SUMMARY
Reorienting of voluntary attention enables the processing of stimuli at previously unattended locations. Although studies have identified a ventral fronto-parietal network underlying attention [1, 2], little is known about whether and how early visual areas are involved in involuntary [3, 4] and even less in voluntary [5] reorienting, and their temporal dynamics are unknown. We used transcranial magnetic stimulation (TMS) over the occipital cortex to interfere with attentional reorienting and study its role and temporal dynamics in this process. Human observers performed an orientation discrimination task, with either valid or invalid attention cueing, across a range of stimulus contrasts. Valid cueing induced a behavioral response gain increase, higher asymptotic performance for attended than unattended locations. During subsequent TMS sessions, observers performed the same task, with high stimulus contrast. Based on phosphene mapping, TMS double pulses were applied at one of various delays to a consistent brain location in retinotopic areas (V1/V2), corresponding to the evoked signal of the target or distractor, in a valid or invalid trial. Thus, the stimulation was identical for the four experimental conditions (valid/invalid cue condition × target/distractor-stimulated). TMS modulation of the target and distractor were both periodic (5 Hz, theta) and out of phase with respect to each other in invalid trials only, when attention had to be disengaged from the distractor and reoriented to the target location. Reorientation of voluntary attention periodically involves V1/V2 at the theta frequency. These results suggest that TMS probes theta phase-reset by attentional reorienting and help link periodic sampling in time and attention reorienting in space.
RESULTS
Voluntary covert attention, in the absence of eye movements, enhances visual processing at the attended location, which is mediated by several areas, including occipital cortex (review [6, 7]). Attentional reorienting allows processing at other locations, critical in an ever-changing environment.
Transcranial magnetic stimulation (TMS) is used to alter the activity of a targeted cortical area at precise moments and test its effects on perceptual or cognitive tasks [8, 9]. To study the role of areas involved in attentional processing, TMS has primarily been used in conjunction with visual search [10–14] and has shown that visual areas receive feedback information from fronto-parietal areas, at post-stimulus delays ≥150 ms. These studies did not explicitly manipulate attention and thus inferred its role in visual search; however, such inference is not warranted—before invoking an attentional explanation, it is important to rule out the effect of visual and physiological factors (e.g., retinal eccentricity and cortical magnification) and to explicitly manipulate attention [15–17]. Other studies have directly manipulated voluntary attention and stimulated attention- related higher-level areas, e.g., FEF, TPJ, and IPS, either to investigate their role during preparatory activity (cue-to-stimulus onset delay; [18, 19]) or in reorienting of attention [20, 21]. Consistent with neurophysiological studies [22], TMS of fronto-parietal regions modulates neuronal activity in occipital cortex (review [23]). So far, no studies have used TMS to directly investigate the role of occipital areas (V1/V2) in the orienting or reorienting of voluntary attention. These areas mediate the coding of orientation and contrast, basic visual dimensions that are task relevant in our study.
Covert spatial attention samples visual information periodically at low frequencies, theta (5–7 Hz) and alpha (8–12 Hz), in detection or discrimination tasks [14, 24–29]. The authors of these studies suggested that this periodicity indicates that attention processes multiple stimuli sequentially, i.e., attention in these tasks is reoriented to different locations, following a low-frequency rhythm. To date, only one study [26] has explicitly manipulated covert attention to assess the behavioral periodicity of attentional sampling with a discrimination task; unfortunately, the main dependent variable was reaction time, which can reflect perceptual processing speed, motor anticipation [30], and criterion [17]. Moreover, although two studies have provided convincing evidence showing that two locations are sampled in alternation [24, 27], whether the periodicity is actually due to a sequential reallocation of attention or to the independent sampling of each location is still largely debated [31]. We note that no study has explicitly manipulated the reorienting of attention to address this point.
