Abstract
Supplemental oxygen, which is routinely administered to preterm infants with pulmonary insufficiency, contributes to bronchopulmonary dysplasia (BPD) in these infants. Hyperoxia also contributes to the development of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) in adults. The mechanisms of oxygen-mediated pulmonary toxicity are not completely understood. Recent studies have suggested an important role for cytochrome P450 (CYP)1A1/1A2 in the protection against hyperoxic lung injury. The role of CYP1B1 in oxygen-mediated pulmonary toxicity has not been studied. In this investigation, we tested the hypothesis that CYP1B1 plays a mechanistic role in oxygen toxicity in pulmonary cells in vitro. In human bronchial epithelial cell line BEAS-2B, hyperoxic treatment for 1–3 days led to decreased cell viability by about 50–80%. Hyperoxic cytotoxicity was accompanied by an increase in levels of reactive oxygen species (ROS) by up to 110%, and an increase of TUNEL-positive cells by up to 4.8-fold. Western blot analysis showed hyperoxia to significantly down-regulated CYP1B1 protein level. Also, there was a decrease of CYP1B1 mRNA by up to 38% and Cyp1b1 promoter activity by up to 65%. On the other hand, CYP1B1 siRNA appeared to rescue the cell viability under hyperoxia stress, and overexpression of CYP1B1 significantly attenuated hyperoxic cytotoxicity after 48 h of incubation. In immortalized lung endothelial cells derived from Cyp1b1-null and wild-type mice, hyperoxia increased caspase 3/7 activities in a time-dependent manner, but endothelial cells lacking the Cyp1b1 gene showed significantly decreased caspase 3/7 activities after 48 and 72 h of incubation, implying that CYP1B1 might promote apoptosis in wild type lung endothelial cells under hyperoxic stress. In conclusion, our results support the hypothesis that CYP1B1 plays a mechanistic role in pulmonary oxygen toxicity, and CYP1B1-mediated apoptosis could be one of the mechanisms of oxygen toxicity. Thus, CYP1B1 could be a novel target for preventative and/or therapeutic interventions against BPD in infants and ALI/ARDS in adults.
Keywords: Cytochrome P450, CYP1B1, hyperoxia, lung, endothelial cells
1. Introduction
Supplemental oxygen, which is frequently administered to premature infants with pulmonary insufficiency, is one of the major risk factors for the development of bronchopulmonary dysplasia (BPD) in premature infants [1]. Hyperoxia also contributes to the development of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) in adults. Infants with BPD often are re-hospitalized before 1 year of age, and have worse neurological outcomes with significantly higher rates of cerebral palsy, spastic dyplegia, and lower cognitive and language scores [2]. BPD has a multifactorial etiology, including genetic predisposition, prematurity, mechanical ventilation, oxygen exposure, and inflammation [3].
Supplemental oxygen, which is often a life-saving therapy for premature neonates and ALI/ARDS patients, may lead to hyperoxia, which in turn is a major risk factor for the development of lung injury that is associated with increased pulmonary permeability, increased inflammatory cell count, and injuries to endothelial and epithelial cells [4]. Multiple studies have shown an involvement of CYP enzymes CYP1A (i.e. CYP1A1 and CYP1A2) in oxygen toxicity [5–8]. CYP is a family of heme-containing proteins that are involved in the metabolism of numerous endogenous substrates and xenobiotics [9]. Induction of CYP1A by β-naphtoflavone (BNF) or 3-methylcholanthrene (MC) prior to hyperoxia protects mice and rats against the toxic effects of oxygen exposure [10, 11]. Meanwhile, pre-treatment of rats with a CYP1A inhibitor, aminobenzotriazole, followed by exposure to 95% O2 leads to severe inflammation, pleural effusions, and severe lung injury [12]. During the initial 48 h of hyperoxia exposure, pulmonary and hepatic CYP1A upregulation is observed [12, 13]. Between 48–60 h, the animals develop severe respiratory distress, accompanied with downregulation of CYP1A [6, 12]. Aryl hydrocarbon receptor (AhR) is a key regulatory transcription factor of many CYP proteins and other important developmental genes, including CYP1A1/2 [14, 15]. Experiments with AhR null mice indicated that the protective effect of CYP1A1/2 on hyperoxic toxicity is dependent on the AhR in the lung [16].
CYP1B1 is the newest member of CYP1 family, described in 1994, after being cloned from a 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated human keratinocyte cell line [17], and shares only 40% homology with CYP1A1 and CYP1A2 [17]. CYP1B1 activates PAH in human lung [18] and inactivates benzo[a]pyrene (BP) in mouse aortic smooth muscle cells [19]. CYP1B1 is endogenously expressed in the lung and other tissues, but not hepatocytes [20]. BNF induces CYP1B1 mRNA, but to a lesser extent than that of CYP1A1 [21, 22]. Thakur et al. [23] have reported that maternal treatment of BP, followed by 7-days of postnatal hyperoxia exposure leads to upregulation of CYP1B1 [23].
CYP1B1 has a conserved DNA sequence and appears in the early stages in ontogenesis [24]. It may play a role in evolution and development. In this investigation, we tested the hypothesis that CYP1B1, which is also regulated by the AHR, plays a mechanistic role in oxygen toxicity in pulmonary cells in vitro.
