Abstract
Cytochrome P450 17A1 (CYP17A1) is an important drug target for castration resistant prostate cancer. It is a bi-functional enzyme, catalyzing production of glucocorticoid precursors by hydroxylation of pregnene- nucleus, and androgen biosynthesis by a second C-C lyase step, at the expense of glucocorticoid production. Cytochrome b5 (cyt b5) is known to be a key regulator of the androgen synthesis reaction in vivo, by a mechanism that is not well understood. Two hypotheses have been proposed for the mechanism by which cyt b5 increases androgen biosynthesis. Cyt b5 could act as an allosteric effector, binding to CYP17A1 and either changing its selective substrate affinity or altering the conformation of the P450 to increase the catalytic rate or decrease unproductive uncoupling channels. Alternatively, cyt b5 could act as a redox donor for supply of the second electron in the P450 cycle, reducing the oxyferrous complex to form the reactive peroxo-intermediate. To understand the mechanism of lyase enhancement by cyt b5, we generated a redox-inactive form of cyt b5, in which the heme is replaced with a Manganese-protoporphyrin IX (Mn-b5), and investigated enhancement of androgen producing lyase reaction by CYP17A1. Given the critical significance of a stable membrane anchor for all of the proteins involved and the need for controlled stoichiometric ratios, we employed the Nanodisc system for this study. The redox inactive form was observed to have no effect on the lyase reaction, while reactions with the normal heme-iron containing cyt b5 were enhanced ~ 5 fold as compared to reactions in the absence of cyt b5. We also performed resonance Raman measurements on ferric CYP17A1 bound to Mn-b5. Upon addition of Mn-b5 to Nanodisc reconstituted CYP17A1, we observed clear evidence for the formation of a b5-CYP17A1 complex, as noted by changes in the porphyrin modes and alteration in the proximal Fe-S vibrational frequency. Thus, although Mn-b5 binds to CYP17A1, it is unable to enhance the lyase reaction, strongly suggesting that cyt b5 has a redox effector role in enhancement of the CYP17A1 mediated lyase reaction necessary for androgen synthesis.
Keywords: CYP17A1, Cytochrome b5, Redox donor, Androgen synthesis, Nanodiscs
1. Introduction
The role of cytochrome b5 in P450 mediated metabolism has been the subject of intense debate for decades. Although its essential role in the fatty acid biosynthetic pathways is well appreciated [1], early work with hepatic drug metabolizing enzymes often yielded conflicting results. For instance, Sato and coworkers suggested that the binding of cytochrome b5 (cyt b5) to cytochrome P450 elicited a structural change in the enzyme which activated product turnover, either by increasing the inherent catalytic rate or by inhibiting non-productive auto-oxidative shunt processes (Fig. 1) [2]. Alternatively, as cyt b5 contains a bis-imidazole coordinated heme, direct transfer of electrons from cyt b5 to P450 has been proposed [2,3]. In this latter role, the relatively high redox potential of cyt b5 (~ 0 mV versus Normal Hydrogen Electrode [4]) suggests that electron transfer to ferric P450 (redox potential ~ −300 mV vs. NHE) is unfavorable. Hence it was suggested that the redox function of cyt b5 involved electron transfer to the ferrous dioxygen intermediate which has a redox potential near 0 mV [5] thus providing the "second electron" in the normal monooxygenase stoichiometry. In an attempt to differentiate between these two roles, Coon and co-workers reconstituted apo-cyt b5 with manganese protoporphyrin IX [6]. They observed no reduced Mn-b5 in the presence of cytochrome P450 reductase (CPR) and NADPH, whereas iron cyt b5 was rapidly reduced. Hence Mn-b5 is incapable of any electron transfer to the P450. From their experiments on various P450 reactions they concluded that cyt b5 effects depend on the specific P450 in question, the substrate being examined, and molar ratio of CPR to P450. This suggested that their observations could not be explained solely by a simple electron transfer role and some effects may also be caused by possible conformational changes caused by cyt b5 binding. While the role of cyt b5 in drug metabolism continues to be explored, a more interesting and potentially critical function of cyt b5, is in human steroid biosynthesis.
