Abstract
BACKGROUND
Emerging evidence indicates that the association between depression and subsequent cardiovascular events is largely mediated by health behaviors. However, it is unclear whether depression is the cause or the consequence of poor health behaviors.
PURPOSE
To examine prospective, bidirectional relationships of depressive symptoms with behavioral and lifestyle factors among patients with coronary heart disease.
METHODS
Depressive symptoms and lifestyle behaviors (physical activity, medication adherence, body mass index, waist-to-hip ratio, sleep quality, and smoking status) were assessed at baseline and 5 years later among a prospective cohort of 667 patients with stable coronary heart disease.
RESULTS
Greater depressive symptoms at baseline predicted poorer lifestyle behaviors 5 years later (less physical activity, lower medication adherence, higher body mass index, higher waist-to-hip ratio, worse sleep quality, and smoking). After adjustment for demographics, cardiac disease severity, comorbidity, and baseline lifestyle behaviors, depressive symptom severity remained predictive of subsequent worsening of physical activity (beta = −0.08; 95% CI= −0.16, −0.01; p = 0.03), medication adherence (beta = −0.16; 95% CI= −0.24, −0.08; p < 0.001), and sleep quality (beta = −0.19; 95% CI = −0.27, −0.11; p < 0.001). Baseline lifestyle behaviors also predicted 5-year change in depressive symptoms, although the associations were attenuated after adjustment for baseline depressive symptoms and covariates.
CONCLUSIONS
Among patients with coronary heart disease, depressive symptoms were linked to a range of lifestyle risk factors and predicted further declines in physical activity, medication adherence, and sleep quality.
Keywords: depression, coronary heart disease, health behaviors, physical activity, medication adherence, sleep quality
Introduction
Depression is an independent risk factor for the development of incident cardiovascular disease (CVD) in healthy populations and adverse outcomes among patients with existing CVD (1–3). Recent evidence indicates that poor health behaviors, particularly physical inactivity, may be the primary mechanisms whereby depression leads to an increased risk of subsequent cardiovascular events (4–8). However, the direction of association between depression and health behaviors, or modifiable lifestyle factors in general, is unclear.
Previous studies have tested unidirectional relationships between depression and lifestyle behaviors and have provided support for both directions of association. A substantial body of literature has shown that lifestyle behaviors predict subsequent depression. For example, smoking and obesity have been associated with a greater risk of clinically-significant depressive symptoms 4 to 17 years later (9–11). Multiple longitudinal studies have linked physical activity to a reduced likelihood of subsequent depression (12), whereas sleep disturbances increase the risk of incident depression (13). Furthermore, randomized trials have demonstrated that exercise is as effective as sertraline for alleviating depressive symptoms (14–16).
Conversely, accumulating evidence suggests that depression is a risk factor for the development of poor health behaviors. A review of 11 longitudinal studies reported robust associations between depression and subsequent physical inactivity (17). Depressive symptoms have been linked to a cluster of behavioral risk factors among patients post-myocardial infarction, including lower adherence to recommendations for diet, exercise, medication regimens, stress reduction, and socializing (18). Other studies indicate that depressive symptoms or a history of major depression elevates the risks of high body mass index (BMI), abdominal obesity, and regular smoking (19–21). Although these studies collectively suggest that bidirectional relationships likely exist between depression and lifestyle behaviors, few studies have formally tested both directions of the association.
We have previously shown in the Heart and Soul Study—a prospective cohort study of 1024 patients with stable coronary heart disease (CHD)—that depressive symptoms were associated with a higher rate of subsequent cardiovascular events, and this association was largely explained by poor health behaviors (4). However, to our knowledge, whether depressive symptoms are the cause or the result of poor lifestyle behaviors has not been examined in patients with CHD. We therefore sought to evaluate the longitudinal, bidirectional relationships of depressive symptoms with behavioral and lifestyle factors (physical activity, medication adherence, body mass index, waist-to-hip ratio [WHR], sleep quality, and smoking) across 5 years in the Heart and Soul Study.
Methods
Participants
The Heart and Soul Study is a prospective cohort study designed to determine how depression and other psychological factors influence cardiovascular outcomes in patients with stable CHD (4). Patients with documented stable CHD were recruited from the San Francisco Veterans Affairs Medical Center, the Veterans Affairs Palo Alto Health Care System, 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco. To be eligible, patients needed to have at least one of the following: a history of myocardial infarction, angiographic evidence of at least 50% stenosis in one or more coronary vessels, evidence of exercise-induced ischemia via treadmill or nuclear testing, a history of coronary revascularization, or coronary artery disease previously noted by an internist or cardiologist. Patients were excluded from the study if they considered themselves incapable of walking 1 block, if they experienced acute coronary syndrome in the prior 6 months, or if they planned to move out of the local area within 3 years.
A total of 1024 participants were enrolled between September 11, 2000 and December 20, 2002. Five years later, 829 participants were alive and 667 (80% of survivors) returned for a 5-year examination. Thus, our final sample was comprised of 667 participants with follow-up data. Compared to participants who did not survive or who dropped out of the study, those with follow-up data tended to be younger, had higher income, were less likely to smoke, and reported less depressive symptoms and more physical activity at baseline. Participants at follow-up were also relatively less likely to have had a stroke, heart failure, or diabetes mellitus. We controlled for these demographic and clinical differences in the analyses. There were no differences in medication adherence, BMI, WHR, or sleep quality based on survival/dropout status. All participants provided written informed consent, and procedures were approved by the appropriate institutional review boards.
