Abstract
Myeloablative conditioning regimens for hematopoietic stem cell transplant (HSCT) are known to affect endocrine function, but little is known regarding reduced intensity conditioning (RIC) regimens. We retrospectively reviewed 114 children and young adults after single RIC HSCT. Analysis was grouped by age (<2y and ≥2y), and diagnosis (HLH/XLP, other immune disorders, metabolic/genetic disorders). All groups displayed short stature by mean height adjusted Z-score (HAZ) before −1.29 and after HSCT (HAZ −1.38, p=0.47). After HSCT, younger children with HLH/XLP grew better (HAZ −3.41 vs −1.65, p= 0.006), while older subjects had worsening (HAZ −0.8 vs −1.01, p= 0.06). Those with steroid therapy beyond standard GVHD prophylaxis were shorter than those without (p 0.04). After HSCT, older subjects with HLH/XLP became thinner with mean BMI Z-score of 1.20 vs. 0.64, p=0.02, likewise in metabolic/genetic disorders (BMI-Z= 0.59 vs. −0.99, p<0.001). BMI increased among younger children in these same groups. Thyroid function was abnormal in 24% (18/76). 25-OH vitamin D levels, were insufficient in 73% (49/65), with low bone mineral density in 8 of 19 evaluable subjects. Despite RIC, children and young adults still have significant late endocrine effects. Further research is required to compare post-transplant endocrine effects after RIC to standard chemotherapy protocols.
Introduction
Hematopoietic stem cell transplant (HSCT) is a potentially curative therapy for patients with many malignancies, primary immune deficiencies, metabolic diseases, and inherited bone marrow failure disorders. Traditionally, stem cell transplantation has involved the use of high dose myeloablative chemotherapy and/or radiation to facilitate successful engraftment or provide disease control in malignancy. Modern HSCT techniques with improved supportive care measures and donor matching have resulted in an increasing number of long-term survivors. Additional advances in the field of HSCT have led to novel, less intense, and theoretically less toxic, reduced intensity chemotherapy (RIC) preparative regimens. Reduced intensity regimens were developed to establish donor engraftment and control of the patients’ primary disease while limiting immediate and late effects to areas such as the endocrine system. Understanding the impact of preparative regimens is important for anticipating development of late effects of these treatments as our long-term survivor population continues to grow.
The endocrine system is known to be extremely susceptible to damage by preparative regimens using high dose chemotherapy and irradiation. However, very little is known regarding the impact of RIC regimens on these late effects 1, 2. Historically, primary hypothyroidism and primary gonadal failure are the most common endocrine outcomes due to injury from chemotherapy and radiation used prior to HSCT 3. In addition, short stature and decreased bone mineral density may develop due to growth hormone deficiency, or hypopituitarism 4.
Knowledge regarding endocrinologic late effects of RIC HSCT is especially lacking in the pediatric population as data are limited. Experience with late effects in adults after HSCT is not directly applicable to children, especially those who are pre-pubertal and still growing.
Here we describe the effects on growth, thyroid function, vitamin D levels and bone density in pediatric patients in follow up after HSCT using reduced intensity preparative regimens that are theoretically less toxic than standard regimens.
Materials and Methods
Subjects
We performed a retrospective review of HSCT patients (birth to 30 years of age) treated at Cincinnati Children’s Hospital Medical Center between January 1, 2003 and July 3, 2012, in order to identify patients who received a reduced intensity preparative regimen without radiation. This research was performed with institutional review board approval. Patients undergoing a single HSCT and surviving at least 1 year were included. Patients were grouped by diagnosis (HLH/XLP, other immune disorders (PID), and metabolic or genetic disorders). Clinical records were reviewed, and data abstracted. Data included patient demographics, clinical and transplant specific data, radiologic and laboratory studies, therapeutic interventions, overall outcomes, and related complications (Table 1).
Table 1.