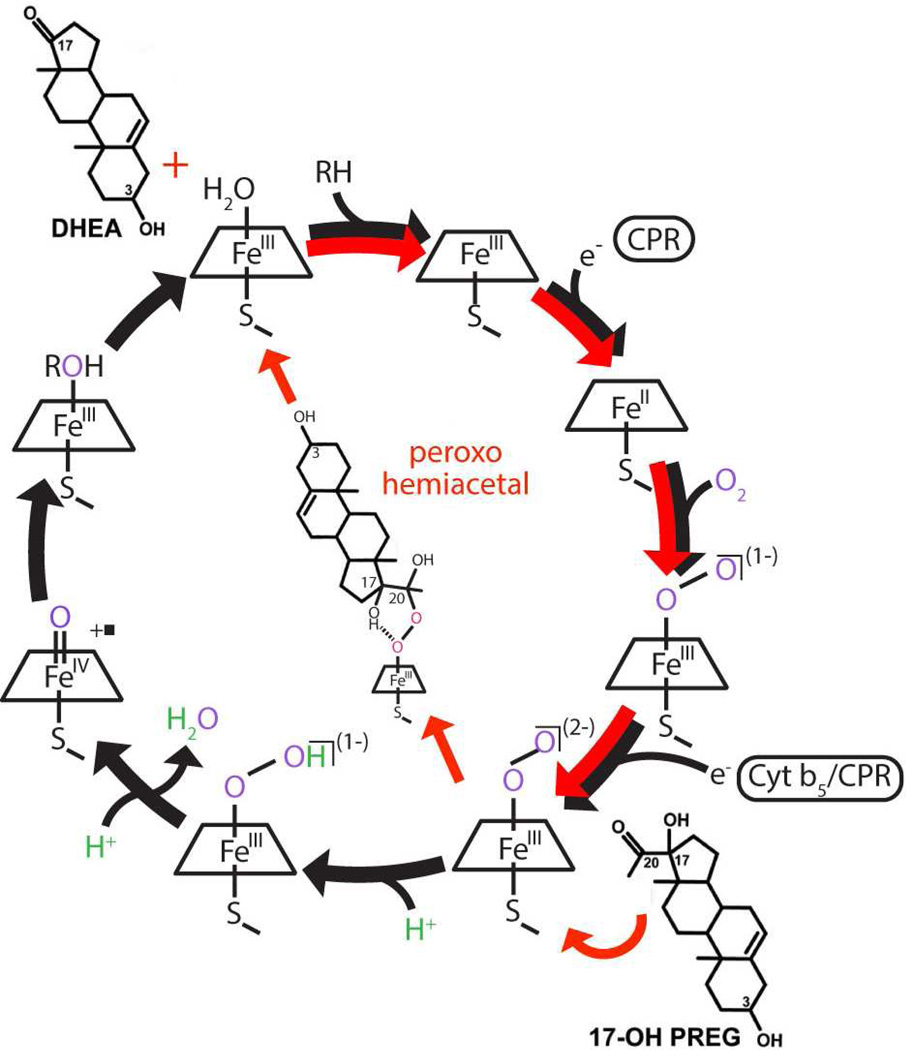
Figure 1.
Cytochrome P450 reaction cycle. Black arrows represent the path followed for CYP17A1 mediated hydroxylation chemistry, while red arrows represent the CYP17A1 mediated carbon-carbon scission reaction, the lyase chemistry. The first reduction is indicated as being carried out by CPR, while the second electron can be donated by either CPR or cytochrome b5.
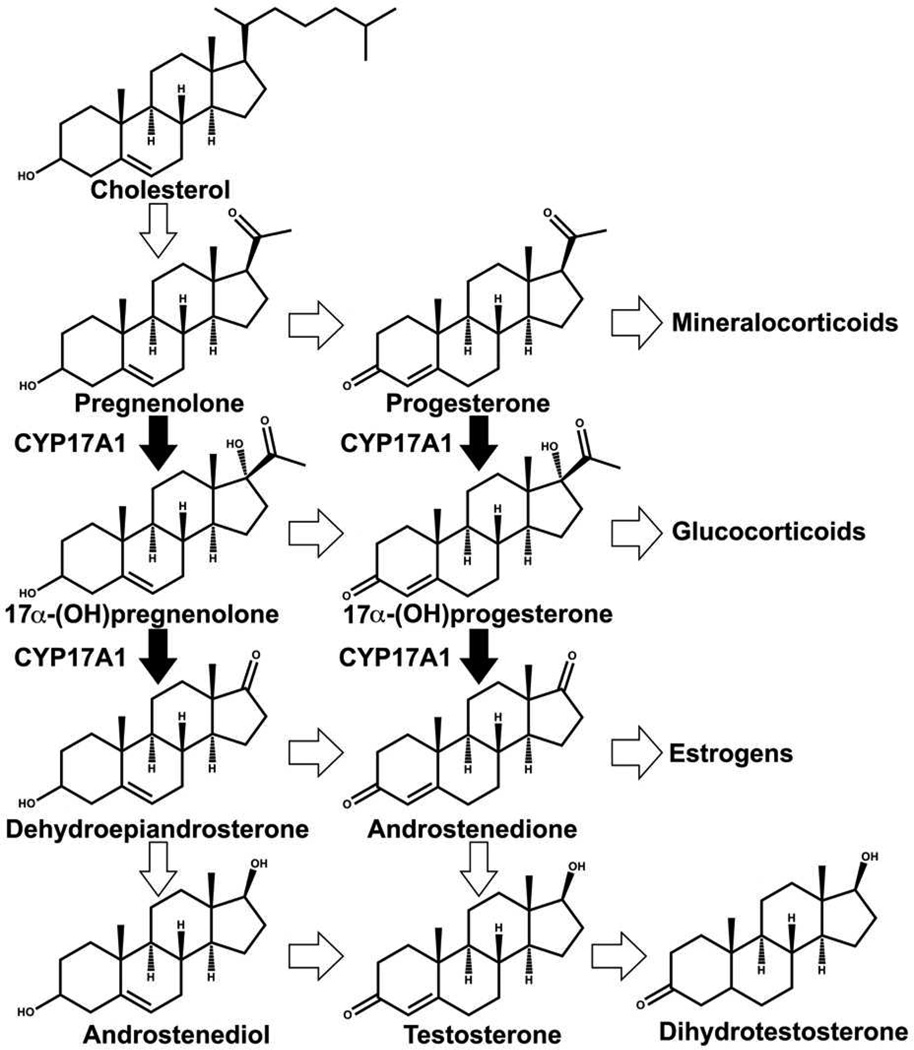
The synthesis of androgens, estrogens and corticosteroids involves multiple P450 catalyzed reactions. Cytochrome P450 17A1 (CYP17A1) is a key player in these reactions, being responsible for catalyzing 17α-hydroxylation of pregnenolone and progesterone followed by C17-C20 bond scission in a separate lyase reaction to form dehydroepiandrosterone (DHEA) and androstenedione (AD) respectively (Fig. 2). The hydroxylation reactions occur throughout a person’s lifetime, and when a person approaches adrenarche, the hydroxylated products can be shunted towards the subsequent step of formation of androgenic precursors by CYP17A1 in addition to the production of glucocorticoids [7]. The presence of membrane bound form of cyt b5 has been known to selectively and significantly enhance the rate of the lyase reaction [8]. Mutations of CYP17A1 surface residues: R347, R358 and K89 which are known to interact with cyt b5, have been shown to cause impairment of lyase activity [9]. In a recent report, significant lyase impairment was shown in mice testicular Leydig cells lacking cyt b5 [10]. Additionally, male patients with mutations causing a lack of cyt b5 present with pseudo hermaphroditism, while high cyt b5 expression in adrenocortical adenomas in Cushing’s syndrome patients has been associated with increased androgen synthesis [11]. Clearly, cyt b5 has a physiological significance in maintaining normal levels of androgen synthesis. The nature of this role has been long debated [8,12–14], with Auchus and coworkers studying lyase activity in recombinant yeast using apo b5 which stimulated the lyase reaction, leading these workers to conclude that there is no redox role [15]. A similar report from Akhtar and coworkers mentions unpublished results demonstrating lyase enhancement in a reconstituted system utilizing Mn-b5 [9]. On the contrary, Estabrook and others demonstrated in an in vitro reconstituted system in the presence of lipids that a zinc substituted derivative of b5 did not stimulate the lyase activity of CYP17A1, and that the previous reports of rate enhancement by apo b5 are