Abstract
Advances in solid drug nanoparticle technologies have resulted in a number of long-acting (LA) formulations with the potential for once monthly or longer administration. Such formulations offer great utility for chronic diseases, particularly when a lack of medication compliance may be detrimental to treatment response. Two such formulations are in clinical development for HIV but the concept of LA delivery has its origins in indications such as schizophrenia and contraception. Many terms have been utilised to describe the LA approach and standardisation would be beneficial. Ultimately, definitions will depend upon specific indications and routes of delivery, but for HIV we propose benchmarks that reflect perceived clinical benefits and available data on patient attitudes. Specifically, we propose dosing intervals of ≥ 1 week, ≥ 1 month or ≥ 6 months, for oral, injectable or implantable strategies, respectively. This review focuses upon the critical importance of potency in achieving the LA outcome for injectable formulations and explores established and emerging technologies that have been employed across indications. Key technological challenges such as the need for consistency and ease of administration for drug combinations, are also discussed. Finally, the review explores the gaps in knowledge regarding the pharmacology of drug release from particulate-based LA injectable suspensions. A number of hypotheses are discussed based upon available data relating to local drug metabolism, active transport systems, the lymphatics, macrophages and patient-specific factors. Greater knowledge of the mechanisms that underpin drug release and protracted exposure will help facilitate further development of this strategy to achieve the promising clinical benefits.
Keywords: HIV, AIDS, nanomedicine, formulation, sustained-release, time-release, controlled-release, extended-release, drug delivery, pharmacokinetics
Graphical Abstract
1. Introduction
The HIV/AIDS epidemic remains a major public health threat and approximately 36.9 million [34.3 million–41.4 million] people worldwide are estimated to be infected. In 2014, AIDS claimed an estimated 1.2 million [980 000–1.6 million] lives globally, with 2 million [1.9 million–2.2 million] people being newly infected in the same year. Worldwide, around 15.8 million people were accessing antiretroviral therapy in June 2015, constituting ~41% of adults and ~32% of children infected with the virus [1]. Antiretroviral therapy (ART) currently involves co-administration of drugs to simultaneously inhibit multiple viral targets, maximising inhibition of viral replication whilst minimising drug resistance. To date, 6 classes of antiretroviral drugs are available: nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), fusion inhibitors, CCR5 antagonists and integrase inhibitors (INIs). Although ART has led to a decline in mortality and morbidity, therapeutic failure occurs in an estimated 8% of treatment naïve and 33% of treatment experienced patients [2]. Antiretroviral drugs also have clinical application in the prevention of HIV infection, and pre-exposure prophylaxis (PrEP) strategies have been developed for subjects at high risk of acquiring the infection. Several factors contribute to heterogeneity in the response to antiretroviral agents, such as viral characteristics, immunological status, and pharmacokinetic variability to drug exposure. Currently available formulations necessitate lifelong, daily dosing and suboptimal adherence places patients at risk of treatment failure and low rates of protection for PrEP [3].
Recently, two antiretroviral drugs have entered clinical development as long-acting (LA) injectable depot formulations. The first of these, developed by Janssen is rilpivirine LA (Edurant®) [4-6] and the second, developed by ViiV Healthcare is cabotegravir LA [7, 8]. Both of these medicines are based upon the same nanotechnology that generates solid drug nanoparticle (SDN) suspensions via the process of wet bead milling (also known as nanomilling; see also section 4.1. below). LA injectable formulations have previously been developed and licensed for other indications such as contraception and schizophrenia (Table 1) [9-11]. The advent of the HIV LA medicines has been greeted with great excitement within the scientific, clinical and patient communities. In the short term, since only single agent LA medicines will be available, the largest impact is likely to be made by their deployment in PrEP [12]. However, it is hoped that the arrival of these medicines will spur further development of fully LA regimens for the treatment of HIV. There are a number of clinical challenges associated with the use of LA injectables, which have already been recently reviewed [13]. These challenges include the management of adverse drug reactions without the ability to easily stop therapy, concerns regarding the potential for injection site reactions (which may be exacerbated by the currently large injection volumes required), and the potential for emergence of viral resistance during protracted periods of sub-therapeutic exposure after therapy discontinuation.
Table 1.
Comparison of selected clinically-available long-acting injections and candidate injections under clinical development
| Technology | Drug name | Route | Dosing interval | Condition | Clinical depot volume |
|---|---|---|---|---|---|
| Suspension-based | |||||
| Solid drug particle | Medroxyprogesterone acetate | SC | 3 monthly | Hormone therapy | 0.65 mL |
| Solid drug particle | Medroxyprogesterone acetate | IM | 3 monthly | Hormone therapy | 1 mL |
| Solid drug particle | Olanzapine | IM | 2-4 weekly | Schizophrenia | max. 2.7 mL |
| Solid drug particle | Paliperidone palmitate | IM | 1 monthly | Schizophrenia | max. 1.5 mL |
| Solid drug particle | Paliperidone palmitate | IM | 3 monthly | Schizophrenia | max. 2.7 mL |
| Microparticle/microsphere | Somatropin | SC | 2-4 weekly | Hormone therapy | max. 1.5 mL |
| Microparticle/microsphere | Leuprolide acetate | IM | 1-3 monthly | Prostate cancer | 1.5 mL |
| Microparticle/microsphere | Naltrexone | IM | 1 monthly | Alcohol dependence | 4 mL |
| Microparticle/microsphere | Risperidone | IM | 2 weekly | Schizophrenia | 2 mL |
| Solid drug particle (undergoing human trials)* | Cabotegravir | IM | 1 quarterly* | HIV therapy and PreP | 2 × 2mL split* |
| Solid drug particle (undergoing human trials)* | Rilpivirine | IM | 1 monthly* | HIV therapy and PreP | 2 × 2mL split* |
| Solution-based | |||||
| Oil-based | Flupenthixol decanoate | IM | 2-4 weekly | Schizophrenia | max. 2 mL |
| Oil-based | Zuclopenthixol decanoate | IM | 2-4 weekly | Schizophrenia | max. 3 mL |
| Oil-based | Testosterone cypionate | IM | 2-4 weekly | Hormone therapy | max. 1.5 mL |
| Oil-based | Estradiol valerate | IM | 1 monthly | Hormone therapy | max. 1 mL |
| In-situ implant | Leuprolide acetate | SC | 1-6 monthly | Prostate cancer | 0.375 mL |
| Early stage solution-based immunotherapies | |||||
| Aqueous concentrated protein (undergoing human trials)* | CCR5 Monoclonal Antibody (PRO-140) | SC | 1-2 weeks* | HIV | 2 × 1mL split* |
| Aqueous concentrated protein (undergoing human trials)* | Broadly neutralising monoclonal antibody (VRC01) | SC | 3-4 weekly* | HIV | TBD |
| Aqueous concentrated protein (undergoing human trials)* | Broadly neutralising monoclonal antibody (VRC01) | IV | 3-4 weekly* | HIV | NA‡ |
| Aqueous concentrated protein (undergoing human trials)* | Anti-CD4 binding site monoclonal Antibody (3BNC117) | IV | 1 monthly* | HIV | NA‡ |
Note that since these formulations are currently still in clinical development, dosing interval and volume should be considered subject to change.
Intravenous infusions have shown long-acting benefits but are not considered depot injections
A major current obstacle to effective roll-out of the LA strategy for HIV therapy is the existence of only two antiretroviral drugs in this format. However, despite the fact that an NNRTI and INI combination has not previously been routinely utilised as a dual therapy oral regimen, the combination of rilpivirine and cabotegravir is currently being intensively studied. Indeed, the LATTE study, which investigated once-daily rilpivirine and cabotegravir as a maintenance therapy in virologically-suppressed adults demonstrated similar efficacy to a conventional oral regimen of efavirenz with two NRTIs at 96 weeks [14]. The very recent announcement that at week 32 in the LATTE 2 study the dual LA combination showed comparable efficacy to oral cabotegravir with dual NRTIs is extremely encouraging, although the data are yet to be published [15].
The range of chronic diseases that are being routinely managed, and the growth in preventative treatments coupled to a continual patient demand for interventions that do not restrict quality of life, has led to a clear clinical need for LA medicines across diseases [16]. This is particularly evident within an increasingly active ageing population. The market for injectable drug delivery devices, including self-injection, is predicted to grow from approximately £8 billion in 2013 to approximately £12 billion in 2018. This is within the context of an estimated total injectable drug market rising to approximately £380 billion by 2020 [17]. LA injections offer increases in patient adherence to therapy, reduction in clinical intervention and improvement in lifestyle.
The purpose of this review is to provide a context for further LA development of antiretroviral drug regimens. Current technologies that have been applied to LA delivery across diseases and indications will be reviewed along with technological challenges that may be important in the context of future development. Special consideration is also given to perceived gaps in knowledge relating to the pharmacology of drug release from an LA depot.
2. The conceptual basis for long-acting drug delivery of antiretroviral drugs
2.1. The key need to consider potency for antiretroviral long-acting medicines
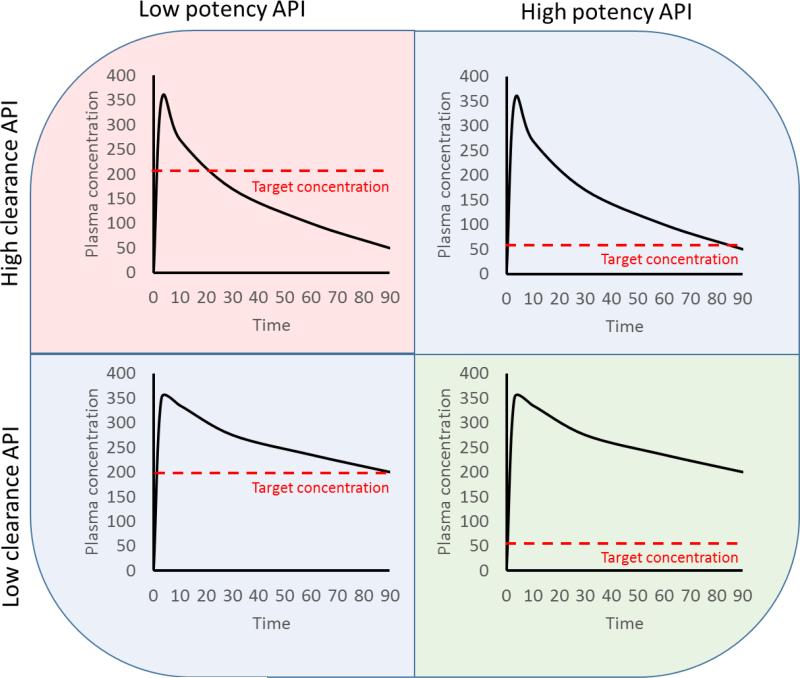
It is tempting to assume that protracted drug release to achieve sustained plasma exposure to the active pharmaceutical ingredient (API) is the predominant consideration in development of an LA drug delivery strategy. However, it should be noted that the plasma and intracellular concentrations required to achieve sustained viral suppression are key considerations for the approach. Indeed, while pharmacokinetic exposure is a prerequisite for success, ultimately it is the potency of the API that drives the concentrations that need to be achieved and for how long. Conceptually, this is described in Figure 1. Ultimately, the amount of drug that is required is dependent upon the potency of the API and while the pharmacokinetic exposure for a drug with high potency may be sufficient to reduce the frequency of dosing, it may not be sufficient for a drug with lower potency. This is important because the technological complexity of achieving a set plasma concentration for a specific duration may or may not be equal for APIs, irrespective of the potency.
