Abstract
This prospective, Post-Authorization Safety Surveillance (PASS) study was carried out in patients with hemophilia A or B and inhibitors treated with FEIBA for 1 year to collect real-world data on safety and effectiveness of FEIBA. The study followed a cohort design and did not make stipulations on treatment or observation schedule, as it was designed to observe routine medical practices based on physicians’ treatment decisions, including whether patients received on-demand or prophylaxis with FEIBA. The attending physician maintained documentation, including medical records, laboratory reports, adverse event reports, and so on and a subject diary was used. Eighty-one patients were treated with FEIBA at 40 sites in 10 countries over a 4-year period. Sixty-nine patients (85.2%) had hemophilia A, two had (2.5%) hemophilia B, and ten (12.3%) had acquired hemophilia A. At baseline 45 patients (55.6%) were prescribed prophylaxis and 36 (44.6%) on-demand treatment. This study was novel in following safety and effectiveness in ‘real world’ on-demand and prophylactic use of FEIBA, and was able to collect data in these rare patients under routine clinical practice.
Keywords: FEIBA, hemophilia, inhibitor, Post-Authorization Surveillance Study, prophylaxis, surgery
Introduction
Alloantibodies neutralizing factor VIII (FVIII) or factor IX (FIX) develop in 20–30% of severe hemophilia A and 1–5% of patients with severe hemophilia B [1]. Inhibitors represent the most challenging complication of hemophilia, and predispose patients to higher rates of morbidity and mortality [2–4]. The preferred treatment of inhibitor patients is immune tolerance induction (ITI); however, approximately 25% of these patients do not respond to standard ITI treatment [5] and, because of its cost, this treatment may be limited in some countries and/or in patients who fail to meet certain criteria (e.g., adults or patients with potential poor prognostic factors) [6–8]. In these patients bypassing agents such as an activated prothrombin complex concentrate (APCC) and activated recombinant FVII (rFVIIa), promoting thrombin generation whereas circumventing the hemostatic cascade [9], offer therapeutic options for treating bleeding episodes, or for prophylaxis treatment of these patients [4,10–12]. Factor eight inhibitor bypassing activity anti-inhibitor coagulant complex (AICC; FEIBA, Baxalta US Inc, West Lake Village, California, USA) is a plasma-derived APCC licensed for more than 35 years, first marketed in 1978, to treat bleeding and cover surgical interventions in hemophilia A and B patients with congenital and acquired hemophilia with inhibitors. More recently, it has been licensed to be used prophylactically to prevent bleeding episodes in patients with congenital hemophilia. FEIBA has been proven well tolerated and effective, with very few thrombotic events [10,13–15]. FEIBA has been approved in more than 60 countries, with a prophylaxis indication in more than 40 countries.
This international postlicensure surveillance study sought to assess the safety and effectiveness of on-demand and/or prophylactic FEIBA treatment in hemophiliacs with inhibitors under the conditions of routine medical practice, independent of whether they received the therapy for treatment or prophylaxis of bleeding episodes in congenital A or B or acquired hemophilia A (AHA), or for perioperative management during minor surgery (e.g., dental surgery or port placement).
Materials and methods
This was a prospective, uncontrolled, open-label, noninterventional surveillance, which began in October 2008 and lasted until 2014 with at least 12 ± 2 months for each treated patient (postobservation follow-up occurred at 24 ± 2 and 36 ± 2 months after enrollment for patients in the United Kingdom).
The study followed a cohort design and did not make stipulations on treatment or observation schedule, as it was designed to observe routine medical practices based on physicians’ treatment decisions, including whether patients received on-demand or prophylactic treatment with FEIBA. On-demand and prophylaxis dosing regimens were left to the judgment of the physicians in charge, who had received the recommendation to remain within the specifications of their locally approved Summary of Product Characteristics (SmPC). Approved prescribing information differs between countries, which was also recorded. All patients or their legal guardians (when they were <18 years of age) were required to sign an informed consent form. The protocol was approved by relevant Ethical Review Boards. During this study, the attending physician maintained documentation, including medical records, laboratory reports, adverse event reports, and so on. For patients carrying out home treatment, a subject diary was provided to capture treatment details. These were specific, based on whether the patient was self-administering prophylaxis or on-demand treatment. As this survey aimed to collect clinical experience from all patients receiving factor eight inhibitor bypassing activity (FEIBA), the eligibility criteria reflected the indications and contraindications mentioned in the SmPC. If the treating physician decided to administer FEIBA outside the label recommendations, the patient could still be documented under the protocol. All patients had congenital hemophilia A or B or AHA with inhibitory autoantibodies to FVIII or FIX.