the result of transfer of the heme group from the P450 to apo b5 forming the holoenzyme [16]. Another property of cyt b5 that complicates all previous reports is the need for an intact membrane to influence the P450 activity [17]. Given the key importance of cyt b5 in the regulation of androgen synthesis, it is imperative that this disparity about the role of cyt b5 be solved in a reproducible system representative of the membrane environment of these proteins. To this end, we employ the Nanodisc system to reconstitute CYP17A1, CPR and cyt b5 in controlled stoichiometric ratios. We reconstituted cyt b5 with manganese protoporphyrin IX and investigated the rate of lyase reaction by CYP17A1 in Nanodiscs, with iron containing cyt b5, or with Mn-protoporphyrin IX substituted form of cyt b5 (Mn-b5) and compared each case versus the rate in the absence of cyt b5. In humans, androgens are primarily derived from the 17α-hydroxypregnenolone substrate, therefore we decided to investigate this reaction to determine whether cyt b5 is a redox donor in CYP17A1 mediated lyase reaction for androgen synthesis. Additionally, we probed the ferric resting state of CYP17A1 in the presence of native substrates to indicate formation of the b5-CYP17A1 complex.
Figure 2.
CYP17A1 catalyzed reactions in steroidogenesis.
2. Materials and methods
2.1 Expression and purification of recombinant proteins, Nanodisc assembly
The expression and purification of CYP17A1 was performed as described [18]. The expression and purification of membrane scaffold protein and cyt b5 were performed as described [19,20]. Nanodiscs with CYP17A1 were assembled as documented [21]. Incorporation of CPR and cyt b5 into purified CYP17A1 Nanodiscs was performed by direct addition of CPR and/or cyt b5 in 2 to 4-fold molar excess, as documented [22–24].
2.2 Reconstitution of cyt b5 with manganese protoporphyrin IX
Cyt b5 was reconstituted with Mn-protoporphyrin IX according to [6], with the following changes: After performing buffer exchange with G-25 column to separate unbound Mn-protoporphyrin IX, the eluate was run through a DEAE-cellulose column, equilibrated with 25 mM Tris acetate (pH 8.0), 1 mM EDTA and 10 mM sodium cholate. A linear salt gradient was formed using the equilibration buffer supplemented with 1M NaCl. Mn-b5 fractions which were characterized on the basis of their observed Rz ratios, were pooled and rigorously dialyzed against 100 mM potassium phosphate buffer (pH 7.4) and flash frozen in liquid nitrogen before being stored at −80 °C until use.