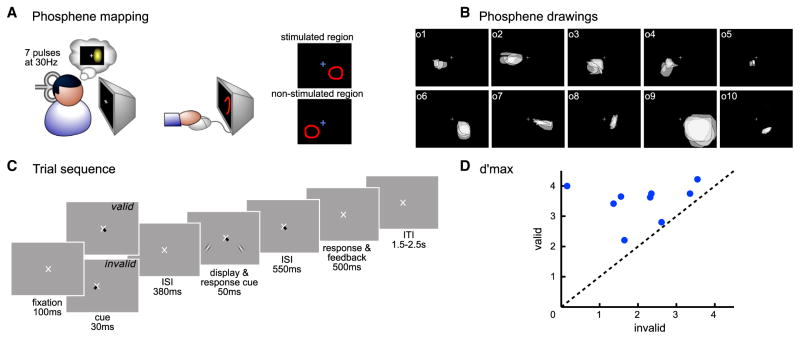
We conducted psychophysics and psychophysics-TMS sessions (see Supplemental Experimental Procedures). In both, we used an orientation discrimination task (see Figure 1B), assessing performance with d′, a perceptual measure of performance, and explicitly manipulated whether covert attention had to be reoriented or not. On valid-cued trials (75% of total trials), observers allocated attention to a single location throughout the trial, whereas on invalid-cued trials (25% of total trials), observers had to first allocate attention to one location and then shift it to another location. In the psychophysics sessions, observers performed the task with stimulus contrasts ranging from 2% to 32%. This allowed us to fit a psychometric function and obtain d′ max, a measure of asymptotic discriminability performance that is independent of an observer’s criterion. In the TMS sessions, the contrast of the stimuli was always set at the level corresponding to d′ max (32% for all observers; Figure 1D). In addition, observers received occipital TMS at various delays either before or after stimulus onset while performing the contrast-contingent orientation discrimination task to investigate the temporal dynamics of early visual cortical areas in attentional reorienting (Figure 2A).
Figure 1. Experimental Paradigm.
(A) Phosphene mapping session. Observers were stimulated in either the right or left occipital pole and drew their perceived phosphene. The phosphene region (“stimulated region”) and the symmetric region in the contralateral visual field (“non-stimulated region”) were used in the main experiment to determine the stimulus location.
(B) Individuals’ phosphenes. Each box represents the phosphenes of one observer. Each translucent shape represents a phosphene drawing for one TMS session.
(C) Trial sequence in both the psychophysics and TMS sessions.
(D) Each dot represents d′ max (d′ at asymptotic performance) for a single observer in the valid condition plotted as a function of the d′ max in the invalid condition. Dots above the diagonal indicate that performance was higher for the valid than invalid cueing conditions.
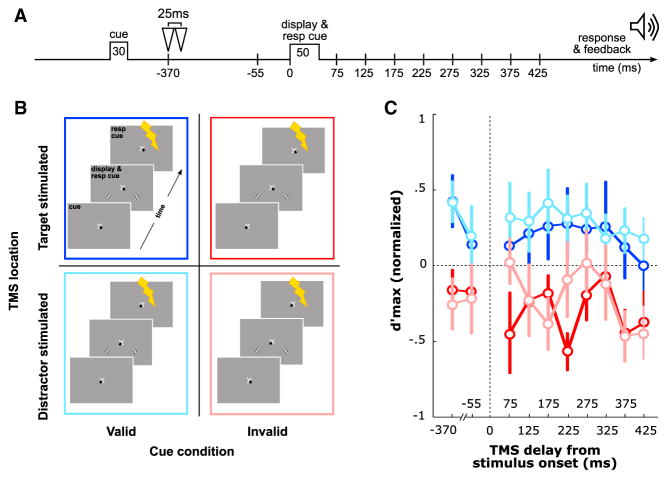
Figure 2. TMS Modulates Performance.
(A) Possible TMS stimulation delays on any given trial. During the TMS session, a double pulse (25 ms interval) was applied over the occipital pole at one of ten possible delays, two before and eight after the stimuli display onset.
(B) Four experimental conditions: (1) valid target-stimulated, (2) valid distractor-stimulated, (3) invalid target-stimulated, and (4) invalid distractor-stimulated.
(C) d′ max as a function of time, for the four experimental conditions; color schema as in (B).
Error bars on plots are ±1 SEM.