Safety was recorded in terms of adverse events, whether related or unrelated to FEIBA. Hemostatic effectiveness was assessed in terms of treatment or prevention of hemorrhagic episodes in patients with hemophilia A or B, and inhibitors. Furthermore, the surveillance attempted to identify practices in managing patients with hemophilia with inhibitors on regular FEIBA prophylaxis. Effectiveness was determined by each physician completing a survey at each surveillance visit with an overall evaluation of the response to therapy or prophylaxis with FEIBA dosing on a four-point scale (ratings included excellent, good, fair, and poor).
Results
The study was initiated in the United Kingdom, France, and Spain in 2008, but later expanded to 40 participating sites in 10 countries (Table 1). The last subject out of the global study was in October 2013 and database lock occurred in February 2014. Seven patients with AHA were also enrolled in a prospective observational study (FEIBHAC – Clinical and Laboratory Evaluation of Anti-Hemorrhagic Treatment with FEIBA in AHA) [16].
Table 1.
Distribution of sites and patients by country
| Country | Sites | Patients | ||
| N | % | N | % | |
| United Kingdom | 10 | 30.0 | 16 | 19.8 |
| France | 12 | 30.0 | 26 | 32.1 |
| Germany | 1 | 2.5 | 7 | 8.6 |
| Belgium | 2 | 5.0 | 3 | 3.7 |
| Spain | 5 | 12.5 | 6 | 7.4 |
| Poland | 2 | 5.0 | 2 | 2.5 |
| Sweden | 1 | 2.5 | 5 | 6.2 |
| Italy | 3 | 7.5 | 10 | 12.4 |
| United States | 3 | 7.5 | 5 | 6.2 |
| Canada | 1 | 2.5 | 1 | 1.2 |
| Overall | 40 | 100.0 | 81 | 100.0 |
Patient characteristics at enrollment
Out of the 92 screened patients, 81 eventually received FEIBA on-demand, on prophylaxis, and/or for perioperative management during minor surgery (Fig. 1). Sixty-nine patients (85.2%) had congenital hemophilia A, two (2.5%) had congenital hemophilia B, and ten (12.3%) had AHA. Six patients dropped out of the study: three due to unrelated death, consent withdrawn in one (patient and guardian unwilling to fill out subject diary), and other reasons in two patients (‘patient moved to another city’ and ‘patient entered into immunotolerance treatment’).
Fig. 1.
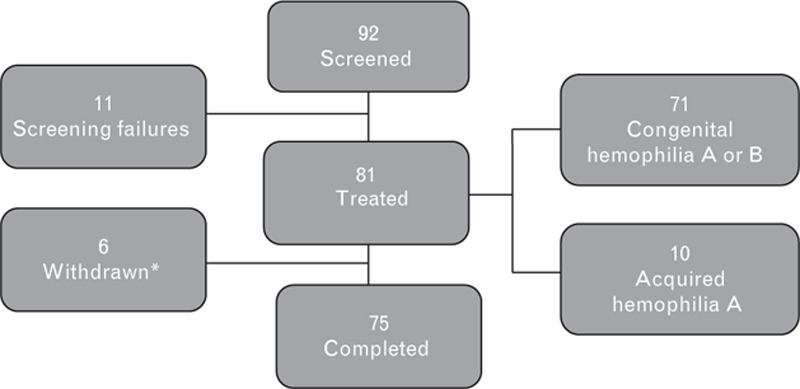
Study population.
There were 71 male patients with congenital hemophilia, of whom 22 were younger than 13 years, and 49 were older than 13 years with a median age of 24.0 years (min–max: 0.0–77.0 years); 10 patients, equally divided between men and women, aged between 59 and 93 years (median age: 84.0 years), suffered from AHA.