2.3 Spectroscopic characterizations of Nanodisc incorporated CYP17A1 and of Mn-b5
Nanodisc incorporated CYP17A1 was characterized by UV-visible spectroscopy in a Cary 300 UV-visible spectrophotometer. The amount of P450 was determined by change in spin shift upon pregnenolone binding using ε390-ε417 = 100 mM−1cm−1 [25]. Mn-b5 was quantified using UV-visible spectroscopy using ε368=50±2 mM−1cm−1 and ε468=38±1.5 mM−1cm−1 [6]. Purity and identity of all proteins was confirmed by denaturing electrophoresis and MALDI-mass spectrometry.
2.5 Determination of NADPH oxidation rates
NADPH oxidation rates and product formation rates were determined with 365 pmoles of CYP17A1 in Nanodiscs reconstituted with 4-fold CPR, with/without the addition of 4-fold cyt b5. The solution was brought to 1 mL by the addition of 100 mM potassium phosphate buffer (pH 7.4) containing 50 mM NaCl and 50 µM 17α-hydroxypregnenolone (Sigma) in a stirred quartz cuvette. The solution was equilibrated at 37°C by incubation for 5 min and reaction was initiated by addition of NADPH solution. The NADPH consumption was monitored by change in absorbance at 340 nm for 15 min and the linearity was verified. At 15 min the reaction was quenched by adding 50 µL 8.9 N sulfuric acid. Each sample was flash frozen in liquid nitrogen and stored at −80°C until product analysis. These reactions were performed in a Cary 300 UV-visible spectrophotometer fitted with magnetic stirrer and Peltier temperature controller.
2.6 Determination of product formation rates
Frozen samples were thawed and extraction of analytes was performed as previously documented [23]. The product analysis was performed by gas chromatography with a DB-17 phenol substituted siloxane column and a flame ionization detector on a Hewlett-Packard 6890 gas chromatograph, and the chromatograms were processed with Grams/32 AI software.
2.7 Preparation of Raman samples
CYP17A1 incorporated in Nanodiscs in 100 mM potassium phosphate buffer (pH 7.4) was concentrated to 200 µM and 2-fold excess of Mn-b5 was added from a 400 µM solution in the same buffer. The resultant solution incubated at 37°C for 15 minutes. Substrate was added to each sample from methanolic stocks such that the final concentration was 420 µM [24]. Finally, ultrapure glycerol was added to a concentration of 15% (v/v); i.e., the final concentration of CYP17A1 was calculated to be 85 µM. The samples were flash frozen in liquid nitrogen and stored at −80°C until analyzed.
2.8 Resonance Raman spectroscopy
To determine the effect of cyt b5 on the heme stretching modes in ferric CYP17A1, we employed the Mn reconstituted form of cyt b5. This was done to prevent interference from the heme of cyt b5 as was done previously by Mak et al. in rR studies on CYP2B4 [26,27]. We acquired resonance Raman spectra after adding 2-fold and 4-fold excess of Mn-b5. The high frequency spectra were acquired using the 406.7 nm excitation line from a Krypton ion laser (Coherent Innova Sabre Ion Laser) and changes in the ν(Fe-S) stretching mode were investigated using the 356.4 nm excitation line from the same laser, a wavelength known to selectively enhance this mode [28]. The RR spectra of all samples were measured using a Spex 1269 spectrometer equipped with a Spec-10 LN liquid nitrogen-cooled detector (Princeton Instruments, NJ). The laser power was adjusted to ~10 mW at the sample. All samples were measured in a spinning NMR tube to avoid local heating and protein degradation. The spectra were collected using a 180° backscattering geometry, with the laser beam being focused as a line image on the sample tube using a cylindrical lens. Spectra were calibrated with data acquired for fenchone and processed with Grams/32 AI software (Galactic Industries, Salem, NH).
3. Results
3.1 Reconstitution and characterization of Mn-b5
Mn-b5 was successfully reconstituted from a purified preparation of apo-cyt b5. The complete reconstitution was confirmed by UV-visible spectroscopy. SDS-PAGE analysis and MALDI-MS using a sinapinic acid matrix confirmed the presence of highly purified and full length protein construct of Mn-b5 as well as cyt b5 (data not shown).
