Abstract
A full description of the terminal architecture of sympathetic axons innervating the gastrointestinal (GI) tract has not been available. To label sympathetic fibers projecting to the gut muscle wall, dextran biotin was injected into the celiac and superior mesenteric ganglia (CSMG) of rats. Nine days post-injection, animals were euthanized, and stomachs and small intestines were processed as whole mounts (submucosa and mucosa removed) to examine CSMG efferent terminals. Myenteric neurons were counterstained with Cuprolinic Blue; catecholaminergic axons were stained immunohistochemically for tyrosine hydroxylase. Essentially all dextran-labeled axons (135 of 136 sampled) were tyrosine hydroxylase-positive. Complete postganglionic arbors (n=154) in the muscle wall were digitized and analyzed morphometrically. Individual sympathetic axons formed complex arbors of varicose neurites within myenteric ganglia/primary plexus and, concomitantly, long rectilinear arrays of neurites within circular muscle/secondary plexus or longitudinal muscle/tertiary plexus. Very few CSMG neurons projected exclusively (i.e. ~100% of an arbor’s varicose branches) to myenteric plexus (~2%) or smooth muscle (~14%). With less stringent inclusion criteria (i.e. ≥ 85% of an axon’s varicose branches), larger minorities of neurons projected predominantly to either myenteric plexus (~13%) or smooth muscle (~27%). The majority (i.e., ~60%) of all individual CSMG postganglionics formed mixed, heterotypic arbors that co-innervated extensively (> 15% of their varicose branches per target) both myenteric ganglia and smooth muscle. The fact that ~87% of all sympathetics projected either extensively or even predominantly to smooth muscle, while simultaneously contacting myenteric plexus, is consistent with the view that these neurons control GI muscle directly, if not exclusively.
Keywords: Autonomic Nervous System, Celiac Ganglion, Enteric Nervous System, Muscularis Externa, Superior Mesenteric Ganglion
Introduction
The sympathetic nervous system (SNS) supplies much of the extrinsic innervation of the gastrointestinal (GI) tract and profoundly influences motility and other GI functions (Furness and Costa, 1974; Furness, 2006a; Jobling, 2012). In spite of the key roles the SNS plays in gut physiology, much about the organization of the sympathetic postganglionic neurons innervating the smooth muscle wall of the GI tract remains unknown. In particular, the morphologies and features of the motor neuron axonal arbors, as these neurite terminals ramify and distribute to modulate function in the gut wall, have not been thoroughly analyzed.
The current incomplete ideas about SNS postganglionic architecture are based on examinations with various protocols that have not evaluated the full distribution or complete morphology of individual postganglionic neurons. Thus the inferred patterns of innervation have been shaped—as well as potentially skewed—by the limitations of the techniques employed. The point is reinforced by the fact that the various patterns of sympathetic organization that have been adduced with different methodologies have been inconsistent.
Classically, one school of thought inferred that sympathetic postganglionic fibers originating in the prevertebral ganglia and projecting to the GI tract innervate directly the smooth muscle fibers of the muscularis externa (Langley, 1903; Hill, 1927). This two-neuron view of the SNS outflow was based largely on the fact that, in other organ systems, autonomic preganglionic fibers originating in the CNS project to postganglionic neurons in prevertebral ganglia, and the axons of those postganglionic neurons then typically innervate smooth muscle effectors directly (Langley, 1903; Hill, 1927; Kuntz, 1934, 1953; Bennett, 1972). With the introduction of tissue protocols that induce catecholamine fluorescence in situ, however, evidence accumulated that the sympathetic postganglionic fibers innervate myenteric ganglion neurons (e.g., Falck et al., 1962; Capurso et al., 1968; Furness, 1970b; Furness and Costa, 1971; Furness and Malmfors, 1971), which were known to project to smooth muscle. In a series of influential investigations beginning with the experiments of Norberg and Sjoqvist, (Norberg, 1964; Norberg and Sjoqvist, 1966; Norberg, 1967), investigators observed that fluorescent catecholaminergic postganglionic fibers in the gut are densely packed in the myenteric plexus, conspicuously less densely packed in the circular muscle than in the myenteric plexus, and almost absent in the longitudinal muscle sheet (Norberg, 1964, 1967; Hollands and Vanov, 1965; Capurso et al., 1968). Such a view was also consistent with both earlier (e.g., Cajal, 1911; Muller, 1911; Carpenter, 1924) and more recent (e.g., Llewellyn-Smith et al., 1984; Wiley and Owyang, 1987; Keast, 1994; Hayakawa et al., 2008) observations from other techniques that suggest sympathetic fibers contact myenteric neurons that perhaps in turn project to smooth muscle fibers.
In spite of widespread illustration of three-neuron chains in reviews, textbooks, and schematics (e.g., Burnstock and Costa, 1973; Brading, 1999; Furness, 2006b; Janig, 2006), the organization has found limited direct support when other methodologies have been used to describe the SNS postganglionic distribution in the gut. Ultrastructural studies have routinely noted that sympathetic axons rarely contact or run in near-proximity to the somata or dendrites of myenteric neurons. Instead, EM assessments indicate that extrinsic sympathetic axons tend to course superficially in the ganglia of the plexuses, where the neurites’ varicosities appear to form appositions with other axons and, tellingly, with sites near the basal lamina and capsule of the plexuses that might allow transmitter diffusion directly to smooth muscle fibers (Gabella, 1970; Manber and Gershon, 1979; Llewellyn-Smith et al., 1981, 1984; Gordon-Weeks, 1982). Similarly, electrophysiological analyses have suggested limited, though certainly some, influences of catecholamines in the myenteric plexus. Some analyses indicated that catecholamines only weakly, if at all, affect the firing patterns or resting potentials of myenteric neurons (Holman et al., 1972; Nishi and North, 1973; Takayanagi et al., 1977). In contrast, however, other experiments have indicated that sympathetic projections to the intestine appear to presynatically inhibit cholinergic potentials in some myenteric neurons (Hirst and McKirdy, 1974), hyperpolarize some myenteric neurons (Galligan and North, 1991; Bian and Galligan, 2007), and potentially modulate reflex circuits within the myenteric plexus (Stebbing et al., 2001).
Reinforcing the conclusion that direct sympathetic innervation of myenteric ganglion cells may be limited, it is also clear that essentially all extrinsic fibers projecting to the different tissues of the gut wall course initially through the myenteric plexus. Thus, even axons that merely traverse the plexus pathways to reach more distal target tissues, including even submucosal sites, might account for an appearance of a concentration of sympathetic fibers (and potential contacts) commonly observed within the plexus. Furthermore, immunohistochemistry of catecholamines and their synthetic enzymes, in contrast to the indirect fluorescence techniques, has regularly described substantial networks of catecholaminergic axons in the circular (though not longitudinal) muscle sheet throughout the rostral-caudal extent of the GI tract (Furness et al., 1990; Tassicker et al., 1999; Phillips et al., 2006, 2009, 2013; Tan et al., 2010).
Most of the apparent discrepancies between the views provided by the different techniques seem to have been predicated on a working assumption that SNS postganglionic projections either innervate smooth muscle fibers directly (i.e., in a two-neuron-outflow pattern) or, instead, innervate the enteric neurons of the plexus and hence smooth muscle indirectly (i.e., in a three-neuron-outflow pattern). Evidence for one pattern has frequently been taken as evidence against the other, and vice versa. The organizational possibilities are not binary, however, and other architectures consistent with both two-neuron pattern and three-neuron pattern would be consistent with all of the previous observations. For example, separate populations of postganglionic fibers might project, respectively, to smooth muscle fibers and to enteric neurons. Or, yet again, individual fibers might course through the plexuses of the gut wall, supplying collaterals or varicosities en passage to myenteric neurons, and then continue on to also directly innervate the smooth muscle.
Interestingly, we are unaware of any morphological experiment that has systematically evaluated such a co-innervation possibility for individual sympathetic fibers in the gut. Presumably, at least in part, it has been relatively impractical to assess this alternative for the sympathetic projections to the gut because the extensively intertwined postganglionic axons projecting to the gut are so densely packed and distributed through such distances and complex tissue elements, that the traditional methodologies (induced fluorescence, ultrastructural analysis, electrophysiology, immunohistochemistry) cannot readily isolate the full reach of individual autonomic axonal arbors in the gut wall.
To circumvent some of the limitations associated with the conventional approaches, the present experiment used a different technique—namely labeling, in any one animal preparation, limited numbers of individual sympathetic postganglionic neuron axonal arbors in their entirety with the anterograde tracer dextran biotin (Walter et al., 2009). The isolated and complete arbors of individually identified axons were then traced, digitally reconstructed, and evaluated morphometrically in whole mounts of the muscularis externa to determine the distribution patterns of single sympathetic nervous system fibers as a means of evaluating and comparing them to the various putative patterns of innervation.
Materials and Methods
Animals
Virgin male rats (Fischer 344; n = 62; Harlan Laboratories, Indianapolis, IN; RRID:RGD_61109) were purchased at 2-months of age and weighed 175–200 g at the time of delivery. The animals were housed in an AAALAC-approved room kept at 20–23°C, on a 12:12 hour light:dark schedule, with access to pelleted chow (Laboratory diet no. 5001; PMI Feeds Inc., Brentwood, MO, USA) and filtered tap water available ad libitum. All Procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (8th ed., The National Academic Press, Washington, D.C.), and approved by the Purdue University Animal Care and Use Committee. Every effort was made to minimize suffering and the number of animals used.
Tracer Injection in the Celiac and Superior Mesenteric Ganglia
After a 2-week acclimation period in the colony, each animal was food-deprived overnight, anesthetized with Isoflurane (Isoflo®; Abbott Laboratories, North Chicago, IL, USA), and injected with Glycopyrrolate (0.2 mg/ml, s.c.; AmericanRegent Inc., Shirley, NY, USA) to reduce salivary, tracheobronchial, and gastric secretions. Each rat was then positioned on its back, and the abdominal cavity was opened by a midline laparotomy. The abdominal organs were draped with sterile saline moistened gauze and gently displaced inside the abdomen to visualize the sympathetic ganglia. To minimize disturbance of organs and surgery time, only the left celiac and superior mesenteric ganglia (CSMG), which are a main source of sympathetic innervation to the stomach and small intestine (Miolan and Niel, 1996; Quinson et al., 2001), were injected with tracer.
More specifically, since the two ganglia are fused in the rat, the posterior pole of the CSMG (Hammer and Santer, 1981; Berthoud and Powley, 1993; Furness, 2014) was impaled using a Nanofil syringe with a beveled 35 gauge needle (NF35BV; World Precision Instruments, Sarasota, FL). The needle was advanced longitudinally, within and parallel to the long axis of the fused ganglia, until the tip rested at the rostral pole of the celiac ganglion part of the CSMG complex (Berthoud and Powley, 1996; Quinson et al., 2001). Dextran biotin, 6 µl of 10K MW lysine-fixable (7.5% concentration; Cat# D1956; Life Technologies, Grand Island, NY; RRID:AB_2307337), was then slowly injected as the needle was gradually retracted in a stepwise fashion within the ganglia. When the injection was completed and the infusion pressure within the CSMG had dissipated, the needle was slowly withdrawn from the ganglia. The displaced organs were then returned to their original position within the abdominal cavity, and the abdominal muscle was closed using interrupted sutures followed by closure of the skin with a single continuous suture.
Prior to being returned to their home cages, rats recovered on a water circulating warming pad until their righting reflexes had completely returned. To minimize post-surgical discomfort and pain, Buprenorphine (0.01 mg/kg; s.c., Buprenex®, Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA) was administered prior to discontinuation of Isoflurane and then again once every 24 h over the following 72 h post-surgery. Also, to prevent post-surgical dehydration, rats were given an injection of warm sterile saline (6 ml; s.c.). Both body weight and intake of food and water were monitored throughout the duration of the recovery period to ensure that rats did not experience any post-surgical malaise.
Control procedures were run to evaluate whether tracer leakage from the CSMG into the surrounding parenchyma or intraperitoneal space might be a source of neural labeling (Fox and Powley, 1989): Following laparotomy and exposure of the left CSMG, as described above, 6 µl of dextran biotin was dispensed over the exterior surface of the CSMG of control rats (n = 4). The complete absence of labeled terminal fields in the gut wall of these four rats indicated that labeled terminals in the ganglia-injected group were a result of tracer being incorporated into the neurons of the left CSMG and transported along their axons to their terminal arbors located in the gut wall and were not a byproduct of tracer leakage.
Tissue Fixation and Dissection
Nine days after tracer injection, rats were weighed, euthanized with a lethal dose of sodium pentobarbital (180 mg/kg, i.p.), and perfused through the left ventricle of the heart with 200 ml of 0.01M PBS at 40°C followed by 500 ml of 4% paraformaldehyde in 0.1 M PBS (pH 7.4) at 4°C. Prior to the perfusion, 0.5 ml of heparin was injected in the left ventricle to facilitate exsanguination.
After fixation, the stomach and small intestine were removed and sampled according to the criteria of Hebel and Stromberg (1976). Specifically, the stomach was divided into dorsal and ventral halves by cutting along the greater and lesser curvatures. Whole mounts of the small intestine consisted of 3 cm long sections from the duodenum (the first 6 cm anal to the pyloric sphincter), mid-jejunum (middle 3 cm of the small intestine), and ileum (the first 6 cm oral to the ileocaecal junction). Intestinal whole mounts were opened by cutting along the longitudinal axis of the mesenteric attachment. The specimens were rinsed under tap water and further fixed overnight in 4% paraformaldehyde in 0.1 M PBS (pH 7.4) at 4°C. Intact muscularis externa whole mounts consisting of the circular and longitudinal muscle layers, with the myenteric plexus sandwiched in-between, were then isolated by removing the mucosa and submucosa from each specimen.
Staining
Free floating whole mounts were rinsed in PBS followed by a 30 min endogenous peroxidase block (methanol:3% H2O2; 4:1). Following additional PBS rinses, whole mounts were rinsed in dH2O and then stained in 0.5% Cuprolinic Blue (quinolinic phthalocyanine; 17052; Polysciences, Inc., Warrington, PA) in 0.05 M sodium acetate buffer containing 1.0 M MgCl2 (pH 4.9) for 2 h in a humidified slide warmer at 38°C. Cuprolinic Blue is a neuron-specific stain that is a pan-neuronal marker for myenteric neurons and is compatible with the dextran biotin protocol (Phillips et al., 2004; Walter et al., 2009). Whole mounts were then rinsed in dH2O, differentiated for 2 min in 0.05 M sodium acetate buffer containing 1.0 M MgCl2 (pH 4.9), and rinsed again in dH2O followed by 3 × 5 min PBS rinses. Whole mounts were then soaked for 3 d in PBS containing 0.5% Triton X-100 and 0.08% Sodium Azide to improve penetration through the muscle sheets. Next, free floating whole mounts were rinsed in PBS for 6 × 5 min followed by a 60 min soak in ABC (prepared according to the manufacturer’s directions; Cat# PK-6100; Vectastain Elite ABC kit; Vector Laboratories; RRID:AB_2336819). Whole mounts were then rinsed in PBS for 6 × 5 min, reacted in DAB solution for 3 min, followed by 6 × 5 min rinses in dH2O. The specimens were mounted on gelatin-coated slides, crushed for 90 min, air-dried overnight, dehydrated in an ascending series of alcohols, cleared in xylene, and coverslipped with Cytoseal (Richard-Allan Scientific, Kalamazoo, MI, USA).