Figure 1.
Diagrammatic representation of the relationship between pharmacokinetics and potency for theoretical long-acting formulations. It can be seen that a prolonged higher pharmacokinetic exposure, with the associated technological challenges, will be required to achieve a long-acting strategy for an API with lower potency compared with an API with higher potency. Ultimately, potency determines how much drug is required and for how long in order to achieve long-acting delivery.
The overwhelming majority of currently available LA injectable formulations available in the clinic were developed for drugs that were already available as oral formulations. Over the past 3 decades the understanding of critical physiochemical and molecular features that are important in the context of drug development for orally delivered drugs has proliferated. This understanding has culminated in the biophysical classification system (BCS) and design rules for optimal bioavailability such as Lipinski's rule of five and its various iterations [18, 19]. Numerous nanotechnological approaches are being explored to augment oral bioavailability and the mechanisms that determine API bioavailability for such advanced formulations may differ from conventional small molecules [20]. Importantly however, the physiochemical and molecular mechanisms that determine drug release for LA injectable formulations are not as well understood (see section 6 below). However, it is likely that the physiochemical properties of an optimal LA injectable drug are different from that of an optimal oral drug and a classification system may warrant development in this area as was recently suggested for topical drugs [21].
Putative differences in optimal physicochemistry are exemplified by the case of paliperidone palmitate. Prodrug strategies have been extensively used by pharmaceutical scientists for decades as an approach to improve aqueous solubility and thereby augment oral bioavailability [22]. However, paliperidone was first approved by the FDA on December 19th 2006 as a once daily oral treatment of schizophrenia and later, on July 31st 2009, paliperidone palmitate was approved as an LA injectable for the acute and maintenance treatment of schizophrenia in adults. Paliperidone was already poorly soluble in water (48.6 μg/mL) with the requirement for a sophisticated oral delivery technology. However, the ‘prodrugging’ of paliperidone with the palmitate moiety further reduced aqueous solubility, resulting in slow intramuscular dissolution and therefore enabling the LA approach [23]. Other examples of the use of a pro-drug to decrease solubility and improve compatibility with the LA approach also exist as for olanzapine pamoate [24]. Often during the drug development process there is a need to compromise potency as medicinal chemists utilise design rules to improve oral bioavailability potential. Importantly therefore, if LA injectable approaches are explored at the outset of development, rather than as a strategy for already licensed oral drugs, there is at least the potential to develop medicines with higher potency that require a lower pharmacokinetic exposure, thereby enabling increased duration of activity while reducing the volume of the depot required. However, the need to start patients on an oral regimen prior to initiating LA regimens (I.e. an “oral lead-in”) to mitigate putative occurrence of adverse drug reactions has been heavily debated in recent years and if the physicochemical properties really do differ markedly then this may pose a challenge for agents explicitly developed for LA.
2.2. A need for standardised nomenclature for long-acting delivery?
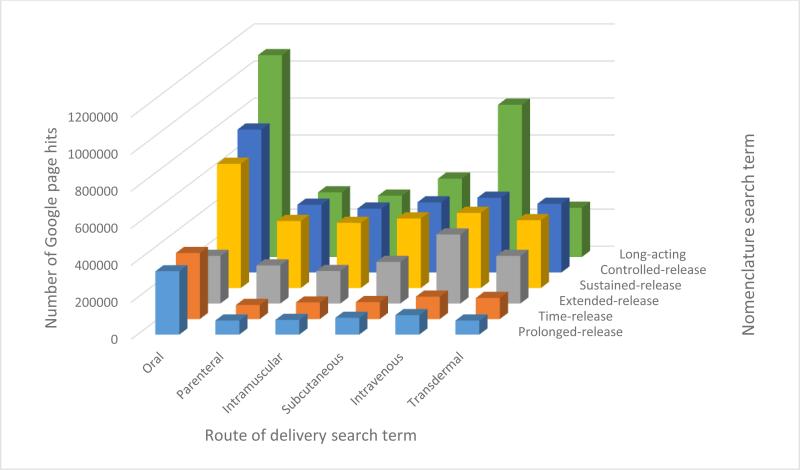
There are a number of terms that are commonly used to describe formulations designed to deliver exposure to therapeutic concentrations of APIs over a protracted period of time. Such terms include time-release, sustained-release, prolonged-release, extended release, controlled release and long-acting delivery to name but a few, and this nomenclature is often used interchangeably within the scientific and commercial literature. This transposable use of terminology complicates the ability to rapidly identify and utilise relevant literature and the authors would like to advocate for a standardised terminology for this particular therapeutic strategy. Therefore, for the purposes of this review, the term long-acting has been applied because it is currently the most widely applied terminology across different routes of delivery (Figure 2), and because the emphasis should be placed upon the duration of therapeutic exposure, which depends upon clearance and potency of the molecule in addition to the “release”.
Figure 2.
Results of a Google search with combinations of different nomenclature used to describe long-acting delivery with different routes of delivery, illustrating the interchangeable use of currently applied terminology.
Irrespective of the specific terminology applied, it is important to recognise that none of the existing terminology specifies the actual duration of therapeutic exposure. Rather, the terms are applied to exemplify that a longer duration of exposure, or a less frequent need for dosing is achievable relative to pre-existent or conventional formulations of the same drug. However, as new technologies emerge and the strategy gains traction, it should be noted that the development of LA formulations may be preferred to their conventional counterparts and thus the LA formulation itself is likely to set the precedent. Importantly, the desired duration is dictated by the specific application for which the formulation is being developed, and is then tempered by what is achievable in terms of the physiochemistry and pharmacology of the API, and the favoured route of administration. This is exemplified by the emergence of an insulin formulation (insulin glargine) for once daily administration that was termed LA because of the inability of its predecessors to achieve daily administration [25]. Since the term LA was expended on insulin glargine there are now reports of an ‘ultra-LA’ insulin formulation (insulin Degludec) that describe medicines with a longer duration of action [26]. Clearly, this poses the question of what an insulin medicine may be termed if ever the challenge of delivering basal insulin for an even longer duration were ever to be surmounted.
2.3. The importance of route of delivery in achieving the long-acting strategy
In the context of HIV therapy and prophylaxis, robust regimens are now already available for delivering therapeutic concentrations of multiple drugs from once-daily oral fixed dose combinations. Therefore, a prerequisite for LA delivery in a HIV context must be considered to enable less frequent dosing than once daily. There are extremely compelling clinical and patient-specific factors that are driving the enthusiasm for LA medicines in HIV. In the context of PreP and HIV therapy, adherence to medication is a major factor that influences the ultimate clinical outcome, and this has been extensively reviewed in the context of LA injectable products previously [27, 28]. Adherence to medication is a major driver of LA injectable development and is in some cases heavily influenced by ‘pill fatigue’. Indeed, despite historical public cautiousness about “nanotechnology”, a recent survey of HIV-positive patients in the USA demonstrated that 61%, 72% and 84% of patients would “definitely or probably try injectable nanoformulated antiretroviral therapy” for weekly, two-weekly or monthly dosing, respectively [29].
While oral drug development in HIV focused for many years on achieving fixed dose combinations available for once-daily dosing of drug regimens, it is difficult to see the utility of any formulation that can deliver drug for greater than a day but less than a week. This is predominantly because of the additional complexity that would be associated with remembering to take the medication in a timely and consistent fashion. Therefore, to have a truly beneficial impact for an oral regimen it is likely that a formulation would need to deliver a minimum of a weekly therapeutic exposure. However, such a formulation would also likely require some forgiveness for administration in order to circumvent issues if a medication were not administered at the same time on the same day each week. Tools such as mobile phone applications or automated calls could be brought to bear to deliver patient reminders for taking a weekly medicine. Current knowledge of the plasma half-life of existing PIs, NNRTIs, entry inhibitors and INIs make it difficult to see how a weekly administration format can be achieved without further API development. Notwithstanding, it should be noted that ultimate performance of antiretroviral therapies are thought to be highly dependent on intracellular drug concentrations in HIV target cells [30, 31], and the active metabolites of some NRTIs are known to have long intracellular half-lives [32, 33]. However, given the enthusiasm for the LA approach in adolescence, it is worthy of consideration that at least some of the NRTI metabolites have been reported to be almost 2-fold lower in patients under the age of 25 [34].
In the context of implants or devices for drug delivery, the invasiveness and associated need for trained healthcare staff to conduct the implantation procedure is likely to mean that a longer duration between administrations will be required to realise this approach. Implants have proven successful in other indications such as contraception [35]. However, it should be noted that the challenge of drug combinations, which are a prerequisite for HIV therapy has not yet been met for implants. Moreover, the plasma concentrations required to mediate the desired effect are lower than those required to suppress HIV replication with currently available antiretroviral drugs. Indeed, peak plasma concentrations of etonogestrel following implantation of IMPLANON® were 813 pg/mL [36], which is lower than those required for adequate viral suppression with antiretroviral drugs, and an implant for HIV therapy would also be required to deliver at least two drugs simultaneously as a minimum. For context, the IC95 concentrations for dolutegravir, rilpivirine and raltegravir are 64 ng/mL (protein binding-adjusted [37]), 20.3 ng/mL (protein binding-adjusted [38]) and 15 ng/mL [39], respectively. Therefore, while conceptually the ability to deliver an efficacious drug combination for >6 months via an implant is extremely appealing, it seems unlikely to be realised in the short- to medium-term without significant advances in current device technologies and/or potency of available antiretroviral drugs. The authors direct the reader to a recent thorough review of implantable drug delivery devices [40].
Taken collectively the authors propose the following table to describe the use of LA terminology in HIV that takes into account routes of administration, patient attitudes (where available) and current clinical paradigms (Table 2).
Table 2.
Proposed timescales to be deemed long-acting for HIV segmented by administration route
| Route of delivery | Oral | Parenteral | Implant / Device |
|---|---|---|---|
| Dosing frequency | ≥ 1 week | ≥ 1month | ≥ 6months |
While it is important to consider developments in oral and implantable LA strategies, the remainder of this review will focus on LA injectable medicines.
