DD255 assay detects D-dimer and FDP with equal specificity, we hypothesize that the use of this unique instrument may allow the precise quantification of each protein form and enable us to draw conclusions regarding the real ratios between these products in plasma samples. Therefore, we were able to provide the first precise evaluation of the ratio of FDP to D-dimer in plasma samples from patients with different diseases. The analysis of the D-dimer immunoreactivity profiles in gel filtration fractions by the DD189-DD255 assay showed that the compositions of the fibrin-derived degradation products may differ greatly not only between patients with different diseases but also between blood samples from patients with the same disease. Although the groups of patients used in the current study were not sufficiently large to draw conclusions regarding regular occurrence, we are in a position to discuss some trends. Firstly, we found that the amount of FDP in the blood of patients with deep vein thrombosis significantly (up to 3.5 times) exceeded the amount of D-dimer. In septic patients and patients undergoing surgical operations, the amounts of FDP and D-dimer were more or less comparable. Meanwhile, in some surgical patients, the amount of D-dimer significantly (up to three-fold) exceeded the amount of FDP.
These results confirm the importance of the accurate selection of the antibodies for the D-dimer assay. To demonstrate the influence of the antibody specificity on the results of the analyte measurements in blood samples, we compared the DD189-Dd255 assay with equal specificity to FDP and D-dimer with the DD162-DD186 assay, which is markedly more specific to FDP than to D-dimer, as well as the DD162-DD186 assay with the DD143-DD195 assay, which is more specific to D-dimer. The results suggested that the measurements obtained using these assays may differ significantly due to the different specificities of the assays to FDP and D-dimer and the different ratios of FDP to D-dimer in different plasma samples. We also expected that the utilization of the D-dimer preparation as a common calibrator may be another source of notable discrepancy between the measurements obtained using these assays.
The gel filtration analysis of a plasma sample from a septic patient clearly demonstrated the influence of the assay specificity on the results of the analysis. The DD162-DD186 assay only detected FDP in the fractions and did not reveal a D-dimer peak. The DD143-DD195 assay detected both peaks and the immunoreactivity in the D-dimer peak was higher than that in the FDP peak.
The measurement of D-dimer in patient plasma by assays with different specificities to FDP and D-dimer as expected led to discrepancies in results. The ‘D-dimer’ levels in plasma samples from patients with deep vein thrombosis (with a prevalence of FDP) detected by the DD162-DD186 assay were incredibly high and almost 100 times higher than those measured by the DD189-Dd255 assay. This observation can be explained by two factors: the high sensitivity of the DD162-DD186 assay to FDP and the poor recognition of the D-dimer compound that was used as the reference material. In other words, FDP yields a significantly higher response in the DD162-DD186 assay than equal amounts of D-dimer. Therefore, D-dimer as a calibrator yielded relatively low signals by the DD162-DD186 assay, whereas the signals obtained with FDP containing samples were incomparably high.
We also demonstrated that patients with the same disease can have different ratios of FDP to D-dimer in blood. Based on the results of the analysis of the limited number of samples utilized in the current study it is difficult to conclude as to whether this observation has additional clinical value. Further studies are necessary for clarity on this point.
In summary, we can conclude that this study demonstrates that the proper selection of antibodies for the D-dimer assay is the most critical factor regarding the precise quantitative immunodetection of cross-linked fibrin degradation products in human blood. It is our belief that only assays with equal specificities to all of the components of cross-linked material will give adequate results. The utilization of antibodies with equal specificities to all FDP forms in all commercial assays will be very helpful for reducing the existing discrepancy between the sample measurements obtained by different assays. It is important in this case that any antigen form (D-dimer, FDP preparation, or pooled plasma) may serve as the common reference material for all such assays. Therefore, the ‘standardization’ of the specificity of the assay antibodies may be considered to be a first and most important step toward D-dimer assay standardization.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood 1991; 78:3114–3124. [PubMed] [Google Scholar]