Fluorescent Immunohistochemistry
To determine if tracer injection into the CSMG inadvertently labeled visceral afferent fibers of passage originating from the dorsal root ganglia (DRG), 12 rats were injected with dextran biotin into the CSMG as described above. Following fixation and dissection of the GI tract into regional whole mounts, a fluorescent double labeling protocol was employed to determine the relationship of dextran filled terminals with the sympathetic innervation of the gut visualized using antibodies to the noradrenaline- (as well as dopamine- and adrenaline-) synthesizing enzyme tyrosine hydroxylase (TH).
Free floating whole mounts were incubated for 5 d in PBS containing 0.5% Triton X-100 and 0.08% sodium azide, followed by a 1 h soak in the same solution to which streptavidin ALEXA Fluor 594 (1:1000; Cat# S11227; Life Technologies; RRID:AB_2313574) had been added. Whole mounts were then rinsed in PBS followed by an overnight block in PBS containing 5% normal goat serum, 2% bovine serum albumin, 0.3% Triton X-100, and 0.08% sodium azide, and then incubated for an additional 24 h in a primary antibody diluted with the same blocking solution. Different whole mounts were exposed to either a mouse TH (1:500; Cat# 22941; ImmunoStar, Hudson, WI; RRID:AB_572268) or a rabbit TH (1:1000; Cat# P40101-0; Pel Freez Biologicals, Rogers, AR; RRID:AB_2313713) antibody. Whole mounts were then rinsed in PBS and 0.3% Triton X-100, and incubated for 2 h at room temperature in the appropriate secondary conjugated to ALEXA Fluor 488 (i.e., either goat anti-mouse, Cat# A11029, RRID:AB10566286 or goat anti-rabbit, Cat# A11034, RRID:AB_10562715; both purchased from Life Technologies) diluted 1:500 with the same solution used for the previous rinses. Finally, double-labeled whole mounts were rinsed in PBS, mounted on gelatin-coated slides, cover-slipped with Dako Fluorescent Mounting Medium (S3023; Dako North America, Inc., Carpinteria, CA), sealed with CoverGrip Coverslip Sealant (23005; Biotium, Inc., Hayward, CA), and stored at 2–8°C in the dark until examined for fluorescent signal.
The degree of co-localization between the injected tracer and the TH antigen was determined using an Olympus DSU (Disk Scanning Unit) spinning disk confocal microscope attached to a BX61 motorized microscope (Olympus, Center Valley, PA). The confocal microscope was controlled using SlideBook digital microscopy software (V5.0; Intelligent Imaging Innovations, Denver, Co). Double-labeled sections were imaged using highly selective filter sets specific for the visualization of ALEXA Fluor 594 (XF102-2; Omega Optical) and ALEXA Fluor 488 (QMAX-Green; Omega Optical, Brattleboro, VT). A 40× water objective lens (NA = 1.15) was used. Extended-focus images or z-series consisting on average of 21 optical sections at an optimized z-increment of 0.38 µm were created. In some cases, z-stacks were compressed into one focal plan (i.e., a maximum value projection). To test for co-localization, a whole mount was systematically scanned for ALEXA Fluor 594. When a well labeled terminal neurite was identified, the upper and lower limits of the terminal were determined, with the experimenter blind to the ALEXA Fluor 488 wavelength, and a z-stack was captured using both filter sets. Each focal plane with the two channels merged was then examined individually for co-localization throughout the entire z-stack.
Antibody Characterization
Table 1 summarizes information about the primary antibodies used in this study to identify the sympathetic motor/tyrosine hydroxylase-positive innervation of the smooth muscle layers of the gut wall. The mouse monoclonal TH antibody (ImmunoStar Cat# 22941, RRID:AB_572268) was raised against TH purified from rat PC12 cells, and the antibody is described as having wide species crossreactivity (manufacturer’s datasheet). The immunogen used to produce the rabbit polyclonal TH antibody (Pel-Freez Biologicals Cat# P40101, RRID:AB_2313713) was SDS-denatured TH from rat pheochromocytoma, and shown by the manufacturer using western blot analysis to be specific for the ≈60 kD TH protein in rat caudate lysate. Immunostaining in the gut wall for both antibodies was consistent with the pattern of sympathetic innervation reported previously in GI whole mounts (Tan et al., 2010; Phillips et al., 2013), which is eliminated following celiac and superior mesenteric ganglionectomy surgery (Phillips et al., 2013). No positive reactions were observed in any whole mounts in which TH antibodies were omitted from the protocol.
Table 1.
Antibodies
| Name | Immunogen | Host | Source | Dilution |
|---|---|---|---|---|
| Tyrosine Hydroxylase | Tyrosine hydroxylase, purified from rat PC12 cells | Mouse | ImmunoStar 22941 RRID:AB_572268 | 1:500 |
| Tyrosine Hydroxylase | SDS-denatured tyrosine hydroxylase, purified from pheochromocytoma | Rabbit | Pel-Freez Biologicals P40101-0 RRID:AB_2313713 | 1:1000 |
Whole Mount Evaluation and Analysis
Criteria for myenteric ganglia
In keeping with the criteria of Bar-Shai and coworkers (2004), two or more myenteric neurons clustered together were considered a ganglion. If a cluster of cell bodies was separated from another cluster of cell bodies by a distance of three or more average neuron diameters, then they were considered two separate ganglia (cf. Berthoud et al., 1997). Also, if a group of neurons was isolated from another group by a string of successive single-file neurons, that was as much as, or more than, three average neuronal diameters in length, then the two groupings were considered to be separate ganglia (cf. Berthoud et al., 1997).
Criteria for myenteric plexus connectives
Enteric circuitry and smooth muscle tissues were distinguished according to commonly used criteria (cf. Scheuermann and Stach, 1984; Furness, 2006a). Briefly, the primary myenteric plexus was considered to be formed of both the myenteric ganglia and the major connectives interconnecting those ganglia (also referred to as interganglionic connectives or interconnective strands). The secondary plexus was defined as those higher order connectives that (a) issue from the myenteric ganglia or interganglionic connectives, (b) lie in a network deep to the primary plexus and in contact with the circular muscle, and (c) consist of fascicles running predominately parallel to the circular muscle fibers. The tertiary plexus was defined as the network of higher order ENS connectives that (a) issue from the myenteric ganglia, interganglionic connectives, or secondary plexus, (b) lie in a network situated at the level of, or just superficial to, the primary plexus and abutting the deeper face of the longitudinal muscle sheet wall, and (c) consist of fascicles running predominately parallel to longitudinal muscle fibers.
The secondary plexus carries axons of extrinsic (and intrinsic) efferent (and afferent) neurons that innervate the circular muscle sheet (Wilson, et al., 1987; Furness, 2006b), whereas the tertiary plexus carries axons that innervate the longitudinal muscle sheet (Llewellyn-Smith et al., 1993). These conventional distinctions and inferences were used in categorizing the tissue targets of the labeled CSMG axonal arbors.
Sympathetic Terminal Arbor Inventories and Morphometry
Initial screening
The whole mounts were first systematically scanned at 400× magnification with brightfield illumination. Once an arbor was identified, it was further inspected to determine whether it satisfied the following four criteria: (a) well labeled, (b) completeness of the axon, (c) minimal tissue artifacts such as folds and tears, and (d) free of multiple independent axons interdigitating or overlapping in a manner that might confuse the evaluation of a given terminal field.
Morphometry
A total of 254 sympathetic axons that satisfied the histological criteria for morphometry were located in the initial screening for staining quality and specimen integrity. Of that inventory, 160 axons were randomly selected for digitization, and individual sympathetic terminal arbors were then subsequently traced, digitally reconstructed, and evaluated quantitatively using a Neurolucida (MicrobrightField Inc., Williston, VT, USA; RRID:nig-0000-10294) workstation employing a Zeiss Axio Imager Z2 microscope (Carl Zeiss Microimaging, Gottingen, Germany) equipped with DIC optics and both a 40× dry and a 63× water immersion long working distance objective.
For each of the digitized sympathetic arbors, the following standardized morphometric measures captured with the Neurolucida software were analyzed for all neurites: total arbor length, parent axon length (using the Strahler Analysis algorithm), varicose neurite length, total number of terminal branches, highest branch order, and two-dimensional terminal field size (using the Convex Hull algorithm [cf. Powley et al., 2012, 2013a, 2013b, 2014]).
Criteria for Sympathetic Postganglionic Innervation or Contacts
Two different indices were used to independently estimate the extent of innervation of, or contact with, tissue targets by sympathetic axons: 1) Consistent with conventional observations (e.g., Falck and Owman, 1966; Furness et al., 2014), beaded segments of sympathetic axons exhibiting substantial numbers of varicosities were assumed to release transmitter to the immediately adjacent tissues from those varicosities. 2) Similarly, consistent with the common assumption (e.g., Levitan and Kaczmarek, 2002), the highest order branches, or distal terminal tips, of sympathetic motor fiber arbors were assumed to be sites of transmitter release.
Assessments of the proportions or extent of innervation patterns of different tissues in the gut wall were developed from the above criteria of postganglionic innervation. In the case of the primary plexus (myenteric ganglia and their primary interconnective strands), both of the measures were recorded. The first measure consisted of counting the number of ganglia that had either a terminal branch within a ganglion or the end of the terminal branch in a primary connective neighboring a ganglion. The second measure involved counting the number of ganglia with varicose fibers passing through or in close proximity. In the case of the myenteric plexus, both measures, that is both a terminal branch and a beaded varicose axon, needed to satisfy a neurite-to-ganglion proximity rule were defined as having a distance from a ganglion of less than or equal to half the average neuron diameter of Cuprolinic blue labeled myenteric neurons. This distance was chosen based on the expected distance of neurotransmitter diffusion (Read and Burnstock, 1969; Furness, 1970b; Gillespie and Maxwell, 1971). The total length of each arbor’s varicose segments located in conjunction with each of the types of tissue (i.e., myenteric ganglia and primary plexus; circular muscle sheet and secondary connectives; longitudinal muscle sheet and tertiary connectives) was also determined and then expressed as a percentage of the total length of all of that arbor’s segments.
Both criteria of postganglionic innervation were similarly used to evaluate the more direct projections to the smooth muscle sheets: 1) Labeled terminal axonal arbor branches and varicose segments of the arbor within the circular muscle or in the secondary plexus in immediate apposition to the muscle were assumed to directly innervate muscle fibers. 2) Similarly, labeled terminal axonal branches and varicose arbor segments in the longitudinal muscle or in the tertiary plexus in immediate apposition with the muscle sheet were assumed to directly innervate longitudinal muscle fibers.
For these estimates of muscle sheet innervation, both the number of terminal branches and the length of varicose arbor segments in association with the muscle sheets were determined and used as separate measurements of degree of innervation to confirm and cross-validate the sympathetic terminal field innervation of muscle in the gut wall. As in the case of the primary myenteric plexus, the proximity rule (i.e. within half an average neuron’s diameter of the putative target tissue) was used.
Final Postganglionic Samples
In the final analysis, after the 160 sympathetic arbors had been traced and digitized, the reconstructions of 154 of the fibers were ultimately used for quantitative comparisons of myenteric ganglion and smooth muscle innervation. These 154 axonal terminal arbors yielded representative samples for each of the five regions prepared as whole mounts (stomach: n = 22; duodenum 0–3 cm: n = 33; duodenum 3–6 cm: n = 29; jejunum: n = 20; ileum: n = 50).
The remaining six of the 160 randomly chosen motor arbors were evaluated separately but not included in quantitative analyses of terminal patterns: two of the six arbors were judged problematic for complete quantitative assessment because the specimens traversed regions with incomplete Cuprolinic Blue staining. The remaining four of the six cases projected to the vasculature rather than the smooth muscle sheets; these vascular efferents were evaluated and are described separately.
Photography
Single high power images were acquired with a Spot Flex camera controlled by Spot Software (V4.7 Advanced Plus; Diagnostic Instruments, Sterling Heights, MI; www.spotimaging.com) mounted on a Leica DMRE. Multi-field composite (or mosaic) images containing the entire area of innervation by a sympathetic axon were generated at high magnification (630×; water immersion objective) with a Leica DFC310 FX digital CD color camera mounted on a Leica DM5500, running Surveyor with Turboscan software (V. 6.0.5.3; Objective Imaging, Cambridge, UK; www.objectiveimaging.com). To capture the varying depth of a neurite within a smooth muscle whole mount, each image in a mosaic typically consisted of multiple focal z-planes that were merged using either Photoshop CS5 (Adobe Systems, San Jose, CA; www.adobe.com) or Helicon Focus Pro X64 software (V5.3.7; HeliconSoft Ltd, Kharkov, Ukraine; www.heliconsoft.com) to generate an all-in-focus image. Photoshop CS5 was also used in final figure production to: 1) apply text and scale bars; 2) adjust brightness, contrast, color, hue, and sharpness; and 3) organize the final layouts of the figures.
Statistics
Statistical analyses and graph generation were accomplished using GraphPad Prism (V. 5.0; GraphPad Software, San Diego, CA, USA; RRID:rid_000081). A one-way ANOVA with Tukey’s post hoc test (corrected for multiple comparisons) was performed to determine significant differences between groups. Data is presented as means ± SEM. Statistical significance was considered p < 0.05.
Results
Co-localization of Dextran Biotin Labeled Terminals with Tyrosine Hydroxylase
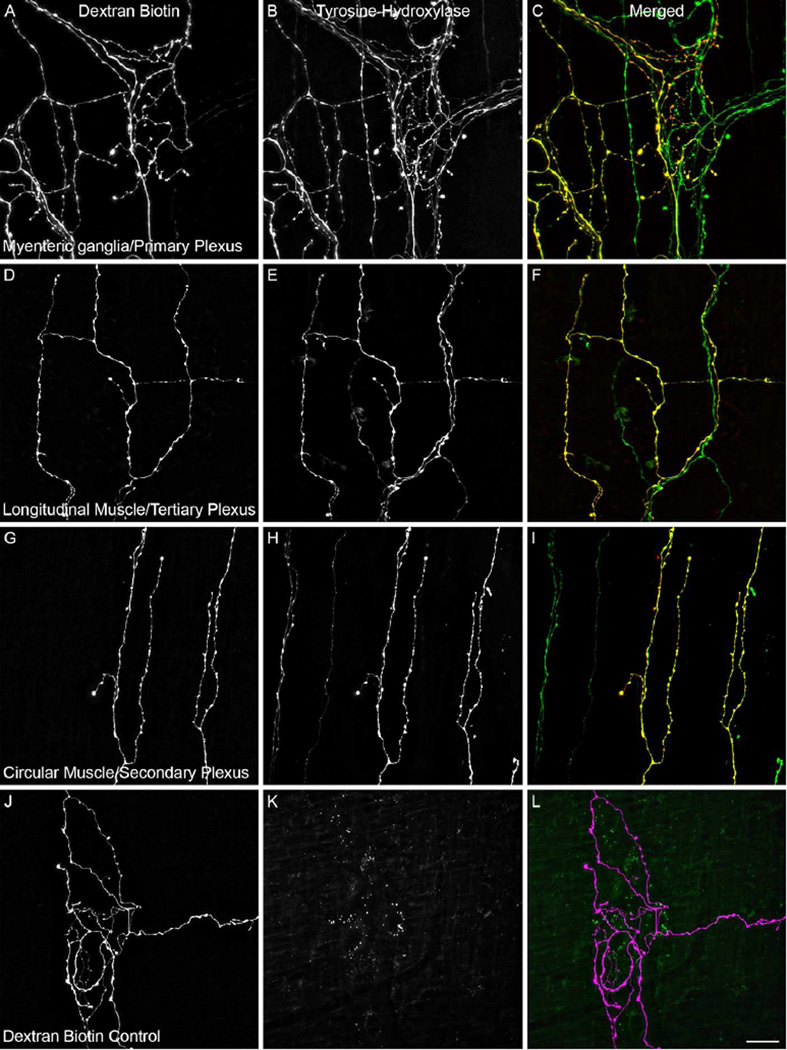
Co-localization analysis was done on 136 dextran labeled terminals located in the muscle wall of jejunal whole mounts. One hundred dextran labeled terminals were evaluated for co-localization with the polyclonal rabbit TH antibody, and an additional 36 dextran labeled endings were evaluated for co-localization with the monoclonal mouse TH antibody. The distribution of arbors sampled consisted of 54 in the myenteric ganglia/1° plexus, 43 in the longitudinal muscle/3° plexus, and 39 in the circular muscle/2° plexus; Figure 1A–I. In all, only one dextran labeled terminal (located in the circular muscle/2° plexus) was found to be TH-negative. Qualitative evaluation of randomly chosen dextran labeled arbors located in the smooth muscle wall of the stomach, duodenum, and ileum were found to be similarly extensively co-localized with TH.