Patient demographics and disease characteristics of children and young adults treated
| Characteristics (n=114) | Number(%)/Median (range) |
|---|---|
| Male | 74(65%) |
| Female | 41(36%) |
| Age (years) | 5.1 (0.2–27) |
| < 18 years | 108 (94%) |
| ≥ 18 years | 7 (6%) |
| Diagnosis | |
| HLH/XLP | 56 (49%) |
| Other PID | 29 (25.5%) |
| BMF/Metabolic/Malignancy | 29 (25.5%) |
| Conditioning | |
| Alemtuzamab /Fludarabine/Melphalan | 114 (100%) |
| Donor Source/HLA Status | |
| Related | |
| Matched (6/6, 8/8) | 27 (23%) |
| Mismatched (7/8) | 1 (1%) |
| Unrelated | |
| Matched (6/6, 8/8) | 56 (50%) |
| Mismatched (4–5/6, 6–7/8) | 30 (26%) |
| Stem cell source | |
| Bone Marrow | 102 (89%) |
| PBSC | 5 (4%) |
| Bone Marrow/Cord Blood | 1 (1%) |
| Cord Blood | 6 (6%) |
| GVHD prophylaxis | |
| CSA/steroids | 22 (19%) |
| CSA/steroids + Methotrexate | 82 (72%) |
| CSA/MMF | 1 (1%) |
| CSA/Methotrexate | 5 (4%) |
| Tacrolimus/steroids | 1 (1%) |
| Methotrexate | 3 (3%) |
| Additional steroid therapy | |
| Yes | 86 (75%) |
| No | 28 (25%) |
RIC, reduced intensity conditioning; HLH, hemophagocytic lymphohistiocystosis; XLP, X-linked lymphoproliferative syndrome; PID, Primary Immune Deficiencies; BMF, bone marrow failure; HLA, human leukocyte antigen; PBSC, peripheral blood stem cell; GVHD, graft versus host disease; CSA, cyclosporine; MMF, mycophenolate mofetil
Therapy
Preparative regimen was alemtuzamab (either as a dose-escalation schedule of 3 mg, 10 mg, 15 mg, 20mg (3 mg, 10 mg, 10 mg, 10 mg in patients less than 10 kg) or as a total of 1mg/kg divided over 5 days), with timing as previously described5 fludarabine (150mg/m2), and melphalan (140mg/m2). Graft versus host disease prophylaxis is shown in Table 1 and was predominantly cyclosporine and steroids (1–2mg/kg/day for up to 2 months), with or without methotrexate. Additional non-routine steroid therapy was defined as glucocorticoids administered either before HSCT or continuing beyond 2 months after HSCT. The majority of patients that received additional non-routine steroid therapy were individuals with HLH/XLP (n=55).
Evaluation of Growth and Endocrine function
Height measurement (without shoes) by stadiometer was performed routinely at every outpatient assessment. Weight was measured and body mass index (kg/m2) was calculated. Thyroid hormone function testing, including thyroid stimulating hormone (TSH), thyroxine (T4), and free T4 levels, was performed prior to bone marrow transplantation. Thyroid hormone testing was also assessed one year following HSCT in a subset of patients (n =77). In 64 patients, 25-OH vitamin D levels were assayed. Serum concentrations of 25-hydroxyvitamin D were determined using ultra-high performance liquid chromatography coupled to electrospray tandem mass spectrometry (UPLC-MS/MS) (Waters, Milford, MA)6. Bone density was assessed by dual-energy X-ray absorptiometry (DXA) in 18 patients and Z-scores were corrected for height-age using an online calculator for the Bone Mineral Density in Childhood Study (BMSCS):http://www.bmdcspublic.com/zscore.httm.
Pubertal hormones were assessed in 8 of the 37 patients over age 12 years and complete pubertal assessments were unavailable. As such, these results were not formally analyzed for this publication.