Of the 71 patients with congenital hemophilia A or B, 45 (63.3%) were treated prophylactically when enrolled in the study, whereas the remaining 36 (36.6%) were treated on-demand (Table 2). Patients on prophylaxis were much younger (median age: 16.0 years, min–max range: 1.0–71.0) than those on on-demand treatment (median age: 38.0, min–max range: 0.0–77.0). In the 59 patients with congenital hemophilia A in whom baseline FVIII measurements had been taken, the median FVIII level was 1.0 IU/dl (min–max range: 0.0–21.0 IU/dl).
Table 2.
Patient demographic characteristics (intent-to-treat population)
| Prophylaxisa | On-demanda | Total | ||
| Type of hemophilia | ||||
| Congenital hemophilia A | 44 | 25 | 69 | |
| Congenital hemophilia B | 1 | 1 | 2 | |
| Acquired hemophilia | 0 | 10 | 10 | |
| Total | 45 | 36 | 81 | |
| Age (years) | ||||
| Mean age | 21.1 | 48.1 | 33.1 | |
| Range | 1.0–71.0 | 0–93.0 | 0–93.0 | |
| Sex | ||||
| Male | 45 | 31 | 76 | |
| Female | 0 | 5 | 5 | |
| Total | 45 | 36 | 81 | |
aRegimen (on-demand/prophylaxis) is intent to treat based on physician prescription at enrollment.
All (100.0%) of the 10 patients with AHA were prescribed an on-demand regimen at baseline. No patients were on prophylaxis for surgery.
Patients with congenital hemophilia A or B reported a total of 135 target joints (in 52/71 patients) as deemed by the physician in charge at enrollment. The majority of these target joints were in the knees (34.8%) or elbows (27.4%), with the remainder in the tarsus (17.8%), shoulder (6.7%), hip (4%), or other joint (9.6%). Most bleeds in this study arm reported in the 12 months prior to enrollment were spontaneous (n = 312; 69.0%) or traumatic (n = 158; 53.5%). Among patients with congenital hemophilia, 35.2% had attempted ITI before enrollment, 25.4% were still on ITI treatment, and 36.6% had never attempted ITI. In patients with AHA, nine out of the ten patients had experienced one to three bleeds in the 12 months prior to enrollment; the remaining patient had experienced seven or more bleeds, which were mostly spontaneous (n = 14; 100.0%) or traumatic (n = 4; 30.0%).
Overall, 57 out of 81 treated patients (70.4%) had been previously treated with FEIBA.
Out of nine patients with previous thromboembolic adverse events, seven (8.6%) had deep vein thrombosis (DVT; five of whom had congenital hemophilia; two had AHA), and one patient each (1.2%) had pulmonary embolism and acute myocardial infarction (both with AHA).
Surveillance period
Treatment
The mean [standard deviation (SD)] time spent on a regular prophylaxis regimen with FEIBA was 267.7 (128.2) days (median 311.0, min–max range 3.0–427.0 days) and 269.8 (132.2) days (median 323.0, min–max range 6.0–427.0 days) on on-demand. The mean (SD) time spent on FEIBA regimen overall was 355.0 (61.3) days (median 367.0, min–max range 74.0–427.0 days). The mean (SD) dose of FEIBA per infusion per kg body weight was 68.4 (22.5) U (median 67.5, min–max range 30.3–135.8 U) as regular prophylaxis and 77.0 (31.0) U (median 75.0, min–max range 33.3–243.8 U) as on-demand. The mean (SD) dose of FEIBA per infusion day per kg body weight was 80.5 (27.8) U (median 76.6, min–max range 38.3–153.6 U) as regular prophylaxis and 104.9 (41.9) U (median 93.6, min–max range 33.3–243.8 U) as on-demand. Patients with congenital hemophilia A or B receiving prophylactic dosing had a median of 170 infusion days per year (annualized, range 41.7–365.3). Fourteen patients on ITI were prophylactically treated with FEIBA: ten of them were treated daily with an average dose of 61.3 U/kg, and the remaining four patients were treated every second day with an average dose of 66.9 U/kg.
The mean infusion rate of FEIBA under routine clinical practice during the study (3.7 U/kg per min, min–max range 0.9–23.5) was higher than that recommended in the SmPC of FEIBA (2.0 U/kg per min). A manual analysis of safety listings did not disclose any adverse events associated with a higher infusion rate. Infusion doses were similar among patients with congenital hemophilia and AHA; however, the mean infusion rate (U/kg per min) was significantly lower in patients with AHA (2.1 U/kg per min, min–max range 1.6–2.4). The mean infusion dose and rate were higher among patients receiving on-demand therapy compared with those on prophylaxis (4.3 vs. 3.4 U/kg per min, respectively) (Table 3).
