Abstract
Sutureless catheter securement systems are intended to eliminate risks associated with sutures. The clinical acceptability of a novel system was investigated compared with the current method of securement for peripherally inserted central catheters (19 facilities using StatLock or sutures) or nontunneled central vascular access devices (3 facilities using StatLock or sutures or HubGuard + Sorbaview Shield). More than 94% of respondents rated the novel system as same, better, or much better than their current product. More than 82% of respondents were willing to replace their current system with the new one.
Keywords: catheters, nontunneled CVAD, PICC, securement device
Today's catheter securement solutions can be grouped into 3 primary categories: sutures, sutureless devices, and tape and dressings. Sutures are the most commonly used securement method for nontunneled central vascular access devices (CVADs).1–3 Sutures are long-lasting and effective at securing catheters. Their use in securing catheters is declining, however, because of concerns about needlestick injuries and infection risk.4,5 To address these issues, manufactured catheter stabilization devices have entered the market and are gaining in acceptance to provide securement.6 Since 2006, various guidelines and standards have recommended the use of such securement devices.6–9
Modern sutureless stabilization/securement devices secure catheters to skin using an adhesive and commonly use the catheter hub suture wings as the key contact hold area. Securement methods employing a dressing only typically use design notches and reinforced nonwoven backing to maximize effectiveness, but remain relatively lower in their securement force in comparison with dedicated rigid securement devices.10 The ultimate goal of each method is to prevent catheter movement from the insertion site and to prevent or minimize potential complications as a result of such catheter movement or dislodgment. A safe and effective securement device for peripherally inserted central catheters (PICCs) and nontunneled CVADs should hold the catheter in place and should be able to resist movement/dislodgment forces ranging from small/minor tugging, common to normal wear, up to the unexpected heavy pull force associated with accidental pull, the pull of a disgruntled or confused patient, or the fall of an intravenous (IV) solution bag. The device should be gentle to wear without causing skin trauma or injuries.
A growing body of clinical literature has described the use of securement, or stabilization, devices for IV catheters to reduce complications, not only for PICCs and nontunneled CVADs but also for short peripheral catheters. From this literature, StatLock devices appear to be the most frequently used sutureless catheter securement device11–19; other devices, such as the Nexiva Closed IV Catheter System with a 3M Tegaderm IV Advanced Securement dressing, Sorbaview dressings, HubGuard catheter securement dressings, and the SecurAcath device also have been the topic of research publications.20–24 Catheter securement reduces phlebitis, infection, catheter migration, and dislodgment.11 Other authors have published papers stating that securement devices help reduce catheter-related bloodstream infections and suture-related needlesticks and improve cost-effectiveness in patients with nontunneled CVADs, including PICCs.4,5 This topic has been reviewed from a clinical standpoint by Gabriel.25 This article describes the evaluation by clinicians of a novel sutureless catheter securement system. The goal was to obtain information on the acceptability from the clinician standpoint of the overall clinical performance of the novel system compared with the current method of PICC or nontunneled CVAD securement.