3.2 NADPH oxidation and catalytic turnover of 17α-hydroxypregnenolone in the presence of cyt b5 or Mn-b5
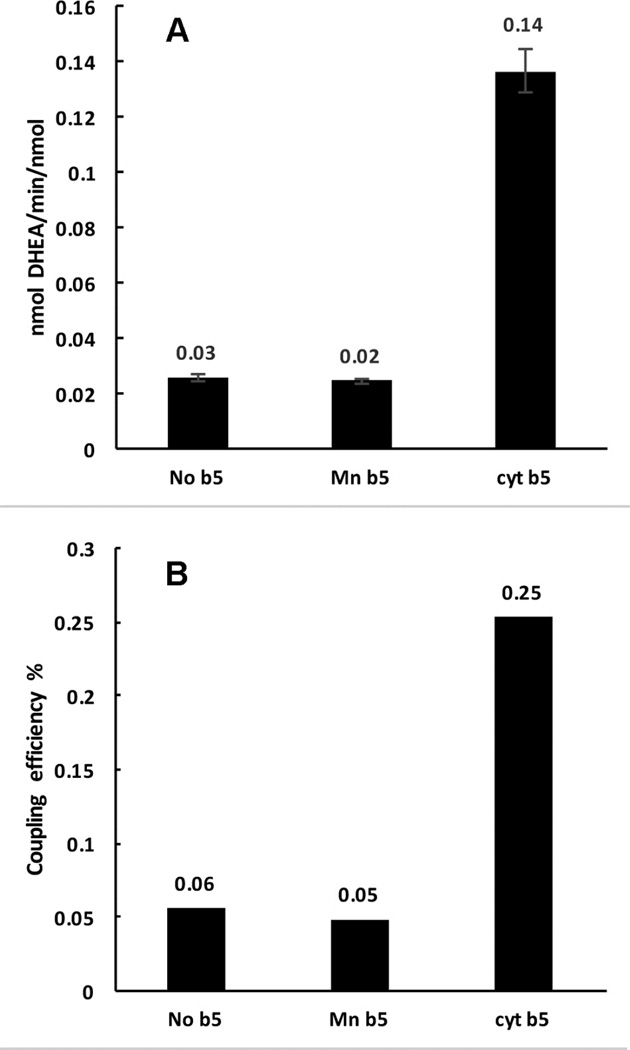
The rates of NADPH oxidation and product formation in the presence of cyt b5 and Mn-b5 were compared to the rates in the absence of any added cyt b5. The presence of cyt b5 increases the product formation rate ~5 fold, while the addition of Mn-b5 does not cause any change (Fig. 3A). The rates of NADPH oxidation were close in value in all three cases, 54±1.3 min−1 for cyt b5, 51±1.0 min−1 for Mn-b5 and 46±1.9 min−1 for reactions in the absence of any b5. The efficiency of coupling was calculated as the ratio of product formation rate to the rate of NADPH consumption. This was seen to be maximal in the reactions with cyt b5, while reactions with no b5 and with Mn-b5 had similar coupling efficiency (Fig. 3B).
Figure 3.
Comparison of CYP17A1 catalyzed lyase reactions with 17α-hydroxypregnenolone as substrate, with no cytochrome b5 present, or with Mn-b5, or native cytochrome b5. (A) DHEA product formation rates (B) Coupling efficiencies (calculated as the percent ratio of amount of product formed to the amount of NADPH consumed).
3.3 Resonance Raman spectroscopy on Mn-b5 binding to ferric CYP17A1
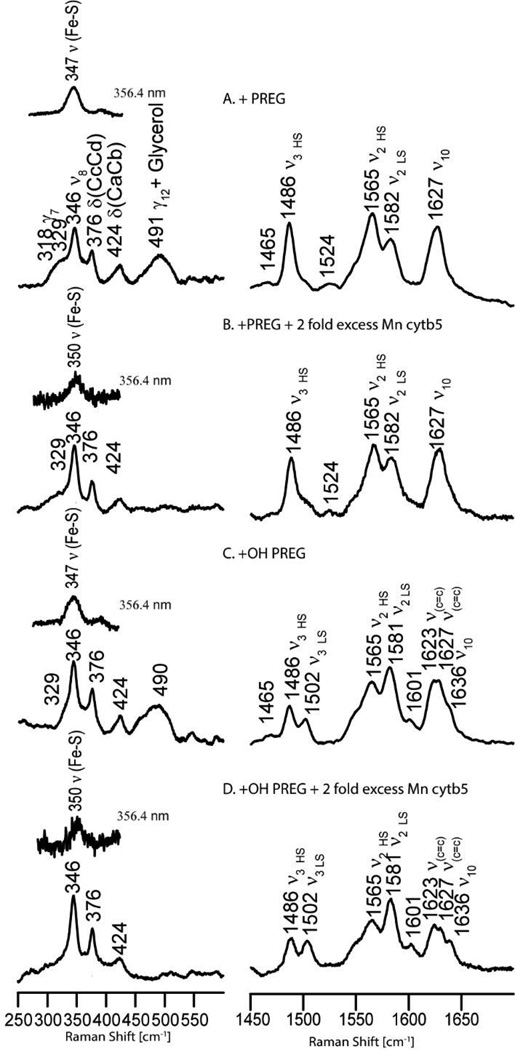
As was reported in an earlier work [29], binding of pregnenolone to substrate-free CYP17A1 causes a spin state conversion from almost pure low spin (LS) to largely high spin (HS) form, while binding of 17α-hydroxypregnenolone generates a lower HS fraction. The persistence of more LS component being attributed to the tendency of the 17α-hydroxy fragment of the substrate to directly interact with the heme iron or promote retention of distal pocket water molecules. In the present work, additions of Mn-b5 to substrate-bound forms of CYP17A1, creating 2-fold excesses relative to the enzyme, cause relatively small increases of the LS component in these samples. This is reflected in the resonance Raman (rR) spectra illustrated in Fig. 4 for the samples bearing a 2-fold excess of Mn-b5. As can be seen in trace B, pregnenolone bound CYP17A1 interacting with Mn-b5 shows a spectrum similar to that for the sample without Mn-b5 (trace A); they both exhibit a strong isolated ν3 mode at 1488 cm−1 and a small signal for LS population near 1500 cm−1. Employing a procedure developed in our laboratory by Mak et al. [30], it is possible to estimate the spin state population using the intensity ratio of IHS/ILS equal to 1.24. Using this value, the HS population of pregnenolone bound CYP17A1 in the presence of a 2-fold excess of Mn-b5 is calculated to be 80%, close to the value observed in the absence of Mn-b5. Similarly, it is noted that the HS component changes from ~57% to ~50% when a 2-fold excess of Mn-b5 was added into 17α-hydroxypregnenolone bound CYP17A1 (Fig. 4 right panel, trace D). These remained nearly the same even in the presence of 4-fold Mn-b5, call of CYP17A1 binding sites for cyt b5 have been occupied (data not shown). In addition to the relatively minor effects on spin state populations, as can be seen in Fig. 4, it is important to emphasize that the frequencies observed for both the low energy and high energy internal modes of the heme prosthetic group do not shift relative to those observed for the corresponding samples without Mn-b5, implying that the interaction with Mn-b5 does not induce significant structural changes of the heme macrocycle or its peripheral substituents in the substrate-bound ferric enzyme. Though minimal effects were observed for spin state populations and heme structure, it is noted that association of CYP17A1 with Mn-b5 increases the strength of Fe-S linkage, demonstrating the formation of a CYP17A1:Mn-b5 complex. This is evident from viewing the inserts for the low-frequency spectra in Fig. 4, where the ν(Fe-S) stretching modes occurring in samples with the excess Mn-b5 appear at 350 cm−1, while those previously reported for the same samples of CYP17A1 in Nanodiscs without Mn-b5 appear at 347 cm−1 [29].