Measures
Depressive symptoms and lifestyle behaviors were assessed at baseline and again 5 years later.
Depressive symptoms
We evaluated depressive symptoms using the 9-item Patient Health Questionnaire (PHQ-9) (22), which assessed the frequency of depressive symptoms corresponding to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for depression (23). Participants reported how often they experienced each of nine depressive symptoms over the past two weeks using the following answer choices: 0 (not at all), 1 (several days), 2 (more than half the days), and 3 (nearly every day). Scores were summed, for a total ranging between 0 and 27. The PHQ-9 has been shown to be a reliable and valid measure for assessing the severity of depressive symptoms in patients with CHD (22,24).
Lifestyle behaviors
Physical activity
Participants were asked, “Which of the following statements best describes how physically active you have been during the last month, that is, done activities such as 15–20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” Answers were scored as follows: 0 = not at all active, 1 = a little active, 2 = fairly active, 3 = quite active, 4 = very active, and 5 = extremely active. Point values ranged from 0 to 5, with higher scores indicating greater physical activity. This one-item measure of physical activity was predictive of subsequent inflammation, insulin resistance, cardiovascular events, and mortality in this cohort (4,25). Self-reported physical activity has been shown to be a valid and reliable assessment of physical activity (26–28).
Medication adherence
Medication adherence was measured by the question, “Overall, in the past month, how often did you take your medications as the doctor prescribed?” Answers were scored as follows: 0 = less than half of the time (<50%), 1 = about half of the time (50%), 2 = most of the time (75%), 3 = nearly all of the time (90%), and 4 = all of the time (100%). Scores ranged from 0 to 4, with higher scores indicating greater medication adherence. Previous research in the Heart and Soul Study has linked this measure of medication nonadherence to concurrent depression (29) and prospective risk of cardiovascular events (30). Self-reported medication adherence is moderately-to-highly concordant with objective measures of adherence (e.g., monitoring using electronic pill caps) (31).
BMI and central adiposity
Diet was not measured in the Heart and Soul Study. BMI and WHR were used instead as indirect indicators of diet and physical activity. Participant height and weight were measured by study personnel and used to calculate BMI (kg/m2). In addition, we examined WHR due to research suggesting that WHR and other indices of central adiposity may be better than BMI for predicting CVD risk and events (32,33). WHR was calculated from measures of waist circumference and hip circumference. Because of sex differences in WHR, all analyses involving WHR were adjusted for sex.
Sleep quality
Participants responded to the following item from the Pittsburgh Sleep Quality Index (PSQI), “During the last month, how would you rate your sleep quality overall?” Possible response choices were: Very bad, fairly bad, good, fairly good, or very good. Responses were coded such that higher scores referred to better sleep quality. This single item (which comprises the entire subjective sleep quality component of the full PSQI scale) has demonstrated high test-retest reliability, convergent and discriminant validity, and the highest correlation with the PSQI global score than the other subscales (34,35).
Smoking
We determined participants’ smoking status based on their responses to a single question: “Do you currently smoke cigarettes?” Self-reported smoking has been shown to be comparable to biochemical assessments of smoking (36).
Covariates
Demographics and medical history were obtained by self-report questionnaire at baseline. To assess baseline cardiac disease severity, resting echocardiography and an exercise treadmill test were performed. Left ventricular ejection fraction was calculated as (end diastolic volume – end systolic volume)/end diastolic volume. Participants were categorized as having diastolic dysfunction if their mitral inflow ratio of peak early-to-late diastolic filling velocity was greater than 0.75 and if their velocity time integral in the pulmonary vein was greater during diastole than during systole (37). Inducible ischemia was defined as the presence of new echocardiographic wall motion abnormalities at peak exercise during treadmill testing that were not present at rest (38).
Data analysis
First, we analyzed the bivariate associations between baseline participant characteristics (demographics, comorbid conditions, and cardiac disease severity) and baseline depressive symptoms, using the established cutoff of PHQ-9 score ≥ 10 to indicate the presence of clinically significant depressive symptoms (22). We used t-tests for continuous variables and chi-squared tests for categorical variables. Participant characteristics that were associated with depressive symptoms at p < 0.10 were subsequently included as covariates in the multivariate analyses.
Next, we used multivariate linear regression to test baseline depressive symptoms as a predictor of change in lifestyle behaviors (i.e., depressive symptoms predicting Year 5 lifestyle behaviors, while adjusting for baseline lifestyle behaviors). Depressive symptoms and lifestyle behaviors were analyzed as continuous variables. Due to differences in measurement scales across the lifestyle behaviors, standardized β coefficients were used to represent standard deviation change in lifestyle behaviors. A series of three models were run for each lifestyle factor. Model 1 was unadjusted, except analyses with WHR were adjusted for sex. Model 2 was adjusted for age, sex, White race, education, and income. Model 3 included additional covariates for left ventricular ejection fraction, comorbid conditions (heart failure, stroke, and diabetes mellitus), and the corresponding baseline lifestyle behavior. Furthermore, based on associations between participant characteristics and specific lifestyle behaviors (see Supplementary Table 1), we also adjusted for history of revascularization in analyses of physical activity and medication adherence, as well as adjusting for hypertension in analyses of BMI and WHR. Because smoking status was assessed as a dichotomous variable, analyses involving smoking status were conducted using logistic regression.