MATERIALS AND METHODS
Novel Securement System
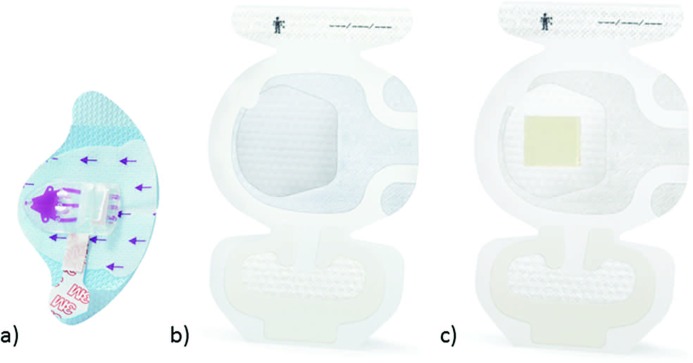
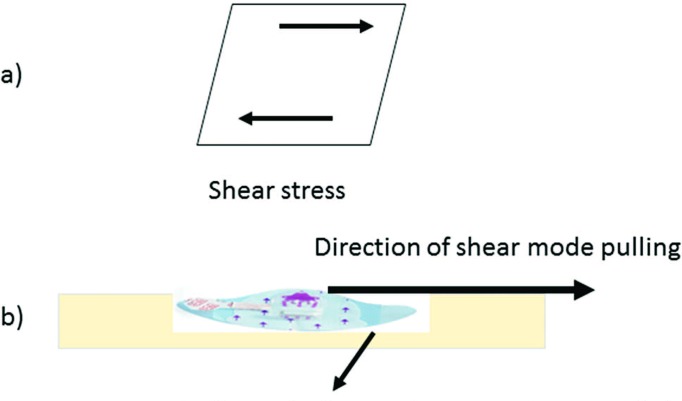
The novel PICC/nontunneled CVAD securement system consists of a securement device and a bordered transparent dressing available with or without chlorhexidine gluconate (CHG). The molded plastic device is integrated onto a breathable base with a gentle silicone adhesive. The soft-cloth-bordered transparent film dressing is made of a thin film backing with a nonlatex adhesive. A large, notched, film-covered soft-cloth tape strip is included in the system. Specifically, the securement device is made of a polycarbonate molded plastic adhered to a laminated nonwoven with a gentle silicone adhesive (Figure 1a). The device has 1 plastic arm, an integrated tape strip on the opposite side of the arm, and vertical securement posts to prevent the catheter hub from moving into the device and to accommodate 1 of the catheter lumens. The device is designed to prevent catheter migration and/or catheter loss and to help stabilize the catheter lumen(s). The securement device is designed to resist catheter dislodgment when the pull occurs in a shear mode (Figure 2).
Figure 1.
(a) Securement device, (b) non-CHG dressing, (c) CHG dressing. Abbreviation: CHG, chlorhexidine gluconate.
Figure 2.
(a) Shear stress, (b) shear stress applied to catheter securement system.
The other component of the securement system is 1 of 2 available securement dressings available either without or with a CHG gel pad integrated into the film of the dressing (Figure 1b and c). The purpose of the dressing is to aid in catheter securement while covering and protecting the catheter insertion site. A number of clinicians require a CHG antimicrobial to further protect the insertion site from skin contaminants. The CHG version of the securement dressing has the same CHG gel pad as existing dressings (3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing). The dressings (Figure 1b and c) are designed for use in combination with the securement device (Figure 1a) and provide additional catheter stabilization. The intended use of the system is to secure the majority of vascular access devices to skin and to cover and protect catheter insertion sites.
Clinician Evaluation
A survey study was implemented requesting clinicians who insert, provide care for, and/or maintain PICCs and nontunneled CVADs to compare the performance of a novel catheter securement system (3M PICC/CVC Securement Device + 3M Tegaderm I.V. Advanced Securement Dressing; 3M Company, St. Paul, MN) versus their current system. For a given period of time (2-3 weeks), the clinicians were asked to use only the novel securement system and to evaluate it by comparing it with their usual experience with the product(s) they typically used up to this point. There was no side-by-side comparison in real time, as would occur in a randomized controlled trial, but rather a comparison of a new system with historical experience.