3. Technologies that have been clinically successful for LA delivery
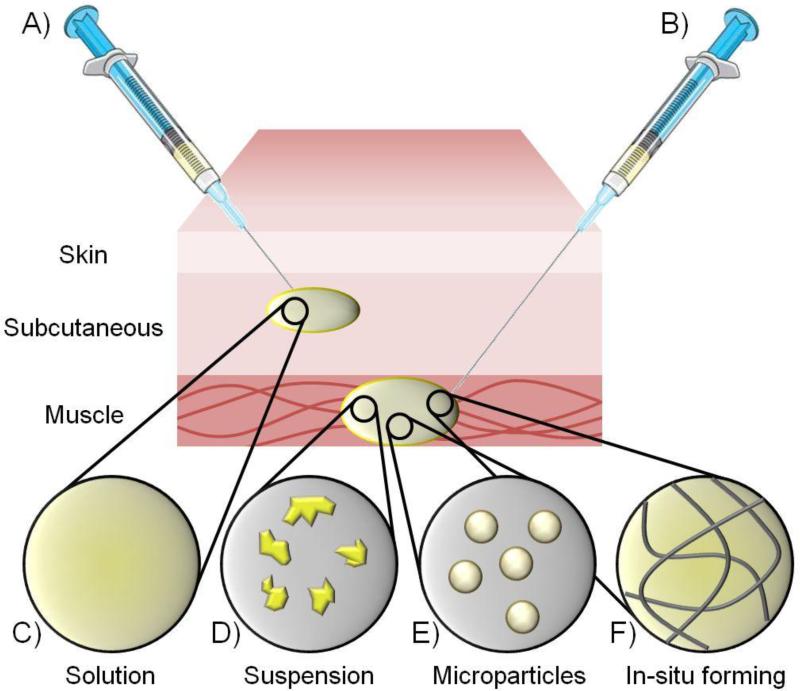
The manufacture of products for LA delivery has been achieved using a variety of differing technologies and a range of new strategies are being investigated. In general, currently marketed products utilise one of two broad formulation approaches (Figure 3); injection of solutions or particle suspensions [41]. Administration predominantly targets intramuscular routes despite subcutaneous injection offering the use of shorter needles and less patient discomfort. This is due to the volume of injected material of up to 5mL in adults and 2 mL in children that can be tolerated [42], allowing a larger dose of drug to be delivered to the depot site. The current most successful LA delivery technology utilises an oil-based vehicle containing dissolved lipophilic drug; the two remaining leading formulation strategies utilise dispersed solids within a liquid matrix (Table 1).
Figure 3.
Schematic representation of the main technologies used for long acting injections. Depots may be delivered A) subcutaneously or B) intramuscularly and utilise either C) oil-based solutions, D) drug particle suspensions, E) drug encapsulated in polymer microparticles, or F) in-situ formation of gels or solid/semi-solid structures.
3.1. Solution-based injections
Despite speculation concerning the actual date of the introduction of intramuscular injections [42], the early to mid-1900s saw the development of injectable solutions of drugs within a range of oil phases. The drug compounds were often palmitate or decanoate esters that facilitated dissolution [43], and oils such as castor oil, soybean oil, peanut oil, cottonseed oil, caprylic and capric triglycerides from coconut or palm seed oils, sesame oil and safflower oil have been regularly used since this time in approved intramuscular formulations [44]. Formulation additives often include small molecule solubilising agents and stabilisers such as antioxidants. These first generation LA formulations were typically developed for antipsychotic therapies [45] and relied upon the injected oil to generate a localised bolus which would slowly disperse and release drug; esterases are believed to chemically cleave the dissolved ingredients to generate the parent active compound [24]. In more recent cases, lipophilic drugs are utilised that do not require localised or plasma activation.
Solutions of lipophilic drugs within injectable oils are relatively simple to manufacture but several issues require optimisation to achieve the desired delivery profile. Release from intramuscular solutions generally allows for drug delivery over a matter of weeks and repeated injection is most commonly required each month [41]. The availability of a range of oils provides the opportunity to modify release kinetics whilst the generation of prodrugs with varying lipophilicity also adds to the available formulation variables [46]. Partition coefficients between the oil solution and the surrounding environment after injection have a direct influence on release kinetics and observed pharmacokinetics [47]. However, the injection site [48, 49] and volume of injected liquid also play important roles [47]. Other non-covalent interactions may also be manipulated to enable tailored drug release, such as the variation in the degree of hydrogen bonding between drug and hydroxyl groups within castor oil through the formation of oil mixtures with varying castor oil content [50].
3.2. Suspension-based injections
The solubility of a drug compound within an injection vehicle such as an oil may be highly restrictive to formulation development, leading to unacceptable injection volumes. In some cases, drug compounds may not possess significant long-term stability in solution, effectively ruling them out for solution-based injections. The suspension of drug particles within a vehicle or the encapsulation of the drug onto a polymeric carrier prior to suspension allows the injection of solids rather than molecular solutions. These two approaches are described separately below.
3.2.1. Drug particle suspensions
The formation of injectable drug particle suspensions is typically achieved in one of two ways. In its simplest conceptual form, the powdered drug may be mixed with a delivery vehicle and milled until drug particles of an appropriate size range (typically < 10 μm) for injection are formed. This process generates a distribution of particle sizes and the breadth of this distribution may affect the overall release profile from the injected material [51]. Delivery vehicles are typically aqueous and include excipients to stabilise the particles. Alternatively, drug crystals may be formed directly within a vehicle through the controlled mixing of two solutions, one of which is a poor solvent for the drug compound. This often necessitates the use of an organic solvent phase that must be removed prior to injection [52]. Injectable drug suspensions were first approved by the FDA at the end of 1960 in the form of an intramuscular contraceptive injection. Drug release from solid drug particles has been achieved for several months due to the slow dissolution of crystals from the suspension, and crystalline prodrugs can maintain release profiles through a combination of dissolution and parent drug formation rates [53].
3.2.2. Microparticle/microsphere suspensions
The entrapment or encapsulation of drugs into polymer matrices at the micron scale offers additional control over drug release profiles [54, 55]. Polymer particles are generated typically using a solvent evaporation technique from emulsified polymer/drug solution [54]. Injected microspheres have been shown to allow tailored release for several months. The added aspect of microsphere matrix erosion at the injection site may be controlled by chemical parameters, such as polymer or copolymer functionality and polymer-drug interactions, and physical properties such as molecular weight, dispersity, microsphere porosity and diameter, degree of crystallinity, glass transition temperature, hydrophilicity and drug distribution throughout the microsphere [56-58]. Despite these variables, initial burst release profiles are often seen from microsphere injections [59] with further identifiable stages of slow and sustained release and a later stage acceleration as the microsphere degrades [60, 61].
Biodegradation of the microsphere is important to allow for clearance of the polymeric matrix material and polyesters dominate the choice of material [62]. Various microsphere morphologies have been employed to optimise drug release including hollow polymer particles and solid matrices to either encapsulate the drug or evenly distribute the API throughout the spherical particle [63]. The first microsphere-containing formulation was approved by the FDA in 1989 for palliative treatment of prostate cancer patients [41].
4. Emerging technologies for LA formulation
The use of solution and suspension-based injections has been successful in introducing many clinical options for the treatment of a relatively narrow range of indications including prostate cancer, hormone moderation and schizophrenia. A number of emerging technologies for LA and injectable therapeutics are either on the brink of translation to clinical adoption or maturing through the stages of commercial development; four of these are detailed below.
4.1. Nanoparticle suspensions
Suspension-based intramuscular injections have typically utilised particles in the < 10 μm diameter range. However, the development of mechanical attrition processes [64] (for example nanomilling technologies and high pressure homogenisation) and non-attrition nanoparticle fabrication (for example emulsion-templated freeze drying) has allowed access to nano-scale solid drug particles (< 1 μm) [65-68]. The rilpivirine LA and cabotegravir LA options [6, 7] that are in extended clinical trials (including evaluation of simultaneous combination in LATTE 2) are based upon this technology.
4.2. Injectable monoliths
Drug eluting implants have been used to provide LA delivery of various therapies for several years. LA contraception has particularly benefitted from the use of sub-dermal injectable implants, providing a ‘forgettable’ solution for several years [69]. Insertion requires trained administration and removal requires a surgical incision, often leading to scarring. In recent years, the formation of biodegradable injectable monoliths has been the subject of considerable interest for the LA delivery of biological drugs [70].
4.3. In situ-forming depots/implants
The limitations of injectable monolithic implants are being addressed using liquid formulations that solidify to form drug depots after injection [71]. These options are known as in situ-forming depot injections and are designed to degrade during use and, therefore, mitigate the need for surgical removal. Injections can be intramuscular or subcutaneous and several solidification mechanisms have been employed, including in situ precipitation, chemical crosslinking and solidification of molten liquids or assembly of liquid crystal [72]. A precipitation mechanism, driven by dispersion of a co-solvent, was employed in the first FDA-approved in situ-forming implant system in 2002, which is available in 1, 3, 4 and 6 month delivery options [73].
4.4. Microneedle delivery
To overcome the intrusive nature and patient acceptability of injections, the development of microneedle technologies has been highly active for nearly two decades [74]. Initially, inorganic and metallic needles up to 900 μm in length were developed and arrays of drug-coated microneedles have been arranged onto patches for transdermal delivery [75]. This format has been extended to include dissolvable/degradable microneedles that deliver drugs in an encapsulated form into the skin [76]. In some studies, the potential for delivery over timescales up to 2 months has been demonstrated [77]; in other cases, delivery of up to several days has been achieved [78, 79]. However, as discussed above, these observations must be tempered against API potency and the resultant target concentrations that need to be achieved.
5. Technological challenges for LA delivery
Current LA technologies have been successful in addressing defined clinical needs within a window of indications. Key challenges in this regard relate to the ultimate manufacture of sterile medicines with maximum drug loading to keep the volume of administration as low as possible. A number of other therapy-specific, manufacturing and administration challenges exist in the extension of these approaches to a broader range of therapeutic needs and these are elaborated in this section of the review.
5.1. Drug combinations
The formulation and manufacturing approaches employed in LA development outlined above require optimisation towards ideal release for each drug compound. The incorporation of two or more drugs into fixed dose LA combinations, an approach that is critical to the success of HIV therapy, is a major challenge. The identification of single vehicles capable of dissolving multiple drugs for solution-based LA injections provides a number of hurdles, especially when attempting to overlay various partition coefficients to maintain the dose ratios over extended periods. Recent reports of bottom-up formation of combination SDNs suggests some potential for future LA formulations using nanosuspension approaches but the tailoring of release profiles is yet to be demonstrated [80, 81]. Equally, the formation of microparticles containing drug combinations using solvent evaporation techniques requires a solvent phase able to generate appropriate drug combinations to match final therapeutic need whilst preventing unequal losses to the aqueous phase during evaporation. In addition, the triphasic release rate profiles observed for microparticles, including the initial ‘burst’ and final increase, presents a significant hurdle to maintaining drug delivery ratios for mixed pharmaceutical payloads.
The range of alternative and emerging technologies also suffer from similar production and manufacturing concerns. For example, the precipitation of multiple drugs from an in-situ forming depot may lead to uneven distribution of drug compounds within the depot and variable or preferential release rates of individual therapy components.
5.2. “Syringeability”
The administration of any LA formulation necessitates the delivery of a dose of drug able to achieve target plasma concentrations for prolonged periods. For liquid formulations, including dispersions, high viscosities may be unavoidable which necessitate the use of wider bore needles than desired, unfavourably high volume or number of simultaneous injections. The generation of low viscosity approaches, new injection methodologies or excipients to enable reduced volumes may aid the application of LA formulations for lower potency drugs or high potency molecules with low ‘processability’.
5.3. Depot consistency, shape and size
Currently, the dimensions that an LA formulation adopts after injection may be highly variable and depend on factors such as the structure of the tissue within the injection site, the physiology of the recipient, the rate of injection and the technology format used. This impacts the drug release kinetics and generates considerable variability [82]. The use of suspension technologies can also have difficulties relating to stability of the drug particles including changes in crystal polymorph and aggregation within the suspension leading to variation in the intended particle size distribution [83].