Statistical analysis
Height-for-age z scores (HAZ) and body mass index z-scores (BMI-Z) were both corrected for age. HAZ and BMI-Z were assessed using the WHO calculation for patients under <= 2 years at the time of transplant and the CDC calculation was used for those greater than 2 years. Both methods rely on a transformation that is only accurate within certain ranges for height-for-age, weight-for-age and weight-for-height. Acceptable ranges for z-scores of each are −5 to 3, −5 to 5 and −4 to 5, respectively. Those patient measurements not within the range for applicable measurements were excluded from analysis. This was done because the calculation of HAZ and BMI-Z are not accurate for measurements that do not satisfy these criteria, and not simply because these values are outliers.
Mean (standard error) values were used to describe HAZ and BMIZ throughout. Other continuous and categorical variables were reported as median (range) and frequency (percent), respectively.. In total, 6 pre-BMT patients were excluded from HAZ analysis and 11 excluded from BMI-Z analysis (Table 2). Only one post-BMT patient was excluded from both HAZ and BMI-Z.
Table 2.
Patients excluded from analysis due to extreme HAZ or BMI
| Patient | Age at HSCT(Y) | Male or Female | Diagnosis | Thyroid status | Steroids Pre-HSCT or >2 mo | Pre-HSCT HAZ < −5 SD | Post-HSCT HAZ < −5 SD | Pre-HSCT BMI > +5 SD | |
|---|---|---|---|---|---|---|---|---|---|
| Extreme HAZ | 1 | 0.42 | M | HLH | NML | Y | Y | Y | N |
| 2 | 0.49 | M | HLH | NML | Y | Y | Y | N | |
| 3 | 0.98 | F | SDS | 1° Hypo | Y | Y | Y | N | |
| 4 | 6.46 | F | SCID | NML | Y | Y | Y | N | |
| 5 | 10.72 | M | Omenn | NML | N | Y | Y | N | |
|
| |||||||||
| Extreme BMI | 6 | 2.13 | M | HLH | NML | Y | N | N | Y |
| 7 | 2.39 | F | HLH | NML | Y | N | N | Y | |
| 8 | 5.4 | M | XLP | NML | Y | N | N | Y | |
| 9 | 8.05 | M | XLP | NML | Y | N | N | Y | |
Abbreviations: HLH: hemophagocytic lymphohistiocytosis, SCID: severe combined immunodeficiency syndrome, SDS: Shwachman-Diamond syndrome, XLP: X-linked lymphoproliferative disorder, NML: normal, Unk: unknown, HAZ: height-for-age Z-score, BMI: body mass index
For change in height assessment, all patients greater than 14 years of age at BMT were excluded, as they might have reached their adult height. Data that change with respect to time after BMT (HAZ, BMIZ, etc) were collected on each patient at two time points, at BMT and at last follow-up. This repeated measure design induces intra-subject correlation. To account for this a multiple linear mixed effects model with unstructured inter-subject correlation was used separately for HAZ and BMI-Z. Variable considered for inclusion into the multivariate model were age (<=2 or >2), gender, additional disease related steroid therapy pre BMT, additional non-routine steroid therapy post BMT, diagnosis, and time of measurement; all variables included an interaction with the time of measurement to assess the variables association with change in HAZ or BMIZ. A guided backwards stepwise procedure was used to identify influential variables. Likelihood ratio tests (LRT) were conducted for each variable along with its interaction with time. A variable was excluded if the LRT yielded a p-value < 0.05. Age, additional disease related steroid therapy pre BMT, BMT diagnosis, and time of measurement variables were included in the model for HAZ. Age, additional disease related steroids pre-BMT, and time of measurement was included in the final BMIZ model. P-values were found using contrast statements in SAS PROC MIXED (SAS, 9.3 Cary, North Carolina). Statistical significance was assessed at the 0.05 level.
Results
Patient Characteristics
In a retrospective review of 772 hematopoietic stem cell transplants consecutively performed at our institution during the study period, we identified 114 individuals (16%) who underwent HSCT using a reduced intensity preparative regimen who met study criteria. Patient demographic and transplant characteristics are shown in Table 1. Median patient age was 5.1 years (range 0.2 to 27) and 41 were female, with median follow-up of 4.0 years (range1–8.8).