Table 3.
Mean dose and infusion rates for anti-inhibitor coagulant complex (intent-to-treat population)
| FEIBA regimen | Prophylaxis | On-demand | Total | |
| Congenital hemophilia A or B | Mean infusion ratea (U/kg per min) (range) | 3.4 (0.9–7.3) | 4.3 (1.4–23.5) | 3.8 (0.9–23.5) |
| N | 19 | 18 | 37 | |
| Mean dose per infusion (U/kg) (range) | 68.2 (30.3–135.8) | 78.0 (33.3–243.8) | 72.1 (30.3–243.8) | |
| N | 46 | 31 | 77 | |
| Acquired hemophilia A | Mean infusion ratea (U/kg per min) (range) | – | 2.1 (1.6–2.4) | 2.1 (1.6–2.4) |
| N | 0 | 7 | 7 | |
| Mean dose per infusion (U/kg) (range) | 76.9 | 73.3 (54.9–83.3) | 73.7 (54.9–83.3) | |
| N | 1b | 9 | 10 | |
| Total | Mean infusion ratea (U/kg per min) (range) | 3.4 (0.9–7.3) | 4.0 (1.4–23.5) | 3.7 (0.9–23.5) |
| N | 19 | 25 | 44 | |
| Mean dose per infusion (U/kg) (range) | 68.4 (30.3–135.8) | 77.0 (33.3–243.8) | 72.3 (30.3–243.8) | |
| N | 47 | 40 | 87 |
Patients with congenital hemophilia A and B prescribed FEIBA prophylaxis had a median of 170 infusion days per year (annualized, range 41.7–365.3).
aMean calculated based on data documented by patients
bOne patient with AHA received one administration on demand which was inadvertently recorded as a prophylactic administration.
Effectiveness
Good or excellent overall final hemostatic effectiveness was rated by the physician in 73 patients (90.1%), whereas the rate was fair in six patients (7.4%); no patients reported poor hemostatic effectiveness. Rates of hemostatic effectiveness were higher among the 30 patients on regular prophylaxis (96.7%) with only one rated fair, compared with 93.1% patients (27/29 with applicable data) on the on-demand regimen.
In patients with congenital hemophilia, the proportion with excellent or good rating was 96.7% in patients with regular prophylaxis as the main regimen and 81.8% of patients with on-demand as the main regimen.
In patients with AHA, the hemostatic effectiveness was excellent in eight (80.0%) and good in two (20.0%).
In congenital hemophilia patients on prophylaxis the median number of bleeds per year was 5.0 (min–max range 0.0–55.1; Table 4): 13 patients (24.5%) experienced no bleeds when on prophylaxis; spontaneous bleeding events occurred in 30 patients (56.6% of prophylaxis patients, 238 events) and traumatic bleeds (including surgery) occurred in 21 patients (39.6%, 73 events). Type of bleed was unknown in 15 patients (28.3%, 65 events).
Table 4.
Results of bleed rate (bleeds per year) during the study (intent-to-treat population)
| FEIBA regimen | Mean | SD | Min | Median | Max | N |
| Congenital hemophilia A or B | ||||||
| Prophylaxis | 9.4 | 13.6 | 0.0 | 5.0 | 55.1 | 53 |
| On-demand | 13.0 | 17.0 | 0.0 | 5.6 | 85.1 | 43 |
| Acquired hemophilia A | ||||||
| On-demand | 4.5 | 4.5 | 0.0 | 3.4 | 14.8 | 1 |
| Total | 10.3 | 14.6 | 0.0 | 4.8 | 85.1 | 106 |
SD, standard deviation.