Finally, to address the possible bidirectional nature of the relationships between depressive symptoms and lifestyle behaviors, we used multivariate linear regression to evaluate the relationship between each lifestyle behavior at baseline and subsequent worsening of depressive symptoms over 5 years. Unstandardized B coefficients were used to represent mean change in PHQ-9 score. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline participant characteristics
Of the 667 participants with stable CHD included in our analyses, 116 (17%) had depressive symptoms (indicated by a PHQ-9 score ≥ 10). The prevalence of depression in this sample was comparable to that of patients hospitalized with acute myocardial infarction (15–20%) (39,40). Less is known about the prevalence of depression in outpatients with CHD. In the National Health Interview Survey of 30,801 adults, the 12-month prevalence of major depression was 9.3% among patients with coronary heart disease and 4.8% in individuals without chronic medical conditions (41).
Participants with depressive symptoms were less likely to have graduated high school and were more likely to be younger, female, and to have an income below $20,000/year (Table 1). Those with depressive symptoms were also more likely to have congestive heart failure and lower left ventricular ejection fraction. Depressive symptoms were not associated with race, other comorbid conditions (hypertension, myocardial infarction, stroke, revascularization, or diabetes mellitus), or other measures of cardiac disease severity (the presence of diastolic dysfunction or inducible ischemia).
Table 1.
Baseline characteristics of 667 participants with stable coronary heart disease, by depressive symptomsa
| Participant characteristic | Depressive Symptoms (N = 116) |
No Depressive Symptoms (N = 551) |
P-value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) years | 62.6 (11.0) | 66.8 (9.9) | <0.001 |
| Male sex, No. (%) | 87 (75.0) | 462 (83.9) | 0.023 |
| White, No. (%) | 68 (58.6) | 330 (59.9) | 0.80 |
| High school graduate, No. (%) | 94 (81.0) | 490 (89.3) | 0.014 |
| Income < $20,000/year, No. (%) | 78 (67.2) | 230 (42.0) | <0.001 |
| Comorbid conditions, No. (%) | |||
| Hypertension | 82 (70.7) | 382 (69.3) | 0.77 |
| Myocardial infarction | 63 (54.8) | 283 (51.5) | 0.52 |
| Stroke | 14 (12.2) | 70 (12.7) | 0.87 |
| Revascularization | 65 (56.5) | 338 (61.3) | 0.34 |
| Congestive heart failure | 27 (23.3) | 71 (13.0) | 0.004 |
| Diabetes mellitus | 32 (27.6) | 125 (22.7) | 0.26 |
| Cardiac disease severity | |||
| Left ventricular ejection fraction, mean (SD) % | 60.6 (9.4) | 62.9 (8.7) | 0.014 |
| Diastolic dysfunction, No. (%) | 10 (10.1) | 50 (9.9) | 0.95 |
| Inducible ischemia, No. (%) | 19 (18.5) | 100 (19.3) | 0.85 |
Patient Health Questionnaire score ≥ 10 versus < 10
Descriptive statistics and correlations
Descriptive statistics for change in depressive symptoms and lifestyle behaviors are provided in Supplementary Table 2. In bivariate correlations (Table 2), baseline depressive symptoms were associated with poorer lifestyle behaviors at Year 5: less physical activity, lower medication adherence, poorer sleep quality, higher WHR (partialed for sex) and BMI, and smoking. Based on Cohen’s guidelines for interpreting Pearson r effect sizes (42), the longitudinal correlations between baseline depressive symptoms and Year 5 lifestyle behaviors were primarily small-to-medium in size (i.e., r = 0.10 – 0.30), whereas there was a medium-to-large effect of −0.39 for baseline depressive symptoms and subsequent sleep quality.
Table 2.
Correlations between depressive symptoms and lifestyle behaviors at baseline and at Year 5
| Baseline Assessments | Mean (SD) or N (%) |
Range | Year 5 Assessments | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Depressive symptoms |
Physical activity |
Medication adherence |
Body mass index |
Waist-to- hip ratioa |
Sleep quality |
Current smoker |
|||
| Depressive symptoms | 4.75 (5.26) | 0 – 27 | 0.66*** | −0.22*** | −0.20*** | 0.14*** | 0.10* | −0.39*** | 0.13*** |
| Physical activity | 2.53 (1.66) | 0 – 5 | −0.24*** | 0.51*** | 0.11** | −0.16*** | −0.10** | 0.12** | −0.12** |
| Medication adherence | 3.56 (0.76) | 0 – 4 | −0.13** | 0.01 | 0.34*** | −0.06 | 0.07† | 0.09* | −0.06 |
| Body mass index | 28.54 (5.07) | 17.56 – 56.05 | 0.13** | −0.19*** | 0.01 | 0.87*** | 0.29*** | −0.05 | −0.07† |
| Waist-to-hip ratioa | 0.95 (0.08) | 0.73 – 1.25 | 0.07† | −0.06 | 0.05 | 0.27*** | 0.56*** | 0.00 | −0.03 |
| Sleep quality | 2.20 (1.09) | 0 – 4 | −0.33*** | 0.12** | 0.06 | −0.06 | −0.13*** | 0.49*** | −0.08* |
| Current smoker | 111 (17%) | 0 (No), 1 (Yes) | 0.16*** | −0.06 | −0.09* | −0.10* | −0.01 | −0.04 | 0.78*** |
p ≤ .001,
p ≤ .01,
p ≤ .05,
p ≤ 0.10
Correlations between depressive symptoms and waist-to-hip ratio were partialed for the effects of sex.