We compared four experimental conditions that differed in terms of the combination of attentional cue validity and TMS location (Figure 2B). Observers were presented with (1) a valid cue and the cortical representation of the target was stimulated with TMS (“valid target-stimulated”); (2) a valid cue and the cortical representation of the distractor was stimulated (“valid distractor-stimulated”); (3) an invalid cue and the cortical representation of the target was stimulated (“invalid target-stimulated”); and (4) an invalid cue and the cortical representation of the distractor was stimulated (“invalid distractor-stimulated”). Critically, prior to stimulus onset, the central cue induced attentional orienting by indicating the target location either in the left or right lower visual field. Given that the response cue appeared simultaneously with the stimulus, in the invalidly cued trials, observers had to reorient attention to the target location. We tested two hypotheses: (1) early visual areas are involved in the reorienting of voluntary attention; (2) voluntary attention reorients periodically at a low frequency.
TMS Effects on Attention
Note that for all analyses reported here, there were no differences between observers stimulated in the right or the left hemispheres (unbalanced ANOVA: F(1,8) < 1); we thus grouped all observers together. Additionally, a repeated-measures two-way ANOVA on the trials in which the TMS double pulses were applied before the stimulus onset showed no significant difference between the valid and invalid conditions, regardless of whether target or distractor were stimulated, and no significant interaction (F(1,8) < 1). These results suggest that TMS on occipital cortex at these particular delays before stimulus onset does not differentially affect the orienting of voluntary attention.
We hypothesized that early visual areas are involved in the reallocation of voluntary attention. To test this hypothesis, we first calculated performance (d′ max, asymptotic performance) in each of the four experimental conditions, at each post-display onset stimulation delay, for each observer separately (see behavioral analysis in Supplemental Experimental Procedures). We plot the mean d′ max for the group and error bars (±1 SEM; Figure 2C).
We conducted a repeated-measures three-way 2 × 2 × 8 ANOVA (cueing validity × TMS location × TMS delay). Consistent with previous voluntary attentional cueing studies (e.g., [32, 33]), performance was better in the validly cued trials than in the invalidly cued trials (F(1,8) = 74.4; p < 0.001), regardless of whether the target or distractor had been stimulated and regardless of the delay, as indicated by the non-significant three-way interaction (F(7,56) < 1). In addition, neither the main effects of TMS location (F(1,8) < 1) and delay (F(7,56) = 1.3) nor their interaction (F(7,56) < 1) were significant. We also analyzed reaction times (secondary dependent variable) for correct responses to rule out any speed-accuracy tradeoff; i.e., reaction times were faster for valid trials than invalid trials (paired t test: t(8) = −4.3, p = 0.0025).
Human observers can switch the allocation of voluntary attention 300 ms after the onset of a central cue [34, 35]. In this experiment, the response cue appeared at the onset of the visual display, inducing attention reorienting during the invalid cue trials only. We were particularly interested in the d′ max values at the earliest post-stimulus TMS delay, 75 ms, as at this delay, information is still undergoing feed forward propagation [36, 37], and observers had not had enough time to fully reorient their attention, i.e., to disengage from the attended location and engage onto the previously unattended location [1, 2]. At this delay, we compared the trials in which the precued location had been stimulated (Figure 2B), i.e., valid target-stimulated versus invalid distractor-stimulated, and trials in which the other location had been stimulated, i.e., valid distractor-stimulated versus invalid target-stimulated. At 75 ms, performance significantly differed between the valid and invalid cueing conditions when the unattended location had been stimulated (paired t test between valid distractor-stimulated and invalid target-stimulated: t(8) = 2.02, p = 0.039), but not when the attended location had been stimulated (paired t test between valid target-stimulated and invalid distractor-stimulated: t(8) = 0.28, p = 0.39; Figure 2C).
