Abstract
Background aims
The PR1 peptide, derived from the leukemia-associated antigens proteinase 3 and neutrophil elastase, is overexpressed on HLA-A2 in acute myeloid leukemia (AML). We developed a T cell receptor (TCR)-like monoclonal antibody (8F4) that binds the PR1/HLA-A2 complex on the surface of AML cells efficiently killing them in vitro and eliminating them in preclinical models. Humanized 8F4 (h8F4) with high affinity for the PR1/HLA-A2 epitope was used to construct an h8F4- chimeric antigen receptor (CAR) that was transduced into T-cells to mediate anti-leukemia activity.
Methods
Human T cells were transduced to express the PR1/HLA-A2-specific CAR (h8F4-CAR-T cells) containing the scFv of h8F4 fused to the intracellular signaling endodomain of CD3 zeta chain through the transmembrane and intracellular costimulatory domain of CD28.
Results
Adult human normal peripheral blood (PB) T cells were efficiently transduced with the h8F4-CAR construct and predominantly displayed an effector memory phenotype with a minor population (12%) of central memory cells in vitro. Umbilical cord blood (UCB) T cells could also be efficiently transduced with the h8F4-CAR. The PB and UCB-derived h8F4-CAR-T cells specifically recognized the PR1/HLA-A2 complex and were capable of killing leukemia cell lines and primary AML blasts in an HLA-A2-dependent manner.
Conclusions
Human adult PB and UCB-derived T cells expressing a CAR derived from the TCR-like 8F4 antibody rapidly and efficiently kill AML in vitro. Our data could lead to a new treatment paradigm for AML in which targeting leukemia stem cells could transfer long-term immunity to protect against relapse.
Keywords: PR1, h8F4, CAR-T cell, AML, UCB
Introduction
Adoptive cellular therapy employs high-affinity B and/or T-cell antigen receptors to eliminate malignant cells and is a promising therapy for hematologic malignancies. This strategy has been most effective in the treatment of adult and pediatric acute lymphoblastic leukemia (ALL), where CAR-expressing T cells redirected against the CD19 antigen have achieved high clinical response rates and durable remissions in patients with relapsed, refractory disease [1, 2]. CAR-T cells are T lymphocytes that are genetically engineered to express the single chain variable fragment (scFv) derived from a B-cell immunoglobulin (Ig) receptor, and the resulting chimeric cell combines the advantages of a highly specific B-cell Ig receptor with the potent cytotoxicity of T-cells. There is an urgent clinical need to translate this biotechnology into therapy for AML, a disease for which the standard treatment strategy has remained unchanged for 30 years despite only a minority of patients achieving durable remissions [3].
We have led the investigation of the leukemia-associated antigen PR1, a human leukocyte antigen-A2*0201 (HLA-A2)-restricted nonameric peptide derived from the parent proteins neutrophil elastase and proteinase 3. These serine proteases are present in the primary azurophilic granules of neutrophils but are overexpressed and mislocalized in myeloid leukemia blasts [4, 5]. Previously, we showed that PR1-specific cytotoxic T-cells can be identified in the peripheral blood of myeloid leukemia patients, mediate cytotoxicity against leukemic blasts in vitro, reduce the leukemic burden in an AML xenograft model, and are associated with the graft-versus-leukemia effect after allogeneic stem cell transplantation (allo-SCT), and [6–9]. Recently, we developed an anti-PR1/HLA-A2 TCR-like antibody, 8F4, that mediates complement-dependent cytolysis of AML blasts and leukemia stem cells in vitro and in vivo [10, 11]. TCR-like antibodies are a unique and novel class of biologics that challenge the paradigm that monoclonal antibodies (mAbs) can recognize only extracellular proteins and, instead, vastly expand the number of potential mAb-binding tumor antigens to include intracellular peptides bound to a surface MHC molecule. TCR-like antibodies are attractive because they can bind targets with affinities that are logs higher than the TCR [12, 13]. The PR1-targeting humanized 8F4, h8F4, will enter an early phase clinical trial for myeloid leukemia patients next year [14].
Herein, we report the incorporation of the h8F4 scFv into a 2nd generation retroviral TCR-like CAR construct and demonstrate efficient transduction of the CAR vector into human healthy donor (HD) peripheral blood mononuclear cells (PBMCs). We show consistent, high expression of the h8F4-CAR on the surface of both CD4+ and CD8+ T-cells. We also show specific binding of the h8F4-CAR to the HLA-A2/PR1 antigen and demonstrate preferential cytotoxicity of h8F4-CAR-T cells against human AML cell lines and primary AML blasts in vitro. In addition, we show efficient transduction and functional cytotoxicity of h8F4-CAR-T cells generated from umbilical cord blood,; a versatile source of cells for adoptive therapy that is more permissive for HLA-mismatching in the allo-SCT setting and that may possess enhanced antitumor efficacy [15, 16].