Baseline depressive symptoms and 5-year change in lifestyle behaviors
Baseline depressive symptoms predicted subsequent poor lifestyle behaviors in regression analyses (Figure 1). After 5 years of follow-up (Table 3), each standard deviation (SD) or 5.3-point increase in baseline depressive symptoms was associated with declines in physical activity, worsening medication adherence, increases in BMI and sex-adjusted WHR, and poorer sleep quality. Baseline depressive symptoms also predicted 43% greater odds of being a smoker at follow-up. After full adjustment for demographics, ejection fraction, comorbid conditions, and baseline lifestyle behaviors, each SD increase in baseline depressive symptoms was associated with 8% of a SD decrease in physical activity 5 years later (p = 0.03), 16% of a SD decrease in medication adherence (p < 0.001), and 19% of a SD decrease in sleep quality (p < 0.001). The associations of baseline depressive symptoms with 5-year WHR and smoking were reduced to non-significance after accounting for demographics, whereas the association with subsequent BMI was explained by comorbid conditions.
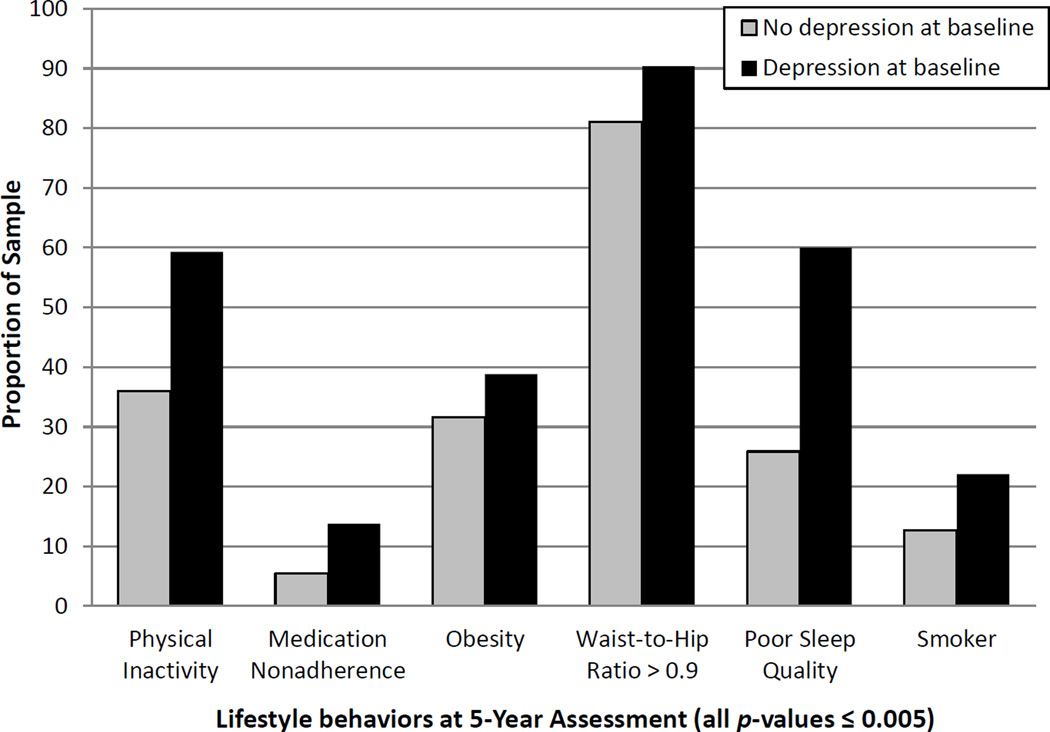
Figure 1. Poor lifestyle behaviors at Year 5 by baseline depressive symptoms (N = 667).
For illustrative purposes, baseline depressive symptoms were dichotomized by Patient Health Questionnaire-9 score (< 10 for no depression versus ≥ 10 for depression). In unadjusted logistic regression analyses, severity of depressive symptoms at baseline was associated with poor health behaviors 5 years later: being “not at all active” or “a little active” (4); taking medications as prescribed 75% or less of the time (29); obesity as defined by body mass index ≥ 30 kg/m2 (49), sleep quality rated as “fairly bad” or “very bad” (70), and smoking (all p-values ≤ 0.005). Adjusting for sex, baseline depressive symptoms also predicted high waist-to-hip ratio at follow-up, based on the World Health Organization’s recommended cutoff of 0.9 for men (49).
Table 3.