- 2.Chen R, Doolittle RF. g-g Cross-linking sites in human and bovine fibrin. Biochemistry 1971; 10:4486–4491. [DOI] [PubMed] [Google Scholar]
- 3.Rowbotham BJ, Carroll P, Whitaker AN, Bunce IH, Cobcroft RG, Elms MJ, et al. Measurement of cross-linked fibrin derivatives - use in the diagnosis of venous thrombosis. Thromb Haemost 1987; 57:59–61. [PubMed] [Google Scholar]
- 4.Bounameaux H, Schneider PA, Reber G, de Moerloose P, Krahenbuhl B. Measurement of plasma D-dimer for diagnosis of deep venous thrombosis. Am J Clin Pathol 1989; 91:82–85. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg JS, Brill-Edwards PA, Demers C, Donovan D, Panju A. D-dimer in patients with clinically suspected pulmonary embolism. Chest 1993; 104:1679–1684. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich J, Schulte H, Schönfeld R, Köhler E, Assmann G. Association of variables of coagulation, fibrinolysis and acute-phase with atherosclerosis in coronary and peripheral arteries and those arteries supplying the brain. Thromb Haemost 1995; 73:374–379. [PubMed] [Google Scholar]
- 7.Salomaa V, Stinson V, Kark JD, Folsom AR, Davis CE, Wu KK. Association of fibrinolytic parameters with early atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation 1995; 91:284–290. [DOI] [PubMed] [Google Scholar]
- 8.Carey MJ, Rodgers GM. Disseminated intravascular coagulation: clinical and laboratory aspects. Am J Hematol 1998; 59:65–73. [DOI] [PubMed] [Google Scholar]
- 9.Angstwurm MW, Dempfle CE, Spannagl M. New disseminated intravascular coagulation score: A useful tool to predict mortality in comparison with Acute Physiology and Chronic Health Evaluation II and Logistic Organ Dysfunction scores. Crit Care Med 2006; 34:314–320. [DOI] [PubMed] [Google Scholar]
- 10.Hesselvik JF, Blombäck M, Brodin B, Maller R. Coagulation, fibrinolysis, and kallikrein systems in sepsis: relation to outcome. Crit Care Med 1989; 17:724–733. [DOI] [PubMed] [Google Scholar]
- 11.Gando S, Nanzaki S, Sasaki S, Aoi K, Kemmotsu O. Activation of the extrinsic coagulation pathway in patients with severe sepsis and septic shock. Crit Care Med 1998; 26:2005–2009. [DOI] [PubMed] [Google Scholar]
- 12.Rocha E, Páramo JA, Fernández FJ, Cuesta B, Hernández M, Paloma MJ, et al. Clotting activation and impairment of fibrinolysis in malignancy. Thromb Res 1989; 54:699–707. [DOI] [PubMed] [Google Scholar]
- 13.Bongard O, Wicky J, Peter R, Simonovska S, Vogel JJ, de Moerloose P, et al. D-dimer plasma measurement in patients undergoing major hip surgery: use in the prediction and diagnosis of postoperative proximal vein thrombosis. Thromb Res 1994; 74:487–493. [DOI] [PubMed] [Google Scholar]
- 14.Wilbur J, Shian B. Diagnosis of deep venous thrombosis and pulmonary embolism. Am Fam Physician 2012; 86:913–919. [PubMed] [Google Scholar]
- 15.Michiels JJ, Freyburger G, van der Graaf F, Janssen M, Oortwijn W, van Beek EJ. Strategies for the safe and effective exclusion and diagnosis of deep vein thrombosis by the sequential use of clinical score, D-dimer testing, and compression ultrasonography. Semin Thromb Hemost 2000; 26:657–667. [DOI] [PubMed] [Google Scholar]
- 16.Crippa L, D’Angelo SV, Tomassini L, Rizzi B, D’Alessandro G, D’Angelo A. The utility and cost-effectiveness of D-dimer measurements in the diagnosis of deep vein thrombosis. Haematologica 1997; 82:446–451. [PubMed] [Google Scholar]
- 17.de Moerloose P, Palareti G, Aguilar C, Legnani C, Reber G, Peetz D. A multicenter evaluation of a new quantitative highly sensitive D-dimer assay for exclusion of venous thromboembolism. Thromb Haemost 2008; 100:505–512. [DOI] [PubMed] [Google Scholar]
- 18.Eichinger S, Minar E, Bialonczyk C, Hirschl M, Quehenberger P, Schneider B, et al. D-dimer levels and risk of recurrent venous thromboembolism. JAMA 2003; 290:1071–1074. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Yoshinaga K, Matsuyama A, Iwasa T, Osoegawa A, Tsujita E, et al. Plasma D-dimer level as a mortality predictor in patients with advanced or recurrent colorectal cancer. Oncology 2012; 83:10–15. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YX, Yang ZM, Feng J, Shan YJ, Wang WL, Mei YQ. High plasma D-dimer level is associated with decreased survival in patients with lung cancer: a meta-analysis. Tumour Biol 2013; 34:3701–3704. [DOI] [PubMed] [Google Scholar]