2. Materials and methods
2.1. Cell culture
BEAS-2B and H358 cells (ATCC, Manassas, VA) were maintained in RPMI 1640 or high-glucose DMEM containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin. The cells were maintained in room air (RA) or exposed to hyperoxia (95% O2 and 5% CO2), as described earlier [25].
Lung endothelial cells were isolated from wild-type and Cyp1b1-null mice as previously described [26, 27]. The EC growth medium was DMEM containing 10% FBS, 2 mM L-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1% nonessential amino acids, 100 µg/ml streptomycin, 100 U/ml penicillin, freshly added heparin at 55 U/ml (Sigma), 100 µg/ml endothelial growth supplement (Sigma), and murine recombinant interferon-γ (R&D Systems, Minneapolis, MN) at 44 U/ml. Cells were incubated at 33°C with 5% CO2 and progressively passaged to larger plates. Cells were normally maintained in 60-mm dishes coated with 1% gelatin prepared in phosphate-buffered saline (PBS) [27].
To establish the CYP1B1-overexpressed cell line, total RNA was isolated from MC-treated BEAS-2B cells using RNeasy Mini Kit (Qiagen, Germantown, MD), and was subjected to reverse transcription (Bio-Rad, Hercules, CA). RT-PCR was performed using 5’-ATGCTAGCGCCGCCACCATGGGCACCAGCCTCAG-3’ and 5’-TAGGTACCCTTATTGGCAAGTTTCCTTGG-3’ as the cloning primers. The open reading frame of human CYP1B1 cDNA was subcloned into pcDNA3.1 between the NheI/KpnI sites. The insert sequence of pCD-CYP1B1 was verified by DNA sequencing. To obtain the stable overexpressed cells, pCD-CYP1B1 or pcDNA3.1 was transfected into H358 cells using SuperFect (Qiagen), and maintained in 500 µg/ml geneticin (Life Technologies). Clones that overexpressed CYP1B1 were screened by CYP1B1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) using immunofluorescence staining, and verified by real-time RT-PCR (data not shown).
2.2. Cell proliferation assays
The conventional trypan blue exclusion assay was performed using 0.4% trypan blue as previously described [28]. 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) (MTT) assay was performed according to the manufacturer’s instruction (ATCC) as previously described [15].
2.3. Intracellular ROS
Intracellular ROS level was quantified according to the manufacturer's instruction (Life Technologies) as previously described [15].
2.4. TUNEL fluorescein assay
TUNEL assay was performed using Click-iT TUNEL Alexa-Fluor Imaging Assay Kit according to the manufacturer's instruction (Life Technologies). Briefly, cells in 96-well black-walled plates (BD Biosciences) were fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, incubated with 50 µl/well of TdT reaction buffer at RT for 10 min, incubated with 50 µl/well of TdT reaction cocktail at 37°C for 60 min, washed twice with 3% BSA in PBS, and incubated with 50 µl/well of the Click-iT reaction cocktail at RT for 30 min. After the final wash with PBS containing 3% BSA, fluorescence was measured with excitation at 495 nm and emission at 519 nm.
2.5. Western blot analysis of CYP1B1 apoprotein
Cell lysates (30 µg protein per well) were resolved in SDS-PAGE and detected with CYP1B1 antibody (Santa Cruz Biotechnology) or β-actin (Actb) antibody (Sigma-Aldrich) by Western blot analyses, as described previously [13, 28].
2.6. qPCR
Total RNA was extracted from the cell lysates using RNeasy Mini Kit (Qiagen). Message RNA was quantified with SuperScript III Two-Step qRT-PCR Kit (Life Technologies). The primer sequences were 5’-CACTGCCAACACCTCTGTCTT-3’ and 5’-CAAGGAGCTCCATGGACTCT-3’ for CYP1B1 [29], 5’-AAACGCATTAACTGGCGAAC-3’ and 5’-GAACTCCAGGAGAACTGCAAA-3’ for human ornithine decarboxylase antizyme OAZ1 [29]. The SYBR Green (Bio-Rad) qPCR condition was 95°C fo r 5 s (denaturation), 65°C for 10 s (annealing), and 72°C for 20 s (extension) for CYP1 B1, and was 95°C for 5 s, 60°C for 10 s, and 72°C for 20 s for OAZ1. The PCR specificity was confirmed with the melting curves. The PCR efficiency was 95.7% for CYP1B1 and 96.5% for OAZ1.
2.7. Cyp1b1 promoter assay
The dual-luciferase assay (Promega, Madison, WI) was performed in BEAS-2B cells double transfected with 2 plasmid constructs using Qiagen SuperFect. The firefly luciferase construct p1.1 contained the rat Cyp1b1 5’-UTR and 1.0 Kb of the proximal 5’-flanking sequence. The renilla luciferase construct was pRL-TK (Promega). Cyp1b1 promoter activities were determined by the dual-luciferase assay, which entailed normalizing the firefly luciferase activities against those of renilla luciferase.
2.8. CYP1B1 siRNA knockdown
Cells were transfected with either ON-TARGETplus hCYP1B1 siRNA or the non-targeting control (Dharmacon, Chicago, IL) using Lipofectamine 2000 (Life Technologies). The siRNA effect was examined by qPCR as described in Materials and methods.