Association between baseline depressive symptoms, entered per standard deviation (SD) [5.3-point] increase, and 5-year change in lifestyle behaviors
| Standardized β (95% CI)b | OR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Modela | Physical activity | Medication adherence | Body Mass Index | Waist-to-hip ratio | Sleep quality | Smoker (vs. Non-smoker) |
| Model 1 | −0.22 (−0.30, −0.14)*** | −0.23 (−0.31, −0.15)*** | 0.15 (0.07, 0.22)*** | 0.08 (0.004, 0.15)* | −0.39 (−0.46, −0.31)*** | 1.43 (1.17, 1.74)*** |
| Model 2 | −0.20 (−0.28, −0.12)*** | −0.21 (−0.29, −0.12)*** | 0.10 (0.02, 0.18)* | 0.06 (−0.01, 0.14) | −0.37 (−0.45, −0.29)*** | 1.16 (0.92, 1.45) |
| Model 3 | −0.08 (−0.16, −0.01)* | −0.16 (−0.24, −0.08)*** | 0.01 (−0.04, 0.05) | 0.02 (−0.05, 0.09) | −0.19 (−0.27, −0.11)*** | 1.26 (0.89, 1.78) |
p ≤ 0.001,
p ≤ 0.01,
p ≤ 0.05
Model 1 was unadjusted, except the analysis for waist-to-hip ratio was adjusted for sex. Model 2 adjusted for demographics: Age, sex, White race, high school graduate, and annual household income < $20,000. Model 3 adjusted for demographics, left ventricular ejection fraction, heart failure, stroke, diabetes mellitus, and baseline lifestyle behavior. Analyses for physical activity and medication adherence also adjusted for history of revascularization, whereas analyses for BMI and WHR included additional adjustment for hypertension.
The standardized β coefficient represents SD change in each lifestyle behavior associated with a 1-SD difference in baseline depressive symptoms (i.e., 5.3 points on the Patient Health Questionnaire-9). The standard deviations for the lifestyle behaviors at Year 5 were 1.74 points for physical activity (0–5 scale), 0.74 points for medication adherence (0–4 scale), 5.44 kg/m2 for BMI, 0.09 for WHR, and 1.22 points for sleep quality (0–4 scale).
Baseline lifestyle behaviors and 5-year change in depressive symptoms
In unadjusted models, poorer baseline lifestyle behaviors (relatively less physical activity, lower medication adherence, higher BMI, poorer sleep quality, and smoking) predicted worsening of depressive symptoms across 5 years (Table 4). Higher baseline WHR was also marginally predictive of 5-year increases in depressive symptoms (p = 0.09). However, baseline lifestyle behaviors were no longer associated with subsequent changes in depressive symptoms after adjusting for baseline depressive symptoms, demographics, ejection fraction, and comorbid conditions.
Table 4.
Association between baseline lifestyle behaviors and 5-year change in depressive symptoms
| Mean Increase in Depressive Symptoms | ||
|---|---|---|
| Predictora | Bb (95% CI) | P-value |
| Less Physical Activity (Per 1-Unit Decrease) | ||
| Model 1 | 0.742 (0.509, 0.976) | <0.001 |
| Model 2 | 0.592 (0.362, 0.822) | <0.001 |
| Model 3 | 0.129 (−0.068, 0.325) | 0.20 |
| Lower Medication Adherence (Per 1-Unit Decrease) | ||
| Model 1 | 0.814 (0.284, 1.343) | 0.003 |
| Model 2 | 0.659 (0.145, 1.174) | 0.01 |
| Model 3 | 0.206 (−0.218, 0.630) | 0.34 |
| Higher Body Mass Index (Per 1-Unit Increase) | ||
| Model 1 | 0.130 (0.054, 0.206) | <0.001 |
| Model 2 | 0.102 (0.026, 0.178) | 0.009 |
| Model 3 | 0.037 (−0.025, 0.099) | 0.25 |
| Higher Waist-to-Hip Ratio (Per 1-Unit Increase) | ||
| Model 1 | 4.921 (−0.713, 10.556) | 0.09 |
| Model 2 | 4.846 (−0.628, 10.321) | 0.08 |
| Model 3 | 1.074 (−3.518, 5.666) | 0.65 |
| Poorer Sleep Quality (Per 1-Unit Decrease) | ||
| Model 1 | 1.474 (1.126, 1.821) | <0.001 |
| Model 2 | 1.348 (1.012, 1.684) | <0.001 |
| Model 3 | 0.201 (−0.113, 0.515) | 0.21 |
| Smoker (vs. Non-Smoker) | ||
| Model 1 | 2.306 (1.240, 3.371) | <0.001 |
| Model 2 | 1.292 (0.201, 2.384) | 0.02 |
| Model 3 | 0.788 (−0.104, 1.680) | 0.08 |
Model 1 was unadjusted, except the analysis for waist-to-hip ratio was adjusted for sex. Model 2 adjusted for demographics: Age, sex, White race, high school graduate, and annual household income < $20,000. Model 3 adjusted for demographics, left ventricular ejection fraction, heart failure, stroke, diabetes mellitus, and baseline depressive symptoms as a continuous variable. Analyses for physical activity and medication adherence also adjusted for history of revascularization, whereas analyses for BMI and WHR included additional adjustment for hypertension.
The unstandardized B coefficient refers to the mean change in depressive symptoms (Patient Health Questionnaire-9 score) across the 5-year period, for each 1-unit difference in baseline lifestyle behaviors or for smokers vs. non-smokers.
Discussion
Previous research has linked depression with modifiable lifestyle behaviors that increase risk of adverse cardiovascular outcomes, but the extent to which depression is the cause or the consequence of lifestyle behaviors is unclear. In the current study, we evaluated the bidirectional associations between depressive symptoms and multiple lifestyle behaviors across five years among 667 patients with stable CHD. The findings support both directions of association, although evidence was stronger for depressive symptoms as a predictor of subsequent health behavior change. Specifically, depressive symptoms were linked to less physical activity, lower medication adherence, higher BMI and WHR, poorer sleep quality, and smoking five years later. After accounting for baseline lifestyle behaviors, demographics, cardiac disease severity, and comorbid conditions, depressive symptoms remained independently predictive of 5-year decline in physical activity, medication adherence, and sleep quality. In contrast, the associations between baseline lifestyle behaviors and 5-year change in depressive symptoms were not significant after accounting for baseline depressive symptoms.