Growth
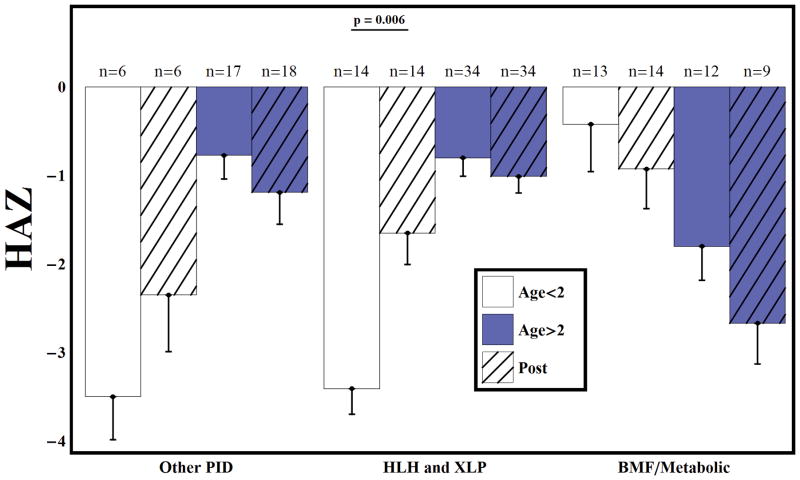
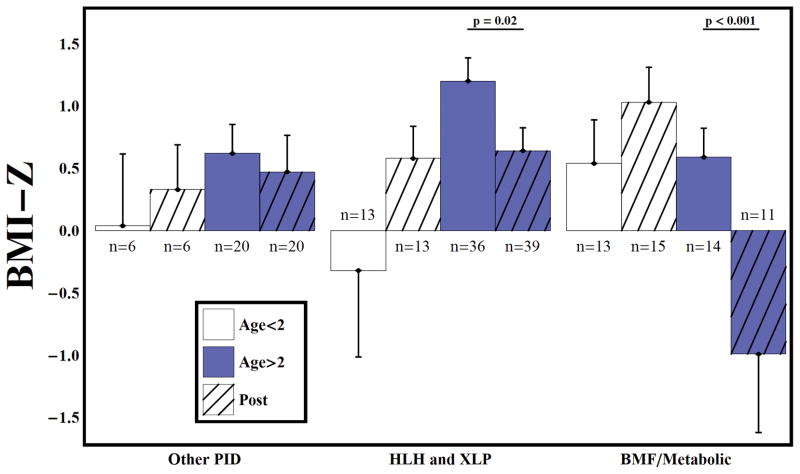
All groups displayed a tendency toward short stature before (HAZ = −1.29) and after HSCT (HAZ = −1.38) (p=0.47). After HSCT, younger children with HLH/XLP demonstrated improved linear growth (HAZ =−3.41 vs −1.65, p= 0.006), while older subjects showed decline in linear growth outcomes (HAZ =−0.8 vs −1.01, p= 0.06), although all diagnostic groups had short stature relative to normal growth in children (Figure 1). Overall, subjects receiving supplemental, non-routine steroid therapy were shorter than those who did not (HAZ = −1.43 vs −1.20, p = 0.04). After HSCT, older subjects with HLH/XLP had decreased BMI (BMI-Z= 1.20 vs. 0.64, p=0.02), similar to metabolic or genetic disorders (BMI-Z= 0.59 vs. −0.99, p<0.001) (Figure 2). There was a trend toward increased BMI-Z over time among younger children in these same groups. No differences in BMI-Z were noted in the PID population. Measurements are shown in Table 2 for 10 children with height and BMI at statistical extremes (≥ 3 or ≤ −5 standard deviations for height, and ≥5 or ≤−5 standard deviations for weight) that that were not included in the statistical analysis.
Figure 1.