Materials and Methods
Reagents
PBMCs were isolated from buffy coat preparations derived from whole blood of healthy volunteer donors (Gulf Coast Regional Blood Center, Houston, TX). HLA-A2 positive fresh UCB units were obtained from the MD Anderson Cord Blood Bank. PBMC and UCB were separated by gradient density centrifugation using Histopaque 1077 (Sigma-Aldrich). HLA-A2*0201 (HLA-A2+) samples were identified by staining blood samples with anti-HLA-A2 antibody (clone BB7.2; BD Pharmingen). AML samples were collected from patients treated at the University of Texas MD Anderson Cancer Center under protocols approved by the Institutional Review Board. HLA status of patients was determined by the MD Anderson HLA Typing Laboratory. T2, K562, and U937 cell lines were obtained from American Type Culture Collection. HLA-A2*0201 transduction of cell lines was performed as previously described using lentiviral vectors [17].
Generation of the HLA-A2/PR1-specific CAR construct
The humanized antibody targeting the PR1/HLA-A2 combined epitope produced by the 8F4 hybridoma was cloned as a single chain (scFv) [18]. The genes coding the VH and VL chains of the monoclonal antibody were cloned by RT-PCR using murine variable domain-specific primers modified to generate SfiI restriction sites at the 5′ end of the amplified VL and 3′ end of the amplified VH.26. Combinatorial scFv genes were generated by splicing-by-overlap PCR and then ligated into SfiI sites of the replicative form of fUSE5 vector phage DNA. To generate the h8F4-CAR, the scFv sequence was cloned in frame with the human IgG1-CH2CH3 domain, CD28 costimulatory domain, and with the ζ chain of the TCR/CD3 complex in the SFG retroviral backbone [19, 20].
Generation and transduction of activated T cells
To generate CAR-T cells, healthy donor PBMCs or UCB were activated on day 0 using 1 μg/ml plate-bound anti-CD3 and anti-CD28 antibodies (clones HIT3a and CD28.2, respectively; BioLegend) in non-tissue culture treated 24-well plates (Falcon). On day 1, 100 IU/ml of rhIL-2 (R&D Systems) was added to the PBMCs and a second 24-well plate was coated with 10 μg/ml recombinant fibronectin fragment (Takara) and left at 4°C overnight. The following day, h8F4-CAR retroviral supernatant was added to the fibronectin plate and the plate was centrifuged at 2000×g for 90 minutes at room temperature. The supernatant was then aspirated and 1–2 million activated T cells were added per well. The T cells were subsequently expanded with rhIL-2 (50 IU/ml) and fresh T-cell medium every 3 days. T-cell medium consisted of 50% RPMI 1640 (HyClone) and 50% Click’s (Irvine Scientific) supplemented with 10% FBS, 100U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine. Transduction efficiency was routinely assessed on day 6.
Phenotypic analysis of h8F4-CAR-T cells
Aliquots of transduced T cells were analyzed by 7-color flow cytometry using a panel of surface molecule specific antibodies, peptide tetramer, and dextramer: CD3 (clone SK7, Biolegend), CD4 (clone RPA-T4; Biolegend), CD8 (clone 3B5; Biolegend), anti-IgG (polyclonal Goat anti-human; Jackson ImmunoResearch), CD45RA (clone MEM-56; Thermo Fisher), and CCR7 (clone 3D12; BD Biosciences); HLA-A*0201 PR1 tetramer (Baylor tetramer core facility), HLA-A*0201 CMV pp65 dextramer and HLA-A*0201 PR1 dextramer (Immudex, Denmark).
Cytotoxicity assay
Freshly transduced h8F4-CAR-T cells were tested for specific cytotoxicity against target cells. Target cells were stained with 5 μg/ml of Calcein AM (Thermo Fisher) for 15 minutes at 37°C, washed, and resuspended at 2×105/ml in culture media. Two-thousand target cells in 10 μl were incubated with effector cells (CAR-transduced or control cytotoxic T-lymphocytes (CTL)) at varying E:T ratios with 5 μl of 0.4% Trypan Blue added into each well of a Terasaki plate to quench the reaction [18, 21]. The BioTek FLx800 Microplate fluorescence reader was used to read the plate. The fluorescence was maximal in wells with targets alone and minimal in those with media alone or 100% dead cells; % specific lysis = (1−(mean value of test)/(mean value of target alone)) × 100.