Figure 4.
Resonance Raman Spectra of CYP17A1 +/− Mn-b5. The right panel shows the high-frequency region of the acquired spectra, while the left panel shows the low frequency region; the spectra were acquired using the 406.7 nm excitation line from a Krypton ion laser, noting that the inset bands in the low frequency region were acquired with the 356.4 nm excitation line from the same laser, a wavelength that selectively enhances the ν(Fe-S) stretching mode [28]. Spectra traces in B and D are the resulting traces after subtraction of spectra obtained for samples containing the equivalent concentration of Mn-b5.
4. Discussion
CYP17A1 is a critical enzyme in steroidogenesis, lying at the branch point of glucocorticoids synthesis and androgen synthesis. It performs hydroxylation on the pregnene- nucleus to form hydroxylated products which can either be diverted to glucocorticoid synthesis, or undergo a C-C bond lyase reaction by CYP17A1 to produce androgens. Cytochrome b5 is known to be the chief regulator of CYP17A1 reactivities in vivo, yet the nature of interactions of CYP17A1 with cyt b5 is not clearly understood. Cyt b5 is known to enhance the lyase reactions from 5 to 10 fold. Whether cyt b5 acts as an allosteric modulator or if it functions as a second electron donor has long been debated [15,31], and conflicting reports from various groups are complicated by the fact that these studies were carried out in uncontrolled conditions where aggregation states and stoichiometries of interactions were not known [15,32,33]. Scott and coworkers have used solution NMR with soluble forms of the proteins to study the conformational changes occurring in CYP17A1 bound to lyase substrates upon cyt b5 binding and associated them with an allosteric role for cyt b5 [12,34], although not directly addressing a possible redox role. The application of Nanodisc technology to this system is therefore an effective solution, enabling us to study reactions in controlled stoichiometries in a native-like membrane environment.
We reconstituted cyt b5 with Mn-protoporphyrin IX, which is known to be redox inactive under these conditions [6], and tested the rate of lyase reaction in 17α-hydroxypregnenolone in the presence and absence of cyt b5, and also in the presence of Mn-b5. Reactions with Mn-b5 showed no enhancement of the rate of DHEA formation, which suggests very strongly that cyt b5 has a definite redox donor role in the CYP17A1 lyase chemistry. Importantly, presence of cyt b5 increases the coupling efficiency about 5-fold. Given the relatively high redox potential of cyt b5 (~0 mV NHE) [4], its redox role is limited to reduction of the oxy-complex [5], giving rise to the nucleophilic peroxo-species, the reactive intermediate responsible for carbon-carbon bond scission [23,35]. An enhanced reduction rate of the oxy-complex is expected to increase the steady-state concentration of the peroxoanion, which in the absence of cyt b5 is depleted through non-productive release of hydrogen peroxide. Our resonance Raman data with Mn-b5 binding to ferric CYP17A1 indicate no major structural changes of the heme planar modes, whereas on the proximal side, Fe-S bond was observed to get strengthened when Mn-b5 was bound. Clearly, Mn-b5 binds to CYP17A1, but is incapable of enhancing the lyase activity, the latter being enhanced if the native iron containing cyt b5 is bound. Taken together, these experiments provide strong evidence that cyt b5 is serving as a redox partner in CYP17A1 catalytic cycle, most likely delivering a second electron to the oxy-complex and facilitating formation of the peroxo-ferric complex, which recent evidence shows to be the catalytically active intermediate in the lyase reaction [35]. This result is in agreement with conclusions on the role of cyt b5 in CYP2B4 catalysis [14,36]. Although our results cannot disprove the presence of functionally important allosteric interactions between cyt b5 and CYP17A1, the lack of any effect of Mn-b5 on the rate of turnover of 17α-hydroxypregnenolone, as compared with 5-fold acceleration of this reaction in the presence of cyt b5, suggests that these allosteric interactions do not play significant role in the human CYP17A1 mediated catalysis of lyase reaction.