Height-for-age z scores before and after RIC HSCT. After HSCT, younger children with HLH/XLP demonstrated improved linear growth (HAZ =−3.41 vs −1.65, p= 0.006), while older subjects showed decline in linear growth outcomes (HAZ =−0.8 vs −1.01, p= 0.06), although all patients remained short relative to normal growth in children (Figure 1). Bars represent mean and standard error. There were 102 subjects on study younger than age 14 at the time of BMT. 6 pre-BMT HAZ measurements were invalid due to CDC/WHO guidelines. 96 had valid pre-BMT HAZ measurement. 6 subjects were missing a post-BMT measurement and 1 was invalid. 95 had a valid post-BMT HAZ measurement.
Figure 2.
Body mass index z-scores before and after RIC HSCT. After HSCT, older subjects with HLH/XLP had decreased BMI (BMI-Z= 1.20 vs. 0.64, p=0.02) which was similar to metabolic or genetic disorders (BMI-Z= 0.59 vs. −0.99, p<0.001). Bars represent mean and standard error. There were 114 subjects on study. 11 pre-BMT measurements were invalid due to CDC/WHO guidelines. 103 and two had valid pre-BMT BMIZ measurement. 12 subjects were missing a post-BMT measurement and 1 was invalid. 103 had a valid post-BMT HAZ measurement.
Thyroid dysfunction
Thyroid function testing (TSH, T4, and free T4) was performed in 51 patients prior to HSCT. Thyroid function was normal in 42 (82%) of those patients while 9 (18%) patients had primary hypothyroidism. Of those with both normal thyroid function prior to HSCT and post-transplant thyroid data (n= 35, 69%), 4 (11%) progressed to having primary hypothyroidism following HSCT. Unfortunately, a significant portion of patients with normal results pre-HSCT (29%) had no thyroid testing post-HSCT. Five patients with abnormal thyroid testing pre-HSCT progressed to have normal thyroid testing post-HSCT; however, all were treated with thyroid hormone replacement at the time of post-HSCT measurement. In total, thyroid tests were performed in 76 subjects following HSCT, and 18 subjects (24%) had abnormal results. Eleven (14%) had evidence of primary hypothyroidism, 5 (7%) had central hypothyroidism, and 2 (3%) had evidence of primary hyperthyroidism. No significant differences were noted between diagnosis groups.
Vitamin D and Bone mineral density
Of the 64 subjects with 25-OH vitamin D levels measured post-transplant, 47 (73%) had insufficiency (<30 ng/mL). More subjects with HLH/XLP had insufficient levels of 25-OH vitamin D (28 of 33, 85%) than subjects with other diagnoses (20 of 32, 63%) (p=0.05). Of note, all but one of the individuals with HLH/XLP (54/55) received additional steroid therapy, either prior to HSCT for the treatment of their underlying disease or after HSCT for GVHD, which may have impacted vitamin D levels. Bone densitometry by DXA scan adjusted for height-age was below −1 SD in 7 of 18 evaluable subjects, with an average Z-score of −1.9 SD (1.4 to −4.3 SD) at median duration after HSCT of 2.1 years. Twelve patients (11%) had a history of fracture, including one patient with two fractures. Fractures included five vertebral compression fractures, four long-bone fractures, three fractures of digits and one clavicular fracture. Vitamin D levels were insufficient (<30 ng/mL) in 9 of 10 of these individuals who were tested. Height-adjusted Z-scores were available in 4 and ranged from 0.18 to −1.4. Five patients had a history of avascular necrosis including the distal and/or proximal femurs in four and the foot in one. One patient required surgical correction.
Discussion
Our study indicates that endocrine complications including short stature, hypothyroidism and vitamin D deficiency still occur after HSCT despite reduced intensity chemotherapy and omission of total body irradiation. As many of these parameters may also be affected by variables such as transplant diagnosis and steroid use, our analysis was structured to address this limitation by looking at disease groups as well as steroid therapy groups.
Impairment of linear growth is a well-recognized complication of HSCT. Myeloablative regimens utilizing radiation therapy have been shown to be one of the most important elements impacting this late effect 3, 7, 8. Impairment of linear growth may also be secondary to damage to neuroendocrine organs like the pituitary and hypothalamus, as well as direct impact on skeletal tissues, including cartilage and growth plates. Poor growth can be compounded by associated thyroid dysfunction, gonadal failure resulting in delayed puberty, or prolonged steroid therapy often utilized after HSCT as well as underlying genetic disease effects.