PICCs
A total of 19 facilities participated in the PICC segment of the study: 2 infusion centers; 1 long-term, acute care facility; 5 infusion/home care centers; 1 physician office; and 10 hospitals. Clinicians used the novel securement system for 2 to 3 weeks to secure and cover their patients' PICC sites, rather than their usual product, which consisted of either
Bard StatLock + film dressing (n = 9)
Bard StatLock + border dressing (n = 60)
Bard StatLock + 3M Tegaderm CHG dressing (n = 20)
Suture + film dressing (n = 4)
After the evaluation period, clinicians used a tool to rate and communicate their experience using the new system compared with their current system. Ninety-seven clinician-completed evaluations met the criteria of applying, removing, and observing at least 2 securement systems. Sixty of the evaluations came from acute care settings (hospitals) and 37 from alternative-site settings (primarily home care). The evaluation involved assessing the following performance characteristics: overall performance; ease of application of the device; ease of application of the system; ease of removal of the device; ease of removal of the system; prevention of catheter migration; overall securement of the system; catheter migration during removal; adhesion properties; residue on skin or catheter; gentleness to skin; patient comfort; and skin redness, itching, irritation, and/or maceration. Additional questions—related to the ability to remove the product without a removal agent, the wear time, and their overall preference compared with their usual system—were also asked. Each respondent did not necessarily answer every question on the form. Each question related to the PICC use of the new product was answered by at least 83 individuals (range: 83-97 respondents).
Nontunneled CVADs
Because the current standard of care for nontunneled CVADs is sutures, it was more difficult to recruit clinicians willing to trial a sutureless device for this application. A total of 3 acute care sites (hospitals) participated in the nontunneled CVAD segment of the study. Clinicians used the novel securement system to secure and cover their patients' nontunneled CVAD sites, rather than their usual product consisting of either
Bard StatLock + film or border dressing or Sorbaview Shield (n = 5)
Sutures or Bard StatLock + film or border dressing (n = 17)
HubGuard − box clamp + Sorbaview Shield (n = 15)
The categories were defined according to what clinicians checked on the form; some used either sutures or StatLock on nontunneled CVADs, so they checked both boxes. After the evaluation period, clinicians used a tool to rate and communicate their experience using the new system compared with their current system. Thirty-seven clinician-completed evaluations met the criteria of applying and observing at least 1 securement system during patient wear. The evaluation involved assessing the same performance characteristics listed above for PICCs. Eleven evaluations were for catheters inserted in the internal jugular vein, and 8 evaluations were for catheters inserted in the subclavian vein. Ten evaluations captured performance data for multiple catheters inserted in both the internal jugular and the subclavian vein. One evaluation was for a catheter inserted in the femoral vein. Additional questions also were asked related to the ability to remove the product without a removal agent, the wear time, and the clinician's overall preference when comparing the product with their usual system. Each respondent did not necessarily answer every question on the form. Each question related to nontunneled CVAD use of the new product was answered by at least 20 individuals (range: 20-37 respondents).
Statistical Analysis
Sample size
The clinician evaluation was designed to give adequate precision in the estimate of clinicians rating the novel securement system the same as or better than their current method of securement.
A sample size of at least 62 evaluators was chosen to provide adequate precision for the proportion of clinicians rating the novel securement system the same as, better, or much better than their current method of securement. Assuming the proportion of positive responses would be 80%, the sample size would provide a 95% confidence interval width of ± 10%.
Minimum criteria for evaluation forms to be included in the PICC data set were (1) a minimum of 2 applications of the system, (2) a minimum of 2 observations of the system during patient wear, and (3) a minimum of 2 removals of the system. Minimum criteria for evaluation forms to be included in the nontunneled CVAD data set were (1) a minimum of 1 application of the system and (2) a minimum of 1 observation of the system during patient wear.
For PICCs, the target sample size was surpassed (97 respondents). For nontunneled CVADs, however, identification and recruitment of sites willing to participate in a sutureless nontunneled CVAD securement evaluation turned out to be a challenge due to resistance from key stakeholders. Although the target number of evaluators was 62, only 42 evaluation forms were returned. Of those 42 forms, 37 met criteria for inclusion. Therefore, the goal of 62 evaluable forms was not attained. Although the projected sample size was not attained, the results provided adequate precision around the proportion because of an overwhelmingly positive response to the novel securement system (97.3%), which led to a smaller confidence interval.26
Analysis methods
The novel securement system evaluation scores were analyzed for PICC and nontunneled CVAD sites separately. A 1-sided 95% confidence interval was calculated using the exact method for the respondents who rated the evaluation system the same, better, or much better than their current method of securement with regard to overall performance.