2.9. ApoTox-Glo Triplex Assay for cytotoxicity and caspase 3/7 activities
Cytotoxicity and caspase 3/7 activities were determined using ApoTox-Glo™ Triplex Assay (Promega) according to the manufacturer's instruction. Briefly, cells in 96-well black-walled plates were incubated with 20 µl of Cytotoxicity Reagent at 37°C for 30 min. Cytot oxicity was determined by fluorescence (excitation at 485 nm and emission at 520 nm). The cells were then incubated with 100 µl of Caspase-Glo 3/7 Reagent at RT for 30 min. The caspase 3/7 activities were determined by bioluminescence.
3. Results
3.1 Cytotoxicity of hyperoxia to BEAS-2B cells
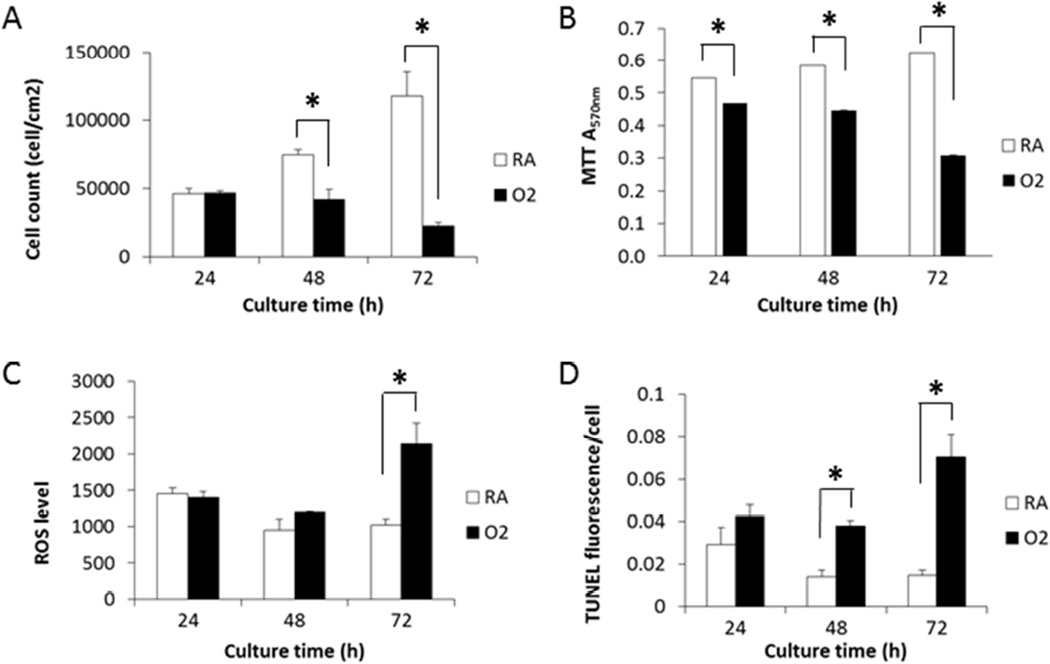
Hyperoxia impairs lung development in premature babies, as well as in newborn mice and other animals [30, 31]. In vitro experiments consistently demonstrated hyperoxic toxicities to pulmonary cell lines such as H358 (unpublished data), H441, and A549 [25]. In BEAS-2B cells, trypan blue exclusion assay showed that the number of live cells increased by about 60% per day under RA conditions (RA) (Figure 1A). Hyperoxia (95% O2 plus 5% CO2) [28] showed no effect on cell proliferation during the first 24 h, but exhibited 44 and 81% inhibition at 48 and 72 h, respectively, based on cell numbers (Figure 1A). The MTT cell proliferation assay measures the activity of NAD(P)H-dependent oxidoreductases which represents the metabolic rate of entire cell population, live and dead, in each well. Hyperoxia decreased the A570nm in the MTT assay of BEAS-2B cells by 14%, 24%, and 51% at 24, 48, and 72 h, respectively (Figure 1B).
Figure 1.
Hyperoxia exhibited cytotoxicity to BEAS-2B cells, which was accompanied by increase of intracellular ROS level and apoptotic cell population. Cells maintained in RA or hyperoxia (O2) condition for 24, 48, and 72 h were subjected to trypan blue exclusion assay (A), MTT assay (B), CM-H2DCF-DA based ROS flow cytometry assay (C), and TUNEL Alexa-Fluor imaging assay (D). (n = 3; *, t-test p < 0.05) Values represent mean ± SEM of at least 3 independent experiments.
According to the literature, hyperoxic cytotoxicity is associated with increased production of ROS [32]. We measured the effect of hyperoxia on intracellular ROS in BEAS-2B cells using CM-H2DCFDA as the probe. ROS converts the fluorescent probe into 5-(and 6-)chloromethyl-2′,7′-dichlorofluorescin (CM-DCF). As anticipated, we found that hyperoxia increased the CM-DCF fluorescence or intracellular ROS by 26% at 48 h and 110% at 72 h (Figure 1C).