The children in our cohort displayed short stature prior to HSCT across all disease groups. In the largest group, children with HLH/XLP, younger individuals had improved growth after HSCT while growth was slower after HSCT in older patients. Poor growth following HSCT in this older population is indeed interesting and consistent in all groups analyzed regardless of original diagnosis. These findings highlight the contrast between younger and older patients with regard to treatment effects. The etiology of this contrast may be multifactorial, but it is perhaps possible that the accumulated effects of longer courses of steroids in older patients prevent rapid growth recovery following HSCT compared to younger children. It would be most interesting to carry the results out until final adult height is achieved to highlight any eventual differences, which is the goal of future ongoing work in a larger cohort.
Overall, steroid treatment resulted in shorter stature; however, numbers were not sufficient to perform a complete analysis of the effects of steroids by diagnostic group. Many children remained short after HSCT (HAZ −1.29 (−5.5 – 0.60) reflecting lasting effects of steroid exposure or development of hormone deficiencies even with reduced intensity chemotherapy. Measurements of growth hormone, IGF-1 or IGF-BP3 levels were not included in routine care after HSCT, and thus our cohort cannot address the mechanism of slower growth which may be important to evaluate in future studies.
It is unclear to what degree growth hormone (GH) deficiency affects growth impairment after HSCT or what role GH replacement has in treatment over time. However, there are some data from the Childhood Cancer Survivor Study that treatment with GH replacement does not increase risk of relapse 9. There remains, however, theoretical concern that GH replacement may increase risk for secondary malignancy in a population that already has a significant susceptibility to malignancy. GH replacement should be considered in patient populations with non-malignant disorders in conjunction with a pediatric endocrinologist.
Similar to growth disturbances, gonadal dysfunction or failure after HSCT has also been strongly associated with the use of radiation and busulfan in the preparative regimen 7. Of note, our current patient cohort had insufficient numbers of pubertal-age children to analyze gonadal function after RIC for HSCT. However, in standard HSCT preparatory regimens, treatment with total body irradiation or alkylating agents (such as busulfan or cyclophosphomide) may result in hypergonadotropic hypogonadism, and recovery of gonadal function is extremely rare 7, 10. Testicular impairment is drug-specific and dose-related. The use of busulfan at 16mg/kg, and cyclophosphomide at 200mg/kg resulted in significant testicular dysfunction in approximately 80% of patients 10. In contrast to females, the age and pubertal status of males at the time of HSCT does not appear to affect the occurrence of testicular dysfunction.
Degree of ovarian dysfunction is also drug-specific and dose-related. However, in females there is a significant role of age and pubertal status in determining the magnitude of damage from alkylating agents. Irreversible ovarian failure can occur in adult women receiving lower doses of both radiation and alkylating chemotherapy agents, (6 Gy of radiation) compared to children and adolescents (10 Gy). The typical doses of total body irradiation delivered during standard HSCT preparative regimens results in ovarian damage in almost all girls over 10 years of age, and in approximately half of those less than 10 years7. Our cohort will be followed in the future for observation of their pubertal development.
Thyroid dysfunction, most commonly primary hypothyroidism, also remains an extremely common complication after HSCT, although autoimmune thyroid disorders, thyroid nodules, or secondary thyroid carcinomas can also occur. Thyroid injury has been associated with the use of myeloablative conditioning regimens where radiation is both dose and delivery dependent. Patients receiving hyperfractionated total body irradiation are less likely to have thyroid dysfunction (15%) compared to those receiving single dose irradiation (89%) 11, 12. However, many of our patients continued to have thyroid dysfunction despite the absence of radiation in the conditioning regimen. In our study, 11% of patients developed new hypothyroidism following HSCT. This is similar to the rate of thyroid disease acquired within the first 3–5 years following HSCT in other studies conducted with non-RIC regimens13, 14, which is a novel finding and suggests similar risks for thyroid disease between RIC and non-RIC regimens.