For all performance characteristics, the proportion of evaluations for which the response was 1 or 2 (worse or much worse), 3 (same as), and 4 or 5 (better or much better) was calculated, as well as the proportion of evaluators for scores of 1 or 2 versus 3, 4, or 5, and for each of the 5 response options (1, 2, 3, 4, 5). In addition, summary statistics (ie, mean, standard deviation, minimum, maximum) were provided for all other questions.
RESULTS
PICC Securement
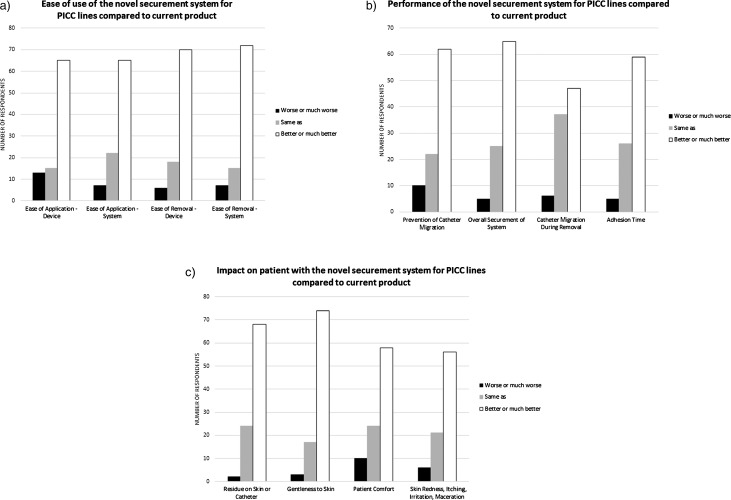
The novel securement system was rated the same, better, or much better for overall performance compared with the current method for PICC securement by 94.7% of the 97 respondents (95% 1-sided lower confidence limit: 89.3%). Seventy-four of 90 respondents (82.2%) were willing to replace their current PICC securement system with the new product. The results met predefined criteria for product acceptance: at least 80% of the evaluators rating the overall performance of the novel securement system the same, better, or much better than StatLock device/dressing combination for PICC securement. The use of sutures for PICC securement was not in the original definition of criteria for product acceptance, since the intention was to compare only with StatLock for the PICC application. However, a small number of users (4 of 97) indicated sutures as a comparator. The data were analyzed both with and without the suture users, and in both cases, the overall results met criteria for product acceptance. The numbers cited above and in Figures 3 and 4 include the suture users. The number of suture users was too small, however, to allow a direct comparison between sutures and the novel securement system.
Figure 3.
(a) Ease-of-use parameters evaluated for the novel securement system applied to PICCs, (b) performance characteristics of the novel securement system applied to PICCs, (c) impact on patient of the novel securement system applied to PICCs. Abbreviation: PICC, peripherally inserted central catheter.
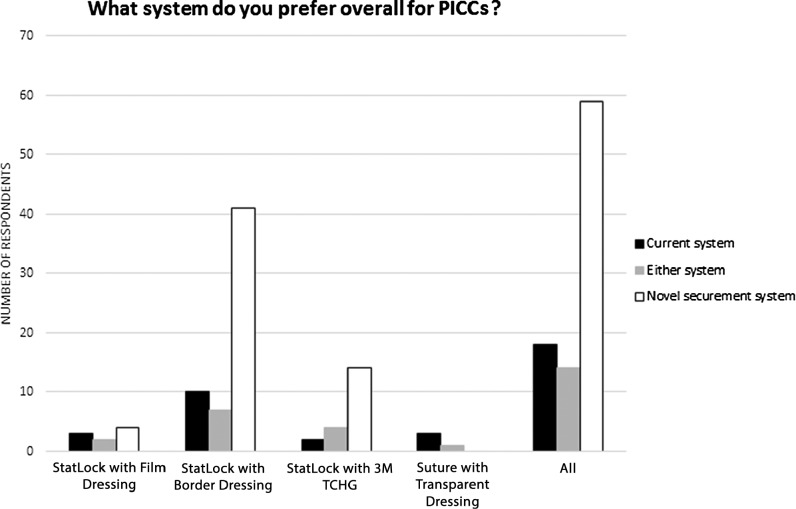
Figure 4.