Since hyperoxia caused cell death (Figure 1A), we performed TUNEL apoptosis assay, a method based on terminal deoxynucleotidyl transferase (TdT)-associated incorporation of dUTPs at the 3’-OH groups of fragmented DNA. Hyperoxia increased dUTP incorporation in the BEAS-2B cells by 1.5-, 2.7-, and 4.8-fold at 24, 48, and 72 h, respectively (Figure 1D), indicating the involvement of apoptosis in the ROS-associated hyperoxic cytotoxicity.
3.2 Hyperoxia downregulated CYP1B1 in BEAS-2B cells
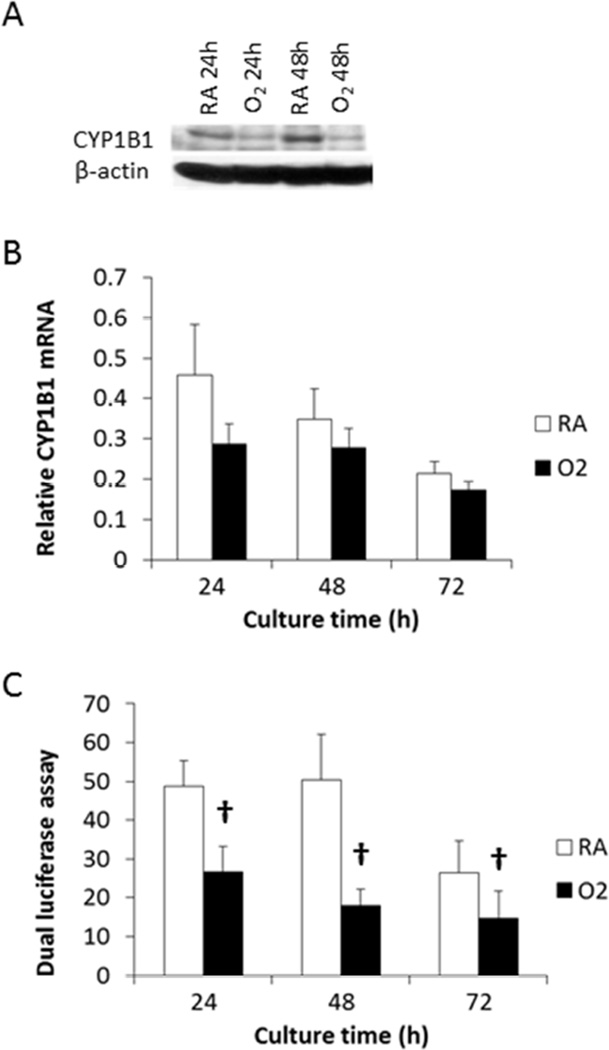
Previous reports indicate that hyperoxia induces CYP1A1 in the lung or cultured pulmonary cells [5, 25]. When BEAS-2B pulmonary cells were exposed to hyperoxia, CYP1B1 apoprotein was significantly downregulated at 24 and 48 h in Western blot analysis (Figure 2A). qPCR indicated that hyperoxia decreased CYP1B1 mRNA level by 38%, 21%, and 19% at 24, 48, and 72 h, respectively (Figure 2B). The reference gene OAZ1 was not affected by hyperoxia, consistent with our previous publication on the effect of hyperoxia in H441 cells [30]. CYP1A1 mRNA was induced by 94% by a 24 h hyperoxic treatment (not shown), consistent with previous observations [25].
Figure 2.
Down-regulation of CYP1B1 by hyperoxia in BEAS-2B cells. Cells maintained in RA or hyperoxia condition were subjected to Western blot analysis of CYP1B1 and β-actin (A), or qPCR of CYP1B1 and the reference gene OAZ1 (B) as compared to room air levels (C) Cells were transfected with p1.1, a firefly luciferase reporter plasmid containing the rat CYP1B1 promoter sequence, together with the pRL-TK renilla luciferase reporter plasmid. The transiently transfected cells were maintained in RA or hyperoxia condition, and subjected to dual-luciferase assay. Two-way ANOVA analysis indicated that hyperoxia significantly decreased CYP1B1 promoter activity (n = 3; †, p < 0.05) at each time point. Values represent mean ± SEM of at least 3 independent experiments.
In order to investigate the mechanisms of downregulation of Cyp1b1a 1.1 Kbp of rat Cyp1b1 promoter sequence was subcloned into pGL3 luciferase reporter system (see Materials and methods). Dual luciferase assay of the p1.1/pRL double transfected BEAS-2B cells indicated that Cyp1b1 promoter activity was down-regulated by hyperoxia by 45–65% at 24, 48, and 72 h (Figure 2C).