Vitamin D deficiency is a widespread issue in the general population in the United States, with reports in the literature suggesting prevalence of 40–50% in the pediatric population 15. In contrast, prevalence in our cohort was higher, with 73% of our patients demonstrating deficiency. Vitamin D deficiency may reflect decreased dietary intake or impaired absorption during HSCT, decreased exercise and/or sun exposure, decreased renal function or increased steroid use in our cohort. While the numbers of patients studied in our cohort are limited, low vitamin D concentrations may be contributing to the decreased bone mineral density by densitometry observed in a few members of our cohort. Additionally, the literature supports an important role for vitamin D in immune function, Therefore, vitamin D deficiency may potentially influence immune reconstitution, GVHD, and/or survival after HSCT, and in our current practice, levels are maintained in the normal range during and after HSCT 16, 17.
The current analysis demonstrates that endocrine complications remain a significant issue after pediatric HSCT despite the use of reduced intensity chemotherapy regimens and avoidance of radiation. This is particularly disappointing in our cohort of patients with non-malignant disorders in whom we anticipate no further risk of disease after HSCT. Prospective follow-up is necessary for the detection of these disorders and in order to improve understanding of how endocrine late effects manifest in this population. Our group proposed recommendations for comprehensive post-transplant endocrine function monitoring (Table 3). Multi-disciplinary follow-up after HSCT, including the involvement of a pediatric endocrinologist, is crucial to maximize long-term outcomes. Our current practice now includes collaborative long-term follow-up in comprehensive joint HSCTand endocrine clinics. Likewise, comparison is needed of post-HSCT endocrine complications after reduced intensity versus standard myeloablative protocols.
Table 3.
Endocrinology monitoring before and after hematopoietic cell transplantation (1–5)
| Clinical | Yearly Laboratory | |
|---|---|---|
| Endocrinology visit | ||
| Growth | Yearly height (every 6 mo under age 10y) | |
| Weight | Yearly weight (every 6 mo under age 10y) | |
| Adrenal | After prolonged corticosteroid usage, slow terminal tapering of steroids; stress doses of steroids during acute illness | Consider ACTH stimulation testing |
| Thyroid | Growth rate | Free T4, TSH |
| Bone | Comprehensive nutrition history and monitoring, with attention to milk intake, vitamins, exercise | 25OH-vitamin D |
| DXA at one year after HSCT, then every 5 years, more often if abnormal | ||
| Gonadal | Pubertal staging History for development of body odor, acne, facial hair, breast size, pubic hair, testes size, menses, erections, libido, sexual function, hot flashes |
Child—x-ray for bone age if early or late Female—LH, FSH, Estradiol, AMH Male—LH, FSH, Testosterone, Inhibin B |
Thyroxine, T4; thyroid-stimulating hormone, TSH; dual-energy X-ray absorptiometry, DXA; luteinizing hormone, LH; follicle stimulating hormone, FSH; anti-mullerian hormone, AMH
1. Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Revista Brasileira de Hematologia e Hemoterapia 2012;34:109–133.
2. BM B, SM S. Endocrine late effects after bone marrow transplant. Br J Haematol 2002;118:58–66.
3. Sanders JE, Hoffmeister PA, Woolfrey AE, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years’ experience; 2009.
4. Ranke MB, Schwarze CP, Dopfer R, et al. Late effects after stem cell transplantation (SCT) in children--growth and hormones. Bone Marrow Transplant 2005;35 Suppl 1:S77–81.
5. Sanders JE. Endocrine complications of high-dose therapy with stem cell transplantation. Pediatric Transplantation 2004;8 Suppl 5:39–50.
Acknowledgments
We thank Cyrus Yang and Ryan Judd for their assistance with data collection.
Footnotes
Conflict of interest.
The authors declare no conflict of interest.