In congenital hemophilia patients on on-demand treatment the median number of bleeds per year was 5.6 (min–max range 0.0–85.1): only 3 patients (7.0%) experienced no bleeds during on-demand treatment periods; spontaneous bleeding events occurred in 29 patients (67.4% of on-demand patients, 217 events); traumatic bleeds (including surgery) occurred in 22 patients (51.2%, 67 events). Type of bleed was unknown in seven patients (16.3%, 11 events). A reduction of bleeding frequency during prophylaxis could not be evaluated because only few patients switched from on-demand to prophylaxis. In any case, the number of patients with 0 bleeds was higher in patients on prophylaxis than on on-demand treatment (24.5 vs. 7.0%, respectively; χ2 7.10, P < 0.01). The median rate of bleeding events among patients with AHA was 3.4 (min–max range 0–14.8).
The mean (SD) FEIBA total dose per bleed was 18 327.4 (19 266.3) U (median 12 384.6, min–max range 805.0–125 000.0 U). The mean (SD) FEIBA total dose per bleed per kg body weight was 365.9 (414.3) U (median 243.8, min–max range 33.3–2 734.4 U).
Safety
Overall three treatment-related serious adverse events (SAEs) were reported in three patients (3.7%), and six treatment-related nonserious adverse events were reported in five patients (6.2%).
The 63 SAEs were reported in 30 patients (37.0%), 17 of which were reported in 8 patients (9.9%) on prophylaxis treatment and 34 were reported in 22 (27.2%) while treated on-demand. Three treatment-related SAEs were reported in three patients (3.7%); all occurred during on-demand treatment. The three treatment-related SAEs reported were: hemarthrosis in a congenital hemophilia patient, Enterobacter cloacae infection of the central venous catheter in a patient with AHA and DVT associated with superficial thrombophlebitis was reported in an 86-year-old female AHA patient concomitantly treated with FEIBA and rFVIIa.
No thrombotic events (except the case of DVT) were reported in patients with congenital hemophilia.
Out of the nine patients with congenital hemophilia or AHA that had experienced previous thromboembolic events, four reported nonproduct-related SAEs, but different from a thromboembolic event: one was fatal (cardiopulmonary failure in a patient with AHA), whereas the other three experienced hemorrhage during the study.
Two additional deaths, both considered unrelated to treatment by the investigator, were reported during the study (one in a patient with AHA, and one in a patient with congenital hemophilia). These were due to pseudomonal sepsis (in a patient with congenital hemophilia), and cardiac failure and lung infection (in a patient with AHA).
The most common SAEs in the total population by system organ class were infections and infestations (nine SAEs in eight patients, 9.9%, mainly associated with indwelling catheters), injury, poisoning and procedural complications (seven SAEs in seven patients, 8.6%), musculoskeletal and connective tissue disorders (nine SAEs in five patients, 6.2%), and nervous system disorders (six SAEs in five patients, 6.2%).
In the analysis by disease, the proportion of patients with adverse events, treatment-related adverse event, SAEs, and suspected ADRs was, as expected, higher in patients with AHA compared with those with congenital hemophilia. This was also reflected in fatal outcomes (two fatal outcomes out of 10 patients among those with AHA, compared with 1 fatal outcome among 71 patients with congenital hemophilia A or B; Table 5).
Table 5.
Patients with serious and non-serious adverse events (Intent to treat population)
| Non-serious adverse event | Related non-serious adverse event | SAE | Related SAE | Deathsa | |
| Congenital hemophilia (N = 71) | |||||
| Patients on prophylaxis | 17 | 2 | 8 | 0 | 0 |
| Patients on on-demand | 17 | 1 | 15 | 1b | 1 |
| Patients on unknown regimen | 8 | 0 | 1 | 0 | 0 |
| Acquired hemophilia (N = 10) | |||||
| Patients on prophylaxis | 0 | 0 | 0 | 0 | 0 |
| Patients on on-demand | 7 | 2 | 7 | 2c | 2 |
| Patients on unknown regimen | 5 | 0 | 4 | 0 | 0 |
SAE, serious adverse event.
aAll deaths were considered unrelated to study drug. Related serious adverse events: bhemarthrosis, ccatheter-related infection, cthrombophlebitis superficial. An 86-year-old woman with AHA developed superficial thrombophlebitis and DVT following co-administration of rFVIIa and FEIBA during the course of the study. Related nonserious adverse events: nausea; allergic pruritus; prolonged prothrombin time; lymphopenia; constipation; pneumonia; hemarthrosis. Note: Patients may have had events in different categories. Therefore, the numbers may not add up to the total. Adverse events with missing seriousness were counted as serious (worst case assumption).