Preference expressed by respondents regarding the system used for PICCs. Abbreviation: PICC, peripherally inserted central catheter.
Figure 3 shows how the individual performance characteristics were rated by the responding clinicians for PICCs. Figure 4 shows overall system preference when respondents compared the new product with their current system for use on PICCs. Although the data analysis was run with same, better, and much better grouped together to verify product acceptance criteria, the results are displayed separating out same from better or much better to illustrate the distribution of the data set and to highlight the number of respondents who preferred the new securement system.
Clinicians also were asked to compare the wear time—the number of days the novel securement system held in place—with that of their existing system for PICC use. Of 88 respondents, 19 answered that the new system had a longer wear time, 66 responded that it had the same wear time, and 3 responded that it had a shorter wear time than their current system. Eighty-two of 86 respondents said that they were able to remove the new product without removal agents (ie, alcohol or adhesive remover), representing a meaningful improvement over StatLock, which requires a removal agent.
Nontunneled CVAD Securement
Of the 37 respondents, 97.3% (95% lower 1-sided confidence limit is 87.8%) rated the novel securement system the same, better, or much better for overall performance compared with their current method for nontunneled CVAD securement. Ninety percent of respondents (27 of 30) were willing to replace their current nontunneled CVAD securement system with the new product. The original criterion for product acceptance was that at least 80% of the evaluators rate the overall performance of the novel securement system the same, better, or much better than the current method for nontunneled CVAD securement—eg, sutures/dressing, StatLock/dressing, or dressing alone. The use of HubGuard for nontunneled CVAD securement was not in the original definition of the criteria for product acceptance because the intention was to compare only with sutures or StatLock for the nontunneled CVAD application. However, a large fraction of respondents (15 of 37) indicated HubGuard as a comparator. The data including these users were analyzed because the number of respondents was smaller than planned. The results met the criteria for product acceptance, with at least 80% of the evaluators rating the overall performance of the novel securement system the same, better, or much better than their current method.
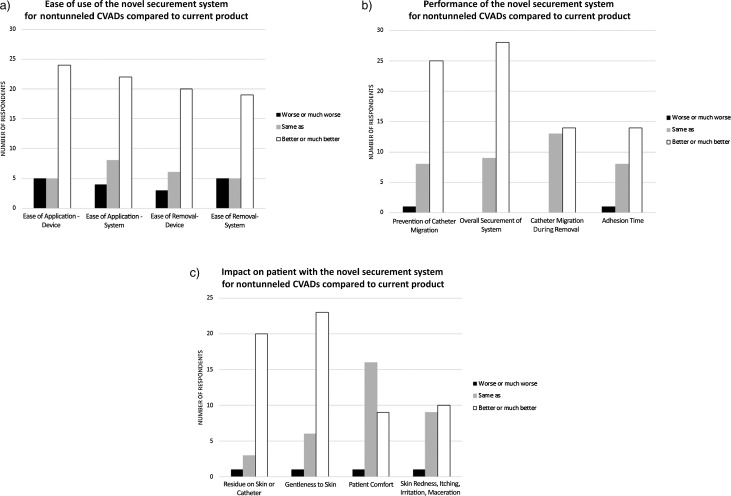
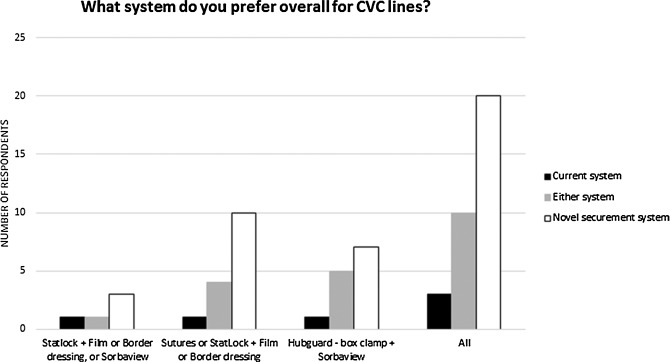
Figure 5 shows how the individual performance characteristics for nontunneled CVADs were rated by responding clinicians. Figure 6 shows overall system preference when respondents compared the new product against their current system for use on nontunneled CVADs.