Figure 1.
Labeled arbors located in the gut wall following injection of dextran into the celiac and superior mesenteric ganglia (CSMG) were determined to be predominately catecholaminergic postganglionic neurites by the presence of the noradrenaline synthesizing enzyme tyrosine hydroxylase (TH). Dextran biotin (left column; A,D,G) labeled neurites were co-localized with TH (middle column; B,E,H) as demonstrated in the merged images (right colum; C,F,I; green neurites are dextran-negative/TH-positive while yellow neurites are dextran-positive/TH-positive) throughout the three target tissues surveyed. ALEXA Fluor 594 labeled dextran-positive neurites (J; and purple labeling in panel L) were absent when the tissue was viewed with the QMAX-Green filter in place (K). Spectral bleed-through was similarly ruled out for the ALEXA Fluor 488 fluorophore by the presence of TH-positive/dextran-negative (green) neurites visible in the merged panels (C,F,I). Scale bar in L = 20 µm.
Omission of the primary resulted in complete loss of TH-positive fibers; Figure 1J–L. Examination of a) dextran-negative/TH-positive arbors in the double labeled material and b) dextran labeled terminals in the primary deletion control material confirmed that the emission spectra of the two fluorophores combined with the filter sets used during the acquisition of the images sufficiently avoided spectral bleed-through artifact and were suitable for co-localization analysis; Figure 1C,F,I, and K.
General Architecture of Sympathetic Postganglionic Terminal Arbors
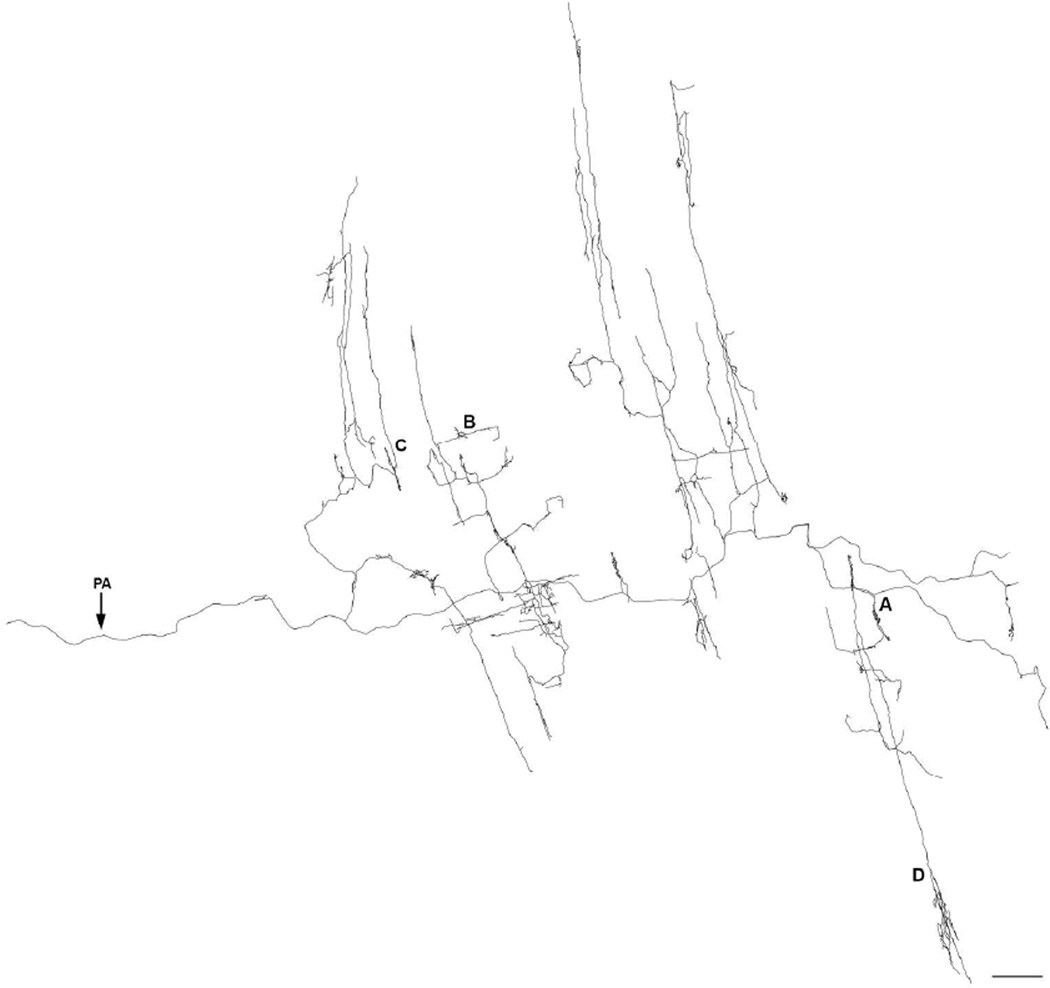
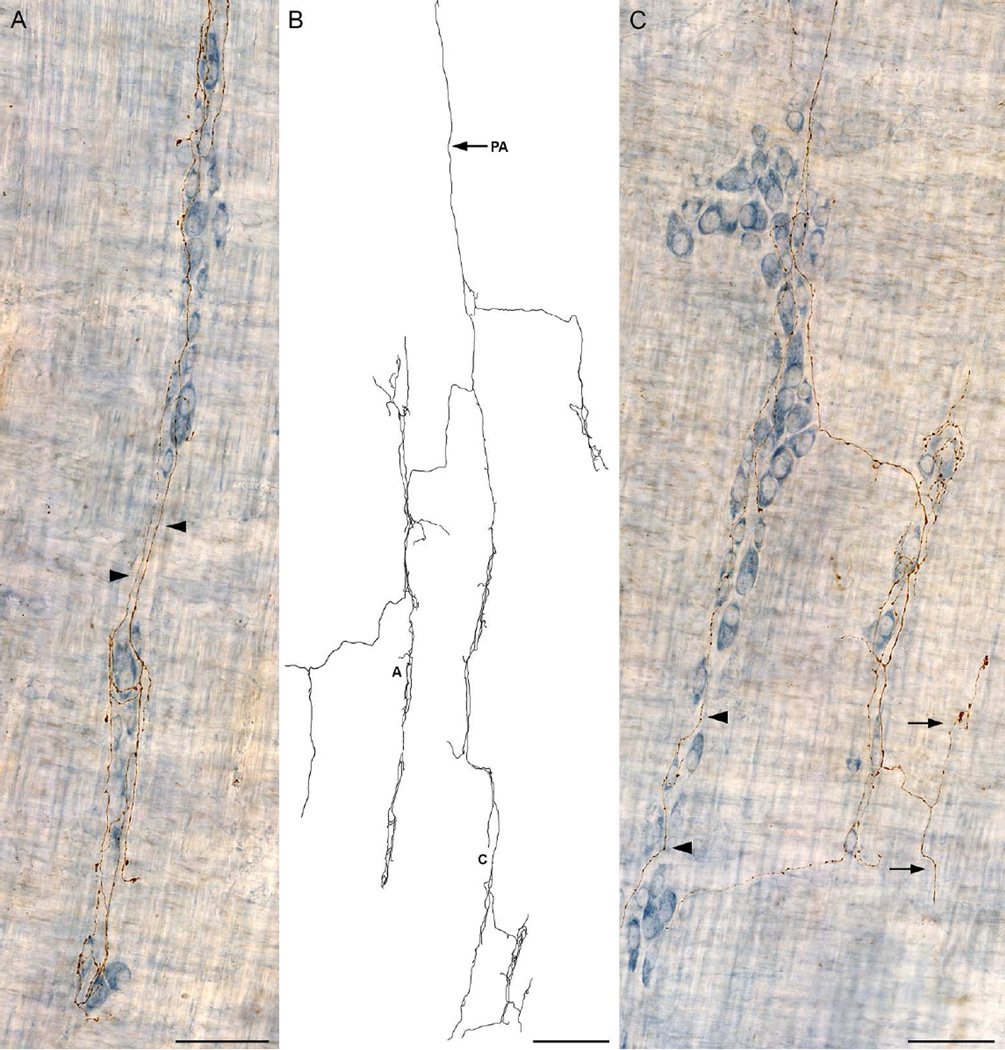
Dextran-biotin labeling of sympathetic axons in whole mounts that preserved the myenteric plexus in situ between the longitudinal and circular muscle sheets provided high-definition delineation of complete motor terminal arbors terminating in the muscle wall of the gut and made it practical to routinely digitize and analyze morphologically individual CMSG axons. Figure 2 illustrates a Neurolucida tracing of a typical CSMG axonal arbor, and Figure 3 contains photomicrographs of different representative sites within the tracing in Figure 2, as designated by the lettering.
Figure 2.
A Neurolucida tracing, collected at 630×, of a single sympathetic neurite with terminal branches and/or varicose fibers of passage located in the myenteric ganglia/1° plexus, the circular muscle/2° plexus, and the longitudinal muscle/3° plexus. The parent axon (PA) is indicated with an arrow. Figure 3 consists of photomicrographs that correspond to the demarcated areas on the tracing. Scale bar = 500 µm.
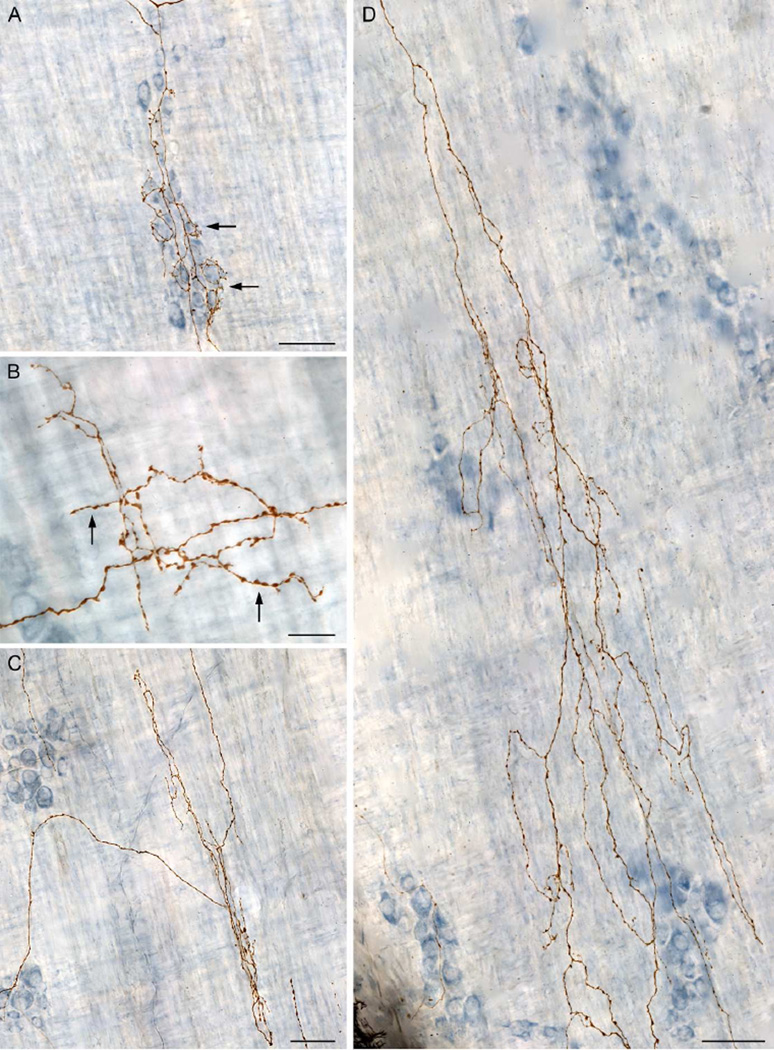
Figure 3.
Images were taken from the traced dextran-biotin labeled ending shown in Figure 2. A: Innervation of the myenteric ganglia/1° plexus by varicose neurites (arrows). B: Varicose fibers terminating in the tertiary plexus (arrows). C: Varicose fibers terminating in the circular muscle/2° plexus. D: Varicose fibers innervating the circular muscle. The dextran-biotin labeled neurites were visualized using DAB (brown) and the myenteric neurons were counterstained with Cuprolinic Blue (blue). Scale bars in A, C, D = 50 µm; B = 20 µm.
In each of the four gut regions sampled (i.e. stomach, duodenum, jejunum and ileum), the basic architecture of individual sympathetic terminal fields throughout the smooth muscle wall consisted of a parent axon entering the gut wall within a bundle or fascicle of fibers, coursing some distance through the myenteric plexus network, and then branching repeatedly to form an extensive terminal array of varicose neurites extending over a large innervation field, making presumptive contacts in multiple different tissues.
The initial screening to identify traceable CSMG arbors produced observations indicating both a need to distinguish different groupings of axonal terminal fields, particularly with reference to the three-neuron and two-neuron models of the sympathetic outflow, and a need to evaluate the disparate ending patterns quantitatively. In brief, one small minority of postganglionic arbors appeared to project strongly to myenteric neurons and the primary plexus, in a configuration consistent with a three-neuron-outflow model, and a second small minority of postganglionic arbors appeared to predominately innervate one or both smooth muscle sheets directly, in a pattern consistent with a two-neuron-outflow model. In contrast, however, a large majority of CSMG postganglionics, as illustrated in Figures 2 and 3, distributed a substantial percentage of their neurites, in parallel, to both the myenteric ganglia/1° plexus and the smooth muscle.