Supplementary Material
Highlights.
Cyt b5 role in human CYP17A1 mediated androgen synthesis was probed in Nanodiscs.
Native cyt b5 enhances androgen synthesis by CYP17A1.
Redox inactive Mn cyt b5 does not enhance androgen synthesis by CYP17A1.
Interactions with Cyt b5 perturb Fe-S and heme Raman modes of CYP17A1.
Cyt b5 acts as a redox donor for CYP17A1 mediated androgen synthesis.
Acknowledgments
This work was supported by National Institutes of Health [GM110428].
Abbreviations
- Cyt b5
cytochrome b5
- Mn-b5
manganese protoporphyrin IX substituted cyt b5
- DHEA
dehydroepiandrosterone
- PREG
pregnenolone
- OH-PREG
17α-hydroxypregenolone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oshino N, Imai Y, Sato R. A function of cytochrome b5 in fatty acid desaturation by rat liver microsomes. J Biochem. 1971;69:155–167. doi: 10.1093/oxfordjournals.jbchem.a129444. [DOI] [PubMed] [Google Scholar]
- 2.Imai Y, Sato R. The roles of cytochrome b5 in a reconstituted N-demethylase system containing cytochrome P-450. Biochem. Biophys. Res. Commun. 1977;75:420–426. doi: 10.1016/0006-291x(77)91059-2. [DOI] [PubMed] [Google Scholar]
- 3.Bonfils C, Balny C, Maurel P. Direct evidence for electron transfer from ferrous cytochrome b5 to the oxyferrous intermediate of liver microsomal cytochrome P-450 LM2. J Biol. Chem. 1981;256:9457–9465. [PubMed] [Google Scholar]
- 4.Rodgers KK, Sligar SG. Surface electrostatics, reduction potentials, and the internal dielectric constant of proteins. J Am. Chem. Soc. 1991;113:9419–9421. [Google Scholar]
- 5.Lipscomb JD, Sligar SG, Namtvedt MJ, Gunsalus IC. Autooxidation and hydroxylation reactions of oxygenated cytochrome P-450cam. J Biol. Chem. 1976:1116–1124. [PubMed] [Google Scholar]
- 6.Morgan ET, Coon MJ. Effects of cytochrome b5 on cytochrome P-450-catalyzed reactions. Studies with manganese-substituted cytochrome b5. Drug Metab. Dispos. 1984;12:358–364. [PubMed] [Google Scholar]
- 7.Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids. 1997;62:133–142. doi: 10.1016/s0039-128x(96)00172-9. [DOI] [PubMed] [Google Scholar]
- 8.Sergeev GV, Gilep AA, Usanov SA. The role of cytochrome b5 structural domains in interaction with cytochromes P450. Biochemistry (Mosc) 2014;79:406–416. doi: 10.1134/S0006297914050046. [DOI] [PubMed] [Google Scholar]
- 9.Lee-Robichaud P, Akhtar ME, Akhtar M. Control of androgen biosynthesis in the human through the interaction of Arg347 and Arg358 of CYP17 with cytochrome b5. Biochem. J. 1998;332:293–296. doi: 10.1042/bj3320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sondhi V, Owen BM, Liu J, Chomic R, Kliewer SA, Hughes BA, Arlt W, Mangelsdorf DJ, Auchus RJ. Impaired 17,20-lyase activity in male mice lacking cytochrome b 5 in Leydig cells. Mol. Endocrinol. 2016;30:469–478. doi: 10.1210/me.2015-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai Y, Yanase T, Hara T, Takayanagi R, Haji M, Nawata H. In-vitro evidence for the regulation of 17,20-lyase activity by cytochrome b5 in adrenocortical adenomas from patients with Cushing’s syndrome. Clin. Endocrinol. (Oxf) 1994;40:205–209. doi: 10.1111/j.1365-2265.1994.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 12.Estrada DF, Laurence JS, Scott EE. Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR. J Biol. Chem. 2013;288:17008–17018. doi: 10.1074/jbc.M113.468926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Huang R, Im SC, Waskell L, Ramamoorthy A. Effects of membrane mimetics on cytochrome P450-cytochrome b5 interactions characterized by NMR spectroscopy. J. Biol. Chem. 2015;290:12705–12718. doi: 10.1074/jbc.M114.597096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Im S-C, Waskell L. The interaction of microsomal cytochrome P450 2B4 with its redox partners, cytochrome P450 reductase and cytochrome b5. Arch. Biochem. Biophys. 2011;507:144–153. doi: 10.1016/j.abb.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol. Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 16.Guryev OL, Gilep AA, Usanov SA, Estabrook RW. Interaction of apo-cytochrome b5 with cytochromes P4503A4 and P45017A: relevance of heme transfer reactions. Biochemistry. 2001;40:5018–5031. doi: 10.1021/bi002305w. [DOI] [PubMed] [Google Scholar]
- 17.Holmans PL, Shet MS, Martin-Wixtrom CA, Fisher CW, Estabrook RW. The high-level expression in Escherichia coli of the membrane-bound form of human and rat cytochrome b5 and studies on their mechanism of function. Arch. Biochem. Biophys. 1994;312:554–565. doi: 10.1006/abbi.1994.1345. [DOI] [PubMed] [Google Scholar]