Temporal Dynamics
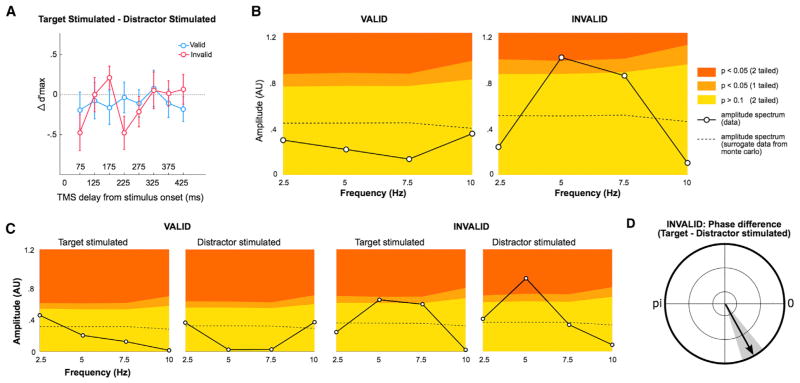
To investigate the temporal dynamics of attentional reorienting, we separately analyzed valid and invalid trials across all post-stimulus TMS delays. We hypothesized that reorienting of attention is periodically-modulated at a low frequency. To test this hypothesis, we first computed a fast Fourier transform (FFT) analysis on the difference between the target-stimulated and the distractor-stimulated trials (see Figures 3A and 3B and behavioral analysis in Supplemental Experimental Procedures). The amplitude spectrum showed a significant peak at 5 Hz, but only in the invalid cueing condition (still significant after false discovery rate [FDR] correction for multiple comparisons, two cueing conditions), indicating that the difference in d′ between target-stimulated and distractor-stimulated trials was periodically modulated at 5 Hz, within the theta frequency range.
Figure 3. TMS Modulation Is Periodic.
(A) Difference in d′ max (normalized) performance between target-stimulated and distractor-stimulated experimental conditions, for both valid (teal) and invalid (magenta) cue condition trials. Error bars on plots are ±1 SEM.
(B) Amplitude spectra obtained from an FFT performed separately on the d′ difference between stimulation conditions (solid lines) for the valid and invalid cue condition trials. The colored background corresponds to the level of significance obtained by a Monte Carlo procedure, under the null hypothesis that the d′ difference does not vary over time. The dashed line corresponds to the amplitude spectrum performed on the surrogate data obtained with the Monte Carlo procedure. The significant peak at 5 Hz in the invalid condition indicates that the magenta curve in (A) is periodically modulated at this specific frequency.
(C) Amplitude spectrum obtained from an FFT performed separately on the four trial conditions (see Figure 2B): target-stimulated or distractor-stimulated, separately for valid and invalid. The same convention is used for the colored background as in (B). The significant peak at 5 Hz in the invalid condition indicates that both the invalid target-stimulated (red) and distractor-stimulated (pink) curves in Figure 3C are periodically modulated at this specific frequency.
(D) Average phase difference of the 5 Hz component between target-stimulated and distractor-stimulated conditions across observers, specifically for the invalid condition. The gray area corresponds to ±1 SEM. The phase difference indicates a significant phase shift between the two curves, i.e., the invalid target-stimulated (red) and distractor-stimulated (pink) curves in Figure 3C.
To further ensure that the periodicity observed in the difference curve (Figure 3A) arises from a periodicity observed in each condition due to reorienting of attention, we performed the same analysis separately for each of the four conditions (Figure 3C). The amplitude spectra showed a significant peak at 5 Hz in the invalid condition only, for both target-stimulated and distractor-stimulated trials (still significant for invalid distractor-stimulated [p = 0.01] and marginally significant [p = 0.07] for invalid distractor-stimulated after FDR correction for multiple comparisons, four experimental conditions), indicating that both conditions were modulated periodically at 5 Hz (Figure 3C).
Additionally, we performed a phase analysis to assess whether the periodicity observed in the difference curve for the invalid condition (Figure 3A) was due to the difference of two out-of-phase periodic curves at 5 Hz (Figure 2C and behavioral analysis in Supplemental Experimental Procedures). We computed the average across observers of the phase difference between target-stimulated (kappa—concentration parameter—1.4) and distractor-stimulated (kappa = 1.6) trials (normalized to a unit length), specifically for the 5 Hz component in the invalid condition (parametric two-sample von Mises distribution test: F = 1.1, p = 0.9, i.e., the two conditions have the same phase distribution; Figure 3D). There was a significant phase difference between the two conditions (parametric Watson-Williams test: F = 6.3, p = 0.02), which indicates a phase shift between these two curves of ~0.5 cycle (Figure 2C). Taken together, these results show that d′ performance in the invalid condition was periodically modulated at 5 Hz and that the two stimulation conditions were phase shifted.