Results
Expression of the h8F4-CAR on primary human T cells
The TCR-like mAb, h8F4, was used to construct an h8F4-CAR that, when transduced into polyclonal T cells, could redirect them to mediate graft-versus-leukemia (GVL) activity. The single chain variable fragments (scFv) of h8F4, including the variable region of light chain 2 (VL2) and variable region of heavy chain (VH), were cloned in frame with the human IgG1-CH2CH3 domain and the ζ chain of the TCR/CD3 complex in the SFG retroviral backbone [19, 20] (Figure 1). The CD28 domain within the construct was included as described [22]. Retroviral supernatant packaged in 293T cells was used to transduce human primary T cells. The transduction efficiency was measured using an anti-IgG antibody, the F(ab)2 fragment against IgG (heavy and light chains), that recognizes the spacer region of the CAR. On day 6 post-transduction, cells were collected and stained with antibodies against the CD3 and the CAR spacer region. As shown in Figure 2A, ~95% of T cells expressed h8F4-CAR on their surface (CD3+/Anti-IgG+) compared to the background level (0.025%) on non-transduced control cells that underwent an identical activation process. Transduced T cells were maintained in culture with rhIL-2 for over 3 weeks. The transduction efficiency of h8F4-CAR remained similar on day 15 and 21 post-transduction, 95.6% and 97.1% respectively (Figure 2B). Moreover, the consistently high expression of h8F4-CAR (> 80%) was achieved in 14 out of 19 different samples. Thus, primary human adult T cells are efficiently transduced with h8F4-CAR and expression remains stably high over time in vitro.
Figure 1. Humanized 8F4-CAR (h8F4) construct.
Schematic plasmid map of humanized 8F4 antibody single chain variable fragments (scFv) containing the PR1/HLA-A2 chimeric antigen receptor (Hu8F4-ScFv-CAR). SP - signal peptide, VL2 variable region of light chain 2 of h8F4, VH variable region of heavy chain of h8F4, L - linker peptide GlyGlyGlyGlySer, CH2CH3 constant region 2 and 3 of human IgG1, CD28 CD28 signaling domain, zeta CD3 zeta chain.
Figure 2. Human adult T cells are efficiently transduced with the h8F4 construct and expression remains stably high over time in vitro.
Peripheral blood T cells were transduced with the h8F4-CAR retrovirus. Transduction efficiency was determined by flow cytometry using an anti-human IgG (heavy and light chain) antibody against the CAR spacer CH2CH3 region and anti-CD3. Non-transduced PBMCs were used as negative control. Representative flow cytometry plots of T cells expressing h8F4-CAR on day 6 (A), day 15, and day 21 (B) post-transduction. (C) Summary of the transduction efficiency from 19 individual experiments on day 6 represented by the percentage of anti-IgG+ T cells. Horizontal bars indicate mean values with SEM.
h8F4-CAR-T cells specifically recognize the PR1/HLA-A2 antigen
To determine whether the antigen receptor maintained recognition of the PR1/HLA-A2 ligand, h8F4-CAR-T cells and non-transduced T cells were stained with CD3 Ab and either PR1/HLA-A2 tetramer (Figure 3A, left panels) or PR1/HLA-A2 dextramer (Figure 3A, right panels). The h8F4-CAR-T-cells showed high avidity for the PR1/HLA-A2 antigen at levels comparable to that seen with the anti-IgG antibody (Figure 2A). When using equivalent concentrations of PR1/HLA-A2 dextramer and CMV pp65/HLA-A2 dextramer in a competition assay, h8F4-CAR transduced T cells demonstrated preferential binding to PR1 and not irrelevant pp65, regardless of whether cells were first incubated with PR1 dextramer (Figure 3B–a) or CMVpp65 dextramer (Figure 3B–b). These data indicate that the h8F4 TCR-like antibody maintains specificity for the HLA-A2/PR1 ligand when expressed on the surface of T-cells as chimeric antigen receptors. Both CD4 and CD8 T cells were transduced with h8F4-CAR equally well (Figure 3C); however, the CD4:CD8 ratio of the CAR-transduced T-cells varied by donor. Among adult PB donors, the percentage of CD4+ CAR cells was 50–60% on average on day 6 post-transduction. The percentage of CD4+ CAR-T cells was higher in UCB-derived cells and was 70% on average, which was expected given the higher average frequency of CD4+ T cells in fresh cord units [23]. As shown in Figure 3D, h8F4-CAR transduced T cells were predominantly effector memory cells (CCR7−/CD45RA−) with 12% displaying a central memory phenotype (CCR7+/CD45RA−).