Criteria Distinguishing Subpopulations of Sympathetic Postganglionics
The fine architecture of sympathetic postganglionics in the muscularis externa of the gut wall has not previously been well characterized, so a working criterion to distinguish between the identified subpopulations was generated. First, the 154 arbors in the random sample of postganglionic neurons that innervated the smooth muscle wall were traced and digitized. Since all branches of these arbors projected to only two types of tissue, the myenteric plexus and/or smooth muscle, the proportions or total lengths of each arbor’s varicose neurite branches in myenteric ganglia/1° plexus and in smooth muscle, respectively, were then calculated and used to plot innervation profiles.
More specifically, for innervation profiles, the composite or combined length of all varicose branch segments and terminal branches was obtained separately for each of the individual 154 arbors. Then, for each arbor, as analyzed below, the separate summed lengths of those varicose neurites in apposition to each of the basic types of target tissues innervated (myenteric ganglia/1° plexus; circular muscle/2° plexus; or longitudinal muscle/3° plexus) were separately expressed as percentages of the respective arbor’s full cumulative length of varicose branches. Further, for the purposes both of generating criteria that distinguished subpopulations and of making general comparisons between the two-neuron and the three-neuron models, the projections to the longitudinal and circular muscle layers were then also combined and collectively considered as innervation of “smooth muscle.”
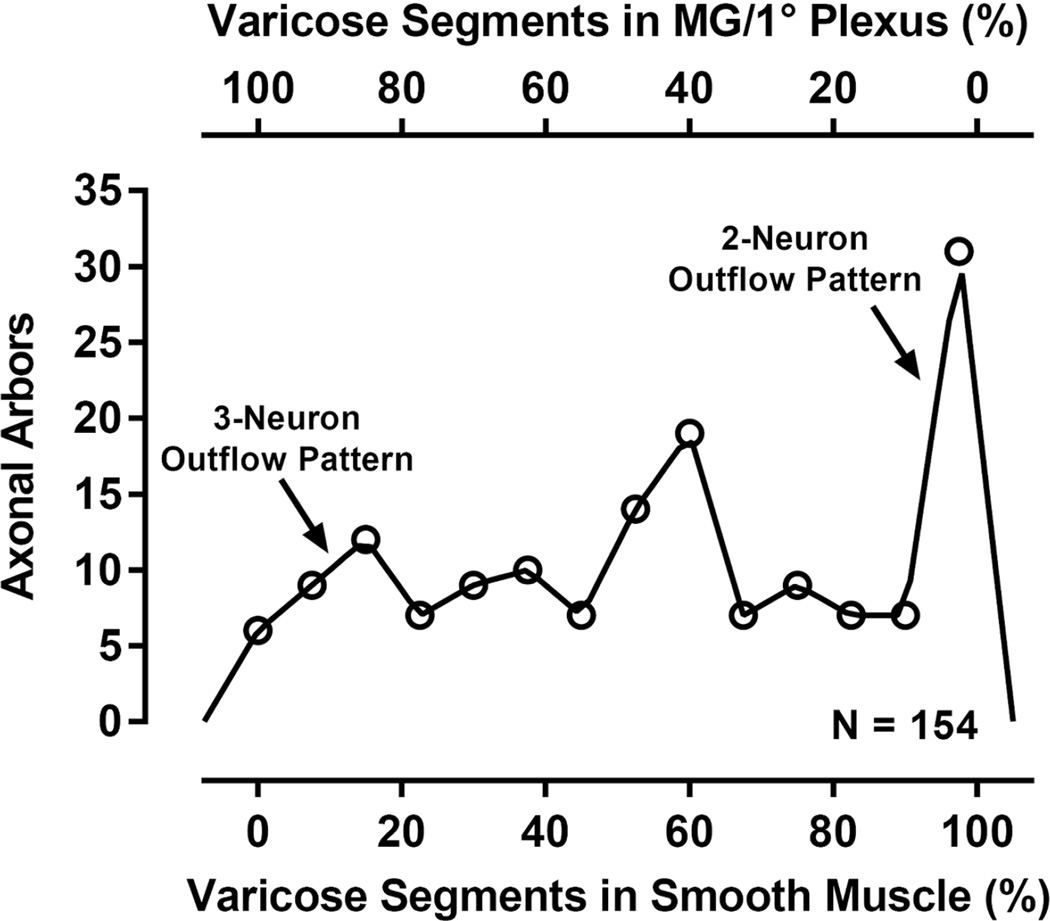
Once the data for the muscle sheets had been combined, the frequency distributions of the numbers of arbors within the sample of 154 cases with different percentages of neurites projected to each of the two broad tissue types (myenteric ganglia/1° plexus; smooth muscle) of the gut wall were examined. Figure 4 illustrates the distribution of all 154 arbors with the different percentages (on the lower x-axis) of their varicose segments projecting specifically to smooth muscle and/or their respective interconnectives. Reciprocally, since all varicose neurites not terminating in muscle terminated in the primary plexus, Figure 4 also illustrates, at the left end of the abscissa (on the upper x-axis), the subpopulation of arbors that project specifically to myenteric ganglia/1° plexus.
Figure 4.
The distribution of the complete sample of 154 CSMG postganglionic arbors (number of arbors on y-axis) plotted as a function of the percent of each arbor’s varicose branches that contacted smooth muscle tissue sites (see lower x-axis) or, conversely, that contacted myenteric ganglion/1° plexus tissue sites (see upper x-axis). The majority of sympathetic arbors did not conform to either a strict two-neuron outflow model or a strict three-neuron outflow model. MG/1° Plexus = myenteric ganglia/1° plexus.
From this neurite distribution in Figure 4, two factors emerged and were used to establish the operationally defined criteria for grouping the 154 arbors examined in the series of specific quantitative comparisons reported below. The most salient factor for identifying subpopulations was that the distribution of arbors was polymodal, including at least three identifiable peaks. The second factor shaping the provisional groupings of the CSMG postganglionic arbors was a set of pragmatic observations about cutoffs that would distinguish the distinct subpopulations that appeared to form clusters in the polymodal distribution of arbor segments illustrated in Figure 4. A strict construction of the commonly used three-neuron and two-neuron ideas of the sympathetic outflow generally might extrapolate to the prediction that an individual arbor would project exclusively to, respectively, the primary myenteric plexus or the smooth muscle, but as Figure 4 indicates, few arbors projected 100% of their varicose neurites to smooth muscle and even fewer arbors projected 0% of their varicose neurites to smooth muscle (and hence 100% to myenteric ganglia/1° plexus).
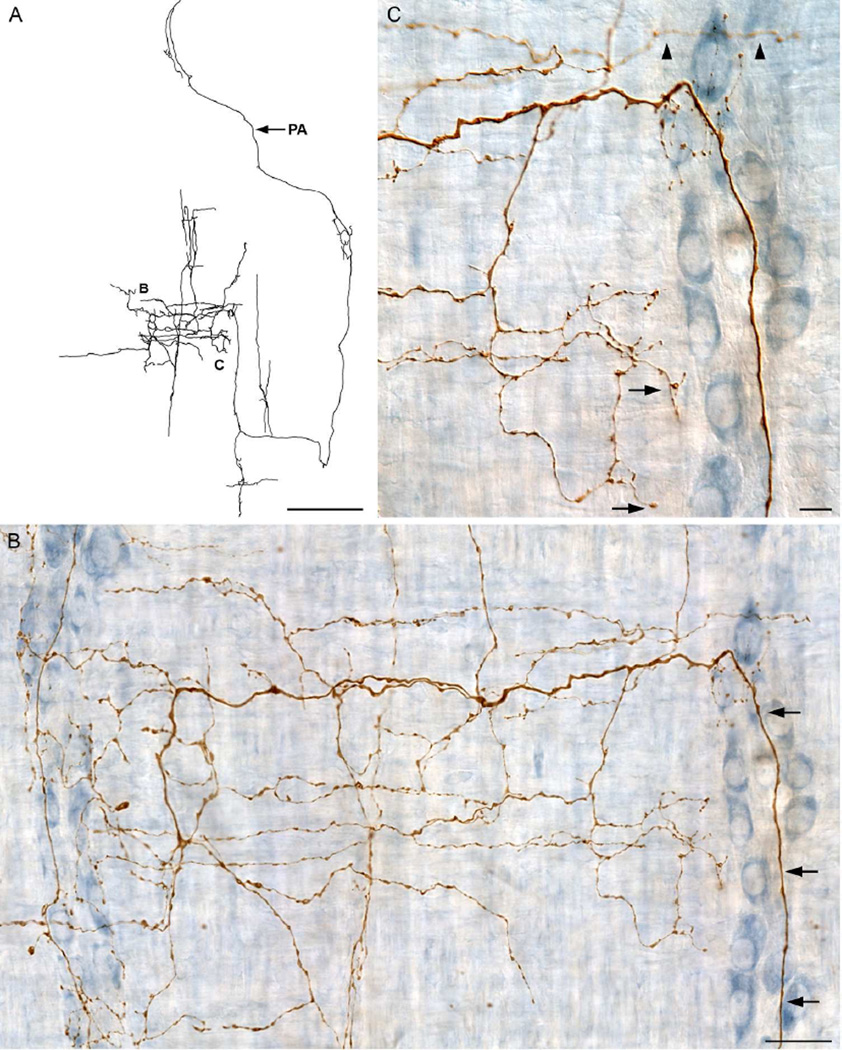
The typical architecture of the postganglionics is illustrated by the axonal terminal arbor in Figure 5 which exemplifies the fact that, even in the case of most of those sympathetic arbors constituting the left-most peak in Figure 4 and projecting predominately to the myenteric ganglia/1° plexus, some of their short branches and/or arrays also typically extended to smooth muscle. Like most of the other arbors in the left-most cluster in Figure 4, the axon in Figure 5 innervated predominately the myenteric ganglia and associated primary plexus, but, nonetheless, the arbor distributed short collateral branches (example designated with arrows in Figure 5C) to the smooth muscle. Specifically, in the case in Figure 5, these collateral varicose branches to the muscle sheet accounted for only 8.5% of the total cumulative length of the fiber’s arbor.
Figure 5.
A dextran-biotin labeled sympathetic axon that innervated predominately the myenteric ganglia/1° plexus. A: Multiple ganglia within the myenteric plexus were innervated with interconnective strand elements (arrowheads) between the ganglia. B: A Neurolucida tracing of the entire terminal from which panels A and C are taken. The neurite consisted of a long parent axon (PA; entering at 12 o’clock) that repeatedly branched to innervate numerous ganglia and interconnective strands of the myenteric plexus. C: Varicose fibers can be seen within the ganglia and passing through the ganglia (arrowheads). A small spur innervates the circular muscle/2° plexus (arrows). Scale bars in A & C = 63 µm; B = 250 µm.
Examination of the left-most and right-most peaks in Figure 4 (corresponding, respectively, to arbors that terminate nearly exclusively in the myenteric ganglia/1° plexus or the smooth muscle) makes it clear that, a restrictive or “exclusive” decision rule requiring that 100% of an arbor’s segments need project to the relevant target tissue, in order for that individual postganglionic to be classified as projecting to a particular target, does not include all of the arbors comprising the two clusters close to the limits of the distribution in Figure 4.
A strategy of defining a less restrictive and more practical cutoff, one that better reflected the clustering patterns in the distribution of Figure 4, was to find the nadir immediately to the right of the left-most peak and the nadir just to the left of the right-most peak and then to use the average of the two nadirs to generate a cutoff criterion. Given that the average of the two nadirs rounded to essentially 15%, we used that value for the cutoff of the left-most peak and the complementary value of 85% as the cutoff of the right-most peak. Arbors with 85% or more of their varicose segments projecting to a particular type of tissue were considered to predominantly innervate that tissue. In contrast, individual postganglionic arbors that comprised the subpopulation in the broad middle peak or cluster in Figure 4, which distributed roughly equivalent proportions of their neurites to the two tissues and did not distribute as much as 85% of their varicose branches to either the myenteric ganglia/1° plexus or the smooth muscle/associated interconnectives, were considered mixed or heterotypic neurons with substantial inputs to both types of tissues and a predominant input to neither type of tissue.
Thus, based on these observed clustering patterns and considerations, we adopted a standard terminology of designating arbors that supplied 85% or more (but less than 100%) of their beaded branch segments to one tissue or the other as predominately innervating that tissue, and more equally distributed arbors that did not issue as much as 85% of their varicose branches to either single tissue type as providing mixed or heterotypic innervation of the two tissues.1 Arbors that projected 100% of their varicose neurites to either one tissue or the other were considered as exclusively innervating that tissue.
A Limited Number of Sympathetic Postganglionic Axons Predominantly (or Even Exclusively) Innervated Myenteric Neurons
Given the widespread use of a three-neuron model of the sympathetic outflow, with the corollary that CSMG axons would presumably project to myenteric ganglion neurons, all 154 sympathetic arbors in the sample were assessed in terms of such a pattern.
When the inclusive criterion (≥ 85% varicose neurites contacting) was used for the myenteric ganglia/1° plexus, a limited minority (i.e. 13%, or 20 of the 154 arbors) innervated, predominately, the myenteric ganglia/1° plexus; Figure 4.
When the varicose arbor distribution criterion was more stringently applied (~100% of varicose neurites contacting), only three axons or 2% of the 154 arbors had terminal branches and varicose fibers of passage exclusively limited to the myenteric ganglia/1° plexus. In the case of these three neurons with all presumptive contacts limited to the myenteric ganglia/1° plexus, innervation of the plexus consisted of 59% of the axon segment length contained within the myenteric ganglia proper and the remaining 41% of the neurite length terminating within the primary interconnective strands of the plexus.
Another Minority of Sympathetic Postganglionic Axons Projected Predominantly (or Even Exclusively) to the Smooth Muscle Sheets
Though the neuronal projections to the two distinct smooth muscle sheets were at times combined for purposes of comparisons and graphing (e.g., Figure 4), for full quantitative assessments, the arbors distributing to smooth muscle were separated into one subset that projected to circular muscle and a second subset that projected to longitudinal muscle.
Postganglionic Phenotype Projecting Predominantly (or Even Exclusively) to Circular Muscle/2° Plexus
When the 15% criterion based on the nadirs around the polymodal clusters, i.e. the criterion used to establish predominant projection patterns, was applied, a separate minority (27%, or 42 of the 154 arbors—those comprising the right-most peak in Figure 4) directly—or predominantly—innervated circular smooth muscle. These cases were typically morphologically simple and usually consisted of a parent axon that extended a short distance from the mesenteric border towards the anti-mesentery and terminated as arrays that projected within the circular muscle/2° plexus, see Table 2.
Table 2.
Distribution of Traced Endings
| Site of Innervation | |||||
|---|---|---|---|---|---|
| Region | Sample Size | Mixed or Heterotypic |
Predominantly Smooth Muscle |
Predominantly MG/1° Plexus |
|
| Stomach | 22 | 15 | 5 | 2 | |
| Duodenum 0–3 | 33 | 19 | 10 | 4 | |
| Duodenum 3–6 | 29 | 21 | 8 | 0 | |
| Mid Jejunum | 20 | 15 | 3 | 2 | |
| Ileum | 50 | 22 | 16 | 12 | |
| Σ | 154 | 92 | 42 | 20 | |
| % | 60 | 27 | 13 | ||
MG/1° Plexus = myenteric ganglia/primary plexus.
If the more stringent ~100% criterion was applied, 14% (22 of the 154 arbors) distributed their varicose branches exclusively to the circular muscle/2° plexus.