3.3 CYP1B1 siRNA protected cells from hyperoxic cytotoxicity
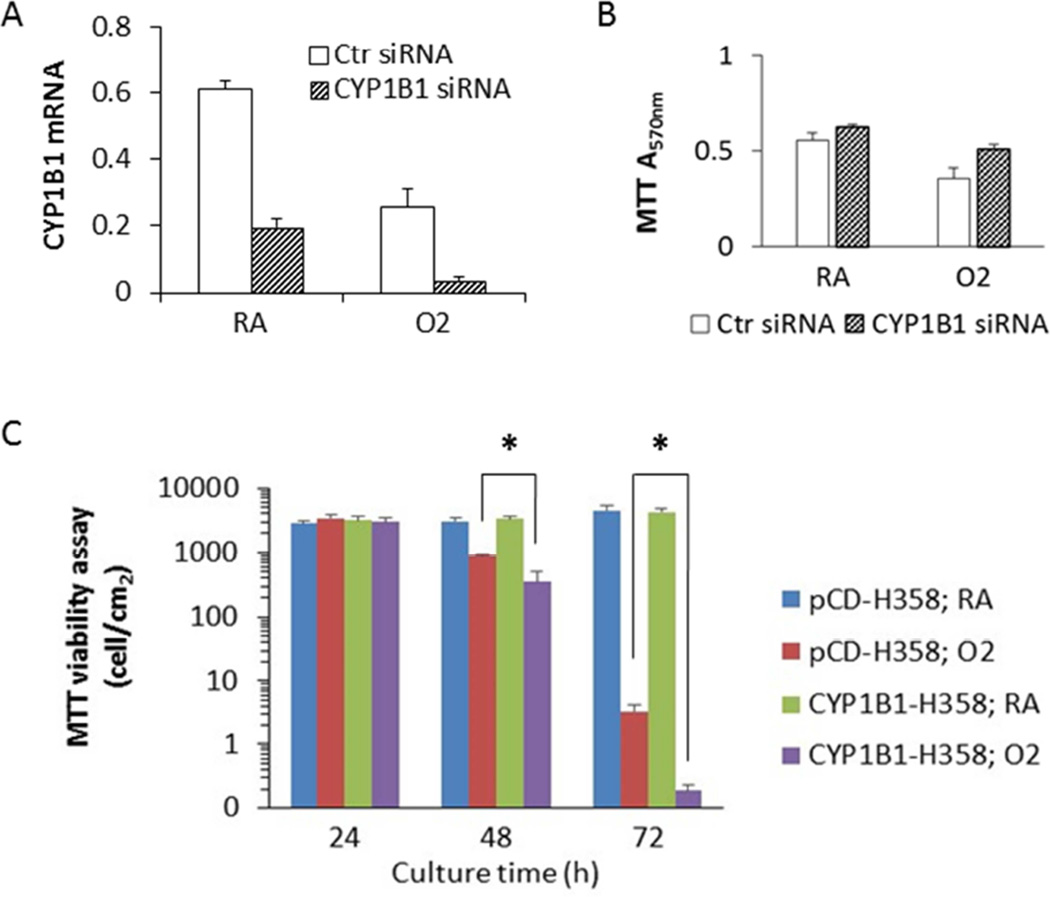
BEAS-2B cells were transiently transfected with either non-specific control siRNA or CYP1B1 siRNA. The qPCR analysis indicated that CYP1B1 siRNA significantly knocked down CYP1B1 mRNA in both room air (21.0%) and hyperoxia (85.5%) conditions (Figure 3A). Cells that were transfected with CYP1B1 siRNA showed elevated the cell viability under hyperoxia conditions by 44% (Figure 3B), suggesting that CYP1B1 siRNA rescued cells from oxygen toxicity, albeit this was not statistically significant.
Figure 3.
CYP1B1 promoted hyperoxic cytotoxicity in human lung cell lines. A. CYP1 B1 siRNA knocked down CYP1B1 mRNA level in both RA and hyperoxia conditions (48 h). B. CYP1B1 siRNA appeared to rescue hyperoxic cytotoxicity in BEAS-2B cells, although this was not statistically significant. . C. Overexpression of CYP1B1 in H358 cells augmented hyperoxic cytotoxicity (n = 3; *, t-test p < 0.05). , Values represent mean ± SEM of at least 3 independent experiments.
3.4 CYP1B1 overexpression promoted hyperoxic cytotoxicity
CYP1B1-H358 cells were generated by stably transfecting CYP1B1 cDNA into H358 cells using a pCDNA vector. The control cells (pCD-H358 cells) were created by transfecting the H358 cells with the empty vector pCDNA3.1(+). The cells were maintained in room air or subjected to hyperoxia for 24, 48, and 72 h. MTT assay measures the total NAD(P)H-dependent oxidoreductase activity in each well. But the two stable cell lines exhibit a different growth rate, resulting in different amount of cells in each well. Therefore, in Figure 3C, we converted the original MTT A570nm readings into cell densities, using a standard curve of MTT A570nm readings versus cell count. We found that CYP1B1-H358 was more susceptible to hyperoxic toxicity (Figure 3C). A 48 h hyperoxic treatment caused a greater decrease in cell viability of CYP1B1-H358 cells, as compared to pCD-H358 cells, and this difference was much more pronounced at 72 h (Figure 3C).