5.4. Formulation sterility and manufacturing
Controlling the sterility of injectable pharmaceuticals is a constant challenge, although one that is overcome using a range of auditable manufacturing processes and equipment [84]. Innovation using injectable micro or nanoparticles and high concentration liquids may lead to the introduction of specialised processing needs with subsequent development of new quality controls. The recent trends towards the production of pre-filled sterile syringes for administration of biopharmaceutical products may offer opportunities to advanced LA products and minimise concerns during drug administration [85].
6. Gaps in knowledge regarding the pharmacology of solid drug nanoparticle LA injectables
As alluded to above (section 2.1.) the key physiochemical properties and molecular mechanisms important in the context of achieved LA delivery have not been extensively discussed or explored within the scientific literature. As such, there exist unanswered questions in terms of the physiological, anatomical, and genetic factors that may influence the extent of bioavailability from a depot and the variability therein. There are also unanswered questions that relate to the impact of environmental factors on drug release. Following oral administration, a drug must survive the molecular processes in the intestinal epithelium, prior to passage via the hepatic portal vein and subsequent first-pass metabolism within the liver. Intuitively one might expect that the pharmacokinetics of a drug after intramuscular administration might be less variable than following oral administration, because these complex mechanisms that evolved to protect the body from exposure to potentially toxic dietary components are circumvented. However, for the majority of LA injectable formulations the pharmacokinetic variability appears to be as large, if not larger than the counterpart oral formulations (Table 3).
Table 3.
Coefficient of Variation (CV%) for pharmacokinetics (Area under the plasma concentration curve; AUC) of selected drugs following administration as oral tablet formulations or LA suspensions.
| Drug | Oral PK variability (AUC CV%) | LA PK variability (AUC CV%) | References |
|---|---|---|---|
| Paliperidone | 35.3 | 40% | [86, 87] |
| Olanzapine | 26% | 50% | [88, 89] |
| Medroxyprogesterone | 52% | 34% | [90, 91] |
| Rilpivirine | 39% | 52%* | [92, 93] |
| Cabotegravir | 27% | 39% | [94] |
Note that the value given represents CV% at a dose of 300mg and the same paper demonstrates a decrease in variability at higher doses falling to 32% at 600mg and 24% at 1200mg, which may indicate a saturable process involved in pharmacokinetic variability.
Irrespective of the route of administration for an API, it is assumed that once the molecule enters the systemic circulation the mechanisms that contribute to its distribution and clearance will remain the same. Therefore, it seems logical to conclude that the predominant reasons for the higher inter-patient variability in pharmacokinetic exposure following LA delivery are governed by the manner in which the drug is absorbed. A potential caveat to this is that there is currently a paucity of information relating to whether intact particles enter the systemic circulation after administration as an LA injectable depot (discussed in more detail below). This is driven by the bioanalytical challenges associated with quantification of dissolved versus particulate drug within the blood – conventional methods of drug detection such a HPLC and LC-MS/MS do not discriminate because of the solvent extraction process necessary in sample preparation, which dissolves this type of nanoparticles. Recently, the authors validated a flow cytometry method for detection of intact nanoparticles in plasma [95] and developed SDNs based on fluorescence resonance energy transfer [80] that may have utility in understanding this fundamental question. Nonetheless, if the majority of the pharmacokinetic variability stems from drug release from the depot there are a number of putative depot-specific physiological, anatomical or environmental factors that may contribute and these are hypothesised in this section of the review.
6.1. A role for drug transporters and metabolic enzymes in LA bioavailability?
As discussed elsewhere in this issue of the journal [96], transporters and drug-metabolism enzymes expressed in the intestinal epithelium and hepatocytes, play a key role in modulating the bioavailability of drugs dosed via the oral route. ABCB1 (P-glycoprotein) remains the best-studied transporter in intestine and there is some evidence that it is variably expressed in skeletal muscle [97]. More robust evidence exists for expression of a number of other well known drug transporters of the ATP-binding cassette (ABC) family in skeletal muscle and these include ABCC1, ABCC4 and ABCC5 [98, 99], all of which have been shown to have antiretroviral substrates [100-102]. This same manuscript demonstrated that other common drug transporters such as ABCC2 (MRP2) and ABCG2 (BCRP) were expressed at low levels in these cells. Transfection of skeletal muscle myoblasts with ABCC1 was demonstrated to protect against statin-related toxicity indicating a functional role for this transporter in skeletal muscle [98].
In liver, the organic anion transporting polypeptide isoforms 1B1 and 1B3 (OATP1B1 and OATP1B3 coded by SLCO1B1 and SLCO1B3) have been shown to play a key role in inter-patient variability in oral pharmacokinetics for many APIs including statins [103] and antiretroviral drugs [104]. A related drug influx transporter, OATP2B1 is also expressed in skeletal muscle and transfection of skeletal muscle myoblasts with OATP2B1 enhanced statin-related toxicity in these cells [98]. While these data are interesting it should be noted that they do not directly demonstrate a role for transporters in facilitating or mitigating drug permeation from muscle to blood. Ultimately, if drug-transporters are important in regulating bioavailability from an LA injectable it seems likely that their expression in capillary endothelium, rather than muscle cells, would be a major mediator. However, while ABCB1 expression appears to vary between capillary endothelial cells from different tissues [105], the authors are not aware of any data that have explicitly investigated expression of transporters in relevant subcutaneous or skeletal muscle-derived capillary endothelium, let alone that derived from gluteal (or deltoid) muscle.
Similarly to transporters, biotransformation of APIs by drug metabolism enzymes in intestinal epithelium and liver is a key determinant of oral bioavailability and pharmacokinetic variability of antiretroviral and other drugs [106-108]. The cytochrome P450 (CYP) enzymes are widely recognised as the predominant family for catalysing phase I metabolism of drugs and the most abundant isoform in liver and intestine is CYP3A4. Other CYP isoforms that play a key role for antiretroviral drugs include CYP3A5, CYP2B6, CYP2C9, and CYP2C19 [109-111] but very little is known about expression of these in muscle or subcutaneous adipose tissue. Interestingly, CYP3A4 and CYP3A5 mRNA and protein expression have been explicitly demonstrated in childhood rhabdomyosarcoma and adjacent healthy tissue [112], as well as quadriceps skeletal muscle from children and adolescents [113]. Numerous relevant CYP isoforms have also been shown to be expressed in human subcutaneous adipose tissue explants, albeit at levels that are far lower than liver [114]. Phase II enzymes of the UDP-glucuronyl transferase (UGT) family also metabolise antiretroviral drugs [115, 116] but their expression in skeletal muscle and adipose tissue is not well understood. Members of the nuclear receptor family of transcription factors that are known to regulate drug transporters and metabolism enzymes are expressed in skeletal muscle [117] and subcutaneous adipose tissue [118], which further highlights the potential metabolic function of this tissue. Moreover, if skeletal muscle (or adipose tissue) does contribute to metabolism of drugs, the presence of nuclear receptors such as PXR and CAR may mean drug-drug interactions at the depot site may warrant consideration if LA combinations are realised.
The capillary architecture and permeability varies from tissue to tissue and the endothelium may be continuous or discontinuous, and continuous endothelium may be fenestrated within tissues such as intestine, kidney or endocrine organs [119]. Muscle capillaries are predominantly continuous, meaning that they are the least permeable of capillaries with many tight junctions between the endothelial cells and fewer intercellular clefts for unrestricted small molecule passage than other types of capillary [120]. Interestingly, it has been estimated that the fractional area of the blood capillary wall that is composed of intercellular cleft and therefore exposed for free diffusion of small molecules and fluids is only 0.4% [121]. Moreover, the width of the intercellular cleft has been determined to vary but appears to be ~20nm at the wider end [121], with a physiological upper limit pore size of 5nm in skeletal muscle [122]. Therefore, this would be expected to restrict the direct access of LA intact nanoparticles (measurable in 100s of nm) directly into the blood. As such, it seems reasonable to speculate that if transporters are expressed within the endothelium they may play an important role in governing the passage of drug from depot directly into blood, and variability therein. Collectively this means the study of the expression of transporters and drug metabolism enzymes specifically in gluteal muscles and their associated blood (or lymph – see below) capillary endothelium may be worthy of further investigation.
6.2. The putative role of the lymphatics in LA bioavailability?
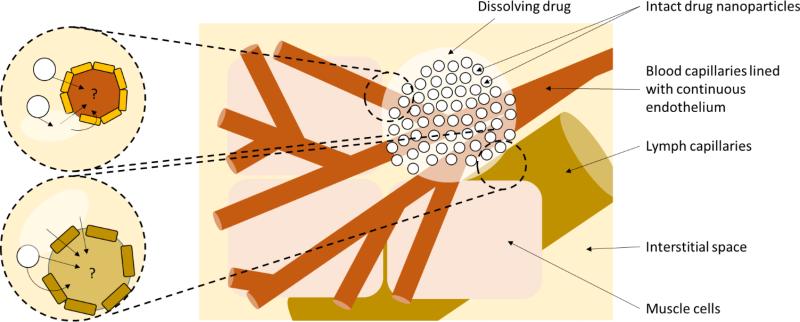
In addition to the blood capillaries, skeletal muscle and subcutaneous tissue is also permeated by a network of lymphatic capillaries (Figure 4) [123, 124]. The lymphatic system is constructed of a web of interspersed vessels and nodes, and serves as a key organ of the immune system [125]. The lymph capillaries are wider in diameter than the blood capillaries [126] and have larger intercellular clefts between their endothelial cells. Indeed, it has been suggested that when the surrounding tissue is hydrated, the endothelial cells may stretch apart to form pores up to 2μm in diameter [127]. Lymph capillaries are also in close association with the interstitial space and have open ends that enable free access to fluid and particulates [128]. Importantly, the lymphatic system has also been explicitly shown to deliver particulates (as well as lipoproteins and macromolecules) from peripheral tissues into the systemic circulation [126], and the lymphatic vessels ultimately empty into the left internal jugular and left subclavian veins. After parenteral delivery, smaller structures (<10nm) enter the systemic circulation predominantly via the blood capillaries whereas structures between 10 - 100nm are optimum for entry into lymphatic vessels (recently reviewed [126]). Indeed, early studies with liposomes showed the critical importance of size [129].
Figure 4.
Diagrammatical representation of the anatomical relationship between blood and lymphatic capillaries. Drug may theoretically enter the systemic circulation through either route since the lymphatics ultimately drain into the eventually empties into the left internal jugular and left subclavian veins. It is currently unknown whether intact nanoparticles enter the systemic circulation or whether dissolved molecule predominates. However, as elaborated in the text, there is a theoretical route for direct entry of nanoparticles either through endothelium endocytic mechanisms or through the intercellular clefts between endothelial cells.
Importantly, particles above 100nm are thought to be less able to permeate lymph [126]. However, it should be noted that although the SDN formulations such as rilpivirine LA and cabotegravir LA have particle diameters measured in 100s of nanometers, they have a large polydispersity in comparison to other nanoparticle technologies meaning that at least some of the rilpivirine LA particles (for early development formulations) were between 10 – 100nm in diameter [5]. Additionally, as larger particles dissolve into molecules, they would also be expected to pass through this size range. Therefore, for this nanoformulation type uniquely, the authors speculate that a combination of mechanisms may contribute to delivery of the drugs to the systemic circulation and that the contribution of individual mechanisms may differ across the dosing interval (i.e. as particles dissolve). It should be noted that concentrations of rilpivirine were reported to be 100 times higher than plasma in the local lymph node a month after intramuscular delivery of the LA formulation [4]. However, intact nanoparticles were not assessed presumably because of the bioanalytical challenges discussed above. The role of the lymphatic system in bioavailability of SDN formulations is not clear but it should be remembered that the flow rate of lymph is much slower than that of blood, which may be beneficial for LA if it is sufficient to provide a meaningful contribution to absorption.