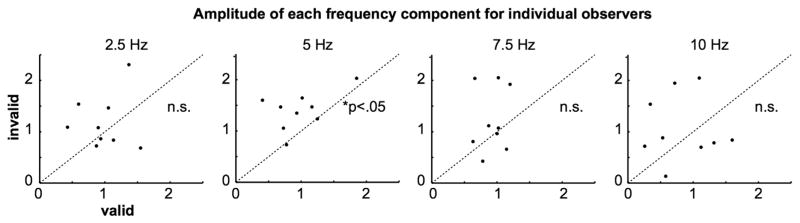
To assess whether this periodicity was present for each observer, we compared the amplitudes of each frequency component for valid and invalid trials. Because the target-stimulated and the distractor-stimulated conditions were both periodically modulated at 5 Hz, we averaged their amplitudes. The amplitude was higher in the invalid than valid condition (Figure 4) only for the 5 Hz component (one-tail paired t test: t(8) = −3.2; p = 0.0066; still significant after FDR correction for multiple comparisons, four frequency components; Figure 3). Note that this comparison was not significant at the other tested frequencies: 2.5, 7.5, and 10 Hz.
Figure 4. Amplitude of Each Frequency Component for Individual Observers.
An FFT was performed for each observer separately for all four conditions (see Figure 3C). Each black dot represents the amplitude of each frequency component for a given observer, averaged across target-stimulated and distractor-stimulated conditions. Only the amplitude of the 5 Hz component was significantly higher in the invalid than the valid cue condition.
DISCUSSION
We explicitly manipulated the orienting and reorienting of voluntary attention using well-established psychophysics protocols [32, 33], in a two-alternative forced choice (2-AFC) orientation discrimination task in conjunction with non-invasive brain stimulation. We assessed the temporal dynamics of attentional orienting and reorienting over early visual areas and their effects on performance. The stimulated areas (V1/V2) are responsible for orientation coding, which mediate orientation discrimination [38, 39]. Critically, we demonstrated that performance in the invalid condition, in which attention had to be reallocated from the distractor location to the target location, was periodically modulated by occipital TMS at the theta frequency (5 Hz). This periodicity was present not only in the averaged data (Figure 3) but also in the data for each individual observer (Figure 4). Additionally, we showed that the periodicity observed in the invalid condition when the target was stimulated was out of phase with the periodicity observed when the distractor was stimulated. This finding demonstrates that early visual cortex processes individual stimulus locations periodically; voluntary attention is reoriented with an inherent theta frequency.
The use of the phosphene mapping procedure allowed us to interfere with the specific retinotopic area involved in the processing of a single stimulus, which could be either the target or distractor, which were presented in different hemifields, and either validly or invalidly cued. This was highly beneficial as this meant that the comparisons of our four experimental conditions involved the exact same TMS stimulation. This optimal control could have not been achieved by using either sham or vertex stimulation, alternative procedures commonly used in TMS research [8, 19, 40] to mimic the TMS coil click but that do not control for all TMS-associated peripheral sensations. Moreover, given that TMS was close to the inter-hemispheric fissure, the noise it produced could not be responsible for the observed effects, as it would not have induced differential effects for the target and distractor that were presented in separate hemifields. Note that phosphene induction is not responsible for the modulation of performance brought about by TMS; had that been the case, the effect would have been the same for all four experimental conditions. Moreover, the combination of the phosphene mapping procedure with the use of neuronavigation granted us excellent precision over the stimulated region of the cortex, as confirmed by the consistency of the phosphenes obtained for each observer across sessions (Figure 1B).