- 18.Gregory M, Mak PJ, Sligar SG, Kincaid JR. Differential hydrogen bonding in human CYP17 dictates hydroxylation versus lyase chemistry. Angew. Chemie. 2013;52:5342–5345. doi: 10.1002/anie.201300760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulrooney SB, Waskell L. High-level expression in Escherichia coli and purification of the membrane-bound form of cytochrome b5. Protein Expr. Purif. 2000;19:173–178. doi: 10.1006/prep.2000.1228. [DOI] [PubMed] [Google Scholar]
- 20.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am. Chem. Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 21.Luthra A, Gregory M, Grinkova YV, Denisov IG, Sligar SG. Nanodiscs in the studies of membrane-bound cytochrome P450 enzymes. Methods Mol. Biol. 2013;987:115–127. doi: 10.1007/978-1-62703-321-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinkova YV, Denisov IG, Sligar SG. Functional reconstitution of monomeric CYP3A4 with multiple cytochrome P450 reductase molecules in Nanodiscs. Biochem. Biophys. Res. Commun. 2010;398:194–198. doi: 10.1016/j.bbrc.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory MC, Denisov IG, Grinkova YV, Khatri Y, Sligar SG. Kinetic solvent isotope effect in human P450 CYP17A1-mediated androgen formation: evidence for a reactive peroxoanion intermediate. J Am. Chem. Soc. 2013;135:16245–16247. doi: 10.1021/ja4086403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatri Y, Gregory MC, Grinkova YV, Denisov IG, Sligar SG. Active site proton delivery and the lyase activity of human CYP17A1. Biochem. Biophys. Res. Commun. 2014;443:179–184. doi: 10.1016/j.bbrc.2013.11.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luthra A, Denisov IG, Sligar SG. Spectroscopic features of cytochrome P450 reaction intermediates. Arch. Biochem. Biophys. 2011;507:26–35. doi: 10.1016/j.abb.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak PJ, Im S-C, Zhang H, Waskell LA, Kincaid JR. Resonance Raman studies of cytochrome P450 2B4 in its interactions with substrates and redox partners. Biochemistry. 2008;47:3950–3963. doi: 10.1021/bi800034b. [DOI] [PubMed] [Google Scholar]
- 27.Gruenke LD, Sun J, Loehr TM, Waskell L. Resonance Raman spectral properties and stability of manganese protoporphyrin IX cytochrome b5. Biochemistry. 1997;36:7114–7125. doi: 10.1021/bi970407p. [DOI] [PubMed] [Google Scholar]
- 28.Champion PM, Stallard BR, Wagner GC, Gunsalus IC. Resonance Raman detection of an Fe-S bond in cytochrome P450cam. J Am. Chem. Soc. 1982;104:5469–5472. [Google Scholar]
- 29.Mak PJ, Gregory MC, Sligar SG, Kincaid JR. Resonance Raman spectroscopy reveals that substrate structure selectively impacts the heme-bound diatomic ligands of CYP17. Biochemistry. 2014;53:90–100. doi: 10.1021/bi4014424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mak PJ, Zhu Q, Kincaid JR. Using resonance Raman cross-section data to estimate the spin state populations of Cytochromes P450. J Raman Spectrosc. 2013;44:1792–1794. doi: 10.1002/jrs.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guryev OL, Gilep AA, Usanov SA, Estabrook RW. Interaction of apo-cytochrome b5 with cytochromes P4503A4 and P45017A: relevance of heme transfer reactions. Biochemistry. 2001;40:5018–5031. doi: 10.1021/bi002305w. [DOI] [PubMed] [Google Scholar]
- 32.Akhtar M, Wright JN, Lee-Robichaud P. A review of mechanistic studies on aromatase (CYP19) and 17-hydroxylase-17,20-lyase (CYP17) J. Steroid Biochem. Mol. Biol. 2011;125:2–12. doi: 10.1016/j.jsbmb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Naffin-Olivos JL, Auchus RJ. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Life Sci. 2006:755–762. doi: 10.1021/bi051623y. [DOI] [PubMed] [Google Scholar]
- 34.Estrada DF, Laurence JS, Scott EE. Cytochrome P450 17A1 interactions with the FMN domain of its reductase as characterized by NMR. J Biol. Chem. 2015;291:3990–4003. doi: 10.1074/jbc.M115.677294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mak PJ, Gregory MC, Denisov IG, Sligar SG, Kincaid JR. Unveiling the crucial intermediates in androgen production. Proc. Natl. Acad. Sci. USA. 2015;112:15856–15861. doi: 10.1073/pnas.1519376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Myshkin E, Waskell L. Role of cytochrome b5 in catalysis by cytochrome P450 2B4. Biochem. Biophys. Res. Commun. 2005;338:499–506. doi: 10.1016/j.bbrc.2005.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.