It should be noted that the lymphatic system has been an area of interest for the delivery of anticancer nanomedicines in recent years [126, 130, 131] and may be worthy of further specific development in the area of antiretroviral therapy, especially considering the role of the lymphatics in HIV pathogenesis [132], the presence of viral reservoirs within lymph nodes [133], and their identification as a key target for eradication strategies [134, 135]. Importantly, subcutaneous delivery (rather than intramuscular delivery) has been at the forefront of gaining access to the lymphatics in cancer and was recently extensively reviewed [136]. It must be acknowledged that if intact nanoparticles do enter the systemic circulation then there may be other routes of elimination that require consideration beyond those for conventional small molecules, and biodistribution may also be influenced by particle-related mechanisms (recently reviewed [137, 138]).
6.3. A role for macrophages in nanoparticle bioavailability?
Interesting recent work from the University of Nebraska Medical Centre demonstrated co-localisation of atazanavir SDNs with macrophages after intramuscular administration to mice [139]. Importantly, these SDNs were produced using techniques analogous to those used in the manufacture of rilpivirine LA and cabotegravir LA. Similar studies have not been conducted with the antiretroviral clinical formulations but other antiretroviral SDNs are known to be taken up into macrophages via endocytic processes [140, 141]. The most compelling data to date on a role for macrophages in drug release comes from work with paliperidone palmitate particles in rats [142]. This superb manuscript documents the inflammatory response to the formulation followed by deep macrophage infiltration and angiogenesis within the depot. Importantly, the manuscript also demonstrated the presence of the particles within macrophages with the most densely loaded macrophages adjacent to the depot, and positively stained macrophages were also demonstrated within the local lymph nodes. While these data are insufficient to demonstrate an absolute role of macrophage in bioavailability following LA administration, this is an area that is certainly worthy of further investigation.
6.4. A role for endocytosis in nanoparticle permeation across capillary endothelium?
The cells of capillary endothelium have been documented to have the structural features associated with endocytosis and transcytosis [119]. Although there is a paucity of data specific to skeletal muscle / subcutaneous blood and lymph capillaries, other continuous capillary endothelial cells have caveolae [143], vesiculo–vacuolar organelles [144], and clathrin-coated architecture [145]. These structures have attracted attention in recent years because of their involvement in nanoparticle uptake and trafficking [146]. Whether these processes contributed to bioavailability of LA depot nanoparticles has not been investigated but this may be worthy of further investigation. It is of interest to note that SDNs exposed to human plasma acquire a protein corona and transferrin (which has been used to augment clathrin- and caveolae-mediated endocytosis [147]) is capable of adsorbing to their surface [95]. Moreover, as mentioned above studies with atazanavir SDNs have explicitly implicated endocytic processes in their macrophage uptake [140].
6.5. Patient factors of putative importance in LA inter-patient variability
The factors that influence the inter-patient variability in pharmacokinetic exposure to orally administered drugs have been well studied and are for the most part drug-specific, involving interrelated factors such as pharmacogenetics [148, 149], body weight [150, 151], gender [152, 153], and drug-drug interactions [154]. As new LA agents become available it will be necessary to determine whether such factors play an equal role in pharmacokinetic variability, or whether other as yet understudied mechanisms contribute or even predominate. As discussed above, although intuitive to expect variability to be lower for LA formulation due to avoidance of molecular processes within intestine, this is not born out by currently available data (Table 3). It is also tempting to speculate that the magnitude of some transporter- or enzyme-mediated drug-drug interactions or pharmacogenetic associations may be lower for the same reason. However, this will ultimately depend upon the contribution of the intestinal mechanisms relative to the role of the liver (or other tissues). In the case of HIV therapy this may be particularly pertinent to interactions with integrase inhibitors that are affected by pH and chelation to metal ions in gut [155, 156].
Other factors for which there is a current paucity of information, relate to whether physical activity, ambient temperature, muscle density / body mass index, or accumulated scar tissue after prolonged therapy may influence depot drug release and therefore pharmacokinetic exposure after LA administration. Data from the 1980s indicated that there may be differences in pharmacokinetic exposure to insulin following exercise after subcutaneous administration, and marked differences in absorption were also noted when patients were at 30°C relative to 10°C ambient temperatures [157]. Although there are currently no data for the emerging HIV LA medicines, other studies have also shown an impact of exercise on pharmacokinetics following parenteral administration (reviewed previously [158, 159]), and this may be worthy of further investigation. If exercise and ambient temperatures do influence drug release then it is possible that factors such as geographical location and occupation may need consideration. The manner in which a depot is administered may also influence the variability in exposure and it is of interest to note that even using image-guided intramuscular administration in a preclinical species, part of the dose ultimately resided subcutaneously [142].
7. Summary and conclusions
The emergence of antiretroviral LA medicines is academically exciting and early signs indicate a high desirability by the HIV patient community. However, while many technologies are discussed in the context of LA delivery it is imperative that the specific indication is considered when interpreting such data. It is important to consider that LA may refer to once daily dosing for one indication versus once monthly (or longer) for another. Therefore, the nomenclature for LA delivery would greatly benefit from standardisation to harmonise strategies in a disease-specific and technology-specific manner. For HIV, it is difficult to see an injectable medicine having real impact unless it can be administered with dose intervals of at least four weeks, preferably a quarter or even longer. There is a current paucity of knowledge regarding the mechanisms that determine ultimate drug delivery into the systemic circulation, which may be critical to future LA development and important for rationalising clinical strategies for their effective use. In addition, the challenges associated with pre-clinical evaluation of candidate LA formulations have also been reviewed recently [46, 160] and the first physiologically-based pharmacokinetic (PBPK) modelling strategies are beginning to emerge [161]. Robust in vitro and in silico methods will help accelerate development while reducing the burden on animal experimentation, which is particularly difficult for LA formulations due to the timescales involved. This is extremely important in the context that a key next step will be to develop truly LA regimens including more than one drug. The recent data from the LATTE 2 study are extremely encouraging for a rilpivirine and cabotegravir combination but a single intramuscular administration would be preferable and NRTIs have stood the test of time as backbone therapies. It is worth noting that if the technological challenges associated with LA formulation of an NRTI backbone can be surmounted, two LA regimens may become available shortly after. Importantly, LA regimens may be well suited to, if not dependent upon, directly-observed therapy, and therefore may be less prone to issues with compliance. The US National INItutes for Health have recently funded the Long-acting / extended-release antiretroviral resource programme (LEAP; www.longactinghiv.org), which aims to accelerate collaboration, development and translation in this area. LEAP constitutes an international network of key stakeholders from industry, academia, charitable organisations and the patient community, aimed at maintaining momentum in this truly exciting area through the provision of workshops, resources and an externally accessible PBPK modelling core.
Acknowledgments
The authors would like to thank the National Institutes for Health (R01AI114405, R24AI118397, 7UM1AI068636), US Agency for International Development (AID-OAA-A-15-00069), Engineering and Physical Sciences Research Council (EP/K002201/1, EP/I038721/1, EP/G066272/1), Medical Research Council (G0800247), European Commission (654190), and Clinton Health Access Initiative for relevant grant support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors are co-inventors of patents relating to the application of nanotechnology to HIV drug delivery and are co-founders of a University of Liverpool start-up company, Tandem Nano Ltd. The authors have also received funding from Merck, Janssen, ViiV Healthcare, AstraZeneca, and Pfizer.
References
- 1.UNAIDS FACT SHEET 2015. 2015 [Google Scholar]
- 2.Palella FJ, Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.DHHS . Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of Health and Human Services; 2010. Panel on Antiretroviral Guidelines for Adult and Adolescents. pp. 1–166. [Google Scholar]
- 4.van 't Klooster G, Hoeben E, Borghys H, Looszova A, Bouche MP, van Velsen F, Baert L. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010;54:2042–2050. doi: 10.1128/AAC.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baert L, van 't Klooster G, Dries W, Francois M, Wouters A, Basstanie E, Iterbeke K, Stappers F, Stevens P, Schueller L, Van Remoortere P, Kraus G, Wigerinck P, Rosier J. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm. 2009;72:502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Williams PE, Crauwels HM, Basstanie ED. Formulation and pharmacology of long-acting rilpivirine. Curr Opin HIV AIDS. 2015;10:233–238. doi: 10.1097/COH.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 7.Andrews CD, Heneine W. Cabotegravir long-acting for HIV-1 prevention. Curr Opin HIV AIDS. 2015;10:258–263. doi: 10.1097/COH.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 8.Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 2015;10:239–245. doi: 10.1097/COH.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera): a highly effective contraceptive option with proven long-term safety. Contraception. 2003;68:75–87. doi: 10.1016/s0010-7824(03)00136-7. [DOI] [PubMed] [Google Scholar]
- 10.De Berardis D, Marini S, Carano A, Lang AP, Cavuto M, Piersanti M, Fornaro M, Perna G, Valchera A, Mazza M, Iasevoli F, Martinotti G, Di Giannantonio M. Efficacy and safety of long acting injectable atypical antipsychotics: a review. Curr Clin Pharmacol. 2013;8:256–264. doi: 10.2174/15748847113089990056. [DOI] [PubMed] [Google Scholar]
- 11.Furiak NM, Ascher-Svanum H, Klein RW, Smolen LJ, Lawson AH, Montgomery W, Conley RR. Cost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the United States: a micro-simulation economic decision model. Curr Med Res Opin. 2011;27:713–730. doi: 10.1185/03007995.2011.554533. [DOI] [PubMed] [Google Scholar]
- 12.Landovitz RJ, Kofron R, McCauley M. The promise and pitfalls of long-acting injectable agents for HIV prevention. Curr Opin HIV AIDS. 2016;11:122–128. doi: 10.1097/COH.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis DA, Boffito M. Long-acting antiviral agents for HIV treatment. Curr Opin HIV AIDS. 2015;10:246–252. doi: 10.1097/COH.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis DA, Brinson CC, Smith GH, de Vente J, Hagins DP, Eron JJ, Griffith SK, Clair MH, Stevens MC, Williams PE, Ford SL, Stancil BS, Bomar MM, Hudson KJ, Smith KY, Spreen WR. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145–1155. doi: 10.1016/S1473-3099(15)00152-8. [DOI] [PubMed] [Google Scholar]
- 15.Healthcare V. [22nd December 2015]; https://www.viivhealthcare.com/media/press-releases/2015/november/viiv healthcare-announces-positive-headline-results-from-a-study-of-two-drug-injectable-regimen-for hiv-maintenance-therapy.aspx.