Figure 3. h8F4-CAR-T cells specifically recognize the PR1/HLA-A2 antigen.
(A) Representative flow cytometry plots demonstrating non-transduced activated control T cells (top) and h8F4-CAR transduced T cells (bottom) stained with either PR1-HLA*02:01 tetramer (left panel) or PR1-HLA*02:01 dextramer (right panel). (B) h8F4-CAR-T cells preferentially bind PR1 dextramer. A dextramer competition assay was performed using equivalent concentrations of PR1-HLA*02:01 dextramer and irrelevant CMVpp65-HLA*02:01 dextramer. h8F4-CAR-T cells were first incubated with PR1 dextramer for 3 minutes before adding an equivalent concentration of CMVpp65 dextramer (a) or in the reverse sequence (b). Results for both dextramer channels are shown after gating on viable, single lymphocytes. (C) Both CD4 and CD8 T cells were transduced equally well with h8F4-CAR virus. Transduction efficiency was analyzed separately for CD4 and CD8 cells by flow cytometry on day 13 post-transduction using anti-human IgG antibody against the CAR spacer CH2CH3 region and anti-CD3. (D) Phenotype of transduced h8F4-CAR-T cells. Phenotype was determined using CCR7 and CD45RA cell surface markers. Left panel: representative plots of PBMCs prior to activation, non-transduced activated control cells, and h8F4-transduced CAR-T cells. Right panel: summary of effector memory (EM) (CCR7−/CD45RA−), central memory (CM) (CCR7+/CD45RA−) and terminally differentiated (TD) effector (CCR7−/CD45RA+) subsets of h8F4-CART cells in 3 different experiments.
Human T cells modified with the high affinity h8F4-CAR mediate anti-leukemic activity
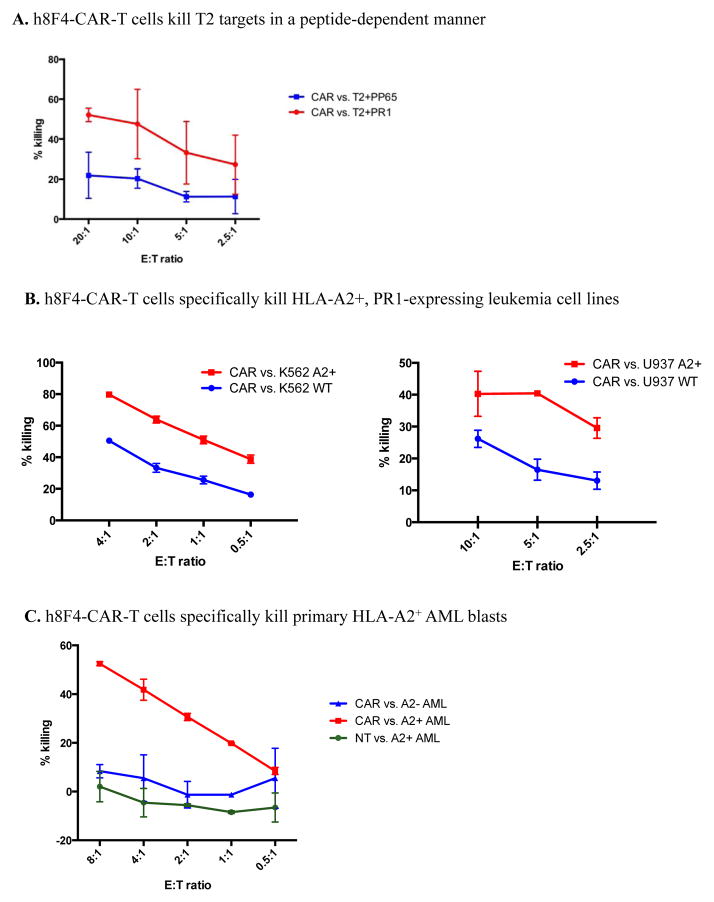
Since h8F4 has higher affinity than the PR1-TCR for the PR1/HLA-A2 ligand [14], we investigated whether h8F4-CAR transduced T cells were capable of killing PR1/HLA-A2 target cells in vitro. As shown in Figure 4A, h8F4-CAR-T cells lysed PR1-pulsed but not control peptide CMV pp65 peptide-pulsed T2 cells, demonstrating that h8F4-CAR-T cells were capable of killing T2 targets in a peptide-dependent manner. Furthermore, h8F4-CAR-T cells lysed the HLA-A2 transduced, PR1-expressing leukemia cell lines U937 (U937-A2) and K562 (K562-A2) but not their wild type counterparts, which express PR1 but not HLA-A2 (Figure 4B). Specific killing of primary patient HLA-A2+ AML blasts by h8F4-CAR-T cells was also demonstrated (Figure 5C), indicating that h8F4-CAR-T cells were capable of killing leukemia cell lines and primary AML blasts in an HLA-A2-dependent manner.