Postganglionic Phenotype Projecting Exclusively or Predominantly to Longitudinal Muscle/3° Plexus
As anticipated from earlier reports, direct innervation of the longitudinal muscle by dextran labeled fiber segments was extremely rare (N = 2; 1% of the 154 arbors) while there was considerable innervation of the tertiary plexus. Since these earlier reports suggest that sympathetic fibers in the tertiary plexus are in such close proximity to longitudinal muscle fibers and express varicosities along the lengths of the terminal branches within the tertiary plexus, the sympathetic innervation within the tertiary plexus is commonly assumed to also target the longitudinal muscle (Read and Burnstock, 1969; Furness, 1970b; Gillespie and Maxwell, 1971; Llewellyn-Smith et al., 1993). This conventional assumption was used for purposes of the present analyses, and thus the innervation of the longitudinal muscle was considered to be a composite of the few fibers in the longitudinal muscle bundles and the varicose fibers running in the tertiary plexus.
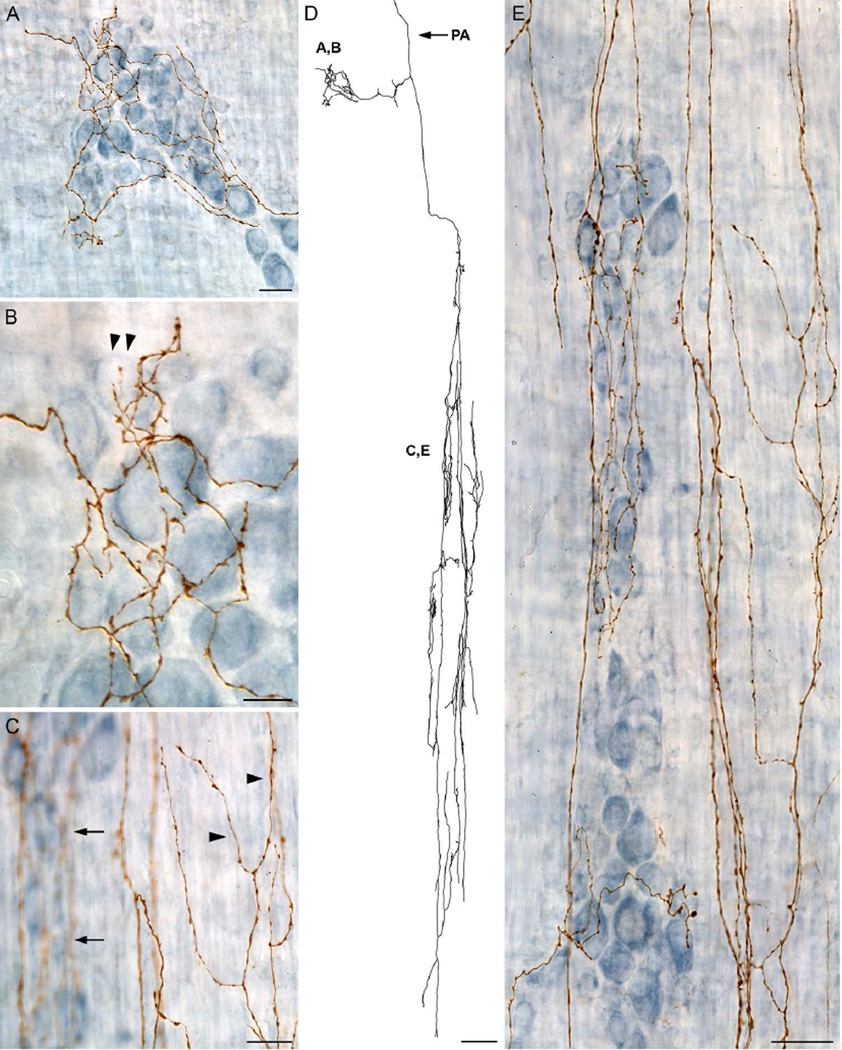
Innervation of the tertiary plexus by dextran labeled fiber segments consisted of branches that tended to have a winding nature as they projected in a predominately longitudinal manner; Figure 3B and 6A. While some axons making up the tertiary plexus were observed within the same focal plane as the myenteric ganglia/1° plexus, other fibers were just-superficial to the ganglia and traversed primarily in the longitudinal axis; Figure 6B. Bifurcating arbors within the tertiary plexus exhibited varicosities along their length and frequently terminated either within the interganglionic space or adjacent to the myenteric ganglia; Figure 6C.
Figure 6.
A dextran-biotin labeled sympathetic axon that innervates the myenteric ganglia/1° plexus, the circular muscle/2° plexus, and the longitudinal muscle/3° plexus. A: The prominent feature of the traced neurite, which is shown in its entirety, was the extensive innervation of the longitudinal muscle/3° plexus between parallel myenteric ganglia (dark blue stained neurons shown in panel B). The parent axon (PA) is indicated with an arrow. B: The smooth large diameter parent axon (arrows) passes through the myenteric ganglia/1° plexus (dark blue neurons), makes a 90 degree turn, and enters the longitudinal muscle/3° plexus where it branches extensively into small caliber varicose axons that run predominately perpendicular to the myenteric ganglia. C: A higher power image of the myenteric ganglia/1° plexus (dark blue neurons), shown in panel B, with the ganglia in focus to illustrate how the small diameter, varicose axons innervating the longitudinal muscle/3° plexus pass both underneath the myenteric ganglia (slightly out of focus axons; arrowheads) and along the border of myenteric ganglia (in focus axons; arrows). Scale bars in A = 250 µm; B = 31 µm; C = 10 µm.
Relevant to the present classifications, the tertiary plexus was never the singular target of any of the analyzed sympathetic axons. Rather, if a neurite innervated the tertiary plexus then it was always the case that the same neurite also innervated one or more other sites.
A Majority of Sympathetic Axons Formed “Mixed” or “Heterotypic” Arbors That Co-innervated the Myenteric Plexus and Smooth Muscle
While modest numbers of CSMG efferents conformed to the expectations that were extrapolated from the three-neuron and the two-neuron chain models of the sympathetic outflow, the majority (60%, or 92 of the 154 cases) of the CSMG postganglionic terminal arbors were more complex and extensive, ending heterotypically in different target tissues at different sites. Specifically, this majority of sympathetic fibers directly innervated both the myenteric ganglia/1° plexus, providing substantial numbers of collaterals or contacts directly to myenteric neurons, and, in parallel, to smooth muscle, by issuing significant numbers of collaterals or contacts directly to a muscle sheet; Figures 2 & 3; also Table 2.
These mixed or heterotypic arbors sorted into three subgroups according to their smooth muscle projections. Notably, a common feature of the three groupings was that the heterotypic endings always included innervation of the myenteric ganglia/1° plexus. The three mixed ending subgroups consisted of endings that innervated (a) the myenteric ganglia/1° plexus and the circular muscle/2° plexus, (b) the myenteric ganglia/1° plexus and the longitudinal muscle/3° plexus, and (c) the myenteric ganglia/1° plexus, the circular muscle/2° plexus, and the longitudinal muscle/3° plexus; see Table 3.
Table 3.
Distribution of Mixed or Heterotypic Arbors in the GI Tract
| Sites of Innervation | |||||
|---|---|---|---|---|---|
| Region | Sample Size |
MG/1° Plexus CM/2° Plexus |
MG/1° Plexus LM/3° Plexus |
MG/1° Plexus CM/2° Plexus LM/3° Plexus |
|
| Stomach | 15 | 2 | 0 | 13 | |
| Duodenum 0–3 | 19 | 1 | 2 | 16 | |
| Duodenum 3–6 | 21 | 3 | 8 | 10 | |
| Mid Jejunum | 15 | 2 | 5 | 8 | |
| Ileum | 22 | 5 | 2 | 15 | |
| Σ | 92 | 13 | 17 | 62 | |
| % | 14 | 19 | 67 | ||
MG/1° Plexus = myenteric ganglia/primary plexus
CM/2° Plexus = circular muscle/secondary plexus
LM/3° Plexus = longitudinal muscle/tertiary plexus
The different groupings or phenotypes of heterotypic sympathetic terminal fields varied in their frequency of occurrence. Using the criterion that only postganglionics with both >15% of their varicose branch lengths in myenteric tissue and also > 15% of their varicose contacts in smooth muscle were considered mixed or heterotypic, it was still the case that the largest group (N = 92) of dextran labeled arbors were mixed endings that innervated different types of sites; see Table 3. As mentioned above, these complex mixed endings always innervated the myenteric ganglia/1° plexus and one or more smooth muscle sheets. In fact, the largest group of mixed endings consisted of neurites that innervated the myenteric ganglia/1° plexus, plus circular muscle/2° plexus, and also longitudinal muscle/3° plexus (N = 62); Figures 3 & Figure 6, followed by neurites that innervated the myenteric ganglia/1° plexus and the longitudinal muscle/3° plexus (N = 17), and lastly, neurites that innervated the myenteric ganglia/1° plexus and the circular muscle/2° plexus (N = 13); Figure 7.
Figure 7.
A dextran-biotin labeled sympathetic axon that innervates the myenteric ganglia/1° plexus and the circular muscle/2° plexus. A: Upon innervating a ganglion, a neurite branches extensively giving off numerous collateral varicose processes. B: A high power image of one pole of the ganglia illustrating the close association between varicose processes and the myenteric neurons that they encircle (arrowheads). C: Innervation of the circular muscle/2° plexus is shown in focus (arrowheads) while the innervation of the myenteric plexus by collaterals from the same parent axon are intentionally shown out of focus (arrows) to illustrate the targeting of the different tissues by the same neurite. D: A tracing of the entire ending highlights the morphology of the long rectilinear multi-branching arrays that run within the circular muscle and superficial to the circular muscle within the secondary plexus. The parent axon (PA) is indicated with an arrow. E: An all-in-focus image of an axonal array innervating the circular muscle/2° plexus with collateral axons produced by the same sympathetic neuron innervating the myenteric plexus which runs parallel to the circular muscle. Scale bars in A & C = 20 µm; B = 10 µm; D = 125 µm; E = 31 µm.
Mixed or Heterotypic Postganglionics Distributed Similar Proportions of Their Arbors to Smooth Muscle and to the Myenteric Plexus
In the case of these heterotypic CSMG postganglionic arbors, the largest subset of sympathetic prostganglionics, the relative proportions distributed to smooth muscle and to the myenteric plexus were compared. For each of the three subgroupings defined by their smooth muscle targets, the myenteric ganglia received the smaller percentage of varicose arbor branches (range from 16% to 26%). An initial evaluation extracted the extent of sympathetic innervation within the various plexuses and muscle (see methods) by identifying the total length devoted to each area and subsequently the proportion of the overall length. Statistical comparisons of the proportional lengths innervating each target were performed using the traced axons that comprised the mixed endings described in Table 3. For the 13 cases in which a neurite innervated both the myenteric ganglia/1° plexus and the circular muscle/2° plexus, a significantly (P < 0.0001) larger percentage of the total arbor length innervated the circular muscle/2° plexus (60%) compared to the myenteric ganglia (16%; P = 0.0002) or the interconnective strands (24%; P = 0.0006). Analysis of the 17 neurites that innervated both the myenteric ganglia/1° plexus compared to the longitudinal muscle/3° plexus revealed an overall significant difference (P = 0.0412), with a greater percentage of the total length innervating the longitudinal muscle/3° plexus (45%) compared to the ganglia (29%; P = 0.1856) and the interconnective strands (26%; P = 0.0483). Conversely, a more uniform pattern of innervation was determined for the 62 heterotypic cases that innervated the myenteric ganglia/1° plexus, circular muscle/2° plexus, and the longitudinal muscle/3° plexus (P = 0.0509) with very similar percentages of overall length innervating each site: ganglia (23%), interconnective strands (29%), circular muscle/2° plexus (27%), and longitudinal muscle/3° plexus (21%).
In addition to the proportion of total arbor length associated with each of the different target tissues, the proportion of the highest order, or terminal, branch innervation was similarly assessed for each site. Although terminal branches are not a direct indication of synaptic modulation due to vesicle release from the varicosities along the axon length, the terminal branches may have some relation to plexus- or site-directed innervation. This analysis was performed on the subclasses of mixed endings; Table 3. The total number of terminal branches within the defined plexuses and circular muscle were summed and a proportion of the overall terminations were calculated. Analysis of the sympathetic arbors that had terminal branches in both the myenteric ganglia/1° plexus and the circular muscle/2° plexus revealed a trend for the circular muscle/2° plexus (46%) to have a greater proportion of terminal branches compared to the ganglia (29%) or the interconnective strands (24%). Analysis of the neurites that had terminal branches in both the myenteric ganglia/1° plexus and the longitudinal muscle/3° plexus revealed an overall significant difference (P = 0.0016), with the largest percentage of terminal branches located in the longitudinal muscle/3° plexus (52%) followed by the ganglia (33%; vs. tertiary plexus, P = 0.1974) and then the interconnective strands (14%; vs. tertiary plexus, P < 0.0001; vs ganglia, P = 0.0272). Lastly, analysis of the terminal branches for the subset of arbors with terminal branches in all three tissue sites revealed a difference (P < 0.0001). There was a similar proportion of terminal branches within the ganglia (35%) and longitudinal muscle/3° plexus (30%), which were both significantly greater compared to the interconnective strands (17%; vs. ganglia, P < 0.0001; vs. tertiary plexus, P = 0.0026) and the circular muscle/2° plexus (18%; vs ganglia, P < 0.0001; vs. tertiary plexus, P = 0.0200); Table 4.
Table 4.
Summary of General Morphometric Parameters
| Region | Total Arbor Length (µm) |
Parent Axon Length (µm) |
Total Number of Terminal Branches |
Highest Branch Order |
Terminal Field Size (µm2) |
|---|---|---|---|---|---|
| Stomach | 77118 ± 17890 |
7456 ± 1454 |
312.9 ± 71.3 |
36.11 ± 3.3 |
30.0 ± 6.5 × 106 |
| Duodenum 0–3 cm |
36887* ± 6682 |
4658* ± 783.3 |
296.6 ± 59.1 |
36.38 ± 3.2 |
7.6* ± 2.0 × 106 |
| Duodenum 3–6 cm |
19580* ± 3013 |
2484* ± 375.4 |
180.6 ± 38.3 |
27.13 ± 3.1 |
2.9* ± 0.9 × 106 |
| Mid- Jejunum |
10263* ± 2342 |
1472*† ± 250.2 |
67.33*† ± 14.5 |
19.83*† ± 2.6 |
2.3* ± 1.2 × 106 |
| Ileum | 12786*† ± 1955 |
1787*† ± 206.3 |
77.88*† ± 12.2 |
18.77*† ± 1.6 |
2.0* ± 0.8 × 106 |
Means ± SEM
Significant difference compared to the stomach
Significant difference compared to duodenum 0–3 cm
Regional Analysis of Myenteric Ganglia Innervation
We and others have shown that there are regional differences in the myenteric plexus located within the smooth muscle wall of the gut (e.g., Karaosmanoglu et al., 1996; Phillips and Powley, 2001), so we sought to determine if these regional differences were also reflected in differences in the sympathetic innervation of the myenteric ganglia. Specifically, we sought to determine if the number of ganglia innervated by individual sympathetic neuronal arbors located in the proximal gut wall (i.e., stomach and proximal duodenum) differed in number from those individual arbors innervating the distal gut (i.e., distal duodenum, mid-jejunum, and ileum).