The periodicity in the invalid condition is consistent with previous studies investigating the temporal dynamics of attention showing that visual information is sampled periodically at the theta frequency [14, 25, 27, 29, 41–43]. For example, Dugué et al. [14] showed that TMS applied over the occipital cortex periodically modulates the performance of observers during a conjunction (L versus T) search task at the theta frequency (~6 Hz). The correlation they observed between the behavioral periodicity and neuronal oscillation (as measured per EEG) suggests that the behavioral effects were due to the intrinsic properties of the system, which processes information periodically, rather than to TMS. Because oscillations reflect periodic cortical excitability, TMS can probe the system at different states. Here, in contrast to the previous studies, we explicitly manipulated the reorienting of attention via the invalid cue. By using TMS to perturb this reallocation process at several moments in time, we showed that attention reorients periodically at the theta frequency, consistent with an intrinsic sampling property of the system. Given our use of eight post-stimulus delays (in four experimental conditions), we could not characterize frequencies ≥10 Hz because of the temporal resolution of the stimulation delays; thus, we cannot rule out that other higher frequencies are related to attentional sampling. In any case, the results clearly show periodicity confined to 5 Hz. The window duration at which TMS pulses were applied (400 ms) limited the resolution of frequency sampling to 2.5 Hz and enabled two cycles of the 5 Hz component, enough to demonstrate periodicity ([44]; Figure 2C). In future studies, we would like to induce several shifts of attention at several moments and locations and employ repetitive TMS [19, 45, 46] to increase temporal resolution, to further explore the link between attentional sampling in time and sequential attentional exploration in space.
Interestingly, we did not observe any periodicity in the valid condition, when attention had already been deployed to the target location and did not need to be reallocated. However, we cannot rule out the existence of periodic attentional sampling for the valid condition. Indeed, it has been shown that attentional sampling occurs even when observers are focused on a single target in a detection task, suggesting that attention may intrinsically be a periodic process [41]. Additionally, the amplitude of such modulation seems less important at the cued than at the uncued location [25].
It is possible that attention may always fluctuate periodically, but with its own spontaneous phase. A single unit recording study in awake-behaving monkeys found that inferior temporal (IT) responses to a stimulus appearing against the backdrop of an existing stimulus showed stronger theta (5 Hz) response modulation after the appearance of a second stimulus, which presumably triggered attentional reorienting. Intriguingly, theta modulation was also observed after the appearance of a single stimulus [43, 47]. Based on that result and on our current finding, we can argue that when the target appeared at the cued location, the attentional oscillation would not have been reset by the reorienting process [1, 2], and TMS would not bear a phase relation with attention. However, when the target appeared at the uncued location, the attentional reorienting process would have theta phase-reset this oscillatory component, which could then be probed with TMS [14, 44, 46, 48]. We note that this explanation does not necessarily entail that attention swings back creating a full cycle of performance modulation. Our usage of “phase-reset” refers to the mathematical description of phase concentration at a particular angle resulting in a spectral peak and does not presuppose either a reset of an ongoing oscillation at the same frequency or additional evoked activity at that frequency, which have not been disentangled [49]. Further research is needed to fully understand the periodicity of attentional sampling and exploration.
To conclude, we used well-established knowledge in psychophysics in conjunction with TMS to investigate the role of early visual areas in the reorienting of voluntary attention. Specifically, TMS disrupted the reorienting of spatial attention at the theta frequency, suggesting that attentional reorienting operates at this frequency. This research furthers our understanding of the link between the periodic sampling of visual information in time and the reallocation of attention in space.
EXPERIMENTAL PROCEDURES
The experimental protocol was in compliance with the safety guidelines for TMS research and was approved by the University Committee on Activities Involving Human Subjects at New York University. A description of the essential experimental procedures and data analyses is presented in the Results section. A complete description can be found in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Manipulating attention reorienting to assess perception in a discrimination task
Identical TMS for all experimental conditions guided by phosphene mapping
Periodic reorienting of voluntary attention at the theta frequency (5 Hz) in V1/V2
Possible link between periodic sampling in time and attention reorienting in space
Acknowledgments
This work was supported by NIH (RO1-EY016200) to M.C., the FYSSEN Foundation to L.D., and NSF (DGE 1342536) to M.R. We want to thank Rufin Van- Rullen as well as the members of the M.C. lab for constructive comments on the manuscript.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2016.04.046.
AUTHOR CONTRIBUTIONS
L.D., M.R., and M.C. designed the study and wrote the paper. L.D. and M.R. conducted the experiments.