Figure 5.
(a) Ease-of-use parameters evaluated for the novel securement system applied to nontunneled CVADs, (b) performance characteristics of the novel securement system applied to nontunneled CVADs, (c) impact on patient of the novel securement system applied to nontunneled CVADs. Abbreviation: CVAD, central vascular access device.
Figure 6.
Preference expressed by respondents regarding the system used for nontunneled CVADs. Abbreviation: CVAD, central vascular access device.
Clinicians were also asked to compare the wear time, as defined previously for PICC securement, of the novel securement system versus their existing system for nontunneled CVAD use. Of 25 respondents, 4 answered that the new system had a longer wear time, 20 responded that it had the same wear time, and 1 responded that it had a shorter wear time than their current system. Twenty-five of 28 respondents said that they were able to remove the new product without removal agents (ie, alcohol or adhesive remover), representing a meaningful improvement when compared with StatLock, which requires a removal agent.
DISCUSSION
Catheter securement issues have serious consequences, and the interaction of patient, practice, and product variables affects securement-related outcomes.27 The novel securement system evaluated in this study was designed to address some gaps in current PICC and nontunneled CVAD securement options. It is suggested that the following requirements must be met by a securement solution, based on current guidelines8 and user feedback:
Securement for the intended time of wear (an increased dressing-change frequency has been associated with an increase in infection, driving the need for longer wear times)
Prevention of catheter migration
Maintenance of skin integrity around the insertion site (skin damage requires action up to and including relocation of the catheter)
Ease of application and removal
Universal use (devices must be usable with more than 1 size or brand of catheter)
Compatibility with common skin preps and other devices
Compatibility with monitoring of the insertion site and the delivery of therapies
Catheter movement can range from small repeated movements, or micromotion, to major movements of outward catheter migration to complete dislodgment. Internal migration deeper into the vessel can also occur with risks of atrial rupture, resulting in cardiac tamponade and death.28 An effective securement device minimizes all such movements and all angles. Many devices available today are successful at securing the catheter hub from small repeated movements, but they are less capable of preventing dislodgment when the catheter is subjected to a sudden, high-impact force. Certain devices use aggressive adhesives that can damage patients' skin unless alcohol or another type of adhesive remover is used to remove them. Some designs require an increased level of catheter mechanical manipulation by the clinician to fit it into the device, and others are designed to fit only 1 type of catheter.