- 21.Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012; 97:1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowlson L, Bacchu S, Paneesha S, McManus A, Randall K, Rose P. Elevated D-dimers are also a marker of underlying malignancy and increased mortality in the absence of venous thromboembolism. J Clin Pathol 2010; 63:818–822. [DOI] [PubMed] [Google Scholar]
- 23.Tomimaru Y, Yano M, Takachi K, Kishi K, Miyashiro I, Ohue M, et al. Plasma D-dimer levels show correlation with number of lymph node metastases in patients with esophageal cancer. J Am Coll Surg 2006; 202:139–145. [DOI] [PubMed] [Google Scholar]
- 24.Mahé I, Bergmann JF, Chassany O, dit-Sollier CB, Simoneau G, Drouet L. COAGFA Group. A multicentric prospective study in usual care: D-dimer and cardiovascular events in patients with atrial fibrillation. Thromb Res 2012; 129:693–699. [DOI] [PubMed] [Google Scholar]
- 25.Kikkert WJ, Claessen BE, Stone GW, Mehran R, Witzenbichler B, Brodie BR, et al. D-dimer levels predict ischemic and hemorrhagic outcomes after acute myocardial infarction: a HORIZONS-AMI biomarker substudy. J Thromb Thrombolysis 2014; 37:155–164. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Distante A, Zizza A, Trimarchi S, Villani M, Salerno Uriarte JA, et al. IRAD-Bio Investigators. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009; 119:2702–2707. [DOI] [PubMed] [Google Scholar]
- 27.Dempfle CE, Zips S, Ergul H, Heene DL. Fibrin Assay Comparative Trial study group. The Fibrin Assay Comparison Trial (FACT): evaluation of 23 quantitative D-dimer assays as basis for the development of D-dimer calibrators. FACT study group. Thromb Haemost 2001; 85:671–678. [PubMed] [Google Scholar]
- 28.Spannagl M, Haverkate F, Reinauer H, Meijer P. The performance of quantitative D-dimer assays in laboratory routine. Blood Coagul Fibrinolysis 2005; 16:439–443. [DOI] [PubMed] [Google Scholar]
- 29.Francis CW, Marder VJ, Barlow GH. Plasmic degradation of crosslinked fibrin. Characterization of new macromolecular soluble complexes and a model of their structure. J Clin Invest 1980; 66:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vila V, Regañon E, Aznar J. Isolation of fibrin(ogen) degradation products from normal plasma by affinity chromatographic study. Clin Chim Acta 1978; 87:245–252. [DOI] [PubMed] [Google Scholar]
- 31.Gaffney PJ, Edgell T, Creighton-Kempsford LJ, Wheeler S, Tarelli E. Fibrin degradation product (FnDP) assays: analysis of standardization issues and target antigens in plasma. Br J Haematol 1995; 90:187–194. [DOI] [PubMed] [Google Scholar]
- 32.Pfitzner SA, Dempfle CE, Matsuda M, Heene DL. Fibrin detected in plasma of patients with disseminated intravascular coagulation by fibrin-specific antibodies consists primarily of high molecular weight factor XIIIa-crosslinked and plasmin-modified complexes partially containing fibrinopeptide A. Thromb Haemost 1997; 78:1069–1078. [PubMed] [Google Scholar]
- 33.Meijer P, Kluft C. The harmonization of quantitative test results of different D-dimer methods. Semin Vasc Med 2005; 5:321–327. [DOI] [PubMed] [Google Scholar]
- 34.Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009; 113:2878–2887. [DOI] [PubMed] [Google Scholar]
- 35.Ellis DR, Eaton AS, Plank MC, Butman BT, Ebert RF. A comparative evaluation of ELISAs for D-dimer and related fibrin(ogen) degradation products. Blood Coagul Fibrinolysis 1993; 4:537–549. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwenhuizen W. A reference material for harmonisation of D-dimer assays. Fibrinogen Subcommittee of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis. Thromb Haemost 1997; 77:1031–1033. [PubMed] [Google Scholar]
- 37.Adema E, Gebert U. Pooled patient samples as reference material for D-Dimer. Thromb Res 1995; 80:85–88. [DOI] [PubMed] [Google Scholar]
- 38.Meijer P, Haverkate F, Kluft C, de Moerloose P, Verbruggen B, Spannagl M. A model for the harmonisation of test results of different quantitative D-dimer methods. Thromb Haemost 2006; 95:567–572. [DOI] [PubMed] [Google Scholar]
- 39.Katrukha AG, Bereznikova AV, Esakova TV, Pettersson K, Lövgren T, Severina ME, et al. Troponin I is released in bloodstream of patients with acute myocardial infarction not in free form but as complex. Clin Chem 1997; 43:1379–1385. [PubMed] [Google Scholar]