References
- 1.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geng JJ, Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci Biobehav Rev. 2013;37:2608– 2620. doi: 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T, Pestilli F, Carrasco M. Transient attention enhances perceptual performance and FMRI response in human visual cortex. Neuron. 2005;45:469–477. doi: 10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinen K, Ruff CC, Bjoertomt O, Schenkluhn B, Bestmann S, Blankenburg F, Driver J, Chambers CD. Concurrent TMS-fMRI reveals dynamic interhemispheric influences of the right parietal cortex during exogenously cued visuospatial attention. Eur J Neurosci. 2011;33:991–1000. doi: 10.1111/j.1460-9568.2010.07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shomstein S, Behrmann M. Cortical systems mediating visual attention to both objects and spatial locations. Proc Natl Acad Sci USA. 2006;103:11387–11392. doi: 10.1073/pnas.0601813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anton-Erxleben K, Carrasco M. Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nat Rev Neurosci. 2013;14:188–200. doi: 10.1038/nrn3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrasco M. Visual attention: the past 25 years. Vision Res. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perini F, Cattaneo L, Carrasco M, Schwarzbach JV. Occipital transcranial magnetic stimulation has an activity-dependent suppressive effect. J Neurosci. 2012;32:12361–12365. doi: 10.1523/JNEUROSCI.5864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silvanto J, Muggleton NG. New light through old windows: moving beyond the “virtual lesion” approach to transcranial magnetic stimulation. Neuroimage. 2008;39:549–552. doi: 10.1016/j.neuroimage.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Kalla R, Muggleton NG, Juan CH, Cowey A, Walsh V. The timing of the involvement of the frontal eye fields and posterior parietal cortex in visual search. Neuroreport. 2008;19:1067–1071. doi: 10.1097/WNR.0b013e328304d9c4. [DOI] [PubMed] [Google Scholar]
- 11.Muggleton NG, Juan CH, Cowey A, Walsh V, O’Breathnach U. Human frontal eye fields and target switching. Cortex. 2010;46:178–184. doi: 10.1016/j.cortex.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Juan CH, Walsh V. Feedback to V1: a reverse hierarchy in vision. Exp Brain Res. 2003;150:259–263. doi: 10.1007/s00221-003-1478-5. [DOI] [PubMed] [Google Scholar]
- 13.Dugué L, Marque P, VanRullen R. Transcranial magnetic stimulation reveals attentional feedback to area V1 during serial visual search. PLoS ONE. 2011;6:e19712. doi: 10.1371/journal.pone.0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugué L, Marque P, VanRullen R. Theta oscillations modulate attentional search performance periodically. J Cogn Neurosci. 2015;27:945–958. doi: 10.1162/jocn_a_00755. [DOI] [PubMed] [Google Scholar]
- 15.Carrasco M, Yeshurun Y. The contribution of covert attention to the set-size and eccentricity effects in visual search. J Exp Psychol Hum Percept Perform. 1998;24:673–692. doi: 10.1037//0096-1523.24.2.673. [DOI] [PubMed] [Google Scholar]
- 16.Joseph JS, Chun MM, Nakayama K. Attentional requirements in a ‘preattentive’ feature search task. Nature. 1997;387:805–807. doi: 10.1038/42940. [DOI] [PubMed] [Google Scholar]
- 17.Carrasco M, McElree B. Covert attention accelerates the rate of visual information processing. Proc Natl Acad Sci USA. 2001;98:5363–5367. doi: 10.1073/pnas.081074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanes L, Quentin R, Tallon-Baudry C, Valero-Cabré A. Causal frequency-specific contributions of frontal spatiotemporal patterns induced by non-invasive neurostimulation to human visual performance. J Neurosci. 2013;33:5000–5005. doi: 10.1523/JNEUROSCI.4401-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thut G, Nietzel A, Pascual-Leone A. Dorsal posterior parietal rTMS affects voluntary orienting of visuospatial attention. Cereb Cortex. 2005;15:628–638. doi: 10.1093/cercor/bhh164. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PCJ, Nobre AC, Rushworth MFS. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 23.Chica AB, Bartolomeo P, Lupiáñez J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behav Brain Res. 2013;237:107–123. doi: 10.1016/j.bbr.