3.5 Knockout of Cyp1b1 gene in lung endothelial cells alleviated hyperoxic toxicity
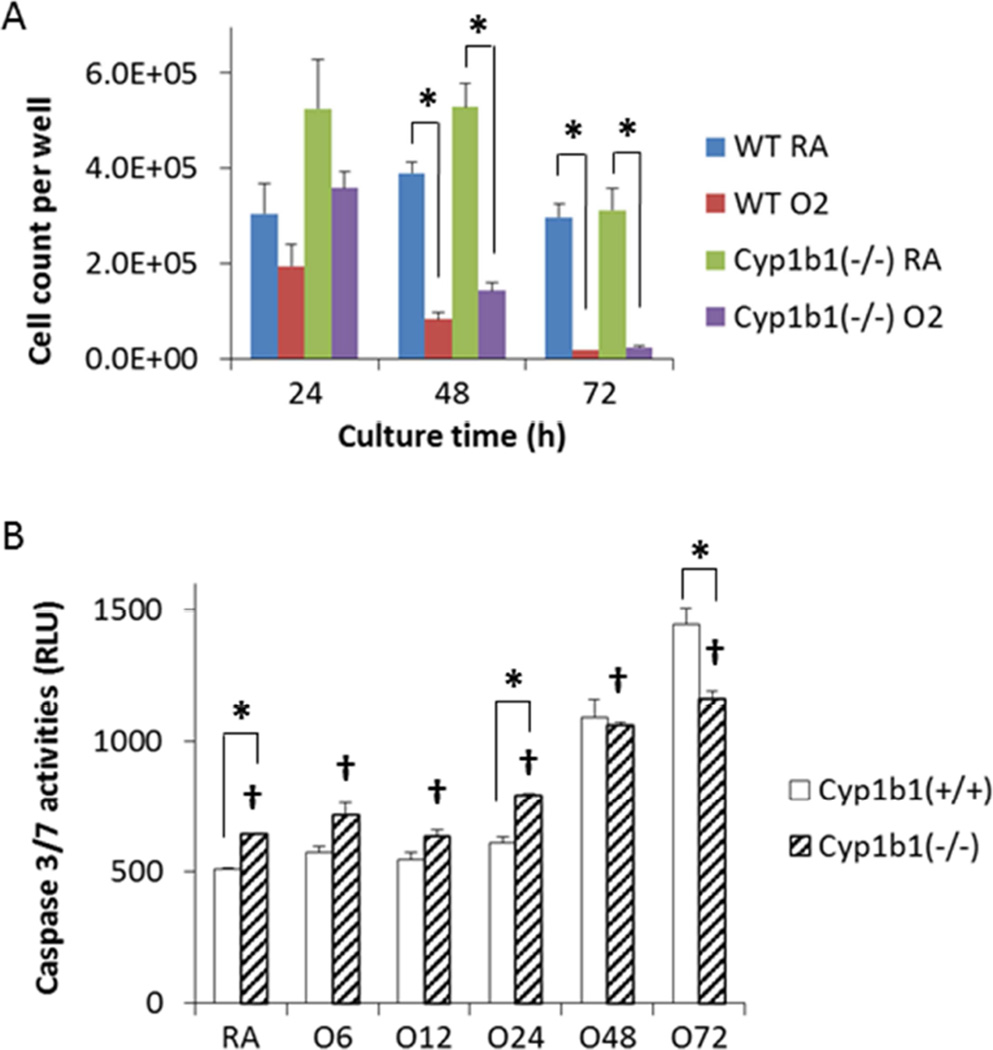
Lung endothelial cell line prepared from wild-type and Cyp1b1-null mice were subjected to RA and hyperoxic condition. Hyperoxic toxicity to cell viability was observed in both cell line (Figure 4A). Although the Cyp1b1−/− cells grew faster than the Cyp1b1+/+ (WT) cells (Figure 4A), the hyperoxia-induced increase of caspase 3/7 activities was lower in the Cyp1b1−/− cells (Figure 4B). The differences became statistically significant at 72 h (Figure 4B), suggesting Cyp1b1 expression promoted apoptosis under hyperoxic stress.
Figure 4.
Lack of CYP1B1 expression decreased the apoptotic activity after 72 h. Lung endothelial cells prepared from wild-type and Cyp1b1-null mice were exposed to high oxygen and viability was assessed. A. Trypan blue exclusion assay exhibited hyperoxic toxicities in both Cyp1b1+/+ (WT) and Cyp1b1−/− cells. B. Two-way ANOVA analysis indicated that lack of CYP1B1 expression significantly reduced the intracellular caspase 3/7 activities promoted by hyperoxia (†, p < 0.05). (n = 3; *, t-test p < 0.05).
DISCUSSION
Lung injury due to prolonged hyperoxia is characterized by increased ROS production [32]. ROS could initiate lung damage by oxidative damage to proteins, lipids, and DNA, which could in turn lead to enhanced expression of pro-inflammatory genes [33]. ROS plays a central role in the subsequent extensive inflammatory response, destruction of the alveolo-capillary barrier, impaired gas exchange, and pulmonary edema [34].
In this study, we focused on the mechanistic role of CYP1B1 in oxygen-mediated pulmonary toxicity using human pulmonary cell lines. Using BEAS-2B cells, we found that the onset of hyperoxic cytotoxicity was most noticeable at 48 h. No significant cell death (Figure 1A), ROS production (Figure 1C), or dUTP incorporation-associated apoptosis (Figure 1D) was observed after 24 h exposure of 95% oxygen. This observation was consistent with our previous observations that CYP1A1 was induced by hyperoxia within 24 h, and this may have led to protection of the cells from hyperoxic toxicity in that time frame [25]. At later time points, CYP1A1 expression declined and there was more cytotoxicity, suggesting that CYP1A1 decrease may have contributed to the toxicity mediated by oxygen [25].