Discussion
The present study aimed to collect real-world data on safety and effectiveness of FEIBA used prophylactically or on-demand in patients with congenital hemophilia or AHA. In fact, because of the rarity of these patients, there is paucity of prospectively collected information and limited evidence on the real use of this bypassing agent, its safety, and effectiveness. Multinational, multicenter, cohort, noninterventional, naturalistic studies can help to ensure consistency in long-term safety and clinical performance on routine use [14,15].
This surveillance cohort showed good or excellent hemostatic effectiveness rated by the physician in more than 90% of total patients at a mean dose of FEIBA per infusion day of 80.5 U/kg body weight, as well as excellent safety results: no thrombotic events reported in any of the 71 patients with congenital hemophilia, and a DVT and superficial thrombophlebitis in one out of the 10 AHA-enrolled patients. The efficacy of prophylactic treatment has been shown previously in randomized controlled clinical trials using FEIBA [11,17].
This FEIBA Post-Authorization Safety Surveillance (PASS) Study is the fourth in FEIBA's history, and the results, as compared to previous PASS studies [13,18–20], show consistency in terms of hemostatic effectiveness. This study's 90.1% good or excellent ratings in all bleeding events were comparable to 82% good or excellent hemostatic effectiveness in 2006, and 81% good or excellent hemostatic effectiveness, including surgical procedures, in 1997 (definitions of effectiveness differed between studies). The product's excellent safety profile, as has been shown throughout the product's 37-year history, was again confirmed in this surveillance [13]. The present study showed safety and effectiveness in a higher number of patients treated prophylactically, with a number of patients with 0 bleeds during the study significantly higher than that in the group treated on-demand (24.5 vs. 7.50%, P < 0.01), which confirms the effectiveness of a prophylactic treatment regimen in the context of uncontrolled, real-world data.
As expected, patients with AHA were older (mean ages were 78.5 vs. 26.7, respectively) and more frail at presentation of the disease because of comorbidities: more adverse events (90.0 vs. 60.6%), SAEs (80.0 vs. 31.0%), and suspected related adverse events (40.0 vs. 5.6%) were observed in patients with AHA than in patients with congenital hemophilia A and B. Bleeding and infusion rates were both lower among those with AHA compared to those with congenital hemophilia A or B. Patients with AHA undergo concomitant administration of immunosuppressive agents to eradicate the auto-antibody. This may influence the disease course in patients with AHA and may explain why bleeding rates were lower compared to patients with congenital hemophilia A or B.
Among patients with congenital hemophilia A or B, adverse events (92.3 vs. 48.8%), SAEs (57.6 vs. 17.7%), and suspected adverse drug reactions (7.6 vs. 4.4%) were reported at a higher rate among those receiving the on-demand regimen compared to those receiving prophylactic dosing, respectively. In particular, no hemophilia B patients experienced any adverse events.
The results of this postauthorization safety study showed that treatment with FEIBA, administered in 81 patients with hemophilia and requiring treatment with inhibitor bypass therapy for bleed resolution or bleed prophylaxis, was well tolerated. Treatment-related adverse reactions occurred in eight patients (9.9%), three of them (3.7%) deemed serious.
A DVT associated with a superficial thrombophlebitis was observed in an 86-year-old woman with AHA following co-administration of rFVIIa and FEIBA, which corresponds to 1.2% of patients. This finding is consistent with previously published experience for patients with AHA [14,16,21,22]. The observed venous thrombosis in this small AHA subgroup may be associated with the older age in the patients with AHA studied; the likelihood of comorbidities as all patients on AHA had other medical conditions apart from AHA itself; and the presence of inherent thrombotic risk factors associated with AHA.
This surveillance provided important details on FEIBA administration in the real world: its prescribed regimen (prophylaxis or on-demand), the type of hemophilia treated (congenital or acquired), and age of the patients. Furthermore, this study provided information on the administration of FEIBA under routine clinical practice: in particular, the mean infusion rate of 3.8 (U/kg per min), with a maximum of 23.5 (U/kg per min), was substantially higher than that recommended in the SmPC of FEIBA (2.0 U/kg/min).