- 16.Lakha F, Henderson C, Glasier A. The acceptability of self-administration of subcutaneous Depo-Provera. Contraception. 2005;72:14–18. doi: 10.1016/j.contraception.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 17. marketsandmarkets.com Injectable Drug Delivery Market by Type (Device, Formulation), Therapeutic (Hormonal, Oncology), Usage Pattern (Curative Care, Immunization), Administration (Skin, Musculoskeletal), End User (Hospital, Home Care Setting) Global Forecast to 2020. 2015 marketsandmarkets.com [Google Scholar]
- 18.Benet LZ. The role of BCS (biopharmaceutics classification system) and BDDCS (biopharmaceutics drug disposition classification system) in drug development. J Pharm Sci. 2013;102:34–42. doi: 10.1002/jps.23359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doak BC, Over B, Giordanetto F, Kihlberg J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem Biol. 2014;21:1115–1142. doi: 10.1016/j.chembiol.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Tatham LM, Rannard SP, Owen A. Nanoformulation strategies for the enhanced oral bioavailability of antiretroviral therapeutics. Ther Deliv. 2015;6:469–490. doi: 10.4155/tde.15.4. [DOI] [PubMed] [Google Scholar]
- 21.Shah VP, Yacobi A, Radulescu FS, Miron DS, Lane ME. A science based approach to topical drug classification system (TCS) Int J Pharm. 2015;491:21–25. doi: 10.1016/j.ijpharm.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Fasinu P, Pillay V, Ndesendo VM, du Toit LC, Choonara YE. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm Drug Dispos. 2011;32:185–209. doi: 10.1002/bdd.750. [DOI] [PubMed] [Google Scholar]
- 23.Gopal S, Gassmann-Mayer C, Palumbo J, Samtani MN, Shiwach R, Alphs L. Practical guidance for dosing and switching paliperidone palmitate treatment in patients with schizophrenia. Curr Med Res Opin. 2010;26:377–387. doi: 10.1185/03007990903482772. [DOI] [PubMed] [Google Scholar]
- 24.Lindenmayer JP. Long-acting injectable antipsychotics: focus on olanzapine pamoate. Neuropsychiatr Dis Treat. 2010;6:261–267. doi: 10.2147/ndt.s3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolli GB, Owens DR. Insulin glargine. Lancet. 2000;356:443–445. doi: 10.1016/S0140-6736(00)02546-0. [DOI] [PubMed] [Google Scholar]
- 26.Zinman B, Fulcher G, Rao PV, Thomas N, Endahl LA, Johansen T, Lindh R, Lewin A, Rosenstock J, Pinget M, Mathieu C. Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: a 16-week, randomised, open-label, phase 2 trial. Lancet. 2011;377:924–931. doi: 10.1016/S0140-6736(10)62305-7. [DOI] [PubMed] [Google Scholar]
- 27.Landovitz RJ, Grinsztejn B. Long-Acting Injectable Preexposure Prophylaxis for HIV Prevention in South Africa: Is There a Will and a Way? J Infect Dis. 2015 doi: 10.1093/infdis/jiv524. [DOI] [PubMed] [Google Scholar]
- 28.Walensky RP, Jacobsen MM, Bekker LG, Parker RA, Wood R, Resch SC, Horstman NK, Freedberg KA, Paltiel AD. Potential Clinical and Economic Value of Long-Acting Preexposure Prophylaxis for South African Women at High-Risk for HIV Infection. J Infect Dis. 2015 doi: 10.1093/infdis/jiv523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams J, Sayles HR, Meza JL, Sayre P, Sandkovsky U, Gendelman HE, Flexner C, Swindells S. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond) 2013;8:1807–1813. doi: 10.2217/nnm.12.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen A, Khoo SH. Intracellular pharmacokinetics of antiretroviral agents. J HIV Ther. 2004;9:97–101. [PubMed] [Google Scholar]
- 31.Hoggard PG, Owen A. The mechanisms that control intracellular penetration of the HIV protease inhibitors. J Antimicrob Chemother. 2003;51:493–496. doi: 10.1093/jac/dkg137. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson L, Yapa HM, Jackson A, Moyle G, Else L, Amara A, Khoo S, Back D, Karolia Z, Higgs C, Boffito M. Plasma Tenofovir, Emtricitabine, and Rilpivirine and Intracellular Tenofovir Diphosphate and Emtricitabine Triphosphate Pharmacokinetics following Drug Intake Cessation. Antimicrob Agents Chemother. 2015;59:6080–6086. doi: 10.1128/AAC.01441-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55:5294–5299. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baheti G, King JR, Acosta EP, Fletcher CV. Age-related differences in plasma and intracellular tenofovir concentrations in HIV-1-infected children, adolescents and adults. AIDS. 2013;27:221–225. doi: 10.1097/QAD.0b013e32835a9a2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer MA. Implanon: a new contraceptive implant. J Obstet Gynecol Neonatal Nurs. 2008;37:361–368. doi: 10.1111/j.1552-6909.2008.00247.x. [DOI] [PubMed] [Google Scholar]
- 36.Huber J, Wenzl R. Pharmacokinetics of Implanon. An integrated analysis. Contraception. 1998;58:85S–90S. doi: 10.1016/s0010-7824(98)00120-6. [DOI] [PubMed] [Google Scholar]
- 37.Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S, Lou Y, Min SS, Goljer I, Culp A, Piscitelli SC, Savina PM. Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans. Antimicrob Agents Chemother. 2013;57:3536–3546. doi: 10.1128/AAC.00292-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azijn H, Tirry I, Vingerhoets J, de Bethune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky LT. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010;54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz A, Gisslen M, Spudich S, Lee E, Jayewardene A, Aweeka F, Price RW. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One. 2009;4:e6877. doi: 10.1371/journal.pone.0006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleiner LW, Wright JC, Wang Y. Evolution of implantable and insertable drug delivery systems. J Control Release. 2014;181:1–10. doi: 10.1016/j.jconrel.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Rhee YS, Park CW, Deluca PP, Mansour HM. Sustained-release injectable drug delivery a review of current and future systems. Pharmaceutical Technology s6. 2010:s8–s13. [Google Scholar]
- 42.Beyea SC, Nicoll LH. Administration of medications via the intramuscular route: an integrative review of the literature and research-based protocol for the procedure. Appl Nurs Res. 1995;8:23–33. doi: 10.1016/s0897-1897(95)80279-7. [DOI] [PubMed] [Google Scholar]
- 43.Park EJ, Amatya S, Kim MS, Park JH, Seol E, Lee H, Shin YH, Na DH. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch Pharm Res. 2013;36:651–659. doi: 10.1007/s12272-013-0105-7. [DOI] [PubMed] [Google Scholar]
- 44.Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201–230. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- 45.Brissos S, Veguilla MR, Taylor D, Balanza-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4:198–219. doi: 10.1177/2045125314540297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen C, Larsen SW, Jensen H, Yaghmur A, Ostergaard J. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert Opin Drug Deliv. 2009;6:1283–1295. doi: 10.1517/17425240903307431. [DOI] [PubMed] [Google Scholar]
- 47.Minto CF, Howe C, Wishart S, Conway AJ, Handelsman DJ. Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and injection volume. J Pharmacol Exp Ther. 1997;281:93–102. [PubMed] [Google Scholar]
- 48.Kinnunen HM, Mrsny RJ. Improving the outcomes of biopharmaceutical delivery via the subcutaneous route by understanding the chemical, physical and physiological properties of the subcutaneous injection site. J Control Release. 2014;182:22–32. doi: 10.1016/j.jconrel.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Yin J, Collier AC, Barr AM, Honer WG, Procyshyn RM. Paliperidone Palmitate Long-Acting Injectable Given Intramuscularly in the Deltoid Versus the Gluteal Muscle: Are They Therapeutically Equivalent? J Clin Psychopharmacol. 2015;35:447–449. doi: 10.1097/JCP.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 50.Fredholt K, Larsen DH, Larsen C. Modification of in vitro drug release rate from oily parenteral depots using a formulation approach. European Journal of Pharmaceutical Sciences. 2000;11:231–237. doi: 10.1016/s0928-0987(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 51.van Hoogevest P, Liu XL, Fahr A. Drug delivery strategies for poorly water-soluble drugs: the industrial perspective. Expert Opin Drug Del. 2011;8:1481–1500. doi: 10.1517/17425247.2011.614228. [DOI] [PubMed] [Google Scholar]
- 52.Patel RM. Parenteral suspension: an overview. International Journal of Current Pharmaceutical Research. 2010;2:4–14. [Google Scholar]
- 53.Wu LF, Janagam DR, Mandrell TD, Johnson JR, Lowe TL. Long-Acting Injectable Hormonal Dosage Forms for Contraception. Pharm Res-Dord. 2015;32:2180–2191. doi: 10.1007/s11095-015-1686-2. [DOI] [PubMed] [Google Scholar]
- 54.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nature Reviews Drug Discovery. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu L, Zhang H, Song W. Current advances in sustained-release injectable preparations. International Journal of Pharmaceutical Sciences and Research. 2012;3:2888–2896. [Google Scholar]
- 56.Freiberg S, Zhu X. Polymer microspheres for controlled drug release. International Journal of Pharmaceutics. 2004;282:1–18. doi: 10.1016/j.ijpharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. Journal of Controlled Release. 2003;90:261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 58.Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Th. 2004;4:35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 59.Deadman CM, Kellaway IW, Yasin M, Dickinson PA, Murdan S. An investigation into the influence of drug lipophilicity on the in vivo absorption profiles from subcutaneous microspheres and in situ forming depots. Journal of Controlled Release. 2007;122:79–85. doi: 10.1016/j.jconrel.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Berchane NS, Carson KH, Rice-Ficht AC, Andrews MJ. Effect of mean diameter and polydispersity of PLG microspheres on drug release: experiment and theory. Int J Pharm. 2007;337:118–126. doi: 10.1016/j.ijpharm.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal P, Rupenthal ID. Injectable implants for the sustained release of protein and peptide drugs. Drug Discov Today. 2013;18:337–349. doi: 10.1016/j.drudis.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Vert M, Schwach G, Engel R, Coudane J. Something new in the field of PLA/GA bioresorbable polymers? J Control Release. 1998;53:85–92. doi: 10.1016/s0168-3659(97)00240-x. [DOI] [PubMed] [Google Scholar]
- 63.Halpern V, Stalter RM, Owen DH, Dorflinger LJ, Lendvay A, Rademacher KH. Towards the development of a longer-acting injectable contraceptive: past research and current trends. Contraception. 2015;92:3–9. doi: 10.1016/j.contraception.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Muller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy Rationale for development and what we can expect for the future. Advanced Drug Delivery Reviews. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 65.Shah DP, Patel B, Shah C. Nanosuspension technology: a innovative slant for drug delivery system and permeability enhancer for poorly water soluble drugs. Journal of Drug Delivery and Therapeutics. 2015;5:10–23. [Google Scholar]