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Landau AN, Fries P. Attention samples stimuli rhythmically. Curr Biol. 2012;22:1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 25.Fiebelkorn IC, Saalmann YB, Kastner S. Rhythmic sampling within and between objects despite sustained attention at a cued location. Curr Biol. 2013;23:2553–2558. doi: 10.1016/j.cub.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song K, Meng M, Chen L, Zhou K, Luo H. Behavioral oscillations in attention: rhythmic α pulses mediated through θ and. J Neurosci. 2014;34:4837–4844. doi: 10.1523/JNEUROSCI.4856-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau AN, Schreyer HM, van Pelt S, Fries P. Distributed attention is implemented through theta-rhythmic gamma modulation. Curr Biol. 2015;25:2332–2337. doi: 10.1016/j.cub.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Dugué L, Vanrullen R. The dynamics of attentional sampling during visual search revealed by Fourier analysis of periodic noise interference. J Vis. 2014;14:11. doi: 10.1167/14.2.11. [DOI] [PubMed] [Google Scholar]
- 29.Dugué L, McLelland D, Lajous M, VanRullen R. Attention searches nonuniformly in space and in time. Proc Natl Acad Sci USA. 2015;112:15214–15219. doi: 10.1073/pnas.1511331112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correa A, Triviño M, Pérez-Dueñas C, Acosta A, Lupiáñez J. Temporal preparation, response inhibition and impulsivity. Brain Cogn. 2010;73:222–228. doi: 10.1016/j.bandc.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 31.VanRullen R. Visual attention: a rhythmic process? Curr Biol. 2013;23:R1110–R1112. doi: 10.1016/j.cub.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Herrmann K, Montaser-Kouhsari L, Carrasco M, Heeger DJ. When size matters: attention affects performance by contrast or response gain. Nat Neurosci. 2010;13:1554–1559. doi: 10.1038/nn.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pestilli F, Ling S, Carrasco M. A population-coding model of attention’s influence on contrast response: Estimating neural effects from psychophysical data. Vision Res. 2009;49:1144–1153. doi: 10.1016/j.visres.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Res. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Stevens ST, Carrasco M. Comparing the time course and efficacy of spatial and feature-based attention. Vision Res. 2007;47:108–113. doi: 10.1016/j.visres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Nowak LG, Munk MH, Girard P, Bullier J. Visual latencies in areas V1 and V2 of the macaque monkey. Vis Neurosci. 1995;12:371–384. doi: 10.1017/s095252380000804x. [DOI] [PubMed] [Google Scholar]
- 37.VanRullen R, Thorpe SJ. The time course of visual processing: from early perception to decision-making. J Cogn Neurosci. 2001;13:454–461. doi: 10.1162/08989290152001880. [DOI] [PubMed] [Google Scholar]
- 38.Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- 39.Ohki K, Chung S, Kara P, Hübener M, Bonhoeffer T, Reid RC. Highly ordered arrangement of single neurons in orientation pinwheels. Nature. 2006;442:925–928. doi: 10.1038/nature05019. [DOI] [PubMed] [Google Scholar]
- 40.Duecker F, de Graaf TA, Jacobs C, Sack AT. Time- and task-dependent non-neural effects of real and sham TMS. PLoS ONE. 2013;8:e73813. doi: 10.1371/journal.pone.0073813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanRullen R, Carlson T, Cavanagh P. The blinking spotlight of attention. Proc Natl Acad Sci USA. 2007;104:19204–19209. doi: 10.1073/pnas.0707316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci USA. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rollenhagen JE, Olson CR. Low-frequency oscillations arising from competitive interactions between visual stimuli in macaque inferotemporal cortex. J Neurophysiol. 2005;94:3368–3387. doi: 10.1152/jn.00158.2005. [DOI] [PubMed] [Google Scholar]
- 44.Vanrullen R, Busch NA, Drewes J, Dubois J. Ongoing EEG Phase as a Trial-by-Trial Predictor of Perceptual and Attentional Variability. Front Psychol. 2011;2:60. doi: 10.3389/fpsyg.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thut G, Miniussi C, Gross J. The functional importance of rhythmic activity in the brain. Curr Biol. 2012;22:R658–R663. doi: 10.1016/j.cub.2012.06.061. [DOI] [PubMed] [Google Scholar]
- 47.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 48.Romei V, Driver J, Schyns PG, Thut G. Rhythmic TMS over parietal cortex links distinct brain frequencies to global versus local visual processing. Curr Biol. 2011;21:334–337. doi: 10.1016/j.cub.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.