Based on prior studies, cardiac patients with depression are known to be relatively less likely to engage in cardiovascular prevention behaviors, including physical activity (17), medication adherence (18,43), smoking cessation (18,44,45), and cardiac rehabilitation (45–47). Our study extends this literature by demonstrating that baseline depressive symptoms were not only associated with the presence of poor lifestyle behaviors 5 years later, but also predicted declines in physical activity, medication adherence, and sleep quality. The effect sizes were mostly small-to-medium in magnitude, yet modest effects can have substantial implications when considered at the population level or as risks accrue across a lifetime. Given the importance of psychosocial factors for determining CVD risk and mortality (3,48), these findings suggest that depressive symptoms may serve as critical targets in efforts to improve health behaviors among patients with CHD.
We found that depressive symptoms predicted some behaviors but not others. Smoking was strongly determined by younger age and indicators of socioeconomic disadvantage, including being an ethnic minority and having low income. WHR is known to vary systematically by age, sex, and ethnicity (49); thus, it was not surprising that depressive symptoms were no longer predictive of WHR after accounting for demographic factors. As expected, BMI was confounded with hypertension and diabetes; adjusting for comorbid conditions eliminated the association between baseline depressive symptoms and subsequent BMI. Our analyses were conservative and perhaps underestimated the links between depressive symptoms and anthropometric measures. For example, it is possible that depressive symptoms prospectively influence obesity, thereby increasing risk for diabetes and hypertension (50).
Prospective studies have shown that obesity, sleep difficulties, and physical activity predict the likelihood of developing major depression (9,10,12,13,51,52). We therefore expected baseline lifestyle behaviors to be significantly predictive of subsequent changes in depressive symptoms, but these associations were explained by baseline depressive symptoms. Our findings may have differed from other prospective studies because many prior studies focused on predicting incident major depression and thus excluded participants with depression at baseline (12,13,52). In contrast, our study examined changes in depressive symptom severity. Consistent with our findings, a meta-analysis showed that obesity was more predictive of subsequent major depression than of increases in depressive symptoms (52). In addition, a 10-year study of adults with depression found that physical activity and depressive symptoms were concurrently associated on each of four waves of assessment, but physical activity did not predict future changes in depressive symptoms (53). Furthermore, our sample was composed of middle-aged and older adults with chronic CHD, rather than younger, healthier participants as in prior research (9,10,54). The effects of lifestyle behaviors on depressive symptoms may differ in sicker patients with chronic medical conditions than in non-clinical populations (53).
Although depressive symptoms and lifestyle behaviors are commonly thought to have reciprocal influences, the current study is only one of several (and perhaps the first with cardiac patients) that have formally tested bidirectional relationships. Previous investigations of bidirectional associations have produced mixed results: bidirectional associations have linked depressive symptoms to high BMI (55), smoking (54), and insomnia (56), but others have found no long-term relationships between depression and physical activity (53) or only a unidirectional association leading from obesity to subsequent depression (51). The mixed findings may be due to differences in lifestyle behaviors, samples (e. g., community-based older adults, young adults, and persons with major depression), follow-up periods spanning from 1 to 10 years, and methodology (e.g., some studies did not adjust for baseline values or physical health).
A number of potential physiological and psychosocial pathways have been proposed to explain the links between depressive symptoms and lifestyle risk factors. There is compelling evidence supporting the role of inflammatory responses in the pathophysiology of depression (57). Physical inactivity, inadequate sleep, and other unhealthy behaviors can lead to elevated inflammation (25) and thus contribute to the development and exacerbation of depressive symptoms. On the other hand, specific symptoms of depression—including loss of interest, hopelessness, fatigue, and trouble remembering—may reduce one’s motivation or ability to maintain physical activity and medication regimens. Patients with elevated depressive symptoms may also have low cardiac self-efficacy (i.e., less confidence in their ability to manage their health), thereby resulting in poor health behaviors and worse clinical outcomes (58). In addition, depression is confounded with other psychosocial factors, such as low social support, that increase the risk of poor health behaviors. Future studies should seek to better understand how depression leads to worse lifestyle behaviors, and vice versa, particularly for chronic conditions in which optimal lifestyle behaviors are critical for disease management.
Evidence from other populations suggests that depression treatment can improve health behaviors, such as antiretroviral medication adherence among persons living with HIV/AIDS (59). It is unclear, however, whether treatment of depressive symptoms will help promote a healthy lifestyle in patients with cardiovascular disease. Randomized clinical trials of antidepressant treatment and psychotherapy for cardiac patients with depression have shown only modest effects on depressive symptoms but no improvements in cardiovascular outcomes (60–62). By contrast, exercise interventions have been effective for ameliorating depressive symptoms, improving cardiovascular biomarkers, and reducing the risk of hospitalizations and mortality (14,63,64). Although our observational study did not find an association between baseline lifestyle behaviors and subsequent changes in depressive symptoms, programs targeting comprehensive lifestyle changes—particularly cardiac rehabilitation—are known to confer benefits for both mental well-being (65) and physical health (65–67).