Figure 4. h8F4-CAR-T cells demonstrate specific cytotoxicity.
(A) T2 target cells were loaded with PR1 peptide or control CMV pp65 peptide and incubated with h8F4-CAR-T cells at the indicated E:T ratios in a 4-hr cytotoxicity assay. (B) HLA-A2 transduced K562 (left panel) and U937 (right panel) leukemia cell lines and their wild type, HLA-A2 negative counterparts were incubated with h8F4-CAR-T cells at the indicated E:T ratios in a 4-hr cytotoxicity assay. (C) Primary human A2+ and A2− AML blasts were incubated with h8F4-CAR-T cells or NT cells at the indicated E:T ratios in a 4-hr cytotoxicity assay. Data are presented as mean percent specific killing of target cells ± SD with experiments done in triplicate.
Figure 5. Human cord blood T cells are efficiently transduced with the h8F4-CAR and demonstrate specific cytotoxicity.
UCB-derived T cells were transduced with the h8F4-CAR virus. (A) Expression of h8F4-CAR on cord blood T cells. Left: representative flow cytometry plot of cord blood T cells expressing h8F4-CAR on day 6 post-transduction. Right: summary of the transduction efficiency from 5 individual experiments. Horizontal bars indicate mean values with SEM. (B) Both CD4 and CD8 cord blood T cells were transduced equally well with the h8F4-CAR virus. Transduction efficiency was analyzed separately for CD4 and CD8 cells on day 6 post-transduction using the anti-spacer antibody. (C) Phenotype of UCB-derived h8F4-CAR-T cells was assessed using CCR7 and CD45RA cell surface markers. (D) Specific cytotoxic activity of h8F4-CAR cord blood T cells. Left: HLA-A2+ and wild type U937 cells were incubated with h8F4-CAR cord blood T cells in a cytotoxicity assay. Right: Primary HLA-A2+ and HLA-A2− AML blasts were incubated with h8F4-CAR cord blood T cells in a cytotoxicity assay at the indicated E:T ratios in a 4-hr cytotoxicity assay. Data are presented as mean percent specific killing of target cells ± SD with experiments done in triplicate.
h8F4-CAR transduced UCB T cells lyse human leukemia target cells
Human UCB T cells were transduced with the h8F4-CAR, and as shown in Figure 5A (left), high expression of h8F4-CAR on UCB T cells was confirmed on day 6 post-transduction (98.3%). Among five different individual UCB samples, four demonstrated high expression of h8F4-CAR with over 85% T-cell transduction (Figure 5A, right). Both CD4 and CD8 cord blood T cells were transduced equally well with h8F4-CAR virus (Figure 5B). As shown in Figure 5C, h8F4-CAR transduced UCB T cells were predominantly effector memory cells (CCR7−/CD45RA−). To measure the specific cytotoxic activity of h8F4-CAR UCB T cells, U937 leukemia cells (both HLA-A2-transduced and wild type) and primary human patient AML blasts were used as targets in a cytotoxicity assay. As shown in Figure 5D, UCB-derived h8F4-CAR-T cells were capable of killing the U937-A2 leukemia cell line and primary AML blasts in an HLA-A2-dependent manner in vitro.