The novel securement system described in this study satisfies the criteria listed above for a securement solution, and it performed well in the opinion of clinical users. The results demonstrate advantages of the novel device in terms of ease of use, product performance, and impact on patients. The novel securement system was rated favorably (better or much better) compared with the other products by a majority of respondents on the ease-of-use parameters (Figures 3a and 5a) and on the performance parameters (Figures 3b and 5b) for both PICCs and nontunneled CVADs. The novel securement system also was rated favorably (better or much better) compared with the other products by a majority of respondents on the parameters having an impact on patients for PICCs (Figure 3c) and nontunneled CVADs (Figure 5c), except on patient comfort for nontunneled CVADs, where it was rated same as by a majority of respondents. A majority of users expressed an overall preference for the novel system compared with their current system (Figure 5 for PICCs, Figure 6 for nontunneled CVADs), with the exception of 3 of the 4 users who used sutures on PICCs. The novel securement system also offers the advantage of not requiring the use of a removal agent. The device provides gentle adhesion because the silicone part can be easily removed in peel mode when the device needs to be removed, yet it is strong enough to ensure securement when submitted to a pull in shear mode.
Some limitations of the study include that data were collected over a relatively brief period of time (2-3 weeks) and that each clinician filled a form summarizing her or his overall experience (not in a patient-specific way, and sometimes checking more than 1 comparator product or even adding comparators that initially were not intended in the study design). The number of respondents for the nontunneled CVADs was smaller than for PICCs (37 vs 97) and from a smaller number of facilities (3 vs 19). However, the parameters assessed and the preference expressed by clinicians clearly surpassed the criteria for product acceptance that had been predefined in the statistical analysis methods. As a result, there can be confidence that the novel securement system is well perceived by users and offers advantages to patients. A clinical study with patient outcomes will be the next step in evaluating the long-term functional performance of the device.
ACKNOWLEDGMENTS
The authors wish to thank Kendra Gregerson and Joe Hommes for their contributions to recruiting and evaluating clinical sites and for implementation of the study. They also thank Lynda Cook, Rita Luettinger, Joni Ekberg, Brenda Caillouet, and Gwendolyn Coney for their help with implementation at facility sites, and Dan DeCabooter, Phong Ha, and Laura Rutledge for providing input into the protocol and the manuscript. Finally, thanks go to the clinical evaluators at the various institutions for their participation in the study.
Footnotes
This study was funded by the 3M Company (products, training, and education on the use of the product, design of the evaluation forms, statistical analysis of the data collected, and preparation of the manuscript). The clinicians received no money from 3M to perform this evaluation, but were provided with product at no cost. All authors are employees of the 3M Company. The 3M Company provided training and education on the use of the product, designed the evaluation forms, performed the analysis of the data collected, and prepared the manuscript.
REFERENCES
- 1.Biggs TC, Mohammed A, Mulvihill D. Importance of securing central venous catheters. Anaesth Intensive Care. 2012;40(2):366–367. [PubMed] [Google Scholar]
- 2.Rupp SM, Apfelbaum JL, Blitt C, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573. [DOI] [PubMed] [Google Scholar]
- 3.Vinjirayer A, Jefferson P, Ball DR. Securing central venous catheters: a comparison of sutures with staples. Emerg Med J. 2004;21(5):582–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal K. Get a hold on costs and safety with securement devices. Nurs Manage. 2005;36(5):52–54. [DOI] [PubMed] [Google Scholar]
- 5.Petree C, Wright DL, Sanders V, Killion JB. Reducing blood stream infections during catheter insertion. Radiol Technol. 2012;83(6):532–540. [PubMed] [Google Scholar]
- 6.Schears G. New standards to improve catheter stabilization and patient and worker safety. J Healthc Risk Manag. 2006;26(4):15–18. [DOI] [PubMed] [Google Scholar]
- 7.Bishop L, Dougherty L, Bodenham A, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol. 2007;29(4):261–278. [DOI] [PubMed] [Google Scholar]
- 8.Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infus Nurs. 2016;39(suppl 1):S1–S159. [Google Scholar]
- 9.O'Grady NP, Alexander M, Burns LA, et al. US Department of Health and Human Services. Guidelines for the prevention of intravascular catheter-related infections. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Accessed February 19, 2016.