Our two dependent measures used to determine ganglia innervation by sympathetic arbors (i.e. terminal branches and varicosities) differed in the number of ganglia estimated to be innervated per axon. Specifically, a greater number of ganglia were found to be innervated by the “varicose neurites” criterion compared to the “terminal branches” criterion; however both measures revealed the same proximal-gut-versus-distal-gut pattern of innervation, with a greater number of ganglia in the proximal gut innervated compared to the distal gut. Analysis of ganglia innervation by terminal branches revealed a significant regional difference (P = 0.0005) with a greater number of ganglia innervated per axon in the stomach compared to the distal gut (vs duodenum 3–6 cm, P = 0.0195; vs. mid-jejunum, P = 0.0468; vs ileum, P = 0.0096), while the proximal duodenum innervation was comparable to the stomach (P > 0.05) but had a greater number of innervated ganglia per axon compared to the distal duodenum (P = 0.0399) and ileum (P = 0.0179); Figure 8. Analysis of ganglia innervated by varicose axons revealed a similar regional pattern (P = 0.0009), with a greater number of ganglia innervated per sympathetic arbor in the stomach compared to the distal intestines (vs. duodenum 3–6 cm, P = 0.0214; vs. mid-jejunum, P = 0.0725; vs. ileum, P = 0.0149), while the proximal duodenum was comparably innervated to the stomach (P > 0.05) but had a greater number of ganglia innervated compared to the distal intestines (vs duodenum 3–6 cm, P = 0.0400; vs mid-jejunum, P = 0.1390; vs. ileum, P = 0.0250); Figure 8.
Figure 8.
A: The number of myenteric ganglia innervated by terminal branches was greatest in the proximal gut and decreased in the distal gut (P = 0.0001). B: The pattern was similar for varicose arbor length (P = 0.0009). Asterisks indicate a significant difference (P < 0.05) compared to the stomach * or duodenum 0–3 cm **; Tukey’s HSD. Mean ± S.E.M.
Sympathetic Innervation of the GI Vasculature
Though most vascular innervation was expected to be in the submucosal sites, a limited number of cases of vascular innervation in the muscularis externa were observed. Individual axons originating from the left CSMG were observed to issue branches in small bundles silhouetting the outer perimeter of blood vessels within the muscle wall of the stomach (N = 4; Figure 9). The blood vessels were distinguished by the pattern of staining outlining the vascular lumen and were made even more evident by varicose sympathetic arbors and terminal branches lining the outer limits; Figure 9. Although it has typically been thought that arteries are supplied by a distinct set of sympathetic fibers, the present experiment observed that a single axon along a blood vessel also formed collateral branches that were associated with neighboring myenteric neurons; Figure 9. Also, the evidence suggested that the vascular innervation, provided by a solitary axon, could project to the circular muscle/2° plexus as well as localized regions of several distinct arterial branches.
Figure 9.
Sympathetic innervation of the blood vessels was observed in some of the whole mounts. A: Sympathetic axons silhouette the outer perimeter of a blood vessel before giving off several collateral branches that extend into the neighboring myenteric ganglia. B: A tracing of the entire ending documents how the parent axon (PA; arrow) bifurcates and the resulting secondary fibers then run parallel in close association to the blood vessel with several collateral branches innervating the nearby myenteric ganglia. C: At higher magnification, the axons in close apposition to the outer wall of a blood vessel are clearly varicose (arrows). D: The innervation of the myenteric ganglia is similarly varicose (arrows). The brown-stained irregular structures in panels A and C are presumably erythrocytes that were not exsanguinated during perfusion and fixation. Scale bars in A = 125 µm; B = 250 µm; C & D = 20 µm.
DISCUSSION
Given the still-incomplete understanding of sympathetic postganglionic axonal organizational patterns in the GI tract, the present study was designed to provide a more comprehensive analysis of the morphology and topography of sympathetic axon terminals located in the smooth muscle wall of the GI tract. Overall, the results indicated that most individual sympathetic CSMG projections to the gut wall have an arbor architecture capable of coordinating or co-activating smooth muscle and myenteric ganglion neuron operations.
In a sample of 154 CSMG neurons, three distinct motor arbor phenotypes innervated the muscularis externa. A substantial majority—from 60% to 84%, depending on criteria—of the sympathetic postganglionic axons were mixed or heterotypic arbors that co-innervated both myenteric ganglion neurons/primary plexus and smooth muscle with substantial projections to both tissues. In contrast, a small percentage of the sympathetic neurons projected exclusively (2%) or predominantly (13%) to the myenteric ganglia/1° plexus, whereas a second and larger minority sample of postganglionics bypassed myenteric ganglia/1° plexus targets and projected directly, either exclusively (14%) or predominantly (27%), to smooth muscle.
Co-localization Controls for CSMG Catecholaminergic Fibers
Since DRG primary afferents course through the prevertebral ganglia (e.g., Strack et al., 1988) and issue collaterals to the postganglionic somata in the ganglia (e.g., Gibbins and Morris, 2006), such visceral afferents could have been inadvertently labeled in an experiment injudiciously selecting the wrong tracer or a problematic injection protocol. Similarly, axons of sympathetic chain ganglia traverse the posterior pole of the CSMG complex and theoretically might be labeled en passage. In a control series of double labeled whole mounts that contained immunohistochemically labeled tyrosine hydroxylase (TH) positive fibers as well as CSMG-dextran labeled fibers, we determined empirically whether the fibers that we routinely labeled with the CSMG injections were catecholaminergic postganglionic neurites by examining the gut wall fibers for co-localization. In the control series, 135 of 136 neurites that were evaluated for co-localization were positive for both dextran and TH, indicating that the population of axonal arbors that we characterized is comprised of sympathetic postganglionic efferents.
The results of the co-localization analysis are corroborated by several other observations. We selected the 10 K dextran tracer because multiple independent experiments (cf. review by Reiner et al., 2000) have indicated that 10K dextran is virtually exclusively an anterograde tracer (in contrast to 3K dextran which is retrogradely transported) taken up at cell bodies and terminals, and only potentially labels fibers of passage that have been damaged. Additionally, the nine-day survival interval we employed was optimized for direct anterograde transport and likely would have been too short an interval for fiber-of-passage or collateral-contact labeling to have been first transported retrogradely to the somata, of say DRG afferents, and then trafficked anterogradely the full distance of any axons projecting to the gut wall. To this point, even in a smaller species (mice) with shorter distances involved, Spencer et al. (2014) used 7 days just for direct transport from the DRG to the wall of the GI tract. Furthermore, we specifically employed fine Nanofil needles and an injection protocol that was designed to minimize adventitial damage to axons and that we have previously validated at other ganglion sites (e.g., nodose). Clearly, the fact that 99% of the fibers we labeled with 10K dextran had TH co-localized with the tracer indicates that the tracer-protocol combination we used selectively targeted sympathetic motor neurons in the CSMG. Consistent with the same basic conclusion, too, is the fact that few of the primary afferents to the intestine that Spencer et al. (2014) labeled with DRG injections of 10K dextran have morphologies similar to the sympathetic efferents we labeled. Finally the fact that our injections did not retrogradely label the intestinofugal neurons located in the myenteric plexus (data not shown) further establishes the anterograde selectivity of our tracer protocol.
Anterograde Tracing for Reassessing the Sympathetic Projections
Given the fact that the present experiment capitalizing on dextran-biotin anterograde tracing has produced evidence for a hybrid perspective concluding that the majority of sympathetic postganglionic projections to the muscularis externa do not conform to either a strict two-neuron view or a literal three-neuron view, it should be stressed that there is a limited amount of research using modern anterograde tracers to investigate the sympathetic nervous system innervation of the gut. Further, most of the work that has used anterograde labeling to identify the sympathetic postganglionic innervation of the GI tract has used an in vitro protocol applying biotinamide to the cut end of a severed mesenteric nerve trunk, a procedure which has the potential to label indiscriminately any cut fiber in the trunk, followed by indirect verification of the sympathetic fibers based on TH-expression (Olsson et al., 2004; Tan et al., 2010; Tassicker et al., 1999). Additionally, those in vitro experiments used whole mounts in which the circular muscle was typically removed, so it was not possible to comprehensively study the sympathetic innervation of the circular muscle/2° plexus (e.g., Olsson et al., 2004; Tan et al., 2010; Tassicker et al., 1999). In light of the evidence that the circular muscle is heavily innervated by postganglionic sympathetic fibers (present results; also see Furness et al., 1990; Tassicker et al., 1999; Olsson et al., 2004; Phillips et al., 2006; 2009; Tan et al., 2010), exclusion of much or all of the circular muscle sheet presumably resulted in the biotinamide surveys missing significant aspects of the topographical organization and distribution of sympathetic terminals. The present investigation overcame prior limitations by direct labeling of sympathetic neurons by tracer injections within the CSMG and the use of intact whole mounts of the complete smooth muscle wall of the gut. In combination, the protocols provided unequivocal labeling of intact sympathetic terminal fields located in the gut wall and made practical detailed qualitative and quantitative analyses of digitally reconstructed terminal arbors.
Regional Sympathetic Morphology
Currently, the characteristics of noradrenergic innervation in the myenteric plexus and smooth muscle layers are most commonly represented schematically and in formal models as distinct neuronal projections to myenteric ganglia/1° plexus, with little indication that the fibers project extensively to smooth muscle or that there are identifying features of an individual axon beyond the simplified organization (Scheuermann and Stach, 1984; Furness, 2006a).
Myenteric Ganglia/1° Plexus
The present study found that 13% (N = 20/154; see Table 2) of the labeled CSMG neuronal arbors projected predominantly to the primary plexus. Consistent with previous reports, a single axon could be traced and seen to innervate a variable number of ganglia along its trajectory (cf. Scheuermann and Stach, 1984). As sympathetic axons coursed from one ganglion to another through the interconnective strands of the primary plexus, some neurite segments contained varicosities and ended as terminal branches (see Furness, 1970b; Mawe et al., 1989). The varicose neurites in both the ganglia and their connectives apparently, in both instances, suggested that the fibers made contacts either on myenteric ganglion cell dendrites or on axons coursing within the interconnectives (Furness, 1970b; Mawe et al., 1989) and associated terminal branches. Also, varicosities that are suggestive of terminal contacts were present within the ganglia (Gabella and Costa, 1969; Olsson et al., 2004) and several en passage varicosities on the same axon could be observed in relation to a single neuronal somata (Gabella, 1971, 1972). Although the typical organization of sympathetic axons around a myenteric neuron has been referred to as basket-like networks (Read and Burnstock, 1968), these were rarely seen in the present observations (see also Llewellyn-Smith et al., 1981). Further, consistent with our observation for the CSMG terminal arbors, Tassicker and coworkers (1999) estimated that basket-like calices of varicosities occur for only 1% of myenteric perikarya.
In contrast to the idea of basket-like or other extensive clustering of the CSMG axonal varicosities in close apposition to individual myenteric neurons, many of the dextran-labeled arbors we evaluated made only limited varicose contacts in close proximity to myenteric perikarya. Additionally, as has been observed in ultrastructural (Gordon-Weeks, 1982; Llewellyn-Smith et al., 1984) and immunohistochemical (Tassicker et al., 1999) surveys, labeled CSMG fibers often appeared to course around or through myenteric ganglia in relatively superficial paths just inside the ganglionic capsule. Some of these superficial CSMG fibers appeared to course through the ganglia without elaborating varicosities; others contained only limited numbers of varicosities in the proximity of myenteric neurons. In some instances, a ganglion only received a single short branch or varicose terminal spur that was juxtaposed to one peripherally located neuron.
This limited and dispersed pattern of innervation was also apparent in the analyses of heterotypic axons with mixed target tissues where only a modest percentage of the overall or total arbor length was comprised of varicose terminal branches (29–35%) and varicose neurite segments (16–29%) in close apposition to the myenteric neurons, a pattern which is in contrast to the view that sympathetic axons preferentially innervate the ganglia (Norberg, 1964; Furness and Costa, 1974; Furness, 2006a). Along with the present finding of a low percentage of fibers exclusively innervating myenteric ganglia, previous ultrastructural identification of direct synapses between adrenergic axons and myenteric neurons (Taxi, 1969) suggests that the prototypical pre- and postsynaptic connections are rarely found, in part because many of the axons course along the outer perimeter of the ganglia (Gordon-Weeks, 1982; Llewellyn-Smith et al., 1984).
Circular muscle/2° plexus
The sympathetic innervation of circular muscle is an area that has typically received relatively limited attention due both to earlier investigations revealing a paucity of catecholamine innervation within the smooth muscle layers and to the proposal that sympathetic innervation modulates myenteric neurons rather than directly influencing the muscle (Norberg, 1964, 1967). In fact, many studies ignored the circular muscle by removing it from their whole mount preparations, thus restricting their observations to only the longitudinal muscle and myenteric plexus (e.g. Furness and Costa, 1971, 1975; Furness and Malmfors, 1971; Baker and Santer, 1989; Tassicker et al., 1999; Olsson et al., 2004; Tan et al., 2010). Even though there has been a relative lack of attention in previous research, evidence for sympathetic neurites within the circular muscle, in a range of species, has still been observed (Capurso et al., 1968; Gabella and Costa, 1969; Baker and Santer 1989; Furness et al. 1990; Tan et al. 2010).
As previously observed in both light microscopy (Gabella and Costa, 1969; Read and Burnstock, 1969; Furness, 1970b; Furness and Malmfors, 1971; Silva et al., 1971; Mawe et al., 1989; Mann and Bell, 1993; Olsson et al., 2004; Phillips et al., 2006; 2009; Tan et al., 2010), and electron microscopy (Gabella and Costa, 1969; Gabella, 1970; Wong, 1977), varicose intramuscular nerve fibers are present amid the bundles of smooth muscle cells in the circular muscle. The main feature of axons in this muscle layer is parallel branches that run along the circular muscle, parallel to the muscle fibers, with varying densities of collateral fibers across samples (Furness, 1970b). Besides neurites within the plane of the smooth muscle, there were also collateral branches that had a similar appearance and orientation as the parallel intramuscular arrays, but were superficial to the circular muscle, and have been considered the secondary plexus (Scheuermann and Stach, 1984; Llewellyn-Smith et al., 1993; Furness, 2006a). While some of the secondary plexus fibers extended into the depth of the circular muscle (Llewellyn-Smith et al., 1993), others ended at the surface of the smooth muscle. At times this connection between the secondary plexus and circular muscle produced distinct features of relatively short bundles of axonal branches that extended between the respective layers.
Although research using histofluorescence to visualize sympathetic nerves has suggested a relatively sparse innervation of the circular muscle compared to the innervation of the ganglia, the present investigation found a greater number of arbors that predominantly innervated the circular muscle/2° plexus (N =42/154; see Table 2) compared to the subset that projected to the myenteric ganglia/1° plexus only.