Discussion
We recently developed a T cell receptor-like murine IgG2a monoclonal antibody, m8F4, which binds to PR1/HLA-A2+ AML, mediates lysis of AML in vitro, and depletes AML in vivo [10, 11]. Mouse 8F4 was humanized - human IgG1 8F4 (h8F4) - and maintains specificity for PR1/HLA-A2 and activity against AML [14]. The h8F4 mAb has high affinity (Kd = 6.5 nM) for the PR1/HLA-A2 conformational epitope [14]. Thus, it is an ideal TCR-like mAb for constructing an 8F4-CAR to transduce T-cells and redirect them to mediate GVL activity against myeloid malignancies. Potential advantages of CAR-modified T cells over monoclonal antibodies include greater cytotoxic potency, active trafficking, passage through the blood-brain barrier, fewer required doses, the potential for long-lived memory and protection against relapse, and increased sensitivity to low antigen density [24, 25]. Disadvantages include the greater potential for on-target, off-tissue toxicity and less control over the dose and schedule as CARs have the potential for immense proliferation in vivo.
To test whether an h8F4-CAR antigen receptor expressed in T-cells mediated anti-leukemia activity in vitro, we generated human adult PB and UCB-derived T cells modified to express PR1/HLA-A2 complex-specific CAR (h8F4-CAR-T). We demonstrated the specificity of h8F4-CAR-T cells, which preferentially bound PR1 dextramer. TCR-like antibodies, such as h8F4, are unique in their binding properties and must have affinity for both the target HLA molecule and the peptide-MHC complex to ensure recognition. We anticipated that h8F4-CAR-T cells would demonstrate low-avidity binding to irrelevant dextramer due to the clustering of CAR receptors on the surface and the inherent HLA-A2 binding requirement as shown in Figure 3B(b). Encouragingly, however, the h8F4-CAR-T cells preferentially bound the relevant PR1 epitope. CAR-modified T cells are potent and do have the potential for on-target, off-tissue toxicity in cases where the antigenic target is co-expressed on normal cells, such as with leukemia-associated antigens. Though we have shown that PR1-targeting strategies, including PR1-specific CTL and the 8F4 mAb, preferentially inhibit leukemia progenitor cells over normal hematopoietic progenitors [5, 10, 11, 21], the potential for h8F4-CAR-T cell toxicity exists. We recognize that clinical application of the h8F4-CAR-T cells may require a system where the construct is either transiently expressed [26, 27] or where the CAR is expressed in tandem with a suicide gene “switch” [19].
In this report, we demonstrated functional specificity of the h8F4-CAR-T cells, which killed PR1-pulsed T2 cells but not control-peptide-pulsed T2 cells and lysed HLA-A2 transduced U937 and K562 leukemia cell lines as well as HLA-A2+ primary AML blasts but not HLA-A2-negative AML or HLA-A2 negative leukemia cell lines. We conclude that T cells expressing a CAR derived from the TCR-like 8F4 antibody rapidly and efficiently kill AML in vitro and that this novel adoptive T cell approach merits further investigation.
Considerable research is focused on identifying and targeting extracellular proteins on the surface of AML blasts using CAR-T cells, and strong preclinical evidence exists for CAR-T cells that target the antigens CD123 and CD33 [28–30]. However, these antigens are coexpressed on the surface of myeloid progenitor cells and additional safeguards will need to be implemented to prevent on-target, off-tissue toxicity, long-term myelosuppression, and consequent infections [30]. Additionally, a predictable challenge to the success of targeting specific leukemia antigens is clonal evolution and intratumoral heterogeneity, from which immune escape variants can emerge to comprise relapsed disease [31, 32]. One solution is to target multiple leukemia antigens simultaneously; however, the limited number of effective antigens currently hinders this approach. We identified 8F4 as the first TCR-like mAb against a leukemia antigen [10]. The successful development and testing of the h8F4-CAR shown here supports the promise of developing a novel T-cell therapy directed against an endogenous self-antigen that is differentially expressed on the surface of leukemia stem cells.
We also demonstrated that T cells derived from UCB could be efficiently transduced with the h8F4-CAR and were capable of killing leukemia cells in a PR1/HLA-A2-dependent manner. UCB lymphocytes are mostly naive T-cells and may be an ideal source for generating h8F4-CAR T cells [15, 16]. Since the first UCB transplant (CBT) was performed in 1988 by Gluckman et al. [33], more than 40,000 patients have received CBT for malignant and non-malignant diseases [34–40]. Importantly, UCB has improved the likelihood of finding a SCT donor for minority populations, who are under-represented in donor registries [41]. Of note, the HLA-A*02:01 allele is common among US African Americans (34–40% of individuals) and US Hispanic individuals (19–23%) in addition to being present at high rates in Caucasians (47% of individuals)[42–44]. To improve the outcome of CBT for patients with AML, the most common disease treated with CBT, graft engineering of donor T cells as demonstrated herein using UCB-derived h8F4-CAR-T cells could increase graft-versus-leukemia (GVL) without increasing graft-versus-host disease (GVHD). In support of this strategy, allogeneic CAR-T cells have already been used in at least 4 different clinical trials without any reports of acute GVHD [1, 2, 45, 46], and we anticipate UCB derived CAR-T cells will carry less risk of GVHD in this setting [47].