- 66.Zhang H, Wang D, Butler R, Campbell NL, Long J, Tan B, Duncalf DJ, Foster AJ, Hopkinson A, Taylor D, Angus D, Cooper AI, Rannard SP. Formation and enhanced biocidal activity of water-dispersable organic nanoparticles. Nat Nanotechnol. 2008;3:506–511. doi: 10.1038/nnano.2008.188. [DOI] [PubMed] [Google Scholar]
- 67.McDonald TO, Giardiello M, Martin P, Siccardi M, Liptrott NJ, Smith D, Roberts P, Curley P, Schipani A, Khoo SH, Long J, Foster AJ, Rannard SP, Owen A. Antiretroviral solid drug nanoparticles with enhanced oral bioavailability: production, characterization, and in vitro-in vivo correlation. Adv Healthc Mater. 2014;3:400–411. doi: 10.1002/adhm.201300280. [DOI] [PubMed] [Google Scholar]
- 68.Du J, Li X, Zhao H, Zhou Y, Wang L, Tian S, Wang Y. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm. 2015;495:738–749. doi: 10.1016/j.ijpharm.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 69.Stoddard A, McNicholas C, Peipert JF. Efficacy and Safety of Long-Acting Reversible Contraception. Drugs. 2011;71:969–980. doi: 10.2165/11591290-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghalanbor Z, Korber M, Bodmeier R. Improved Lysozyme Stability and Release Properties of Poly(lactide-co-glycolide) Implants Prepared by Hot-Melt Extrusion. Pharm Res-Dord. 2010;27:371–379. doi: 10.1007/s11095-009-0033-x. [DOI] [PubMed] [Google Scholar]
- 71.Lambert WJ, Peck KD. Development of an in-Situ Forming Biodegradable Poly-Lactide-Co-Glycolide System for the Controlled-Release of Proteins. Journal of Controlled Release. 1995;33:189–195. [Google Scholar]
- 72.Agarwal P, Rupenthal ID. Injectable implants for the sustained release of protein and peptide drugs. Drug Discov Today. 2013;18:337–349. doi: 10.1016/j.drudis.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 73.Cox MC, Scripture CD, Figg WD. Leuprolide acetate given by a subcutaneous extended-release injection: less of a pain? Expert Rev Anticanc. 2005;5:605–611. doi: 10.1586/14737140.5.4.605. [DOI] [PubMed] [Google Scholar]
- 74.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Advanced Drug Delivery Reviews. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donnelly RF, Garland MJ, Morrow DIJ, Migalska K, Singh TRR, Majithiya R, Woolfson AD. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. Journal of Controlled Release. 2010;147:333–341. doi: 10.1016/j.jconrel.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Hong XY, Wei LM, Wu F, Wu ZZ, Chen LZ, Liu ZG, Yuan WE. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des Dev Ther. 2013;7:945–952. doi: 10.2147/DDDT.S44401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res-Dord. 2006;23:1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 78.Kochhar JS, Lim WXS, Zou S, Foo WY, Pan J, Kang LF. Microneedle Integrated Transdermal Patch for Fast Onset and Sustained Delivery of Lidocaine. Mol Pharmaceut. 2013;10:4272–4280. doi: 10.1021/mp400359w. [DOI] [PubMed] [Google Scholar]
- 79.Lee IC, He JS, Tsai MT, Lin KC. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J Mater Chem B. 2015;3:276–285. doi: 10.1039/c4tb01555j. [DOI] [PubMed] [Google Scholar]
- 80.McDonald TO, Martin P, Patterson JP, Smith D, Giardiello M, Marcello M, See V, O'Reilly RK, Owen A, Rannard S. Multicomponent Organic Nanoparticles for Fluorescence Studies in Biological Systems. Adv Funct Mater. 2012;22:2469–2478. [Google Scholar]
- 81.Giardiello M, McDonald TO, Martin P, Owen A, Rannard SP. Facile synthesis of complex multi-component organic and organic-magnetic inorganic nanocomposite particles. J Mater Chem. 2012;22:24744–24752. [Google Scholar]
- 82.Kempe S, Mader K. In situ forming implants -an attractive formulation principle for parenteral depot formulations. Journal of Controlled Release. 2012;161:668–679. doi: 10.1016/j.jconrel.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 83.Yang YH, Corona A, Schubert B, Reeder R, Henson MA. The effect of oil type on the aggregation stability of nanostructured lipid carriers. J Colloid Interf Sci. 2014;418:261–272. doi: 10.1016/j.jcis.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 84.Gapp G, Holzknecht P. Risk analysis of sterile production plants: a new and simple, workable approach. PDA J Pharm Sci Technol. 2011;65:217–226. doi: 10.5731/pdajpst.2011.00693. [DOI] [PubMed] [Google Scholar]
- 85.Badkar A, Wolf A, Bohack L, Kolhe P. Development of biotechnology products in pre-filled syringes: technical considerations and approaches. AAPS PharmSciTech. 2011;12:564–572. doi: 10.1208/s12249-011-9617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boom S, Talluri K, Janssens L, Remmerie B, De Meulder M, Rossenu S, van Osselaer N, Eerdekens M, Cleton A. Single-and multiple-dose pharmacokinetics and dose proportionality of the psychotropic agent paliperidone extended release. J Clin Pharmacol. 2009;49:1318–1330. doi: 10.1177/0091270009339190. [DOI] [PubMed] [Google Scholar]
- 87.Si T, Su Y, Liu Y, Zhang H, Li H, Rui Q, Shu L. Pharmacokinetics and tolerability of paliperidone palmitate injection in Chinese subjects. Hum Psychopharmacol. 2014;29:203–210. doi: 10.1002/hup.2388. [DOI] [PubMed] [Google Scholar]
- 88.Markowitz JS, DeVane CL, Malcolm RJ, Gefroh HA, Wang JS, Zhu HJ, Donovan JL. Pharmacokinetics of olanzapine after single-dose oral administration of standard tablet versus normal and sublingual administration of an orally disintegrating tablet in normal volunteers. J Clin Pharmacol. 2006;46:164–171. doi: 10.1177/0091270005283839. [DOI] [PubMed] [Google Scholar]
- 89.Mitchell M, Kothare P, Bergstrom R, Zhao F, Jen KY, Walker D, Johnson J, McDonnell D. Single-and multiple-dose pharmacokinetic, safety, and tolerability profiles of olanzapine long-acting injection: an open-label, multicenter, nonrandomized study in patients with schizophrenia. Clin Ther. 2013;35:1890–1908. doi: 10.1016/j.clinthera.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 90.Johansson ED, Johansen PB, Rasmussen SN. Medroxyprogesterone acetate pharmacokinetics following oral high-dose administration in humans: a bioavailability evaluation of a new MPA tablet formulation. Acta Pharmacol Toxicol (Copenh) 1986;58:311–317. doi: 10.1111/j.1600-0773.1986.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 91.Sierra-Ramirez JA, Lara-Ricalde R, Lujan M, Velazquez-Ramirez N, Godinez-Victoria M, Hernadez-Munguia IA, Padilla A, Garza-Flores J. Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg) Contraception. 2011;84:565–570. doi: 10.1016/j.contraception.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 92.Crauwels HM, van Heeswijk RP, Buelens A, Stevens M, Boven K, Hoetelmans RM. Impact of food and different meal types on the pharmacokinetics of rilpivirine. J Clin Pharmacol. 2013;53:834–840. doi: 10.1002/jcph.107. [DOI] [PubMed] [Google Scholar]
- 93.Verloes R, Deleu S, Niemeijer N, Crauwels H, Meyvisch P, Williams P. Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in healthy volunteers. HIV Med. 2015;16:477–484. doi: 10.1111/hiv.12247. [DOI] [PubMed] [Google Scholar]
- 94.Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y, Gould E, Stevens M, Piscitelli S. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr. 2014;67:487–492. doi: 10.1097/QAI.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 95.Liptrott NJ, Giardiello M, Hunter JW, Tatham L, Tidbury LR, Siccardi M, Rannard S, Owen A. Flow cytometric analysis of the physical and protein-binding characteristics of solid drug nanoparticle suspensions. Nanomedicine (Lond) 2015;10:1407–1421. doi: 10.2217/nnm.14.77. [DOI] [PubMed] [Google Scholar]
- 96.Bendayan R. Role and modulation of drug transporters in HIV/AIDS therapy. Advanced Drug Delivery Reviews. doi: 10.1016/j.addr.2016.05.001. In Press. [DOI] [PubMed] [Google Scholar]
- 97.Garberoglio C, Dudas M, Casper ES, Bertino J, Cordoncardo C. Expression of P-Glycoprotein in Normal Muscle-Cells and Myogenic Tumors. Arch Pathol Lab Med. 1992;116:1055–1061. [PubMed] [Google Scholar]
- 98.Knauer MJ, Urquhart BL, Meyer zu Schwabedissen HE, Schwarz UI, Lemke CJ, Leake BF, Kim RB, Tirona RG. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106:297–306. doi: 10.1161/CIRCRESAHA.109.203596. [DOI] [PubMed] [Google Scholar]
- 99.Flens MJ, Zaman GJR, vanderValk P, Izquierdo MA, Schroeijers AB, Scheffer GL, vanderGroep P, deHaas M, Meijer CJLM, Scheper RJ. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996;148:1237–1247. [PMC free article] [PubMed] [Google Scholar]
- 100.Srinivas RV, Middlemas D, Flynn P, Fridland A. Human immunodeficiency virus protease inhibitors serve as substrates for multidrug transporter proteins MDR1 and MRP1 but retain antiviral efficacy in cell lines expressing these transporters. Antimicrob Agents Chemother. 1998;42:3157–3162. doi: 10.1128/aac.42.12.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 102.Jorajuria S, Dereuddre-Bosquet N, Becher F, Martin S, Porcheray F, Garrigues A, Mabondzo A, Benech H, Grassi J, Orlowski S, Dormont D, Clayette P. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther. 2004;9:519–528. [PubMed] [Google Scholar]
- 103.Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- 104.Hartkoorn RC, Kwan WS, Shallcross V, Chaikan A, Liptrott N, Egan D, Sora ES, James CE, Gibbons S, Bray PG, Back DJ, Khoo SH, Owen A. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20:112–120. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holmstock N, Annaert P, Augustijns P. Boosting of HIV protease inhibitors by ritonavir in the intestine: the relative role of cytochrome P450 and P-glycoprotein inhibition based on Caco-2 monolayers versus in situ intestinal perfusion in mice. Drug Metab Dispos. 2012;40:1473–1477. doi: 10.1124/dmd.112.044677. [DOI] [PubMed] [Google Scholar]
- 107.Olagunju A, Schipani A, Siccardi M, Egan D, Khoo S, Back D, Owen A. CYP3A4*22 (c.522-191 C>T; rs35599367) is associated with lopinavir pharmacokinetics in HIV-positive adults. Pharmacogenet Genomics. 2014;24:459–463. doi: 10.1097/FPC.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 108.Olsen L, Oostenbrink C, Jorgensen FS. Prediction of cytochrome P450 mediated metabolism. Adv Drug Deliv Rev. 2015;86:61–71. doi: 10.1016/j.addr.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 109.Josephson F, Allqvist A, Janabi M, Sayi J, Aklillu E, Jande M, Mahindi M, Burhenne J, Bottiger Y, Gustafsson LL, Haefeli WE, Bertilsson L. CYP3A5 genotype has an impact on the metabolism of the HIV protease inhibitor saquinavir. Clin Pharmacol Ther. 2007;81:708–712. doi: 10.1038/sj.clpt.6100117. [DOI] [PubMed] [Google Scholar]
- 110.Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, Bogner JR, Rockstroh J, Esser S, Jaeger H, Harrer T, Mauss S, van Lunzen J, Skoetz N, Jetter A, Groneuer C, Fatkenheuer G, Khoo SH, Egan D, Back DJ, Owen A. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother. 2008;61:914–918. doi: 10.1093/jac/dkn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hirani VN, Raucy JL, Lasker JM. Conversion of the HIV protease inhibitor nelfinavir to a bioactive metabolite by human liver CYP2C19. Drug Metab Dispos. 2004;32:1462–1467. doi: 10.1124/dmd.104.001743. [DOI] [PubMed] [Google Scholar]