Also, in the case of the majority of CSMG postganglionics that were clearly heterotypic arbors with multiple target tissues, calculations suggest that the circular muscle receives an extensive percentage of varicose sympathetic fiber length (27–60%) compared to the ganglia (16–29%). Further, while these observations may not directly equate to density, it is the case that the direct physiological responses on the smooth muscle layers may not require a high density of noradrenergic innervation due to the fact that smooth muscle cells can pass the change in potential between adjoining membranes to neighboring cells (Furness, 1970a) through interconnected gap junctions (Gabella, 1973; Baluk and Gabella, 1987; Brock and Cunnane, 1992), electrical coupling (Gabella, 1973), and/or fibroblasts (Baluk and Gabella, 1987).
Longitudinal muscle/3° plexus
As opposed to those studies that thoroughly investigated the circular muscle, some early ultrastructural research suggested a complete lack of sympathetic nerve fibers within the longitudinal muscle sheet (Gabella, 1973; Baluk and Gabella, 1987), while other evidence has shown the existence of the rare axon penetrating the first muscle cell layer or residing within shallow grooves in the sheet (Llewellyn-Smith et al., 1993). In line with this observation, we observed longitudinal muscle innervation (see also Gabella and Costa, 1969; Read and Burnstock, 1969; Furness, 1970b; Furness and Malmfors, 1971; Silva et al., 1971; Mawe et al., 1989; Mann and Bell, 1993; Olsson et al., 2004; Tan et al., 2010), consisting of one or two short collateral terminal fiber segments running longitudinally. Despite the paucity of direct longitudinal muscle innervation there were instances, analogous to the relationship between the secondary plexus and circular muscle fibers, in which fibers coursed in a longitudinal array superficial to the myenteric plexus. Although this pattern of innervation may be suggestive of a longitudinal effector the functional relevance is unknown. On the other hand, early research considered the nervous tissue in the tertiary plexus as neuroeffectors of the longitudinal muscle (Read and Burnstock, 1969; Furness, 1970b; Gillespie and Maxwell, 1971). Ultrastructural observations also indicate that this plexus of fibers is indeed selectively juxtaposed with longitudinal muscle cells (Llewellyn-Smith et al., 1993). The meandering tertiary plexus axons in the present dextran-labeled series were observed as either short wavy fibers with several varicose branches protruding into the internodal area, a wandering expansion across the zone between two adjacent ganglia, or a protracted extension across several internodal regions.
Multiple Target Mechanisms for Complex Integration
The present study found that the largest group of sympathetic axons (60%; N = 92/154; see Figure 4; Table 2) consisted of mixed endings that branched extensively to innervate multiple sites in both the myenteric ganglia/1° plexus and the smooth muscle wall of the gut. It should be noted, too, that the estimate of 60% is a conservative one, achieved when the stringency of the criteria used to classify an arbor as innervating exclusively either the myenteric ganglia/1° plexus or the smooth muscle targets was relaxed to require only “predominant” innervation. If the more stringent criteria for exclusive projections were applied to the 154 sympathetic arbors assessed, however, over 84% of all terminal arbors would have been classified as heterotypic or mixed-tissue projecting axons.
Consistent with earlier research that has been mostly discounted or overlooked, we observed fibers exiting the myenteric ganglia/1° plexus and extending into the circular muscle/2° plexus (cf. Capurso et al., 1968; Silva et al., 1971), and longitudinal muscle/3° plexus (cf. Capurso et al., 1968; Furness, 1970b). Along with the myenteric ganglia/1° plexus fibers that branched and continued to project to either one of the muscle sheets, we also observed single fibers that projected through the myenteric ganglia/1° plexus and continued to both secondary (presumptive circular muscle projection) and tertiary (provisional longitudinal muscle projection) plexuses, which confirmed a previously postulated pattern (Scheuermann and Stach, 1984). Also, various terminal field patterns and sizes within the different plexuses and circular muscle were observed. This distributed arrangement of sympathetic fibers is suggestive of a complex mixed-target-site model for modulating GI function, which appears to simultaneously incorporate both the classical two-neuron-outflow view (Hill, 1927; Langley, 1903) and the three-neuron chain in autonomic circuitry (Norberg, 1967).
Even more specifically, the robust co-innervation of myenteric ganglia/1° plexus and one or both smooth muscle sheets by the majority of individual CSMG postganglionics clearly has an architecture that may provide a “hardwired” cross-linkage of the myenteric plexus and smooth muscle effectors. The structure might provide an autonomic analogue of the alpha-gamma linkages that operate in the striated musculature.
Modulatory Mechanisms of CSMG Postganglionics
The presently observed co-connectivity of individual, morphologically unique axons projecting both in the different plexuses and the muscle sheets, could alternatively be an example in which structurally different collateral branches from the same axon produce unique functional consequences due to the release of disparate transmitters (Kandel and Gardener, 1972; Burnstock, 1976). The sympathetic nervous system employs neurochemical coding (Costa and Furness, 1984; Gibbins, 1991, 1992), and prevertebral neurons synthesize multiple transmitters and/or neuromodulators (Kandel and Gardner, 1972; Burnstock, 1976; Hokfelt et al., 1977). These mixes of neurotransmitters or neuromodulators could suggest distinct functional properties.
Besides neurochemical coding, the various innervation patterns that were observed in the present work could be structured to process the incoming signal in a frequency-dependent manner (Grossman et al., 1973). Such distinct morphology could create different strengths of spatially and temporally organized nerve impulses that modulate the smooth muscle patterns involved in motility. Additionally, it is the case that vesicle release from varicosities relies on the frequency of a nerve impulse, as the presence of an action potential does not always correspond to the discharge of a vesicle from all varicosities (Brock and Cunnane, 1987). In fact, there is a low probability of vesicle release from any given varicosity (Brock and Cunnane, 1992). Varicosities could filter the incoming signal, just as different diameter axons or branch points along the axon modify the conduction of the nerve impulse (Waxman, 1972).
Ganglia and Muscle Modulatory Mechanisms
The present comparisons of the proportions of axonal segment lengths and terminal branches within myenteric ganglia/1° plexus to those within the circular muscle/2° plexus and the longitudinal muscle/3° plexus, showed that the myenteric neurons receive a small proportion of varicose fiber length (see Figure 7) and “terminal endings”, which suggests that, quantitatively, the predominant target for sympathetic innervation is the smooth muscle. This organizational analysis suggests a substantial role for direct sympathetic modulation of smooth muscle inhibition. Two types of inhibitory mechanisms have been previously suggested, whereby the myenteric ganglia and muscle input either reinforce one another or operate under distinct circumstances (Silva et al., 1971). Whether the mechanism at any given period is direct, indirect, or synergistic could depend on how abruptly an inhibitory response must occur, or it could fluctuate depending on the existing level of tension in the smooth muscle at the time of sympathetic transmission. The complexity of the direct and indirect mechanism are in line with the Gabella and Costa (1969) finding, which suggested the existence of both the classical or two-neuron view of preganglionic neuron to postganglionic neuron to muscle, and the three-neuron chain, involving an intercalated “postganglionic” myenteric neuron. The present investigation illustrates the possibility that the sympathetic nervous system may have separate pools of neurons consistent with each of these candidate views of connectivity, while also showing that the majority of neurons were a complex mixture of both.
An additional caveat about varicosities of postganglionic fibers further complicates the interpretation of the extensive co-innervation patterns of the sympathetic projections to the muscularis externa. Noradrenergic varicosities have morphological features more like “junctions” (absence of closed synaptic clefts and post-synaptic thickenings, etc.) than like synapses and, correspondingly, appear to operate with junctional neurotransmission rather than synaptic transmission (Goyal and Chaudhury, 2013). Further, in the terminology of Goyal and Chaudbury, sympathetic junctions vary in separation from their target(s) from “close” to “wide” junctions. Thus, the CSMG axon varicosities associated with the myenteric plexus and those innervating GI smooth muscle fibers might differ in their proximity or “closeness” to their effector tissues. Finally, given the respective volumes or masses of the different effector tissues, the sympathetic varicosities might have different neurotransmission efficacies in the different tissues that the axons co-innervate. So little experimental work has been done on the transmitter efficiencies of noradrenergic varicosities articulating with different target tissues that it is difficult to gauge how such factors might affect sympathetic efficacies in various tissues, but such issues will need to be addressed before a full understanding of the sympathetic co-innervation patterns can be developed.
So, although it is parsimonious to assume separate neuronal classes are responsible for simple direct and indirect projections modifying the activity of the muscularis externa, given the data, it would appear as though the sympathetic neurite modulation occurs at various levels, through interactions with myenteric neurons, direct neuromuscular junctions in muscle sheets, as well as potentially with parasympathetic varicosities, glial cells, and fibroblast cells. Adding to the apparent complexity of the sympathetic circuitry is the presently observed heterotypic or mixed-target axons. Such a highly intricate system suggests that the postganglionic sympathetic terminal input to the GI tract affects—and could perhaps integrate the operation of—multiple target tissues. Such operations would have parallels to the complex processing that occurs within the prevertebral ganglia.
Individual sympathetic postganglionic neurons, labeled by injections of the anterograde tracer dextran, projected directly to both the gastrointestinal smooth muscle sheets and the local myenteric ganglia throughout the stomach and small intestine. This widespread co-innervation pattern suggests that sympathetic fibers may directly influence smooth muscle while contributing to myenteric-plexus-muscle coordination.
Acknowledgments
The present experiment is based on a dissertation submitted by GCW in partial fulfillment of the Ph.D., PULSe Program, Purdue University, December, 2014, and GCW thanks his committee members, Terry L. Powley, Edward A. Fox, Jean-Christophe Rochet and John R. Burgess for their time and input over the course of his graduate work.
Grant Sponsor: National Institutes of Health (NIH)
National Institute of Diabetes and Digestive and Kidney Diseases
Grant Number: DK027627
Footnotes
Conflict of Interest Statement
The Authors declare no conflict of interest.
Resources Cited
RRID:RGD_61109
RRID:AB_2307337
RRID:AB_2336819
RRID:nig-0000-10294
RRID:rid_000081
RRID:AB_572268
RRID:AB_2313713
RRID:AB_2313574
RRID:AB10566286
RRID:AB_10562715
Footnote 1: Given that the choice of what to use as cutoff criteria was arbitrary, we also explored the consequences of using cutoffs of <10% and <20%, instead of the <15% criterion based on an average of the nadirs. The 10% and 20% variants changed the absolute numbers of arbors considered to fall in the different peaks, of course, but these alternative criteria did not materially change the patterns discussed below.
Furthermore, as a practical assessment of different criteria, variant cutoffs much smaller or larger than 15% for the clusters of neurons at the two limits of the innervation distribution seemed particularly problematic. Consideration of the left-most peak in Figure 4 illustrates this issue: As already indicated, more stringent cutoff criterion (i.e. <15%, and particularly <10%) to define sympathetic postganglionic arbors that “predominantly” innervated the primary plexus dramatically diminished the size of the subset of neurons that could be construed as consistent with a three-neuron outflow model. But then conversely, relaxing the criteria to include postganglionics with more than 15% of their varicose segments outside the primary plexus as still “practically projecting to the primary plexus” seemed incautious because it assumed that as much as, say, 20% or more of the total neurite length of the arbor could be summarily minimized, if not discounted.
Role of Authors
GCW participated in the design, data collection, statistical analyses, figure production and writing of the manuscript. RJP contributed to the design, development of the tracer protocol, statistical analyses, figure production and writing of the manuscript. JLM contributed to the development of the tracer protocol, surgery and ganglion injections, and development of the manuscript. TLP participated in the design, analyses of the data, and preparation of the manuscript.