Several limitations should be considered when interpreting the findings of this study. First, causal conclusions cannot be drawn due to the observational nature of the study. We were careful in adjusting for potential confounding variables, but it remains possible that other factors were responsible for the prospective associations between depressive symptoms and lifestyle behaviors. Second, this study lacked direct measures of diet and sleep hygiene. BMI, WHR, and sleep quality—although generally seen as modifiable lifestyle factors—are not health behaviors per se but the results of health behaviors. Third, with the exception of BMI and WHR, lifestyle behaviors were measured by self-report using single items. The items for physical activity and medication adherence predicted future cardiovascular events in this cohort (4,30), yet the findings would be strengthened if more comprehensive, psychometrically-validated scales were used. Furthermore, self-report measures are susceptible to recall or response biases (68). To the extent that depression influences self-report of health behaviors, the discordance between subjective and objective measures could result in over- or under-estimated effects. For example, a meta-analysis found that the association between depression and medication nonadherence was weaker when adherence was measured using pharmacy refill records compared to self-report and electronic cap measures (69). It is unclear whether self-reported adherence is overestimated or whether it captures behaviors (e.g., incorrectly-timed doses or not ingesting medications) that cannot be determined from objective measures. Further work is needed to compare the links between depression and health behaviors using different types of self-report and objective measures. Finally, although this sample was racially diverse and had a range of diagnoses, women and more severe cases of CHD were underrepresented. Only those participants who survived and were healthy enough to return for the 5-year exam were included in this analysis. Thus, the findings may not generalize to less healthy patients or to other populations.
In summary, psychological and behavioral factors have long been implicated in the development and prognosis of CHD. This study demonstrates that depressive symptoms predict long-term worsening of health behaviors that are critical for disease management. Likewise, poor health behaviors and high BMI are prospectively linked to increases in depressive symptoms, although the associations are relatively less robust. These bidirectional findings are especially important given the growing evidence that lifestyle behaviors may be largely responsible for the adverse cardiovascular events and mortality associated with depression. Considering the intricate link between depressive symptoms and lifestyle behaviors, future work should evaluate whether depression treatment leads to downstream changes in health behaviors and improvements in cardiovascular outcomes.
Supplementary Material
Acknowledgments
Funding: Nancy Sin was supported by T32AG000212 and F32AG048698 from the National Institute on Aging. The Heart and Soul Study was funded by the Department of Veteran Affairs (Epidemiology Merit Review Program), Washington, DC; grant R01 HL-079235 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland; the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), Princeton, New Jersey; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, New York; and the Ischemia Research and Education Foundation, South San Francisco, California.
Footnotes
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: The authors declare that they have no conflict of interest.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327–354. doi: 10.1146/annurev-clinpsy-050212-185526. [DOI] [PubMed] [Google Scholar]
- 2.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 3.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a Risk Factor for Mortality in Patients With Coronary Heart Disease: A Meta-analysis. Psychosom Med. 2004;66(6):802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 4.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. J Am Med Assoc. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Win S, Parakh K, Eze-Nliam CM, Gottdiener JS, Kop WJ, Ziegelstein RC. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011;97(6):500–505. doi: 10.1136/hrt.2010.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye S, Muntner P, Shimbo D, Judd SE, Richman J, Davidson KW, et al. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the REGARDS (Reason for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2013;61(6):622–630. doi: 10.1016/j.jacc.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummett BH, Babyak MA, Siegler IC, Mark DB, Williams RB, Barefoot JC. Effect of smoking and sedentary behavior on the association between depressive symptoms and mortality from coronary heart disease. Am J Cardiol. 2003;92(5):529–532. doi: 10.1016/s0002-9149(03)00719-7. [DOI] [PubMed] [Google Scholar]
- 8.Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol. 2008;52(25):2156–2162. doi: 10.1016/j.jacc.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 9.Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Läksy K, et al. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes. 2006;30(3):520–527. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- 10.Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP. Cigarette smoking predicts development of depressive symptoms among US adolescents. Ann Behav Med. 1997;19(1):42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- 11.Vogelzangs N, Kritchevsky SB, Beekman AT, Brenes GA, Newman AB, Satterfield S, et al. Obesity and Onset of Significant Depressive symptoms. J Clin Psychiatry. 2010;71(4):391–399. doi: 10.4088/JCP.08m04743blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46(5):397–411. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, et al. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease: results from the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) study. J Am Coll Cardiol. 2012;60(12):1053–1063. doi: 10.1016/j.jacc.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman BM, Babyak MA, Craighead WE, Sherwood A, Doraiswamy PM, Coons MJ, et al. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med. 2011;73(2):127–133. doi: 10.1097/PSY.0b013e31820433a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 17.Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31(4):306–315. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160(12):1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]
- 19.Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107(5):1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 20.Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al. Smoking, smoking cessation, and major depression. J Am Med Assoc. 1990;264(12):1546–1549. [PubMed] [Google Scholar]