Conclusion
In this PASS study, FEIBA confirmed safety and effectiveness in 81 patients treated in a real-world setting. The data from this PASS study provided further information on the use of FEIBA given as prophylaxis or on-demand in two different diseases: congenital hemophilia and AHA. Moreover, these real-world findings must be confirmed by further studies to evaluate the safety of infusion rates faster than that recommended in the SmPC. Finally this study demonstrates the importance of collecting data in these rare patients in routine clinical practice settings.
Acknowledgements
The authors gratefully acknowledge the contributions of Manfred Pirck, David Jackson (from CROM Source), Norma Guzman-Becerra, and Borislava Pavlova of Baxalta Innovations AG/Baxalta US Inc., and all investigators who participated: G. Dolan, C. Grimley, S. Evans, S. Pavord, D. Perry, A. Roy, J. Hanley, K. Talks, H. Rangarajan, R.C. Tait, A. Alvi, J. Wilde, K. Khair, T. Lambert, A. Rafowicz, A. Lienhart, C. Rothschild, M.F. Torchet, M. Trossaert, S. Girault, C. Oudot, S. Claeyssens, P. Moreau, V. Guérin, Y. Gruel, A.S. Valentin, A. Borel-Derlon, P. Gautier, C. Berger, J. Oldenburg, C. Hermans, J.M. Minon, E. Rongé-Collard, V. Jiménez Yuste, T. Alvarez Roman, R. Parra, C. Alonso, A.R. Cid, R. Pérez Garrido, R.J. Nunez Vazquez, J.A. López, F.J. Battle Fonrodona, A. Klukowska, P. Laguna, T. Robak, J. Trelinski, K. Chojnowski, A. Olsson, V. Radulovic, E. Santagostino, M.E. Mancuso, A. Rocino, E. Zanon, B. Brandolin, D. Ritchie, N.A. Dower, A. Soni, D.J. Park, L. Hsieh, G. Puthenveetil, J. Stites, V. Wang, M.D. Nguyen, S.L. Carpenter, B. Wicklund, D.B. Wilson, and L. Boggio.
Baxalta Innovations GmbH/Baxalta US Inc. sponsored the observational study discussed.
Conflicts of interest
R.N., A.N., A.G., and J.D. are full-time employees of Baxalta US Inc/Baxalta Innovations GmbH; V.R. was an employee of Baxalta U.S. Inc. when this study took place; C.N., S.V., and F.B. were investigators in this study, which was sponsored by Baxalta US Inc/Baxalta Innovations GmbH.
Footnotes
Vadim Romanov and Alessandro Gringeri are to be considered senior authors.
Vadim Romanov was an employee of the company now known as Baxalta US Inc. at the time that this document was drafted.
Please see the Acknowledgements section for a comprehensive list of investigators.
References
- 1.Franchini M, Coppola A, Tagliaferri A, Lippi G. FEIBA versus NovoSeven in hemophilia patients with inhibitors. Semin Thromb Hemost 2013; 39:772–778. [DOI] [PubMed] [Google Scholar]
- 2.Coppola A, Santoro C, Tagliaferri A, Franchini M, Di Minno G. Understanding inhibitor development in haemophilia A: towards clinical prediction and prevention strategies. Haemophilia 2010; 16 (Suppl 1):13–19. [DOI] [PubMed] [Google Scholar]
- 3.Leissinger CA. Prevention of bleeds in hemophilia patients with inhibitors: emerging data and clinical direction. Am J Hematol 2004; 77:187–193. [DOI] [PubMed] [Google Scholar]
- 4.Konkle BA, Ebbesen LS, Erhardtsen E, Bianco RP, Lissitchkov T, Rusen L, Serban MA. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost 2007; 5:1904–1913. [DOI] [PubMed] [Google Scholar]
- 5.Hay CRM. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012; 119:1335–1344. [DOI] [PubMed] [Google Scholar]
- 6.Gringeri A, Musso R, Mazzucconi MG, Piseddu G, Schiavoni M, Pignoloni P, Mannucci PM. RITS-FITNHES Study Group. Immune tolerance induction with a high purity von Willebrand factor/VIII complex concentrate in haemophilia A patients with inhibitors at high risk of a poor response. Haemophilia 2007; 13:373–379. [DOI] [PubMed] [Google Scholar]
- 7.DiMichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia 2007; 13 (Suppl 1):1–22. [DOI] [PubMed] [Google Scholar]