Literature Cited
- Baker DM, Santer RM. Image analysis of the sympathetic innervation of the myenteric plexus in the small intestine of mammalian species. Comp Biochem Physiol C. 1989;94(2):527–531. doi: 10.1016/0742-8413(89)90108-4. [DOI] [PubMed] [Google Scholar]
- Baluk P, Gabella G. Scanning electron microscopy of the muscle coat of the guinea-pig small intestine. Cell Tissue Res. 1987;250(3):551–561. doi: 10.1007/BF00218946. [DOI] [PubMed] [Google Scholar]
- Bar-Shai A, Maayan C, Vromen A, Udassin R, Nissan A, Freund HR, Hanani M. Decreased density of ganglia and neurons in the myenteric plexus of familial dysautonomia patients. J Neurol Sci. 2004;220(1–2):89–94. doi: 10.1016/j.jns.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Autonomic neuromuscular transmission. viii. Cambridge Eng.: University Press; 1972. p. 273. [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195(2):183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42(2):153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35(1):80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bian X, Galligan JJ. α2-Adrenoceptors couple to inhibition of R-type calcium currents in myenteric neurons. Neurogastroenterol Motil. 2007;19:845–855. doi: 10.1111/j.1365-2982.2007.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. The autonomic nervous system and its effectors. viii. Oxford ; Malden, MA, USA: Blackwell Science; 1999. p. 370. [Google Scholar]
- Brock JA, Cunnane TC. Relationship between the nerve action potential and transmitter release from sympathetic postganglionic nerve terminals. Nature. 1987;326(6113):605–607. doi: 10.1038/326605a0. [DOI] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Electrophysiology of neuroeffector transmission in smooth muscle. In: Burnstock G, Hoyle CHV, editors. Autonomic neuroeffector mechanisms. xi. Chur, Switzerland; Philadelphia: Harwood Academic Publishers; 1992. pp. 121–214. [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976;1(4):239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Costa M. Inhibitory innervation of the gut. Gastroenterology. 1973;64(1):141–144. [PubMed] [Google Scholar]
- Cajal SRY. Histologie du Systeme Nerveux de l’Homme et des Vertébrés. Maloine, Paris, France: 1911. [Google Scholar]
- Capurso L, Friedmann CA, Parks AG. Adrenergic fibres in the human intestine. Gut. 1968;9(6):678–682. doi: 10.1136/gut.9.6.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter FW. A note on the connections, in the mamalian myenteric plexus, between the enteric neurons and extrinsic nerve fibers. Anat Rec. 1924;28(2):149–156. [Google Scholar]
- Costa M, Furness JB. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984;13(3):911–919. doi: 10.1016/0306-4522(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Falck B, Owman CH. Histochemistry of monoaminergic mechanisms in peripheral neurons. In: von Euler US, Rosell S, Uvnäs Br, editors. Mechanisms of release of biogenic amines; proceedings of an international Wenner-Gren Center symposium held in Stockholm, February 1965; Symposium Publications Division; Oxford, New York. 1966. pp. 59–70. [Google Scholar]
- Falck B, Hillarp N-A, Thieme G, Torp A. Fluorescence of catecholamines and related compounds condensed with formaldehyde. J Histochem Cytochem. 1962;10:348–354. [Google Scholar]
- Fox EA, Powley TL. False-positive artifacts of tracer strategies distort autonomic connectivity maps. Brain Res Brain Res Rev. 1989;14(1):53–77. doi: 10.1016/0165-0173(89)90009-x. [DOI] [PubMed] [Google Scholar]
- Furness JB. The excitatory input to a single smooth muscle cell. Pflugers Archiv : European journal of physiology. 1970a;314(1):1–13. doi: 10.1007/BF00587042. [DOI] [PubMed] [Google Scholar]
- Furness JB. The origin and distribution of adrenergic nerve fibres in the guinea-pig colon. Histochemie. 1970b;21(4):295–306. doi: 10.1007/BF00280899. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system. xiii. Malden, Mass.: Blackwell Pub; 2006a. p. 274. [Google Scholar]
- Furness JB. The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci. 2006b;130(1–2):1–5. doi: 10.1016/j.autneu.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Furness JB. Peripheral autonomic nervous system. In: Paxinos G, editor. The Rat Nervous System. 4th. Amsterdam: Elsevier; 2014. pp. 61–76. [Google Scholar]
- Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. Morphology and distribution of intrinsic adrenergic neurones in the proximal colon of the guinea-pig. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1971;120(3):346–363. doi: 10.1007/BF00324897. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M. The adrenergic innervation of the gastrointestinal tract. Ergeb Physiol. 1974;69(0):2–51. [PubMed] [Google Scholar]
- Furness JB, Costa M. The use of glyoxylic acid for the fluorescence histochemical demonstration of peripheral stores of noradrenaline and 5-hydroxytryptamine in whole mounts. Histochemistry. 1975;41(4):335–352. doi: 10.1007/BF00490076. [DOI] [PubMed] [Google Scholar]
- Furness JB, Lloyd KC, Sternini C, Walsh JH. Projections of substance P, vasoactive intestinal peptide and tyrosine hydroxylase immunoreactive nerve fibres in the canine intestine, with special reference to the innervation of the circular muscle. Arch Histol Cytol. 1990;53(2):129–140. doi: 10.1679/aohc.53.129. [DOI] [PubMed] [Google Scholar]
- Furness JB, Malmfors T. Aspects of the arrangement of the adrenergic innervation in guinea-pig as revealed by the fluorescence histochemical method applied to stretched, air-dried preparations. Histochemie. 1971;25(4):297–309. doi: 10.1007/BF00278223. [DOI] [PubMed] [Google Scholar]
- Gabella G. Electron microscopic observations on the innervation of the intestinal inner muscle layer. Experientia. 1970;26(1):44–46. doi: 10.1007/BF01900382. [DOI] [PubMed] [Google Scholar]
- Gabella G. Synapses of adrenergic fibres. Experientia. 1971;27(3):280–281. doi: 10.1007/BF02138146. [DOI] [PubMed] [Google Scholar]
- Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat. 1972;111(Pt 1):69–97. [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Cellular structures and electrophysiological behaviour. Fine structure of smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973;265(867):7–16. doi: 10.1098/rstb.1973.0004. [DOI] [PubMed] [Google Scholar]
- Gabella G, Costa M. Adrenergic innervation of the intestinal smooth musculature. Experientia. 1969;25(4):395–396. doi: 10.1007/BF01899944. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, North RA. Opioid, 5-HT1A and α2 receptors localized to subsets of guinea-pig myenteric neurons. J Auton Nerv Syst. 1991;32:1–12. doi: 10.1016/0165-1838(91)90229-v. [DOI] [PubMed] [Google Scholar]
- Gibbins IL. Vasomotor, pilomotor and secretomotor neurons distinguished by size and neuropeptide content in superior cervical ganglia of mice. J Auton Nerv Syst. 1991;34(2–3):171–183. doi: 10.1016/0165-1838(91)90083-f. [DOI] [PubMed] [Google Scholar]
- Gibbins IL. Vasoconstrictor, vasodilator and pilomotor pathways in sympathetic ganglia of guinea-pigs. Neuroscience. 1992;47(3):657–672. doi: 10.1016/0306-4522(92)90174-z. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Morris JL. Structure of peripheral synapses: autonomic ganglia. Cell Tissue Res. 2006;326(2):205–220. doi: 10.1007/s00441-006-0233-1. [DOI] [PubMed] [Google Scholar]
- Gillespie JS, Maxwell JD. Adrenergic innervation of sphincteric and nonsphincteric smooth muscle in the rat intestine. J Histochem Cytochem. 1971;19(11):676–681. doi: 10.1177/19.11.676. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks PR. Noradrenergic and non-noradrenergic nerves containing small granular vesicles in Auerbach's plexus of the guinea-pig: evidence against the presence of noradrenergic synapses. Neuroscience. 1982;7(11):2925–2936. doi: 10.1016/0306-4522(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Chaudhury A. Structure activity relationship of synaptic anad junctional neurotransmission. Auton Neurosci. 2013;176:11–31. doi: 10.1016/j.autneu.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y, Spira ME, Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- Hamer DW, Santer RM. Anatomy and blood supply of the coeliac-superior mesenteric ganglion complex of the rat. Anat Embryol. 1981;162(3):353–362. doi: 10.1007/BF00299978. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M. Fine structural survey of tyrosine hydroxylase immunoreactive terminals in the myenteric ganglion of the rat duodenum. J Chem Neuroanat. 2008;36:191–196. doi: 10.1016/j.jchemneu.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy of the laboratory rat. viii. Baltimore: Williams & Wilkins; 1976. p. 173. [Google Scholar]
- Hill CJ. A contribution to our knowledge of the enteric plexuses. Phil Trans R Soc Long B. 1927;215:355–387. [Google Scholar]
- Hirst GD, McKirdy HC. A nervous mechanism for descending inhibition in guinea-pig small intestine. J Physiol. 1974;238(1):129–143. doi: 10.1113/jphysiol.1974.sp010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Elfvin LG, Elde R, Schultzberg M, Goldstein M, Luft R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc Natl Acad Sci U S A. 1977;74(8):3587–3591. doi: 10.1073/pnas.74.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands BC, Vanov S. Localization of catechol amines in visceral organs and ganglia of the rat, guinea-pig and rabbit. British journal of pharmacology and chemotherapy. 1965;25(2):307–316. doi: 10.1111/j.1476-5381.1965.tb02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman ME, Hirst GD, Spence I. Preliminary studies of the neurones of Auerbach's plexus using intracellular microelectrodes. The Australian journal of experimental biology and medical science. 1972;50(7):795–801. doi: 10.1038/icb.1972.76. [DOI] [PubMed] [Google Scholar]
- Jänig W. The integrative action of the autonomic nervous system : neurobiology of homeostasis. xxi. Cambridge, UK ; New York: Cambridge University Press; 2006. p. 610. [Google Scholar]
- Jobling P. Visceral Motoneurons. In: Mai JrK, Paxinos G., editors. The human nervous system. xi. Amsterdam; Boston: Elsevier Academic Press; 2012. pp. 499–519. [Google Scholar]
- Kandel ER, Gardner D. The synaptic actions mediated by the different branches of a single neuron. Research publications - Association for Research in Nervous and Mental Disease. 1972;50:91–146. [PubMed] [Google Scholar]
- Karaosmanoglu T, Aygun B, Wade PR, Gershon MD. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: an evaluation of markers used to count neurons. Anat Rec. 1996;244(4):470–480. doi: 10.1002/(SICI)1097-0185(199604)244:4<470::AID-AR5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kuntz A. The autonomic nervous system. Philadelphia: Lea & Febiger; 1934. p. 697. [Google Scholar]
- Kuntz A. The autonomic nervous system. Philadelphia: Lea & Febiger; 1953. p. 605. [Google Scholar]
- Langley JN. The autonomic nervous system. Brain. 1903;26(1):1–26. [Google Scholar]
- Llewellyn-Smith IJ, Costa M, Furness JB, Bornstein JC. Structure of the tertiary component of the myenteric plexus in the guinea-pig small intestine. Cell Tissue Res. 1993;272(3):509–516. doi: 10.1007/BF00318557. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Furness JB, O'Brien PE, Costa M. Noradrenergic nerves in human small intestine. Distribution and ultrastructure. Gastroenterology. 1984;87(3):513–529. [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Wilson AJ, Furness JB, Costa M, Rush RA. Ultrastructural identification of noradrenergic axons and their distribution within the enteric plexuses of the guinea-pig small intestine. J Neurocytol. 1981;10(2):331–352. doi: 10.1007/BF01257975. [DOI] [PubMed] [Google Scholar]
- Manber L, Gershon MD. A reciprocal adrenergic-cholinergic axoaxonic synapse in the mammalian gut. Am J Physiol. 1979;236(6):E738–E745. doi: 10.1152/ajpendo.1979.236.6.E738. [DOI] [PubMed] [Google Scholar]
- Mann R, Bell C. Distribution and origin of aminergic neurones in dog small intestine. J Auton Nerv Syst. 1993;43(2):107–115. doi: 10.1016/0165-1838(93)90347-w. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Schemann M, Wood JD, Gershon MD. Immunocytochemical analysis of potential neurotransmitters present in the myenteric plexus and muscular layers of the corpus of the guinea pig stomach. Anat Rec. 1989;224(3):431–442. doi: 10.1002/ar.1092240312. [DOI] [PubMed] [Google Scholar]
- Miolan JP, Niel JP. The mammalian sympathetic prevertebral ganglia: integrative properties and role in the nervous control of digestive tract motility. J Auton Nerv Syst. 1996;58(3):125–138. doi: 10.1016/0165-1838(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Müller LR. Die darminnervation. Dtsch Arch Klin Med. 1911;105:1–43. [Google Scholar]
- Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg KA. Adrenergic Innervation of the Intestinal Wall Studied by Fluorescence Microscopy. Int J Neuropharmacol. 1964;3:379–382. doi: 10.1016/0028-3908(64)90067-x. [DOI] [PubMed] [Google Scholar]
- Norberg KA. Transmitter histochemistry of the sympathetic adrenergic nervous system. Brain Res. 1967;5(2):125–170. doi: 10.1016/0006-8993(67)90084-4. [DOI] [PubMed] [Google Scholar]
- Norberg KA, Sjoqvist F. New possibilities for adrenergic modulation of ganglionic transmission. Pharmacol Rev. 1966;18(1):743–751. [PubMed] [Google Scholar]
- Olsson C, Costa M, Brookes SJ. Neurochemical characterization of extrinsic innervation of the guinea pig rectum. J Comp Neurol. 2004;470(4):357–371. doi: 10.1002/cne.20000. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133(1–2):99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Hudson CN, Powley TL. Sympathetic axonopathies and hyperinnervation in the small intestine smooth muscle of aged Fischer 344 rats. Auton Neurosci. 2013;179(1–2):108–121. doi: 10.1016/j.autneu.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434(3):358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Rhodes BS, Powley TL. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of Fischer 344 rats. Anat Embryol (Berl) 2006;211(6):673–683. doi: 10.1007/s00429-006-0123-z. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Walter GC, Ringer BE, Higgs KM, Powley TL. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp Neurol. 2009;220(1):109–119. doi: 10.1016/j.expneurol.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Baronowsky EA, Gilbert JM, Hudson CN, Martin FN, Mason JK, McAdams JL, Phillips RJ. Vagal afferent innervation of the lower esophageal sphincter. Auton Neurosci. 2013a;177(2):129–142. doi: 10.1016/j.autneu.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Gilbert JM, Baronowsky EA, Billingsley CN, Martin FN, Phillips RJ. Vagal sensory innervation of the gastric sling muscle and antral wall: implications for gastro-esophageal reflux disease? Neurogastroenterol Motil. 2012;24(10):e526–e537. doi: 10.1111/nmo.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Hudson CN, McAdams JL, Baronowsky EA, Martin FN, Mason JK, Phillips RJ. Organization of vagal afferents in pylorus: mechanoreceptors arrayed for high sensitivity and fine spatial resolution? Auton Neurosci. 2014;183:36–48. doi: 10.1016/j.autneu.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Mittal RK, Baronowsky EA, Hudson CN, Martin FN, McAdams JL, Mason JK, Phillips RJ. Architecture of vagal motor units controlling striated muscle of esophagus: peripheral elements patterning peristalsis? Auton Neurosci. 2013b;179(1–2):90–98. doi: 10.1016/j.autneu.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinson N, Robbins HL, Clark MJ, Furness JB. Locations and innervation of cell bodies of sympathetic neurons projecting to the gastrointestinal tract in the rat. Arch Histol Cytol. 2001;64(3):281–294. doi: 10.1679/aohc.64.281. [DOI] [PubMed] [Google Scholar]
- Read JB, Burnstock G. Comparative histochemical studies of adrenergic nerves in the enteric plexuses of vertebrate large intestine. Comparative biochemistry and physiology. 1968;27(2):505–517. doi: 10.1016/0010-406x(68)90247-8. [DOI] [PubMed] [Google Scholar]
- Read JB, Burnstock G. Adrenergic innervation of the gut musculature in vertebrates. Histochemie. 1969;17(3):263–272. doi: 10.1007/BF00309871. [DOI] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103(1):23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Scheuermann DW, Stach W. Fluorescence microscopic study of the architecture and structure of an adrenergic network in the plexus myentericus (Auerbach), plexus submucosus externus (Schabadasch) and plexus submucosus internus (Meissner) of the porcine small intestine. Acta Anat (Basel) 1984;119(1):49–59. doi: 10.1159/000145861. [DOI] [PubMed] [Google Scholar]
- Silva DG, Ross G, Osborne LW. Adrenergic innervation of the ileum of the cat. Am J Physiol. 1971;220(2):347–352. doi: 10.1152/ajplegacy.1971.220.2.347. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Kyloh M, Duffield M. Identification of different types of spinal afferent nerve endings that encode noxious and innocuous stimuli in the large intestine using a novel anterograde tracing technique. PLoS One. 9(11):e112466. doi: 10.1371/journal.pone.0112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing MJ, Johnson PJ, Vremec MA, Bornstein JC. Role of α2-adrenoceptors in the sympathetic inhibition of motility reflexes of guinea-pig ileum. J Physiol. 2001;534(2):465–478. doi: 10.1111/j.1469-7793.2001.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Marubiou LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988;455(1):187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Takayanagi I, Sato T, Takagi K. Effects of sympathetic nerve stimulation on electrical activity of Auerbach's plexus and intestinal smooth muscle tone. The Journal of pharmacy and pharmacology. 1977;29(6):376–377. doi: 10.1111/j.2042-7158.1977.tb11343.x. [DOI] [PubMed] [Google Scholar]
- Tan LL, Bornstein JC, Anderson CR. The neurochemistry and innervation patterns of extrinsic sensory and sympathetic nerves in the myenteric plexus of the C57Bl6 mouse jejunum. Neuroscience. 2010;166(2):564–579. doi: 10.1016/j.neuroscience.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Tassicker BC, Hennig GW, Costa M, Brookes SJ. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tissue Res. 1999;295(3):437–452. doi: 10.1007/s004410051250. [DOI] [PubMed] [Google Scholar]
- Taxi J. Morphological and cytochemical studies on the synapses in the autonomic nervous system. Prog Brain Res. 1969;31:5–20. doi: 10.1016/S0079-6123(08)63223-9. [DOI] [PubMed] [Google Scholar]
- Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods. 2009;178(1):1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. Regional differentiation of the axon: a review with special reference to the concept of the multiplex neuron. Brain Res. 1972;47(2):269–288. doi: 10.1016/0006-8993(72)90639-7. [DOI] [PubMed] [Google Scholar]
- Wiley J, Owyang C. Neuropeptide Y inhibits cholinergic transmission in the isolated guinea pig colon: Mediation through α-adrenergic receptors. Proc Natl Acad Sci. 1987;84:2047–2051. doi: 10.1073/pnas.84.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC. Ultrastructural localization of adrenergic nerve terminals in the circular muscle layer and muscularis mucosae of the rat duodenum after acute treatment with 6-hydroxydopamine. J Anat. 1977;124(Pt 3):637–642. [PMC free article] [PubMed] [Google Scholar]