In summary, we have successfully developed a CAR-T cell based on the construct of a TCR-like monoclonal antibody that targets an intracellular leukemia-associated antigen. We showed that this approach is feasible using peripheral blood as well as UCB T cells. This method could improve upon the existing cellular approaches to the therapy of leukemia and other malignancies.
Acknowledgments
This study was supported by research funding from NCI CA100632 (to JJM); NCI CA148600 (to JJM and EJS); Leukemia and Lymphoma Society 6030-12 (to JJM); P30CA16672 (to KCD); Leukemia and Lymphoma Society 7262-08 (to JJM).
Footnotes
Authorship statement: H.R.G., S.LJ., E.T., X.D., H.H., and L.St J. performed experiments. H.R.G., S.LJ., E.T. and H.H. analyzed data. A.S., G.D. and B.S. provided reagents. H.R.G., S.L.J.,, E.T., G.A., K.R., and E.J.S. wrote the paper. Q.M. and J.J.M. designed the research and wrote the paper. We have no conflict of interests.
References
- 1.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. The New England journal of medicine. 2015;373(12):1136–52. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 4.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88(7):2450–7. [PubMed] [Google Scholar]
- 5.Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90(7):2529–34. [PubMed] [Google Scholar]
- 6.Ma Q, Wang C, Jones D, Quintanilla KE, Li D, Wang Y, et al. Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model. Cytotherapy. 2010;12(8):1056–62. doi: 10.3109/14653249.2010.506506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molldrem JJ, Lee PP, Wang C, Champlin RE, Davis MM. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low-frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer research. 1999;59(11):2675–81. [PubMed] [Google Scholar]
- 8.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nature medicine. 2000;6(9):1018–23. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 9.Rezvani K, Price DA, Brenchley JM, Kilical Y, Gostick E, Sconocchia G, et al. Transfer of PR1-specific T-cell clones from donor to recipient by stem cell transplantation and association with GvL activity. Cytotherapy. 2007;9(3):245–51. doi: 10.1080/14653240701218524. [DOI] [PubMed] [Google Scholar]
- 10.Sergeeva A, Alatrash G, He H, Ruisaard K, Lu S, Wygant J, et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. 2011;117(16):4262–72. doi: 10.1182/blood-2010-07-299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sergeeva A, He H, Ruisaard K, St John L, Alatrash G, Clise-Dwyer K, et al. Activity of 8F4a T-cell receptor-like anti-PR1/HLA-A2 antibody, against primary human AML in vivo. Leukemia. 2016 doi: 10.1038/leu.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, Ahmed M, Tassev DV, Hasan A, Kuo TY, Guo HF, et al. Affinity maturation of T-cell receptor-like antibodies for Wilms tumor 1 peptide greatly enhances therapeutic potential. Leukemia. 2015;29(11):2238–47. doi: 10.1038/leu.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong S, Malecek K, Johnson LA, Yu Z, Vega-Saenz de Miera E, Darvishian F, et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(17):6973–8. doi: 10.1073/pnas.1221609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sergeeva A, Sang M, Ruisaard K, St John L, Clise-Dwyer K, Lu S, et al. Humanized anti-PR1/HLA-A2 antibody h8F4 induces apoptosis of human acute myeloid leukemia. Keystone Symposia, Antibodies as drugs; Whistler, Canada. 2016. [Google Scholar]
- 15.Hiwarkar P, Qasim W, Ricciardelli I, Gilmour K, Quezada S, Saudemont A, et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood. 2015;126(26):2882–91. doi: 10.1182/blood-2015-06-654780. [DOI] [PubMed] [Google Scholar]
- 16.Pegram HJ, Purdon TJ, van Leeuwen DG, Curran KJ, Giralt SA, Barker JN, et al. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29(2):415–22. doi: 10.1038/leu.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Cruz TG, Liu S, Khalili JS, Whittington M, Zhang M, Overwijk W, et al. Natural splice variant of MHC class I cytoplasmic tail enhances dendritic cell-induced CD8+ T-cell responses and boosts anti-tumor immunity. PloS one. 2011;6(8):e22939. doi: 10.1371/journal.pone.0022939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Sukhumalchandra P, Enyenihi AA, St John LS, Hunsucker SA, Mittendorf EA, et al. A novel HLA-A*0201 restricted peptide derived from cathepsin G is an effective immunotherapeutic target in acute myeloid leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(1):247–57. doi: 10.1158/1078-0432.CCR-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160-70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108(12):3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alatrash G, Mittendorf EA, Sergeeva A, Sukhumalchandra P, Qiao N, Zhang M, et al. Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy. Journal of immunology. 2012;189(11):5476–84. doi: 10.4049/jimmunol.1201221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011;121(5):1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Experimental hematology. 2003;31(8):708–14. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 24.Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs) Oncoimmunology. 2012;1(6):863–73. doi: 10.4161/onci.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert opinion on biological therapy. 2011;11(7):855–73. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krug C, Wiesinger M, Abken H, Schuler-Thurner B, Schuler G, Dorrie J, et al. A GMP-compliant protocol to expand and transfect cancer patient T cells with mRNA encoding a tumor-specific chimeric antigen receptor. Cancer immunology, immunotherapy : CII. 2014;63(10):999–1008. doi: 10.1007/s00262-014-1572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutsky K, Song DG, Lynn R, Smith JB, Poussin M, Figini M, et al. Rigorous optimization and validation of potent RNA CAR T cell therapy for the treatment of common epithelial cancers expressing folate receptor. Oncotarget. 2015;6(30):28911–28. doi: 10.18632/oncotarget.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343–54. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–47. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014;28(8):1596–605. doi: 10.1038/leu.2014.62. [DOI] [PubMed] [Google Scholar]
- 31.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klippel ZK, Chou J, Towlerton AM, Voong LN, Robbins P, Bensinger WI, et al. Immune escape from NY-ESO-1-specific T-cell therapy via loss of heterozygosity in the MHC. Gene therapy. 2014;21(3):337–42. doi: 10.1038/gt.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gluckman E, Devergie A, Bourdeau-Esperou H, Thierry D, Traineau R, Auerbach A, et al. Transplantation of umbilical cord blood in Fanconi’s anemia. Nouvelle revue francaise d’hematologie. 1990;32(6):423–5. [PubMed] [Google Scholar]
- 34.Laporte JP, Gorin NC, Rubinstein P, Lesage S, Portnoi MF, Barbu V, et al. Cord-blood transplantation from an unrelated donor in an adult with chronic myelogenous leukemia. The New England journal of medicine. 1996;335(3):167–70. doi: 10.1056/NEJM199607183350304. [DOI] [PubMed] [Google Scholar]
- 35.Kurtzberg J, Laughlin M, Graham ML, Smith C, Olson JF, Halperin EC, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. The New England journal of medicine. 1996;335(3):157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 36.Wagner JE, Kernan NA, Steinbuch M, Broxmeyer HE, Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995;346(8969):214–9. doi: 10.1016/s0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- 37.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90(12):4665–78. [PubMed] [Google Scholar]
- 38.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. The New England journal of medicine. 2001;344(24):1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. The New England journal of medicine. 2004;351(22):2265–75. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 40.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 41.Kelly SS, Sola CB, de Lima M, Shpall E. Ex vivo expansion of cord blood. Bone marrow transplantation. 2009;44(10):673–81. doi: 10.1038/bmt.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Galarza FF, Takeshita LY, Santos EJ, Kempson F, Maia MH, da Silva AL, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic acids research. 2015;43(Database issue):D784–8. doi: 10.1093/nar/gku1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis JM, Henson V, Slack R, Ng J, Hartzman RJ, Katovich Hurley C. Frequencies of HLA-A2 alleles in five U.S. population groups. Predominance Of A*02011 and identification of HLA-A*0231. Human immunology. 2000;61(3):334–40. doi: 10.1016/s0198-8859(99)00155-x. [DOI] [PubMed] [Google Scholar]
- 44.Cao K, Hollenbach J, Shi X, Shi W, Chopek M, Fernandez-Vina MA. Analysis of the frequencies of HLA-A B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Human immunology. 2001;62(9):1009–30. doi: 10.1016/s0198-8859(01)00298-1. [DOI] [PubMed] [Google Scholar]
- 45.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(10):1112–21. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lund TC, Boitano AE, Delaney CS, Shpall EJ, Wagner JE. Advances in umbilical cord blood manipulation-from niche to bedside. Nature reviews Clinical oncology. 2015;12(3):163–74. doi: 10.1038/nrclinonc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]