- 21.Vogelzangs N, Kritchevsky SB, Beekman ATF, Newman AB, Satterfield S, Simonsick EM, et al. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65(12):1386–1393. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a Brief Depression Severity Measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 24.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study) Am J Cardiol. 2005;96(8):1076–1081. doi: 10.1016/j.amjcard.2005.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvie JL, Whooley MA, Regan MC, Sin NL, Cohen BE. Effect of Physical Activity Level on Biomarkers of Inflammation and Insulin Resistance Over 5 Years in Outpatients With Coronary Heart Disease (from the Heart and Soul Study) Am J Cardiol. 2014;114(8):1192–1197. doi: 10.1016/j.amjcard.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aadahl M, Kjær M, Kristensen JH, Mollerup B, Jørgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14(3):422–428. doi: 10.1097/HJR.0b013e3280128d00. [DOI] [PubMed] [Google Scholar]
- 27.Gill DP, Jones GR, Zou G, Speechley M. Using a single question to assess physical activity in older adults: a reliability and validity study. BMC Med Res Methodol. 2012;12(20) doi: 10.1186/1471-2288-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowles HR, FitzGerald SJ, Morrow JR, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160(3):279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 29.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165(21):2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167(16):1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004;42(7):649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- 32.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist: hip ratio as predictors of cardiovascular risk—a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 33.Dalton M, Cameron AJ, Zimmet PZ, Shaw JE, Jolley D, Dunstan DW, et al. Waist circumference, waist–hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003;254(6):555–563. doi: 10.1111/j.1365-2796.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Carpenter JS, Andrykowski MA. Psychometric evaluation of the pittsburgh sleep quality index. J Psychosom Res. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 36.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren X, Ristow B, Na B, Ali S, Schiller NB, Whooley MA. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am J Cardiol. 2007;99(12):1643–1647. doi: 10.1016/j.amjcard.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gehi AK, Ali S, Na B, Schiller NB, Whooley MA. Inducible ischemia and the risk of recurrent cardiovascular events in outpatients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2008;168(13):1423–1428. doi: 10.1001/archinte.168.13.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, et al. Prevalence of Depression in Survivors of Acute Myocardial Infarction. J Gen Intern Med. 2006 Jan;21(1):30–38. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichtman JH, Bigger JT, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, et al. Depression and Coronary Heart Disease Recommendations for Screening, Referral, and Treatment: A Science Advisory From the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 41.Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409–416. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Rieckmann N, Gerin W, Kronish IM, Burg MM, Chaplin WF, Kong G, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol. 2006;48(11):2218–2222. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 44.Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres-Finnerty N, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Arch Intern Med. 2008;168(2):186–191. doi: 10.1001/archinternmed.2007.60. [DOI] [PubMed] [Google Scholar]
- 45.Kronish IM, Rieckmann N, Halm EA, Shimbo D, Vorchheimer D, Haas DC, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med. 2006;21(11):1178–1183. doi: 10.1111/j.1525-1497.2006.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swardfager W, Herrmann N, Marzolini S, Saleem M, Farber SB, Kiss A, et al. Major depressive disorder predicts completion, adherence, and outcomes in cardiac rehabilitation: a prospective cohort study of 195 patients with coronary artery disease. J Clin Psychiatry. 2011;72(9):1181–1188. doi: 10.4088/JCP.09m05810blu. [DOI] [PubMed] [Google Scholar]
- 47.McGrady A, McGinnis R, Badenhop D, Bentle M, Rajput M. Effects of depression and anxiety on adherence to cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2009;29(6):358–364. doi: 10.1097/HCR.0b013e3181be7a8f. [DOI] [PubMed] [Google Scholar]
- 48.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132(9):873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. Waist circumference and waist-hip ratio: Report of a WHO expert consultation. 2008
- 50.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 51.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes. 2003;27(4):514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 52.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 53.Harris AH, Cronkite R, Moos R. Physical activity, exercise coping, and depression in a 10-year cohort study of depressed patients. J Affect Disord. 2006;93(1):79–85. doi: 10.1016/j.jad.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking: a longitudinal investigation. Arch Gen Psychiatry. 1998;55(2):161–166. doi: 10.1001/archpsyc.55.2.161. [DOI] [PubMed] [Google Scholar]
- 55.Pan A, Sun Q, Czernichow S, Kivimaki M, Okereke OI, Lucas M, et al. Bidirectional association between depression and obesity in middle-aged and older women. Int J Obes. 2012;36(4):595–602. doi: 10.1038/ijo.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sivertsen B, Salo P, Mykletun A, Hysing M, Pallesen S, Krokstad S, et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74(7):758–765. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 57.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar U, Ali S, Whooley MA. Self-efficacy as a marker of cardiac function and predictor of heart failure hospitalization and mortality in patients with stable coronary heart disease: findings from the Heart and Soul Study. Health Psychol. 2009;28(2):166–173. doi: 10.1037/a0013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sin NL, DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med. 2014;47(3):259–269. doi: 10.1007/s12160-013-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. J Am Med Assoc. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. J Am Med Assoc. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 63.Blumenthal JA, Babyak MA, O’Connor C, Keteyian S, Landzberg J, Howlett J, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. J Am Med Assoc. 2012;308(5):465–474. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rutledge T, Redwine LS, Linke SE, Mills PJ. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med. 2013;75(4):335–349. doi: 10.1097/PSY.0b013e318291d798. [DOI] [PubMed] [Google Scholar]
- 66.Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123(21):2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536. [DOI] [PubMed] [Google Scholar]
- 67.Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121(1):63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Troiano RP, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 69.Grenard JL, Munjas BA, Adams JL, Suttorp M, Maglione M, McGlynn EA, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caska CM, Hendrickson BE, Wong MH, Ali S, Neylan T, Whooley MA. Anger expression and sleep quality in patients with coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2009;71(3):280–285. doi: 10.1097/PSY.0b013e31819b6a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.