The mechanisms by which hyperoxia caused downregulation of CYP1B1 at the protein (Figure. 2A) and mRNA levels (Figure. 2B) is not completely understood, but probably entailed attenuation of CYP1B1 gene at the transcriptional level based on our transient transfection experiments using the luciferase reporter gene (Figure. 2C). However, that fact that CYP1B1 mRNA repression by hyperoxia was modest relative to that of CYP1B1 protein could be explained by a combination of transcriptional and post-transcriptional/translational mechanisms. It is possible that ROS produced under hyperoxic conditions may have decreased the stability of CYP1B1 protein [35].
The downregulation of CYP1B1 might be a protective mechanism because we found that CYP1B1 promoted hyperoxic cytotoxicity. This idea is supported by our observation that overexpression of CYP1B1 in the cells led to increased cytotoxicity caused by hyperoxic incubation for 48 h or longer (Figure 3C). Furthermore, knock-down of CYP1B1 mRNA rescued the cells from toxicity after 48 h hyperoxia (Figure. 3A&B), although this was statistically significant. Conditional deletion of CYP1B1 in endothelial cells significantly decreased hyperoxia-mediated apoptosis (Figure 4B). The fact that endothelial cells lacking CYP1B1 showed lesser apoptosis and toxicity than wild type cells supports the hypothesis that CYP1B1 in pulmonary endothelial cells in vivo contributes to the pro-oxidant effects under hyperoxic conditions. Our recent studies showing decreased hyperoxic lung injury in mice lacking the gene for Cyp1b1 (Veith et al., 2016, unpublished results) lends further credence to this hypothesis.
Tang et al. reported that Cyp1b1 deficiency in retinal endothelial cells showed increased oxidative stress and protection against abnormal angiogenesis, and this was due increased production of thrombospondin-2 (TSP-2), an inhibitor of angiogenesis [36].
In summary, the results of this investigation support the hypothesis that CYP1B1 plays a mechanistic role in pulmonary oxygen toxicity, and CYP1B1-mediated apoptosis could be one of the mechanisms of oxygen toxicity. CYP1B1 could thus be one of the targets for preventative and/or therapeutic interventions against BPD in infants and ALI/ARDS in adults.
Supplementary Material
Highlights.
In human lung cells, hyperoxia caused downregulation of CYP1B1 protein and mRNA
Cells expressing human CYP1B1 displayed greater susceptible to oxygen toxicity
Lung endothelial cells from mice lacking CYP1B1 showed attenuation oxygen toxicity
CYP1B1 could be a novel target for prevention of hyperoxic lung injury in humans
Acknowledgments
This work was supported in part by R01 grants ES-019689, ES-009132, HL-112516, HL129794 to BM, HL-088343 to XIC, K08HL127103 to KL, EY022883, NIH P30 EY016665, Environmental Protection Agency 83573701 to NS, and DK090249 to CRJ.
Abbreviations
- BPD
bronchopulmonary dysplasia
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- CYP
cytochrome P450
- ROS
reactive oxygen species
- AHR
arylhydrocarbon receptor
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- BNF
β-naphtoflavone
- MC
3-methylcholanthrene
- BP
benzo[a]pyrene
- MTT
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123:1562–1573. doi: 10.1542/peds.2008-1962. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, Laptook AR, Bell EF, Stoll BJ, Newman N, Hale EC, Bara R, Walsh MC. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88:509–515. doi: 10.1016/j.earlhumdev.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013;305:L893–L905. doi: 10.1152/ajplung.00267.2013. [DOI] [PubMed] [Google Scholar]
- 4.Olivant Fisher A, Husain K, Wolfson MR, Hubert TL, Rodriguez E, Shaffer TH, Theroux MC. Hyperoxia during one lung ventilation: inflammatory and oxidative responses. Pediatr Pulmonol. 2012;47:979–986. doi: 10.1002/ppul.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang W, Couroucli XI, Wang L, Barrios R, Moorthy B. Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo. Biochem Biophys Res Commun. 2011;407:79–85. doi: 10.1016/j.bbrc.2011.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couroucli XI, Welty SE, Geske RS, Moorthy B. Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol Pharmacol. 2002;61:507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- 7.Lingappan K, Jiang W, Wang L, Couroucli XI, Moorthy B. Sex-specific differences in hyperoxic lung injury in mice: role of cytochrome P450 (CYP)1A. Toxicology. 2015;331:14–23. doi: 10.1016/j.tox.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Lingappan K, Jiang W, Couroucli XI, Welty SE, Shivanna B, Barrios R, Wang G, Firoze Khan M, Gonzalez FJ, Jackson Roberts L, Moorthy B. Disruption of cytochrome P4501A2 in mice leads to increased susceptibility to hyperoxic lung injury. Free Radic Biol Med. 2015;82:147–159. doi: 10.1016/j.freeradbiomed.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen L, Oostenbrink C, Jorgensen FS. Prediction of cytochrome P450 mediated metabolism. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Sprong C, Janssen YM, Borm PJ. The role of reactive oxygen species, cytokines and cytochrome P450 in pulmonary damage due to hyperoxia. Med Hypotheses. 1991;34:296–299. doi: 10.1016/0306-9877(91)90045-z. [DOI] [PubMed] [Google Scholar]