- 10.Rutledge LF, DeCabooter DP, Walters SA, Bernatchez SF. Catheter securement systems: comparison of two investigational devices to a sutureless securement device, a securement dressing, and sutures in a pig model. Intensive Care Med Exp. 2015;3(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alekseyev S, Byrne M, Carpenter A, Franker C, Kidd C, Hulton L. Prolonging the life of a patient's IV: an integrative review of intravenous securement devices. Medsurg Nurs. 2012;21(5):285–292. [PubMed] [Google Scholar]
- 12.Stephenson C. The advantages of a precision-engineered securement device for fixation of arterial pressure-monitoring catheters. J Assoc Vasc Access. 2005;10(3):130–132. [Google Scholar]
- 13.Frey AM, Schears GJ. Why are we stuck on tape and suture? A review of catheter securement devices. J Infus Nurs. 2006;29(1):34–38. [DOI] [PubMed] [Google Scholar]
- 14.Schears GJ, Liebig C, Frey AM, et al. StatLock Catheter Securement Device Significantly Reduces Central Venous Catheter Complications. Oakbrook Terrace, IL: Joint Commission on Accreditation of Health Care Organizations; 2000. Patient Safety Initiative 2000: Spotlight on Solutions. http://www.fda.gov/ohrms/dockets/dailys/02/Oct02/102902/01p-0120-1.pdf. Accessed February 22, 2016. [Google Scholar]
- 15.Schears GJ. Summary of product trials for 10,164 patients: comparing an intravenous stabilizing device to tape. J Infus Nurs. 2006;29(4):225–231. [DOI] [PubMed] [Google Scholar]
- 16.Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371–384. [DOI] [PubMed] [Google Scholar]
- 17.Royer T. Improving short peripheral IV outcomes: a clinical trial of two securement methods. J Assoc Vasc Access. 2003;8(4):45–49. [Google Scholar]
- 18.Smith B. Peripheral intravenous catheter dwell times: a comparison of 3 securement methods for implementation of a 96-hour scheduled change protocol. J Infus Nurs. 2006;29(1):14–17. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto AJ, Solomon JA, Soulen MC, et al. Sutureless securement device reduces complications of peripherally inserted central venous catheters. J Vasc Interv Radiol. 2002;13(1):77–81. [DOI] [PubMed] [Google Scholar]
- 20.Delp J, Hadaway L. New product decisions—the process and outcome for a community health system. J Assoc Vasc Access. 2011;16(2):74–84. [Google Scholar]
- 21.McNeill EE, Hines NL, Phariss R. A clinical trial of a new all-in-one peripheral-short catheter. J Assoc Vasc Access. 2009;14(1):46–51. [Google Scholar]
- 22.Flippo PL, Lee J. Clinical evaluation of the Sorbaview SHIELD securement device used on peripheral intravenous catheters in the acute care setting. J Assoc Vasc Access. 2011;16(2):95–98, 100–102. [Google Scholar]
- 23.Cordovani D, Cooper RM. A prospective trial on a new sutureless securement device for central venous catheters. Can J Anaesth. 2013;60(5):504–505. [DOI] [PubMed] [Google Scholar]
- 24.Egan GM, Siskin GP, Weinmann R, Galloway MM. A prospective postmarket study to evaluate the safety and efficacy of a new peripherally inserted central catheter stabilization system. J Infus Nurs. 2013;36(3):181–188. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel J. Vascular access devices: securement and dressings. Nurs Stand. 2010;24(52):41–46. [DOI] [PubMed] [Google Scholar]
- 26.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413. [Google Scholar]
- 27.Macklin D, Blackburn PL. Central venous catheter securement: using the healthcare and technology synergy model to take a closer look. J Assoc Vasc Access. 2015;20(1):45–50. [Google Scholar]
- 28.Pittiruti M, Lamperti M. Late cardiac tamponade in adults secondary to tip position in the right atrium: an urban legend? A systematic review of the literature. J Cardiothorac Vasc Anesth. 2015;29(2):491–495. [DOI] [PubMed] [Google Scholar]