References
- 1.Sanders JE. Endocrine complications of high-dose therapy with stem cell transplantation. Pediatric Transplantation. 2004;8(Suppl 5):39–50. doi: 10.1111/j.1398-2265.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker K, Ness K, Weisdorf D, Francisco L, Sun C, Forman S, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Leukemia. 2010;24(12):2039–47. doi: 10.1038/leu.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afify Z, Shaw P, Clavano-Harding A, Cowell C. Growth and endocrine function in children with acute myeloid leukaemia after bone marrow transplantation using busulfan/cyclophosphamide. Bone Marrow Transplant. 2000;25(10):1087–92. doi: 10.1038/sj.bmt.1702384. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak CC, Gracia CR, Sanders JE, Cheng EY, Baker KS, Pulsipher MA, et al. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: endocrine challenges-thyroid dysfunction, growth impairment, bone health, & reproductive risks. Biol Blood Marrow Transplant. 2011;17(12):1725–38. doi: 10.1016/j.bbmt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh RA, Rao MB, Gefen A, Bellman D, Mehta PA, Khandelwal P, et al. Experience with Alemtuzumab, Fludarabine, and Melphalan Reduced-Intensity Conditioning Hematopoietic Cell Transplantation in Patients with Nonmalignant Diseases Reveals Good Outcomes and That the Risk of Mixed Chimerism Depends on Underlying Disease, Stem Cell Source, and Alemtuzumab Regimen. Biology of Blood and Marrow Transplantation. 2015;21(8):1460–1470. doi: 10.1016/j.bbmt.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding S, Schoenmakers I, Jones K, Koulman A, Prentice A, Volmer D. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Analytical and bioanalytical chemistry. 2010;398(2):779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen A, Bekassy AN, Gaiero A, Faraci M, Zecca S, Tichelli A, et al. Endocrinological late complications after hematopoietic SCT in children. Bone Marrow Transplant. 2008;41(Suppl 2):S43–8. doi: 10.1038/bmt.2008.54. [DOI] [PubMed] [Google Scholar]
- 8.Cohen A, Rovelli A, Bakker B, Uderzo C, van Lint M-T, Esperou H, et al. Final Height of Patients Who Underwent Bone Marrow Transplantation for Hematological Disorders During Childhood: A Study by the Working Party for Late Effects-EBMT. Blood. 1999;93(12):4109–4115. [PubMed] [Google Scholar]
- 9.Patterson BC, Chen Y, Sklar CA, Neglia J, Yasui Y, Mertens A, et al. Growth Hormone Exposure as a Risk Factor for the Development of Subsequent Neoplasms of the Central Nervous System: A Report From the Childhood Cancer Survivor Study. The Journal of Clinical Endocrinology & Metabolism. 2014;99(6):2030–2037. doi: 10.1210/jc.2013-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders J, Hawley J, Levy W, Gooley T, Buckner C, Deeg H, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87(7):3045–3052. [PubMed] [Google Scholar]
- 11.Borgström B, Bolme P. Thyroid function in children after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1994;13(1):59–64. [PubMed] [Google Scholar]
- 12.Boulad F, Bromley M, Black P, Heller G, Sarafoglou K, Gillio A, et al. Thyroid dysfunction following bone marrow transplantation using hyperfractionated radiation. Bone Marrow Transplant. 1995;15(1):71–6. [PubMed] [Google Scholar]
- 13.Jung MH, Cho KS, Lee JW, Chung NG, Cho B, Suh BK, et al. Endocrine Complications after Hematopoietic Stem Cell Transplantation during Childhood and Adolescence. Journal of Korean Medical Science. 2009;24(6):1071–1077. doi: 10.3346/jkms.2009.24.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders JE, Hoffmeister PA, Woolfrey AE, Carpenter PA, Storer BE, Storb RF, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years’ experience. Blood. 2009;113(2):306–308. doi: 10.1182/blood-2008-08-173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 16.Chun RF, Adams JS, Hewison M. Back to the future: a new look at ‘old’ vitamin D. The Journal of endocrinology. 2008;198(2):261–9. doi: 10.1677/JOE-08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. The Journal of steroid biochemistry and molecular biology. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]