- 112.Molina-Ortiz D, Camacho-Carranza R, Gonzalez-Zamora JF, Shalkow-Kalincovstein J, Cardenas-Cardos R, Nosti-Palacios R, Vences-Mejia A. Differential expression of cytochrome P450 enzymes in normal and tumor tissues from childhood rhabdomyosarcoma. PLoS One. 2014;9:e93261. doi: 10.1371/journal.pone.0093261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molina-Ortiz D, González-Zamora JF, Camacho-Carranza R, Lopez-Acosta O, Colin-Martinez O, Domínguez-Ramírez AM, Vences-Mejía A. Xenobiotic-Metabolizing Enzymes in Skeletal Muscle of Children and Adolescents Pharmacology & Pharmacy. 2013;4:231–239. [Google Scholar]
- 114.Ellero S, Chakhtoura G, Barreau C, Langouet S, Benelli C, Penicaud L, Beaune P, de Waziers I. Xenobiotic-metabolizing cytochromes p450 in human white adipose tissue: expression and induction. Drug Metab Dispos. 2010;38:679–686. doi: 10.1124/dmd.109.029249. [DOI] [PubMed] [Google Scholar]
- 115.Ribaudo HJ, Daar ES, Tierney C, Morse GD, Mollan K, Sax PE, Fischl MA, Collier AC, Haas DW. Impact of UGT1A1 Gilbert variant on discontinuation of ritonavir-boosted atazanavir in AIDS Clinical Trials Group Study A5202. J Infect Dis. 2013;207:420–425. doi: 10.1093/infdis/jis690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Belanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine. Drug Metab Dispos. 2009;37:1793–1796. doi: 10.1124/dmd.109.027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishimura M, Naito S, Yokoi T. Tissue-specific mRNA expression profiles of human nuclear receptor subfamilies. Drug Metab Pharmacokinet. 2004;19:135–149. doi: 10.2133/dmpk.19.135. [DOI] [PubMed] [Google Scholar]
- 118.Schote AB, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: expression and correlations. Mol Immunol. 2007;44:1436–1445. doi: 10.1016/j.molimm.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 119.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 120.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovascular Research. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 121.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 122.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. Journal of angiogenesis research. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Skalak TC, Schmid-Schonbein GW, Zweifach BW. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvascular research. 1984;28:95–112. doi: 10.1016/0026-2862(84)90032-3. [DOI] [PubMed] [Google Scholar]
- 124.Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89:297–310. doi: 10.1002/(SICI)1520-6017(200003)89:3<297::AID-JPS2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 125.Bogle G, Dunbar PR. T cell responses in lymph nodes, Wiley interdisciplinary reviews. Systems biology and medicine. 2010;2:107–116. doi: 10.1002/wsbm.47. [DOI] [PubMed] [Google Scholar]
- 126.Trevaskis NL, Kaminskas LM, Porter CJ. From sewer to saviour -targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 127.Scallan J, Huxley VH, Korthuis RJ. Capillary Fluid Exchange: Regulation, Functions, and Pathology. Morgan & Claypool Life Sciences; San Rafael (CA): 2010. Chapter 3The Lymphatic Vasculature. [PubMed] [Google Scholar]
- 128.Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. The journal of investigative dermatology. Symposium proceedings / the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research. 2000;5:14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 129.Oussoren C, Zuidema J, Crommelin DJ, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid compostion and lipid dose. Biochim Biophys Acta. 1997;1328:261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 130.Zhang XY, Lu WY. Recent advances in lymphatic targeted drug delivery system for tumor metastasis. Cancer biology & medicine. 2014;11:247–254. doi: 10.7497/j.issn.2095-3941.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie Y, Bagby TR, Cohen MS, Forrest ML. Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv. 2009;6:785–792. doi: 10.1517/17425240903085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lederman MM, Margolis L. The lymph node in HIV pathogenesis. Seminars in immunology. 2008;20:187–195. doi: 10.1016/j.smim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horiike M, Iwami S, Kodama M, Sato A, Watanabe Y, Yasui M, Ishida Y, Kobayashi T, Miura T, Igarashi T. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423:107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 134.Grossman CI, Ross AL, Auerbach JD, Ananworanich J, Dube K, Tucker JD, Noseda V, Possas C, Rausch DM, A.S.T.a.H.I.V.C.i. International Towards Multidisciplinary HIV-Cure Research: Integrating Social Science with Biomedical Research. Trends in microbiology. 2016;24:5–11. doi: 10.1016/j.tim.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, Anderson J, Perkey K, Stevenson M, Perelson AS, Douek DC, Haase AT, Schacker TW. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McLennan DN, Porter CJ, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug discovery today. Technologies. 2005;2:89–96. doi: 10.1016/j.ddtec.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 137.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao QH, Qiu LY. An overview of the pharmacokinetics of polymer-based nanoassemblies and nanoparticles. Curr Drug Metab. 2013;14:832–839. doi: 10.2174/138920021131400104. [DOI] [PubMed] [Google Scholar]
- 139.Martinez-Skinner AL, Arainga MA, Puligujja P, Palandri DL, Baldridge HM, Edagwa BJ, McMillan JM, Mosley RL, Gendelman HE. Cellular Responses and Tissue Depots for Nanoformulated Antiretroviral Therapy. PLoS One. 2015;10:e0145966. doi: 10.1371/journal.pone.0145966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guo D, Zhang G, Wysocki TA, Wysocki BJ, Gelbard HA, Liu XM, McMillan JM, Gendelman HE. Endosomal trafficking of nanoformulated antiretroviral therapy facilitates drug particle carriage and HIV clearance. J Virol. 2014;88:9504–9513. doi: 10.1128/JVI.01557-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liptrott N, Martin P, Giardiello M, McDonald T, Rannard S, Owen A. Solid Drug Nanoparticle Dispersions for Improved delivery of Efavirenz to Macrophages. BPS Winter Meeting; London. 2012.p. P008. [Google Scholar]
- 142.Darville N, van Heerden M, Vynckier A, De Meulder M, Sterkens P, Annaert P, Van den Mooter G. Intramuscular administration of paliperidone palmitate extended-release injectable microsuspension induces a subclinical inflammatory reaction modulating the pharmacokinetics in rats. J Pharm Sci. 2014;103:2072–2087. doi: 10.1002/jps.24014. [DOI] [PubMed] [Google Scholar]
- 143.Sowa G. Caveolae, caveolins, cavins, and endothelial cell function: new insights. Frontiers in physiology. 2012;2:120. doi: 10.3389/fphys.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stan RV. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. Journal of cellular and molecular medicine. 2007;11:621–643. doi: 10.1111/j.1582-4934.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azizi PM, Zyla RE, Guan S, Wang C, Liu J, Bolz SS, Heit B, Klip A, Lee WL. Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells. Mol Biol Cell. 2015;26:740–750. doi: 10.1091/mbc.E14-08-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cleal K, He L, Watson PD, Jones AT. Endocytosis, intracellular traffic and fate of cell penetrating peptide based conjugates and nanoparticles. Curr Pharm Des. 2013;19:2878–2894. doi: 10.2174/13816128113199990297. [DOI] [PubMed] [Google Scholar]
- 147.Chang J, Paillard A, Passirani C, Morille M, Benoit JP, Betbeder D, Garcion E. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res. 2012;29:1495–1505. doi: 10.1007/s11095-011-0624-1. [DOI] [PubMed] [Google Scholar]
- 148.Mahungu TW, Johnson MA, Owen A, Back DJ. The impact of pharmacogenetics on HIV therapy. Int J STD AIDS. 2009;20:145–151. doi: 10.1258/ijsa.2008.008369. [DOI] [PubMed] [Google Scholar]
- 149.Owen A, Pirmohamed M, Khoo SH, Back DJ. Pharmacogenetics of HIV therapy. Pharmacogenet Genomics. 2006;16:693–703. doi: 10.1097/01.fpc.0000236338.41799.57. [DOI] [PubMed] [Google Scholar]
- 150.Cressey TR, Urien S, Capparelli EV, Best BM, Buranabanjasatean S, Limtrakul A, Rawangban B, Sabsanong P, Treluyer JM, Jourdain G, Stek A, Lallemant M, Mirochnick M. Impact of body weight and missed doses on lopinavir concentrations with standard and increased lopinavir/ritonavir doses during late pregnancy. J Antimicrob Chemother. 2015;70:217–224. doi: 10.1093/jac/dku367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dhoro M, Zvada S, Ngara B, Nhachi C, Kadzirange G, Chonzi P, Masimirembwa C. CYP2B6*6, CYP2B6*18, Body weight and sex are predictors of efavirenz pharmacokinetics and treatment response: population pharmacokinetic modeling in an HIV/AIDS and TB cohort in Zimbabwe. BMC pharmacology & toxicology. 2015;16:4. doi: 10.1186/s40360-015-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Floridia M, Giuliano M, Palmisano L, Vella S. Gender differences in the treatment of HIV infection. Pharmacol Res. 2008;58:173–182. doi: 10.1016/j.phrs.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 153.Nicolson TJ, Mellor HR, Roberts RR. Gender differences in drug toxicity. Trends Pharmacol Sci. 2010;31:108–114. doi: 10.1016/j.tips.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 154.Tittle V, Bull L, Boffito M, Nwokolo N. Pharmacokinetic and pharmacodynamic drug interactions between antiretrovirals and oral contraceptives. Clin Pharmacokinet. 2015;54:23–34. doi: 10.1007/s40262-014-0204-8. [DOI] [PubMed] [Google Scholar]
- 155.Moss DM, Siccardi M, Murphy M, Piperakis MM, Khoo SH, Back DJ, Owen A. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother. 2012;56:3020–3026. doi: 10.1128/AAC.06407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song I, Borland J, Arya N, Wynne B, Piscitelli S. Pharmacokinetics of dolutegravir when administered with mineral supplements in healthy adult subjects. J Clin Pharmacol. 2015;55:490–496. doi: 10.1002/jcph.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ronnemaa T, Koivisto VA. Combined effect of exercise and ambient temperature on insulin absorption and postprandial glycemia in type I patients. Diabetes Care. 1988;11:769–773. doi: 10.2337/diacare.11.10.769. [DOI] [PubMed] [Google Scholar]
- 158.Khazaeinia T, Ramsey AA, Tam YK. The effects of exercise on the pharmacokinetics of drugs. J Pharm Pharm Sci. 2000;3:292–302. [PubMed] [Google Scholar]
- 159.Ciccone CD. Basic pharmacokinetics and the potential effect of physical therapy interventions on pharmacokinetic variables. Physical therapy. 1995;75:343–351. doi: 10.1093/ptj/75.5.343. [DOI] [PubMed] [Google Scholar]
- 160.Weng Larsen S, Larsen C. Critical factors influencing the in vivo performance of long-acting lipophilic solutions--impact on in vitro release method design. AAPS J. 2009;11:762–770. doi: 10.1208/s12248-009-9153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rajoli RK, Back DJ, Rannard S, Freel Meyers CL, Flexner C, Owen A, Siccardi M. Physiologically Based Pharmacokinetic Modelling to Inform Development of Intramuscular Long-Acting Nanoformulations for HIV. Clin Pharmacokinet. 2015;54:639–650. doi: 10.1007/s40262-014-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]