- 11.Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B. Attenuation of hyperoxic lung injury by the CYP1A inducer beta-naphthoflavone. Toxicol Sci. 2005;87:204–212. doi: 10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- 12.Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE. Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome P-450 inhibitor, 1-aminobenzotriazole. J Pharmacol Exp Ther. 2000;292:553–560. [PubMed] [Google Scholar]
- 13.Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- 14.Shivanna B, Zhang W, Jiang W, Welty SE, Couroucli XI, Wang L, Moorthy B. Functional deficiency of aryl hydrocarbon receptor augments oxygen toxicity-induced alveolar simplification in newborn mice. Toxicol Appl Pharmacol. 2013;267:209–217. doi: 10.1016/j.taap.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Patel A, Chu C, Jiang W, Wang L, Welty SE, Moorthy B, Shivanna B. Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: Mechanistic roles of antioxidant enzymes and RelB. Toxicol Appl Pharmacol. 2015;286:92–101. doi: 10.1016/j.taap.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- 17.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- 18.Uppstad H, Ovrebo S, Haugen A, Mollerup S. Importance of CYP1A1 and CYP1B1 in bioactivation of benzo[a]pyrene in human lung cell lines. Toxicol Lett. 2010;192:221–228. doi: 10.1016/j.toxlet.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Moorthy B, Miller KP, Jiang W, Williams ES, Kondraganti SR, Ramos KS. Role of cytochrome P4501B1 in benzo[a]pyrene bioactivation to DNA-binding metabolites in mouse vascular smooth muscle cells: evidence from 32P-postlabeling for formation of 3-hydroxybenzo[a]pyrene and benzo[a]pyrene-3,6-quinone as major proximate genotoxic intermediates. J Pharmacol Exp Ther. 2003;305:394–401. doi: 10.1124/jpet.102.044271. [DOI] [PubMed] [Google Scholar]
- 20.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- 21.Bussmann UA, Bussmann LE, Baranao JL. An aryl hydrocarbon receptor agonist amplifies the mitogenic actions of estradiol in granulosa cells: evidence of involvement of the cognate receptors. Biol Reprod. 2006;74:417–426. doi: 10.1095/biolreprod.105.043901. [DOI] [PubMed] [Google Scholar]
- 22.Cheung CY, Chen J, Chang TK. Evaluation of a real-time polymerase chain reaction method for the quantification of CYP1B1 gene expression in MCF-7 human breast carcinoma cells. J Pharmacol Toxicol Methods. 2004;49:97–104. doi: 10.1016/j.vascn.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Thakur VS, Liang YW, Lingappan K, Jiang W, Wang L, Barrios R, Zhou G, Guntupalli B, Shivanna B, Maturu P, Welty SE, Moorthy B, Couroucli XI. Increased susceptibility to hyperoxic lung injury and alveolar simplification in newborn rats by prenatal administration of benzo[a]pyrene. Toxicol Lett. 2014;230:322–332. doi: 10.1016/j.toxlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhary D, Jansson I, Schenkman JB, Sarfarazi M, Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch Biochem Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 25.Bhakta KY, Jiang W, Couroucli XI, Fazili IS, Muthiah K, Moorthy B. Regulation of cytochrome P4501A1 expression by hyperoxia in human lung cell lines: Implications for hyperoxic lung injury. Toxicol Appl Pharmacol. 2008;233:169–178. doi: 10.1016/j.taap.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci U S A. 1999;96:1977–1982. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Scheef EA, Gurel Z, Sorenson CM, Jefcoate CR, Sheibani N. CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. Am J Physiol Cell Physiol. 2010;298:C665–C678. doi: 10.1152/ajpcell.00153.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiwari KK, Chu C, Couroucli X, Moorthy B, Lingappan K. Differential concentration-specific effects of caffeine on cell viability, oxidative stress, and cell cycle in pulmonary oxygen toxicity in vitro. Biochem Biophys Res Commun. 2014;450:1345–1350. doi: 10.1016/j.bbrc.2014.06.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang TK, Chen J, Pillay V, Ho JY, Bandiera SM. Real-time polymerase chain reaction analysis of CYP1B1 gene expression in human liver. Toxicol Sci. 2003;71:11–19. doi: 10.1093/toxsci/71.1.11. [DOI] [PubMed] [Google Scholar]
- 30.Shivanna B, Chu C, Welty SE, Jiang W, Wang L, Couroucli XI, Moorthy B. Omeprazole attenuates hyperoxic injury in H441 cells via the aryl hydrocarbon receptor. Free Radic Biol Med. 2011;51:1910–1917. doi: 10.1016/j.freeradbiomed.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buczynski BW, Maduekwe ET, O'Reilly MA. The role of hyperoxia in the pathogenesis of experimental BPD. Semin Perinatol. 2013;37:69–78. doi: 10.1053/j.semperi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–757. [PubMed] [Google Scholar]
- 33.Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–431. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 34.Gore A, Muralidhar M, Espey MG, Degenhardt K, Mantell LL. Hyperoxia sensing: from molecular mechanisms to significance in disease. J Immunotoxicol. 2010;7:239–254. doi: 10.3109/1547691X.2010.492254. [DOI] [PubMed] [Google Scholar]
- 35.Savas U, Carstens CP, Jefcoate CR. Biological oxidations and P450 reactions. Archives of Biochemistry and Biophysics. 1997;347:181–192. doi: 10.1006/abbi.1997.0339. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.