- 8.Astermark J, Morado M, Rocino A, et al. Current European practice in immune tolerance induction therapy in patients with haemophilia and inhibitors. Haemophilia 2006; 12:363–371. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman M, Dargaud Y. Mechanisms and monitoring of bypassing agent therapy. J Thromb Haemost 2012; 10:1478–1485. [DOI] [PubMed] [Google Scholar]
- 10.Antunes SV, Tangada S, Stasyshyn O, Mamonov V, Phillips J, Guzman-Becerra N, et al. Randomized comparison of prophylaxis and on-demand regimens with FEIBA NF in the treatment of haemophilia A and B with inhibitors. Haemophilia 2014; 20:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gringeri A, Leissinger C, Cortesi PA, Jo H, Fusco F, Riva S, et al. Health-related quality of life in patients with haemophilia and inhibitors on prophylaxis with antiinhibitor complex concentrate: results from the Pro-FEIBA study. Haemophilia 2013; 19:736–743. [DOI] [PubMed] [Google Scholar]
- 12.National Hemophilia Foundation. MASAC recommendation regarding prophylaxis with bypassing agents in patients with hemophilia and high titer inhibitors. MASAC 220. 10-6-2013. National Hemophilia Foundation (NHF). https://www.hemophilia.org/sites/default/files/document/files/masac220.pdf [Accessed 21 December 2015]. [Google Scholar]
- 13.Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA®): 10-year compilation of thrombotic adverse events. Haemophilia 2002; 8:83–90. [DOI] [PubMed] [Google Scholar]
- 14.Berg R, Gringeri A, Füllenhals E, Reininger AJ. Thrombotic and embolic events associated with the use of FEIBA in congenital hemophilia: a cumulative review over four decades [abstract]. Haemophilia 2014; 20 (Suppl 3):50.24762275 [Google Scholar]
- 15.Gough S. Postmarketing surveillance: a UK/European perspective. Curr Med Res Opin 2005; 21:565–570. [DOI] [PubMed] [Google Scholar]
- 16.Borg JY, Négrier C, Durieu I, Dolimier E, Masquelier AM, Lévesque H. FEIBHAC Study Group. FEIBA in the treatment of acquired haemophilia A: results from the prospective multicentre French ’FEIBA dans l’hémophilie A acquise’ (FEIBHAC) registry. Haemophilia 2015; 21:330–337. [DOI] [PubMed] [Google Scholar]
- 17.Antunes S, Tangada S, Stasyshyn O, Mamonov V, Phillips J, Guzman-Becerra N, et al. A prospective, open-label, randomized, parallel study with AICC to evaluate the efficacy and safety of prophylactic versus on-demand treatment in haemophilia A or B subjects with inhibitors. Presented at the XXIV Congress of the International Society on Thrombosis and Haemostasis: 29 June–4July 2013, Amsterdam, The Netherlands. Poster: PB 4. 58-6. [Google Scholar]
- 18.Hilgartner M, Aledort L, Andes A, Gill J. Efficacy and safety of vapor-heated antiinhibitor coagulant complex in hemophilia patients. FEIBA Study Group. Transfusion 1990; 30:626–630. [DOI] [PubMed] [Google Scholar]
- 19.Negrier C, Goudemand J, Sultan Y, Bertrand M, Rothschild C, Lauroua P. Multicenter retrospective study on the utilization of FEIBA in France in patients with factor VIII and factor IX inhibitors. French FEIBA Study Group. Factor Eight Bypassing Activity. Thromb Haemost 1997; 77:1113–1119. [PubMed] [Google Scholar]
- 20.DiMichele D, Negrier C. A retrospective postlicensure survey of FEIBA efficacy and safety. Haemophilia 2006; 12:352–362. [DOI] [PubMed] [Google Scholar]
- 21.Baudo F, Collins P, Huth-Kuehne A, Lévesque H, Marco P, Nemes L, et al. Management of bleeding in acquired hemophilia A (AHA): results from the European Acquired Hemophilia (EACH2) Registry. Blood 2012; 120:39–46. [DOI] [PubMed] [Google Scholar]
- 22.Knoebl P, Marco P, Baudo F, Collins P, Huth-Kühne A, Nemes L, et al. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. J Thromb Haemost 2012; 10:622–631. [DOI] [PubMed] [Google Scholar]


